The Critical Balance Between Quiescence and Reactivation of Neural Stem Cells
Abstract
1. Introduction
2. NSC Quiescent States, Activation and the Niche Influence
3. Alterations in mRNA Processing During Quiescence
4. Promotion and Maintenance of Quiescence Through Notch Signalling
5. Bone Morphogenetic Proteins in Quiescence and Regeneration
6. Hippo Signalling Maintains NSC Quiescence
7. Nutrient-Dependent Reactivation Through the InR/PI3K/Akt Cascade
8. SUMOylation and NSC Reactivation
9. Lysosomal Activity and Autophagy in Quiescence and Reactivation
10. Metabolic Shifts Between NSC Quiescence and Reactivation
11. Cellular Protrusions and Adhesion in Quiescence and Reactivation
12. Mechanisms Behind qNSC Reactivation upon Injury
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Knoblich, J.A. Asymmetric cell division: Recent developments and their implications for tumour biology. Nat. Rev. Mol. Cell Biol. 2010, 11, 849–860. [Google Scholar] [CrossRef]
- Pan, D. The hippo signaling pathway in development and cancer. Dev. Cell 2010, 19, 491–505. [Google Scholar] [CrossRef]
- Otsuki, L.; Brand, A.H. Quiescent Neural Stem Cells for Brain Repair and Regeneration: Lessons from Model Systems. Trends Neurosci. 2020, 43, 213–226. [Google Scholar] [CrossRef]
- Homem, C.C.; Knoblich, J.A. Drosophila neuroblasts: A model for stem cell biology. Development 2012, 139, 4297–4310. [Google Scholar] [CrossRef]
- Urbán, N.; Blomfield, I.M.; Guillemot, F. Quiescence of Adult Mammalian Neural Stem Cells: A Highly Regulated Rest. Neuron 2019, 104, 834–848. [Google Scholar] [CrossRef]
- Ding, W.Y.; Huang, J.; Wang, H. Waking up quiescent neural stem cells: Molecular mechanisms and implications in neurodevelopmental disorders. PLoS Genet. 2020, 16, e1008653. [Google Scholar] [CrossRef]
- Matsubara, S.; Matsuda, T.; Nakashima, K. Regulation of Adult Mammalian Neural Stem Cells and Neurogenesis by Cell Extrinsic and Intrinsic Factors. Cells 2021, 10, 1145. [Google Scholar] [CrossRef]
- Paik, J.H.; Ding, Z.; Narurkar, R.; Ramkissoon, S.; Muller, F.; Kamoun, W.S.; Chae, S.S.; Zheng, H.; Ying, H.; Mahoney, J.; et al. FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis. Cell Stem Cell 2009, 5, 540–553. [Google Scholar] [CrossRef]
- Renault, V.M.; Rafalski, V.A.; Morgan, A.A.; Salih, D.A.; Brett, J.O.; Webb, A.E.; Villeda, S.A.; Thekkat, P.U.; Guillerey, C.; Denko, N.C.; et al. FoxO3 regulates neural stem cell homeostasis. Cell Stem Cell 2009, 5, 527–539. [Google Scholar] [CrossRef]
- Spéder, P.; Brand, A.H. Systemic and local cues drive neural stem cell niche remodelling during neurogenesis in. Elife 2018, 7. [Google Scholar] [CrossRef]
- Britton, J.S.; Edgar, B.A. Environmental control of the cell cycle in Drosophila: Nutrition activates mitotic and endoreplicative cells by distinct mechanisms. Development 1998, 125, 2149–2158. [Google Scholar] [CrossRef]
- Sousa-Nunes, R.; Yee, L.L.; Gould, A.P. Fat cells reactivate quiescent neuroblasts via TOR and glial insulin relays in Drosophila. Nature 2011, 471, 508–512. [Google Scholar] [CrossRef]
- Ito, K.; Hotta, Y. Proliferation pattern of postembryonic neuroblasts in the brain of Drosophila melanogaster. Dev. Biol. 1992, 149, 134–148. [Google Scholar] [CrossRef]
- Gage, F.H. Adult neurogenesis in mammals. Science 2019, 364, 827–828. [Google Scholar] [CrossRef]
- Grégoire, C.A.; Goldenstein, B.L.; Floriddia, E.M.; Barnabé-Heider, F.; Fernandes, K.J. Endogenous neural stem cell responses to stroke and spinal cord injury. Glia 2015, 63, 1469–1482. [Google Scholar] [CrossRef]
- Lin, R.; Iacovitti, L. Classic and novel stem cell niches in brain homeostasis and repair. Brain Res. 2015, 1628, 327–342. [Google Scholar] [CrossRef]
- Lie, D.C.; Colamarino, S.A.; Song, H.J.; Désiré, L.; Mira, H.; Consiglio, A.; Lein, E.S.; Jessberger, S.; Lansford, H.; Dearie, A.R.; et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature 2005, 437, 1370–1375. [Google Scholar] [CrossRef]
- Ahn, S.; Joyner, A.L. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature 2005, 437, 894–897. [Google Scholar] [CrossRef]
- Ortega, F.; Gascon, S.; Masserdotti, G.; Deshpande, A.; Simon, C.; Fischer, J.; Dimou, L.; Chichung Lie, D.; Schroeder, T.; Berninger, B. Oligodendrogliogenic and neurogenic adult subependymal zone neural stem cells constitute distinct lineages and exhibit differential responsiveness to Wnt signalling. Nat. Cell Biol. 2013, 15, 602–613. [Google Scholar] [CrossRef]
- Sohn, J.; Orosco, L.; Guo, F.; Chung, S.H.; Bannerman, P.; Mills Ko, E.; Zarbalis, K.; Deng, W.; Pleasure, D. The subventricular zone continues to generate corpus callosum and rostral migratory stream astroglia in normal adult mice. J. Neurosci. 2015, 35, 3756–3763. [Google Scholar] [CrossRef]
- Tong, C.K.; Fuentealba, L.C.; Shah, J.K.; Lindquist, R.A.; Ihrie, R.A.; Guinto, C.D.; Rodas-Rodriguez, J.L.; Alvarez-Buylla, A. A Dorsal SHH-Dependent Domain in the V-SVZ Produces Large Numbers of Oligodendroglial Lineage Cells in the Postnatal Brain. Stem Cell Rep. 2015, 5, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Chaker, Z.; Makarouni, E.; Doetsch, F. The Organism as the Niche: Physiological States Crack the Code of Adult Neural Stem Cell Heterogeneity. Annu. Rev. Cell Dev. Biol. 2024, 40, 381–406. [Google Scholar] [CrossRef] [PubMed]
- Encinas, J.M.; Michurina, T.V.; Peunova, N.; Park, J.H.; Tordo, J.; Peterson, D.A.; Fishell, G.; Koulakov, A.; Enikolopov, G. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell 2011, 8, 566–579. [Google Scholar] [CrossRef]
- Bond, A.M.; Ming, G.L.; Song, H. Adult Mammalian Neural Stem Cells and Neurogenesis: Five Decades Later. Cell Stem Cell 2015, 17, 385–395. [Google Scholar] [CrossRef]
- Labusch, M.; Mancini, L.; Morizet, D.; Bally-Cuif, L. Conserved and Divergent Features of Adult Neurogenesis in Zebrafish. Front. Cell Dev. Biol. 2020, 8, 525. [Google Scholar] [CrossRef] [PubMed]
- Lübke, L.; Zhang, G.; Strähle, U.; Rastegar, S. Expression Is Associated with Quiescent Neural Stem Cells during Constitutive and Reactive Neurogenesis in the Adult Zebrafish Telencephalon. Brain Sci. 2022, 12, 284. [Google Scholar] [CrossRef]
- Llorens-Bobadilla, E.; Zhao, S.; Baser, A.; Saiz-Castro, G.; Zwadlo, K.; Martin-Villalba, A. Single-Cell Transcriptomics Reveals a Population of Dormant Neural Stem Cells that Become Activated upon Brain Injury. Cell Stem Cell 2015, 17, 329–340. [Google Scholar] [CrossRef]
- Foley, T.; Thetiot, M.; Bally-Cuif, L. Neural Stem Cell Regulation in Zebrafish. Annu. Rev. Genet. 2024, 58, 249–272. [Google Scholar] [CrossRef]
- Cavallucci, V.; Fidaleo, M.; Pani, G. Neural Stem Cells and Nutrients: Poised Between Quiescence and Exhaustion. Trends Endocrinol. Metab. 2016, 27, 756–769. [Google Scholar] [CrossRef]
- Gujar, M.R.; Gao, Y.; Teng, X.; Ding, W.Y.; Lin, J.; Tan, Y.S.; Chew, L.Y.; Toyama, Y.; Wang, H. Patronin/CAMSAP promotes reactivation and regeneration of Drosophila quiescent neural stem cells. EMBO Rep. 2023, 24, e56624. [Google Scholar] [CrossRef]
- Caron, A.; Trzuskot, L.; Lindsey, B.W. Uncovering the spectrum of adult zebrafish neural stem cell cycle regulators. Front. Cell Dev. Biol. 2022, 10, 941893. [Google Scholar] [CrossRef]
- Gao, Y.; Tan, Y.S.; Lin, J.; Chew, L.Y.; Aung, H.Y.; Palliyana, B.; Gujar, M.R.; Lin, K.Y.; Kondo, S.; Wang, H. SUMOylation of Warts kinase promotes neural stem cell reactivation. Nat. Commun. 2024, 15, 8557. [Google Scholar] [CrossRef] [PubMed]
- Blasco-Chamarro, L.; Fariñas, I. Fine-tuned Rest: Unveiling the Regulatory Landscape of Adult Quiescent Neural Stem Cells. Neuroscience 2023, 525, 26–37. [Google Scholar] [CrossRef]
- Barros, C. Created in BioRender. 2025. Available online: https://BioRender.com/gzahgln (accessed on 4 March 2025).
- Grandel, H.; Kaslin, J.; Ganz, J.; Wenzel, I.; Brand, M. Neural stem cells and neurogenesis in the adult zebrafish brain: Origin, proliferation dynamics, migration and cell fate. Dev. Biol. 2006, 295, 263–277. [Google Scholar] [CrossRef]
- Martynoga, B.; Mateo, J.L.; Zhou, B.; Andersen, J.; Achimastou, A.; Urbán, N.; van den Berg, D.; Georgopoulou, D.; Hadjur, S.; Wittbrodt, J.; et al. Epigenomic enhancer annotation reveals a key role for NFIX in neural stem cell quiescence. Genes Dev. 2013, 27, 1769–1786. [Google Scholar] [CrossRef] [PubMed]
- Alessio, N.; Aprile, D.; Cappabianca, S.; Peluso, G.; Di Bernardo, G.; Galderisi, U. Different Stages of Quiescence, Senescence, and Cell Stress Identified by Molecular Algorithm Based on the Expression of Ki67, RPS6, and Beta-Galactosidase Activity. Int. J. Mol. Sci. 2021, 22, 3102. [Google Scholar] [CrossRef] [PubMed]
- Marqués-Torrejón, M.; Williams, C.A.C.; Southgate, B.; Alfazema, N.; Clements, M.P.; Garcia-Diaz, C.; Blin, C.; Arranz-Emparan, N.; Fraser, J.; Gammoh, N.; et al. LRIG1 is a gatekeeper to exit from quiescence in adult neural stem cells. Nat. Commun. 2021, 12, 2594. [Google Scholar] [CrossRef]
- Cheung, T.H.; Rando, T.A. Molecular regulation of stem cell quiescence. Nat. Rev. Mol. Cell Biol. 2013, 14, 329–340. [Google Scholar] [CrossRef]
- Sood, C.; Justis, V.T.; Doyle, S.E.; Siegrist, S.E. Notch signaling regulates neural stem cell quiescence entry and exit in Drosophila. Development 2022, 149. [Google Scholar] [CrossRef]
- Tsuji, T.; Hasegawa, E.; Isshiki, T. Neuroblast entry into quiescence is regulated intrinsically by the combined action of spatial Hox proteins and temporal identity factors. Development 2008, 135, 3859–3869. [Google Scholar] [CrossRef]
- Bossing, T.; Udolph, G.; Doe, C.Q.; Technau, G.M. The embryonic central nervous system lineages of Drosophila melanogaster. I. Neuroblast lineages derived from the ventral half of the neuroectoderm. Dev. Biol. 1996, 179, 41–64. [Google Scholar] [CrossRef] [PubMed]
- Otsuki, L.; Brand, A.H. Cell cycle heterogeneity directs the timing of neural stem cell activation from quiescence. Science 2018, 360, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Otsuki, L.; Brand, A.H. Dorsal-Ventral Differences in Neural Stem Cell Quiescence Are Induced by p57. Dev. Cell 2019, 49, 293–300.e293. [Google Scholar] [CrossRef]
- McKeown, C.R.; Cline, H.T. Nutrient restriction causes reversible G2 arrest in. Development 2019, 146. [Google Scholar] [CrossRef]
- Luo, Y.; Coskun, V.; Liang, A.; Yu, J.; Cheng, L.; Ge, W.; Shi, Z.; Zhang, K.; Li, C.; Cui, Y.; et al. Single-cell transcriptome analyses reveal signals to activate dormant neural stem cells. Cell 2015, 161, 1175–1186. [Google Scholar] [CrossRef]
- Chavali, M.; Klingener, M.; Kokkosis, A.G.; Garkun, Y.; Felong, S.; Maffei, A.; Aguirre, A. Non-canonical Wnt signaling regulates neural stem cell quiescence during homeostasis and after demyelination. Nat. Commun. 2018, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Rigo, P.; Ahmed-de-Prado, S.; Johnston, R.L.; Choudhury, C.; Guillemot, F.; Harris, L. Sequential transcriptional programs underpin activation of quiescent hippocampal stem cells. bioRxiv 2024. [Google Scholar] [CrossRef]
- Labusch, M.; Thetiot, M.; Than-Trong, E.; Morizet, D.; Coolen, M.; Varet, H.; Legendre, R.; Ortica, S.; Mancini, L.; Bally-Cuif, L. Prosaposin maintains adult neural stem cells in a state associated with deep quiescence. Stem Cell Rep. 2024, 19, 515–528. [Google Scholar] [CrossRef]
- Paul, A.; Chaker, Z.; Doetsch, F. Hypothalamic regulation of regionally distinct adult neural stem cells and neurogenesis. Science 2017, 356, 1383–1386. [Google Scholar] [CrossRef]
- Lugert, S.; Basak, O.; Knuckles, P.; Haussler, U.; Fabel, K.; Gotz, M.; Haas, C.A.; Kempermann, G.; Taylor, V.; Giachino, C. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell 2010, 6, 445–456. [Google Scholar] [CrossRef]
- van Praag, H.; Kempermann, G.; Gage, F.H. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 1999, 2, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Shingo, T.; Gregg, C.; Enwere, E.; Fujikawa, H.; Hassam, R.; Geary, C.; Cross, J.C.; Weiss, S. Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science 2003, 299, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Chaker, Z.; Segalada, C.; Kretz, J.A.; Acar, I.E.; Delgado, A.C.; Crotet, V.; Moor, A.E.; Doetsch, F. Pregnancy-responsive pools of adult neural stem cells for transient neurogenesis in mothers. Science 2023, 382, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Gengatharan, A.; Malvaut, S.; Marymonchyk, A.; Ghareghani, M.; Snapyan, M.; Fischer-Sternjak, J.; Ninkovic, J.; Gotz, M.; Saghatelyan, A. Adult neural stem cell activation in mice is regulated by the day/night cycle and intracellular calcium dynamics. Cell 2021, 184, 709–722.e13. [Google Scholar] [CrossRef]
- Alonso, M.I.; Gato, A. Cerebrospinal fluid and neural stem cell niche control. Neural Regen. Res. 2018, 13, 1546–1547. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Q.; Haydar, T.F.; Bordey, A. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat. Neurosci. 2005, 8, 1179–1187. [Google Scholar] [CrossRef]
- Young, S.Z.; Taylor, M.M.; Bordey, A. Neurotransmitters couple brain activity to subventricular zone neurogenesis. Eur. J. Neurosci. 2011, 33, 1123–1132. [Google Scholar] [CrossRef]
- Marymonchyk, A.; Rodriguez-Aller, R.; Willis, A.; Beaupre, F.; Warsi, S.; Snapyan, M.; Clavet-Fournier, V.; Lavoie-Cardinal, F.; Kaplan, D.R.; Miller, F.D.; et al. Neural stem cell quiescence and activation dynamics are regulated by feedback input from their progeny under homeostatic and regenerative conditions. Cell Stem Cell 2025, 32, 445–462.e9. [Google Scholar] [CrossRef]
- Walker, J.V.; Zhuang, H.; Singer, D.; Illsley, C.S.; Kok, W.L.; Sivaraj, K.K.; Gao, Y.; Bolton, C.; Liu, Y.; Zhao, M.; et al. Transit amplifying cells coordinate mouse incisor mesenchymal stem cell activation. Nat. Commun. 2019, 10, 3596. [Google Scholar] [CrossRef]
- Tavazoie, M.; Van der Veken, L.; Silva-Vargas, V.; Louissaint, M.; Colonna, L.; Zaidi, B.; Garcia-Verdugo, J.M.; Doetsch, F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell 2008, 3, 279–288. [Google Scholar] [CrossRef]
- Calvo, C.F.; Fontaine, R.H.; Soueid, J.; Tammela, T.; Makinen, T.; Alfaro-Cervello, C.; Bonnaud, F.; Miguez, A.; Benhaim, L.; Xu, Y.; et al. Vascular endothelial growth factor receptor 3 directly regulates murine neurogenesis. Genes Dev. 2011, 25, 831–844. [Google Scholar] [CrossRef]
- Crouch, E.E.; Liu, C.; Silva-Vargas, V.; Doetsch, F. Regional and stage-specific effects of prospectively purified vascular cells on the adult V-SVZ neural stem cell lineage. J. Neurosci. 2015, 35, 4528–4539. [Google Scholar] [CrossRef] [PubMed]
- Ottone, C.; Krusche, B.; Whitby, A.; Clements, M.; Quadrato, G.; Pitulescu, M.E.; Adams, R.H.; Parrinello, S. Direct cell-cell contact with the vascular niche maintains quiescent neural stem cells. Nat. Cell Biol. 2014, 16, 1045–1056. [Google Scholar] [CrossRef]
- Navarro Negredo, P.; Yeo, R.W.; Brunet, A. Aging and Rejuvenation of Neural Stem Cells and Their Niches. Cell Stem Cell 2020, 27, 202–223. [Google Scholar] [CrossRef] [PubMed]
- Gage, F.H.; Temple, S. Neural stem cells: Generating and regenerating the brain. Neuron 2013, 80, 588–601. [Google Scholar] [CrossRef] [PubMed]
- Kalamakis, G.; Brüne, D.; Ravichandran, S.; Bolz, J.; Fan, W.; Ziebell, F.; Stiehl, T.; Catalá-Martinez, F.; Kupke, J.; Zhao, S.; et al. Quiescence Modulates Stem Cell Maintenance and Regenerative Capacity in the Aging Brain. Cell 2019, 176, 1407–1419.e1414. [Google Scholar] [CrossRef]
- Ruetz, T.J.; Pogson, A.N.; Kashiwagi, C.M.; Gagnon, S.D.; Morton, B.; Sun, E.D.; Na, J.; Yeo, R.W.; Leeman, D.S.; Morgens, D.W.; et al. CRISPR-Cas9 screens reveal regulators of ageing in neural stem cells. Nature 2024, 634, 1150–1159. [Google Scholar] [CrossRef]
- Rossi, A.; Coum, A.; Madelenat, M.; Harris, L.; Miedzik, A.; Strohbuecker, S.; Chai, A.; Fiaz, H.; Chaouni, R.; Faull, P.; et al. Neural stem cells alter nucleocytoplasmic partitioning and accumulate nuclear polyadenylated transcripts during quiescence. bioRxiv 2021. [Google Scholar] [CrossRef]
- Suh, E.J.; Remillard, M.Y.; Legesse-Miller, A.; Johnson, E.L.; Lemons, J.M.; Chapman, T.R.; Forman, J.J.; Kojima, M.; Silberman, E.S.; Coller, H.A. A microRNA network regulates proliferative timing and extracellular matrix synthesis during cellular quiescence in fibroblasts. Genome Biol. 2012, 13, R121. [Google Scholar] [CrossRef]
- Martinez, I.; Hayes, K.E.; Barr, J.A.; Harold, A.D.; Xie, M.; Bukhari, S.I.A.; Vasudevan, S.; Steitz, J.A.; DiMaio, D. An Exportin-1-dependent microRNA biogenesis pathway during human cell quiescence. Proc. Natl. Acad. Sci. USA 2017, 114, E4961–E4970. [Google Scholar] [CrossRef]
- Lai, S.L.; Doe, C.Q. Transient nuclear Prospero induces neural progenitor quiescence. Elife 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Joy, T.; Hirono, K.; Doe, C.Q. The RanGEF Bj1 promotes prospero nuclear export and neuroblast self-renewal. Dev. Neurobiol. 2015, 75, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Carmody, S.R.; Wente, S.R. mRNA nuclear export at a glance. J. Cell Sci. 2009, 122, 1933–1937. [Google Scholar] [CrossRef] [PubMed]
- Medina, R.; Zaidi, S.K.; Liu, C.G.; Stein, J.L.; van Wijnen, A.J.; Croce, C.M.; Stein, G.S. MicroRNAs 221 and 222 bypass quiescence and compromise cell survival. Cancer Res. 2008, 68, 2773–2780. [Google Scholar] [CrossRef]
- Leucht, C.; Stigloher, C.; Wizenmann, A.; Klafke, R.; Folchert, A.; Bally-Cuif, L. MicroRNA-9 directs late organizer activity of the midbrain-hindbrain boundary. Nat. Neurosci. 2008, 11, 641–648. [Google Scholar] [CrossRef]
- Katz, S.; Cussigh, D.; Urbán, N.; Blomfield, I.; Guillemot, F.; Bally-Cuif, L.; Coolen, M. A Nuclear Role for miR-9 and Argonaute Proteins in Balancing Quiescent and Activated Neural Stem Cell States. Cell Rep. 2016, 17, 1383–1398. [Google Scholar] [CrossRef]
- Artavanis-Tsakonas, S.; Muskavitch, M.A. Notch: The past, the present, and the future. Curr. Top. Dev. Biol. 2010, 92, 1–29. [Google Scholar] [CrossRef]
- Wharton, K.A.; Johansen, K.M.; Xu, T.; Artavanis-Tsakonas, S. Nucleotide sequence from the neurogenic locus notch implies a gene product that shares homology with proteins containing EGF-like repeats. Cell 1985, 43, 567–581. [Google Scholar] [CrossRef]
- Kopczynski, C.C.; Alton, A.K.; Fechtel, K.; Kooh, P.J.; Muskavitch, M.A. Delta, a Drosophila neurogenic gene, is transcriptionally complex and encodes a protein related to blood coagulation factors and epidermal growth factor of vertebrates. Genes Dev. 1988, 2, 1723–1735. [Google Scholar] [CrossRef]
- Salazar, J.L.; Yamamoto, S. Integration of Drosophila and Human Genetics to Understand Notch Signaling Related Diseases. Adv. Exp. Med. Biol. 2018, 1066, 141–185. [Google Scholar] [CrossRef]
- Kawai, H.; Kawaguchi, D.; Kuebrich, B.D.; Kitamoto, T.; Yamaguchi, M.; Gotoh, Y.; Furutachi, S. Area-Specific Regulation of Quiescent Neural Stem Cells by Notch3 in the Adult Mouse Subependymal Zone. J. Neurosci. 2017, 37, 11867–11880. [Google Scholar] [CrossRef] [PubMed]
- Imayoshi, I.; Sakamoto, M.; Yamaguchi, M.; Mori, K.; Kageyama, R. Essential roles of Notch signaling in maintenance of neural stem cells in developing and adult brains. J. Neurosci. 2010, 30, 3489–3498. [Google Scholar] [CrossRef]
- Hu, N.; Zou, L. Multiple functions of Hes genes in the proliferation and differentiation of neural stem cells. Ann. Anat. 2022, 239, 151848. [Google Scholar] [CrossRef]
- Than-Trong, E.; Ortica-Gatti, S.; Mella, S.; Nepal, C.; Alunni, A.; Bally-Cuif, L. Neural stem cell quiescence and stemness are molecularly distinct outputs of the Notch3 signalling cascade in the vertebrate adult brain. Development 2018, 145. [Google Scholar] [CrossRef] [PubMed]
- Chapouton, P.; Skupien, P.; Hesl, B.; Coolen, M.; Moore, J.C.; Madelaine, R.; Kremmer, E.; Faus-Kessler, T.; Blader, P.; Lawson, N.D.; et al. Notch activity levels control the balance between quiescence and recruitment of adult neural stem cells. J. Neurosci. 2010, 30, 7961–7974. [Google Scholar] [CrossRef] [PubMed]
- Kizil, C. Mechanisms of Pathology-Induced Neural Stem Cell Plasticity and Neural Regeneration in Adult Zebrafish Brain. Curr. Pathobiol. Rep. 2018, 6, 71–77. [Google Scholar] [CrossRef]
- März, M.; Schmidt, R.; Rastegar, S.; Strähle, U. Regenerative response following stab injury in the adult zebrafish telencephalon. Dev. Dyn. 2011, 240, 2221–2231. [Google Scholar] [CrossRef]
- Kim, H.K.; Lee, D.W.; Kim, E.; Jeong, I.; Kim, S.; Kim, B.J.; Park, H.C. Notch Signaling Controls Oligodendrocyte Regeneration in the Injured Telencephalon of Adult Zebrafish. Exp. Neurobiol. 2020, 29, 417–424. [Google Scholar] [CrossRef]
- Meyers, E.A.; Kessler, J.A. TGF-β Family Signaling in Neural and Neuronal Differentiation, Development, and Function. Cold Spring Harb. Perspect. Biol. 2017, 9. [Google Scholar] [CrossRef]
- Kanai, M.I.; Kim, M.J.; Akiyama, T.; Takemura, M.; Wharton, K.; O’Connor, M.B.; Nakato, H. Regulation of neuroblast proliferation by surface glia in the Drosophila larval brain. Sci. Rep. 2018, 8, 3730. [Google Scholar] [CrossRef]
- Bonaguidi, M.A.; Wheeler, M.A.; Shapiro, J.S.; Stadel, R.P.; Sun, G.J.; Ming, G.L.; Song, H. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell 2011, 145, 1142–1155. [Google Scholar] [CrossRef]
- Miyazono, K.; Kamiya, Y.; Morikawa, M. Bone morphogenetic protein receptors and signal transduction. J. Biochem. 2010, 147, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Mira, H.; Andreu, Z.; Suh, H.; Lie, D.C.; Jessberger, S.; Consiglio, A.; San Emeterio, J.; Hortigüela, R.; Marqués-Torrejón, M.A.; Nakashima, K.; et al. Signaling through BMPR-IA regulates quiescence and long-term activity of neural stem cells in the adult hippocampus. Cell Stem Cell 2010, 7, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhang, X.; Li, Z.; Liu, C.; Liu, Q.; Chai, H.; Yao, H.; Luo, Y.; Li, S.; Li, C. Characteristics of quiescent adult neural stem cells induced by the bFGF/BMP4 combination or BMP4 alone. Front. Cell Neurosci. 2024, 18, 1391556. [Google Scholar] [CrossRef] [PubMed]
- Marcy, G.; Foucault, L.; Babina, E.; Capeliez, T.; Texeraud, E.; Zweifel, S.; Heinrich, C.; Hernandez-Vargas, H.; Parras, C.; Jabaudon, D.; et al. Single-cell analysis of the postnatal dorsal V-SVZ reveals a role for Bmpr1a signaling in silencing pallial germinal activity. Sci. Adv. 2023, 9, eabq7553. [Google Scholar] [CrossRef]
- Lim, D.A.; Tramontin, A.D.; Trevejo, J.M.; Herrera, D.G.; García-Verdugo, J.M.; Alvarez-Buylla, A. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron 2000, 28, 713–726. [Google Scholar] [CrossRef]
- Zhang, G.; Lübke, L.; Chen, F.; Beil, T.; Takamiya, M.; Diotel, N.; Strähle, U.; Rastegar, S. Neuron-Radial Glial Cell Communication via BMP/Id1 Signaling Is Key to Long-Term Maintenance of the Regenerative Capacity of the Adult Zebrafish Telencephalon. Cells 2021, 10, 2794. [Google Scholar] [CrossRef]
- Zhang, G.; Ferg, M.; Lübke, L.; Takamiya, M.; Beil, T.; Gourain, V.; Diotel, N.; Strähle, U.; Rastegar, S. Bone morphogenetic protein signaling regulates Id1-mediated neural stem cell quiescence in the adult zebrafish brain via a phylogenetically conserved enhancer module. Stem Cells 2020, 38, 875–889. [Google Scholar] [CrossRef]
- Halder, G.; Johnson, R.L. Hippo signaling: Growth control and beyond. Development 2011, 138, 9–22. [Google Scholar] [CrossRef]
- Harvey, K.; Tapon, N. The Salvador-Warts-Hippo pathway - an emerging tumour-suppressor network. Nat. Rev. Cancer 2007, 7, 182–191. [Google Scholar] [CrossRef]
- Fan, W.; Jurado-Arjona, J.; Alanis-Lobato, G.; Péron, S.; Berger, C.; Andrade-Navarro, M.A.; Falk, S.; Berninger, B. The transcriptional co-activator Yap1 promotes adult hippocampal neural stem cell activation. EMBO J. 2023, 42, e110384. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Berger, C. Hippo pathway regulates neural stem cell quiescence. Cell Cycle 2016, 15, 1525–1526. [Google Scholar] [CrossRef] [PubMed]
- Lavado, A.; Park, J.Y.; Paré, J.; Finkelstein, D.; Pan, H.; Xu, B.; Fan, Y.; Kumar, R.P.; Neale, G.; Kwak, Y.D.; et al. The Hippo Pathway Prevents YAP/TAZ-Driven Hypertranscription and Controls Neural Progenitor Number. Dev. Cell 2018, 47, 576–591.e578. [Google Scholar] [CrossRef]
- Luo, J.; Li, P. Context-dependent transcriptional regulations of YAP/TAZ in stem cell and differentiation. Stem Cell Res. Ther. 2022, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Wang, Y.; Zhang, P.; Chen, H.; Xu, Z.; Jiao, J.; Yuan, Z. BMP2-SMAD signaling represses the proliferation of embryonic neural stem cells through YAP. J. Neurosci. 2014, 34, 12039–12048. [Google Scholar] [CrossRef]
- Ding, R.; Weynans, K.; Bossing, T.; Barros, C.S.; Berger, C. The Hippo signalling pathway maintains quiescence in Drosophila neural stem cells. Nat. Commun. 2016, 7, 10510. [Google Scholar] [CrossRef]
- Shi, Z.; Jiao, S.; Zhou, Z. STRIPAK complexes in cell signaling and cancer. Oncogene 2016, 35, 4549–4557. [Google Scholar] [CrossRef]
- Schulte, J.; Sepp, K.J.; Jorquera, R.A.; Wu, C.; Song, Y.; Hong, P.; Littleton, J.T. DMob4/Phocein regulates synapse formation, axonal transport, and microtubule organization. J. Neurosci. 2010, 30, 5189–5203. [Google Scholar] [CrossRef]
- Couzens, A.L.; Knight, J.D.; Kean, M.J.; Teo, G.; Weiss, A.; Dunham, W.H.; Lin, Z.Y.; Bagshaw, R.D.; Sicheri, F.; Pawson, T.; et al. Protein interaction network of the mammalian Hippo pathway reveals mechanisms of kinase-phosphatase interactions. Sci. Signal 2013, 6, rs15. [Google Scholar] [CrossRef]
- Gil-Ranedo, J.; Gonzaga, E.; Jaworek, K.J.; Berger, C.; Bossing, T.; Barros, C.S. STRIPAK Members Orchestrate Hippo and Insulin Receptor Signaling to Promote Neural Stem Cell Reactivation. Cell Rep. 2019, 27, 2921–2933.e2925. [Google Scholar] [CrossRef]
- Ribeiro, P.S.; Josué, F.; Wepf, A.; Wehr, M.C.; Rinner, O.; Kelly, G.; Tapon, N.; Gstaiger, M. Combined functional genomic and proteomic approaches identify a PP2A complex as a negative regulator of Hippo signaling. Mol. Cell 2010, 39, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Chai, P.C.; Liu, Z.; Chia, W.; Cai, Y. Hedgehog signaling acts with the temporal cascade to promote neuroblast cell cycle exit. PLoS Biol. 2013, 11, e1001494. [Google Scholar] [CrossRef]
- Yu, F.X.; Guan, K.L. The Hippo pathway: Regulators and regulations. Genes Dev. 2013, 27, 355–371. [Google Scholar] [CrossRef]
- Moya, I.M.; Halder, G. Hippo-YAP/TAZ signalling in organ regeneration and regenerative medicine. Nat. Rev. Mol. Cell Biol. 2019, 20, 211–226. [Google Scholar] [CrossRef]
- Zhao, X.; Le, T.P.; Erhardt, S.; Findley, T.O.; Wang, J. Hippo-Yap Pathway Orchestrates Neural Crest Ontogenesis. Front. Cell Dev. Biol. 2021, 9, 706623. [Google Scholar] [CrossRef] [PubMed]
- Riley, S.E.; Feng, Y.; Hansen, C.G. Hippo-Yap/Taz signalling in zebrafish regeneration. NPJ Regen. Med. 2022, 7, 9. [Google Scholar] [CrossRef]
- Klatt Shaw, D.; Saraswathy, V.M.; Zhou, L.; McAdow, A.R.; Burris, B.; Butka, E.; Morris, S.A.; Dietmann, S.; Mokalled, M.H. Localized EMT reprograms glial progenitors to promote spinal cord repair. Dev. Cell 2021, 56, 613–626.e617. [Google Scholar] [CrossRef]
- Florindo, C.; Mimoso, J.M.; Palma, S.L.; Gonçalves, C.; Silvestre, D.; Campinho, M.; Tavares, Á. Mob4 is required for neurodevelopment in zebrafish. MicroPubl. Biol. 2023, 2023. [Google Scholar] [CrossRef]
- Santos, I.B.; Garrido-Maraver, J.; Gonçalves, C.; Oliveira, B.I.; Tavares, Á.A. Role of MOB4 in Cell Proliferation and Neurogenesis. BioChem 2023, 3, 182–196. [Google Scholar] [CrossRef]
- Lee, M.S.; Wan, J.; Goldman, D. Tgfb3 collaborates with PP2A and notch signaling pathways to inhibit retina regeneration. Elife 2020, 9. [Google Scholar] [CrossRef]
- Vinayagam, A.; Kulkarni, M.M.; Sopko, R.; Sun, X.; Hu, Y.; Nand, A.; Villalta, C.; Moghimi, A.; Yang, X.; Mohr, S.E.; et al. An Integrative Analysis of the InR/PI3K/Akt Network Identifies the Dynamic Response to Insulin Signaling. Cell Rep. 2016, 16, 3062–3074. [Google Scholar] [CrossRef]
- Homem, C.C.; Repic, M.; Knoblich, J.A. Proliferation control in neural stem and progenitor cells. Nat. Rev. Neurosci. 2015, 16, 647–659. [Google Scholar] [CrossRef]
- Chell, J.M.; Brand, A.H. Nutrition-responsive glia control exit of neural stem cells from quiescence. Cell 2010, 143, 1161–1173. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Y.; Zhu, W.G. Applications of post-translational modifications of FoxO family proteins in biological functions. J. Mol. Cell Biol. 2011, 3, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Chen, G.; Zhu, H.; Zhong, Y.; Yang, Z.; Jian, Z.; Xiong, X. Metabolic and proteostatic differences in quiescent and active neural stem cells. Neural Regen. Res. 2024, 19, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.E.; Pollina, E.A.; Vierbuchen, T.; Urbán, N.; Ucar, D.; Leeman, D.S.; Martynoga, B.; Sewak, M.; Rando, T.A.; Guillemot, F.; et al. FOXO3 shares common targets with ASCL1 genome-wide and inhibits ASCL1-dependent neurogenesis. Cell Rep. 2013, 4, 477–491. [Google Scholar] [CrossRef]
- Kobayashi, T.; Kageyama, R. Lysosomes and signaling pathways for maintenance of quiescence in adult neural stem cells. FEBS J. 2021, 288, 3082–3093. [Google Scholar] [CrossRef]
- Zhou, Y.; Bond, A.M.; Shade, J.E.; Zhu, Y.; Davis, C.O.; Wang, X.; Su, Y.; Yoon, K.J.; Phan, A.T.; Chen, W.J.; et al. Autocrine Mfge8 Signaling Prevents Developmental Exhaustion of the Adult Neural Stem Cell Pool. Cell Stem Cell 2018, 23, 444–452.e444. [Google Scholar] [CrossRef]
- Khalifeh-Soltani, A.; Ha, A.; Podolsky, M.J.; McCarthy, D.A.; McKleroy, W.; Azary, S.; Sakuma, S.; Tharp, K.M.; Wu, N.; Yokosaki, Y.; et al. α8β1 integrin regulates nutrient absorption through an Mfge8-PTEN dependent mechanism. Elife 2016, 5. [Google Scholar] [CrossRef]
- Klotz, L.O.; Sánchez-Ramos, C.; Prieto-Arroyo, I.; Urbánek, P.; Steinbrenner, H.; Monsalve, M. Redox regulation of FoxO transcription factors. Redox Biol. 2015, 6, 51–72. [Google Scholar] [CrossRef]
- Lehtinen, M.K.; Yuan, Z.; Boag, P.R.; Yang, Y.; Villén, J.; Becker, E.B.; DiBacco, S.; de la Iglesia, N.; Gygi, S.; Blackwell, T.K.; et al. A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell 2006, 125, 987–1001. [Google Scholar] [CrossRef] [PubMed]
- Schlueter, P.J.; Peng, G.; Westerfield, M.; Duan, C. Insulin-like growth factor signaling regulates zebrafish embryonic growth and development by promoting cell survival and cell cycle progression. Cell Death Differ. 2007, 14, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, Y.; Monson, C.; Duan, C.; Wu, Y.; Gao, C.; Yakar, S.; Sadler, K.C.; LeRoith, D. The role of insulin receptor signaling in zebrafish embryogenesis. Endocrinology 2008, 149, 5996–6005. [Google Scholar] [CrossRef]
- Gence, L.; Fernezelian, D.; Meilhac, O.; Rastegar, S.; Bascands, J.L.; Diotel, N. Insulin signaling promotes neurogenesis in the brain of adult zebrafish. J. Comp. Neurol. 2023, 531, 1812–1827. [Google Scholar] [CrossRef]
- Huang, J.; Wang, H. Hsp83/Hsp90 Physically Associates with Insulin Receptor to Promote Neural Stem Cell Reactivation. Stem Cell Rep. 2018, 11, 883–896. [Google Scholar] [CrossRef] [PubMed]
- Spéder, P.; Brand, A.H. Gap junction proteins in the blood-brain barrier control nutrient-dependent reactivation of Drosophila neural stem cells. Dev. Cell 2014, 30, 309–321. [Google Scholar] [CrossRef]
- Li, S.; Koe, C.T.; Tay, S.T.; Tan, A.L.K.; Zhang, S.; Zhang, Y.; Tan, P.; Sung, W.K.; Wang, H. Erratum: An intrinsic mechanism controls reactivation of neural stem cells by spindle matrix proteins. Nat. Commun. 2017, 8, 1298. [Google Scholar] [CrossRef]
- Choksi, S.P.; Southall, T.D.; Bossing, T.; Edoff, K.; de Wit, E.; Fischer, B.E.; van Steensel, B.; Micklem, G.; Brand, A.H. Prospero acts as a binary switch between self-renewal and differentiation in Drosophila neural stem cells. Dev. Cell 2006, 11, 775–789. [Google Scholar] [CrossRef]
- Callan, M.A.; Clements, N.; Ahrendt, N.; Zarnescu, D.C. Fragile X Protein is required for inhibition of insulin signaling and regulates glial-dependent neuroblast reactivation in the developing brain. Brain Res. 2012, 1462, 151–161. [Google Scholar] [CrossRef]
- Callan, M.A.; Cabernard, C.; Heck, J.; Luois, S.; Doe, C.Q.; Zarnescu, D.C. Fragile X protein controls neural stem cell proliferation in the Drosophila brain. Hum. Mol. Genet. 2010, 19, 3068–3079. [Google Scholar] [CrossRef]
- Queiroz, L.Y.; Kageyama, R.; Cimarosti, H.I. SUMOylation effects on neural stem cells self-renewal, differentiation, and survival. Neurosci. Res. 2024, 199, 1–11. [Google Scholar] [CrossRef]
- Bernstock, J.D.; Peruzzotti-Jametti, L.; Leonardi, T.; Vicario, N.; Ye, D.; Lee, Y.J.; Maric, D.; Johnson, K.R.; Mou, Y.; Van Den Bosch, A.; et al. SUMOylation promotes survival and integration of neural stem cell grafts in ischemic stroke. EBioMedicine 2019, 42, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Guo, Z.; Wu, H.; Wang, X.; Yang, L.; Shi, X.; Du, J.; Tang, B.; Li, W.; Zhang, Y. SUMOylation represses Nanog expression via modulating transcription factors Oct4 and Sox2. PLoS ONE 2012, 7, e39606. [Google Scholar] [CrossRef]
- Fernández-Lloris, R.; Osses, N.; Jaffray, E.; Shen, L.N.; Vaughan, O.A.; Girwood, D.; Bartrons, R.; Rosa, J.L.; Hay, R.T.; Ventura, F. Repression of SOX6 transcriptional activity by SUMO modification. FEBS Lett. 2006, 580, 1215–1221. [Google Scholar] [CrossRef]
- Marelli, E.; Hughes, J.; Scotting, P.J. SUMO-dependent transcriptional repression by Sox2 inhibits the proliferation of neural stem cells. PLoS ONE 2024, 19, e0298818. [Google Scholar] [CrossRef]
- Nowak, M.; Hammerschmidt, M. Ubc9 regulates mitosis and cell survival during zebrafish development. Mol. Biol. Cell 2006, 17, 5324–5336. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Piao, W.; Takamura, T.; Kori, H.; Miyachi, H.; Kitano, S.; Iwamoto, Y.; Yamada, M.; Imayoshi, I.; Shioda, S.; et al. Enhanced lysosomal degradation maintains the quiescent state of neural stem cells. Nat. Commun. 2019, 10, 5446. [Google Scholar] [CrossRef]
- Leeman, D.S.; Hebestreit, K.; Ruetz, T.; Webb, A.E.; McKay, A.; Pollina, E.A.; Dulken, B.W.; Zhao, X.; Yeo, R.W.; Ho, T.T.; et al. Lysosome activation clears aggregates and enhances quiescent neural stem cell activation during aging. Science 2018, 359, 1277–1283. [Google Scholar] [CrossRef]
- Calatayud-Baselga, I.; Casares-Crespo, L.; Franch-Ibáñez, C.; Guijarro-Nuez, J.; Sanz, P.; Mira, H. Autophagy drives the conversion of developmental neural stem cells to the adult quiescent state. Nat. Commun. 2023, 14, 7541. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Rangel, C.; Reynolds, M.M.; Caldwell, M.C.; Johns, M.; Nayak, M.; Welsh, C.J.; McDermott, S.; Datta, S. Drosophila perlecan modulates FGF and hedgehog signals to activate neural stem cell division. Dev. Biol. 2003, 253, 247–257. [Google Scholar] [CrossRef]
- Barrett, A.L.; Krueger, S.; Datta, S. Branchless and Hedgehog operate in a positive feedback loop to regulate the initiation of neuroblast division in the Drosophila larval brain. Dev. Biol. 2008, 317, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Zavortink, M.; Froldi, F.; Golenkina, S.; Lam, T.; Cheng, L.Y. Glial Hedgehog signalling and lipid metabolism regulate neural stem cell proliferation in Drosophila. EMBO Rep. 2021, 22, e52130. [Google Scholar] [CrossRef]
- Meda, F.; Gauron, C.; Rampon, C.; Teillon, J.; Volovitch, M.; Vriz, S. Nerves Control Redox Levels in Mature Tissues Through Schwann Cells and Hedgehog Signaling. Antioxid. Redox Signal. 2016, 24, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Berg, D.A.; Zhu, Y.; Shin, J.Y.; Song, J.; Bonaguidi, M.A.; Enikolopov, G.; Nauen, D.W.; Christian, K.M.; Ming, G.L.; et al. Single-Cell RNA-Seq with Waterfall Reveals Molecular Cascades underlying Adult Neurogenesis. Cell Stem Cell 2015, 17, 360–372. [Google Scholar] [CrossRef]
- Knobloch, M.; Pilz, G.A.; Ghesquière, B.; Kovacs, W.J.; Wegleiter, T.; Moore, D.L.; Hruzova, M.; Zamboni, N.; Carmeliet, P.; Jessberger, S. A Fatty Acid Oxidation-Dependent Metabolic Shift Regulates Adult Neural Stem Cell Activity. Cell Rep. 2017, 20, 2144–2155. [Google Scholar] [CrossRef]
- Knobloch, M.; Braun, S.M.; Zurkirchen, L.; von Schoultz, C.; Zamboni, N.; Araúzo-Bravo, M.J.; Kovacs, W.J.; Karalay, O.; Suter, U.; Machado, R.A.; et al. Metabolic control of adult neural stem cell activity by Fasn-dependent lipogenesis. Nature 2013, 493, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Candelario, K.M.; Thomas, K.; Wang, R.; Wright, K.; Messier, A.; Cunningham, L.A. Hypoxia inducible factor-1alpha (HIF-1alpha) is required for neural stem cell maintenance and vascular stability in the adult mouse SVZ. J. Neurosci. 2014, 34, 16713–16719. [Google Scholar] [CrossRef]
- Kawase, K.; Nakamura, Y.; Wolbeck, L.; Takemura, S.; Zaitsu, K.; Ando, T.; Jinnou, H.; Sawada, M.; Nakajima, C.; Rydbirk, R.; et al. Significance of birth in the maintenance of quiescent neural stem cells. Sci. Adv. 2025, 11, eadn6377. [Google Scholar] [CrossRef]
- Noguchi, H.; Castillo, J.G.; Nakashima, K.; Pleasure, S.J. Suppressor of fused controls perinatal expansion and quiescence of future dentate adult neural stem cells. Elife 2019, 8. [Google Scholar] [CrossRef]
- Daynac, M.; Tirou, L.; Faure, H.; Mouthon, M.A.; Gauthier, L.R.; Hahn, H.; Boussin, F.D.; Ruat, M. Hedgehog Controls Quiescence and Activation of Neural Stem Cells in the Adult Ventricular-Subventricular Zone. Stem Cell Rep. 2016, 7, 735–748. [Google Scholar] [CrossRef]
- Gonzalez-Reyes, L.E.; Chiang, C.C.; Zhang, M.; Johnson, J.; Arrillaga-Tamez, M.; Couturier, N.H.; Reddy, N.; Starikov, L.; Capadona, J.R.; Kottmann, A.H.; et al. Sonic Hedgehog is expressed by hilar mossy cells and regulates cellular survival and neurogenesis in the adult hippocampus. Sci. Rep. 2019, 9, 17402. [Google Scholar] [CrossRef]
- Gherghina, L.-Y.; Tang, J.L.Y.; Brand, A.H. Quiescent neural stem cells transiently become ‘neurons’ to coordinate reactivation. bioRxiv 2024. [Google Scholar] [CrossRef]
- Deng, Q.; Tan, Y.S.; Chew, L.Y.; Wang, H. Msps governs acentrosomal microtubule assembly and reactivation of quiescent neural stem cells. EMBO J. 2021, 40, e104549. [Google Scholar] [CrossRef] [PubMed]
- Truman, J.W.; Bate, M. Spatial and temporal patterns of neurogenesis in the central nervous system of Drosophila melanogaster. Dev. Biol. 1988, 125, 145–157. [Google Scholar] [CrossRef]
- Gujar, M.R.; Gao, Y.; Teng, X.; Deng, Q.; Lin, K.Y.; Tan, Y.S.; Toyama, Y.; Wang, H. Golgi-dependent reactivation and regeneration of Drosophila quiescent neural stem cells. Dev. Cell 2023, 58, 1933–1949.e1935. [Google Scholar] [CrossRef]
- Lin, K.Y.; Gujar, M.R.; Lin, J.; Ding, W.Y.; Huang, J.; Gao, Y.; Tan, Y.S.; Teng, X.; Christine, L.S.L.; Kanchanawong, P.; et al. Astrocytes control quiescent NSC reactivation via GPCR signaling-mediated F-actin remodeling. Sci. Adv. 2024, 10, eadl4694. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Mandal, S. Mitochondrial Control of Stem Cell State and Fate: Lessons From. Front. Cell Dev. Biol. 2021, 9, 606639. [Google Scholar] [CrossRef]
- Anvarian, Z.; Mykytyn, K.; Mukhopadhyay, S.; Pedersen, L.B.; Christensen, S.T. Cellular signalling by primary cilia in development, organ function and disease. Nat. Rev. Nephrol. 2019, 15, 199–219. [Google Scholar] [CrossRef]
- Singla, V.; Reiter, J.F. The primary cilium as the cell’s antenna: Signaling at a sensory organelle. Science 2006, 313, 629–633. [Google Scholar] [CrossRef]
- Khatri, P.; Obernier, K.; Simeonova, I.K.; Hellwig, A.; Hölzl-Wenig, G.; Mandl, C.; Scholl, C.; Wölfl, S.; Winkler, J.; Gaspar, J.A.; et al. Proliferation and cilia dynamics in neural stem cells prospectively isolated from the SEZ. Sci. Rep. 2014, 4, 3803. [Google Scholar] [CrossRef]
- Han, Y.G.; Spassky, N.; Romaguera-Ros, M.; Garcia-Verdugo, J.M.; Aguilar, A.; Schneider-Maunoury, S.; Alvarez-Buylla, A. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat. Neurosci. 2008, 11, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.K.; Han, Y.G.; Shah, J.K.; Obernier, K.; Guinto, C.D.; Alvarez-Buylla, A. Primary cilia are required in a unique subpopulation of neural progenitors. Proc. Natl. Acad. Sci. USA 2014, 111, 12438–12443. [Google Scholar] [CrossRef] [PubMed]
- Breunig, J.J.; Sarkisian, M.R.; Arellano, J.I.; Morozov, Y.M.; Ayoub, A.E.; Sojitra, S.; Wang, B.; Flavell, R.A.; Rakic, P.; Town, T. Primary cilia regulate hippocampal neurogenesis by mediating sonic hedgehog signaling. Proc. Natl. Acad. Sci. USA 2008, 105, 13127–13132. [Google Scholar] [CrossRef]
- Jeanson, L.; Copin, B.; Papon, J.F.; Dastot-Le Moal, F.; Duquesnoy, P.; Montantin, G.; Cadranel, J.; Corvol, H.; Coste, A.; Désir, J.; et al. RSPH3 Mutations Cause Primary Ciliary Dyskinesia with Central-Complex Defects and a Near Absence of Radial Spokes. Am. J. Hum. Genet. 2015, 97, 153–162. [Google Scholar] [CrossRef]
- Coquand, L.; Victoria, G.S.; Tata, A.; Carpentieri, J.A.; Brault, J.B.; Guimiot, F.; Fraisier, V.; Baffet, A.D. CAMSAPs organize an acentrosomal microtubule network from basal varicosities in radial glial cells. J. Cell Biol. 2021, 220. [Google Scholar] [CrossRef] [PubMed]
- Richard, C.A.; Seum, C.; Gonzalez-Gaitan, M. Microtubule polarity determines the lineage of embryonic neural precursor in zebrafish spinal cord. Commun. Biol. 2024, 7, 439. [Google Scholar] [CrossRef]
- Jurisch-Yaksi, N.; Yaksi, E.; Kizil, C. Radial glia in the zebrafish brain: Functional, structural, and physiological comparison with the mammalian glia. Glia 2020, 68, 2451–2470. [Google Scholar] [CrossRef]
- Alexandre, P.; Reugels, A.M.; Barker, D.; Blanc, E.; Clarke, J.D. Neurons derive from the more apical daughter in asymmetric divisions in the zebrafish neural tube. Nat. Neurosci. 2010, 13, 673–679. [Google Scholar] [CrossRef]
- Barros, C.S.; Franco, S.J.; Müller, U. Extracellular matrix: Functions in the nervous system. Cold Spring Harb. Perspect. Biol. 2011, 3, a005108. [Google Scholar] [CrossRef]
- Yeo, R.W.; Zhou, O.Y.; Zhong, B.L.; Sun, E.D.; Navarro Negredo, P.; Nair, S.; Sharmin, M.; Ruetz, T.J.; Wilson, M.; Kundaje, A.; et al. Chromatin accessibility dynamics of neurogenic niche cells reveal defects in neural stem cell adhesion and migration during aging. Nat. Aging 2023, 3, 866–893. [Google Scholar] [CrossRef]
- Morizur, L.; Chicheportiche, A.; Gauthier, L.R.; Daynac, M.; Boussin, F.D.; Mouthon, M.A. Distinct Molecular Signatures of Quiescent and Activated Adult Neural Stem Cells Reveal Specific Interactions with Their Microenvironment. Stem Cell Rep. 2018, 11, 565–577. [Google Scholar] [CrossRef]
- Codega, P.; Silva-Vargas, V.; Paul, A.; Maldonado-Soto, A.R.; Deleo, A.M.; Pastrana, E.; Doetsch, F. Prospective identification and purification of quiescent adult neural stem cells from their in vivo niche. Neuron 2014, 82, 545–559. [Google Scholar] [CrossRef]
- Wang, D.Y.; Luo, A.F.; Bai, Q.R.; Gong, X.L.; Zheng, Y.; Shen, Q.; Hu, X.L.; Wang, X.M. VCAM1 Labels a Subpopulation of Neural Stem Cells in the Adult Hippocampus and Contributes to Spatial Memory. Stem Cell Rep. 2020, 14, 1093–1106. [Google Scholar] [CrossRef] [PubMed]
- Porlan, E.; Martí-Prado, B.; Morante-Redolat, J.M.; Consiglio, A.; Delgado, A.C.; Kypta, R.; López-Otín, C.; Kirstein, M.; Fariñas, I. MT5-MMP regulates adult neural stem cell functional quiescence through the cleavage of N-cadherin. Nat. Cell Biol. 2014, 16, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Kokovay, E.; Wang, Y.; Kusek, G.; Wurster, R.; Lederman, P.; Lowry, N.; Shen, Q.; Temple, S. VCAM1 is essential to maintain the structure of the SVZ niche and acts as an environmental sensor to regulate SVZ lineage progression. Cell Stem Cell 2012, 11, 220–230. [Google Scholar] [CrossRef]
- Van Camp, J.K.; Beckers, S.; Zegers, D.; Van Hul, W. Wnt signaling and the control of human stem cell fate. Stem Cell Rev. Rep. 2014, 10, 207–229. [Google Scholar] [CrossRef]
- van Amerongen, R.; Bowman, A.N.; Nusse, R. Developmental stage and time dictate the fate of Wnt/beta-catenin-responsive stem cells in the mammary gland. Cell Stem Cell 2012, 11, 387–400. [Google Scholar] [CrossRef]
- Bowman, A.N.; van Amerongen, R.; Palmer, T.D.; Nusse, R. Lineage tracing with Axin2 reveals distinct developmental and adult populations of Wnt/β-catenin-responsive neural stem cells. Proc. Natl. Acad. Sci. USA 2013, 110, 7324–7329. [Google Scholar] [CrossRef]
- Austin, S.H.L.; Gabarró-Solanas, R.; Rigo, P.; Paun, O.; Harris, L.; Guillemot, F.; Urbán, N. Wnt/β-catenin signalling is dispensable for adult neural stem cell homeostasis and activation. Development 2021, 148. [Google Scholar] [CrossRef]
- Simões, A.R.; Neto, M.; Alves, C.S.; Santos, M.B.; Fernández-Hernández, I.; Veiga-Fernandes, H.; Brea, D.; Durá, I.; Encinas, J.M.; Rhiner, C. Damage-responsive neuro-glial clusters coordinate the recruitment of dormant neural stem cells in Drosophila. Dev. Cell 2022, 57, 1661–1675.e1667. [Google Scholar] [CrossRef]
- Sokol, S.Y. Maintaining embryonic stem cell pluripotency with Wnt signaling. Development 2011, 138, 4341–4350. [Google Scholar] [CrossRef]
- Hikasa, H.; Sokol, S.Y. Phosphorylation of TCF proteins by homeodomain-interacting protein kinase 2. J. Biol. Chem. 2011, 286, 12093–12100. [Google Scholar] [CrossRef]
- Ferrer-Vaquer, A.; Piliszek, A.; Tian, G.; Aho, R.J.; Dufort, D.; Hadjantonakis, A.K. A sensitive and bright single-cell resolution live imaging reporter of Wnt/ss-catenin signaling in the mouse. BMC Dev. Biol. 2010, 10, 121. [Google Scholar] [CrossRef]
- Garcia-Corzo, L.; Calatayud-Baselga, I.; Casares-Crespo, L.; Mora-Martinez, C.; Julian Escribano-Saiz, J.; Hortiguela, R.; Asenjo-Martinez, A.; Jordan-Pla, A.; Ercoli, S.; Flames, N.; et al. The transcription factor LEF1 interacts with NFIX and switches isoforms during adult hippocampal neural stem cell quiescence. Front. Cell Dev. Biol. 2022, 10, 912319. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, B.W.; Aitken, G.E.; Tang, J.K.; Khabooshan, M.; Douek, A.M.; Vandestadt, C.; Kaslin, J. Midbrain tectal stem cells display diverse regenerative capacities in zebrafish. Sci. Rep. 2019, 9, 4420. [Google Scholar] [CrossRef]
- Harrison, N.J.; Connolly, E.; Gascón Gubieda, A.; Yang, Z.; Altenhein, B.; Losada Perez, M.; Moreira, M.; Sun, J.; Hidalgo, A. Regenerative neurogenic response from glia requires insulin-driven neuron-glia communication. Elife 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Hesse, R.G.; Kouklis, G.K.; Ahituv, N.; Pomerantz, J.H. The human ARF tumor suppressor senses blastema activity and suppresses epimorphic tissue regeneration. Elife 2015, 4. [Google Scholar] [CrossRef]
- Radak, M.; Fallahi, H. The Epigenetic Regulation of Quiescent in Stem Cells. Glob. Med. Genet. 2023, 10, 339–344. [Google Scholar] [CrossRef]
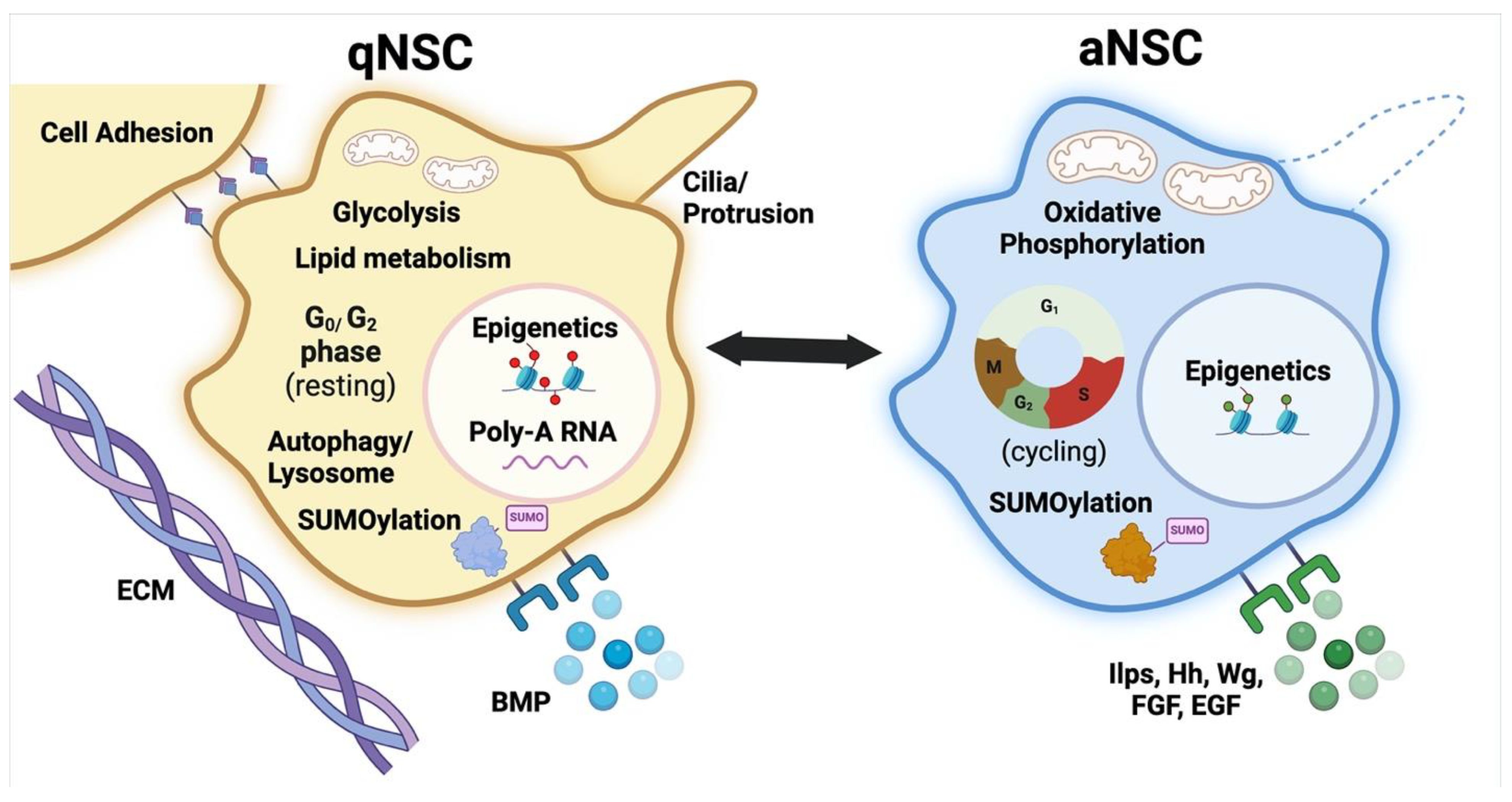
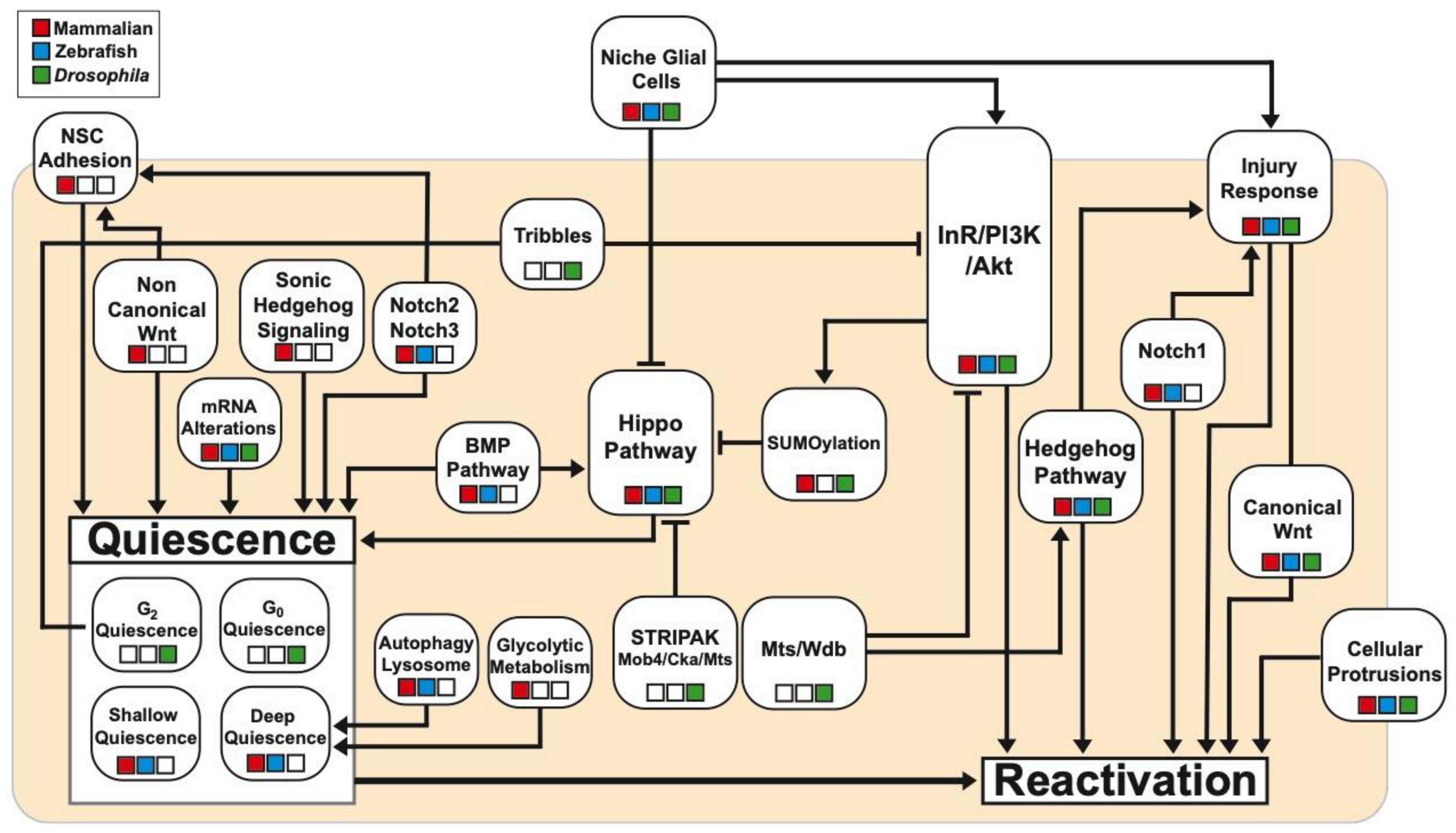
| Drosophila | Mammals | Zebrafish | |
|---|---|---|---|
| Quiescent states, activation and niche influence | G0 and G2 Quiescence: [3,41,43,44] | Shallow and Deep Quiescence: [3,5,7,25,27,35,36,37,39,40] [46,47,48,160]; Niche Influence: [22,51,52,53,54,55,56,57,58,59,60,61,62,63,64,158]; Activation and Aging: [65,66,67,68] | Shallow and Deep Quiescence: [49] |
| mRNA Alterations | Nucleocytoplasmic portioning of poly(A) RNA and mRNA regulates qNSC and prime for reactivation: [69,72,73] | Nucleocytoplasmic portioning of poly(A) RNA and mRNA regulates qNSC and prime for reactivation: [69,70,71,75] | Nucleocytoplasmic portioning of poly(A) RNA and mRNA regulates qNSC and prime for reactivation: [74,76,77] |
| Notch Signalling | Regulates quiescence: [40] | Varied Notch1, Notch2 and Notch3 in quiescence and reactivation: [5,7,46,82,83,84] | Varied Notch1, Notch2 and Notch3 in quiescence and reactivation: [25,28,85,86,87,88,89] |
| BMP/Id1 | Not known in NSC quiescence/ activation regulation. | Induces quiescence including through expression of Id1-3: [92,93,94,95,96,97] | Induces quiescence through expression of Id1; linked to Notch: [25,98,99] |
| Hippo Pathway | Maintains quiescence through Hippo/Salvador/Wats/Mats/Yorkie cascade: [2,32,100,107] | Maintains quiescence through MST1-2/SAV1/LATS1-2/MOB1/YAP/TAZ cascade: [2,8,100,102,103,104,105,106,114,115] | Maintains quiescence through Mst2/Sav1/Lats1-2/MOB1/Yap/Taz cascade: [25,26,116,117,118] |
| STRIPAK | Mob4 and Cka associate PP2A/Mts to inactivate Hippo Pathway PP2A/Mts and Wdb inactivate InR/PI3K/Akt pathway: [108,109,110,111,112,113] | Not implicated in quiescence or reactivation of NSCs. | Present within quiescent Müller glia cells and in response to injury, but limited knowledge in NSCs: [119,120,121] |
| Insulin Pathway | InR/PI3K/Akt/TOR pathway leads to reactivation of NSCs, including through inhibition of FOXO: [6,43,122,123,124,136,137,138,140,141] | InR/PI3K/Akt/mTOR pathway leads to reactivation of NSCs, including through inhibition of FOXO3: [5,7,9,29,92,125,126,127,128,129,130,131,132] | Limited knowledge. insra and insrb highly conserved: [133,134,135] |
| SUMOylation | smt3 and Ubc9 SUMOylate Wts leading to reactivation: [32] | Implicated in self-renewal and differentiation of NSCs: [142,143,144,145] | Ubc9 is present in early development and proliferative zones at later stages. Little known in NSCs: [146,147] |
| Lysosomal and Autophagy | Limited knowledge. | Lysosomal activity and autophagy are involved in maintaining NSC quiescence: [129,148,149,150] | Prosaposin (Psap) has been implicated in deep quiescence in zebrafish: [49] |
| Metabolism | Early lipid intake correlates to reactivation, modulated by Hedgehog: [113,151,152,153] | Fatty acid metabolism enriched in qNSCs; switch to oxidative metabolism required for reactivation; birth is associated with metabolic changes: [5,8,9,27,37,160] [29,155,156,157,159] | Limited knowledge; positive feedback loop of H2O2 contributes to regeneration through Hedgehog: [154] |
| Cellular Protusions and Adhesions | Cellular protrusions are hallmark of qNSCs; promotes reactivation through Golgi apparatus and Actin: [30,124,132,138,163,164,165,166,167,168,169] | Primary cilia are a hallmark of deep qNSCs; adhesions are highly important in determining quiescence or reactivation: [3,9,46,47,68,95,170,171,172,173,174,175,181,183,184,185,186,190] | Limited knowledge. Patronin is conserved in active radial glial cells; cilia are present and involved in retaining NSC stemness: [176,177,178,179] |
| Injury/Regeneration | Neuro-glial response coordinated by injury. Promotes Wg/Wnt distribution: [30,191] | NSCs responsive to canonical WNT signalling; Non-canonical Wnt signaling implicated in quiescence; TCFs; Canonical Wnt signaling required for brain repair: [5,7,29,47,67,189,190,194,195] | Highly adept at regeneration. Notch, BMP, Hedgehog, YAP/TAZ and InR pathways implicated in repair; ARF likely suppresses regeneration within mammalian system: [31,87,88,89,117,118,196,197,198] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elkin, A.M.; Robbins, S.; Barros, C.S.; Bossing, T. The Critical Balance Between Quiescence and Reactivation of Neural Stem Cells. Biomolecules 2025, 15, 672. https://doi.org/10.3390/biom15050672
Elkin AM, Robbins S, Barros CS, Bossing T. The Critical Balance Between Quiescence and Reactivation of Neural Stem Cells. Biomolecules. 2025; 15(5):672. https://doi.org/10.3390/biom15050672
Chicago/Turabian StyleElkin, Adam M., Sarah Robbins, Claudia S. Barros, and Torsten Bossing. 2025. "The Critical Balance Between Quiescence and Reactivation of Neural Stem Cells" Biomolecules 15, no. 5: 672. https://doi.org/10.3390/biom15050672
APA StyleElkin, A. M., Robbins, S., Barros, C. S., & Bossing, T. (2025). The Critical Balance Between Quiescence and Reactivation of Neural Stem Cells. Biomolecules, 15(5), 672. https://doi.org/10.3390/biom15050672






