Post-Translational Modifications in Multiple Myeloma: Mechanisms of Drug Resistance and Therapeutic Opportunities
Abstract
1. Introduction
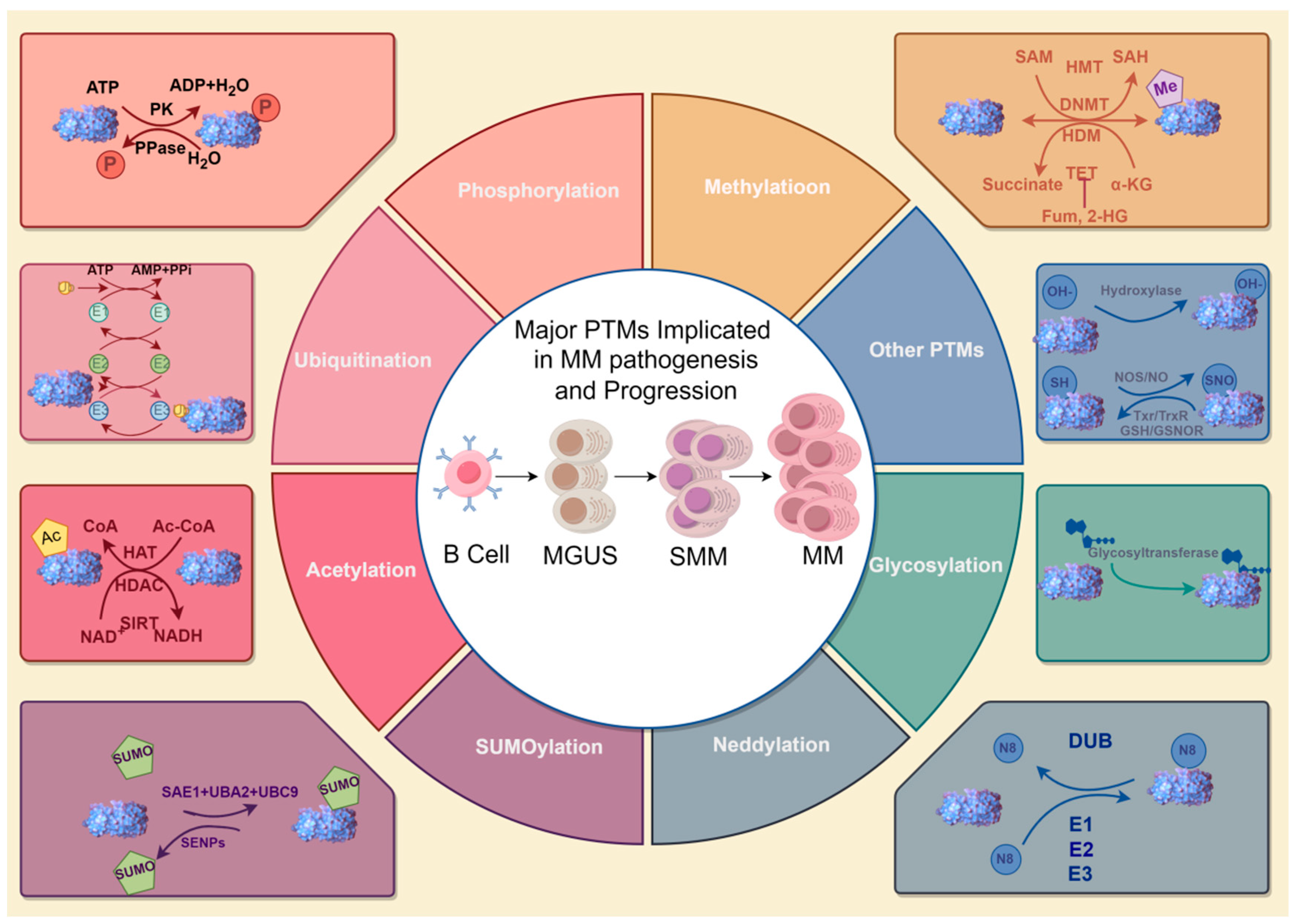
2. Major PTMs Implicated in MM Pathogenesis and Progression
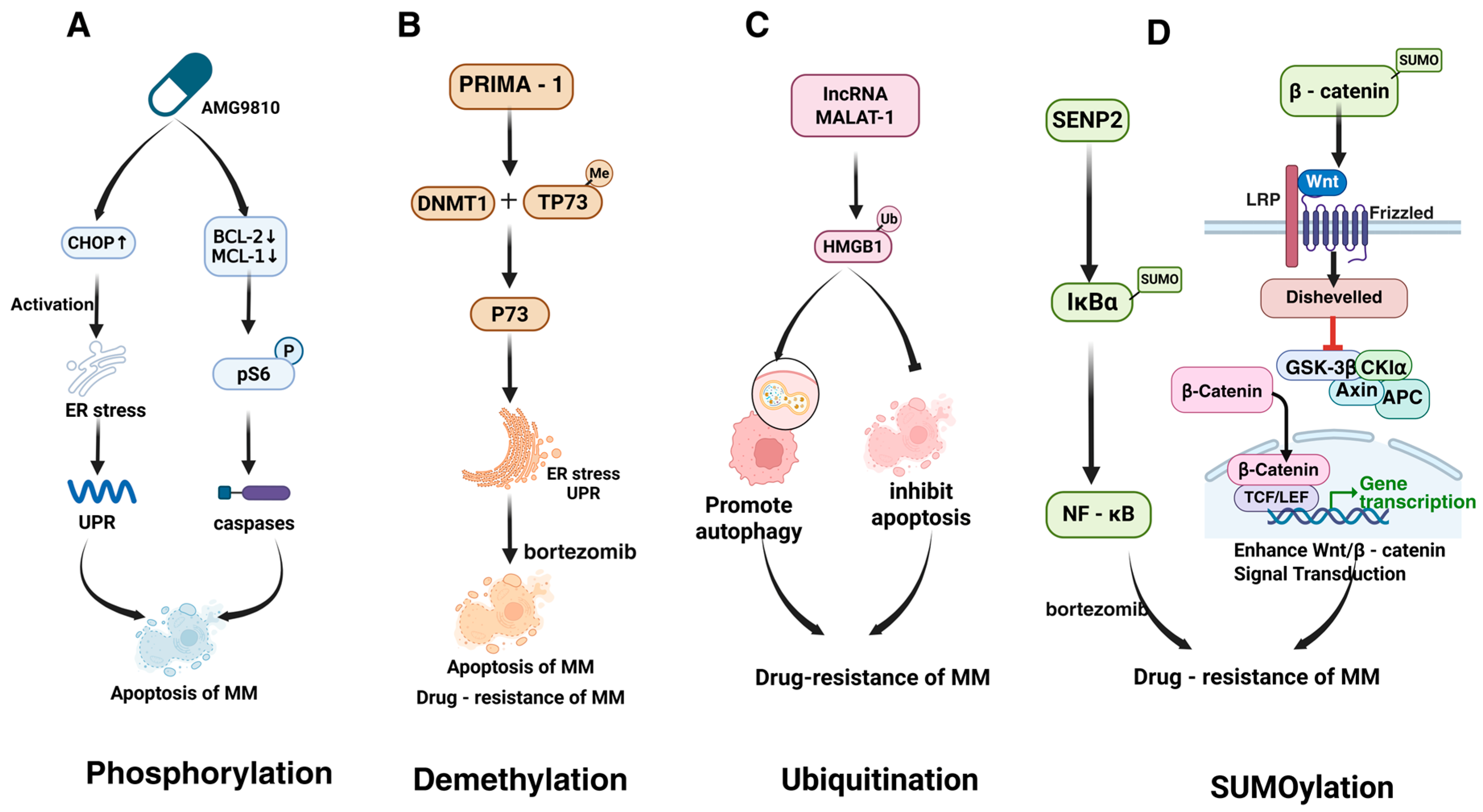
2.1. Phosphorylation
2.2. Acetylation
2.3. Ubiquitination
2.4. Methylation
2.5. SUMOylation
2.6. Neddylation
2.7. Glycosylation
2.8. Other PTMs
| PTM Type | Key Proteins/Molecules | Mechanism in MM | References |
|---|---|---|---|
| Phosphorylation | |||
| STAT3 (Tyr705) | Phosphorylated STAT3 activates NF-κB/STAT3 signaling, promoting proliferation, survival, and immune evasion. | [28,29] | |
| AMPK/mTOR | Phosphorylation regulates metabolic reprogramming; hyperactivation supports MM survival under stress. | [30,82] | |
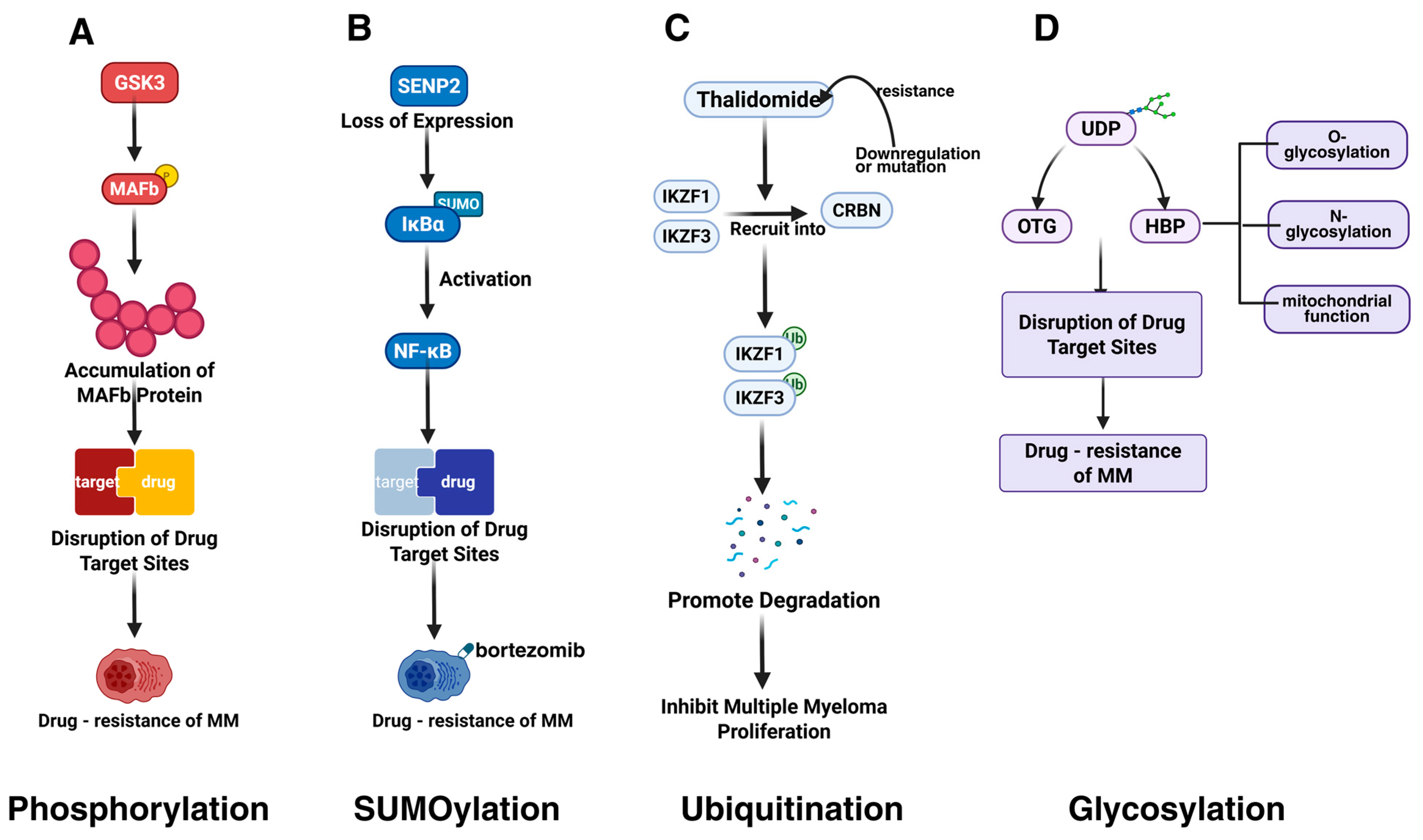
| MAFb | GSK3-mediated phosphorylation stabilizes MAFb, disrupting proteasome inhibitor targets and inducing resistance. | [83] | |
| Akt/GSK-3β | Akt phosphorylates and inactivates GSK-3β, stabilizing c-Myc to drive drug resistance. | [84] | |
| MKK4/7-JNK | GCK-induced phosphorylation activates RAS-mutant MAPK signaling, promoting adaptive resistance. | [29] | |
| Acetylation | |||
| ADA2B (SAGA complex) | Acetylates histones to regulate c-Myc expression, sustaining oncogenic programs. | [37] | |
| KDM6A | Modulates H3K27 acetylation to suppress immune recognition genes, enabling immune evasion. | [38,39] | |
| YWHAZ | Acetylation by ENO1 enhances mitophagy, supporting metabolic adaptation in MM progression. | [40] | |
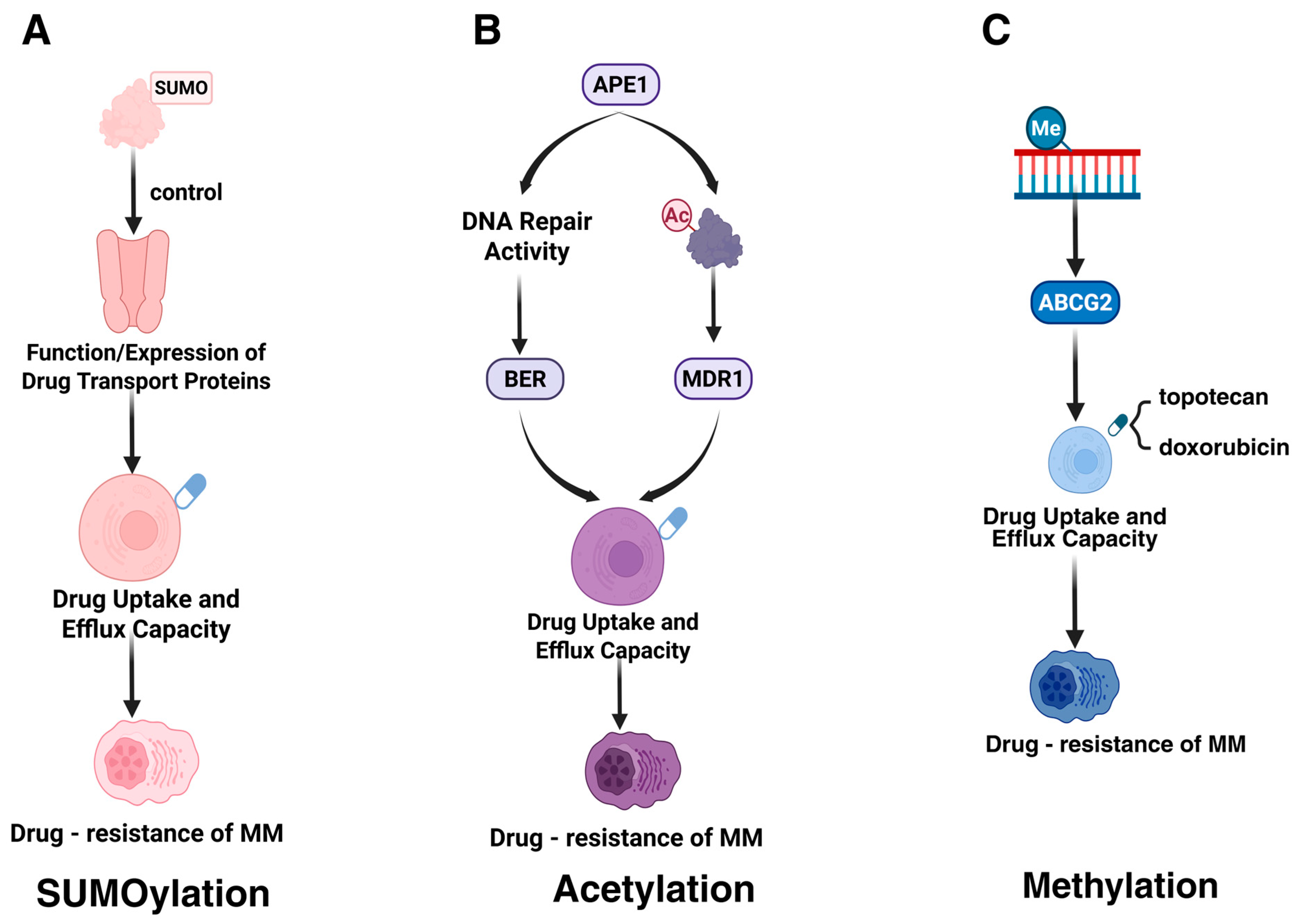
| APE1 (K6/K7) | Acetylation enhances DNA repair via BER and upregulates MDR1, promoting melphalan resistance. | [36] | |
| Ubiquitination | |||
| CRL4CRBN | IMiDs recruit CRBN to ubiquitinate IKZF1/3 for proteasomal degradation; mutations confer resistance. | [48] | |
| IKZF1/IKZF3 | Degradation by IMiDs suppresses MM survival; loss of ubiquitination leads to IMiD resistance. | [85,86] | |
| HMGB1 | MALAT-1-induced ubiquitination promotes autophagy and inhibits apoptosis, driving drug resistance. | [87] | |
| BRCC36 | Cleaves K63-ubiquitin chains on CRBN, stabilizing it to enhance IMiD sensitivity. | [49] | |
| NEK2/USP7/TRIP13 | USP7 deubiquitinates NEK2, stabilizing it to promote chromosomal instability and PI resistance. | [51,88] | |
| Methylation | |||
| MMSET (NSD2) | Catalyzes H3K36me2, dysregulating tumor suppressor genes and promoting MM progression. | [55,56] | |
| SOCS1 | Methylation silences SOCS1, enhancing cytokine signaling and resistance to apoptosis. | [60] | |
| EGLN3 | Methylation of EGLN3 (prolyl hydroxylase) correlates with hypoxia adaptation and poor prognosis. | [79] | |
| SUMOylation | |||
| β-catenin | SUMOylation stabilizes β-catenin, activating Wnt signaling to drive proliferation and drug resistance. | [65] | |
| IκBα | SENP2 deficiency increases SUMOylation of IκBα, activating NF-κB and bortezomib resistance. | [89] | |
| IRF4/c-Myc | SUMOylation stabilizes IRF4/c-Myc; inhibition by TAK-981 restores lenalidomide sensitivity. | [90] | |
| Neddylation | |||
| CRLs (Cullin-RING ligases) | Neddylation activates CRLs; inhibition by MLN4924 stabilizes pro-apoptotic proteins (e.g., NOXA). | [69,91] | |
| REDD1 | Neddylation blockade stabilizes REDD1, inhibiting PI3K/AKT/mTOR and overcoming resistance. | [91] | |
| Glycosylation | |||
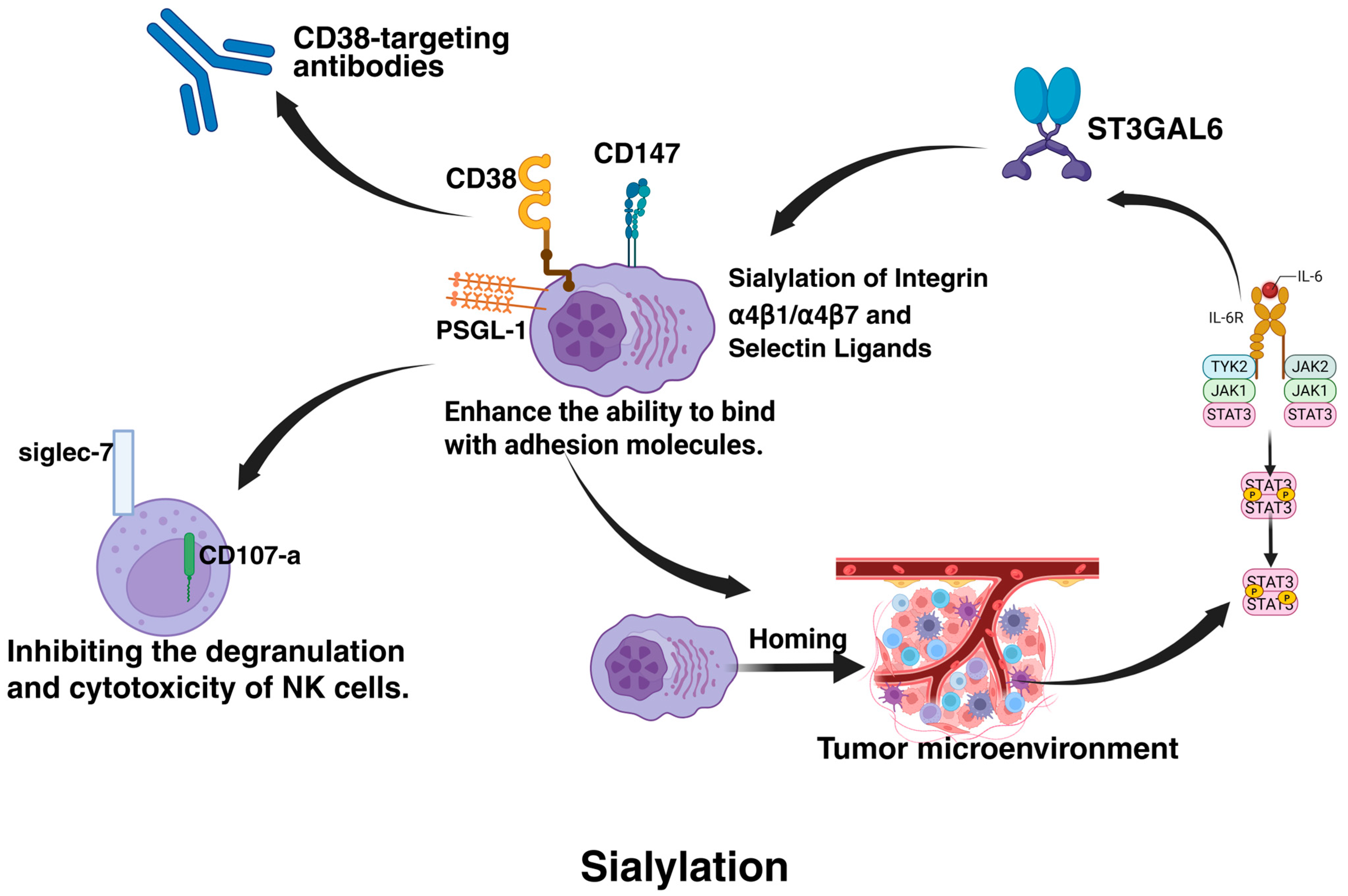
| α4β1/α4β7 integrins | Sialylation enhances adhesion to bone marrow stroma, promoting CAM-DR and drug sanctuary. | [92,93,94] | |
| CD38/PSGL-1 | Sialylation masks CD38 epitopes (daratumumab resistance); PSGL-1 binds Siglec-7 to suppress NK activity. | [76,93] | |
| IgG (Fab region) | Altered glycosylation in the Fab region correlates with disease progression and bone loss. | [72,78] | |
| Hydroxylation | |||
| HIF-1α | Hydroxylation stabilizes HIF-1α under hypoxia, promoting tumor adaptation and survival. | [79] | |
| Nitrosylation | |||
| STAT3/NF-κB | S-nitrosylation inhibits STAT3/NF-κB activity; SNAC reverses hyperactivation to restore drug sensitivity. | [81] | |
| Deubiquitination | |||
| USP14/UCHL5 | Deubiquitinate misfolded proteins to reduce proteotoxic stress, conferring PI resistance. | [53] | |
| Deacetylation | |||
| HDAC1 | Deacetylates histones and non-histones (e.g., HPV), enhancing DNA repair and resistance to DNA damage. | [95] | |
3. PTM Targeting Therapies in MM
3.1. PIs
3.2. IMiDs
3.3. Histone Deacetylase (HDAC) Inhibitors
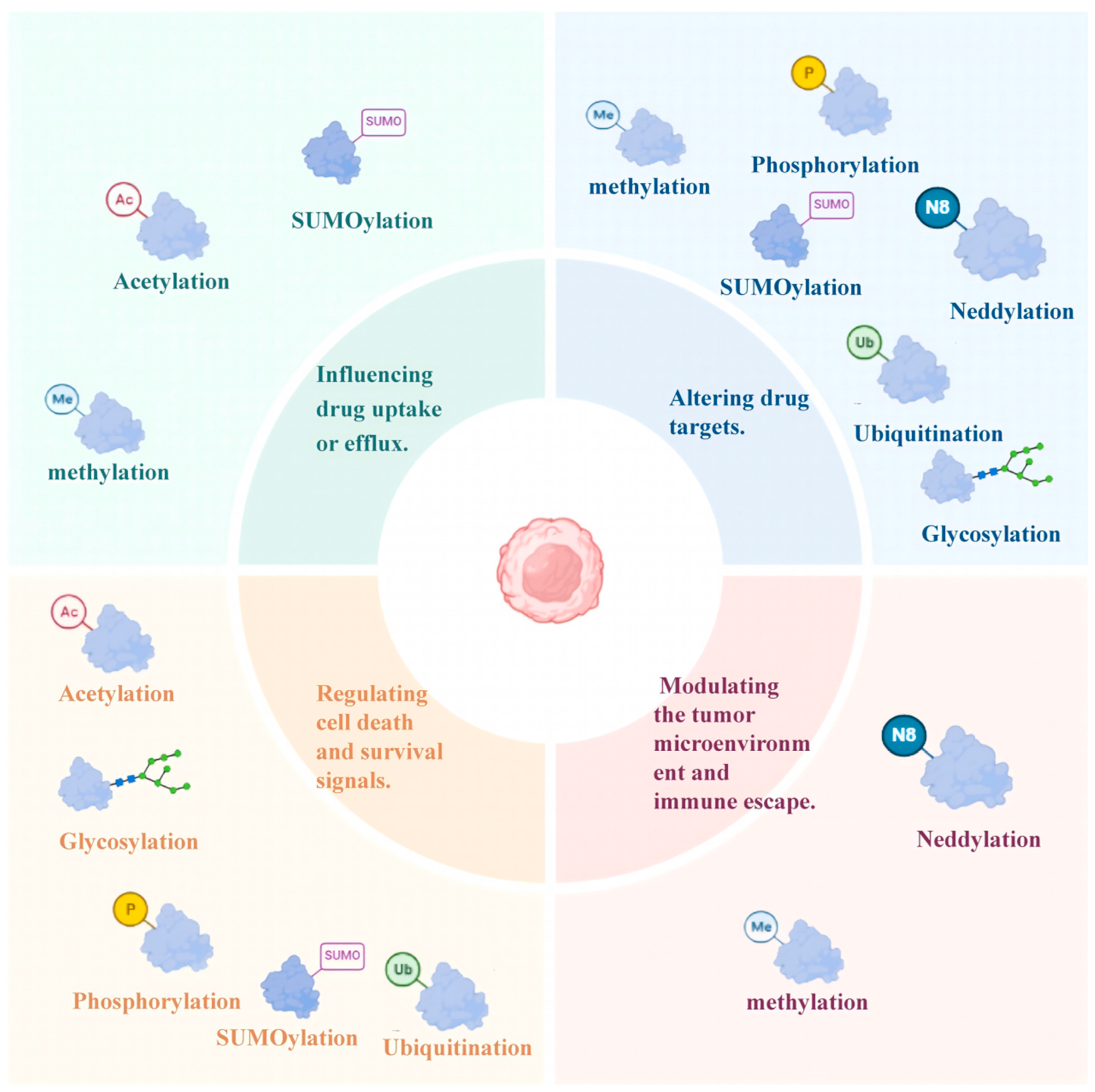
4. Role of PTMs in Drug Resistance Mechanisms in MM
4.1. Influencing Drug Uptake or Efflux
4.1.1. SUMOylation
4.1.2. Acetylation
4.1.3. Methylation
4.2. Altering Drug Targets
4.2.1. Phosphorylation
4.2.2. SUMOylation
4.2.3. Ubiquitination
4.2.4. Deubiquitination
4.2.5. Glycosylation
4.2.6. Neddylation
4.3. Regulating Cell Death and Survival Signals
4.3.1. Phosphorylation
4.3.2. Demethylation
4.3.3. Ubiquitination
4.3.4. Deubiquitination
4.3.5. Glycosylation
4.3.6. SUMOylation
4.3.7. Deacetylation
4.4. Modulating the Tumor Microenvironment and Immune Escape
4.4.1. Sialylation
4.4.2. Methylation
4.4.3. Neddylation
5. Overcoming Drug Resistance in MM via Targeting PTMs
| Strategy Category | Drug/Technology Name | Targeted PTM | Target/Pathway | Mechanism of Action | Ref |
|---|---|---|---|---|---|
| FDA-Approved Drugs | |||||
| Tyrosine Kinase Inhibitors (TKI) | Dasatinib | Phosphorylation | Src, BCR-ABL | Inhibits Src and BCR-ABL kinase activity, blocks PI3K/AKT and MAPK phosphorylation signaling, reverses resistance to PIs (PIs, e.g., bortezomib) and IMiDs (IMiDs, e.g., lenalidomide). | [135,136,137,138] |
| Tyrosine Kinase Inhibitors (TKI) | Imatinib | Phosphorylation | BCR-ABL | Binds specifically to the ATP-binding site of the BCR-ABL fusion protein, inhibits STAT5 and RAS/MAPK phosphorylation, and suppresses MM (MM) cell proliferation. | [135,136,137] |
| mTOR Inhibitor | Everolimus | Phosphorylation | mTORC1 (not mTORC2) | Inhibits mTORC1 phosphorylation activity, downregulates 4EBP1 and S6K1 signaling, blocks protein synthesis, and enhances sensitivity to PIs. | [135,136,137] |
| HDAC Inhibitor | Panobinostat | Acetylation, SUMOylation (indirect) | HDAC1/2/3/6 | Inhibits HDAC6-mediated deacetylation of SUMOylation enzymes (e.g., SENP1), increases histone (H3K9/K14) and non-histone (e.g., HSP90) acetylation, and activates pro-apoptotic genes (BIM, NOXA). | [139,140,141,142,143] |
| HDAC Inhibitor | Vorinostat | Acetylation | HDAC1/2/3 (Class I) | Selectively inhibits Class I HDACs, enhances histone H3/H4 acetylation, promotes RelA acetylation to block its nuclear translocation, and reverses NF-κB-mediated drug resistance. | [141,142,143] |
| Preclinical Inhibitors | |||||
| SUMO E1 Inhibitor | TAK-981 | SUMOylation | SUMO E1 enzyme (SAE1/SAE2) | Covalently inhibits SUMO activation, reduces SUMO modification of IRF4 and c-Myc, and enhances CRBN-dependent degradation induced by lenalidomide. | [90,144,145,146] |
| Neddylation Inhibitor | MLN4924 | Ubiquitination (via Neddylation) | NEDD8-activating enzyme (NAE) | Inhibits neddylation of CRL complexes, leading to accumulation of pro-apoptotic proteins (NOXA, BIM) and stabilization of IκBα to suppress NF-κB signaling. | [69,147] |
| Glycosylation Inhibitor | OGT inhibitors (e.g., OSMI-1) | Glycosylation (O-GlcNAcylation) | O-GlcNAc transferase (OGT) | Reduces O-GlcNAc modification of β-catenin (Ser112) and c-Myc, inhibits Wnt/β-catenin and MYC signaling, and reverses bortezomib resistance. | [129,148] |
| Gene Editing Technologies | |||||
| CRISPR/Cas9 | Ubc9/USP7 knockout | SUMOylation, Ubiquitination | Ubc9 (SUMO E2), USP7 | Knockout of Ubc9 blocks SUMO-PML modification and inhibits DNA repair; knockout of USP7 stabilizes p53 and PTEN protein levels, inducing apoptosis. | [149,150,151,152,153,154,155] |
| siRNA/shRNA | SAE2/USP14 targeting | SUMOylation, Ubiquitination | SAE2, USP14 | siRNA-mediated SAE2 silencing inhibits SUMO activation; shRNA-mediated USP14 silencing enhances ubiquitinated protein degradation and downregulates IRF4. | [156,157,158,159,160,161] |
| ASO | MDM2/USP7 targeting | Ubiquitination | MDM2, USP7 | Antisense oligonucleotides (ASOs) suppress MDM2 or USP7 expression, stabilize ubiquitination levels of p53 or PTEN, and promote apoptosis. | [162,163] |
| Novel Therapeutic Modalities | |||||
| PROTACs | IRF4/c-Myc degraders | Ubiquitination | IRF4, c-Myc | Bifunctional molecules recruit CRBN or VHL to induce ubiquitination and degradation of IRF4 or c-Myc, directly eliminating drug resistance-associated transcription factors. | [164,165,166,167,168] |
| LYSOTACs | Wnt pathway-targeted degraders | Lysosomal degradation | LRP6, β-catenin | Target LRP6 or β-catenin for lysosomal degradation, inhibits Wnt signaling, and restore bortezomib sensitivity. | [169,170,171,172,173,174,175,176] |
| Combination Therapies | |||||
| SUMO inhibitor + PI | TAK-981 + Bortezomib | SUMOylation + Ubiquitination | SUMO pathway + Proteasome | TAK-981 induces misfolded protein accumulation, synergizing with bortezomib to activate the UPR (UPR) and ER stress, triggering apoptosis. | [70,111] |
| HDAC inhibitor + IMiD | Panobinostat + Lenalidomide | Acetylation + Ubiquitination | HDACs + CRBN | Panobinostat upregulates CRBN expression, enhancing lenalidomide-induced ubiquitination and degradation of IKZF1/3, thereby suppressing the IRF4-MYC axis. | [40,84,177] |
5.1. FDA-Approved Drugs Targeting PTMs
5.2. Preclinical Inhibitors Targeting PTMs
5.3. Gene Editing Technologies
5.3.1. CRISPR/Cas9
5.3.2. Oligonucleotide-Based Interventions
5.4. Novel Therapeutic Modalities
5.4.1. Proteolysis-Targeting Chimeras (PROTACs)
5.4.2. Lysosome-Targeting Chimeras (LYSOTACs)
5.5. Combination Therapies
5.6. Personalized Therapy
5.6.1. Personalized CAR-T Therapy
5.6.2. Targeted Interventions in Epigenetic Modifiers and Personalized Therapy
6. The Challenges in Targeting PTMs for Therapy
6.1. Complexity and Dynamic Nature of PTMs
6.2. Heterogeneity and Plasticity of MM Cells
6.3. Technological Limitations
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABC | ATP Binding Cassette |
| ASOs | Antisense Oligonucleotides |
| BER | Base Excision Repair |
| BiTEs | Bispecific T-Cell Engagers |
| BCMA | B Cell Maturation Antigen |
| BMM | Bone Marrow Microenvironment |
| CAM DR | Cell Adhesion-Mediated Drug Resistance |
| CAR-T | Chimeric Antigen Receptor T-Cell |
| CHOP | C EBP Homologous Protein |
| CIN | Chromosomal Instability |
| CRBN | Cereblon |
| CRLs | Cullin-RING Ligases |
| DHA | Docosahexaenoic Acid |
| DUBs | Deubiquitinating Enzymes |
| EPA | Eicosapentaenoic Acid |
| ER | Endoplasmic Reticulum |
| GWAS | Genome-Wide Association Studies |
| HATs | Histone Acetyltransferases |
| HBP | Hexosamine Biosynthesis Pathway |
| HDACs | Histone Deacetylases |
| HIF-1α | Hypoxia Inducible Factor 1 Alpha |
| IMiDs | Immunomodulatory Drugs |
| IRF4 | Interferon Regulatory Factor 4 |
| ISS | International Staging System |
| LLPS | Liquid-Liquid Phase Separation |
| LYSOTACs | Lysosome-Targeting Chimeras |
| mAbs | Monoclonal Antibodies |
| MDR1 | Multidrug Resistance Protein 1 |
| MM | Multiple Myeloma |
| mTOR | Mammalian Target of Rapamycin |
| NAE | NEDD8-Activating Enzyme |
| NF-κB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells |
| OGT | O-GlcNAc Transferase |
| OS | Overall Survival |
| PFS | Progression-Free Survival |
| PIs | Proteasome Inhibitors |
| PROTACs | Proteolysis-Targeting Chimeras |
| PTMs | Post Translational Modifications |
| RRMM | Relapsed Refractory Multiple Myeloma |
| SENPs | SUMO-Specific Proteases |
| SNAC | S-Nitroso-N-Acetylcysteine |
| SOCS1 | Suppressor of Cytokine Signaling 1 |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| STs | Sialyltransferases |
| TKIs | Tyrosine Kinase Inhibitors |
| TRPV1 | Transient Receptor Potential Vanilloid 1 |
| UCHs | Ubiquitin C-Terminal Hydrolases |
| UPR | Unfolded Protein Response |
| UPS | Ubiquitin-Proteasome System |
| USPs | Ubiquitin-Specific Proteases |
| Wnt | Wingless Integrated Signaling Pathway |
References
- Kazandjian, D. Multiple myeloma epidemiology and survival: A unique malignancy. Semin. Oncol. 2016, 43, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Brigle, K.; Rogers, B. Pathobiology and Diagnosis of Multiple Myeloma. Semin. Oncol. Nurs. 2017, 33, 225–236. [Google Scholar] [CrossRef]
- Rodriguez-Otero, P.; Ailawadhi, S.; Arnulf, B.; Patel, K.; Cavo, M.; Nooka, A.K.; Manier, S.; Callander, N.; Costa, L.J.; Vij, R.; et al. Ide-cel or Standard Regimens in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2023, 388, 1002–1014. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.A.; Mouhieddine, T.H.; Ortiz, R.J.; Richter, J. Revisiting the role of alkylating agents in multiple myeloma: Up-to-date evidence and future perspectives. Crit. Rev. Oncol. Hematol. 2023, 187, 104040. [Google Scholar] [CrossRef] [PubMed]
- Esma, F.; Salvini, M.; Troia, R.; Boccadoro, M.; Larocca, A.; Pautasso, C. Melphalan hydrochloride for the treatment of multiple myeloma. Expert. Opin. Pharmacother. 2017, 18, 1127–1136. [Google Scholar] [CrossRef]
- Stewart, A.K.; Rajkumar, S.V.; Dimopoulos, M.A.; Masszi, T.; Špička, I.; Oriol, A.; Hájek, R.; Rosiñol, L.; Siegel, D.S.; Mihaylov, G.G.; et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N. Engl. J. Med. 2015, 372, 142–152. [Google Scholar] [CrossRef]
- Scott, K.; Hayden, P.J.; Will, A.; Wheatley, K.; Coyne, I. Bortezomib for the treatment of multiple myeloma. Cochrane Database Syst. Rev. 2016, 4, Cd010816. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Goldschmidt, H.; Niesvizky, R.; Joshua, D.; Chng, W.J.; Oriol, A.; Orlowski, R.Z.; Ludwig, H.; Facon, T.; Hajek, R.; et al. Carfilzomib or bortezomib in relapsed or refractory multiple myeloma (ENDEAVOR): An interim overall survival analysis of an open-label, randomised, phase 3 trial. Lancet Oncol. 2017, 18, 1327–1337. [Google Scholar] [CrossRef]
- Raza, S.; Safyan, R.A.; Lentzsch, S. Immunomodulatory Drugs (IMiDs) in Multiple Myeloma. Curr. Cancer Drug Targets 2017, 17, 846–857. [Google Scholar] [CrossRef]
- Ricciuti, G.; Falcone, A.; Cascavilla, N.; Martinelli, G.; Cerchione, C. Autologous stem cell transplantation in multiple myeloma. Panminerva Med. 2020, 62, 220–224. [Google Scholar] [CrossRef]
- Friedman, K.M.; Garrett, T.E.; Evans, J.W.; Horton, H.M.; Latimer, H.J.; Seidel, S.L.; Horvath, C.J.; Morgan, R.A. Effective Targeting of Multiple B-Cell Maturation Antigen-Expressing Hematological Malignances by Anti-B-Cell Maturation Antigen Chimeric Antigen Receptor T Cells. Hum. Gene Ther. 2018, 29, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Liu, D. Antibody-drug conjugates in clinical trials for lymphoid malignancies and multiple myeloma. J. Hematol. Oncol. 2019, 12, 94. [Google Scholar] [CrossRef] [PubMed]
- Viola, D.; Dona, A.; Caserta, E.; Troadec, E.; Besi, F.; McDonald, T.; Ghoda, L.; Gunes, E.G.; Sanchez, J.F.; Khalife, J.; et al. Daratumumab induces mechanisms of immune activation through CD38+ NK cell targeting. Leukemia 2021, 35, 189–200. [Google Scholar] [CrossRef]
- Cai, H.; Kakiuchi-Kiyota, S.; Hendricks, R.; Zhong, S.; Liu, L.; Adedeji, A.O.; Chan, P.; Schutten, M.M.; Kamath, A.V.; Ovacik, M.A. Nonclinical Pharmacokinetics, Pharmacodynamics, and Translational Model of RO7297089, A Novel Anti-BCMA/CD16A Bispecific Tetravalent Antibody for the Treatment of Multiple Myeloma. Aaps J. 2022, 24, 100. [Google Scholar] [CrossRef]
- Dunphy, K.; Dowling, P.; Bazou, D.; O’Gorman, P. Current Methods of Post-Translational Modification Analysis and Their Applications in Blood Cancers. Cancers 2021, 13, 1930. [Google Scholar] [CrossRef] [PubMed]
- Wirth, M.; Schick, M.; Keller, U.; Krönke, J. Ubiquitination and Ubiquitin-Like Modifications in Multiple Myeloma: Biology and Therapy. Cancers 2020, 12, 3764. [Google Scholar] [CrossRef]
- Issa, M.E.; Takhsha, F.S.; Chirumamilla, C.S.; Perez-Novo, C.; Vanden Berghe, W.; Cuendet, M. Epigenetic strategies to reverse drug resistance in heterogeneous multiple myeloma. Clin. Epigenetics 2017, 9, 17. [Google Scholar] [CrossRef]
- Alzrigat, M.; Párraga, A.A.; Jernberg-Wiklund, H. Epigenetics in multiple myeloma: From mechanisms to therapy. Semin. Cancer Biol. 2018, 51, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Hammarén, H.M.; Savitski, M.M.; Baek, S.H. Control of protein stability by post-translational modifications. Nat. Commun. 2023, 14, 201. [Google Scholar] [CrossRef]
- Pan, S.; Chen, R. Pathological implication of protein post-translational modifications in cancer. Mol. Asp. Med. 2022, 86, 101097. [Google Scholar] [CrossRef]
- Powley, I.R.; Hughes, M.A.; Cain, K.; MacFarlane, M. Caspase-8 tyrosine-380 phosphorylation inhibits CD95 DISC function by preventing procaspase-8 maturation and cycling within the complex. Oncogene 2016, 35, 5629–5640. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qian, C.; Cao, X. Post-Translational Modification Control of Innate Immunity. Immunity 2016, 45, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Rott, R.; Szargel, R.; Shani, V.; Hamza, H.; Savyon, M.; Abd Elghani, F.; Bandopadhyay, R.; Engelender, S. SUMOylation and ubiquitination reciprocally regulate α-synuclein degradation and pathological aggregation. Proc. Natl. Acad. Sci. USA 2017, 114, 13176–13181. [Google Scholar] [CrossRef]
- Dikic, I.; Schulman, B.A. An expanded lexicon for the ubiquitin code. Nat. Rev. Mol. Cell Biol. 2023, 24, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.T. Protein phosphatase 1--targeted in many directions. J. Cell Sci. 2002, 115, 241–256. [Google Scholar] [CrossRef]
- Singh, V.; Ram, M.; Kumar, R.; Prasad, R.; Roy, B.K.; Singh, K.K. Phosphorylation: Implications in Cancer. Protein J. 2017, 36, 1–6. [Google Scholar] [CrossRef]
- Pawson, T.; Scott, J.D. Signaling through scaffold, anchoring, and adaptor proteins. Science 1997, 278, 2075–2080. [Google Scholar] [CrossRef]
- Li, L.; Hu, X.; Nkwocha, J.; Sharma, K.; Kmieciak, M.; Mann, H.; Zhou, L.; Grant, S. Non-canonical role for the ataxia-telangiectasia-Rad3 pathway in STAT3 activation in human multiple myeloma cells. Cell Oncol 2023, 46, 1369–1380. [Google Scholar] [CrossRef]
- Li, S.; Fu, J.; Yang, J.; Ma, H.; Bhutani, D.; Mapara, M.Y.; Marcireau, C.; Lentzsch, S. Targeting the GCK pathway: A novel and selective therapeutic strategy against RAS-mutated multiple myeloma. Blood 2021, 137, 1754–1764. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Li, Q.; Gao, S.; Liu, S.; Ma, J.; Xie, Y.; Wang, J.; Cao, Z.; Liu, Z. Inositol Polyphosphate 4-Phosphatase Type II Is a Tumor Suppressor in Multiple Myeloma. Front. Oncol. 2021, 11, 785297. [Google Scholar] [CrossRef]
- Murphy, C.S.; DeMambro, V.E.; Fadel, S.; Fairfield, H.; Garter, C.A.; Rodriguez, P.; Qiang, Y.W.; Vary, C.P.H.; Reagan, M.R. Inhibition of Acyl-CoA Synthetase Long Chain Isozymes Decreases Multiple Myeloma Cell Proliferation and Causes Mitochondrial Dysfunction. bioRxiv 2024. [Google Scholar] [CrossRef]
- Jia, Y.; Yu, X.; Liu, R.; Shi, L.; Jin, H.; Yang, D.; Zhang, X.; Shen, Y.; Feng, Y.; Zhang, P.; et al. PRMT1 methylation of WTAP promotes multiple myeloma tumorigenesis by activating oxidative phosphorylation via m6A modification of NDUFS6. Cell Death Dis. 2023, 14, 512. [Google Scholar] [CrossRef]
- Yao, Y.; Park, W.D.; Morelli, E.; Samur, M.K.; Kwiatkowski, N.P.; Shirasaki, R.; Xu, Y.; Chakraborty, C.; Nabet, B.; Ches, M.; et al. Aberrant CDK7 Activity Drives the Cell Cycle and Transcriptional Dysregulation to Support Multiple Myeloma Growth: An Attractive Molecular Vulnerability. Blood 2021, 138, 2687. [Google Scholar] [CrossRef]
- Varland, S.; Osberg, C.; Arnesen, T. N-terminal modifications of cellular proteins: The enzymes involved, their substrate specificities and biological effects. Proteomics 2015, 15, 2385–2401. [Google Scholar] [CrossRef]
- Choudhary, C.; Weinert, B.T.; Nishida, Y.; Verdin, E.; Mann, M. The growing landscape of lysine acetylation links metabolism and cell signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 536–550. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhang, L.; Li, M.; Du, J.; Zhou, L.; Yang, S.; Zeng, L.; Li, Z.; Wang, G.; Wang, D. Functional analysis of the involvement of apurinic/apyrimidinic endonuclease 1 in the resistance to melphalan in multiple myeloma. BMC Cancer 2014, 14, 11. [Google Scholar] [CrossRef]
- Chen, Y.C.; Bhaskara, G.B.; Lu, Y.; Lin, K.; Dent, S.Y.R. The SAGA acetyltransferase module is required for the maintenance of MAF and MYC oncogenic gene expression programs in multiple myeloma. Genes. Dev. 2024, 38, 738–754. [Google Scholar] [CrossRef] [PubMed]
- Dupéré-Richer, D.; Riva, A.; Barwick, B.G.; Maji, S.; Casellas Román, H.; Li, J.; De, U.; Sobh, A.; Quickstad, G.; Piper, C.; et al. KDM6A regulates immune response genes in multiple myeloma. Blood 2024, 144, 1508–1520. [Google Scholar] [CrossRef]
- Ezponda, T.; Dupéré-Richer, D.; Will, C.M.; Small, E.C.; Varghese, N.; Patel, T.; Nabet, B.; Popovic, R.; Oyer, J.; Bulic, M.; et al. UTX/KDM6A Loss Enhances the Malignant Phenotype of Multiple Myeloma and Sensitizes Cells to EZH2 inhibition. Cell Rep. 2017, 21, 628–640. [Google Scholar] [CrossRef]
- Gao, X.; Feng, Q.; Zhang, Q.; Zhang, Y.; Hu, C.; Zhang, L.; Zhang, H.; Wang, G.; Hu, K.; Ma, M.; et al. Targeting enolase 1 reverses bortezomib resistance in multiple myeloma through YWHAZ/Parkin axis. J. Biomed. Sci. 2025, 32, 9. [Google Scholar] [CrossRef]
- Gu, Z.; Xia, J.; Xu, H.; Frech, I.; Tricot, G.; Zhan, F. NEK2 Promotes Aerobic Glycolysis in Multiple Myeloma Through Regulating Splicing of Pyruvate Kinase. J. Hematol. Oncol. 2017, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.Y.; Stewart, A.K.; Sooknanan, R.R.; Henderson, G.; Hawley, T.S.; Reimold, A.M.; Glimcher, L.H.; Baumann, H.; Malek, L.T.; Hawley, R.G. Identification of c-myc promoter-binding protein and X-box binding protein 1 as interleukin-6 target genes in human multiple myeloma cells. Int. J. Oncol. 1999, 15, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Deng, Z.; Ding, P.; Qiang, W.; Lu, Y.; Gao, S.; Hu, Y.; Yang, Y.; Du, J.; Gu, C. A novel protein encoded by circHNRNPU promotes multiple myeloma progression by regulating the bone marrow microenvironment and alternative splicing. J. Exp. Clin. Cancer Res. 2022, 41, 85. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, X.L.; Wang, H.F.; Guo, T.; Yao, J.; Jiang, Z.S.; Pei, Q. The prognostic significance of ubiquitination-related genes in multiple myeloma by bioinformatics analysis. BMC Med. Genom. 2024, 17, 164. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Cho, J.; Song, E.J. Ubiquitin-proteasome system (UPS) as a target for anticancer treatment. Arch. Pharm. Res. 2020, 43, 1144–1161. [Google Scholar] [CrossRef]
- Maimaitiyiming, Y.; Yang, T.; Wang, Q.Q.; Feng, Y.; Chen, Z.; Björklund, M.; Wang, F.; Hu, C.; Hsu, C.H.; Naranmandura, H. Heat Treatment Promotes Ubiquitin-Mediated Proteolysis of SARS-CoV-2 RNA Polymerase and Decreases Viral Load. Research 2022, 2022, 9802969. [Google Scholar] [CrossRef]
- Maimaitiyiming, Y.; Wang, Q.Q.; Yang, C.; Ogra, Y.; Lou, Y.; Smith, C.A.; Hussain, L.; Shao, Y.M.; Lin, J.; Liu, J.; et al. Hyperthermia Selectively Destabilizes Oncogenic Fusion Proteins. Blood Cancer Discov. 2021, 2, 388–401. [Google Scholar] [CrossRef]
- Gao, S.; Geng, C.; Song, T.; Lin, X.; Liu, J.; Cai, Z.; Cang, Y. Activation of c-Abl Kinase Potentiates the Anti-myeloma Drug Lenalidomide by Promoting DDA1 Protein Recruitment to the CRL4 Ubiquitin Ligase. J. Biol. Chem. 2017, 292, 3683–3691. [Google Scholar] [CrossRef]
- Wang, B.; Li, M.; Cao, D.; Sun, Q.; Yu, W.; Ma, J.; Ren, H.; Xu, G.; Zhou, L. Lys-63-specific deubiquitinase BRCC36 enhances the sensitivity of multiple myeloma cells to lenalidomide by inhibiting lysosomal degradation of cereblon. Cell Mol. Life Sci. 2024, 81, 349. [Google Scholar] [CrossRef]
- Jia, H.; Liu, C.; Ge, F.; Xiao, C.; Lu, C.; Wang, T.; He, Q. Identification of ubiquitinated proteins from human multiple myeloma U266 cells by proteomics. Biomed. Environ. Sci. 2011, 24, 422–430. [Google Scholar] [CrossRef]
- Li, C.; Xia, J.; Franqui-Machin, R.; Chen, F.; He, Y.; Ashby, T.C.; Teng, F.; Xu, H.; Liu, D.; Gai, D.; et al. TRIP13 modulates protein deubiquitination and accelerates tumor development and progression of B cell malignancies. J. Clin. Investig. 2021, 131, e146893. [Google Scholar] [CrossRef] [PubMed]
- Lub, S.; Maes, K.; Menu, E.; De Bruyne, E.; Vanderkerken, K.; Van Valckenborgh, E. Novel strategies to target the ubiquitin proteasome system in multiple myeloma. Oncotarget 2016, 7, 6521–6537. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Ozcan, U. Unfolded protein response signaling and metabolic diseases. J. Biol. Chem. 2014, 289, 1203–1211. [Google Scholar] [CrossRef]
- Kim, H.J.; Ha, S.; Lee, H.Y.; Lee, K.J. ROSics: Chemistry and proteomics of cysteine modifications in redox biology. Mass. Spectrom. Rev. 2015, 34, 184–208. [Google Scholar] [CrossRef] [PubMed]
- Berardi, A.; Kaestner, C.L.; Ghitti, M.; Quilici, G.; Cocomazzi, P.; Li, J.; Ballabio, F.; Zucchelli, C.; Knapp, S.; Licht, J.D.; et al. The C-terminal PHDVC5HCH tandem domain of NSD2 is a combinatorial reader of unmodified H3K4 and tri-methylated H3K27 that regulates transcription of cell adhesion genes in multiple myeloma. Nucleic Acids Res. 2025, 53, gkae1121. [Google Scholar] [CrossRef] [PubMed]
- Popovic, R.; Martinez-Garcia, E.; Giannopoulou, E.G.; Zhang, Q.; Zhang, Q.; Ezponda, T.; Shah, M.Y.; Zheng, Y.; Will, C.M.; Small, E.C.; et al. Histone methyltransferase MMSET/NSD2 alters EZH2 binding and reprograms the myeloma epigenome through global and focal changes in H3K36 and H3K27 methylation. PLoS Genet. 2014, 10, e1004566. [Google Scholar] [CrossRef]
- de Krijger, I.; van der Torre, J.; Peuscher, M.H.; Eder, M.; Jacobs, J.J.L. H3K36 dimethylation by MMSET promotes classical non-homologous end-joining at unprotected telomeres. Oncogene 2020, 39, 4814–4827. [Google Scholar] [CrossRef]
- Wang, L.; Lin, N.; Li, Y. The PI3K/AKT signaling pathway regulates ABCG2 expression and confers resistance to chemotherapy in human multiple myeloma. Oncol. Rep. 2019, 41, 1678–1690. [Google Scholar] [CrossRef]
- Niebudek, K.; Balcerczak, E.; Mirowski, M.; Pietrzak, J.; Zawadzka, I.; Żebrowska-Nawrocka, M. The contribution of ABCG2 G34A and C421A polymorphisms to multiple myeloma susceptibility. Onco Targets Ther. 2019, 12, 1655–1660. [Google Scholar] [CrossRef]
- Wang, M.M.; Zhu, Q.; Ren, Z.H.; Zou, L.F.; Dou, H.J.; Hu, J.P. Arsenic trioxide induces socs-1 gene demethylation in myeloma cell lines. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2008, 16, 1064–1068. [Google Scholar]
- Furukawa, Y.; Kikuchi, J. Epigenetic mechanisms of cell adhesion-mediated drug resistance in multiple myeloma. Int. J. Hematol. 2016, 104, 281–292. [Google Scholar] [CrossRef]
- Driscoll, J.J.; Pelluru, D.; Lefkimmiatis, K.; Fulciniti, M.; Prabhala, R.H.; Greipp, P.R.; Barlogie, B.; Tai, Y.T.; Anderson, K.C.; Shaughnessy, J.D., Jr.; et al. The sumoylation pathway is dysregulated in multiple myeloma and is associated with adverse patient outcome. Blood 2010, 115, 2827–2834. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Qian, J.; Yang, Y.; Gu, C. Novel insights into the impact of the SUMOylation pathway in hematological malignancies (Review). Int. J. Oncol. 2021, 59, 73. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, L.; Jiang, D.; Wei, W.; Nasir, M.F.; Khan, M.S.; Yousafi, Q.; Liu, X.; Fu, X.; Li, X.; et al. Sumoylation as an Emerging Target in Therapeutics against Cancer. Curr. Pharm. Des. 2020, 26, 4764–4776. [Google Scholar] [CrossRef]
- Huang, H.J.; Zhou, L.L.; Fu, W.J.; Zhang, C.Y.; Jiang, H.; Du, J.; Hou, J. β-catenin SUMOylation is involved in the dysregulated proliferation of myeloma cells. Am. J. Cancer Res. 2015, 5, 309–320. [Google Scholar] [PubMed]
- Weger, S.; Hammer, E.; Heilbronn, R. SUMO-1 modification regulates the protein stability of the large regulatory protein Rep78 of adeno associated virus type 2 (AAV-2). Virology 2004, 330, 284–294. [Google Scholar] [CrossRef]
- Wu, S.; Yu, L. Targeting cullin-RING ligases for cancer treatment: Rationales, advances and therapeutic implications. Cytotechnology 2016, 68, 1–8. [Google Scholar] [CrossRef]
- Ying, J.; Zhang, M.; Qiu, X.; Lu, Y. Targeting the neddylation pathway in cells as a potential therapeutic approach for diseases. Cancer Chemother. Pharmacol. 2018, 81, 797–808. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, Y. Cullin-RING Ligases as attractive anti-cancer targets. Curr. Pharm. Des. 2013, 19, 3215–3225. [Google Scholar] [CrossRef]
- Driscoll, J.J.; Dechowdhury, R. Therapeutically targeting the SUMOylation, Ubiquitination and Proteasome pathways as a novel anticancer strategy. Target. Oncol. 2010, 5, 281–289. [Google Scholar] [CrossRef]
- Petillo, S.; Capuano, C.; Molfetta, R.; Fionda, C.; Mekhloufi, A.; Pighi, C.; Antonangeli, F.; Zingoni, A.; Soriani, A.; Petrucci, M.T.; et al. Immunomodulatory effect of NEDD8-activating enzyme inhibition in Multiple Myeloma: Upregulation of NKG2D ligands and sensitization to Natural Killer cell recognition. Cell Death Dis. 2021, 12, 836. [Google Scholar] [CrossRef] [PubMed]
- Langerhorst, P.; Baerenfaenger, M.; Kulkarni, P.; Nadal, S.; Wijnands, C.; Post, M.A.; Noori, S.; vanDuijn, M.M.; Joosten, I.; Dejoie, T.; et al. N-linked glycosylation of the M-protein variable region: Glycoproteogenomics reveals a new layer of personalized complexity in multiple myeloma. Clin. Chem. Lab. Med. 2024, 62, 1626–1635. [Google Scholar] [CrossRef]
- Zhang, Z.; Westhrin, M.; Bondt, A.; Wuhrer, M.; Standal, T.; Holst, S. Serum protein N-glycosylation changes in multiple myeloma. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 960–970. [Google Scholar] [CrossRef]
- Renfrow, M.B.; Mackay, C.L.; Chalmers, M.J.; Julian, B.A.; Mestecky, J.; Kilian, M.; Poulsen, K.; Emmett, M.R.; Marshall, A.G.; Novak, J. Analysis of O-glycan heterogeneity in IgA1 myeloma proteins by Fourier transform ion cyclotron resonance mass spectrometry: Implications for IgA nephropathy. Anal. Bioanal. Chem. 2007, 389, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Smith, A.D.; Poulsen, K.; Kilian, M.; Julian, B.A.; Mestecky, J.; Novak, J.; Renfrow, M.B. Naturally occurring structural isomers in serum IgA1 o-glycosylation. J. Proteome Res. 2012, 11, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Mittermayr, S.; Lê, G.N.; Clarke, C.; Millán Martín, S.; Larkin, A.M.; O’Gorman, P.; Bones, J. Polyclonal Immunoglobulin G N-Glycosylation in the Pathogenesis of Plasma Cell Disorders. J. Proteome Res. 2017, 16, 748–762. [Google Scholar] [CrossRef]
- Westhrin, M.; Kovcic, V.; Zhang, Z.; Moen, S.H.; Nedal, T.M.V.; Bondt, A.; Holst, S.; Misund, K.; Buene, G.; Sundan, A.; et al. Monoclonal immunoglobulins promote bone loss in multiple myeloma. Blood 2020, 136, 2656–2666. [Google Scholar] [CrossRef]
- Lauc, G.; Huffman, J.E.; Pučić, M.; Zgaga, L.; Adamczyk, B.; Mužinić, A.; Novokmet, M.; Polašek, O.; Gornik, O.; Krištić, J.; et al. Loci associated with N-glycosylation of human immunoglobulin G show pleiotropy with autoimmune diseases and haematological cancers. PLoS Genet. 2013, 9, e1003225. [Google Scholar] [CrossRef]
- Hatzimichael, E.; Dasoula, A.; Shah, R.; Syed, N.; Papoudou-Bai, A.; Coley, H.M.; Dranitsaris, G.; Bourantas, K.L.; Stebbing, J.; Crook, T. The prolyl-hydroxylase EGLN3 and not EGLN1 is inactivated by methylation in plasma cell neoplasia. Eur. J. Haematol. 2010, 84, 47–51. [Google Scholar] [CrossRef]
- Shin, D.H.; Chun, Y.S.; Lee, D.S.; Huang, L.E.; Park, J.W. Bortezomib inhibits tumor adaptation to hypoxia by stimulating the FIH-mediated repression of hypoxia-inducible factor-1. Blood 2008, 111, 3131–3136. [Google Scholar] [CrossRef]
- Kim, J.; Choi, S.; Saxena, N.; Singh, A.K.; Singh, I.; Won, J.S. Regulation of STAT3 and NF-κB activations by S-nitrosylation in multiple myeloma. Free Radic. Biol. Med. 2017, 106, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.S.; Fairfield, H.; DeMambro, V.E.; Fadel, S.; Gartner, C.A.; Karam, M.; Potts, C.; Rodriguez, P.; Qiang, Y.W.; Hamidi, H.; et al. Inhibition of acyl-CoA synthetase long-chain isozymes decreases multiple myeloma cell proliferation and causes mitochondrial dysfunction. Mol. Oncol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Qiang, Y.W.; Ye, S.; Huang, Y.; Chen, Y.; Van Rhee, F.; Epstein, J.; Walker, B.A.; Morgan, G.J.; Davies, F.E. MAFb protein confers intrinsic resistance to proteasome inhibitors in multiple myeloma. BMC Cancer 2018, 18, 724. [Google Scholar] [CrossRef] [PubMed]
- Hirano, M.; Imai, Y.; Kaito, Y.; Murayama, T.; Sato, K.; Ishida, T.; Yamamoto, J.; Ito, T.; Futami, M.; Ri, M.; et al. Small-molecule HDAC and Akt inhibitors suppress tumor growth and enhance immunotherapy in multiple myeloma. J. Exp. Clin. Cancer Res. 2021, 40, 110. [Google Scholar] [CrossRef]
- Krönke, J.; Udeshi, N.D.; Narla, A.; Grauman, P.; Hurst, S.N.; McConkey, M.; Svinkina, T.; Heckl, D.; Comer, E.; Li, X.; et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science 2014, 343, 301–305. [Google Scholar] [CrossRef]
- Krönke, J.; Hurst, S.N.; Ebert, B.L. Lenalidomide induces degradation of IKZF1 and IKZF3. Oncoimmunology 2014, 3, e941742. [Google Scholar] [CrossRef]
- Gao, D.; Lv, A.E.; Li, H.P.; Han, D.H.; Zhang, Y.P. LncRNA MALAT-1 Elevates HMGB1 to Promote Autophagy Resulting in Inhibition of Tumor Cell Apoptosis in Multiple Myeloma. J. Cell Biochem. 2017, 118, 3341–3348. [Google Scholar] [CrossRef]
- Tibullo, D.; Giallongo, C.; Romano, A.; Vicario, N.; Barbato, A.; Puglisi, F.; Parenti, R.; Amorini, A.M.; Wissam Saab, M.; Tavazzi, B.; et al. Mitochondrial Functions, Energy Metabolism and Protein Glycosylation are Interconnected Processes Mediating Resistance to Bortezomib in Multiple Myeloma Cells. Biomolecules 2020, 10, 696. [Google Scholar] [CrossRef]
- Kukkula, A.; Ojala, V.K.; Mendez, L.M.; Sistonen, L.; Elenius, K.; Sundvall, M. Therapeutic Potential of Targeting the SUMO Pathway in Cancer. Cancers 2021, 13, 4402. [Google Scholar] [CrossRef]
- Du, L.; Liu, W.; Pichiorri, F.; Rosen, S.T. SUMOylation inhibition enhances multiple myeloma sensitivity to lenalidomide. Cancer Gene Ther. 2023, 30, 567–574. [Google Scholar] [CrossRef]
- Gu, Y.; Kaufman, J.L.; Bernal, L.; Torre, C.; Matulis, S.M.; Harvey, R.D.; Chen, J.; Sun, S.Y.; Boise, L.H.; Lonial, S. MLN4924, an NAE inhibitor, suppresses AKT and mTOR signaling via upregulation of REDD1 in human myeloma cells. Blood 2014, 123, 3269–3276. [Google Scholar] [CrossRef] [PubMed]
- Daly, J.; Sarkar, S.; Natoni, A.; Stark, J.C.; Riley, N.M.; Bertozzi, C.R.; Carlsten, M.; O’Dwyer, M.E. Targeting hypersialylation in multiple myeloma represents a novel approach to enhance NK cell-mediated tumor responses. Blood Adv. 2022, 6, 3352–3366. [Google Scholar] [CrossRef] [PubMed]
- Natoni, A.; Macauley, M.S.; O’Dwyer, M.E. Targeting Selectins and Their Ligands in Cancer. Front. Oncol. 2016, 6, 93. [Google Scholar] [CrossRef]
- Natoni, A.; Smith, T.A.G.; Keane, N.; McEllistrim, C.; Connolly, C.; Jha, A.; Andrulis, M.; Ellert, E.; Raab, M.S.; Glavey, S.V.; et al. E-selectin ligands recognised by HECA452 induce drug resistance in myeloma, which is overcome by the E-selectin antagonist, GMI-1271. Leukemia 2017, 31, 2642–2651. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, S.; Xie, Y.; Jiang, H.; Guo, J.; Wang, Y.; Peng, Z.; Hu, M.; Wang, M.; Wang, J.; et al. Deacetylation induced nuclear condensation of HP1γ promotes multiple myeloma drug resistance. Nat. Commun. 2023, 14, 1290. [Google Scholar] [CrossRef]
- Gandolfi, S.; Laubach, J.P.; Hideshima, T.; Chauhan, D.; Anderson, K.C.; Richardson, P.G. The proteasome and proteasome inhibitors in multiple myeloma. Cancer Metastasis Rev. 2017, 36, 561–584. [Google Scholar] [CrossRef]
- Narayanan, S.; Cai, C.Y.; Assaraf, Y.G.; Guo, H.Q.; Cui, Q.; Wei, L.; Huang, J.J.; Ashby, C.R., Jr.; Chen, Z.S. Targeting the ubiquitin-proteasome pathway to overcome anti-cancer drug resistance. Drug Resist. Updat. 2020, 48, 100663. [Google Scholar] [CrossRef]
- Pigneux, A.; Mahon, F.X.; Moreau-Gaudry, F.; Uhalde, M.; de Verneuil, H.; Lacombe, F.; Reiffers, J.; Milpied, N.; Praloran, V.; Belloc, F. Proteasome inhibition specifically sensitizes leukemic cells to anthracyclin-induced apoptosis through the accumulation of Bim and Bax pro-apoptotic proteins. Cancer Biol. Ther. 2007, 6, 603–611. [Google Scholar] [CrossRef][Green Version]
- Gomez-Bougie, P.; Wuillème-Toumi, S.; Ménoret, E.; Trichet, V.; Robillard, N.; Philippe, M.; Bataille, R.; Amiot, M. Noxa up-regulation and Mcl-1 cleavage are associated to apoptosis induction by bortezomib in multiple myeloma. Cancer Res. 2007, 67, 5418–5424. [Google Scholar] [CrossRef]
- Saj, F.; Nisha, Y.; Ganesan, P.; Kayal, S.; Kar, R.; Halanaik, D.; Dubashi, B. Efficacy and safety of pomalidomide, bortezomib, and dexamethasone combination chemotherapy for newly diagnosed multiple myeloma: POMACE Phase II Study. Blood Cancer J. 2023, 13, 45. [Google Scholar] [CrossRef]
- Jannuzzi, A.T.; Arslan, S.; Yilmaz, A.M.; Sari, G.; Beklen, H.; Méndez, L.; Fedorova, M.; Arga, K.Y.; Karademir Yilmaz, B.; Alpertunga, B. Higher proteotoxic stress rather than mitochondrial damage is involved in higher neurotoxicity of bortezomib compared to carfilzomib. Redox Biol. 2020, 32, 101502. [Google Scholar] [CrossRef] [PubMed]
- Karademir, B.; Sari, G.; Jannuzzi, A.T.; Musunuri, S.; Wicher, G.; Grune, T.; Mi, J.; Hacioglu-Bay, H.; Forsberg-Nilsson, K.; Bergquist, J.; et al. Proteomic approach for understanding milder neurotoxicity of Carfilzomib against Bortezomib. Sci. Rep. 2018, 8, 16318. [Google Scholar] [CrossRef] [PubMed]
- Haq, M.; Pellegrini, M.V.; Thumallapally, N. Ixazomib. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2025. [Google Scholar]
- Barankiewicz, J.; Salomon-Perzyński, A.; Misiewicz-Krzemińska, I.; Lech-Marańda, E. CRL4(CRBN) E3 Ligase Complex as a Therapeutic Target in Multiple Myeloma. Cancers 2022, 14, 4492. [Google Scholar] [CrossRef]
- Mark, T.M.; Coleman, M.; Niesvizky, R. Preclinical and clinical results with pomalidomide in the treatment of relapsed/refractory multiple myeloma. Leuk. Res. 2014, 38, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Siegel, D.S.; Schiller, G.J.; Samaras, C.; Sebag, M.; Berdeja, J.; Ganguly, S.; Matous, J.; Song, K.; Seet, C.S.; Talamo, G.; et al. Pomalidomide, dexamethasone, and daratumumab in relapsed refractory multiple myeloma after lenalidomide treatment. Leukemia 2020, 34, 3286–3297. [Google Scholar] [CrossRef]
- Ramaiah, M.J.; Tangutur, A.D.; Manyam, R.R. Epigenetic modulation and understanding of HDAC inhibitors in cancer therapy. Life Sci. 2021, 277, 119504. [Google Scholar] [CrossRef]
- Sivaraj, D.; Green, M.M.; Gasparetto, C. Panobinostat for the management of multiple myeloma. Future Oncol. 2017, 13, 477–488. [Google Scholar] [CrossRef]
- Berdeja, J.G.; Laubach, J.P.; Richter, J.; Stricker, S.; Spencer, A.; Richardson, P.G.; Chari, A. Panobinostat From Bench to Bedside: Rethinking the Treatment Paradigm for Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2021, 21, 752–765. [Google Scholar] [CrossRef]
- Richardson, P.G.; Harvey, R.D.; Laubach, J.P.; Moreau, P.; Lonial, S.; San-Miguel, J.F. Panobinostat for the treatment of relapsed or relapsed/refractory multiple myeloma: Pharmacology and clinical outcomes. Expert. Rev. Clin. Pharmacol. 2016, 9, 35–48. [Google Scholar] [CrossRef]
- Heynen, G.; Baumgartner, F.; Heider, M.; Patra, U.; Holz, M.; Braune, J.; Kaiser, M.; Schäffer, I.; Bamopoulos, S.A.; Ramberger, E.; et al. SUMOylation inhibition overcomes proteasome inhibitor resistance in multiple myeloma. Blood Adv. 2023, 7, 469–481. [Google Scholar] [CrossRef]
- Turner, J.G.; Gump, J.L.; Zhang, C.; Cook, J.M.; Marchion, D.; Hazlehurst, L.; Munster, P.; Schell, M.J.; Dalton, W.S.; Sullivan, D.M. ABCG2 expression, function, and promoter methylation in human multiple myeloma. Blood 2006, 108, 3881–3889. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Fang, Y.; Wu, X.; Xu, T.; Hu, T.; Xu, Y.; Ma, P.; Wang, Q.; Shu, Y. The emerging roles of SUMOylation in the tumor microenvironment and therapeutic implications. Exp. Hematol. Oncol. 2023, 12, 58. [Google Scholar] [CrossRef]
- van Andel, H.; Kocemba, K.A.; Spaargaren, M.; Pals, S.T. Aberrant Wnt signaling in multiple myeloma: Molecular mechanisms and targeting options. Leukemia 2019, 33, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- Beider, K.; Rosenberg, E.; Dimenshtein-Voevoda, V.; Sirovsky, Y.; Vladimirsky, J.; Magen, H.; Ostrovsky, O.; Shimoni, A.; Bromberg, Z.; Weiss, L.; et al. Blocking of Transient Receptor Potential Vanilloid 1 (TRPV1) promotes terminal mitophagy in multiple myeloma, disturbing calcium homeostasis and targeting ubiquitin pathway and bortezomib-induced unfolded protein response. J. Hematol. Oncol. 2020, 13, 158. [Google Scholar] [CrossRef] [PubMed]
- Petillo, S.; Sproviero, E.; Loconte, L.; Cuollo, L.; Zingoni, A.; Molfetta, R.; Fionda, C.; Soriani, A.; Cerboni, C.; Petrucci, M.T.; et al. NEDD8-activating enzyme inhibition potentiates the anti-myeloma activity of natural killer cells. Cell Death Dis. 2023, 14, 438. [Google Scholar] [CrossRef]
- Yuan, S.; Liu, Z.; Xu, Z.; Liu, J.; Zhang, J. High mobility group box 1 (HMGB1): A pivotal regulator of hematopoietic malignancies. J. Hematol. Oncol. 2020, 13, 91. [Google Scholar] [CrossRef]
- Li, S.; Bode, A.M.; Zhu, F.; Liu, K.; Zhang, J.; Kim, M.O.; Reddy, K.; Zykova, T.; Ma, W.Y.; Carper, A.L.; et al. TRPV1-antagonist AMG9810 promotes mouse skin tumorigenesis through EGFR/Akt signaling. Carcinogenesis 2011, 32, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, V.; Kumar, S. PI3K/AKT/mTOR pathway in multiple myeloma: From basic biology to clinical promise. Leuk. Lymphoma 2018, 59, 2524–2534. [Google Scholar] [CrossRef]
- Vega, M.; Chen, Y.; Shi, Y.; Gera, J.; Lichtenstein, A. Turnover of the mTOR inhibitor, DEPTOR, and downstream AKT phosphorylation in multiple myeloma cells, is dependent on ERK1-mediated phosphorylation. J. Biol. Chem. 2022, 298, 101750. [Google Scholar] [CrossRef]
- Jiang, F.; Tang, X.; Tang, C.; Hua, Z.; Ke, M.; Wang, C.; Zhao, J.; Gao, S.; Jurczyszyn, A.; Janz, S.; et al. HNRNPA2B1 promotes multiple myeloma progression by increasing AKT3 expression via m6A-dependent stabilization of ILF3 mRNA. J. Hematol. Oncol. 2021, 14, 54. [Google Scholar] [CrossRef]
- Isa, R.; Horinaka, M.; Tsukamoto, T.; Mizuhara, K.; Fujibayashi, Y.; Taminishi-Katsuragawa, Y.; Okamoto, H.; Yasuda, S.; Kawaji-Kanayama, Y.; Matsumura-Kimoto, Y.; et al. The Rationale for the Dual-Targeting Therapy for RSK2 and AKT in Multiple Myeloma. Int. J. Mol. Sci. 2022, 23, 2919. [Google Scholar] [CrossRef]
- Damiano, J.S.; Hazlehurst, L.A.; Dalton, W.S. Cell adhesion-mediated drug resistance (CAM-DR) protects the K562 chronic myelogenous leukemia cell line from apoptosis induced by BCR/ABL inhibition, cytotoxic drugs, and gamma-irradiation. Leukemia 2001, 15, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, J.; Furukawa, Y. Toll-like receptor CD180 and the bone marrow microenvironment as therapeutic targets in multiple myeloma. Rinsho Ketsueki 2020, 61, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Garssen, J.; Redegeld, F. The efficacy of bortezomib in human multiple myeloma cells is enhanced by combination with omega-3 fatty acids DHA and EPA: Timing is essential. Clin. Nutr. 2021, 40, 1942–1953. [Google Scholar] [CrossRef]
- Liu, Y.; Liao, S.; Bennett, S.; Tang, H.; Song, D.; Wood, D.; Zhan, X.; Xu, J. STAT3 and its targeting inhibitors in osteosarcoma. Cell Prolif. 2021, 54, e12974. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Shirazi, F.; Singh, R.K.; Kuiatse, I.; Wang, H.; Lee, H.C.; Berkova, Z.; Berger, A.; Hyer, M.; Chattopadhyay, N.; et al. Ubiquitin-activating enzyme inhibition induces an unfolded protein response and overcomes drug resistance in myeloma. Blood 2019, 133, 1572–1584. [Google Scholar] [CrossRef]
- Xu, X.; Peng, Q.; Jiang, X.; Tan, S.; Yang, W.; Han, Y.; Oyang, L.; Lin, J.; Shen, M.; Wang, J.; et al. Altered glycosylation in cancer: Molecular functions and therapeutic potential. Cancer Commun. 2024, 44, 1316–1336. [Google Scholar] [CrossRef]
- Itkonen, H.M.; Poulose, N.; Steele, R.E.; Martin, S.E.S.; Levine, Z.G.; Duveau, D.Y.; Carelli, R.; Singh, R.; Urbanucci, A.; Loda, M.; et al. Inhibition of O-GlcNAc Transferase Renders Prostate Cancer Cells Dependent on CDK9. Mol. Cancer Res. 2020, 18, 1512–1521. [Google Scholar] [CrossRef]
- Xie, H.; Gu, Y.; Wang, W.; Wang, X.; Ye, X.; Xin, C.; Lu, M.; Reddy, B.A.; Shu, P. Silencing of SENP2 in Multiple Myeloma Induces Bortezomib Resistance by Activating NF-κB Through the Modulation of IκBα Sumoylation. Sci. Rep. 2020, 10, 766. [Google Scholar] [CrossRef]
- Natoni, A.; Farrell, M.L.; Harris, S.; Falank, C.; Kirkham-McCarthy, L.; Macauley, M.S.; Reagan, M.R.; O’Dwyer, M. Sialyltransferase inhibition leads to inhibition of tumor cell interactions with E-selectin, VCAM1, and MADCAM1, and improves survival in a human multiple myeloma mouse model. Haematologica 2020, 105, 457–467. [Google Scholar] [CrossRef]
- Natoni, A.; Bohara, R.; Pandit, A.; O’Dwyer, M. Targeted Approaches to Inhibit Sialylation of Multiple Myeloma in the Bone Marrow Microenvironment. Front. Bioeng. Biotechnol. 2019, 7, 252. [Google Scholar] [CrossRef] [PubMed]
- Glavey, S.V.; Manier, S.; Natoni, A.; Sacco, A.; Moschetta, M.; Reagan, M.R.; Murillo, L.S.; Sahin, I.; Wu, P.; Mishima, Y.; et al. The sialyltransferase ST3GAL6 influences homing and survival in multiple myeloma. Blood 2014, 124, 1765–1776. [Google Scholar] [CrossRef]
- Nguyen, H.P.; Le, A.Q.; Liu, E.; Cesarano, A.; DiMeo, F.; Perna, F.; Kapur, R.; Walker, B.A.; Tran, N.T. Protein arginine methyltransferase 1 is a therapeutic vulnerability in multiple myeloma. Front. Immunol. 2023, 14, 1239614. [Google Scholar] [CrossRef]
- Mashimo, K.; Tsubaki, M.; Takeda, T.; Asano, R.; Jinushi, M.; Imano, M.; Satou, T.; Sakaguchi, K.; Nishida, S. RANKL-induced c-Src activation contributes to conventional anti-cancer drug resistance and dasatinib overcomes this resistance in RANK-expressing multiple myeloma cells. Clin. Exp. Med. 2019, 19, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Laukkanen, S.; Veloso, A.; Yan, C.; Oksa, L.; Alpert, E.J.; Do, D.; Hyvärinen, N.; McCarthy, K.; Adhikari, A.; Yang, Q.; et al. Therapeutic targeting of LCK tyrosine kinase and mTOR signaling in T-cell acute lymphoblastic leukemia. Blood 2022, 140, 1891–1906. [Google Scholar] [CrossRef]
- Frassanito, M.A.; De Veirman, K.; Desantis, V.; Di Marzo, L.; Vergara, D.; Ruggieri, S.; Annese, T.; Nico, B.; Menu, E.; Catacchio, I.; et al. Halting pro-survival autophagy by TGFβ inhibition in bone marrow fibroblasts overcomes bortezomib resistance in multiple myeloma patients. Leukemia 2016, 30, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Hegedüs, L.; Szücs, K.D.; Kudla, M.; Heidenreich, J.; Jendrossek, V.; Peña-Llopis, S.; Garay, T.; Czirok, A.; Aigner, C.; Plönes, T.; et al. Nintedanib and Dasatinib Treatments Induce Protective Autophagy as a Potential Resistance Mechanism in MPM Cells. Front. Cell Dev. Biol. 2022, 10, 852812. [Google Scholar] [CrossRef]
- He, Y.; Fang, Y.; Zhang, M.; Zhao, Y.; Tu, B.; Shi, M.; Muhitdinov, B.; Asrorov, A.; Xu, Q.; Huang, Y. Remodeling “cold” tumor immune microenvironment via epigenetic-based therapy using targeted liposomes with in situ formed albumin corona. Acta Pharm. Sin. B 2022, 12, 2057–2073. [Google Scholar] [CrossRef]
- El Omari, N.; Bakrim, S.; Khalid, A.; Abdalla, A.N.; Almalki, W.H.; Lee, L.H.; Ardianto, C.; Ming, L.C.; Bouyahya, A. Molecular mechanisms underlying the clinical efficacy of panobinostat involve Stochasticity of epigenetic signaling, sensitization to anticancer drugs, and induction of cellular cell death related to cellular stresses. Biomed. Pharmacother. 2023, 164, 114886. [Google Scholar] [CrossRef]
- Renzini, A.; D’Onghia, M.; Coletti, D.; Moresi, V. Histone Deacetylases as Modulators of the Crosstalk Between Skeletal Muscle and Other Organs. Front. Physiol. 2022, 13, 706003. [Google Scholar] [CrossRef]
- Bajbouj, K.; Al-Ali, A.; Ramakrishnan, R.K.; Saber-Ayad, M.; Hamid, Q. Histone Modification in NSCLC: Molecular Mechanisms and Therapeutic Targets. Int. J. Mol. Sci. 2021, 22, 1701. [Google Scholar] [CrossRef] [PubMed]
- Parveen, R.; Harihar, D.; Chatterji, B.P. Recent histone deacetylase inhibitors in cancer therapy. Cancer 2023, 129, 3372–3380. [Google Scholar] [CrossRef]
- Bjorklund, C.C.; Lu, L.; Kang, J.; Hagner, P.R.; Havens, C.G.; Amatangelo, M.; Wang, M.; Ren, Y.; Couto, S.; Breider, M.; et al. Rate of CRL4(CRBN) substrate Ikaros and Aiolos degradation underlies differential activity of lenalidomide and pomalidomide in multiple myeloma cells by regulation of c-Myc and IRF4. Blood Cancer J. 2015, 5, e354. [Google Scholar] [CrossRef] [PubMed]
- Di Bacco, A.; Bahlis, N.J.; Munshi, N.C.; Avet-Loiseau, H.; Masszi, T.; Viterbo, L.; Pour, L.; Ganly, P.; Cavo, M.; Langer, C.; et al. c-MYC expression and maturity phenotypes are associated with outcome benefit from addition of ixazomib to lenalidomide-dexamethasone in myeloma. Eur. J. Haematol. 2020, 105, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Franssen, L.E.; Nijhof, I.S.; Couto, S.; Levin, M.D.; Bos, G.M.J.; Broijl, A.; Klein, S.K.; Ren, Y.; Wang, M.; Koene, H.R.; et al. Cereblon loss and up-regulation of c-Myc are associated with lenalidomide resistance in multiple myeloma patients. Haematologica 2018, 103, e368–e371. [Google Scholar] [CrossRef]
- McMillin, D.W.; Jacobs, H.M.; Delmore, J.E.; Buon, L.; Hunter, Z.R.; Monrose, V.; Yu, J.; Smith, P.G.; Richardson, P.G.; Anderson, K.C.; et al. Molecular and cellular effects of NEDD8-activating enzyme inhibition in myeloma. Mol. Cancer Ther. 2012, 11, 942–951. [Google Scholar] [CrossRef]
- Xia, M.; Wang, S.; Qi, Y.; Long, K.; Li, E.; He, L.; Pan, F.; Guo, Z.; Hu, Z. Inhibition of O-GlcNAc transferase sensitizes prostate cancer cells to docetaxel. Front. Oncol. 2022, 12, 993243. [Google Scholar] [CrossRef]
- Liu, J.; Song, T.; Zhou, W.; Xing, L.; Wang, S.; Ho, M.; Peng, Z.; Tai, Y.T.; Hideshima, T.; Anderson, K.C.; et al. A genome-scale CRISPR-Cas9 screening in myeloma cells identifies regulators of immunomodulatory drug sensitivity. Leukemia 2019, 33, 171–180. [Google Scholar] [CrossRef]
- Bohl, S.R.; Schmalbrock, L.K.; Bauhuf, I.; Meyer, T.; Dolnik, A.; Szyska, M.; Blätte, T.J.; Knödler, S.; Röhner, L.; Miller, D.; et al. Comprehensive CRISPR-Cas9 screens identify genetic determinants of drug responsiveness in multiple myeloma. Blood Adv. 2021, 5, 2391–2402. [Google Scholar] [CrossRef]
- Escrivá-Fernández, J.; Cueto-Ureña, C.; Solana-Orts, A.; Lledó, E.; Ballester-Lurbe, B.; Poch, E. A CRISPR interference strategy for gene expression silencing in multiple myeloma cell lines. J. Biol. Eng. 2023, 17, 34. [Google Scholar] [CrossRef]
- Grillone, K.; Ascrizzi, S.; Cremaschi, P.; Amato, J.; Polerà, N.; Croci, O.; Rocca, R.; Riillo, C.; Conforti, F.; Graziano, R.; et al. An unbiased lncRNA dropout CRISPR-Cas9 screen reveals RP11-350G8.5 as a novel therapeutic target for multiple myeloma. Blood 2024, 144, 1705–1721. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Zhang, C.; Jiang, Y.; Liu, H.; Huang, H.; Guo, D. Therapeutic status and the prospect of CRISPR/Cas9 gene editing in multiple myeloma. Future Oncol. 2020, 16, 1125–1136. [Google Scholar] [CrossRef]
- Agathanggelou, A.; Smith, E.; Davies, N.J.; Kwok, M.; Zlatanou, A.; Oldreive, C.E.; Mao, J.; Da Costa, D.; Yadollahi, S.; Perry, T.; et al. USP7 inhibition alters homologous recombination repair and targets CLL cells independently of ATM/p53 functional status. Blood 2017, 130, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, M.; Jiang, J.; Wan, Y.; Li, X.; Zhang, M.; Xiao, F.; Zhong, L.; Zhong, H.; Qin, Z.; et al. Targeting deubiquitinase USP7-mediated stabilization of XPO1 contributes to the anti-myeloma effects of selinexor. J. Transl. Med. 2025, 23, 62. [Google Scholar] [CrossRef]
- Baumann, V.; Lorenzer, C.; Thell, M.; Winkler, A.M.; Winkler, J. RNAi-Mediated Knockdown of Protein Expression. Methods Mol. Biol. 2017, 1654, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.; Dey, P.; De, A. Recent advancements in targeted protein knockdown technologies-emerging paradigms for targeted therapy. Explor. Target. Antitumor Ther. 2023, 4, 1227–1248. [Google Scholar] [CrossRef] [PubMed]
- Marschall, A.L.; Dübel, S.; Böldicke, T. Specific in vivo knockdown of protein function by intrabodies. MAbs 2015, 7, 1010–1035. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Y.; Pang, Z.; Guo, F.; Qin, Q.; Yin, T.; Sang, Y.; Feng, C.; Li, X.; Jiang, L.; et al. Knockdown of SUMO-activating enzyme subunit 2 (SAE2) suppresses cancer malignancy and enhances chemotherapy sensitivity in small cell lung cancer. J. Hematol. Oncol. 2015, 8, 67. [Google Scholar] [CrossRef]
- Aristodemou, A.E.N.; Rueda, D.S.; Taylor, G.P.; Bangham, C.R.M. The transcriptome of HTLV-1-infected primary cells following reactivation reveals changes to host gene expression central to the proviral life cycle. PLoS Pathog. 2023, 19, e1011494. [Google Scholar] [CrossRef]
- Jia, Y.; Zhao, J.; Yu, T.; Zhang, X.; Qi, X.; Hao, T.; Jin, Z.; Zhao, X. PSMC3 promotes RNAi by maintaining AGO2 stability through USP14. Cell Mol. Biol. Lett. 2022, 27, 111. [Google Scholar] [CrossRef]
- Deng, J.; Liao, S.; Chen, C.; Han, F.; Lei, S.; Lai, X.; Ye, K.; Han, Q.; E, F.; Lu, C.; et al. Specific intracellular retention of circSKA3 promotes colorectal cancer metastasis by attenuating ubiquitination and degradation of SLUG. Cell Death Dis. 2023, 14, 750. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; He, X.Y.; Xiao, C.M.; Lin, Q.; Wang, M.Y.; Liu, C.Y.; Kong, L.Y.; Chen, Z.; Xia, Y.Z. Circular RNA-encoded oncogenic PIAS1 variant blocks immunogenic ferroptosis by modulating the balance between SUMOylation and phosphorylation of STAT1. Mol. Cancer 2024, 23, 207. [Google Scholar] [CrossRef]
- Ishida, T.; Ciulli, A. E3 Ligase Ligands for PROTACs: How They Were Found and How to Discover New Ones. SLAS Discov. 2021, 26, 484–502. [Google Scholar] [CrossRef]
- An, S.; Fu, L. Small-molecule PROTACs: An emerging and promising approach for the development of targeted therapy drugs. EBioMedicine 2018, 36, 553–562. [Google Scholar] [CrossRef]
- Ma, L.; Wang, J.; Zhang, Y.; Fang, F.; Ling, J.; Chu, X.; Zhang, Z.; Tao, Y.; Li, X.; Tian, Y.; et al. BRD4 PROTAC degrader MZ1 exerts anticancer effects in acute myeloid leukemia by targeting c-Myc and ANP32B genes. Cancer Biol. Ther. 2022, 23, 1–15. [Google Scholar] [CrossRef]
- Fiskus, W.; Mill, C.P.; Perera, D.; Birdwell, C.; Deng, Q.; Yang, H.; Lara, B.H.; Jain, N.; Burger, J.; Ferrajoli, A.; et al. BET proteolysis targeted chimera-based therapy of novel models of Richter Transformation-diffuse large B-cell lymphoma. Leukemia 2021, 35, 2621–2634. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Rong, Q.; Ye, L.; Fang, B.; Zhao, Y.; Sun, Y.; Zhou, H.; Wang, D.; He, J.; Cui, Z.; et al. Discovery of a Novel Orally Bioavailable FLT3-PROTAC Degrader for Efficient Treatment of Acute Myeloid Leukemia and Overcoming Resistance of FLT3 Inhibitors. J. Med. Chem. 2024, 67, 7197–7223. [Google Scholar] [CrossRef]
- Ahn, G.; Riley, N.M.; Kamber, R.A.; Wisnovsky, S.; Moncayo von Hase, S.; Bassik, M.C.; Banik, S.M.; Bertozzi, C.R. Elucidating the cellular determinants of targeted membrane protein degradation by lysosome-targeting chimeras. Science 2023, 382, eadf6249. [Google Scholar] [CrossRef] [PubMed]
- Banik, S.M.; Pedram, K.; Wisnovsky, S.; Ahn, G.; Riley, N.M.; Bertozzi, C.R. Lysosome-targeting chimaeras for degradation of extracellular proteins. Nature 2020, 584, 291–297. [Google Scholar] [CrossRef]
- Su, L.Y.; Tian, Y.; Zheng, Q.; Cao, Y.; Yao, M.; Wang, S.; Xu, W.; Xi, C.; Clocchiatti, A.; Nie, G.; et al. Anti-tumor immunotherapy using engineered bacterial outer membrane vesicles fused to lysosome-targeting chimeras mediated by transferrin receptor. Cell Chem. Biol. 2024, 31, 1219–1230.e1215. [Google Scholar] [CrossRef]
- Malarvannan, M.; Unnikrishnan, S.; Monohar, S.; Ravichandiran, V.; Paul, D. Design and optimization strategies of PROTACs and its Application, Comparisons to other targeted protein degradation for multiple oncology therapies. Bioorg Chem. 2025, 154, 107984. [Google Scholar] [CrossRef]
- Song, J.; Hu, M.; Zhou, J.; Xie, S.; Li, T.; Li, Y. Targeted protein degradation in drug development: Recent advances and future challenges. Eur. J. Med. Chem. 2023, 261, 115839. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.Q.; Xiao, H.T.; Yang, F.; Wang, Y.D.; Li, P.; Zheng, Z.G. Advancing targeted protein degradation for metabolic diseases therapy. Pharmacol. Res. 2023, 188, 106627. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Cui, J.; Chen, H.; Yu, B.; Long, S. Recent progress in degradation of membrane proteins by PROTACs and alternative targeted protein degradation techniques. Eur. J. Med. Chem. 2023, 262, 115911. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.; Zhou, B.; Zhang, B.; Ren, H.; Zhu, L.; Zheng, G.; Ge, M.; Ge, J. Recent advances in the development of EGFR degraders: PROTACs and LYTACs. Eur. J. Med. Chem. 2022, 239, 114533. [Google Scholar] [CrossRef]
- Richardson, P.G.; Hungria, V.T.; Yoon, S.S.; Beksac, M.; Dimopoulos, M.A.; Elghandour, A.; Jedrzejczak, W.W.; Guenther, A.; Nakorn, T.N.; Siritanaratkul, N.; et al. Panobinostat plus bortezomib and dexamethasone in previously treated multiple myeloma: Outcomes by prior treatment. Blood 2016, 127, 713–721. [Google Scholar] [CrossRef]
- Kotani, H.; Oshima, H.; Boucher, J.C.; Yamano, T.; Sakaguchi, H.; Sato, S.; Fukuda, K.; Nishiyama, A.; Yamashita, K.; Ohtsubo, K.; et al. Dual inhibition of SUMOylation and MEK conquers MYC-expressing KRAS-mutant cancers by accumulating DNA damage. J. Biomed. Sci. 2024, 31, 68. [Google Scholar] [CrossRef]
- Charliński, G.; Vesole, D.H.; Jurczyszyn, A. Rapid Progress in the Use of Immunomodulatory Drugs and Cereblon E3 Ligase Modulators in the Treatment of Multiple Myeloma. Cancers 2021, 13, 4666. [Google Scholar] [CrossRef]
- Hosen, N. Identifying and targeting multiple myeloma-specific antigens resulting from post-translational protein modifications by CAR-T cell therapies. Rinsho Ketsueki 2023, 64, 427–431. [Google Scholar] [CrossRef]
- CAR T-cell Efficacy in Solid Tumors Is Affected by N-glycosylation. Cancer Discov. 2022, 12, 598. [CrossRef]
- Munshi, N.C.; Anderson, L.D., Jr.; Shah, N.; Madduri, D.; Berdeja, J.; Lonial, S.; Raje, N.; Lin, Y.; Siegel, D.; Oriol, A.; et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2021, 384, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Garcia, E.; Popovic, R.; Min, D.J.; Sweet, S.M.; Thomas, P.M.; Zamdborg, L.; Heffner, A.; Will, C.; Lamy, L.; Staudt, L.M.; et al. The MMSET histone methyl transferase switches global histone methylation and alters gene expression in t(4;14) multiple myeloma cells. Blood 2011, 117, 211–220. [Google Scholar] [CrossRef]
- Xie, Z.; Bi, C.; Chooi, J.Y.; Chan, Z.L.; Mustafa, N.; Chng, W.J. MMSET regulates expression of IRF4 in t(4;14) myeloma and its silencing potentiates the effect of bortezomib. Leukemia 2015, 29, 2347–2354. [Google Scholar] [CrossRef]
- Dolloff, N.G.; Talamo, G. Targeted therapy of multiple myeloma. Adv. Exp. Med. Biol. 2013, 779, 197–221. [Google Scholar] [CrossRef]
- San-Miguel, J.F.; Hungria, V.T.M.; Yoon, S.S.; Beksac, M.; Dimopoulos, M.A.; Elghandour, A.; Jedrzejczak, W.W.; Guenther, A.; Na Nakorn, T.; Siritanaratkul, N.; et al. Panobinostat plus bortezomib and dexamethasone: Impact of dose intensity and administration frequency on safety in the PANORAMA 1 trial. Br. J. Haematol. 2017, 179, 66–74. [Google Scholar] [CrossRef]
- San-Miguel, J.F.; Hungria, V.T.; Yoon, S.S.; Beksac, M.; Dimopoulos, M.A.; Elghandour, A.; Jedrzejczak, W.W.; Günther, A.; Nakorn, T.N.; Siritanaratkul, N.; et al. Overall survival of patients with relapsed multiple myeloma treated with panobinostat or placebo plus bortezomib and dexamethasone (the PANORAMA 1 trial): A randomised, placebo-controlled, phase 3 trial. Lancet Haematol. 2016, 3, e506–e515. [Google Scholar] [CrossRef] [PubMed]
- Laubach, J.P.; Moreau, P.; San-Miguel, J.F.; Richardson, P.G. Panobinostat for the Treatment of Multiple Myeloma. Clin. Cancer Res. 2015, 21, 4767–4773. [Google Scholar] [CrossRef] [PubMed]
- Gkotzamanidou, M.; Terpou, E.; Kentepozidis, N.; Terpos, E. Targeting the Interplay between HDACs and DNA Damage Repair for Myeloma Therapy. Int. J. Mol. Sci. 2021, 22, 406. [Google Scholar] [CrossRef]
- Guo, J.; Chai, X.; Mei, Y.; Du, J.; Du, H.; Shi, H.; Zhu, J.K.; Zhang, H. Acetylproteomics analyses reveal critical features of lysine-ε-acetylation in Arabidopsis and a role of 14-3-3 protein acetylation in alkaline response. Stress. Biol. 2022, 2, 1. [Google Scholar] [CrossRef]
- Ji, Y.; Wu, Z.; Dai, Z.; Sun, K.; Wang, J.; Wu, G. Nutritional epigenetics with a focus on amino acids: Implications for the development and treatment of metabolic syndrome. J. Nutr. Biochem. 2016, 27, 1–8. [Google Scholar] [CrossRef]
- Auner, H.W.; Cenci, S. Recent advances and future directions in targeting the secretory apparatus in multiple myeloma. Br. J. Haematol. 2015, 168, 14–25. [Google Scholar] [CrossRef]
- Casado, P.; Alcolea, M.P.; Iorio, F.; Rodríguez-Prados, J.C.; Vanhaesebroeck, B.; Saez-Rodriguez, J.; Joel, S.; Cutillas, P.R. Phosphoproteomics data classify hematological cancer cell lines according to tumor type and sensitivity to kinase inhibitors. Genome Biol. 2013, 14, R37. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Lin, T.C.; Bi, X.; Lu, G.; Dawson, B.C.; Miranda, R.; Medeiros, L.J.; McNiece, I.; McCarty, N. TRIM44 promotes quiescent multiple myeloma cell occupancy and survival in the osteoblastic niche via HIF-1α stabilization. Leukemia 2019, 33, 469–486. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Roy, M.; Liang, L.; Cao, W.; Hu, B.; Li, Y.; Xiao, X.; Wang, H.; Ye, M.; Sun, S.; et al. Deubiquitylase USP12 induces pro-survival autophagy and bortezomib resistance in multiple myeloma by stabilizing HMGB1. Oncogene 2022, 41, 1298–1308. [Google Scholar] [CrossRef]
- Nikesitch, N.; Ling, S.C. Molecular mechanisms in multiple myeloma drug resistance. J. Clin. Pathol. 2016, 69, 97–101. [Google Scholar] [CrossRef]
- van de Donk, N.W.; Bloem, A.C.; van der Spek, E.; Lokhorst, H.M. New treatment strategies for multiple myeloma by targeting BCL-2 and the mevalonate pathway. Curr. Pharm. Des. 2006, 12, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Ge, F.; Tao, S.; Bi, L.; Zhang, Z.; Zhang, X. Proteomics: Addressing the challenges of multiple myeloma. Acta Biochim. Biophys. Sin. (Shanghai) 2011, 43, 89–95. [Google Scholar] [CrossRef]
- Kratka, K.; Sistik, P.; Olivkova, I.; Kusnierova, P.; Svagera, Z.; Stejskal, D. Mass Spectrometry-Based Proteomics in Clinical Diagnosis of Amyloidosis and Multiple Myeloma: A Review (2012–2024). J. Mass. Spectrom. 2025, 60, e5116. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, S.; Xu, J.; Cui, W.; Jin, H.; Wang, X.; Maimaitiyiming, Y. Post-Translational Modifications in Multiple Myeloma: Mechanisms of Drug Resistance and Therapeutic Opportunities. Biomolecules 2025, 15, 702. https://doi.org/10.3390/biom15050702
Hu S, Xu J, Cui W, Jin H, Wang X, Maimaitiyiming Y. Post-Translational Modifications in Multiple Myeloma: Mechanisms of Drug Resistance and Therapeutic Opportunities. Biomolecules. 2025; 15(5):702. https://doi.org/10.3390/biom15050702
Chicago/Turabian StyleHu, Shuoyang, Jirun Xu, Weiyan Cui, Haoran Jin, Xiaoyu Wang, and Yasen Maimaitiyiming. 2025. "Post-Translational Modifications in Multiple Myeloma: Mechanisms of Drug Resistance and Therapeutic Opportunities" Biomolecules 15, no. 5: 702. https://doi.org/10.3390/biom15050702
APA StyleHu, S., Xu, J., Cui, W., Jin, H., Wang, X., & Maimaitiyiming, Y. (2025). Post-Translational Modifications in Multiple Myeloma: Mechanisms of Drug Resistance and Therapeutic Opportunities. Biomolecules, 15(5), 702. https://doi.org/10.3390/biom15050702






