Molecular and Biophysical Perspectives on Dormancy Breaking: Lessons from Yeast Spore
Abstract
1. Introduction
| Feature | S. cerevisiae (Budding Yeast) | Refs | S. pombe (Fission Yeast) | Refs |
|---|---|---|---|---|
| Life Cycle Characteristics | ||||
| Natural state | Predominantly diploid | [21] | Predominantly haploid | |
| Mating type | MATa and MATα | h- and h+ | ||
| Sporulation Conditions | ||||
| Primary trigger | Nitrogen depletion with non-fermentable carbon source | [22] | Nitrogen depletion | |
| Spore Structure | ||||
| Ascus wall after sporulation | Remains intact | Digested by glucanases (Agn2, Eng2) | [23,24,25] | |
| Spore wall composition | Four layers: mannan, β-1,3-glucan, chitosan, dityrosine | [15,26,27] | Primarily glucan and chitosan (exact composition not fully characterized) | [28,29,30] |
| Spore connection | Interspore bridges connect sibling spores | [31] | No interspore bridges | |
| Spore surface | Ridged proteinaceous layer | [32] | Characteristic outward projection | [33,34] |
| Metabolic Features | ||||
| Trehalose accumulation | Significant increase | [35,36] | ~1000-fold increase compared to vegetative cells | [37,38,39] |
| Glycogen accumulation | Present | [35,36] | ~40-fold increase compared to vegetative cells | [37,39] |
| Adenosine triphosphate (ATP) levels | Substantial (~3–4 mM) | [35] | Relatively high compared to residual ascus | [40] |
| Transcription activity | ~5% of vegetative cells | [41] | Not precisely quantified | |
| Protein filament formation | Acetyl-CoA synthetase Acs1 forms filaments | [42] | Not known | |
| Germination Process | ||||
| Typical duration | 4–6 h | [43,44,45,46] | 10–12 h | [37,47,48,49] |
| Primary trigger | Glucose (Cyclic adenosine monophosphate-protein kinase A (cAMP-PKA) pathway) | [50] | Glucose (cAMP-PKA pathway) | [37,47,51] |
| Initial stages | Spore uncoating, polarized growth | [43,44,45,46] | Bright-to-dark transition, isotropic swelling | [37,47,48] |
| Polarization mechanism | Prepolarized to grow away from interspore bridges | [52] | Random polarization with dynamic polar cap movement | [48] |
| Polarization proteins | Cdc10, Bud8, Bud5 | [52,53] | GTP-bound Cdc42, Bud6, Bgs4, Cdc42 GAP (Rga6) | [48,54,55] |
| Cell growth pattern | Polarized growth -> Non-polarized growth -> Budding | [43,44,45,46] | Isotropic swelling -> Germ tube formation (outgrowth) | [37,47,48,49] |
| Required Nutrients for Complete Germination | ||||
| Glucose | Required for fast response | [44,50,56,57] | Essential | [58] |
| Additional nutrients | Required for later stages | [44,50,57] | Copper and iron ions required for outgrowth | [59,60] |
| Molecular Regulators | ||||
| Key signaling pathway | cAMP-PKA pathway | [50] | cAMP-PKA pathway | [37,47,51] |
| Trehalase | Nth1, Nth2 | Ntp1 | [37,38] | |
| Cell cycle regulators | Not required for early germination stage (Cdc28, Cdc37, Cdc4, Cdc34, Cdc7, Cdc24) | [50] | Not known | |
| Actin role | Essential for polarized growth | [46] | Essential for germ tube formation | [47] |
| Histone dynamics | Not known | Hht1 (H3) expression decreases during germination | [61] | |
| Heat shock protein | Hsp42 | [62] | Not known | |
| Biophysical Properties | ||||
| Particle mobility in dormant spores | Restricted (~50–150 nm particles) | [62] | Restricted (~40 nm and ~50–150 nm particles) | [37] |
| Small protein diffusion | Not fully documented | Relatively free diffusion | [37] | |
| pH changes during germination | Dormant spores: ~5.9 -> Vegetative cells: ~7.4 | [62,63] | Not known | |
| Ecological Context | ||||
| Natural habitat | Fruits, insect vectors, forest niches | [64,65,66] | Not well characterized (honey?) | [67] |
| Spore survival advantage | High survival in the insect gut | [64] | High survival in the insect gut | [64] |
| Germination pattern | Commonly sibling spore mating | [52,68] | Single spores |
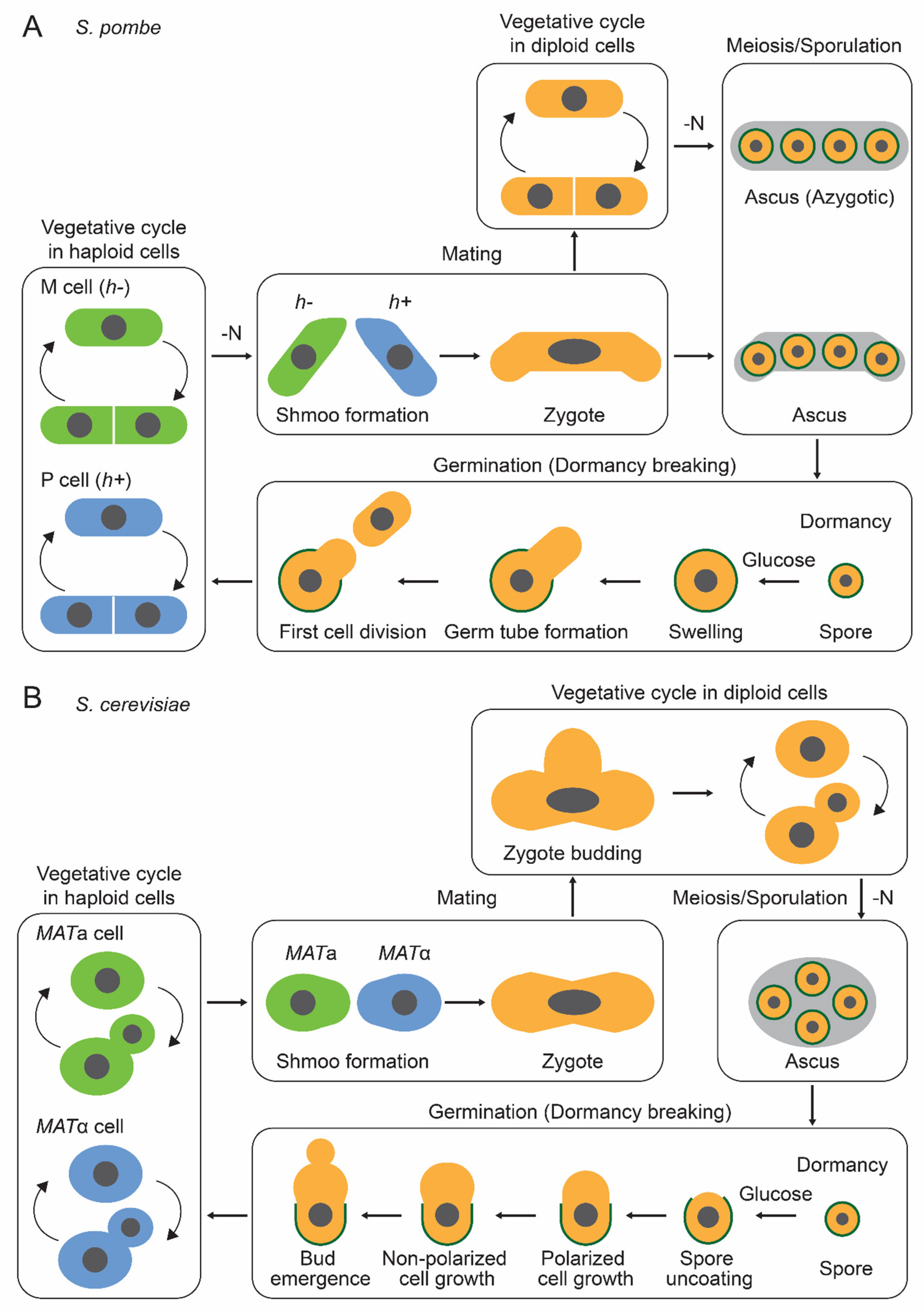
2. Dormancy in Yeast Cells
2.1. S. pombe
2.1.1. Enhanced Stress Resistance of S. pombe Spores
2.1.2. Metabolic Changes in S. pombe Spores
2.2. S. cerevisiae
2.2.1. Enhanced Stress Resistance of S. cerevisiae Spores
2.2.2. Reduced Metabolic Activities in S. cerevisiae Spores
2.2.3. Carbohydrate Accumulation in S. cerevisiae Spores
2.2.4. Ecological Significance of S. cerevisiae Spores
3. Dormancy Breaking in Yeast Cells
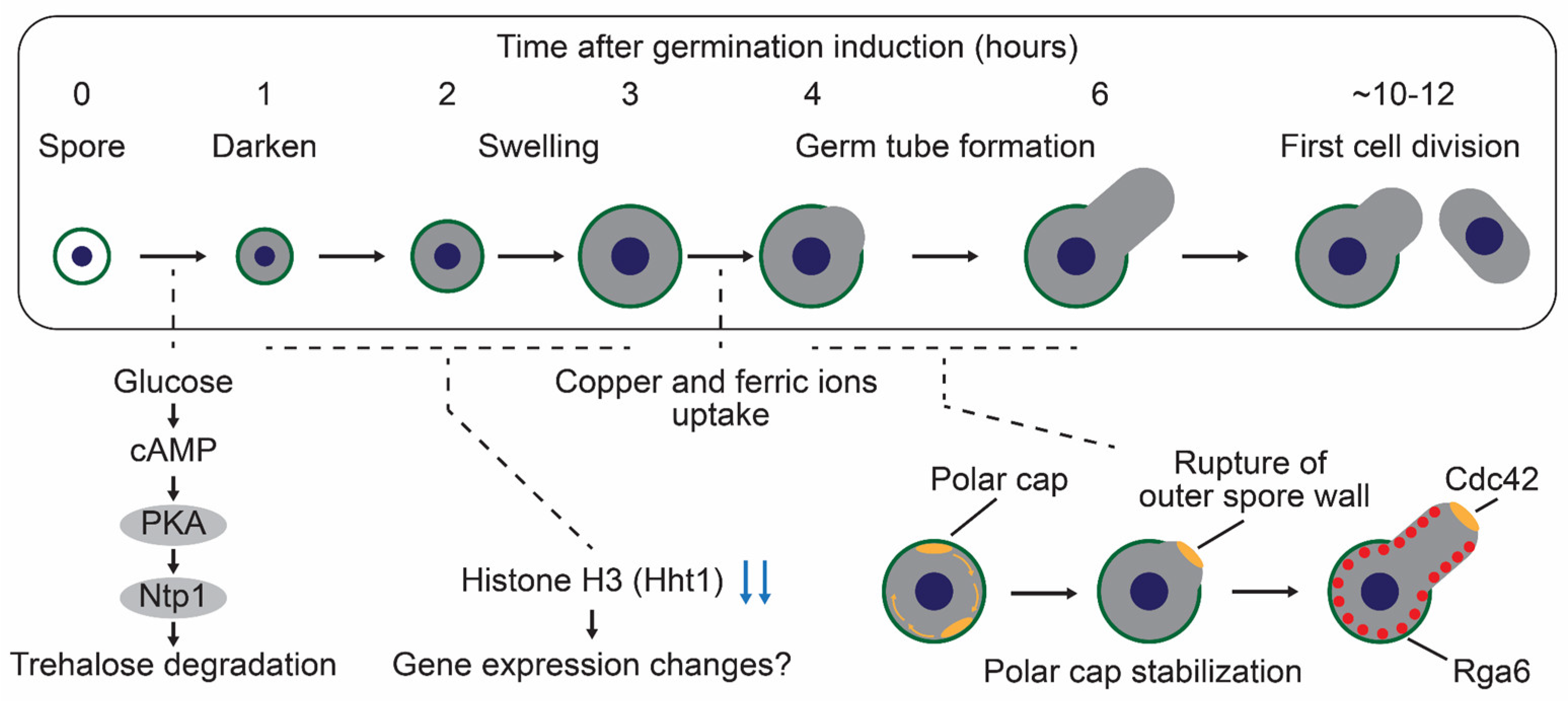
3.1. S. pombe
3.1.1. Glucose-Sensing and Trehalose Degradation Pathway During Germination in S. pombe
3.1.2. Gene Expression Landscape During Germination in S. pombe
3.1.3. Nutrients Required for Germination in S. pombe
3.1.4. Morphological Changes During Germination in S. pombe
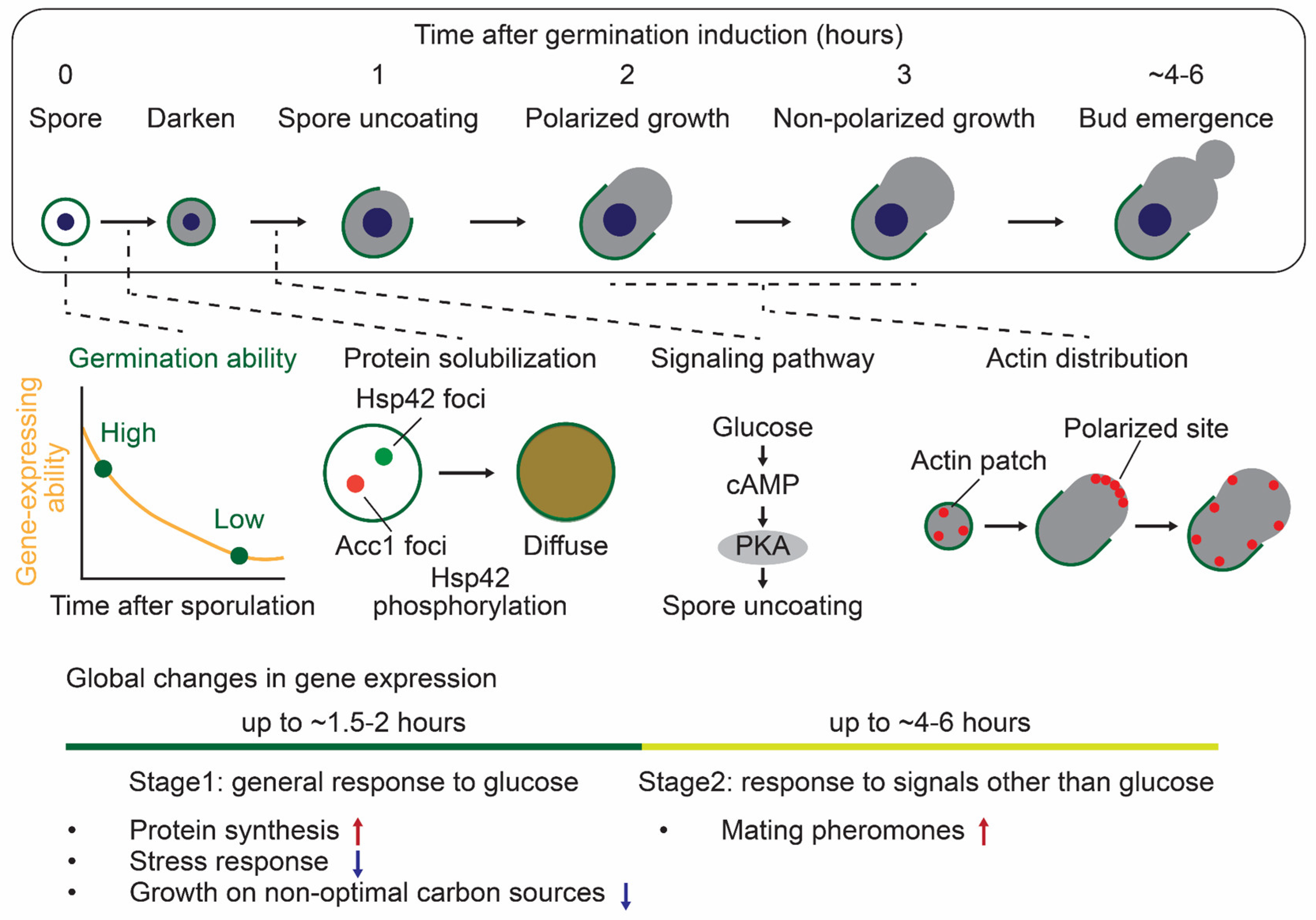
3.2. S. cerevisiae
3.2.1. Characteristics for S. cerevisiae Germination: Intact Ascus and Interspore Bridge
3.2.2. Methods to Monitor S. cerevisiae Germination
3.2.3. Molecular Mechanisms Regulating S. cerevisiae Germination
3.2.4. Transcriptome, Proteome, and Phosphoproteome Profiles During S. cerevisiae Germination
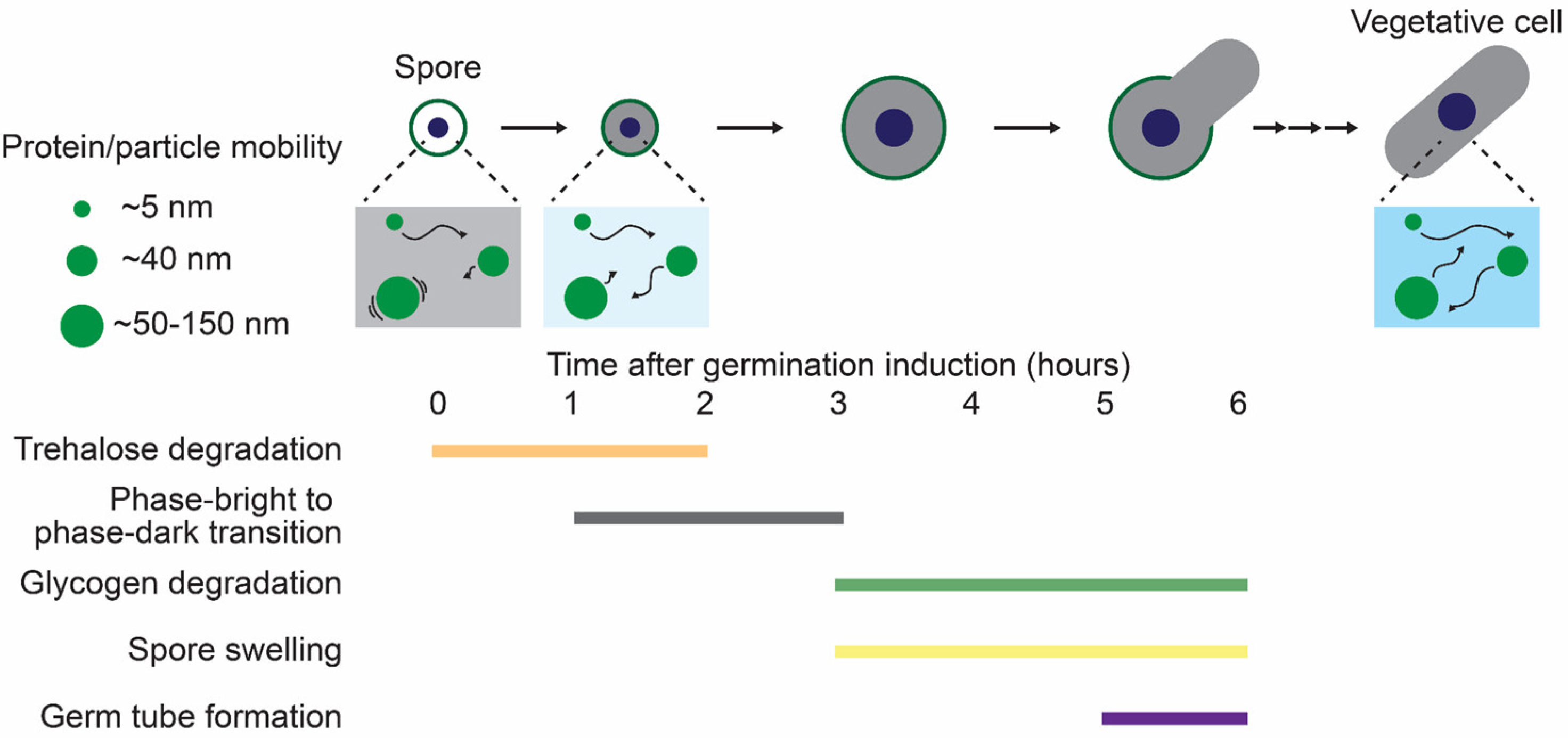
4. The Biophysical Properties of the Cytoplasm During Dormancy
4.1. Overview
4.1.1. What Cellular Processes Are Affected by Cytoplasmic Properties?
4.1.2. Homeostasis Mechanisms Regulating Cytoplasmic Properties
4.1.3. Methods to Evaluate the Cytoplasmic Properties
4.2. Dormancy and Dormancy Breaking
4.2.1. The Biophysical Properties of the Cytoplasm in Dormant Yeast Spores
4.2.2. The Biophysical Properties of the Cytoplasm During Spore Germination
4.2.3. The Mechanisms Regulating Cytoplasmic Properties During Dormancy and Germination
5. Summary and Perspective
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| cAMP-PKA | cyclic Adenosine MonoPhosphate-Protein Kinase A |
| FRAP | Fluorescence Recovery After Photobleaching |
| FCS | Fluorescence Correlation Spectroscopy |
| FLIP | Fluorescence Loss in Photobleaching |
| FDAP | Fluorescence Decay After Photostimulation |
| LLPS | Liquid-Liquid Phase Separation |
| GEMs | Genetically Encoded Multimeric nanoparticles |
| GAP | GTPase-Activating Proteins |
| TOR | Target Of Rapamycin |
| RNAPII | RNA Polymerase II |
| BSA | Bovine Serum Albumin |
References
- Lennon, J.T.; Jones, S.E. Microbial Seed Banks: The Ecological and Evolutionary Implications of Dormancy. Nat. Rev. Microbiol. 2011, 9, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Rittershaus, E.S.C.; Baek, S.-H.; Sassetti, C.M. The Normalcy of Dormancy: Common Themes in Microbial Quiescence. Cell Host Microbe 2013, 13, 643–651. [Google Scholar] [CrossRef]
- Setlow, P. Germination of Spores of Bacillus Species: What We Know and Do Not Know. J. Bacteriol. 2014, 196, 1297–1305. [Google Scholar] [CrossRef]
- Park, H.-S.; Yu, J.-H. Genetic Control of Asexual Sporulation in Filamentous Fungi. Curr. Opin. Microbiol. 2012, 15, 669–677. [Google Scholar] [CrossRef]
- Zhao, Y.; Lin, J.; Fan, Y.; Lin, X. Life Cycle of Cryptococcus Neoformans. Annu. Rev. Microbiol. 2019, 73, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Née, G.; Xiang, Y.; Soppe, W.J. The Release of Dormancy, a Wake-up Call for Seeds to Germinate. Curr. Opin. Plant Biol. 2017, 35, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Keilin, D. The Leeuwenhoek Lecture—The Problem of Anabiosis or Latent Life: History and Current Concept. Proc. R. Soc. Lond. Ser. B-Biol. Sci. 1959, 150, 149–191. [Google Scholar] [CrossRef]
- Clegg, J.S. Cryptobiosis—A Peculiar State of Biological Organization. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2001, 128, 613–624. [Google Scholar] [CrossRef]
- Hibshman, J.D.; Clegg, J.S.; Goldstein, B. Mechanisms of Desiccation Tolerance: Themes and Variations in Brine Shrimp, Roundworms, and Tardigrades. Front. Physiol. 2020, 11, 592016. [Google Scholar] [CrossRef]
- Sogame, Y.; Kikawada, T. Current Findings on the Molecular Mechanisms Underlying Anhydrobiosis in Polypedilum Vanderplanki. Curr. Opin. Insect Sci. 2017, 19, 16–21. [Google Scholar] [CrossRef]
- Wharton, D.A. Anhydrobiosis. Curr. Biol. 2015, 25, R1114–R1116. [Google Scholar] [CrossRef] [PubMed]
- Sipiczki, M. Where Does Fission Yeast Sit on the Tree of Life? Genome Biol. 2000, 1, 1–4. [Google Scholar] [CrossRef]
- Wood, V.; Gwilliam, R.; Rajandream, M.-A.; Lyne, M.; Lyne, R.; Stewart, A.; Sgouros, J.; Peat, N.; Hayles, J.; Baker, S.; et al. The Genome Sequence of Schizosaccharomyces Pombe. Nature 2002, 415, 871–880. [Google Scholar] [CrossRef] [PubMed]
- van Werven, F.J.; Amon, A. Regulation of Entry into Gametogenesis. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 3521–3531. [Google Scholar] [CrossRef] [PubMed]
- Neiman, A.M. Sporulation in the Budding Yeast Saccharomyces Cerevisiae. Genetics 2011, 189, 737–765. [Google Scholar] [CrossRef]
- Ohtsuka, H.; Imada, K.; Shimasaki, T.; Aiba, H. Sporulation: A Response to Starvation in the Fission Yeast Schizosaccharomyces Pombe. MicrobiologyOpen 2022, 11, e1303. [Google Scholar] [CrossRef]
- Sun, S.; Gresham, D. Cellular Quiescence in Budding Yeast. Yeast Chichester Engl. 2021, 38, 12–29. [Google Scholar] [CrossRef]
- Yanagida, M. Cellular Quiescence: Are Controlling Genes Conserved? Trends Cell Biol. 2009, 19, 705–715. [Google Scholar] [CrossRef]
- Walker, R.M.; Sanabria, V.C.; Youk, H. Microbial Life in Slow and Stopped Lanes. Trends Microbiol. 2024, 32, 650–662. [Google Scholar] [CrossRef]
- Li, L.; Miles, S.; Melville, Z.; Prasad, A.; Bradley, G.; Breeden, L.L. Key Events during the Transition from Rapid Growth to Quiescence in Budding Yeast Require Posttranscriptional Regulators. Mol. Biol. Cell 2013, 24, 3697–3709. [Google Scholar] [CrossRef]
- Peter, J.; De Chiara, M.; Friedrich, A.; Yue, J.-X.; Pflieger, D.; Bergström, A.; Sigwalt, A.; Barre, B.; Freel, K.; Llored, A.; et al. Genome Evolution across 1011 Saccharomyces Cerevisiae Isolates. Nature 2018, 556, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Freese, E.B.; Chu, M.I.; Freese, E. Initiation of Yeast Sporulation by Partial Carbon, Nitrogen, or Phosphate Deprivation. J. Bacteriol. 1982, 149, 840–851. [Google Scholar] [CrossRef]
- Dekker, N.; van Rijssel, J.; Distel, B.; Hochstenbach, F. Role of the α-Glucanase Agn2p in Ascus-Wall Endolysis Following Sporulation in Fission Yeast. Yeast 2007, 24, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Encinar del Dedo, J.; Dueñas, E.; Arnáiz, Y.; del Rey, F.; Vázquez de Aldana, C.R. β-Glucanase Eng2 Is Required for Ascus Wall Endolysis after Sporulation in the Fission Yeast Schizosaccharomyces Pombe. Eukaryot. Cell 2009, 8, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; King, M.C. A Quality Control Mechanism Linking Meiotic Success to Release of Ascospores. PLoS ONE 2013, 8, e82758. [Google Scholar] [CrossRef]
- Briza, P.; Winkler, G.; Kalchhauser, H.; Breitenbach, M. Dityrosine Is a Prominent Component of the Yeast Ascospore Wall. A Proof of Its Structure. J. Biol. Chem. 1986, 261, 4288–4294. [Google Scholar] [CrossRef]
- Briza, P.; Ellinger, A.; Winkler, G.; Breitenbach, M. Chemical Composition of the Yeast Ascospore Wall. The Second Outer Layer Consists of Chitosan. J. Biol. Chem. 1988, 263, 11569–11574. [Google Scholar] [CrossRef]
- Liu, J.; Tang, X.; Wang, H.; Balasubramanian, M. Bgs2p, a 1,3-β-Glucan Synthase Subunit, Is Essential for Maturation of Ascospore Wall in Schizosaccharomyces Pombe. FEBS Lett. 2000, 478, 105–108. [Google Scholar] [CrossRef]
- Tougan, T.; Chiba, Y.; Kakihara, Y.; Hirata, A.; Nojima, H. Meu10 Is Required for Spore Wall Maturation in Schizosaccharomyces Pombe. Genes Cells 2002, 7, 217–231. [Google Scholar] [CrossRef]
- Arellano, M.; Cartagena-Lirola, H.; Nasser Hajibagheri, M.A.; Durán, A.; Henar Valdivieso, M. Proper Ascospore Maturation Requires the Chs1+ Chitin Synthase Gene in Schizosaccharomyces Pombe. Mol. Microbiol. 2000, 35, 79–89. [Google Scholar] [CrossRef]
- Coluccio, A.; Neiman, A.M. Interspore Bridges: A New Feature of the Saccharomyces Cerevisiae Spore Wall. Microbiology 2004, 150, 3189–3196. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, G.; Li, Z.-J.; Liu, Y.-S.; Gao, X.-D.; Nakanishi, H. Studies on the Proteinaceous Structure Present on the Surface of the Saccharomyces Cerevisiae Spore Wall. J. Fungi 2023, 9, 392. [Google Scholar] [CrossRef]
- Tahara, Y.O.; Miyata, M.; Nakamura, T. Quick-Freeze, Deep-Etch Electron Microscopy Reveals the Characteristic Architecture of the Fission Yeast Spore. J. Fungi 2021, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Teraguchi, E.; Imada, K.; Tahara, Y.O.; Nakamura, S.; Miyata, M.; Kagiwada, S.; Nakamura, T. The Fission Yeast RNA-Binding Protein Meu5 Is Involved in Outer Forespore Membrane Breakdown during Spore Formation. J. Fungi 2020, 6, 284. [Google Scholar] [CrossRef] [PubMed]
- Walther, T.; Létisse, F.; Peyriga, L.; Alkim, C.; Liu, Y.; Lardenois, A.; Martin-Yken, H.; Portais, J.-C.; Primig, M.; François, J.M. Developmental Stage-Dependent Metabolic Regulation during Meiotic Differentiation in Budding Yeast. BMC Biol. 2014, 12, 60. [Google Scholar] [CrossRef]
- Kane, S.M.; Roth, R. Carbohydrate Metabolism During Ascospore Development in Yeast. J. Bacteriol. 1974, 118, 8–14. [Google Scholar] [CrossRef]
- Sakai, K.; Kondo, Y.; Goto, Y.; Aoki, K. Cytoplasmic Fluidization Contributes to Breaking Spore Dormancy in Fission Yeast. Proc. Natl. Acad. Sci. USA 2024, 121, e2405553121. [Google Scholar] [CrossRef]
- Beltran, F.F.; Castillo, R.; Vicente-Soler, J.; Cansado, J.; Gacto, M. Role for Trehalase during Germination of Spores in the Fission Yeast Schizosaccharomyces Pombe. FEMS Microbiol. Lett. 2000, 193, 117–121. [Google Scholar] [CrossRef]
- Inoue, H.; Shimoda, C. Changes in Trehalose Content and Trehalase Activity during Spore Germination in Fission Yeast, Schizosaccharomyces Pombe. Arch. Microbiol. 1981, 129, 19–22. [Google Scholar] [CrossRef]
- Takaine, M.; Ueno, M.; Kitamura, K.; Imamura, H.; Yoshida, S. Reliable Imaging of ATP in Living Budding and Fission Yeast. J. Cell Sci. 2019, 132, jcs230649. [Google Scholar] [CrossRef]
- Brengues, M.; Pintard, L.; Lapeyre, B. mRNA Decay Is Rapidly Induced after Spore Germination of Saccharomyces Cerevisiae. J. Biol. Chem. 2002, 277, 40505–40512. [Google Scholar] [CrossRef] [PubMed]
- Hugener, J.; Xu, J.; Wettstein, R.; Ioannidi, L.; Velikov, D.; Wollweber, F.; Henggeler, A.; Matos, J.; Pilhofer, M. FilamentID Reveals the Composition and Function of Metabolic Enzyme Polymers during Gametogenesis. Cell 2024, 187, 3303–3318.e18. [Google Scholar] [CrossRef]
- Joseph-Strauss, D.; Zenvirth, D.; Simchen, G.; Barkai, N. Spore Germination in Saccharomyces Cerevisiae: Global Gene Expression Patterns and Cell Cycle Landmarks. Genome Biol. 2007, 8, R241. [Google Scholar] [CrossRef]
- Geijer, C.; Joseph-Strauss, D.; Simchen, G.; Barkai, N.; Hohmann, S. Saccharomyces Cerevisiae Spore Germination. In Dormancy and Resistance in Harsh Environments; Lubzens, E., Cerda, J., Clark, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 29–41. ISBN 978-3-642-12422-8. [Google Scholar]
- Sando, N.; Oguchi, T.; Nagano, M.; Osumi, M. Morphological Changes in Ascospores of Saccharomyces Cerevisiae During Aerobic and Anaerobic Germination. J. Gen. Appl. Microbiol. 1980, 26, 403–412. [Google Scholar] [CrossRef]
- Kono, K.; Matsunaga, R.; Hirata, A.; Suzuki, G.; Abe, M.; Ohya, Y. Involvement of Actin and Polarisome in Morphological Change during Spore Germination of Saccharomyces Cerevisiae. Yeast 2005, 22, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, M.; Shimoda, C. The Cyclic AMP/PKA Signal Pathway Is Required for Initiation of Spore Germination in Schizosaccharomyces Pombe. Yeast 2001, 18, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Bonazzi, D.; Julien, J.-D.; Romao, M.; Seddiki, R.; Piel, M.; Boudaoud, A.; Minc, N. Symmetry Breaking in Spore Germination Relies on an Interplay between Polar Cap Stability and Spore Wall Mechanics. Dev. Cell 2014, 28, 534–546. [Google Scholar] [CrossRef]
- Tsuyuzaki, H.; Ujiie, R.; Sato, M. Wake-up Alarm: Virtual Time-Lapse Gene Expression Landscape Illuminates Mechanisms Underlying Dormancy Breaking of Germinating Spores. Curr. Genet. 2021, 67, 519–534. [Google Scholar] [CrossRef]
- Herman, P.K.; Rine, J. Yeast Spore Germination: A Requirement for Ras Protein Activity during Re-entry into the Cell Cycle. EMBO J. 1997, 16, 6171–6181. [Google Scholar] [CrossRef]
- Maeda, T.; Watanabe, Y.; Kunitomo, H.; Yamamoto, M. Cloning of the Pka1 Gene Encoding the Catalytic Subunit of the cAMP-Dependent Protein Kinase in Schizosaccharomyces Pombe. J. Biol. Chem. 1994, 269, 9632–9637. [Google Scholar] [CrossRef]
- Heasley, L.R.; Singer, E.; Cooperman, B.J.; McMurray, M.A. Saccharomyces Spores Are Born Prepolarized to Outgrow Away from Spore–Spore Connections and Penetrate the Ascus Wall. Yeast 2021, 38, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Cooperman, B.; McMurray, M. Roles for the Canonical Polarity Machinery in the de Novo Establishment of Polarity in Budding Yeast Spores. Mol. Biol. Cell 2025, 36(3), 1–17. [Google Scholar] [CrossRef]
- Cortés, J.C.G.; Carnero, E.; Ishiguro, J.; Sánchez, Y.; Durán, A.; Ribas, J.C. The Novel Fission Yeast (1,3)β-D-Glucan Synthase Catalytic Subunit Bgs4p Is Essential during Both Cytokinesis and Polarized Growth. J. Cell Sci. 2005, 118, 157–174. [Google Scholar] [CrossRef]
- Wei, W.; Zheng, B.; Zheng, S.; Wu, D.; Chu, Y.; Zhang, S.; Wang, D.; Ma, X.; Liu, X.; Yao, X.; et al. The Cdc42 GAP Rga6 Promotes Monopolar Outgrowth of Spores. J. Cell Biol. 2022, 222, e202202064. [Google Scholar] [CrossRef] [PubMed]
- Donnini, C.; Artoni, N.; Marmiroli, N. Germination Conditions That Require Mitochondrial Function in Saccharomyces Cerevisiae: Utilization of Acetate and Galactose. J. Bacteriol. 1986, 168, 1250–1253. [Google Scholar] [CrossRef]
- Xu, G.; West, T.P. Nutritional and Physiological Factors Affecting Germination of Heterothallic Saccharomyces Cerevisiae Ascospores. Microbios 1992, 72, 27–34. [Google Scholar]
- Shimoda, C. Differential Effect of Glucose and Fructose on Spore Germination in the Fission Yeast, Schizosaccharomyces Pombe. Can. J. Microbiol. 1980, 26, 741–745. [Google Scholar] [CrossRef]
- Plante, S.; Normant, V.; Ramos-Torres, K.M.; Labbé, S. Cell-Surface Copper Transporters and Superoxide Dismutase 1 Are Essential for Outgrowth during Fungal Spore Germination. J. Biol. Chem. 2017, 292, 11896–11914. [Google Scholar] [CrossRef]
- Plante, S.; Labbé, S. Spore Germination Requires Ferrichrome Biosynthesis and the Siderophore Transporter Str1 in Schizosaccharomyces Pombe. Genetics 2019, 211, 893–911. [Google Scholar] [CrossRef]
- Tsuyuzaki, H.; Hosokawa, M.; Arikawa, K.; Yoda, T.; Okada, N.; Takeyama, H.; Sato, M. Time-Lapse Single-Cell Transcriptomics Reveals Modulation of Histone H3 for Dormancy Breaking in Fission Yeast. Nat. Commun. 2020, 11, 1265. [Google Scholar] [CrossRef]
- Plante, S.; Moon, K.-M.; Lemieux, P.; Foster, L.J.; Landry, C.R. Breaking Spore Dormancy in Budding Yeast Transforms the Cytoplasm and the Solubility of the Proteome. PLOS Biol. 2023, 21, e3002042. [Google Scholar] [CrossRef] [PubMed]
- Barton, J.K.; den Hollander, J.A.; Lee, T.M.; MacLaughlin, A.; Shulman, R.G. Measurement of the Internal pH of Yeast Spores by 31P Nuclear Magnetic Resonance. Proc. Natl. Acad. Sci. USA 1980, 77, 2470–2473. [Google Scholar] [CrossRef] [PubMed]
- Coluccio, A.E.; Rodriguez, R.K.; Kernan, M.J.; Neiman, A.M. The Yeast Spore Wall Enables Spores to Survive Passage through the Digestive Tract of Drosophila. PLoS ONE 2008, 3, e2873. [Google Scholar] [CrossRef] [PubMed]
- Knight, S.J.; Goddard, M.R. Sporulation in Soil as an Overwinter Survival Strategy in Saccharomyces Cerevisiae. FEMS Yeast Res. 2016, 16, fov102. [Google Scholar] [CrossRef]
- Stefanini, I.; Dapporto, L.; Legras, J.-L.; Calabretta, A.; Di Paola, M.; De Filippo, C.; Viola, R.; Capretti, P.; Polsinelli, M.; Turillazzi, S.; et al. Role of Social Wasps in Saccharomyces Cerevisiae Ecology and Evolution. Proc. Natl. Acad. Sci. USA 2012, 109, 13398–13403. [Google Scholar] [CrossRef]
- Brysch-Herzberg, M.; Jia, G.-S.; Seidel, M.; Assali, I.; Du, L.-L. Insights into the Ecology of Schizosaccharomyces Species in Natural and Artificial Habitats. Antonie Van Leeuwenhoek 2022, 115, 661–695. [Google Scholar] [CrossRef]
- McClure, A.W.; Jacobs, K.C.; Zyla, T.R.; Lew, D.J. Mating in Wild Yeast: Delayed Interest in Sex after Spore Germination. Mol. Biol. Cell 2018, 29, 3119–3127. [Google Scholar] [CrossRef]
- Mata, J.; Lyne, R.; Burns, G.; Bähler, J. The Transcriptional Program of Meiosis and Sporulation in Fission Yeast. Nat. Genet. 2002, 32, 143–147. [Google Scholar] [CrossRef]
- Krapp, A.; Hamelin, R.; Armand, F.; Chiappe, D.; Krapp, L.; Cano, E.; Moniatte, M.; Simanis, V. Analysis of the S. pombe Meiotic Proteome Reveals a Switch from Anabolic to Catabolic Processes and Extensive Post-Transcriptional Regulation. Cell Rep. 2019, 26, 1044–1058.e5. [Google Scholar] [CrossRef]
- Sivakova, B.; Wagner, A.; Kretova, M.; Jakubikova, J.; Gregan, J.; Kratochwill, K.; Barath, P.; Cipak, L. Quantitative Proteomics and Phosphoproteomics Profiling of Meiotic Divisions in the Fission Yeast Schizosaccharomyces Pombe. Sci. Rep. 2024, 14, 23105. [Google Scholar] [CrossRef]
- Billmyre, R.B.; Eickbush, M.T.; Craig, C.J.; Lange, J.J.; Wood, C.; Helston, R.M.; Zanders, S.E. Genome-Wide Quantification of Contributions to Sexual Fitness Identifies Genes Required for Spore Viability and Health in Fission Yeast. PLOS Genet. 2022, 18, e1010462. [Google Scholar] [CrossRef] [PubMed]
- Ucisik-Akkaya, E.; Leatherwood, J.K.; Neiman, A.M. A Genome-Wide Screen for Sporulation-Defective Mutants in Schizosaccharomyces Pombe. G3 GenesGenomesGenetics 2014, 4, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Fukunishi, K.; Miyakubi, K.; Hatanaka, M.; Otsuru, N.; Hirata, A.; Shimoda, C.; Nakamura, T. The Fission Yeast Spore Is Coated by a Proteinaceous Surface Layer Comprising Mainly Isp3. Mol. Biol. Cell 2014, 25, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-J.; Osakada, H.; Kojidani, T.; Haraguchi, T.; Hiraoka, Y. Lipid Droplet Dynamics during Schizosaccharomyces Pombe Sporulation and Their Role in Spore Survival. Biol. Open 2017, 6, 217–222. [Google Scholar] [CrossRef]
- Ünal, E.; Kinde, B.; Amon, A. Gametogenesis Eliminates Age-Induced Cellular Damage and Resets Life Span in Yeast. Science 2011, 332, 1554–1557. [Google Scholar] [CrossRef]
- Dawes, I.W.; Hardie, I.D. Selective Killing of Vegetative Cells in Sporulated Yeast Cultures by Exposure to Diethyl Ether. Mol. Gen. Genet. MGG 1974, 131, 281–289. [Google Scholar] [CrossRef]
- Ho, K.H.; Miller, J.J. Free Proline Content and Sensitivity to Desiccation and Heat during Yeast Sporulation and Spore Germination. Can. J. Microbiol. 1978, 24, 312–320. [Google Scholar] [CrossRef]
- Briza, P.; Breitenbach, M.; Ellinger, A.; Segall, J. Isolation of Two Developmentally Regulated Genes Involved in Spore Wall Maturation in Saccharomyces Cerevisiae. Genes Dev. 1990, 4, 1775–1789. [Google Scholar] [CrossRef]
- Suda, Y.; Rodriguez, R.K.; Coluccio, A.E.; Neiman, A.M. A Screen for Spore Wall Permeability Mutants Identifies a Secreted Protease Required for Proper Spore Wall Assembly. PLoS ONE 2009, 4, e7184. [Google Scholar] [CrossRef]
- Pammer, M.; Briza, P.; Ellinger, A.; Schuster, T.; Stucka, R.; Feldmann, H.; Breitenbach, M. DIT101 (CSD2, CAL1), a cell cycle-regulated yeast gene required for synthesis of chitin in cell walls and chitosan in spore walls. Yeast 1992, 8, 1089–1099. [Google Scholar] [CrossRef]
- Maire, T.; Allertz, T.; Betjes, M.A.; Youk, H. Dormancy-to-death Transition in Yeast Spores Occurs Due to Gradual Loss of Gene-expressing Ability. Mol. Syst. Biol. 2020, 16, e9245. [Google Scholar] [CrossRef] [PubMed]
- Sawadogo, M.; Roeder, R.G. Energy Requirement for Specific Transcription Initiation by the Human RNA Polymerase II System. J. Biol. Chem. 1984, 259, 5321–5326. [Google Scholar] [CrossRef]
- Takaine, M.; Imamura, H.; Yoshida, S. High and Stable ATP Levels Prevent Aberrant Intracellular Protein Aggregation in Yeast. eLife 2022, 11, e67659. [Google Scholar] [CrossRef]
- Bell, W.; Klaassen, P.; Ohnacker, M.; Boller, T.; Herweijer, M.; Schoppink, P.; Vanderzee, P.; Wiemken, A. Characterization of the 56-kDa Subunit of Yeast Trehalose-6-Phosphate Synthase and Cloning of Its Gene Reveal Its Identity with the Product of CIF1, a Regulator of Carbon Catabolite Inactivation. Eur. J. Biochem. 1992, 209, 951–959. [Google Scholar] [CrossRef] [PubMed]
- De Silva-Udawatta, M.N.; Cannon, J.F. Roles of Trehalose Phosphate Synthase in Yeast Glycogen Metabolism and Sporulation. Mol. Microbiol. 2001, 40, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Sutter, B.M.; Ye, X.; Tu, B.P. Trehalose Is a Key Determinant of the Quiescent Metabolic State That Fuels Cell Cycle Progression upon Return to Growth. Mol. Biol. Cell 2010, 21, 1982–1990. [Google Scholar] [CrossRef]
- Ewald, J.C.; Kuehne, A.; Zamboni, N.; Skotheim, J.M. The Yeast Cyclin-Dependent Kinase Routes Carbon Fluxes to Fuel Cell Cycle Progression. Mol. Cell 2016, 62, 532–545. [Google Scholar] [CrossRef]
- Zhao, G.; Chen, Y.; Carey, L.; Futcher, B. Cyclin-Dependent Kinase Co-Ordinates Carbohydrate Metabolism and Cell Cycle in S. cerevisiae. Mol. Cell 2016, 62, 546–557. [Google Scholar] [CrossRef]
- Reuter, M.; Bell, G.; Greig, D. Increased Outbreeding in Yeast in Response to Dispersal by an Insect Vector. Curr. Biol. 2007, 17, R81–R83. [Google Scholar] [CrossRef]
- Johnke, R.; Padilla, G.M. Germination and Outgrowth of Schizosaccharomyces Pombe Spores Isolated by a Simple Batch Centrifugation Technique. Microbiology 1979, 115, 255–258. [Google Scholar] [CrossRef]
- Sakai, K.; Aoki, K.; Goto, Y. Live-Cell Fluorescence Imaging and Optogenetic Control of PKA Kinase Activity in Fission Yeast Schizosaccharomyces Pombe. Yeast 2024, 41, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Li, J.; Young, D. The Schizosaccharomyces Pombe Pka1 Gene, Encoding a Homolog of cAMP-Dependent Protein Kinase. Gene 1994, 151, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, C.S. Glucose Sensing via the Protein Kinase A Pathway in Schizosaccharomyces Pombe. Biochem. Soc. Trans. 2005, 33, 257–260. [Google Scholar] [CrossRef]
- Cansado, J.; Soto, T.; Fernandez, J.; Vicente-Soler, J.; Gacto, M. Characterization of Mutants Devoid of Neutral Trehalase Activity in the Fission Yeast Schizosaccharomyces Pombe: Partial Protection from Heat Shock and High-Salt Stress. J. Bacteriol. 1998, 180, 1342–1345. [Google Scholar] [CrossRef]
- Blázquez, M.A.; Stucka, R.; Feldmann, H.; Gancedo, C. Trehalose-6-P Synthase Is Dispensable for Growth on Glucose but Not for Spore Germination in Schizosaccharomyces Pombe. J. Bacteriol. 1994, 176, 3895–3902. [Google Scholar] [CrossRef]
- Gallo Castro, D.; Martin, S.G. Differential GAP Requirement for Cdc42-GTP Polarization during Proliferation and Sexual Reproduction. J. Cell Biol. 2018, 217, 4215–4229. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Conti, S.F.; Naylor, H.B. Fine Structure of Microorganisms. J. Bacteriol. 1958, 76, 406–416. [Google Scholar] [CrossRef]
- Plante, S.; Landry, C.R. Purification of Yeast Spores to Investigate Their Dynamics of Activation. Curr. Protoc. Microbiol. 2020, 59, e123. [Google Scholar] [CrossRef]
- Choih, S.J.; Ferro, A.J.; Shapiro, S.K. Function of S-Adenosylmethionine in Germinating Yeast Ascospores. J. Bacteriol. 1977, 131, 63–68. [Google Scholar] [CrossRef]
- Rousseau, P.; Halvorson, H.O.; Bulla, L.A.; Julian, G.S. Germination and Outgrowth of Single Spores of Saccharomyces Cerevisiae Viewed by Scanning Electron and Phase-Contrast Microscopy. J. Bacteriol. 1972, 109, 1232–1238. [Google Scholar] [CrossRef]
- Tingle, M.A.; Küenzi, M.T.; Halvorson, H.O. Germination of Yeast Spores Lacking Mitochondrial Deoxyribonucleic Acid. J. Bacteriol. 1974, 117, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Munder, M.C.; Midtvedt, D.; Franzmann, T.; Nüske, E.; Otto, O.; Herbig, M.; Ulbricht, E.; Müller, P.; Taubenberger, A.; Maharana, S.; et al. A pH-Driven Transition of the Cytoplasm from a Fluid- to a Solid-like State Promotes Entry into Dormancy. eLife 2016, 5, e09347. [Google Scholar] [CrossRef]
- Geijer, C.; Pirkov, I.; Vongsangnak, W.; Ericsson, A.; Nielsen, J.; Krantz, M.; Hohmann, S. Time Course Gene Expression Profiling of Yeast Spore Germination Reveals a Network of Transcription Factors Orchestrating the Global Response. BMC Genomics 2012, 13, 554. [Google Scholar] [CrossRef] [PubMed]
- Luby-Phelps, K.; Taylor, D.L.; Lanni, F. Probing the Structure of Cytoplasm. J. Cell Biol. 1986, 102, 2015–2022. [Google Scholar] [CrossRef]
- Moeendarbary, E.; Valon, L.; Fritzsche, M.; Harris, A.R.; Moulding, D.A.; Thrasher, A.J.; Stride, E.; Mahadevan, L.; Charras, G.T. The Cytoplasm of Living Cells Behaves as a Poroelastic Material. Nat. Mater. 2013, 12, 253–261. [Google Scholar] [CrossRef]
- Etoc, F.; Balloul, E.; Vicario, C.; Normanno, D.; Liße, D.; Sittner, A.; Piehler, J.; Dahan, M.; Coppey, M. Non-Specific Interactions Govern Cytosolic Diffusion of Nanosized Objects in Mammalian Cells. Nat. Mater. 2018, 17, 740–746. [Google Scholar] [CrossRef]
- Di Rienzo, C.; Piazza, V.; Gratton, E.; Beltram, F.; Cardarelli, F. Probing Short-Range Protein Brownian Motion in the Cytoplasm of Living Cells. Nat. Commun. 2014, 5, 5891. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, K.; Fujiwara, K.; Ikenaga, M.; Nakajo, N.; Yanagisawa, M.; Mizuno, D. Universal Glass-Forming Behavior of in Vitro and Living Cytoplasm. Sci. Rep. 2017, 7, 15143. [Google Scholar] [CrossRef]
- Parry, B.R.; Surovtsev, I.V.; Cabeen, M.T.; O’Hern, C.S.; Dufresne, E.R.; Jacobs-Wagner, C. The Bacterial Cytoplasm Has Glass-like Properties and Is Fluidized by Metabolic Activity. Cell 2014, 156, 183–194. [Google Scholar] [CrossRef]
- Joyner, R.P.; Tang, J.H.; Helenius, J.; Dultz, E.; Brune, C.; Holt, L.J.; Huet, S.; Müller, D.J.; Weis, K. A Glucose-Starvation Response Regulates the Diffusion of Macromolecules. eLife 2016, 5, e09376. [Google Scholar] [CrossRef]
- Xie, Y.; Shu, T.; Liu, T.; Spindler, M.-C.; Mahamid, J.; Hocky, G.M.; Gresham, D.; Holt, L.J. Polysome Collapse and RNA Condensation Fluidize the Cytoplasm. Mol. Cell 2024, 84, 2698–2716.e9. [Google Scholar] [CrossRef] [PubMed]
- Marini, G.; Nüske, E.; Leng, W.; Alberti, S.; Pigino, G. Reorganization of Budding Yeast Cytoplasm upon Energy Depletion. Mol. Biol. Cell 2020, 31, 1232–1245. [Google Scholar] [CrossRef]
- Xie, J.; Najafi, J.; Le Borgne, R.; Verbavatz, J.-M.; Durieu, C.; Sallé, J.; Minc, N. Contribution of Cytoplasm Viscoelastic Properties to Mitotic Spindle Positioning. Proc. Natl. Acad. Sci. USA 2022, 119, e2115593119. [Google Scholar] [CrossRef]
- Sunde, E.P.; Setlow, P.; Hederstedt, L.; Halle, B. The Physical State of Water in Bacterial Spores. Proc. Natl. Acad. Sci. USA 2009, 106, 19334–19339. [Google Scholar] [CrossRef]
- Buitink, J.; Leprince, O. Intracellular Glasses and Seed Survival in the Dry State. C. R. Biol. 2008, 331, 788–795. [Google Scholar] [CrossRef]
- Sakurai, M.; Furuki, T.; Akao, K.; Tanaka, D.; Nakahara, Y.; Kikawada, T.; Watanabe, M.; Okuda, T. Vitrification Is Essential for Anhydrobiosis in an African Chironomid, Polypedilum Vanderplanki. Proc. Natl. Acad. Sci. USA 2008, 105, 5093–5098. [Google Scholar] [CrossRef] [PubMed]
- Hengherr, S.; Heyer, A.G.; Köhler, H.-R.; Schill, R.O. Trehalose and Anhydrobiosis in Tardigrades—Evidence for Divergence in Responses to Dehydration. FEBS J. 2008, 275, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Molines, A.T.; Lemière, J.; Gazzola, M.; Steinmark, I.E.; Edrington, C.H.; Hsu, C.-T.; Real-Calderon, P.; Suhling, K.; Goshima, G.; Holt, L.J.; et al. Physical Properties of the Cytoplasm Modulate the Rates of Microtubule Polymerization and Depolymerization. Dev. Cell 2022, 57, 466–479.e6. [Google Scholar] [CrossRef]
- Miermont, A.; Waharte, F.; Hu, S.; McClean, M.N.; Bottani, S.; Léon, S.; Hersen, P. Severe Osmotic Compression Triggers a Slowdown of Intracellular Signaling, Which Can Be Explained by Molecular Crowding. Proc. Natl. Acad. Sci. USA 2013, 110, 5725–5730. [Google Scholar] [CrossRef]
- Alric, B.; Formosa-Dague, C.; Dague, E.; Holt, L.J.; Delarue, M. Macromolecular Crowding Limits Growth under Pressure. Nat. Phys. 2022, 18, 411–416. [Google Scholar] [CrossRef]
- Delarue, M.; Brittingham, G.P.; Pfeffer, S.; Surovtsev, I.V.; Pinglay, S.; Kennedy, K.J.; Schaffer, M.; Gutierrez, J.I.; Sang, D.; Poterewicz, G.; et al. mTORC1 Controls Phase Separation and the Biophysical Properties of the Cytoplasm by Tuning Crowding. Cell 2018, 174, 338–349.e20. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.; Yamada, M.; Kunida, K.; Yasuda, S.; Matsuda, M. Processive Phosphorylation of ERK MAP Kinase in Mammalian Cells. Proc. Natl. Acad. Sci. USA 2011, 108, 12675–12680. [Google Scholar] [CrossRef] [PubMed]
- Boyd-Shiwarski, C.R.; Shiwarski, D.J.; Griffiths, S.E.; Beacham, R.T.; Norrell, L.; Morrison, D.E.; Wang, J.; Mann, J.; Tennant, W.; Anderson, E.N.; et al. WNK Kinases Sense Molecular Crowding and Rescue Cell Volume via Phase Separation. Cell 2022, 185, 4488–4506.e20. [Google Scholar] [CrossRef]
- Persson, L.B.; Ambati, V.S.; Brandman, O. Cellular Control of Viscosity Counters Changes in Temperature and Energy Availability. Cell 2020, 183, 1572–1585.e16. [Google Scholar] [CrossRef]
- Neurohr, G.E.; Terry, R.L.; Lengefeld, J.; Bonney, M.; Brittingham, G.P.; Moretto, F.; Miettinen, T.P.; Vaites, L.P.; Soares, L.M.; Paulo, J.A.; et al. Excessive Cell Growth Causes Cytoplasm Dilution And Contributes to Senescence. Cell 2019, 176, 1083–1097.e18. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T.; Nagai, T. Quantitative Measurement of Intracellular Protein Dynamics Using Photobleaching or Photoactivation of Fluorescent Proteins. Microscopy 2014, 63, 403–408. [Google Scholar] [CrossRef]
- Wirtz, D. Particle-Tracking Microrheology of Living Cells: Principles and Applications. Annu. Rev. Biophys. 2009, 38, 301–326. [Google Scholar] [CrossRef]
- Bonucci, M.; Shu, T.; Holt, L.J. How It Feels in a Cell. Trends Cell Biol. 2023, 33, 924–938. [Google Scholar] [CrossRef]
- Hernandez, C.M.; Duran-Chaparro, D.C.; van Eeuwen, T.; Rout, M.P.; Holt, L.J. Development and Characterization of 50 Nanometer Diameter Genetically Encoded Multimeric Nanoparticles. bioRxiv 2024. bioRxiv:2024.07.05.602291. [Google Scholar]
- Shu, T.; Szórádi, T.; Kidiyoor, G.R.; Xie, Y.; Herzog, N.L.; Bazley, A.; Bonucci, M.; Keegan, S.; Saxena, S.; Ettefa, F.; et al. nucGEMs Probe the Biophysical Properties of the Nucleoplasm. bioRxiv 2021. bioRxiv:2021.11.18.469159. [Google Scholar]
- Chambers, J.E.; Zubkov, N.; Kubánková, M.; Nixon-Abell, J.; Mela, I.; Abreu, S.; Schwiening, M.; Lavarda, G.; López-Duarte, I.; Dickens, J.A.; et al. Z-A1-Antitrypsin Polymers Impose Molecular Filtration in the Endoplasmic Reticulum after Undergoing Phase Transition to a Solid State. Sci. Adv. 2022, 8, eabm2094. [Google Scholar] [CrossRef] [PubMed]
- Serrano, A.; Puerner, C.; Plumb, E.; Chevalier, L.; Elferich, J.; Sinn, L.R.; Grigorieff, N.; Ralser, M.; Delarue, M.; Bassilana, M.; et al. Dynamic Cytoplasmic Fluidity during Morphogenesis in a Human Fungal Pathogen. bioRxiv 2024. bioRxiv:2024.11.16.623909. [Google Scholar]
- McLaughlin, G.A.; Langdon, E.M.; Crutchley, J.M.; Holt, L.J.; Forest, M.G.; Newby, J.M.; Gladfelter, A.S. Spatial Heterogeneity of the Cytosol Revealed by Machine Learning-Based 3D Particle Tracking. Mol. Biol. Cell 2020, 31, 1498–1511. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Lakonishok, M.; Serpinskaya, A.S.; Gelfand, V.I. A Novel Mechanism of Bulk Cytoplasmic Transport by Cortical Dynein in Drosophila Ovary. eLife 2022, 11, e75538. [Google Scholar] [CrossRef]
- Vibhute, M.A.; Schaap, M.H.; Maas, R.J.M.; Nelissen, F.H.T.; Spruijt, E.; Heus, H.A.; Hansen, M.M.K.; Huck, W.T.S. Transcription and Translation in Cytomimetic Protocells Perform Most Efficiently at Distinct Macromolecular Crowding Conditions. ACS Synth. Biol. 2020, 9, 2797–2807. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, J.-H.; Phong, C.; Ferrell, J.E. Viscosity-Dependent Control of Protein Synthesis and Degradation. Nat. Commun. 2024, 15, 2149. [Google Scholar] [CrossRef]
- Petrovska, I.; Nüske, E.; Munder, M.C.; Kulasegaran, G.; Malinovska, L.; Kroschwald, S.; Richter, D.; Fahmy, K.; Gibson, K.; Verbavatz, J.-M.; et al. Filament Formation by Metabolic Enzymes Is a Specific Adaptation to an Advanced State of Cellular Starvation. eLife 2014, 3, e02409. [Google Scholar] [CrossRef]
- Guo, M.; Ehrlicher, A.J.; Jensen, M.H.; Renz, M.; Moore, J.R.; Goldman, R.D.; Lippincott-Schwartz, J.; Mackintosh, F.C.; Weitz, D.A. Probing the Stochastic, Motor-Driven Properties of the Cytoplasm Using Force Spectrum Microscopy. Cell 2014, 158, 822–832. [Google Scholar] [CrossRef]
- Torrino, S.; Oldham, W.M.; Tejedor, A.R.; Burgos, I.S.; Nasr, L.; Rachedi, N.; Fraissard, K.; Chauvet, C.; Sbai, C.; O’Hara, B.P.; et al. Mechano-Dependent Sorbitol Accumulation Supports Biomolecular Condensate. Cell 2024, 188, 447–464.e20. [Google Scholar] [CrossRef]
- Gade, V.R.; Heinrich, S.; Paloni, M.; Gómez-García, P.A.; Dzanko, A.; Oswald, A.; Marchand, D.; Khawaja, S.; Barducci, A.; Weis, K. Polysomes and mRNA Control the Biophysical Properties of the Eukaryotic Cytoplasm. bioRxiv 2024. bioRxiv:2024.11.14.623620. [Google Scholar]
- Dorone, Y.; Boeynaems, S.; Flores, E.; Jin, B.; Hateley, S.; Bossi, F.; Lazarus, E.; Pennington, J.G.; Michiels, E.; Decker, M.D.; et al. A Prion-like Protein Regulator of Seed Germination Undergoes Hydration-Dependent Phase Separation. Cell 2021, 184, 4284–4298.e27. [Google Scholar] [CrossRef] [PubMed]
- Seike, T.; Sakata, N.; Matsuda, F.; Furusawa, C. Elevated Sporulation Efficiency in Fission Yeast Schizosaccharomyces Japonicus Strains Isolated from Drosophila. J. Fungi 2021, 7, 350. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakai, K.; Kondo, Y.; Aoki, K.; Goto, Y. Molecular and Biophysical Perspectives on Dormancy Breaking: Lessons from Yeast Spore. Biomolecules 2025, 15, 701. https://doi.org/10.3390/biom15050701
Sakai K, Kondo Y, Aoki K, Goto Y. Molecular and Biophysical Perspectives on Dormancy Breaking: Lessons from Yeast Spore. Biomolecules. 2025; 15(5):701. https://doi.org/10.3390/biom15050701
Chicago/Turabian StyleSakai, Keiichiro, Yohei Kondo, Kazuhiro Aoki, and Yuhei Goto. 2025. "Molecular and Biophysical Perspectives on Dormancy Breaking: Lessons from Yeast Spore" Biomolecules 15, no. 5: 701. https://doi.org/10.3390/biom15050701
APA StyleSakai, K., Kondo, Y., Aoki, K., & Goto, Y. (2025). Molecular and Biophysical Perspectives on Dormancy Breaking: Lessons from Yeast Spore. Biomolecules, 15(5), 701. https://doi.org/10.3390/biom15050701






