The Association Between Myokines, Inflammation, and Nutritional Status in Patients with Multiple Sclerosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Aim
2.2. Methods
2.3. Nutritional Status Assessment
2.4. Nutritional Indices
2.5. Biochemical Parameters
2.6. Statistical Analysis
3. Results
3.1. Comparison of MS Patients and Healthy Controls
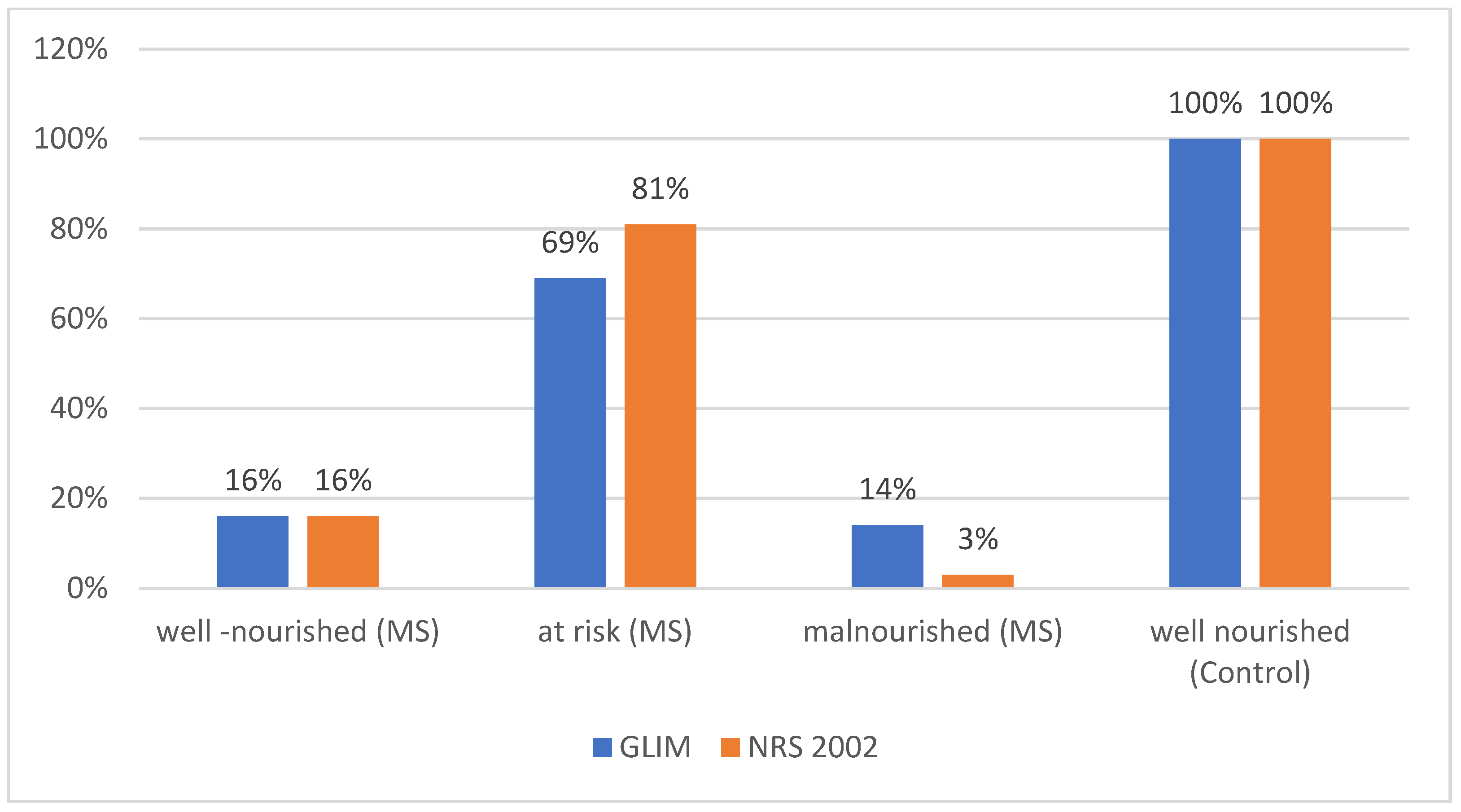
3.2. Comparison of Malnourished MS Patients and Patients at Risk
4. Discussion
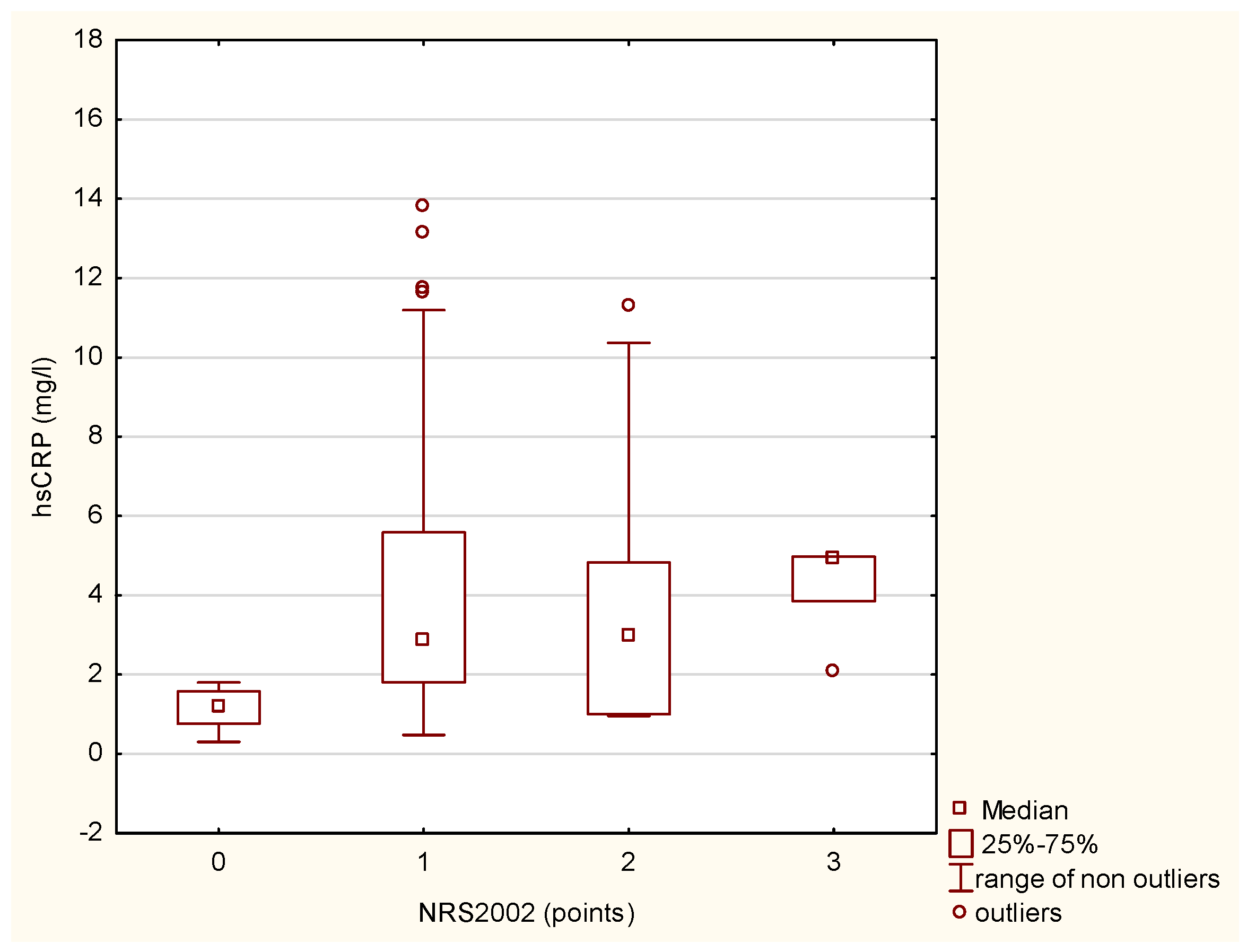
4.1. Nutritional Status and Inflammation
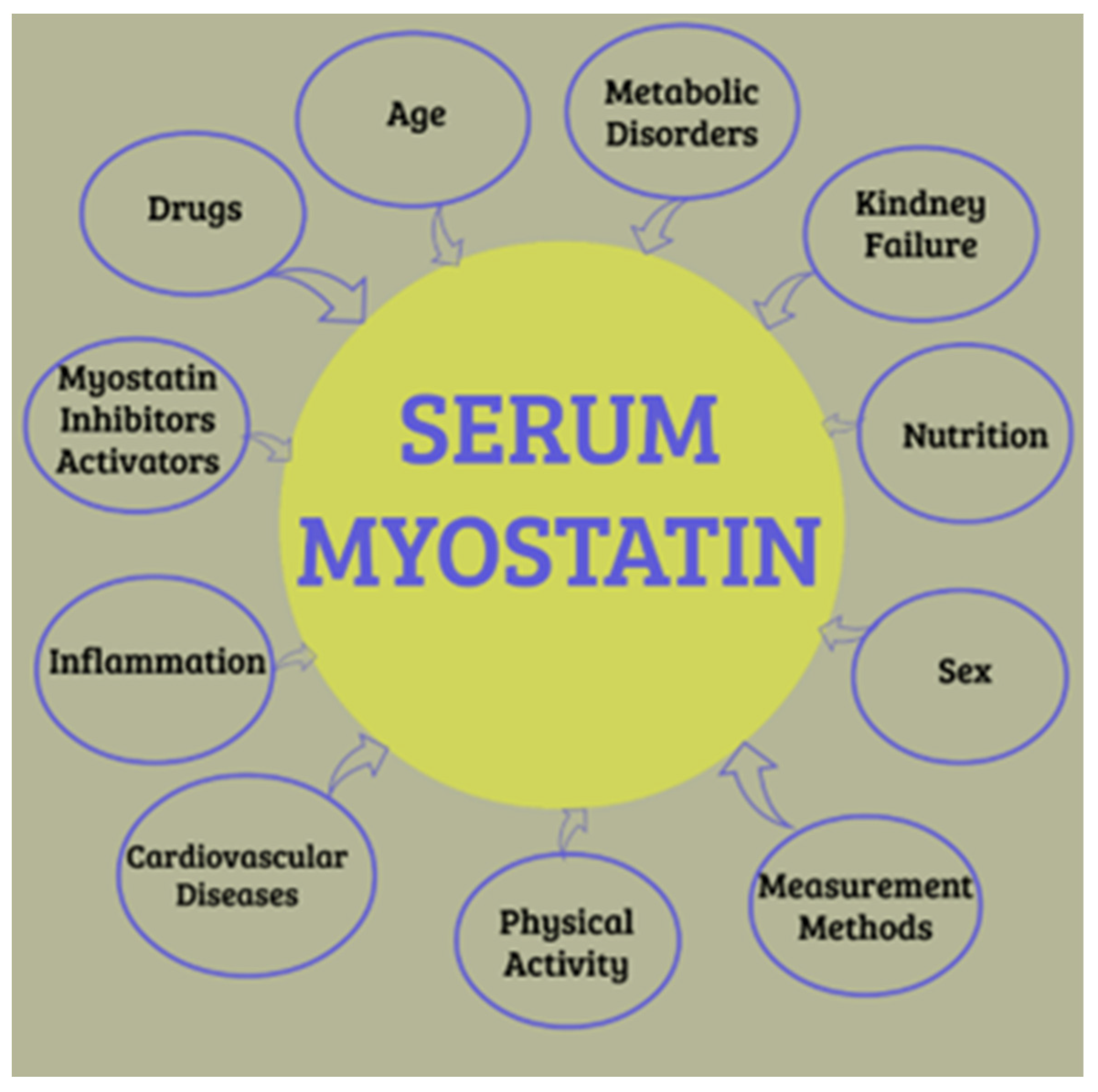
4.2. Myokines, Body Composition, and Inflammation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noseworthy, J.H.; Lucchinetti, C.; Rodriguez, M.; Weinshenker, B.G. Multiple Sclerosis. N. Engl. J. Med. 2000, 343, 938–952. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yu, K.; Shyh-Chang, N.; Jiang, Z.; Liu, T.; Ma, S.; Luo, L.; Guang, L.; Liang, K.; Ma, W.; et al. Pathogenesis of Sarcopenia and the Relationship with Fat Mass: Descriptive Review. J. Cachexia Sarcopenia Muscle 2022, 13, 781–794. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Jensen, G.L.; Ballesteros-Pomar, M.D.; Blaauw, R.; Correia, M.I.T.D.; Cuerda, C.; Evans, D.C.; Fukushima, R.; Ochoa Gautier, J.B.; Gonzalez, M.C.; et al. Guidance for Assessment of the Inflammation Etiologic Criterion for the GLIM Diagnosis of Malnutrition: A Modified Delphi Approach. Clin. Nutr. 2024, 43, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jiang, W.; Li, G.; Ding, Y.; Li, H.; Sun, J.; Chen, Y.; Wang, S.; Zhang, G. Inflammatory and Nutritional Markers as Indicators for Diagnosing and Assessing Disease Activity in MS and NMOSD. J. Inflamm. Res. 2024, 17, 10065–10078. [Google Scholar] [CrossRef]
- Arhire, L.I.; Mihalache, L.; Covasa, M. Irisin: A Hope in Understanding and Managing Obesity and Metabolic Syndrome. Front. Endocrinol. 2019, 10, 524. [Google Scholar] [CrossRef]
- Mazur-Bialy, A.I.; Pocheć, E.; Zarawski, M. Anti-Inflammatory Properties of Irisin, Mediator of Physical Activity, Are Connected with TLR4/MyD88 Signaling Pathway Activation. Int. J. Mol. Sci. 2017, 18, 701. [Google Scholar] [CrossRef]
- Zhang, Q.-X.; Zhang, S.-N.; Zhang, L.-J.; Zhang, D.-Q.; Yang, L. Irisin Levels in the Serum and Cerebrospinal Fluid of Patients with Multiple Sclerosis and the Expression and Distribution of Irisin in Experimental Autoimmune Encephalomyelitis. Clin. Exp. Immunol. 2021, 206, 208–215. [Google Scholar] [CrossRef]
- Piccirillo, R. Exercise-Induced Myokines with Therapeutic Potential for Muscle Wasting. Front. Physiol. 2019, 10, 287. [Google Scholar] [CrossRef]
- Azman, K.F.; Zakaria, R. Recent Advances on the Role of Brain-Derived Neurotrophic Factor (BDNF) in Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 6827. [Google Scholar] [CrossRef]
- Nociti, V.; Romozzi, M. The Role of BDNF in Multiple Sclerosis Neuroinflammation. Int. J. Mol. Sci. 2023, 24, 8447. [Google Scholar] [CrossRef]
- Neeper, S.A.; Góauctemez-Pinilla, F.; Choi, J.; Cotman, C. Exercise and Brain Neurotrophins. Nature 1995, 373, 109. [Google Scholar] [CrossRef]
- White, L.J.; Castellano, V. Exercise and Brain Health—Implications for Multiple Sclerosis: Part 1—Neuronal Growth Factors. Sports Med. 2008, 38, 91–100. [Google Scholar] [CrossRef]
- Xue, B.; Waseem, S.M.A.; Zhu, Z.; Alshahrani, M.A.; Nazam, N.; Anjum, F.; Habib, A.H.; Rafeeq, M.M.; Nazam, F.; Sharma, M. Brain-Derived Neurotrophic Factor: A Connecting Link Between Nutrition, Lifestyle, and Alzheimer’s Disease. Front. Neurosci. 2022, 16, 925991. [Google Scholar] [CrossRef] [PubMed]
- Elliott, B.; Renshaw, D.; Getting, S.; Mackenzie, R. The Central Role of Myostatin in Skeletal Muscle and Whole Body Homeostasis. Acta Physiol. 2012, 205, 324–340. [Google Scholar] [CrossRef] [PubMed]
- Baig, M.H.; Ahmad, K.; Moon, J.S.; Park, S.-Y.; Ho Lim, J.; Chun, H.J.; Qadri, A.F.; Hwang, Y.C.; Jan, A.T.; Ahmad, S.S.; et al. Myostatin and Its Regulation: A Comprehensive Review of Myostatin Inhibiting Strategies. Front. Physiol. 2022, 13, 876078. [Google Scholar] [CrossRef] [PubMed]
- Leal, L.G.; Lopes, M.A.; Batista, M.L., Jr. Physical Exercise-Induced Myokines and Muscle-Adipose Tissue Crosstalk: A Review of Current Knowledge and the Implications for Health and Metabolic Diseases. Front. Physiol. 2018, 9, 1307. [Google Scholar] [CrossRef]
- Ginevičienė, V.; Jakaitienė, A.; Pranckevičienė, E.; Milašius, K.; Utkus, A. Variants in the Myostatin Gene and Physical Performance Phenotype of Elite Athletes. Genes 2021, 12, 757. [Google Scholar] [CrossRef]
- Brandt, C.; Nielsen, A.R.; Fischer, C.P.; Hansen, J.; Pedersen, B.K.; Plomgaard, P. Plasma and Muscle Myostatin in Relation to Type 2 Diabetes. PLoS ONE 2012, 7, e37236. [Google Scholar] [CrossRef]
- Karczewski, J.; Zielińska, A.; Staszewski, R.; Eder, P.; Dobrowolska, A. Metabolic Link between Obesity and Autoimmune Diseases. Eur. Cytokine Netw. 2021, 32, 64–72. [Google Scholar] [CrossRef]
- Mogiłko, N.; Malgorzewicz, S. Prevalence of Poor Nutrition Status in Multiple Sclerosis Patients Assessed by Different Diagnostic Tools. Acta Biochim. Pol. 2023, 70, 343–345. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of Multiple Sclerosis: 2017 Revisions of the McDonald Criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Nazeri, M.; Bazrafshan, H.; Abolhasani Foroughi, A. Serum Inflammatory Markers in Patients with Multiple Sclerosis and Their Association with Clinical Manifestations and MRI Findings. Acta Neurol. Belg. 2022, 122, 1187–1193. [Google Scholar] [CrossRef]
- Ma, C.; Ding, H.; Deng, Y.; Liu, H.; Xiong, X.; Yang, Y. Irisin: A New Code Uncover the Relationship of Skeletal Muscle and Cardiovascular Health During Exercise. Front. Physiol. 2021, 12, 620608. [Google Scholar] [CrossRef]
- Tu, W.-J.; Qiu, H.-C.; Liu, Q.; Li, X.; Zhao, J.-Z.; Zeng, X. Decreased Level of Irisin, a Skeletal Muscle Cell-Derived Myokine, Is Associated with Post-Stroke Depression in the Ischemic Stroke Population. J. Neuroinflamm. 2018, 15, 133. [Google Scholar] [CrossRef] [PubMed]
- Koźma-Śmiechowicz, M.A.; Gajewski, B.; Fortak, P.; Gajewska, K.; Nowicki, M. Physical Activity, Body Composition, Serum Myokines and the Risk of Death in Hemodialysis Patients. Medicina 2023, 59, 2020. [Google Scholar] [CrossRef]
- Kerschensteiner, M.; Gallmeier, E.; Behrens, L.; Leal, V.V.; Misgeld, T.; Klinkert, W.E.F.; Kolbeck, R.; Hoppe, E.; Oropeza-Wekerle, R.-L.; Bartke, I.; et al. Activated Human T Cells, B Cells, and Monocytes Produce Brain-Derived Neurotrophic Factor In Vitro and in Inflammatory Brain Lesions: A Neuroprotective Role of Inflammation? J. Exp. Med. 1999, 189, 865–870. [Google Scholar] [CrossRef]
- Weinstock-Guttman, B.; Zivadinov, R.; Tamaño-Blanco, M.; Abdelrahman, N.; Badgett, D.; Durfee, J.; Hussein, S.; Feichter, J.; Patrick, K.; Benedict, R.; et al. Immune Cell BDNF Secretion Is Associated with White Matter Volume in Multiple Sclerosis. J. Neuroimmunol. 2007, 188, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Patanella, A.K.; Zinno, M.; Quaranta, D.; Nociti, V.; Frisullo, G.; Gainotti, G.; Tonali, P.A.; Batocchi, A.P.; Marra, C. Correlations between Peripheral Blood Mononuclear Cell Production of BDNF, TNF-Alpha, IL-6, IL-10 and Cognitive Performances in Multiple Sclerosis Patients. J. Neurosci. Res. 2010, 88, 1106–1112. [Google Scholar] [CrossRef]
- Murillo-Saich, J.D.; Vazquez-Villegas, M.L.; Ramirez-Villafaña, M.; Saldaña-Cruz, A.M.; Aceves-Aceves, J.A.; Gonzalez-Lopez, L.; Guma, M.; Gamez-Nava, J.I. Association of Myostatin, a Cytokine Released by Muscle, with Inflammation in Rheumatoid Arthritis: A Cross-Sectional Study. Medicine 2021, 100, e24186. [Google Scholar] [CrossRef]
- Su, C.; Hu, S.; Sun, Y.; Zhao, J.; Dai, C.; Wang, L.; Xu, G.; Tang, C. Myostatin Induces Tumor Necrosis Factor-α Expression in Rheumatoid Arthritis Synovial Fibroblasts through the PI3K–Akt Signaling Pathway. J. Cell. Physiol. 2019, 234, 9793–9801. [Google Scholar] [CrossRef]
- Zhang, L.; Rajan, V.; Lin, E.; Hu, Z.; Han, H.Q.; Zhou, X.; Song, Y.; Min, H.; Wang, X.; Du, J.; et al. Pharmacological Inhibition of Myostatin Suppresses Systemic Inflammation and Muscle Atrophy in Mice with Chronic Kidney Disease. FASEB J. 2011, 25, 1653–1663. [Google Scholar] [CrossRef] [PubMed]
- Baczek, J.; Silkiewicz, M.; Wojszel, Z.B. Myostatin as a Biomarker of Muscle Wasting and Other Pathologies-State of the Art and Knowledge Gaps. Nutrients 2020, 12, 2401. [Google Scholar] [CrossRef] [PubMed]

| (median 1.77) | (median 1.73) | ||
| Min-Max: 0.4–5.01 | Min-Max: 0.4–4.67 | ||
| Parameters | MS (n= 92) | Healthy Control (n= 75) | p-Value |
| Age (yr.) | 46.57 ± 11.7 | 51.2 ± 13.2 | p = 0.05 |
| (median:46) | (median:61) | ||
| Min-Max 24–71 | Min-Max 35–70 | ||
| Body weight (kg) | 74.50 ± 16.73 | 74.83 ± 14.70 | p = 0.78 |
| (median: 73.60) | (median: 73.60) | ||
| Min-Max: 45.00–130.00 | Min-Max: 49.40–120.00 | ||
| Fat mass (kg) | 23.77 ± 10.17 | 25.72 ± 9.32 | p = 0.45 |
| (median: 24.83) | (median: 24.83) | ||
| Min-Max: 5.80–65.90 | Min-Max: 7.60–44.00 | ||
| Muscle mass (kg) | 30.20 ± 7.40 | 28.90 ± 6.90 | p = 0.35 |
| (median: 29.70) | (median: 28.90) | ||
| Min-Max: 16.00–50.00 | Min-Max: 15.00–47.00 | ||
| Body fat (%) | 31.3 ± 8.7 | 35.4 ± 7.4 | p = 0.61 |
| (median: 31.7) | (median: 33.0) | ||
| Min-Max: 13.2–50.7 | Min-Max: 13–50 | ||
| Total water (L) | 37.1 ± 8.2 | 35.4 ± 7.4 | |
| (median: 34.6) | (median: 33.7) | ||
| Min-Max: 24.4–59.8 | Min-Max: 14.5–52.4 | ||
| BMI | 25.83 ± 5.05 | 27.35 ± 4.35 | p = 0.12 |
| (median: 25.60) | (median: 27.10) | ||
| Min-Max: 16.70–43.40 | Min-Max: 20.30–37.80 | ||
| UBWL (kg) | 1.75 ± 2.53 | 0 | p = 0.00 |
| (median 1.75) | (median: 0) | ||
| Min-Max: 3.31–6.81 | Min-Max: 0 | ||
| Total cholesterol (mg/dl) | 200.2 ± 45.6 | 198.3 ± 42.3 | p = 0.65 |
| (median: 201.0) | (median: 198.3) | ||
| Min-Max: 140.0–300.0 | Min-Max: 130.0–290.0 | ||
| HDL cholesterol (mg/dl) | 61.9 ± 16.4 | 57.9 ± 14.3 | p = 0.13 |
| (median: 60.8) | (median: 68) | ||
| Min-Max: 37.8–131 | Min-Max: 22.8–87 | ||
| LDL cholesterol (mg/dl) | 130 ± 39.8 | 69.8 ± 26.2 | p = 0.04 |
| (median: 124) | (median: 69.8) | ||
| Min-Max: 53–242 | Min-Max: 40–177 | ||
| Albumin (g/L) | 39.4 ± 4.0 | 41.2 ± 3.8 | p = 0.02 |
| (median: 40.0) | (median: 41.2) | ||
| Min-Max: 25.0–47.0 | Min-Max: 28.0–45.0 | ||
| hsCRP (mg/L) | 4.4 ± 13.4 | 2.1 ± 7.2 | p = 0.03 |
| (median: 2.6) | (median: 2.1) | ||
| Min-Max: 0.5–40.0 | Min-Max: 0.2–20.0 | ||
| Irisin (ng/mL) | 497.21 ± 690.1 | 765.85 ± 799.35 | p = 0.02 |
| (median: 181.54) | (median: 369.39) | ||
| Min-Max: 15.84–2714.38 | Min-Max: 15.9–2704.4 | ||
| IL-6 (pg/mL) | 1.86 ± 3.12 | 1.32 ± 1.22 | p = 0.49 |
| (median: 0.97) | (median: 1.32) | ||
| Min-Max: 0.22–21.8 | Min-Max: 0.30–3.76 | ||
| BDNF (pg/mL) | 387.47 ± 647.82 | 365.77 ± 436.21 | p = 0.90 |
| (median: 387.47) | (median: 365.77) | ||
| Min-Max: 0.40–1683.11 | Min-Max: 0.41–1238.19 | ||
| Myostatin (ng/mL) | 1.77 ± 1.62 | 1.73 ± 1.47 | p = 0.93 |
| (median: 1.77) | (median: 1.73) | ||
| Min-Max: 0.4–5.01 | Min-Max: 0.4–4.67 |
| (median 1.77) | (median 1.73) | (median 1.73) | ||
| Min-Max: 0.00–5.01 | Min-Max: 0.00–4.67 | Min-Max: 0.4–4.67 | ||
| Parameters | At Risk (n= 62) | Malnourished (n= 13) | Healthy Control (n= 75) | * p-Value at Risk vs. Malnourished |
| Age (yr.) | 46.35 ± 12.63 | 38.69 ± 10.26 | 51.2 ± 13.2 ** | p = 0.04 |
| (median 46.35) | (median 38.69) | (median 61) | ||
| Min-Max: 21.09–71.61 | Min-Max: 18.17–59.21 | Min-Max: 35–70 | ||
| Body weight (kg) | 79.38 ± 15.91 | 55.45 ± 8.71 | 74.83 ± 14.70 ** | p = 0.000001 |
| (median 79.38) | (median 55.45) | (median: 73.60) | ||
| Min-Max: 47.56–111.20 | Min-Max: 38.03–72.87 | Min-Max: 49.40–120.00 | ||
| Fat mass (kg) | 27.29 ± 9.39 | 13.53 ± 6.10 | 25.72 ± 9.32 ** | p = 0.000003 |
| (median 27.29) | (median 13.53) | (median: 24.83) | ||
| Min-Max: 8.51–46.07 | Min-Max: 1.33–25.73 | Min-Max: 7.60–44.00 | ||
| Muscle Mass (kg) | 28.87 ± 7.31 | 22.79 ± 3.94 | 28.90 ± 6.90 ** | p = 0.005 |
| (median 28.87) | (median 22.79) | (median: 28.90) | ||
| Min-Max: 14.25–43.49 | Min-Max: 14.91–30.67 | Min-Max: 15.00–47.00 | ||
| Body fat (%) | 34.23 ± 8.07 | 24.68 ± 7.34 | 35.4 ± 7.4 ** | p = 0.0002 |
| (median 34.23) | (median 24.68) | (median 33) | ||
| Min-Max: 18.09–50.37 | Min-Max: 10.00–39.36 | Min-Max: 13–50 | ||
| Total water (L) | 38.07 ± 8.88 | 30.75 ± 4.85 | 35.4 ± 7.4 | p = 0.005 |
| (median 38.07) | (median 30.75) | (median 33.7) | ||
| Min-Max: 20.31–55.83 | Min-Max: 21.05–40.45 | Min-Max: 14.5–52.4 | ||
| BMI (kg/m2) | 27.52 ± 4.74 | 19.53 ± 1.84 | 27.35 ± 4.35 ** | p = 0.000 |
| (median 27.52) | (median 19.53) | (median: 27.10) | ||
| Min-Max: 18.04–37.00 | Min-Max: 15.85–23.21 | Min-Max: 20.30–37.80 | ||
| Body weight loss (kg) | 0.00 ± 0.00 | 1.75 ± 2.53 | 0 ** | p = 0.000 |
| (median 0.00) | (median 1.75) | (median 0) | ||
| Min-Max: 0.00–0.00 | Min-Max: 3.31–6.81 | Min-Max: 0 | ||
| Total cholesterol (mg/dl) | 224.37 ± 47.40 | 194.77 ± 46.64 | 198.3 ± 42.3 ** | p = 0.045 |
| (median 224.37) | (median 194.77) | (median: 198.3) | ||
| Min-Max: 129.57–319.17 | Min-Max: 101.49–288.05 | Min-Max: 130.0–290.0 | ||
| HDL cholesterol (mg/dl) | 61.10 ± 16.76 | 66.98 ± 20.99 | 57.9 ± 14.3 | p = 0.278 |
| (median 61.10) | (median 66.98) | (median 68) | ||
| Min-Max: 27.58–94.62 | Min-Max: 25.00–108.96 | Min-Max: 22.8–87 | ||
| LDL cholesterol (mg/dl) | 137.43 ± 37.82 | 110.92 ± 34.04 | 68.1 ± 27.1 ** | p = 0.026 |
| (median 137.43) | (median 110.92) | (median 66.5) | ||
| Min-Max: 61.79–213.07 | Min-Max: 42.84–179 | Min-Max: 23–177 | ||
| hsCRP (mg/L) | 2.88 ± 2.20 | 4.96 ± 3.20 | 2.1 ± 7.2 ** | p = 0.006 |
| (median 2.88) | (median 4.96) | (median: 2.1) | ||
| Min-Max: 0.21–7.28 | Min-Max: 0.20–11.36 | Min-Max: 0.2–20.0 | ||
| Irisin (ng/mL) | 237.14 ± 233.67 | 75.65 ± 85.28 | 765.85 ± 799.35 ** | p = 0.020 |
| (median 237.14) | (median 75.65) | (median: 369.39) | ||
| Min-Max: 15.11–704.48 | Min-Max: 15.40–246.21 | Min-Max: 15.9–2704.4 | ||
| IL-6 (pg/mL) | 1.97 ± 3.38 | 1.32 ± 1.22 | 1.32 ± 1.22 | p = 0.494 |
| (median 1.97) | (median 1.32) | (median: 1.32) | ||
| Min-Max: 0.00–8.73 | Min-Max: 0.00–3.76 | Min-Max: 0.30–3.76 | ||
| BDNF (pg/mL) | 387.47 ± 647.82 | 365.77 ± 436.21 | 365.77 ± 436.21 | p = 0.909 |
| (median 387.47) | (median 365.77) | (median: 365.77) | ||
| Min-Max: 0.00–1683.11 | Min-Max: 0.00–1238.19 | Min-Max: 0.41–1238.19 | ||
| Myostatin (ng/mL) | 1.77 ± 1.62 | 1.73 ± 1.47 | 1.73 ± 1.47 | p = 0.935 |
| (median 1.77) | (median 1.73) | (median: 1.73) | ||
| Min-Max: 0.00–5.01 | Min-Max: 0.00–4.67 | Min-Max: 0.4–4.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mogiłko, N.; Małgorzewicz, S. The Association Between Myokines, Inflammation, and Nutritional Status in Patients with Multiple Sclerosis. Biomolecules 2025, 15, 703. https://doi.org/10.3390/biom15050703
Mogiłko N, Małgorzewicz S. The Association Between Myokines, Inflammation, and Nutritional Status in Patients with Multiple Sclerosis. Biomolecules. 2025; 15(5):703. https://doi.org/10.3390/biom15050703
Chicago/Turabian StyleMogiłko, Natalia, and Sylwia Małgorzewicz. 2025. "The Association Between Myokines, Inflammation, and Nutritional Status in Patients with Multiple Sclerosis" Biomolecules 15, no. 5: 703. https://doi.org/10.3390/biom15050703
APA StyleMogiłko, N., & Małgorzewicz, S. (2025). The Association Between Myokines, Inflammation, and Nutritional Status in Patients with Multiple Sclerosis. Biomolecules, 15(5), 703. https://doi.org/10.3390/biom15050703





