Regulation of Matrix Metalloproteinases by Wine-Derived Compounds: Implications for Cancer Therapy
Abstract
1. Introduction
2. Role of Wine-Derived Compounds for MMP Regulation in Cancer Pathology
2.1. Progress of Metastasis by ECM Degradation
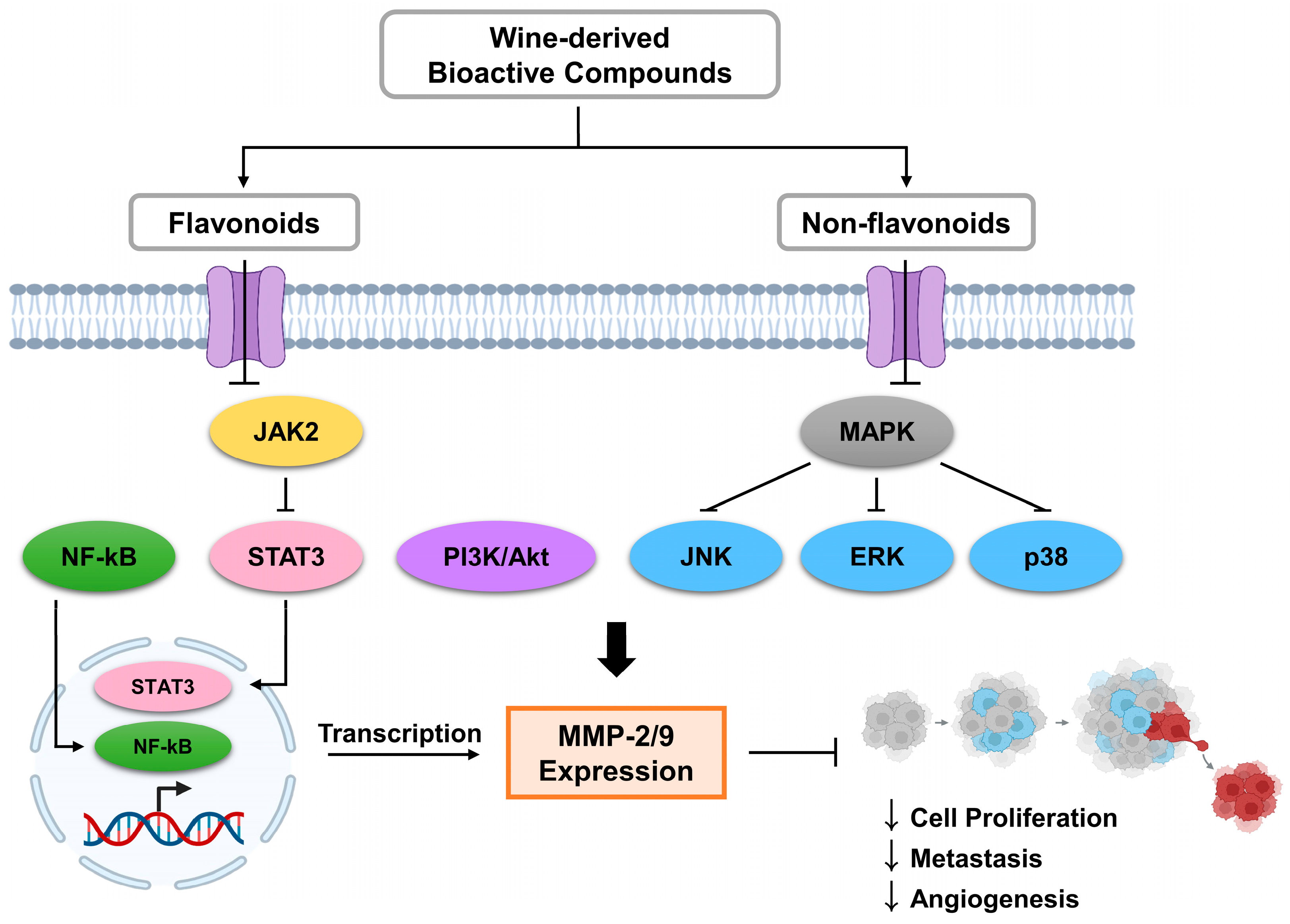
2.2. Wine Compounds as Signaling Pathway Inhibitors
2.3. Anti-Cancer Effects of Wine
3. Bioactive Compounds in Wine as Regulators of MMP-2 and MMP-9
3.1. Flavonoids
3.2. Non-Flavonoids
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MMP | Matrix metalloproteinase |
| HDL | High-density lipoprotein |
| ECM | Extracellular matrix |
| GSE | Grape seed extract |
| MAPK | Mitogen-activated protein kinase |
| ERK | Extracellular signal-regulated kinase |
| CoA | Coenzyme A |
| NF-κB | Nuclear factor-kappa B |
| NSCLC | Non-small cell lung cancer |
| COX-2 | Cyclooxygenase-2 |
| TNF-α | Tumor necrosis factor |
| TIMP-1 | Tissue inhibitor of metalloproteinase-1 |
| TIMP-2 | Tissue inhibitor of metalloproteinase-2 |
| VEGF | Vascular endothelial growth factor |
| SCI | Spinal cord injury |
References
- Rumgay, H.; Shield, K.; Charvat, H.; Ferrari, P.; Sornpaisarn, B.; Obot, I.; Islami, F.; Lemmens, V.E.P.P.; Rehm, J.; Soerjomataram, I. Global Burden of Cancer in 2020 Attributable to Alcohol Consumption: A Population-Based Study. Lancet. Oncol. 2021, 22, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Rumgay, H.; Murphy, N.; Ferrari, P.; Soerjomataram, I. Alcohol and Cancer: Epidemiology and Biological Mechanisms. Nutrients 2021, 13, 3173. [Google Scholar] [CrossRef]
- Pflaum, T.; Hausler, T.; Baumung, C.; Ackermann, S.; Kuballa, T.; Rehm, J.; Lachenmeier, D.W. Carcinogenic Compounds in Alcoholic Beverages: An Update. Arch. Toxicol. 2016, 90, 2349–2367. [Google Scholar] [CrossRef]
- Korn, A.R.; Reedy, J.; Brockton, N.T.; Kahle, L.L.; Mitrou, P.; Shams-White, M.M. The 2018 World Cancer Research Fund/American Institute for Cancer Research Score and Cancer Risk: A Longitudinal Analysis in the NIH-AARP Diet and Health Study. Cancer Epidemiol. Biomark. Prev. 2022, 31, 1983–1992. [Google Scholar] [CrossRef]
- Rehm, J.; Shield, K.D.; Weiderpass, E. Alcohol Consumption. A Leading Risk Factor for Cancer. Chem. Biol. Interact. 2020, 331, 109280. [Google Scholar] [CrossRef] [PubMed]
- Buljeta, I.; Pichler, A.; Šimunović, J.; Kopjar, M. Beneficial Effects of Red Wine Polyphenols on Human Health: Comprehensive Review. Curr. Issues Mol. Biol. 2023, 45, 782–798. [Google Scholar] [CrossRef] [PubMed]
- Santos-Buelga, C.; González-Manzano, S.; González-Paramás, A.M. Wine, Polyphenols, and Mediterranean Diets. What Else Is There to Say? Molecules 2021, 26, 5537. [Google Scholar] [CrossRef]
- Karunarathna, I.; De Alvis, K.; Gunasena, P.; Jayawardana, A. Unraveling the Complex Relationship between Alcohol and Cancer Risk: Effects of Alcohol on Health. 2024. Available online: https://www.researchgate.net/publication/382801225_Unraveling_the_Complex_Relationship_Between_Alcohol_and_Cancer_Risk_Effects_of_Alcohol_on_Health (accessed on 20 May 2025). [CrossRef]
- Arranz, S.; Chiva-Blanch, G.; Valderas-Martínez, P.; Medina-Remón, A.; Lamuela-Raventós, R.M.; Estruch, R. Wine, Beer, Alcohol and Polyphenols on Cardiovascular Disease and Cancer. Nutrients 2012, 4, 759–781. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, Y. Absorption and metabolism of red wine polyphenols and their potential health benefits in cardiovascular function. Am. J. Clin. Nutr. 2012, 95, 1496–1497. [Google Scholar] [CrossRef]
- Miraldi, E.; Baini, G.; Biagi, M.; Cappellucci, G.; Giordano, A.; Vaccaro, F.; Bertelli, A.A.E. Wine, Polyphenols, and the Matrix Effect: Is Alcohol Always the Same? Int. J. Mol. Sci. 2024, 25, 9796. [Google Scholar] [CrossRef]
- Anderson, L.A.; Cantwell, M.M.; Watson, R.G.P.; Johnston, B.T.; Murphy, S.J.; Ferguson, H.R.; McGuigan, J.; Comber, H.; Reynolds, J.V.; Murray, L.J. The Association Between Alcohol and Reflux Esophagitis, Barrett’s Esophagus, and Esophageal Adenocarcinoma. Gastroenterology 2009, 136, 799–805. [Google Scholar] [CrossRef]
- Cháirez-Ramírez, M.H.; de la Cruz-López, K.G.; García-Carrancá, A. Polyphenols as Antitumor Agents Targeting Key Players in Cancer-Driving Signaling Pathways. Front. Pharmacol. 2021, 12, 710304. [Google Scholar] [CrossRef]
- Postigo, V.; García, M.; Crespo, J.; Canonico, L.; Comitini, F.; Ciani, M. Bioactive Properties of Fermented Beverages: Wine and Beer. Fermentation 2025, 11, 234. [Google Scholar] [CrossRef]
- Clarke, S.; Bosman, G.; du Toit, W.; Aleixandre-Tudo, J.L. White wine phenolics: Current methods of analysis. J. Sci. Food Agric. 2023, 103, 7–25. [Google Scholar] [CrossRef]
- Reboredo-Rodríguez, P.; González-Barreiro, C.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gándara, J. Effects of sugar concentration processes in grapes and wine aging on aroma compounds of sweet wines—A review. Crit. Rev. Food Sci. Nutr. 2025, 55, 1053–1073. [Google Scholar] [CrossRef]
- Figueiredo-González, M.; Cancho-Grande, B.; Simal-Gándara, J. Effects on colour and phenolic composition of sugar concentration processes in dried-on-or dried-off-vine grapes and their aged or not natural sweet wines. Trends Food Sci. Technol. 2013, 31, 36–54. [Google Scholar] [CrossRef]
- Farhan, M.; Rizvi, A. The Pharmacological Properties of Red Grape Polyphenol Resveratrol: Clinical Trials and Obstacles in Drug Development. Nutrition 2023, 15, 4486. [Google Scholar] [CrossRef]
- Foroughi Gilvaee, M.; Martirosyan, D.; Mashayekhnia, M.; Maadi, M.; Sarvendani, M.; Maghsoumi, M. Exploring the Potential of Bioactive Compounds in Preventing Cancer Growth and Progression: A Comprehensive Review. Bioact. Compd. Health Dis. 2024, 7, 302–324. [Google Scholar] [CrossRef]
- Neveu, V.; Perez-Jimenez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef] [PubMed]
- El Rayess, Y.; Nehme, N.; Azzi Achkouty, S.; Julien, S.G. Wine Phenolic Compounds: Chemistry, Functionality and Health Benefits. Antioxidants 2024, 13, 1312. [Google Scholar] [CrossRef]
- Ragusa, A.; Centonze, C.; Grasso, M.; Latronico, M.; Mastrangelo, P.; Sparascio, F.; Fanizzi, F.; Maffia, M.A. Comparative Study of Phenols in Apulian Italian Wines. Foods 2017, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Rastija, V.; Srečnik, G.; Marica-Medi’c-Šari’c. Polyphenolic Composition of Croatian Wines with Different Geographical Origins. Food Chem. 2009, 115, 54–60. [Google Scholar] [CrossRef]
- Beara, I.N.; Torović, L.D.; Pintać, D.Đ.; Majkić, T.M.; Orčić, D.Z.; Mimica-Dukić, N.M.; Lesjak, M.M. Polyphenolic Profile, Antioxidant and Neuroprotective Potency of Grape Juices and Wines from Fruška Gora Region (Serbia). Int. J. Food Prop. 2017, 20, S2552–S2568. [Google Scholar] [CrossRef]
- Luki’c, I.; Radeka, S.; Budi’c Leto, I.; Bubola, M.; Vrhovsek, U. Targeted UPLC-QqQ-MS/MS Profiling of Phenolic Compounds for Differentiation of Monovarietal Wines and Corroboration of Particular Varietal Typicity Concepts. Food Chem. 2019, 300, 125251. [Google Scholar] [CrossRef]
- Khan, A.; Khan, A.; Khan, M.A.; Malik, Z.; Massey, S.; Parveen, R.; Mustafa, S.; Shamsi, A.; Husain, S.A. Phytocompounds targeting epigenetic modulations: An assessment in cancer. Front. Pharmacol. 2024, 14, 1273993. [Google Scholar] [CrossRef]
- Flanzy, C. Oenologie: Fondements Scientifiques et Technologiques; Collection Sciences et Techniques Agroalimentaires; Tec and DocLavoisier: Paris, France, 1998. [Google Scholar]
- Waterhouse, A.L. Wine Phenolics. Ann. N. Y. Acad. Sci. 2002, 957, 21–36. [Google Scholar] [CrossRef]
- Evers, M.S.; Roullier-Gall, C.; Morge, C.; Sparrow, C.; Gobert, A.; Alexandre, H. Vitamins in Wine: Which, What for, and How Much? Compr. Rev. Food Sci. Food Saf. 2021, 20, 2991–3035. [Google Scholar] [CrossRef]
- Ribereau-Gayon, J.; Peynaud, E.; Ribereau-Gayon, P.; Sudraud, P. Caractertes des Vins. Maturation du Raisin. Levures et Bacteries. Traite d’Oenologie. Sci. Et Tech. De Vin 1975, 2, 87. [Google Scholar]
- Moreno, J.; Peinado, R. Enological Chemistry; Academic Press: New York, NY, USA, 2012. [Google Scholar]
- Castor, J.G.B. The B-complex vitamins of musts and wines as microbial growth factors. Appl. Microbiol. 1953, 1, 97–102. [Google Scholar] [CrossRef]
- Abdel-Hamid, N.M.; Abass, S.A. Matrix Metalloproteinase Contribution in Management of Cancer Proliferation, Metastasis and Drug Targeting. Mol. Biol. Rep. 2021, 48, 6525–6538. [Google Scholar] [CrossRef]
- Shoari, A. Potential of MMP-2 and MMP-9 Gelatinase Blockade as a Therapeutic Strategy in Fibrosarcoma Treatment: A Decadal Review. Targets 2024, 2, 104–125. [Google Scholar] [CrossRef]
- Verma, R.P.; Hansch, C. Matrix Metalloproteinases (MMPs): Chemical–Biological Functions and (Q)SARs. Bioorg. Med. Chem. 2007, 15, 2223–2268. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.B.; Nair, B.G.; Perry, J.J.P.; Martin, D.B.C. Recent Insights into Natural Product Inhibitors of Matrix Metalloproteinases. Medchemcomm 2019, 10, 2024–2037. [Google Scholar] [CrossRef]
- Lenci, E.; Cosottini, L.; Trabocchi, A. Novel Matrix Metalloproteinase Inhibitors: An Updated Patent Review (2014–2020). Expert. Opin. Ther. Pat. 2021, 31, 509–523. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Ding, D.; Wolter, W.R.; Pérez, R.L.; Champion, M.M.; Mahasenan, K.V.; Hesek, D.; Lee, M.; Schroeder, V.A.; Jones, J.I.; et al. Validation of Matrix Metalloproteinase-9 (MMP-9) as a Novel Target for Treatment of Diabetic Foot Ulcers in Humans and Discovery of a Potent and Selective Small-Molecule MMP-9 Inhibitor That Accelerates Healing. J. Med. Chem. 2018, 61, 8825–8837. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.K.; Jeong, K.C.; Kim, Y.K.; Jung, S.T. Role of Matrix Metalloproteinase (MMP) 2 and MMP-9 in Soft Tissue Sarcoma. Clin. Orthop. Surg. 2014, 6, 443. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef]
- Li, H.; Qiu, Z.; Li, F.; Wang, C. The Relationship between MMP-2 and MMP-9 Expression Levels with Breast Cancer Incidence and Prognosis. Oncol. Lett. 2017, 14, 5865–5870. [Google Scholar] [CrossRef]
- Mannello, F.; Tonti, G.; Papa, S. Matrix Metalloproteinase Inhibitors as Anticancer Therapeutics. Curr. Cancer Drug Targets 2005, 5, 285–298. [Google Scholar] [CrossRef]
- Mustafa, S.; Koran, S.; Alomair, L. Insights Into the Role of Matrix Metalloproteinases in Cancer and Its Various Therapeutic Aspects: A Review. Front. Mol. Biosci. 2022, 9, 896099. [Google Scholar] [CrossRef]
- Di Martino, J.S.; Akhter, T.; Bravo-Cordero, J.J. Remodeling the ECM: Implications for Metastasis and Tumor Dormancy. Cancers 2021, 13, 4916. [Google Scholar] [CrossRef] [PubMed]
- Winer, A.; Adams, S.; Mignatti, P. Matrix Metalloproteinase Inhibitors in Cancer Therapy: Turning Past Failures Into Future Successes. Mol. Cancer Ther. 2018, 17, 1147–1155. [Google Scholar] [CrossRef]
- Edelman, E.J.; Fiellin, D.A. Alcohol Use. Ann. Intern. Med. 2016, 165, 379. [Google Scholar] [CrossRef] [PubMed]
- Teissedre, P.-L.; Rasines-Perea, Z.; Ruf, J.C.; Stockley, C.; Antoce, A.O.; Romano, R.; Fradera, U.; Kosti, R.I. Effects of Alcohol Consumption in General, and Wine in Particular, on the Risk of Cancer Development: A Review. OENO One 2020, 54, 813–832. [Google Scholar] [CrossRef]
- Shoari, A.; Ashja Ardalan, A.; Dimesa, A.M.; Coban, M.A. Targeting Invasion: The Role of MMP-2 and MMP-9 Inhibition in Colorectal Cancer Therapy. Biomolecules 2025, 15, 35. [Google Scholar] [CrossRef] [PubMed]
- Laronha, H.; Caldeira, J. Structure and Function of Human Matrix Metalloproteinases. Cells 2020, 9, 1076. [Google Scholar] [CrossRef]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef]
- Mlynarczyk, G.; Gudowska-Sawczuk, M.; Mroczko, B.; Bruczko-Goralewska, M.; Romanowicz, L.; Tokarzewicz, A. Higher Content but No Specific Activity in Gelatinase B (MMP-9) Compared with Gelatinase A (MMP-2) in Human Renal Carcinoma. Cancers 2023, 15, 5475. [Google Scholar] [CrossRef]
- Jabło’nska-Trypu’c, A.; Matejczyk, M.; Rosochacki, S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J. Enzym. Inhib. Med. Chem. 2016, 31, 177–183. [Google Scholar] [CrossRef]
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argáez, V.; Lara-Riegos, J.; Ramírez-Camacho, M.A.; Alvarez-Sánchez, M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef]
- Szczygielski, O.; Dąbrowska, E.; Niemyjska, S.; Przylipiak, A.; Zajkowska, M. Targeting Matrix Metalloproteinases and Their Inhibitors in Melanoma. Int. J. Mol. Sci. 2024, 25, 13558. [Google Scholar] [CrossRef] [PubMed]
- Hedayati, N.; Safari, M.H.; Milasi, Y.E.; Kahkesh, S.; Farahani, N.; Khoshnazar, S.M.; Dorostgou, Z.; Alaei, E.; Alimohammadi, M.; Rahimzadeh, P.; et al. Modulation of the PI3K/Akt signaling pathway by resveratrol in cancer: Molecular mechanisms and therapeutic opportunity. Discov. Oncol. 2025, 16, 669. [Google Scholar] [CrossRef] [PubMed]
- Pavitra, E.; Kancharla, J.; Gupta, V.K.; Prasad, K.; Sung, J.Y.; Kim, J.; Huh, Y.S. The role of NF-κB in breast cancer initiation, growth, metastasis, and resistance to chemotherapy. Biomed. Pharmacother. 2023, 163, 114822. [Google Scholar] [CrossRef] [PubMed]
- Stefani, C.; Miricescu, D.; Stanescu-Spinu, I.I.; Nica, R.I.; Greabu, M.; Totan, A.R.; Jinga, M. Growth factors, PI3K/AKT/mTOR and MAPK signaling pathways in colorectal cancer pathogenesis: Where are we now? Int. J. Mol. Sci. 2021, 22, 10260. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef]
- Shi, A.; Liu, L.; Li, S.; Qi, B. Natural products targeting the MAPK-signaling pathway in cancer: Overview. Cancer Res. Clin. Oncol. 2024, 150, 6. [Google Scholar] [CrossRef]
- Suzuki, T.; Ohishi, T.; Tanabe, H.; Miyoshi, N.; Nakamura, Y. Anti-inflammatory effects of dietary polyphenols through inhibitory activity against metalloproteinases. Molecules 2023, 28, 5426. [Google Scholar] [CrossRef]
- Zhan, X.Z.; Bo, Y.W.; Zhang, Y.; Zhang, H.D.; Shang, Z.H.; Yu, H.; Chen, X.L.; Kong, X.T.; Zhao, W.Z.; Teimonen, T.; et al. Luteolin inhibits diffuse large B-cell lymphoma cell growth through the JAK2/STAT3 signaling pathway. Front. Pharmacol. 2025, 16, 1545779. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Wang, W.W.; Huang, J.; Zhu, W.G. Ellagic acid induces esophageal squamous cell carcinoma cell apoptosis by modulating SHP-1/STAT3 signaling. Kaohsiung J. Med. Sci. 2020, 36, 699–704. [Google Scholar] [CrossRef]
- Zughaibi, T.A.; Suhail, M.; Tarique, M.; Tabrez, S. Targeting PI3K/Akt/mTOR pathway by different flavonoids: A cancer chemopreventive approach. Int. J. Mol. Sci. 2021, 22, 12455. [Google Scholar] [CrossRef]
- Ferraz da Costa, D.C.; Pereira Rangel, L.; Quarti, J.; Santos, R.A.; Silva, J.L.; Fialho, E. Bioactive Compounds and Metabolites from Grapes and Red Wine in Breast Cancer Chemoprevention and Therapy. Molecules 2020, 25, 3531. [Google Scholar] [CrossRef] [PubMed]
- Majrashi, T.A.; Alshehri, S.A.; Alsayari, A.; Muhsinah, A.B.; Alrouji, M.; Alshahrani, A.M.; Shamsi, A.; Atiya, A. Insight into the biological roles and mechanisms of phytochemicals in different types of cancer: Targeting cancer therapeutics. Nutrients 2023, 15, 1704. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Mao, Y.; Chen, H.; Lin, Y.; Hu, Z.; Wu, J.; Xu, X.; Xu, X.; Qin, J.; Xie, L. Apigenin promotes apoptosis, inhibits invasion and induces cell cycle arrest of T24 human bladder cancer cells. Cancer Cell Int. 2013, 13, 54. [Google Scholar] [CrossRef] [PubMed]
- Amor, S.; Châlons, P.; Aires, V.; Delmas, D. Polyphenol Extracts from Red Wine and Grapevine: Potential Effects on Cancers. Diseases 2018, 6, 106. [Google Scholar] [CrossRef] [PubMed]
- Dinicola, S.; Pasqualato, A.; Cucina, A.; Coluccia, P.; Ferranti, F.; Canipari, R.; Catizone, A.; Proietti, S.; D’Anselmi, F.; Ricci, G.; et al. Grape Seed Extract Suppresses MDA-MB231 Breast Cancer Cell Migration and Invasion. Eur. J. Nutr. 2014, 53, 421–431. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Ran, Z.; Hou, L.; Guo, H.; Wang, K.; Li, X. Expression of VEGF, COX-2 and MMP-9 in breast cancer and their relationship with ultrasound findings. Int. J. Clin. Exp. Pathol. 2018, 11, 4264–4269. [Google Scholar]
- Islam, M.d.T.; Jang, N.H.; Lee, H.J. Natural Products as Regulators against Matrix Metalloproteinases for the Treatment of Cancer. Biomedicines 2024, 12, 794. [Google Scholar] [CrossRef]
- Rollin, J.; Régina, S.; Vourc’h, P.; Iochmann, S.; Bléchet, C.; Reverdiau, P.; Gruel, Y. Influence of MMP-2 and MMP-9 promoter polymorphisms on gene expression and clinical outcome of non-small cell lung cancer. Lung Cancer. 2007, 56, 273–280. [Google Scholar] [CrossRef]
- Pawłowski, W.; Caban, M.; Lewandowska, U. Cancer Prevention and Treatment with Polyphenols: Type IV Collagenase-Mediated Mechanisms. Cancers 2024, 16, 3193. [Google Scholar] [CrossRef]
- Liskova, A.; Samec, M.; Koklesova, L.; Samuel, S.M.; Zhai, K.; Al-Ishaq, R.K.; Abotaleb, M.; Nosal, V.; Kajo, K.; Ashrafizadeh, M.; et al. Flavonoids against the SARS-CoV-2 Induced Inflammatory Storm. Biomed. Pharmacother. 2021, 138, 111430. [Google Scholar] [CrossRef] [PubMed]
- Bonfili, L.; Cecarini, V.; Amici, M.; Cuccioloni, M.; Angeletti, M.; Keller, J.N.; Eleuteri, A.M. Natural Polyphenols as Proteasome Modulators and Their Role as Anti-cancer Compounds. FEBS J. 2008, 275, 5512–5526. [Google Scholar] [CrossRef]
- Hazafa, A.; Rehman, K.U.; Jahan, N.; Jabeen, Z. The Role of Polyphenol (Flavonoids) Compounds in the Treatment of Cancer Cells. Nutr. Cancer 2020, 72, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Tait, S.; Salvati, A.L.; Desideri, N.; Fiore, L. Antiviral Activity of Substituted Homoisoflavonoids on Enteroviruses. Antiviral Res. 2006, 72, 252–255. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Kim, J.K.; Park, S.U. Quercetin and Its Role in Biological Functions: An Updated Review. EXCLI J. 2018, 17, 856–863. [Google Scholar] [PubMed]
- Tang, S.M.; Deng, X.T.; Zhou, J.; Li, Q.P.; Ge, X.X.; Miao, L. Pharmacological Basis and New Insights of Quercetin Action in Respect to Its Anti-Cancer Effects. Biomed. Pharmacother. 2020, 121, 109604. [Google Scholar] [CrossRef]
- Asgharian, P.; Tazekand, A.P.; Hosseini, K.; Forouhandeh, H.; Ghasemnejad, T.; Ranjbar, M.; Hasan, M.; Kumar, M.; Beirami, S.M.; Tarhriz, V.; et al. Potential Mechanisms of Quercetin in Cancer Prevention: Focus on Cellular and Molecular Targets. Cancer Cell. Int. 2022, 22, 257. [Google Scholar] [CrossRef]
- Tang, H.; Kuang, Y.; Wu, W.; Peng, B.; Fu, Q. Quercetin Inhibits the Metabolism of Arachidonic Acid by Inhibiting the Activity of CYP3A4, Thereby Inhibiting the Progression of Breast Cancer. Mol. Med. 2023, 29, 127. [Google Scholar] [CrossRef]
- Jia, L.; Huang, S.; Yin, X.; Zan, Y.; Guo, Y.; Han, L. Quercetin Suppresses the Mobility of Breast Cancer by Suppressing Glycolysis through Akt-MTOR Pathway Mediated Autophagy Induction. Life Sci. 2018, 208, 123–130. [Google Scholar] [CrossRef]
- Rajendran, P. Unveiling the Power of Flavonoids: A Dynamic Exploration of Their Impact on Cancer through Matrix Metalloproteinases Regulation. BioMedicine. Pharmacother. 2024, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Chien, H.; Wang, K.; Chang, Y.; Hsieh, Y.; Yu, N.; Yang, S.; Lin, H. Kaempferol Suppresses Cell Migration through the Activation of the ERK Signaling Pathways in ARPE-19 Cells. Environ. Toxicol. 2019, 34, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; An, Y.; He, X.; Zhang, D.; He, W. Protection of Kaempferol on Oxidative Stress-Induced Retinal Pigment Epithelial Cell Damage. Oxid. Med. Cell. Longev. 2018, 2018, 1610751. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Liu, J.; Du, R.; Yu, Q.; Gong, L.; Jiang, H.; Rong, R. Qualitative and Quantitative Analysis for the Chemical Constituents of Tetrastigma Hemsleyanum Diels et Gilg Using Ultra-High Performance Liquid Chromatography/Hybrid Quadrupole-Orbitrap Mass Spectrometry and Preliminary Screening for Anti-Influenza Virus Components. J. Evid. Based Complement. Altern. Med. 2019, 2019, 1–14. [Google Scholar]
- Costa, F.F. Non-coding RNAs: New players in eukaryotic biology. Gene 2015, 357, 83–94. [Google Scholar] [CrossRef]
- Lin, C.W.; Chen, P.N.; Chen, M.K.; Yang, W.E.; Tang, C.H.; Yang, S.F.; Hsieh, Y.S. Kaempferol Reduces Matrix Metalloproteinase-2 Expression by Down-Regulating ERK1/2 and the Activator Protein-1 Signaling Pathways in Oral Cancer Cells. PLoS ONE 2013, 8, e80883. [Google Scholar] [CrossRef]
- Ju, P.; Ho, Y.; Chen, P.; Lee, H.; Lai, S.; Yang, S.; Yeh, C. Kaempferol Inhibits the Cell Migration of Human Hepatocellular Carcinoma Cells by Suppressing MMP-9 and Akt Signaling. Environ. Toxicol. 2021, 36, 1981–1989. [Google Scholar] [CrossRef]
- Rahmani, A.; Almatroudi, A.; Allemailem, K.; Alwanian, W.; Alharbi, B.; Alrumaihi, F.; Khan, A.; Almatroodi, S. Myricetin: A Significant Emphasis on Its Anticancer Potential via the Modulation of Inflammation and Signal Transduction Pathways. Int. J. Mol. Sci. 2023, 24, 9665. [Google Scholar] [CrossRef]
- Barzegar, A. Antioxidant Activity of Polyphenolic Myricetin in Vitro Cell- Free and Cell-Based Systems. Mol. Biol. Res. Commun. 2016, 5, 87–95. [Google Scholar]
- Grenier, D.; Chen, H.; Ben Lagha, A.; Fournier-Larente, J.; Morin, M.-P. Dual Action of Myricetin on Porphyromonas Gingivalis and the Inflammatory Response of Host Cells: A Promising Therapeutic Molecule for Periodontal Diseases. PLoS ONE 2015, 10, e0131758. [Google Scholar] [CrossRef]
- Ci, Y.; Zhang, Y.; Liu, Y.; Lu, S.; Cao, J.; Li, H.; Zhang, J.; Huang, Z.; Zhu, X.; Gao, J.; et al. Myricetin Suppresses Breast Cancer Metastasis through Down-regulating the Activity of Matrix Metalloproteinase (MMP)-2/9. Phytother. Res. 2018, 32, 1373–1381. [Google Scholar] [CrossRef]
- Shimoi, K.; Okada, H.; Furugori, M.; Goda, T.; Takase, S.; Suzuki, M.; Hara, Y.; Yamamoto, H.; Kinae, N. Intestinal Absorption of Luteolin and Luteolin 7-O. -β-glucoside in Rats and Humans. FEBS Lett. 1998, 438, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Formica, J.V.; Regelson, W. Review of the Biology of Quercetin and Related Bioflavonoids. Food Chem. Toxicol. 1995, 33, 1061–1080. [Google Scholar] [CrossRef]
- Çelik, M.; Ünal, F.; Yüzbaşıoğlu, D.; Yılmaz, S.; Aksoy, H.; Karaman, Ş. Effects of Thymus Kotschyanus Var. Glabrescens Boiss. Extract on Mitomycin-C Induced Chromosomal Aberrations and Sister Chromatid Exchanges in Human Lymphocytes. Cytotechnology 2006, 51, 99–104. [Google Scholar] [CrossRef]
- Yamamoto, H.; Sakakibara, J.; Nagatsu, A.; Sekiya, K. Inhibitors of Arachidonate Lipoxygenase from Defatted Perilla Seed. J. Agric. Food Chem. 1998, 46, 862–865. [Google Scholar] [CrossRef]
- Yao, X.; Jiang, W.; Yu, D.; Yan, Z. Luteolin Inhibits Proliferation and Induces Apoptosis of Human Melanoma Cells in Vivo and in Vitro by Suppressing MMP-2 and MMP-9 through the PI3K/AKT Pathway. Food Funct. 2019, 10, 703–712. [Google Scholar] [CrossRef]
- Duthie, G.; Crozier, A. Plant-derived phenolic antioxidants. Curr. Opin. Clin. Nutr. Metab. Care 2000, 3, 447–451. [Google Scholar] [CrossRef]
- Allemailem, K.S.; Almatroudi, A.; Alharbi, H.O.A.; AlSuhaymi, N.; Alsugoor, M.H.; Aldakheel, F.M.; Khan, A.A.; Rahmani, A.H. Apigenin: A bioflavonoid with a promising role in disease prevention and treatment. Biomedicines 2024, 12, 1353. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Qi, M.; Li, P.; Zhan, Y.; Shao, H. Apigenin in cancer therapy: Anti-cancer effects and mechanisms of action. Cell Biosci. 2017, 7, 50. [Google Scholar] [CrossRef]
- He, J.; Xu, Q.; Wang, M.; Li, C.; Qian, X.; Shi, Z.; Liu, L.Z.; Jiang, B.H. Oral administration of apigenin inhibits metastasis through AKT/P70S6K1/MMP-9 pathway in orthotopic ovarian tumor model. Int. J. Mol. Sci. 2012, 13, 7271–7282. [Google Scholar] [CrossRef]
- Tanabe, H.; Suzuki, T.; Ohishi, T.; Isemura, M.; Nakamura, Y.; Unno, K. Effects of Epigallocatechin-3-Gallate on Matrix Metalloproteinases in Terms of Its Anticancer Activity. Molecules 2023, 28, 525. [Google Scholar] [CrossRef]
- Jung, H.A.; Jung, M.J.; Kim, J.Y.; Chung, H.Y.; Choi, J.S. Inhibitory Activity of Flavonoids FromPrunus Davidiana and Other Flavonoids on Total ROS and Hydroxyl Radical Generation. Arch. Pharm. Res. 2003, 26, 809–815. [Google Scholar] [CrossRef]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and Beyond. Am. J. Clin. Nutr. 2005, 81, 215S–217S. [Google Scholar] [CrossRef]
- Papież, M.A.; Baran, J.; Bukowska-Straková, K.; Wiczkowski, W. Antileukemic Action of (−)-Epicatechin in the Spleen of Rats with Acute Myeloid Leukemia. Food Chem. Toxicol. 2010, 48, 3391–3397. [Google Scholar] [CrossRef]
- Wu, A.; He, Y.; Zhou, H.; Huang, N.; Xu, H.; Xia, J.; Zengbo, L.; Huang, M. Downregulation of MMP-9 by Epicatechin Can Improve the Radiosensitivity of Non-Small Cell Lung Cancer. J. Cancer Res. Ther. 2024, 20, 1284–1292. [Google Scholar] [CrossRef]
- Ramalho, S.A.; Gualberto, N.C.; Neta, M.T.S.L.; Batista, R.A.; Araújo, S.M.; Moreira, J.d.J.d.S.; Narain, N. Catechin and Epicatechin Contents in Wines Obtained from Brazilian Exotic Tropical Fruits. Food Nutr. Sci. 2014, 5, 449–457. [Google Scholar] [CrossRef][Green Version]
- Yang, C.; Wang, H. Cancer Preventive Activities of Tea Catechins. Molecules 2016, 21, 1679. [Google Scholar] [CrossRef]
- Demeule, M.; Brossard, M.; Pagé, M.; Gingras, D.; Béliveau, R. Matrix Metalloproteinase Inhibition by Green Tea Catechins. Biochim. Biophys. Acta. 2000, 1478, 51–60. [Google Scholar] [CrossRef]
- Sazuka, M.; Imazawa, H.; Shoji, Y.; Mita, T.; Hara, Y.; Isemura, M. Inhibition of Collagenases from Mouse Lung Carcinoma Cells by Green Tea Catechins and Black Tea Theaflavins. Biosci. Biotechnol. Biochem. 1997, 61, 1504–1506. [Google Scholar] [CrossRef]
- Huang, H.S.; Liaw, E.T. Extraction optimization of flavonoids from Hypericum formosanum and matrix metalloproteinase-1 inhibitory activity. Molecules 2017, 22, E2172. [Google Scholar] [CrossRef]
- Meng, Q.-F.; Zhang, Z.; Wang, Y.-J.; Chen, W.; Li, F.-F.; Yue, L.-T.; Zhang, C.-J.; Li, H.; Zhang, M.; Wang, C.-C.; et al. Astilbin ameliorates experimental autoimmune myasthenia gravis by decreased Th17 cytokines and up-regulated T regulatory cells. J. Neuroimmunol. 2016, 298, 138–145. [Google Scholar] [CrossRef]
- Yu, J.; Xiao, Z.; Zhao, R.; Lu, C.; Zhang, Y. Astilbin emulsion improves guinea pig lesions in a psoriasis-like model by suppressing IL-6 and IL-22 via p38 MAPK. Mol. Med. Rep. 2017, 17, 3789–3796. [Google Scholar] [CrossRef]
- Chen, F.; Zhu, X.; Sun, Z.; Ma, Y. Astilbin Inhibits High Glucose-Induced Inflammation and Extracellular Matrix Accumulation by Suppressing the TLR4/MyD88/NF-κB Pathway in Rat Glomerular Mesangial Cells. Front. Pharmacol. 2018, 9, 1187. [Google Scholar] [CrossRef]
- Shubina, V.S.; Shatalin, Y. V Antioxidant and Iron-Chelating Properties of Taxifolin and Its Condensation Product with Glyoxylic Acid. J. Food Sci. Technol. 2017, 54, 1467–1475. [Google Scholar] [CrossRef]
- El-Nashar, H.A.S.; Aly, S.H.; Ahmadi, A.; El-Shazly, M. The Impact of Polyphenolics in the Management of Breast Cancer: Mechanistic Aspects and Recent Patents. Recent. Pat. Anticancer. Drug Discov. 2022, 17, 358–379. [Google Scholar] [CrossRef]
- Xie, J.; Pang, Y.; Wu, X. Taxifolin Suppresses the Malignant Progression of Gastric Cancer by Regulating the AhR/CYP1A1 Signaling Pathway. Int. J. Mol. Med. 2021, 48, 197. [Google Scholar] [CrossRef]
- Garrido, J.; Borges, F. Wine and Grape Polyphenols: A Chemical Perspective. Food Res. Int. 2013, 54, 1844–1858. [Google Scholar] [CrossRef]
- Spagnuolo, C.; Russo, G.L.; Orhan, I.E.; Habtemariam, S.; Daglia, M.; Sureda, A.; Nabavi, S.F.; Devi, K.P.; Loizzo, M.R.; Tundis, R.; et al. Genistein and Cancer: Current Status, Challenges, and Future Directions. Adv. Nutr. 2015, 6, 408–419. [Google Scholar] [CrossRef]
- Rahman Mazumder, M.d.A.; Hongsprabhas, P. Genistein as Antioxidant and Antibrowning Agents in in Vivo and in Vitro: A Review. Biomed. Pharmacother. 2016, 82, 379–392. [Google Scholar] [CrossRef]
- Yuliawati, D.; Mintaroem, K.; Sutrisno, S. Inhibitory Effect of Genistein on MMP-2 and MMP-9 Expression through Suppressing NF-kB Activity in Peritoneum of Murine Model of Endometriosis. Asian Pac. J. Reprod. 2018, 7, 261. [Google Scholar] [CrossRef]
- Stabrauskiene, J.; Kopustinskiene, D.M.; Lazauskas, R.; Bernatoniene, J. Naringin and Naringenin: Their Mechanisms of Action and the Potential Anticancer Activities. Biomedicines 2022, 10, 1686. [Google Scholar] [CrossRef]
- Aroui, S.; Aouey, B.; Chtourou, Y.; Meunier, A.C.; Fetoui, H.; Kenani, A. Naringin Suppresses Cell Metastasis and the Expression of Matrix Metalloproteinases (MMP-2 and MMP-9) via the Inhibition of ERK-P38-JNK Signaling Pathway in Human Glioblastoma. Chem. Biol. Interact. 2016, 244, 195–203. [Google Scholar] [CrossRef]
- Lian, G.Y.; Wang, Q.M.; Mak, T.S.K.; Huang, X.R.; Yu, X.Q.; Lan, H.-Y. Inhibition of Tumor Invasion and Metastasis by Targeting TGF-β-Smad-MMP2 Pathway with Asiatic Acid and Naringenin. Mol. Ther. Oncolytics 2021, 20, 277–289. [Google Scholar] [CrossRef]
- Esghaei, M.; Ghaffari, H.; Rahimi Esboei, B.; Ebrahimi Tapeh, Z.; Bokharaei Salim, F.; Motevalian, M. Evaluation of anticancer activity of camellia sinensis in the caco-2 colorectal cancer cell line. Asian Pac. J. Cancer Prev. 2018, 19, 1697–1701. [Google Scholar]
- Chen, Y.; Chang, Y.; Wang, K.; Chen, P.; Hseu, Y.; Chen, K.; Yeh, K.; Chen, C.; Hsu, L. Naringenin Inhibited Migration and Invasion of Glioblastoma Cells through Multiple Mechanisms. Environ. Toxicol. 2019, 34, 233–239. [Google Scholar] [CrossRef]
- Bao, L.; Liu, F.; Guo, H.; Li, Y.; Tan, B.; Zhang, W.; Peng, Y. Naringenin Inhibits Proliferation, Migration, and Invasion as Well as Induces Apoptosis of Gastric Cancer SGC7901 Cell Line by Downregulation of AKT Pathway. Tumour Biol. 2016, 37, 11365–11374. [Google Scholar] [CrossRef]
- Lee, E.J.; Oh, S.Y.; Sung, M.K. Luteolin exerts anti-tumor activity through the suppression of epidermal growth factor receptor-mediated pathway in MDAMB-231 ER-negative breast cancer cells. Food Chem. Toxicol. 2012, 50, 4136–4143. [Google Scholar] [CrossRef]
- Esmaeili, M.A.; Alilou, M. Naringenin attenuates CCl induced hepatic inflammation by the activation of Nrf2 mediated pathway in rats. Clin. Exp. Pharmacol. Physiol. 2014, 41, 416–422. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Kanno, S.; Shouji, A.; Tomizawa, A.; Hiura, T.; Osanai, Y.; Ujibe, M. Inhibitory effect of naringin on lipopolysaccharide (LPS)-induced endotoxin shock in mice and nitric oxide production in RAW 264.7 macrophages. Life Sci. 2006, 78, 673–681. [Google Scholar] [CrossRef]
- Chtourou, Y.; Aouey, B.; Kebieche, M.; Fetoui, H. Protective role of naringin against cisplatin induced oxidative stress, inflammatory response and apoptosis in rat striatum via suppressing ROS-mediated NF-kB and P53 signaling pathways. Chem. Biol. Interact. 2015, 239, 76–86. [Google Scholar] [CrossRef]
- Chtourou, Y.; Gargouri, B.; Kebieche, M.; Fetoui, H. Naringin abrogates cis-platin induced cognitive deficits and cholinergic dysfunction through the downregulation of AChE expression and iNOS signaling pathways in hippocampus of aged rats. J. Mol. Neurosci. 2015, 56, 349–362. [Google Scholar] [CrossRef]
- Kim, H.K.; Jeong, T.S.; Lee, M.K.; Park, Y.B.; Choi, M.S. Lipid-lowering efficacy of hesperetin metabolites in high-cholesterol fed rats. Clin. Chim. Acta. 2003, 327, 129–137. [Google Scholar] [CrossRef]
- Taheri, A.; Mobaser, S.E.; Golpour, P.; Nourbakhsh, M.; Tavakoli Yaraki, M.; Yarahmadi, S.; Nourbakhsh, M. Hesperetin attenuates the expression of markers of adipose tissue fibrosis in pre-adipocytes. BMC Complement. Med. Ther. 2023, 23, 315. [Google Scholar] [CrossRef]
- Ye, L.; Chan, F.L.; Chen, S.; Leung, L.K. The citrus flavonone hesperetin inhibits growth of aromatase-expressing MCF-7 tumor in ovariectomized athymic mice. J. Nutr. Biochem. 2012, 23, 1230–1237. [Google Scholar] [CrossRef]
- Li, F.; Ye, L.; Mei Lin, S.; Leung, L.K. Dietary flavones and flavonones display differential effects on aromatase (CYP19) transcription in the breast cancer cells MCF-7. Mol. Cell. Endocrinol. 2011, 344, 51–58. [Google Scholar] [CrossRef]
- Yunita, E.; Muflikhasari, H.A.; Ilmawati, G.P.N.; Meiyanto, E.; Hermawan, A. Hesperetin alleviates doxorubicin-induced migration in 4T1 breast cancer cells. Futur. J. Pharm. Sci. 2020, 6, 23. [Google Scholar] [CrossRef]
- Sohel, M.; Sultana, H.; Sultana, T.; Al Amin, M.; Aktar, S.; Ali, M.C.; Rahim, Z.B.; Hossain, M.A.; Al Mamun, A.; Amin, M.N.; et al. Chemotherapeutic potential of hesperetin for cancer treatment, with mechanistic insights: A comprehensive review. Heliyon 2022, 8, e08815. [Google Scholar] [CrossRef]
- Urminská, D.; Jedináková, N. Beer as A Source of Hop Prenylated Flavonoids, Compounds with Antioxidant, Chemoprotective and Phytoestrogen Activity. J. Microbiol. Biotechnol. Food Sci. 2021, 11, e4426. [Google Scholar] [CrossRef]
- Magalhães, P.J.; Carvalho, A.B.; Gonçalves, L.M.; Pacheco, J.G.; Guido, L.F.; Brányik, T.; Rodrigues, P.G.; Kuncová, G.; Dostálek, P.; Barros, A.A. The Impact of Xanthohumol on a Brewing Yeast’s Viability, Vitality and Metabolite Formation. J. Inst. Brew. 2011, 117, 368–376. [Google Scholar] [CrossRef]
- Girisa, S.; Saikia, Q.; Bordoloi, D.; Banik, K.; Monisha, J.; Daimary, U.D.; Verma, E.; Ahn, K.S.; Kunnumakkara, A.B. Xanthohumol from Hop: Hope for Cancer Prevention and Treatment. IUBMB Life 2021, 73, 1016–1044. [Google Scholar] [CrossRef]
- Pritchard, S.; Nicolson, M.; Lloret, C.; McKay, J.; Ross, V.; Kerr, K.; Murray, G.; McLeod, H. Expression of Matrix Metalloproteinases 1, 2, 9 and Their Tissue Inhibitors in Stage II Non-Small Cell Lung Cancer: Implications for MMP Inhibition Therapy. Oncol. Rep. 2001, 8, 421–424. [Google Scholar] [CrossRef]
- Cai, X.; Zhu, H.; Li, Y. PKCζ, MMP-2 and MMP-9 Expression in Lung Adenocarcinoma and Association with a Metastatic Phenotype. Mol. Med. Rep. 2017, 16, 8301–8306. [Google Scholar] [CrossRef]
- Sławińska-Brych, A.; Mizerska-Kowalska, M.; Król, S.K.; Stepulak, A.; Zdzisińska, B. Xanthohumol Impairs the PMA-Driven Invasive Behaviour of Lung Cancer Cell Line A549 and Exerts Anti-EMT Action. Cells 2021, 10, 1484. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, X.; Cao, Z.; Zhao, Z.; Zhang, Y. Isoxanthohumol Exerts Anticancer Activity against Drug-Resistant Thyroid Cancer Cells by Inhibiting Cell Migration and Invasion, Apoptosis Induction and Targeting PI3K/AKT/m-TOR Signaling Pathway. Trop. J. Pharm. Res. 2022, 20, 1151–1157. [Google Scholar] [CrossRef]
- Chojnacka, K.; Owczarek, K.; Caban, M.; Sosnowska, D.; Kajszczak, D.; Lewandowska, U. Chemoprotective Effects of Japanese Quince (Chaenomeles japonica L.) Phenol Leaf Extract on Colon Cancer Cells through the Modulation of Extracellular Signal-Regulated Kinases/AKT Signaling Pathway. J. Physiol. Pharmacol. 2022, 73, 41–52. [Google Scholar]
- Yue, S.J.; Zhang, P.X.; Zhu, Y.; Li, N.G.; Chen, Y.Y.; Li, J.J.; Zhang, S.; Jin, R.Y.; Yan, H.; Shi, X.Q.; et al. A Ferulic Acid Derivative FXS-3 Inhibits Proliferation and Metastasis of Human Lung Cancer A549 Cells via Positive JNK Signaling Pathway and Negative ERK/P38, AKT/MTOR and MEK/ERK Signaling Pathways. Molecules 2019, 24, 2165. [Google Scholar] [CrossRef]
- Caban, M.; Owczarek, K.; Podsedek, A.; Sosnowska, D.; Lewandowska, U. Spent Hops Extract (Humulus lupulus L.) Attenuates Inflammation and Angiogenesis of the Retina via the Nuclear Factor-KappaB and Protein Kinase B/Extracellular Signal-Regulated Kinase Pathways. J. Physiol. Pharmacol. 2023, 74, 537–549. [Google Scholar]
- Rentzsch, M.; Wilkens, A.; Winterhalter, P. Non-Flavonoid Phenolic Compounds. In Wine Chemistry and Biochemistry; Springer: New York, NY, USA; pp. 509–527.
- Niemetz, R.; Gross, G.G. Enzymology of Gallotannin and Ellagitannin Biosynthesis. Phytochemistry 2005, 66, 2001–2011. [Google Scholar] [CrossRef]
- Kim, S.H.; Jun, C.D.; Suk, K.; Choi, B.J.; Lim, H.; Park, S.; Lee, S.H.; Shin, H.Y.; Kim, D.-K.; Shin, T.Y. Gallic Acid Inhibits Histamine Release and Pro-Inflammatory Cytokine Production in Mast Cells. Toxicol. Sci. 2006, 91, 123–131. [Google Scholar] [CrossRef]
- Chen, Y.; Chang, L. Gallic Acid Downregulates Matrix Metalloproteinase-2 (MMP-2) and MMP-9 in Human Leukemia Cells with Expressed Bcr/Abl. Mol. Nutr. Food Res. 2012, 56, 1398–1412. [Google Scholar] [CrossRef] [PubMed]
- Meng, M. Gallic Acid Suppresses the Migration and Invasion of PC-3 Human Prostate Cancer Cells via Inhibition of Matrix Metalloproteinase-2 and -9 Signaling Pathways. Oncol. Rep. 2011, 26, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Talcott, S.T.; Lee, J.-H. Ellagic Acid and Flavonoid Antioxidant Content of Muscadine Wine and Juice. J. Agric. Food Chem. 2002, 50, 3186–3192. [Google Scholar] [CrossRef] [PubMed]
- Heber, D. Multitargeted Therapy of Cancer by Ellagitannins. Cancer Lett. 2008, 269, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, Z.Y.; Mo, S.L.; Loo, T.Y.; Wang, D.M.; Luo, H.B.; Yang, D.P.; Chen, Y.-L.; Shen, J.G.; Chen, J.P. Ellagic Acid, a Phenolic Compound, Exerts Anti-Angiogenesis Effects via VEGFR-2 Signaling Pathway in Breast Cancer. Breast Cancer Res. Treat. 2012, 134, 943–955. [Google Scholar] [CrossRef]
- Umesalma, S.; Sudhandiran, G. Ellagic Acid Prevents Rat Colon Carcinogenesis Induced by 1, 2 Dimethyl Hydrazine through Inhibition of AKT-Phosphoinositide-3 Kinase Pathway. Eur. J. Pharmacol. 2011, 660, 249–258. [Google Scholar] [CrossRef]
- Gerloff, A.; Singer, M.V.; Feick, P. Beer and Its Non-Alcoholic Compounds: Role in Pancreatic Exocrine Secretion, Alcoholic Pancreatitis and Pancreatic Carcinoma. Int. J. Environ. Res. Public. Health 2010, 7, 1093–1104. [Google Scholar] [CrossRef]
- Chung, Y.C.; Lu, L.C.; Tsai, M.H.; Chen, Y.J.; Chen, Y.Y.; Yao, S.P.; Hsu, C.P. The Inhibitory Effect of Ellagic Acid on Cell Growth of Ovarian Carcinoma Cells. J. Evid. Based Complement. Altern. Med. 2013, 2013, 306705. [Google Scholar] [CrossRef]
- Zhang, H.M.; Zhao, L.; Li, H.; Xu, H.; Chen, W.W.; Tao, L. Research Progress on the Anticarcinogenic Actions and Mechanisms of Ellagic Acid. Cancer Biol. Med. 2014, 11, 92–100. [Google Scholar]
- Ceci, C.; Tentori, L.; Atzori, M.; Lacal, P.; Bonanno, E.; Scimeca, M.; Cicconi, R.; Mattei, M.; De Martino, M.; Vespasiani, G.; et al. Ellagic Acid Inhibits Bladder Cancer Invasiveness and In Vivo Tumor Growth. Nutrients 2016, 8, 744. [Google Scholar] [CrossRef]
- Srigopalram, S.; Jayraaj, I.A.; Kaleeswaran, B.; Balamurugan, K.; Ranjithkumar, M.; Kumar, T.S.; Park, J.I.; Nou, I.S. Ellagic Acid Normalizes Mitochondrial Outer Membrane Permeabilization and Attenuates Inflammation-Mediated Cell Proliferation in Experimental Liver Cancer. Appl. Biochem. Biotechnol. 2014, 173, 2254–2266. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Zhan, J.; Wang, G.; Zhao, X.; Huang, W.; Zhou, G. The Red Wine Component Ellagic Acid Induces Autophagy and Exhibits Anti-lung Cancer Activity in Vitro and in Vivo. J. Cell Mol. Med. 2019, 23, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Ekeuku, S.O.; Pang, K.L.; Chin, K.Y. Effects of Caffeic Acid and Its Derivatives on Bone: A Systematic Review. Drug. Des. Devel. Ther. 2021, 15, 259–275. [Google Scholar] [CrossRef]
- Gu, W.; Yang, Y.; Zhang, C.; Zhang, Y.; Chen, L.; Shen, J.; Li, G.; Li, Z.; Li, L.; Li, Y.; et al. Caffeic Acid Attenuates the Angiogenic Function of Hepatocellular Carcinoma Cells via Reduction in JNK-1-Mediated HIF-1α Stabilization in Hypoxia. RSC Adv. 2016, 6, 82774–82782. [Google Scholar] [CrossRef]
- Yeh, C.B.; Hsieh, M.J.; Hsieh, Y.H.; Chien, M.H.; Chiou, H.L.; Yang, S.F. Antimetastatic Effects of Norcantharidin on Hepatocellular Carcinoma by Transcriptional Inhibition of MMP-9 through Modulation of NF-κB Activity. PLoS ONE 2012, 7, e31055. [Google Scholar] [CrossRef]
- Pei, K.; Ou, J.; Huang, J.; Ou, S. Coumaric Acid and Its Conjugates: Dietary Sources, Pharmacokinetic Properties and Biological Activities. J. Sci. Food Agric. 2016, 96, 2952–2962. [Google Scholar] [CrossRef]
- Jaganathan, S.K. Events Associated with Apoptotic Effect of P-Coumaric Acid in HCT-15 Colon Cancer Cells. World J. Gastroenterol. 2013, 19, 7726. [Google Scholar] [CrossRef]
- Ferreira, P.S.; Victorelli, F.D.; Fonseca-Santos, B.; Chorilli, M. A Review of Analytical Methods for p-Coumaric Acid in Plant-Based Products, Beverages, and Biological Matrices. Crit. Rev. Anal. Chem. 2019, 49, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. P-Coumaric Acid as An Active Ingredient in Cosmetics: A Review Focusing on Its Antimelanogenic Effects. Antioxidants 2019, 8, 275. [Google Scholar] [CrossRef]
- Hu, X.; Yang, Z.; Liu, W.; Pan, Z.; Zhang, X.; Li, M.; Liu, X.; Zheng, Q.; Li, D. The Anti- Tumor Effects of p-Coumaric Acid on Melanoma A375 and B16 Cells. Front. Oncol. 2020, 10, 558414. [Google Scholar] [CrossRef]
- Mozaffari Godarzi, S.; Valizade Gorji, A.; Gholizadeh, B.; Mard, S.A.; Mansouri, E. Antioxidant Effect of P-Coumaric Acid on Interleukin 1-β and Tumor Necrosis Factor-α in Rats with Renal Ischemic Reperfusion. Nefrología 2020, 40, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Pragasam, S.J.; Venkatesan, V.; Rasool, M. Immunomodulatory and Anti-Inflammatory Effect of p-Coumaric Acid, a Common Dietary Polyphenol on Experimental Inflammation in Rats. Inflammation 2013, 36, 169–176. [Google Scholar] [CrossRef]
- Yoon, J.H.; Youn, K.; Ho, C.T.; Karwe, M.V.; Jeong, W.S.; Jun, M. Coumaric Acid and Ursolic Acid from Corni fructus Attenuated β-Amyloid Induced Toxicity through Regulation of the NF-κB Signaling Pathway in PC12 Cells. J. Agric. Food Chem. 2014, 62, 4911–4916. [Google Scholar] [CrossRef] [PubMed]
- Behrangi, N.; Namvar, N.; Ataei, M.; Dizaji, S.; Javdani, G.; Sanati, M.H. MMP-9 Gene Expression Variation by Ingesting Tart Cherry and P-Coumaric Acid During Remyelination in the Cuprizone Mouse Model. Acta Med. Iran. 2017, 55, 539–549. [Google Scholar]
- Jayasooriya, R.G.P.T.; Lee, Y.G.; Kang, C.H.; Lee, K.T.; Choi, Y.H.; Park, S.Y.; Hwang, J.-K.; Kim, G.Y. Piceatannol Inhibits MMP-9-Dependent Invasion of Tumor Necrosis Factor-α-Stimulated DU145 Cells by Suppressing the Akt-Mediated Nuclear Factor-ΚB Pathway. Oncol. Lett. 2013, 5, 341–347. [Google Scholar] [CrossRef]
- Lü, L.; Tang, D.; Wang, L.; Huang, L.; Jiang, G.; Xiao, X.; Zeng, F. Gambogic Acid Inhibits TNF-α-Induced Invasion of Human Prostate Cancer PC3 Cells in Vitro through PI3K/Akt and NF-ΚB Signaling Pathways. Acta. Pharmacol. Sin. 2012, 33, 531–541. [Google Scholar] [CrossRef]
- Mimeault, M.; Batra, S.K. Potential Applications of Curcumin and Its Novel Synthetic Analogs and Nanotechnology-Based Formulations in Cancer Prevention and Therapy. Chin. Med. 2011, 6, 31. [Google Scholar] [CrossRef]
- Song, N.; Hwang, M.K.; Heo, Y.S.; Lee, K.W.; Lee, H.J. Piceatannol Suppresses the Metastatic Potential of MCF10A Human Breast Epithelial Cells Harboring Mutated H-Ras by Inhibiting MMP-2 Expression. Int. J. Mol. Med. 2013, 32, 775–784. [Google Scholar] [CrossRef]
- Signorelli, P.; Ghidoni, R. Resveratrol as an Anticancer Nutrient: Molecular Basis, Open Questions and Promises. J. Nutr. Biochem. 2005, 16, 449–466. [Google Scholar] [CrossRef]
- Brisdelli, F.; D’Andrea, G.; Bozzi, A. Resveratrol: A Natural Polyphenol with Multiple Chemopreventive Properties (Review). Curr. Drug Metab. 2009, 10, 530–546. [Google Scholar] [CrossRef]
- Gweon, E.J.; Kim, S.-J. Resveratrol Attenuates Matrix Metalloproteinase-9 and -2- Regulated Differentiation of HTB94 Chondrosarcoma Cells through the P38 Kinase and JNK Pathways. Oncol. Rep. 2014, 32, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Gweon, E.J.; Kim, S.J. Resveratrol Induces MMP-9 and Cell Migration via the P38 Kinase and PI-3K Pathways in HT1080 Human Fibrosarcoma Cells. Oncol. Rep. 2013, 29, 826–834. [Google Scholar] [CrossRef]
- Reddy, H.L.; Dayan, A.D.; Cavagnaro, J.; Gad, S.; Li, J.; Goodrich, R.P. Toxicity Testing of a Novel Riboflavin-Based Technology for Pathogen Reduction and White Blood Cell Inactivation. Transfus. Med. Rev. 2008, 22, 133–153. [Google Scholar] [CrossRef] [PubMed]
- Belin, M.W.; Lim, L.; Rajpal, R.K.; Hafezi, F.; Gomes, J.A.P.; Cochener, B. Corneal Cross-Linking: Current USA Status: ReportFrom the Cornea Society. Cornea 2018, 37, 1218–1225. [Google Scholar] [CrossRef]
- Vo, H.V.T.; Kim, N.; Lee, H.J. Vitamin Bs as Potent Anticancer Agents through MMP-2/9 Regulation. Front. Biosci. 2025, 30, 24072. [Google Scholar] [CrossRef]
- Machado, D.; Shishido, S.M.; Queiroz, K.C.S.; Oliveira, D.N.; Faria, A.L.C.; Catharino, R.R.; Spek, C.A.; Ferreira, C.V. Irradiated Riboflavin Diminishes the Aggressiveness of Melanoma In Vitro and In Vivo. PLoS ONE 2013, 8, e54269. [Google Scholar] [CrossRef]
- Walkey, C.J.; Kitts, D.D.; Liu, Y.; van Vuuren, H.J.J. Bioengineering Yeast to Enhance Folate Levels in Wine. Process Biochem. 2015, 50, 205–210. [Google Scholar] [CrossRef]
- Cieślik, E.; Cieślik, I. Occurrence and Significance of Folic Acid. Pteridines 2018, 29, 187–195. [Google Scholar] [CrossRef]
- Kao, C.H.; Sun, L.M.; Chen, Y.S.; Lin, C.L.; Liang, J.A.; Kao, C.H.; Weng, M.W. Risk of Nongenitourinary Cancers in Patients with Spinal Cord Injury: A Population-based Cohort Study. Medicine 2016, 95, e2462. [Google Scholar] [CrossRef]
- Miranpuri, G.S.; Nguyen, J.; Moreno, N.; Yutuc, N.A.; Kim, J.; Buttar, S.; Brown, G.R.; Sauer, S.E.; Singh, C.K.; Kumar, S.; et al. Folic acid modulates matrix metalloproteinase-9 expression following spinal cord injury. Ann. Neurosci. 2019, 26, 60–65. [Google Scholar] [CrossRef]
- Aatif, M. Current understanding of polyphenols to enhance bioavailability for better therapies. Biomedicines 2023, 11, 2078. [Google Scholar] [CrossRef]
- Grabarczyk, M.; Justyńska, W.; Czpakowska, J.; Smolińska, E.; Bielenin, A.; Glabinski, A.; Szpakowski, P. Role of plant phytochemicals: Resveratrol, curcumin, luteolin and quercetin in demyelination, neurodegeneration, and epilepsy. Antioxidants 2024, 13, 1364. [Google Scholar] [CrossRef]
- Pervin, M.; Unno, K.; Takagaki, A.; Isemura, M.; Nakamura, Y. Function of green tea catechins in the brain: Epigallocatechin gallate and its metabolites. Int. J. Mol. Sci. 2019, 20, 3630. [Google Scholar] [CrossRef] [PubMed]


| Bioactive Compounds | Amounts in Wine (mg/L) | Refs. | |
|---|---|---|---|
| Flavonoids | Quercetin | 8.3 | [9,20] |
| Kaempferol | 2.3 | [9] | |
| Myricetin | 8.3 | [9] | |
| Luteolin | 0.2–7.2 | [21,22] | |
| Apigenin | 0.2 | [21,23] | |
| Epicatechin | 3.3 | [24] | |
| Epigallocatechin gallate | 5–20 | [9] | |
| Taxifolin | 0.65–9.6 | [21,25] | |
| Genistein | 0.01 | [9,26] | |
| Naringenin | 0.1–19.8 | [21,23] | |
| Naringin | 7.5 | [9] | |
| Hesperetin | 0.5 | [9] | |
| Xanthohumol | 0.002–1.2 | [9] | |
| Isoxanthohumol | 0.04–3.4 | [9] | |
| Non-flavonoids | Gallic acid | 2–130 | [21,27] |
| Ellagic acid | 8.9 | [24] | |
| Caffeic acid | 0.3–26 | [21,27] | |
| p-coumaric acid | 0.4–15 | [9] | |
| Piceatannol | 5.8 | [9] | |
| Resveratrol | 0.5–7 | [21,28] | |
| Riboflavin | 0.0085–0.1349 | [29,30] | |
| Folic acid | 0.0004–0.0045 | [29,30,31,32] |
| Compounds | MMP-2 Activity/Expression | MMP-9 Activity/Expression | Cell Lines/ Animals | Refs. |
|---|---|---|---|---|
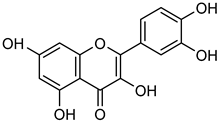 Quercetin | Down regulation (↓/↓) | Down regulation (↓/↓) | MDA-MB-231 cells | [64,81,82] |
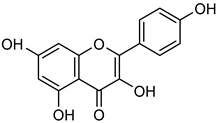 Kaempferol | Down regulation (↓/-) | Down regulation (↓/-) | A2780 cells OVCAR-3 cells SK-Hep-1 cells Huh-7 cells | [83,84,85,86,87,88,89] |
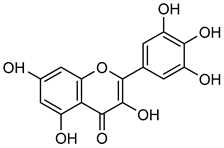 Myricetin | Down regulation (↓/↓) | Down regulation (↓/↓) | MDA-Mb-231Br cells | [90,91,92,93] |
| Compounds | MMP-2 Activity/Expression | MMP-9 Activity/Expression | Cell Lines/Animals | Refs. |
|---|---|---|---|---|
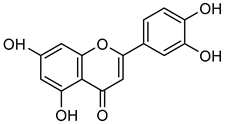 Luteolin | Down regulation (-/↓) | Down regulation (-/↓) | A375 cells | [98] |
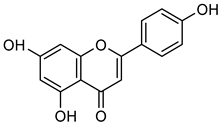 Apigenin | Not applicable | Down regulation (-/↓) | OVCAR-3 cells | [102] |
| Compounds | MMP-2 Activity/Expression | MMP-9 Activity/Expression | Cell Lines/ Animals | Refs. |
|---|---|---|---|---|
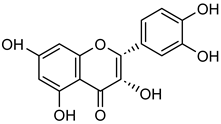 Epicatechin | Not applicable | Down regulation (↓/-) | H1299 cells A549 cells | [106,107] |
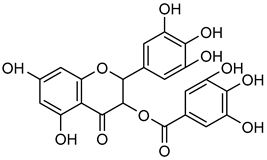 EGCG | Down regulation (↓/-) | Down regulation (↓/-) | OVCAR-3 cells | [110,111] |
| Compounds | MMP-2 Activity/Expression | MMP-9 Activity/Expression | Cell Lines/Animals | Refs. |
|---|---|---|---|---|
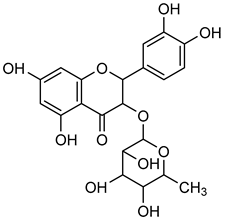 Astilbin | Down regulation (-/↓) | Down regulation (-/↓) | HBZY-1 cells | [115] |
 Taxifolin | Down regulation (-/↓) | Down regulation (-/↓) | NCI-N87 cells AGS cells | [118] |
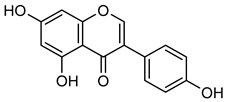 Genistein | Down regulation (↓/-) | Down regulation (↓/-) | MDA-Mb-231Br cells | [121,122] |
| Compounds | MMP-2 Activity/Expression | MMP-9 Activity/Expression | Cell Lines/ Animals | Refs. |
|---|---|---|---|---|
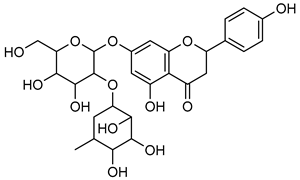 Naringenin | Down regulation (↓/↓) | Down regulation (↓/↓) | MDA-MB-231 cells SGC-7901 cells A549 cells U87 cells | [124,125,126,127,128,129,130,131,132,133] |
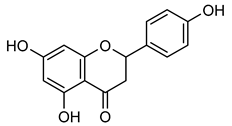 Naringin | Down regulation (↓/↓) | Down regulation (↓/↓) | U87 cells | [125] |
 Hesperctin | Down regulation (↓/↓) | Down regulation (↓/↓) | MCF-7 cells 4T1 cells | [137,138,139,140] |
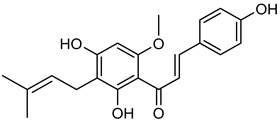 Xanthohumol | Down regulation (↓/-) | Down regulation (↓/↓) | A549 cells | [146] |
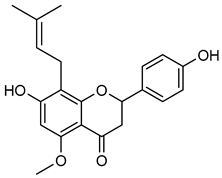 Isoxanthohumo | Down regulation (-/↓) | Down regulation (-/↓) | MDA-MB-231 cells MonoMac6 cells | [147,150] |
| Compounds | MMP-2 Activity/Expression | MMP-9 Activity/Expression | Cell Lines/ Animals | Refs. |
|---|---|---|---|---|
 Gallic acid | Down regulation (↓/-) | Down regulation (↓/-) | K562 cells PC-3 cells | [154,155,156] |
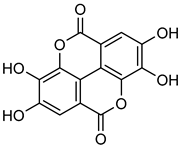 Ellagic acid | Down regulation (-/↓) | Down regulation (-/↓) | A2780 cells | [166] |
| Compounds | MMP-2 Activity/Expression | MMP-9 Activity/Expression | Cell Lines/ Animals | Ref. |
|---|---|---|---|---|
 Caffeic acid | Down regulation (↓/-) | Down regulation (↓/-) | HCC cells | [152] |
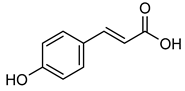 p-coumaric acid | Not Applicable | Down regulation (-/↓) | Mice | [178] |
| Compounds | MMP-2 Activity/Expression | MMP-9 Activity/Expression | Cell Lines/ Animals | Refs. |
|---|---|---|---|---|
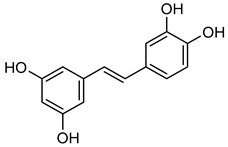 Piceatannol | Down regulation (↓/-) | Down regulation (↓/-) | MCF10A cells HPC cells | [179,180,181,182,183] |
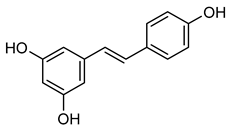 Resveratrol | Down regulation (↓/↓) | Down regulation (↓/↓) | HTB94 cells | [186,187] |
| Compounds | MMP-2 Activity/Expression | MMP-9 Activity/Expression | Cell Lines/ Animals | Refs. |
|---|---|---|---|---|
 Riboflavin | Down regulation (↓/↓) | Down regulation (↓/↓) | MCF10A cells | [191] |
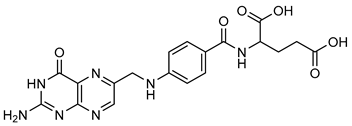 Folic Acid | Down regulation (-/↓) | Down regulation (↓/↓) | Sprague Dawley Rats | [193,194] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.T.; Vo, H.V.T.; Lee, H.J. Regulation of Matrix Metalloproteinases by Wine-Derived Compounds: Implications for Cancer Therapy. Biomolecules 2025, 15, 781. https://doi.org/10.3390/biom15060781
Islam MT, Vo HVT, Lee HJ. Regulation of Matrix Metalloproteinases by Wine-Derived Compounds: Implications for Cancer Therapy. Biomolecules. 2025; 15(6):781. https://doi.org/10.3390/biom15060781
Chicago/Turabian StyleIslam, Md. Towhedul, Ha Vy Thi Vo, and Hyuck Jin Lee. 2025. "Regulation of Matrix Metalloproteinases by Wine-Derived Compounds: Implications for Cancer Therapy" Biomolecules 15, no. 6: 781. https://doi.org/10.3390/biom15060781
APA StyleIslam, M. T., Vo, H. V. T., & Lee, H. J. (2025). Regulation of Matrix Metalloproteinases by Wine-Derived Compounds: Implications for Cancer Therapy. Biomolecules, 15(6), 781. https://doi.org/10.3390/biom15060781








