The Hepatoprotective Properties of Gentiopicroside, Sweroside, and Swertiamarin Against Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) †
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culturing
2.2. Phytochemicals and Arachidonic Acid Preparation
2.3. MTT Assay for Measuring the Cell Viability of Cells Pre-Treated with Phytochemicals Gentiopicroside, Sweroside, and Silymarin in the Presence of Arachidonic Acid
2.4. The Seahorse Assay for Assessing the Mitochondrial Function of Cells Pre-Treated with Gentiana Species and Phytochemicals in the Presence of Arachidonic Acid
2.5. The DCF Assay for Assessing the ROS Produced by Cells Pre-Treated with Gentian spp. and Single Compounds: Gentiopicroside, Sweroside, Swertiamarin, and Silymarin in the Presence of Arachidonic Acid
2.6. An Annexin V-FITC PI Assay for Investigating Apoptosis in Hepatocytes Pre-Treated with Gentiana Macrophylla and Single Compounds: Gentiopicroside, Prior to Arachidonic Acid Exposure
2.7. Statistics
3. Results
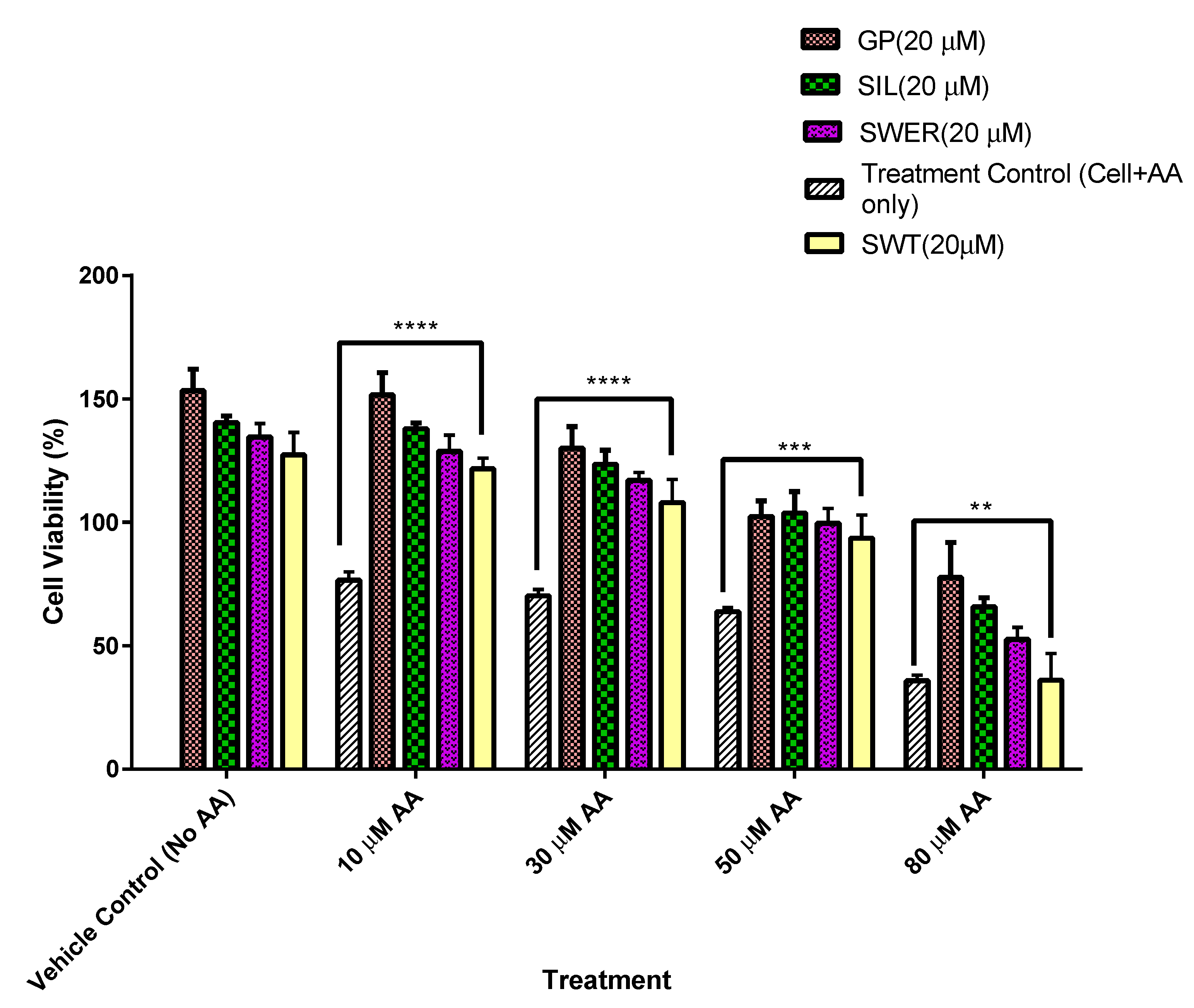
3.1. A Comparison of the Cytotoxic Effects of Fatty Acid on Single Compounds: Gentiopicroside, Sweroside, and Silymarin Pre-Treated Hepatocytes (HepG2)
3.2. A Comparison of the Cytotoxic Effects of Fatty Acid on Single Compounds: Gentiopicroside, Sweroside, and Silymarin Pre-Treated THLE-2 Cells (THLE-2)
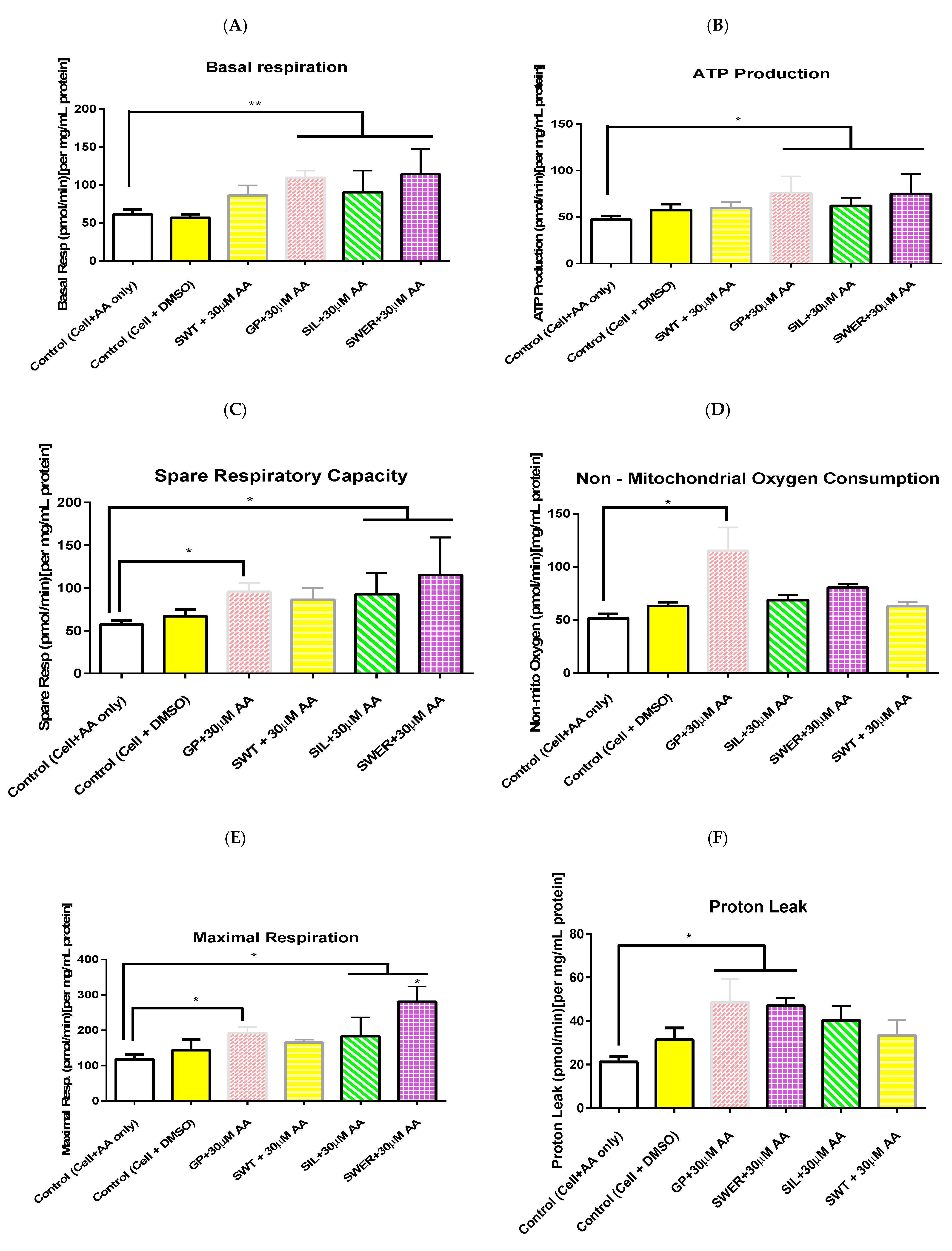
3.3. A Comparison of the Effects of Single Compounds: Gentiopicroside, Sweroside, and Silymarin Pre-Treatment on Hepatocyte Mitochondrial Function in the Presence of Arachidonic Acid
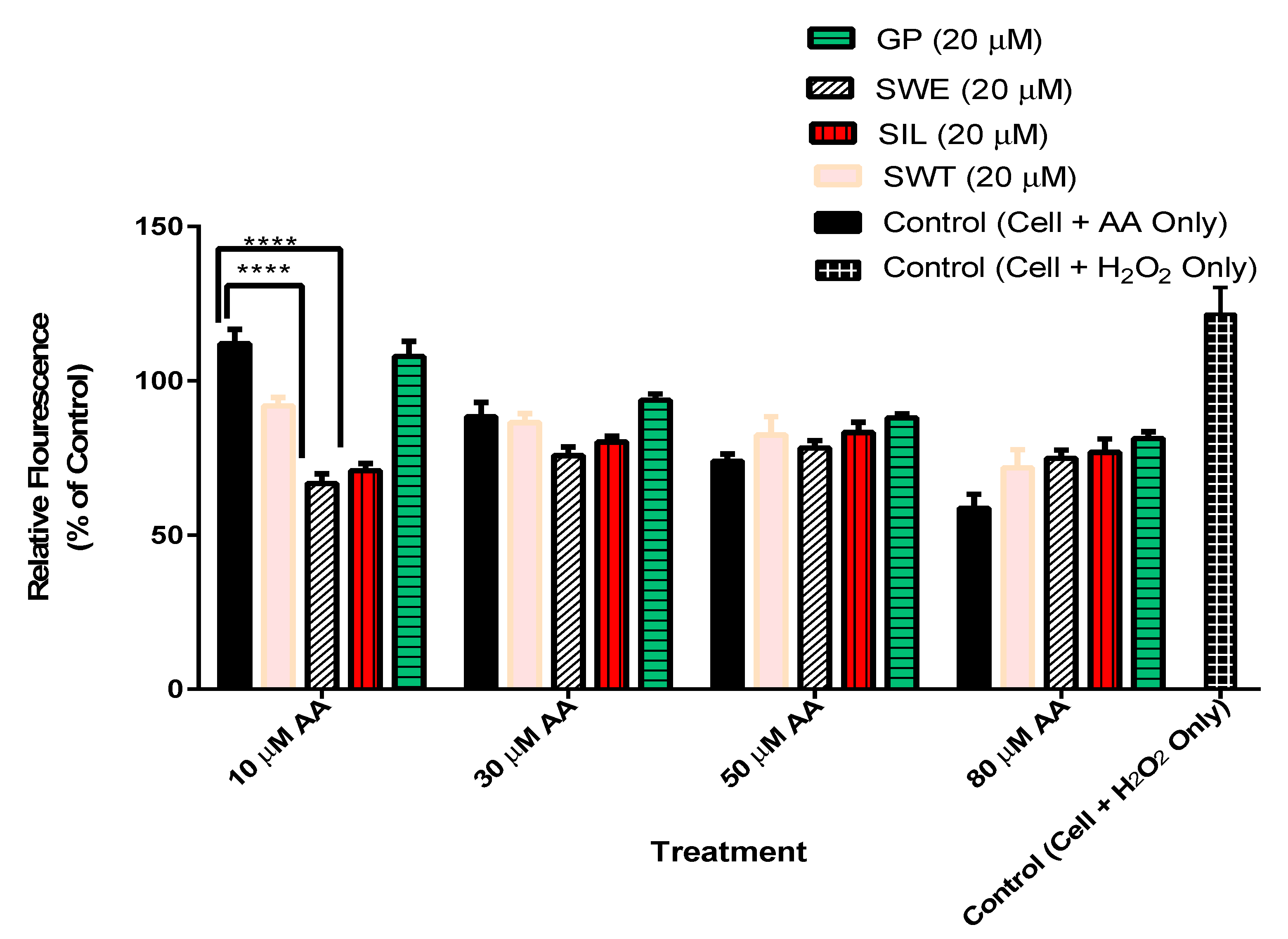
3.4. The Effect of Phytochemicals Gentiopicroside, Sweroside, Swertiamarin, and Silymarin Pre-Treatment on Hepatocyte ROS Production in the Presence of Arachidonic Acid
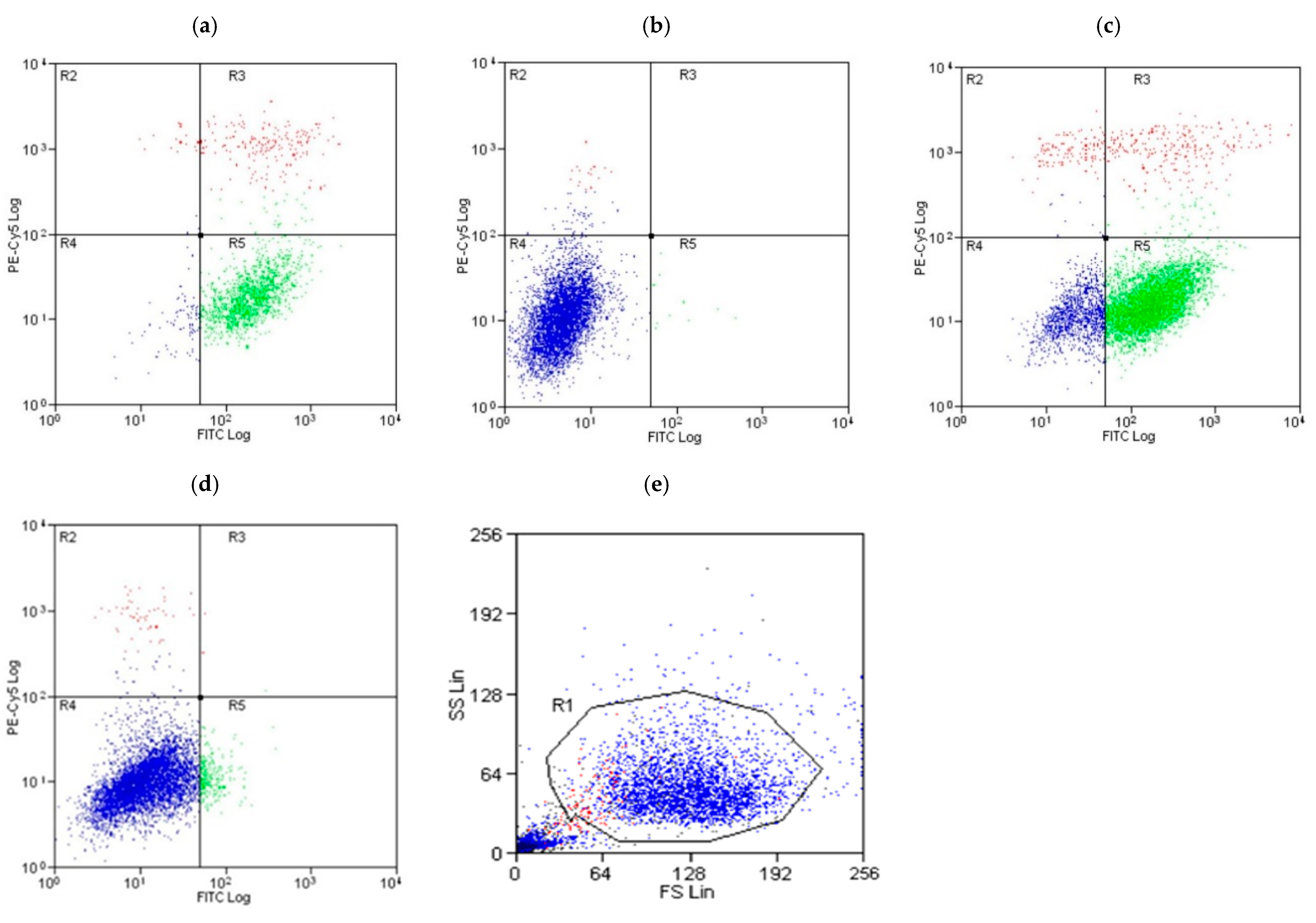
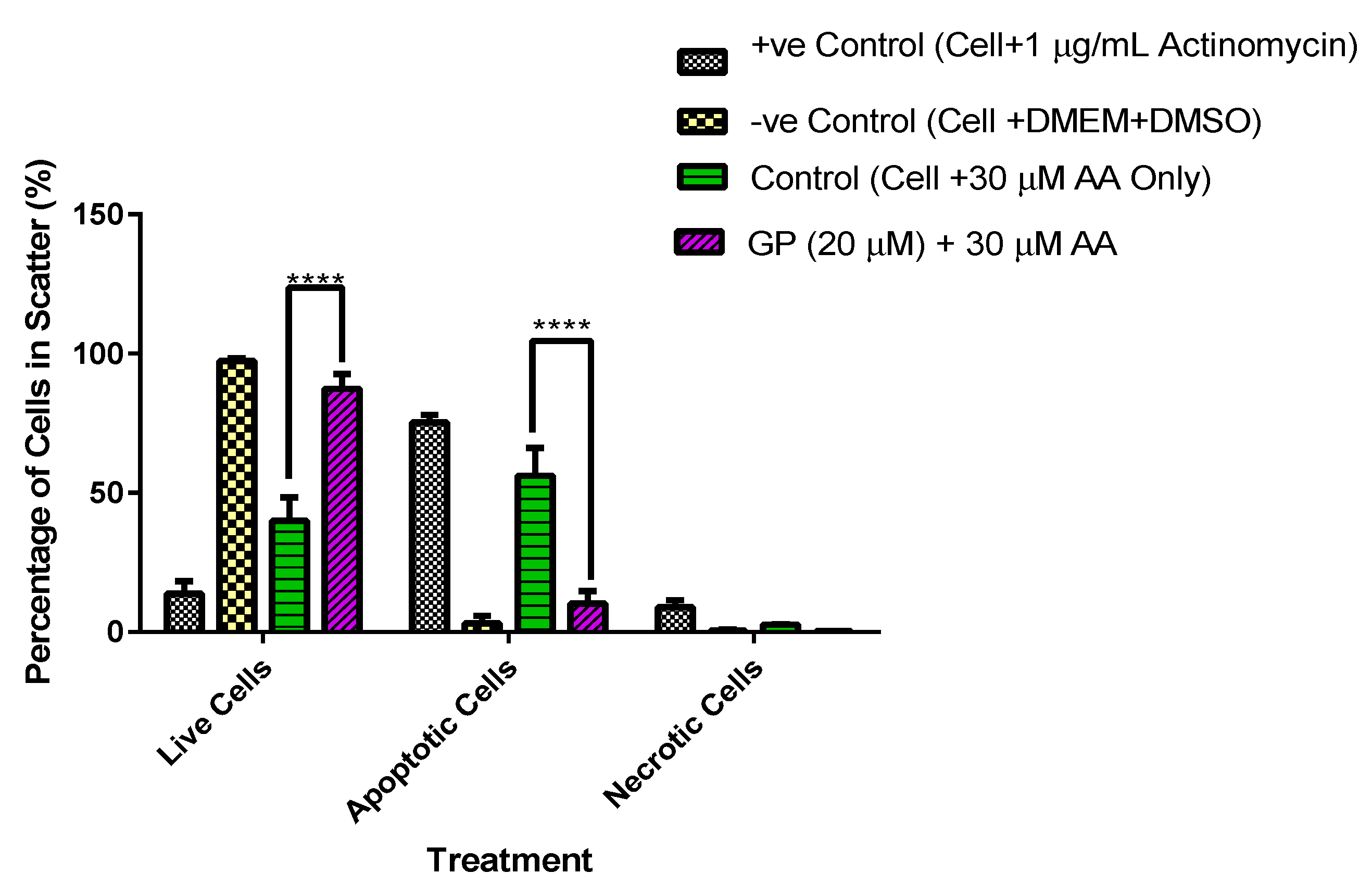
3.5. A Comparative Assessment of Hepatocyte (HepG2) Protection via Apoptosis and Necrosis Prevention by Gentiana Macrophylla and Gentiopicroside
4. Discussion
5. Conclusions
6. Study Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Pan, Z.; Derbala, M.; AlNaamani, K.; Ghazinian, H.; Fan, J.G.; Eslam, M. MAFLD Criteria Are Better than MASLD Criteria at Predicting the Risk of Chronic Kidney Disease. Ann. Hepatol. 2024, 29, 101512. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.D.; Targher, G.; Byrne, C.D.; Somers, V.; Kim, S.U.; Chahal, C.A.A.; Wong, V.W.S.; Cai, J.; Shapiro, M.D.; Eslam, M.; et al. An International Multidisciplinary Consensus Statement on MAFLD and the Risk of CVD. Hepatol. Int. 2023, 17, 773–791. [Google Scholar] [CrossRef] [PubMed]
- Kaya, E.; Yilmaz, Y. Metabolic-Associated Fatty Liver Disease (MAFLD): A Multi-Systemic Disease Beyond the Liver. J. Clin. Transl. Hepatol. 2022, 10, 329–338. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Campbell-Sargent, C.; Mirshahi, F.; Rizzo, W.B.; Contos, M.J.; Sterling, R.K.; Luketic, V.A.; Shiffman, M.L.; Clore, J.N. Nonalcoholic Steatohepatitis: Association of Insulin Resistance and Mitochondrial Abnormalities. Gastroenterology 2001, 120, 1183–1192. [Google Scholar] [CrossRef]
- Marchesini, G.; Brizi, M.; Morselli-Labate, A.M.; Bianchi, G.; Bugianesi, E.; McCullough, A.J.; Forlani, G.; Melchionda, N. Association of Nonalcoholic Fatty Liver Disease with Insulin Resistance. Am. J. Med. 1999, 107, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Ciociola, E.; Dutta, T.; Sasidharan, K.; Kovooru, L.; Noto, F.R.; Pennisi, G.; Petta, S.; Mirarchi, A.; Maurotti, S.; Scopacasa, B.; et al. MARC1 Downregulation Reduces Hepatocyte Lipid Content by Increasing Beta-Oxidation. Running Title: MARC1 Downregulation Leads to Higher β-Oxidation. Clin. Mol. Hepatol. 2024, 31, 464–478. [Google Scholar]
- Karkucinska-Wieckowska, A.; Simoes, I.C.M.; Kalinowski, P.; Lebiedzinska-Arciszewska, M.; Zieniewicz, K.; Milkiewicz, P.; Górska-Ponikowska, M.; Pinton, P.; Malik, A.N.; Krawczyk, M.; et al. Mitochondria, Oxidative Stress and Nonalcoholic Fatty Liver Disease: A Complex Relationship. Eur. J. Clin. Investig. 2022, 52, e13622. [Google Scholar] [CrossRef]
- Li, Z.Z.; Berk, M.; McIntyre, T.M.; Gores, G.J.; Feldstein, A.E. The Lysosomal-Mitochondrial Axis in Free Fatty Acid–Induced Hepatic Lipotoxicity. Hepatology 2008, 47, 1495–1503. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, J.; Shang, J.; Zhang, L. Prevention of Free Fatty Acid-Induced Hepatic Lipotoxicity in HepG2 Cells by Magnesium Isoglycyrrhizinate in Vitro. Pharmacology 2009, 84, 183–190. [Google Scholar] [CrossRef]
- Passaro, A.; Sanz, J.M.; Naumovski, N.; Sergi, D. The Complex Interplay between Oxinflammation, Mitochondrial Dysfunction and Lipotoxicity: Focus on Their Role in the Pathogenesis of Skeletal Muscle Insulin Resistance and Modulation by Dietary Fatty Acids. Adv. Redox Res. 2024, 11, 100100. [Google Scholar] [CrossRef]
- Scorrano, L.; Penzo, D.; Petronilli, V.; Pagano, F.; Bernardi, P. Arachidonic Acid Causes Cell Death through the Mitochondrial Permeability Transition. Implications for Tumor Necrosis Factor-α Apoptotic Signaling. J. Biol. Chem. 2001, 276, 12035–12040. [Google Scholar] [CrossRef] [PubMed]
- Hugo, C.; Asante, I.; Sadybekov, A.; Katritch, V.; Yassine, H.N. Development of Calcium-Dependent Phospholipase A2 Inhibitors to Target Cellular Senescence and Oxidative Stress in Neurodegenerative Diseases. Antioxid. Redox Signal. 2024, 41, 1100–1116. [Google Scholar] [CrossRef]
- Zhu, Z.-L.; Chen, M.-L. Hepatoprotective Effect on Aerial Parts of Gentiana manshurica Kitag. Lishizhen Med. Mater. Medica Res. 2007, 12, 090. [Google Scholar]
- Dong, Q.; Wang, Z.; Hu, N.; Tie, F.; Liu, Z.; Sun, Y.; Wang, Y.; Tan, N.; Wang, H. Total Iridoid Glycosides from Swertia Mussotii Franch. Alleviate Cholestasis Induced by α-Naphthyl Isothiocyanate through Activating the Farnesoid X Receptor and Inhibiting Oxidative Stress. Int. J. Mol. Sci. 2024, 25, 10607. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Ge, Y.B.; Li, J.; Zhang, Y.; Huang, X.J.; Chen, G.X. The Distribution, Uses, and Characteristic Components of Gentianaceae Plants in China. World J. Tradit. Chin. Med. 2021, 7, 287–298. [Google Scholar] [CrossRef]
- Tian, C.; Zhang, T.; Wang, L.; Shan, Q.; Jiang, L. The Hepatoprotective Effect and Chemical Constituents of Total Iridoids and Xanthones Extracted from Swertia Mussotii Franch. J. Ethnopharmacol. 2014, 154, 259–266. [Google Scholar] [CrossRef]
- Hamza, A.A.; Gamel, M.; Abdalla, A.; Abdalla, Y.; Amin, A. Gentiana Lutea Attenuates Hepatotoxicity Induced by Ketoconazole in Rats by Fortifying the Cellular Antioxidant Defense System. J. Basic Appl. Zool. 2023, 84, 1–12. [Google Scholar] [CrossRef]
- Tang, X.; Yang, Q.; Yang, F.; Gong, J.; Han, H.; Yang, L.; Wang, Z. Target Profiling Analyses of Bile Acids in the Evaluation of Hepatoprotective Effect of Gentiopicroside on ANIT-Induced Cholestatic Liver Injury in Mice. J. Ethnopharmacol. 2016, 194, 63–71. [Google Scholar] [CrossRef]
- Verma, D.; Thakur, S.; Shrimal, H.; Kumar, S.; Das, J.; Sarkar, B.; Kapoor, D.N.; Deb, P.K. UPLC-QTOF-MS Based Targeted Metabolomics to Unravel the Hepatoprotective Marker Compounds of Swertia chirayita. Nat. Prod. Res. 2024; Online ahead of print. [Google Scholar] [CrossRef]
- Saller, R.; Meier, R.; Brignoli, R. The Use of Silymarin in the Treatment of Liver Diseases. Drugs 2001, 61, 2035–2063. [Google Scholar] [CrossRef]
- Dhande, D.; Dhok, A.; Anjankar, A.; Nagpure, S. Silymarin as an Antioxidant Therapy in Chronic Liver Diseases: A Comprehensive Review. Cureus 2024, 16, e67083. [Google Scholar] [CrossRef] [PubMed]
- Okiljević, B.; Martić, N.; Govedarica, S.; Andrejić Višnjić, B.; Bosanac, M.; Baljak, J.; Pavlić, B.; Milanović, I.; Rašković, A. Cardioprotective and Hepatoprotective Potential of Silymarin in Paracetamol-Induced Oxidative Stress. Pharmaceutics 2024, 16, 520. [Google Scholar] [CrossRef] [PubMed]
- Boateng, A. Hepatoprotective Properties of Gentiana SPP: Against Non-Alcoholic Fatty Liver Disease (NAFLD). Ph.D. Thesis, University of Westminster Biomedical Sciences, London, UK, 2018. [Google Scholar] [CrossRef]
- Xu, Z.; Lin, Z.; Zeng, J.; Chen, R.; Li, C.; Xiao, H.; Huang, H.; Xu, S.; Lan, T. Gentiopicroside Ameliorates Glucose and Lipid Metabolism in T2DM by Activating PI3K/AKT Pathway Via FGFR1. Preprint 2021. [Google Scholar] [CrossRef]
- Faisal, Z.; Mohos, V.; Fliszár-Nyúl, E.; Valentová, K.; Káňová, K.; Lemli, B.; Kunsági-Máté, S.; Poór, M. Interaction of Silymarin Components and Their Sulfate Metabolites with Human Serum Albumin and Cytochrome P450 (2C9, 2C19, 2D6, and 3A4) Enzymes. Biomed. Pharmacother. 2021, 138, 111459. [Google Scholar] [CrossRef] [PubMed]
- Gyamfi, D.; Everitt, H.E.; Tewfik, I.; Clemens, D.L.; Patel, V.B. Hepatic Mitochondrial Dysfunction Induced by Fatty Acids and Ethanol. Free Radic. Biol. Med. 2012, 53, 2131–2145. [Google Scholar] [CrossRef]
- Choi, R.Y.; Nam, S.J.; Lee, H.I.; Lee, J.; Leutou, A.S.; Ri Ham, J.; Lee, M.K. Gentiopicroside Isolated from Gentiana Scabra Bge. Inhibits Adipogenesis in 3T3-L1 Cells and Reduces Body Weight in Diet-Induced Obese Mice. Bioorg. Med. Chem. Lett. 2019, 29, 1699–1704. [Google Scholar] [CrossRef]
- Lian, L.H.; Wu, Y.L.; Wan, Y.; Li, X.; Xie, W.X.; Nan, J.X. Anti-Apoptotic Activity of Gentiopicroside in d-Galactosamine/Lipopolysaccharide-Induced Murine Fulminant Hepatic Failure. Chem. Biol. Interact. 2010, 188, 127–133. [Google Scholar] [CrossRef]
- Zhao, L.; Ye, J.; Wu, G.T.; Peng, X.J.; Xia, P.F.; Ren, Y. Gentiopicroside Prevents Interleukin-1 Beta Induced Inflammation Response in Rat Articular Chondrocyte. J. Ethnopharmacol. 2015, 172, 100–107. [Google Scholar] [CrossRef]
- Mihailović, V.; Mihailović, M.; Uskoković, A.; ArambaŠić, J.; MiŠić, D.; Stanković, V.; Katanić, J.; Mladenović, M.; Solujić, S.; Matić, S. Hepatoprotective Effects of Gentiana asclepiadea L. Extracts against Carbon Tetrachloride Induced Liver Injury in Rats. Food Chem. Toxicol. 2013, 52, 83–90. [Google Scholar] [CrossRef]
- Shah, F.; Louise-May, S.; Greene, N. Chemotypes Sensitivity and Predictivity of in Vivo Outcomes for Cytotoxic Assays in THLE and HepG2 Cell Lines. Bioorg. Med. Chem. Lett. 2014, 24, 2753–2757. [Google Scholar] [CrossRef]
- Paradies, G.; Paradies, V.; Ruggiero, F.M.; Petrosillo, G. Oxidative Stress, Cardiolipin and Mitochondrial Dysfunction in Nonalcoholic Fatty Liver Disease. World J. Gastroenterol. WJG 2014, 20, 14205. [Google Scholar] [CrossRef]
- Wei, Y.; Rector, R.S.; Thyfault, J.P.; Ibdah, J.A. Nonalcoholic Fatty Liver Disease and Mitochondrial Dysfunction. World J. Gastroenterol. WJG 2008, 14, 193. [Google Scholar] [CrossRef] [PubMed]
- Jastroch, M.; Divakaruni, A.S.; Mookerjee, S.; Treberg, J.R.; Brand, M.D. Mitochondrial Proton and Electron Leaks. Essays Biochem. 2010, 47, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Stuart, J.A.; Brindle, K.M.; Harper, J.A.; Brand, M.D. Mitochondrial Proton Leak and the Uncoupling Proteins. J. Bioenerg. Biomembr. 1999, 31, 517–524. [Google Scholar] [CrossRef]
- Trimble, M.W.; Kaul, N.; Wildà, J.E.; Bowmanà, J.P.; Han, M.-O.; Chun, J.-A.; Lee, W.-H.; Lee, J.-W.; Chung, C.-H. The Inhibitory Effect of the Components of Cornus Officinalis on Melanogenesis. Int. J. Cosmet. Sci. 2008, 30, 383–384. [Google Scholar] [CrossRef]
- Gülçin, I.; Elias, R.; Gepdiremen, A.; Taoubi, K.; Köksal, E. Antioxidant Secoiridoids from Fringe Tree (Chionanthus virginicus L.). Wood Sci. Technol. 2009, 43, 195–212. [Google Scholar] [CrossRef]
- Hyun, A.J.; Hae, Y.C.; Yokozawa, T.; Youn, C.K.; Sook, K.H.; Jae, S.C. Alaternin and Emodin with Hydroxyl Radical Inhibitory and/or Scavenging Activities and Hepatoprotective Activity on Tacrine-Induced Cytotoxicity in HepG2 Cells. Arch. Pharm. Res. 2004, 27, 947–953. [Google Scholar] [CrossRef]
- Angeli, J.P.F.; Barcelos, G.R.M.; Serpeloni, J.M.; Barbosa Júnior, F.; Nersesyan, A.; Mantovani, M.S. Evaluation of the Genotoxic and Anti-Genotoxic Activities of Silybin in Human Hepatoma Cells (HepG2). Mutagenesis 2010, 25, 223–229. [Google Scholar] [CrossRef]
- Bio Klamt, F.; Gottfried, C.; Tramontina, F.; Dal-Pizzol, F.; Conte Da Frota, M.L.; Cla¤, J.; Moreira, F.; Dias, R.D.; Moriguchi, E.; Wofchuk, S.; et al. Time-Related Increase in Mitochondrial Superoxide Production, Biomolecule Damage and Antioxidant Enzyme Activities in Cortical Astrocyte Cultures. NeuroReport 2002, 13, 1515–1518. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Chen, Q.; Galleano, M.; Cederbaum, A.I. Cytotoxicity and Apoptosis Produced by Arachidonic Acid in Hep G2 Cells Overexpressing Human Cytochrome P4502E1. J. Biol. Chem. 1997, 272, 14532–14541. [Google Scholar] [CrossRef]
- Cao, Y.; Pearman, A.T.; Zimmerman, G.A.; McIntyre, T.M.; Prescott, S.M. Intracellular Unesterified Arachidonic Acid Signals Apoptosis. Proc. Natl. Acad. Sci. USA 2000, 97, 11280–11285. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boateng, A.O.; Patel, V.B.; Bligh, S.W.A. The Hepatoprotective Properties of Gentiopicroside, Sweroside, and Swertiamarin Against Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Biomolecules 2025, 15, 726. https://doi.org/10.3390/biom15050726
Boateng AO, Patel VB, Bligh SWA. The Hepatoprotective Properties of Gentiopicroside, Sweroside, and Swertiamarin Against Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Biomolecules. 2025; 15(5):726. https://doi.org/10.3390/biom15050726
Chicago/Turabian StyleBoateng, Anthony O., Vinood B. Patel, and S. W. Annie Bligh. 2025. "The Hepatoprotective Properties of Gentiopicroside, Sweroside, and Swertiamarin Against Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)" Biomolecules 15, no. 5: 726. https://doi.org/10.3390/biom15050726
APA StyleBoateng, A. O., Patel, V. B., & Bligh, S. W. A. (2025). The Hepatoprotective Properties of Gentiopicroside, Sweroside, and Swertiamarin Against Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Biomolecules, 15(5), 726. https://doi.org/10.3390/biom15050726






