Regulation of Protein Synthesis at the Translational Level: Novel Findings in Cardiovascular Biology
Abstract
1. Introduction
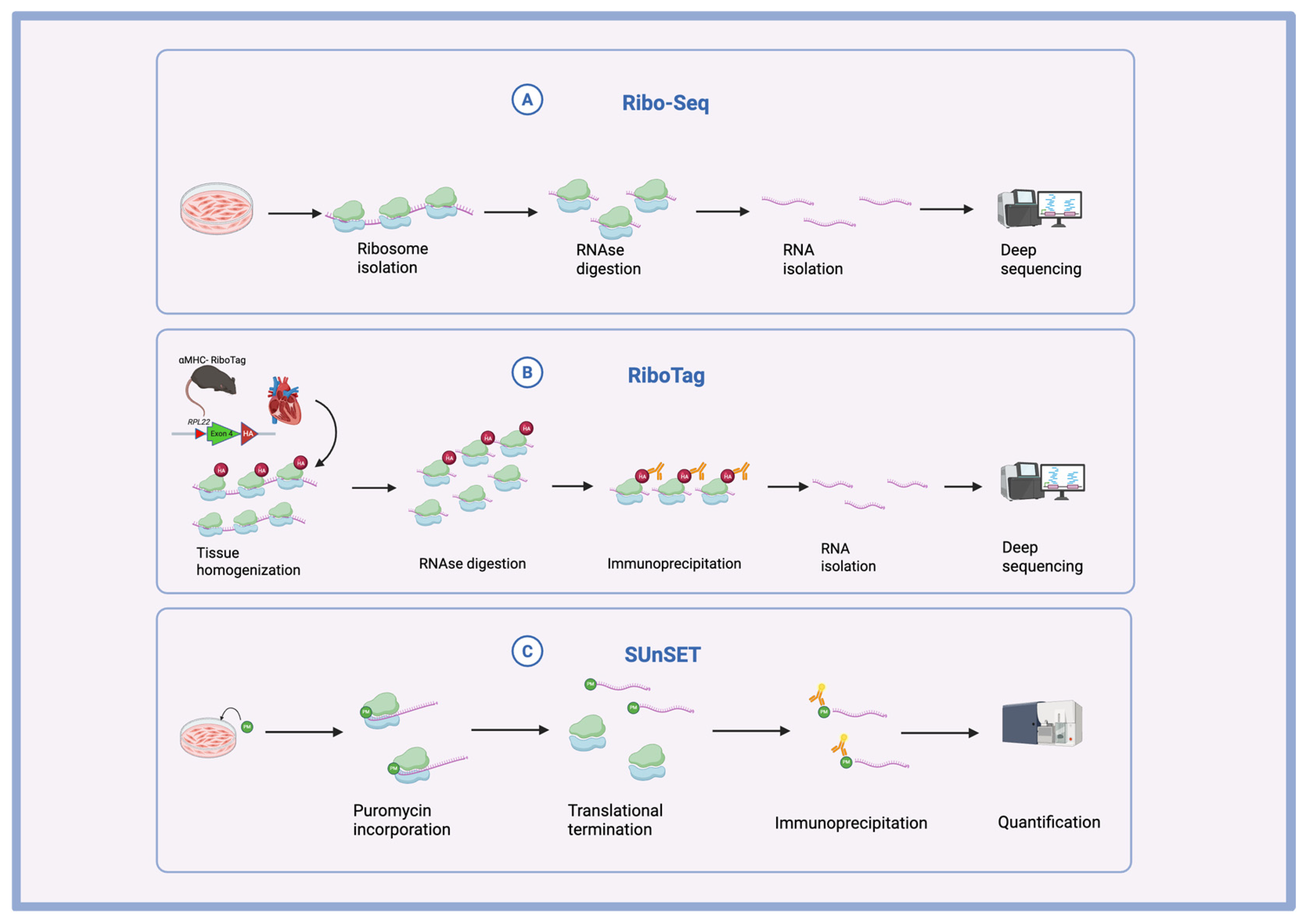
2. Main Methods of Translational Study
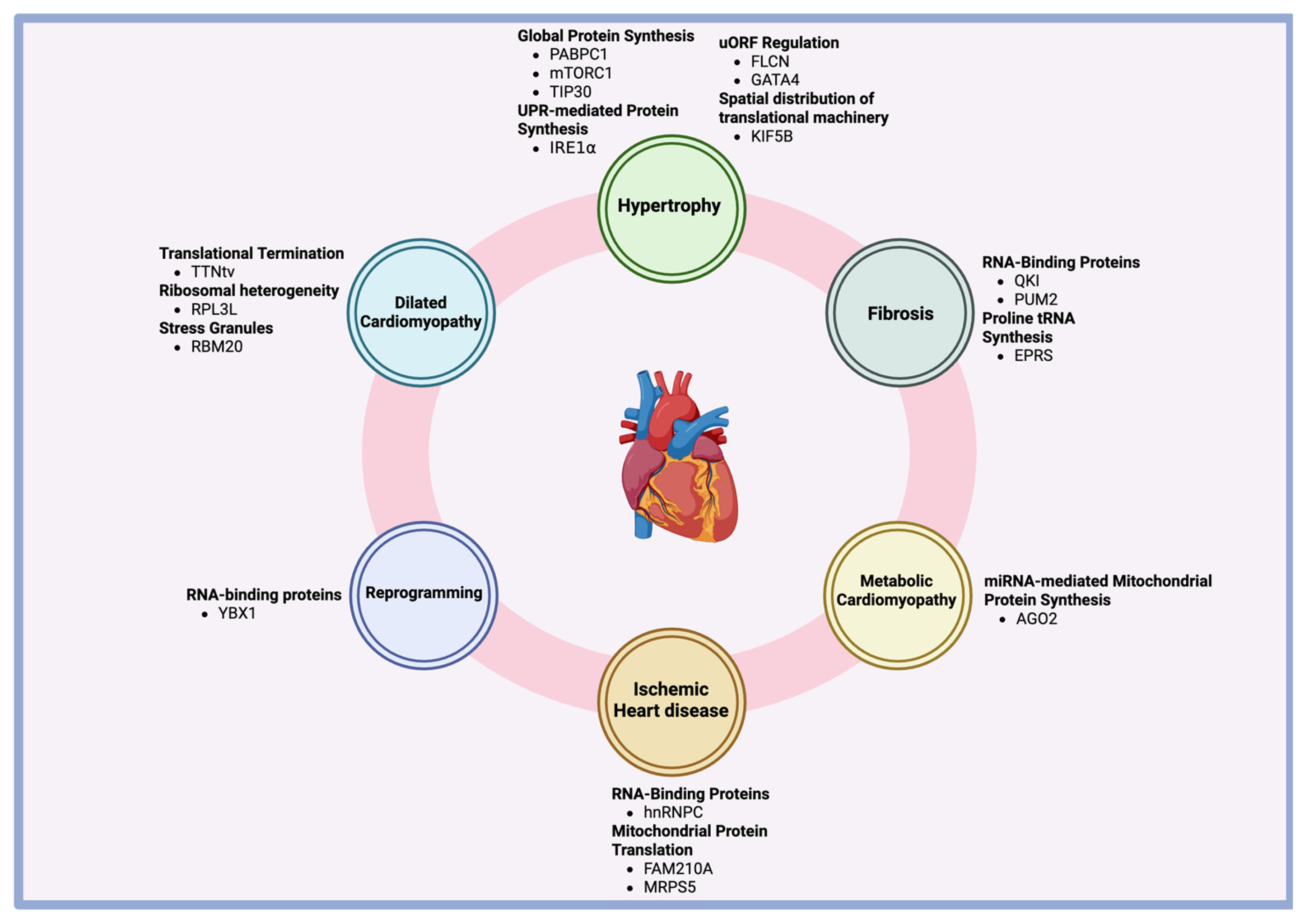
3. Translational Regulation of Cardiac Hypertrophy
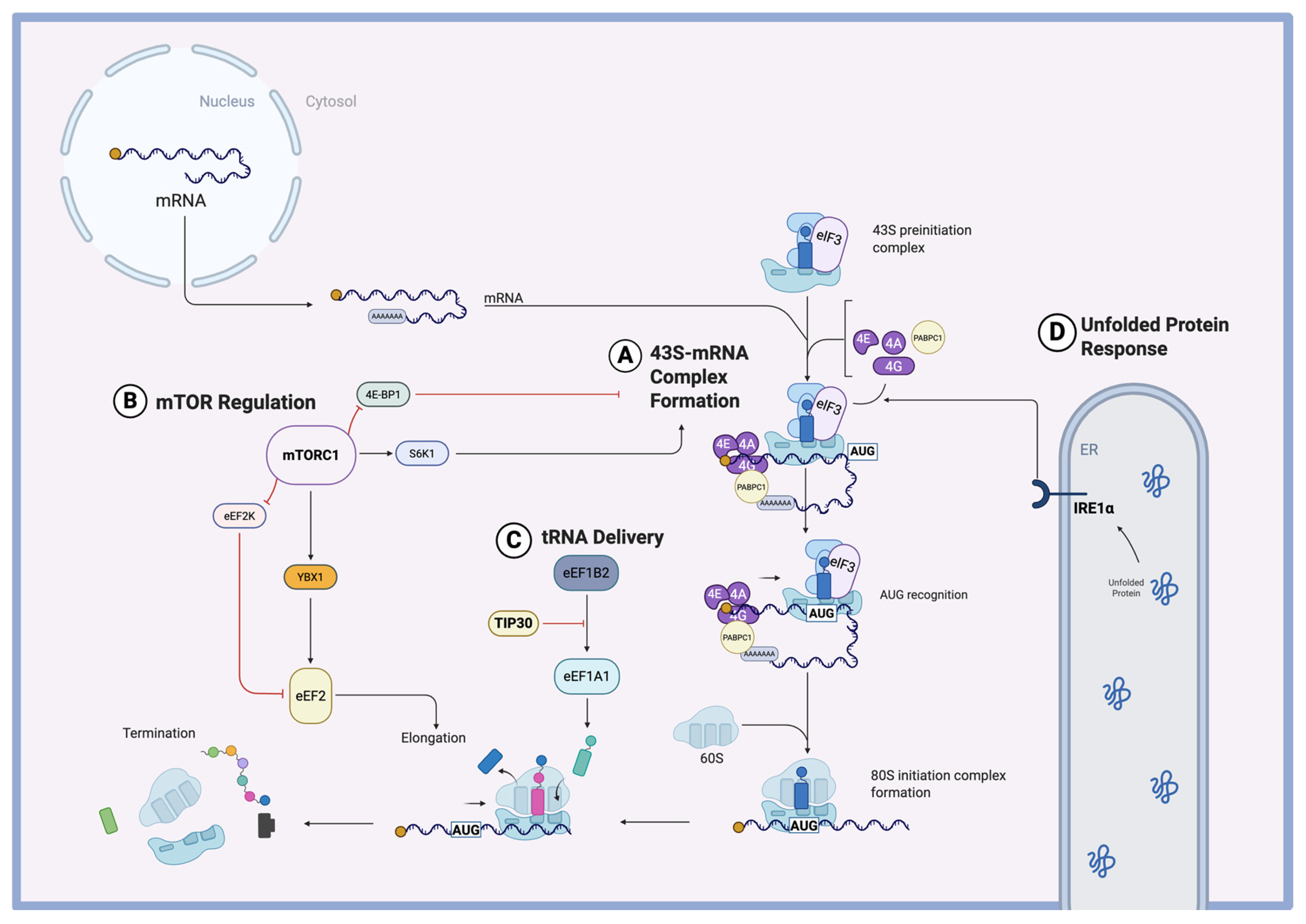
3.1. Translational Complex Components in Hypertrophy
3.2. Translational Initiation in Hypertrophy
3.3. Translational Elongation in Hypertrophy
3.4. The Distinction Between Pressure Overload and Adrenergic Stress Hypertrophy
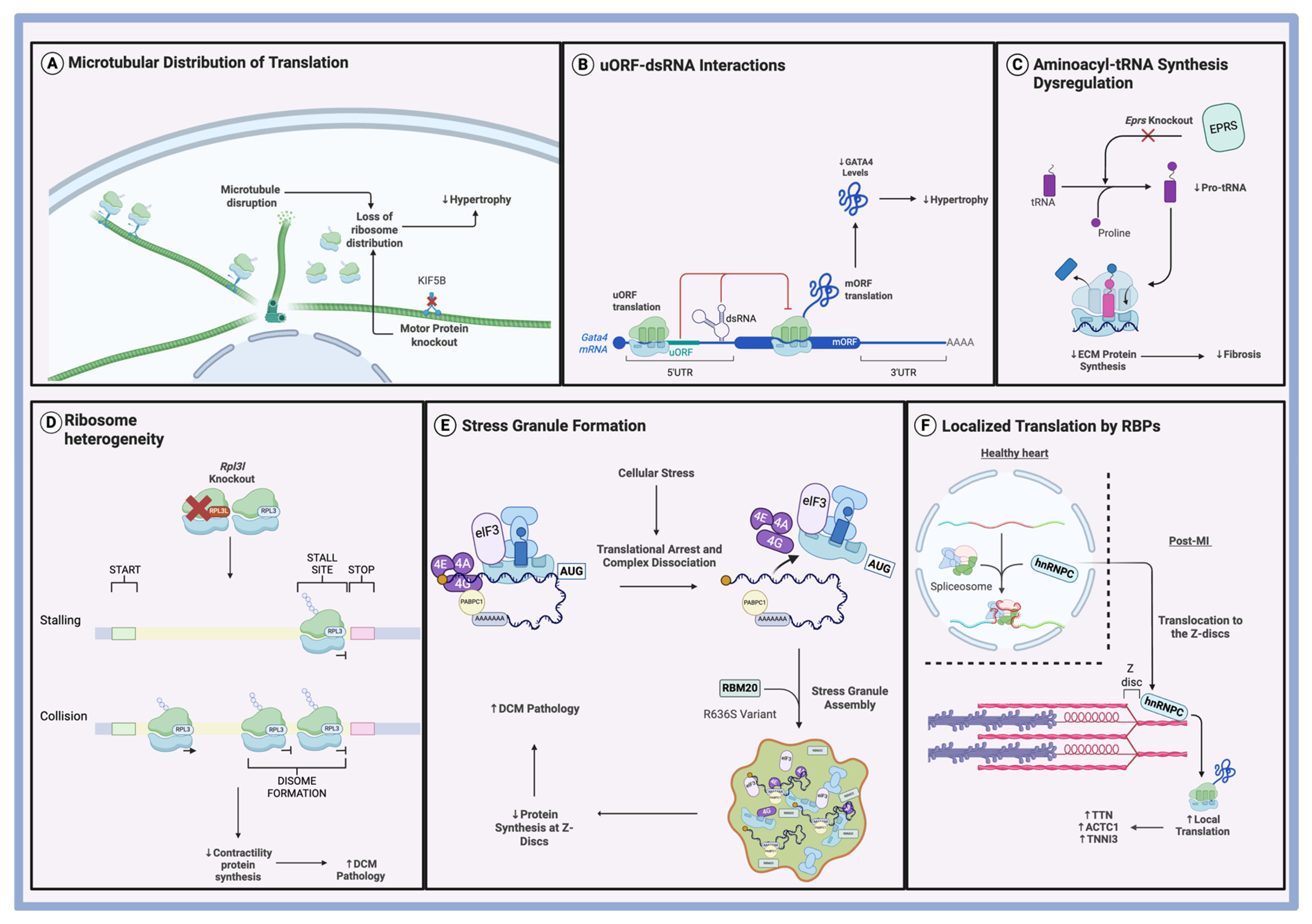
3.5. The Role of uORFs in Hypertrophy
4. Translational Regulation of Cardiac Fibrosis
4.1. RNA-Binding Proteins in Cardiac Fibrosis
4.2. tRNA Synthesis in Cardiac Fibrosis
5. Translational Regulation of Dilated Cardiomyopathy
5.1. Protein Truncating Variants in DCM
5.2. Ribosome Heterogeneity in DCM
5.3. Stress Granules in DCM
6. Translational Regulation of Ischemic Heart Disease
6.1. RNA-Binding Proteins in Ischemic Heart Disease
6.2. Mitochondrial Protein Translation in Ischemic Heart Disease
7. Translational Regulation of Diabetic Cardiomyopathy
8. Translational Regulation of Fibroblast-Cardiomyocyte Reprogramming
9. Clinical Relevance
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schafer, S.; Adami, E.; Heinig, M.; Rodrigues, K.E.C.; Kreuchwig, F.; Silhavy, J.; van Heesch, S.; Simaite, D.; Rajewsky, N.; Cuppen, E.; et al. Translational regulation shapes the molecular landscape of complex disease phenotypes. Nat. Commun. 2015, 6, 7200. [Google Scholar] [CrossRef] [PubMed]
- Schwanhäusser, B.; Busse, D.; Li, N.; Dittmar, G.; Schuchhardt, J.; Wolf, J.; Chen, W.; Selbach, M. Global quantification of mammalian gene expression control. Nature 2011, 473, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Buszczak, M.; Signer, R.A.J.; Morrison, S.J. Cellular differences in protein synthesis regulate tissue homeostasis. Cell 2014, 159, 242–251. [Google Scholar] [CrossRef]
- Raghavan, A.; Orgilvie, R.; Reilly, C.; Abelson, M.; Raghavan, S.; Vasdewani, J.; Krathwohl, M.; Bohjanen, P. Genome-wide analysis of mRNA decay in resting and activated primary human T lymphocytes. Nucleic Acids Res. 2002, 30, 5529–5538. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.R.; Ilinykh, A.; Ivey, M.J.; Kuwabara, J.T.; D’Antoni, M.L.; Debuque, R.; Chandran, A.; Wang, L.; Arora, K.; Rosenthal, N.A.; et al. Revisiting Cardiac Cellular Composition. Circ. Res. 2016, 118, 400–409. [Google Scholar] [CrossRef]
- Doroudgar, S.; Hofmann, C.; Boileau, E.; Malone, B.; Riechert, E.; Gorska, A.A.; Jakobi, T.; Sandmann, C.; Jürgensen, L.; Kmietczyk, V.; et al. Monitoring Cell-Type–Specific Gene Expression Using Ribosome Profiling In Vivo During Cardiac Hemodynamic Stress. Circ. Res. 2019, 125, 431–448. [Google Scholar] [CrossRef]
- Chothani, S.; Schäfer, S.; Adami, E.; Viswanathan, S.; Widjaja, A.A.; Langley, S.R.; Tan, J.; Wang, M.; Quaife, N.M.; Jian Pua, C.; et al. Widespread Translational Control of Fibrosis in the Human Heart by RNA-Binding Proteins. Circulation 2019, 140, 937–951. [Google Scholar] [CrossRef]
- Lewis, S.E.; Kelly, F.J.; Goldspink, D.F. Pre- and post-natal growth and protein turnover in smooth muscle, heart and slow- and fast-twitch skeletal muscles of the rat. Biochem. J. 1984, 217, 517–526. [Google Scholar] [CrossRef]
- van Heesch, S.; Witte, F.; Schneider-Lunitz, V.; Schulz, J.F.; Adami, E.; Faber, A.B.; Kirchner, M.; Maatz, H.; Blachut, S.; Sandmann, C.-L.; et al. The Translational Landscape of the Human Heart. Cell 2019, 178, 242–260.e29. [Google Scholar] [CrossRef]
- Schmidt, E.K.; Clavarino, G.; Ceppi, M.; Pierre, P. SUnSET, a nonradioactive method to monitor protein synthesis. Nat. Methods. 2009, 6, 275–277. [Google Scholar] [CrossRef]
- Ingolia, N.T.; Ghaemmaghami, S.; Newman, J.R.S.; Weissman, J.S. Genome-Wide Analysis in Vivo of Translation with Nucleotide Resolution Using Ribosome Profiling. Science 2009, 324, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Zhang, Y.; Ma, Q.; Gu, F.; Day, D.S.; He, A.; Zhou, B.; Li, J.; Stevens, S.M.; Romo, D.; et al. Interrogating translational efficiency and lineage-specific transcriptomes using ribosome affinity purification. Proc. Natl. Acad. Sci. USA 2013, 110, 15395–15400. [Google Scholar] [CrossRef] [PubMed]
- Chothani, S.; Adami, E.; Ouyang, J.F.; Viswanathan, S.; Hubner, N.; Cook, S.A.; Schafer, S.; Rackham, O.J.L. deltaTE: Detection of Translationally Regulated Genes by Integrative Analysis of Ribo-Seq and RNA-Seq Data. Curr. Protoc. Mol. Biol. 2019, 129, e108. [Google Scholar] [CrossRef] [PubMed]
- Meijer, H.A.; Thomas, A.A.M. Control of eukaryotic protein synthesis by upstream open reading frames in the 5′-untranslated region of an mRNA. Biochem. J. 2002, 367 Pt 1, 1–11. [Google Scholar] [CrossRef]
- Silva, J.; Fernandes, R.; Romão, L. Translational Regulation by Upstream Open Reading Frames and Human Diseases. In The mRNA Metabolism in Human Disease; Romão, L., Ed.; Springer: Cham, Switzerland, 2019; pp. 99–116. [Google Scholar] [CrossRef]
- Liang, X.H.; Shen, W.; Sun, H.; Migawa, M.T.; Vickers, T.A.; Crooke, S.T. Translation efficiency of mRNAs is increased by antisense oligonucleotides targeting upstream open reading frames. Nat. Biotechnol. 2016, 34, 875–880. [Google Scholar] [CrossRef]
- Bencun, M.; Spreyer, L.; Boileau, E.; Eschenbach, J.; Frey, N.; Dieterich, C.; Völkers, M. A novel uORF regulates folliculin to promote cell growth and lysosomal biogenesis during cardiac stress. Sci. Rep. 2025, 15, 3319. [Google Scholar] [CrossRef]
- Sanz, E.; Yang, L.; Su, T.; Morris, D.R.; McKnight, G.S.; Amieux, P.S. Cell-type-specific isolation of ribosome-associated mRNA from complex tissues. Proc. Natl. Acad. Sci. USA 2009, 106, 13939–13944. [Google Scholar] [CrossRef]
- Heiman, M.; Schaefer, A.; Gong, S.; Peterson, J.D.; Day, M.; Ramsey, K.E.; Suárez-Fariñas, M.; Schwarz, C.; Stephan, D.A.; Surmeier, D.J.; et al. A translational profiling approach for the molecular characterization of CNS cell types. Cell 2008, 135, 738–748. [Google Scholar] [CrossRef]
- Heineke, J.; Molkentin, J.D. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat. Rev. Mol. Cell Biol. 2006, 7, 589–600. [Google Scholar] [CrossRef]
- Nakamura, M.; Sadoshima, J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat. Rev. Cardiol. 2018, 15, 387–407. [Google Scholar] [CrossRef]
- McDermott, P.J.; Baicu, C.F.; Wahl, S.R.; Van Laer, A.O.; Zile, M.R. In vivo measurements of the contributions of protein synthesis and protein degradation in regulating cardiac pressure overload hypertrophy in the mouse. Mol. Cell Biochem. 2012, 367, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Wang, Y. mRNA Metabolism in Cardiac Development and Disease: Life After Transcription. Physiol. Rev. 2020, 100, 673–694. [Google Scholar] [CrossRef] [PubMed]
- Garlick, P.J.; McNurlan, M.A.; Preedy, V.R. A rapid and convenient technique for measuring the rate of protein synthesis in tissues by injection of [3H]phenylalanine. Biochem. J. 1980, 192, 719–723. [Google Scholar] [CrossRef]
- Vogel, C.; Marcotte, E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012, 13, 227–232. [Google Scholar] [CrossRef]
- Spruill, L.S.; Baicu, C.F.; Zile, M.R.; McDermott, P.J. Selective translation of mRNAs in the left ventricular myocardium of the mouse in response to acute pressure overload. J. Mol. Cell. Cardiol. 2008, 44, 69–75. [Google Scholar] [CrossRef]
- Siehl, D.; Chua, B.H.; Lautensack-Belser, N.; Morgan, H.E. Faster protein and ribosome synthesis in thyroxine-induced hypertrophy of rat heart. Am. J. Physiol. Cell Physiol. 1985, 248, C309–C319. [Google Scholar] [CrossRef]
- Scarborough, E.A.; Uchida, K.; Vogel, M.; Erlitzki, N.; Iyer, M.; Phyo, S.A.; Bogush, A.; Kehat, I.; Prosser, B.L. Microtubules orchestrate local translation to enable cardiac growth. Nat. Commun. 2021, 12, 1547. [Google Scholar] [CrossRef]
- Li, C.; Li, S.; Zhang, G.; Li, Q.; Song, W.; Wang, X.; Cook, J.A.; van der Stoel, M.; Wright, B.W.; Altamirano, F.; et al. IRE1α Mediates the Hypertrophic Growth of Cardiomyocytes Through Facilitating the Formation of Initiation Complex to Promote the Translation of TOP-Motif Transcripts. Circulation 2024, 150, 1010–1029. [Google Scholar] [CrossRef]
- Chorghade, S.; Seimetz, J.; Emmons, R.; Yang, J.; Bresson, S.M.; Lisio, M.D.; Parise, G.; Conrad, N.K.; Kalsotra, A. Poly(A) tail length regulates PABPC1 expression to tune translation in the heart. eLife 2017, 6, e24139. [Google Scholar] [CrossRef]
- Sonenberg, N.; Hinnebusch, A.G. Regulation of translation initiation in eukaryotes: Mechanisms and biological targets. Cell 2009, 136, 731–745. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, M.; Jiang, Q. PABPC1—mRNA stability, protein translation and tumorigenesis. Front. Oncol. 2022, 12, 1025291. [Google Scholar] [CrossRef] [PubMed]
- Houser, S.R.; Margulies, K.B.; Murphy, A.M.; Spinale, F.G.; Francis, G.S.; Prabhu, S.D.; Rockman, H.A.; Kass, D.A.; Molkentin, J.D.; Sussman, M.A.; et al. Animal Models of Heart Failure. Circ. Res. 2012, 111, 131–150. [Google Scholar] [CrossRef] [PubMed]
- Safaee, N.; Kozlov, G.; Noronha, A.M.; Xie, J.; Wilds, C.J.; Gehring, K. Interdomain allostery promotes assembly of the poly(A) mRNA complex with PABP and eIF4G. Mol. Cell 2012, 48, 375–386. [Google Scholar] [CrossRef]
- Zoncu, R.; Sabatini, D.M.; Efeyan, A. mTOR: From growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 2011, 12, 21–35. [Google Scholar] [CrossRef]
- Zhang, D.; Contu, R.; Latronico, M.V.G.; Zhang, J.; Rizzi, R.; Catalucci, D.; Miyamoto, S.; Huang, K.; Ceci, M.; Gu, Y.; et al. MTORC1 regulates cardiac function and myocyte survival through 4E-BP1 inhibition in mice. J. Clin. Investig. 2010, 120, 2805–2816. [Google Scholar] [CrossRef]
- Thoreen, C.C.; Chantranupong, L.; Keys, H.R.; Wang, T.; Gray, N.S.; Sabatini, D.M. A unifying model for mTORC1-mediated regulation of mRNA translation. Nature 2012, 485, 109–113. [Google Scholar] [CrossRef]
- Walter, P.; Ron, D. The unfolded protein response: From stress pathway to homeostatic regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef]
- Buscà, R.; Pouysségur, J.; Lenormand, P. ERK1 and ERK2 Map kinases: Specific roles or functional redundancy? Front. Cell Dev. Biol. 2016, 4, 53. [Google Scholar] [CrossRef]
- Tamai, T.; Yamaguchi, O.; Hikoso, S.; Takeda, T.; Taneike, M.; Oka, T.; Oyabu, J.; Murakawa, T.; Nakayama, H.; Uno, Y.; et al. Rheb (Ras Homologue Enriched in Brain)-dependent Mammalian Target of Rapamycin Complex 1 (mTORC1) Activation Becomes Indispensable for Cardiac Hypertrophic Growth after Early Postnatal Period *. J. Biol. Chem. 2013, 288, 10176–10187. [Google Scholar] [CrossRef]
- Varma, E.; Burghaus, J.; Schwarzl, T.; Sekaran, T.; Gupta, P.; Górska, A.A.; Hofmann, C.; Stroh, C.; Jürgensen, L.; Kamuf-Schenk, V.; et al. Translational control of Ybx1 expression regulates cardiac function in response to pressure overload in vivo. Basic Res. Cardiol. 2023, 118, 25. [Google Scholar] [CrossRef]
- Mordovkina, D.; Lyabin, D.N.; Smolin, E.A.; Sogorina, E.M.; Ovchinnikov, L.P.; Eliseeva, I. Y-Box Binding Proteins in mRNP Assembly, Translation, and Stability Control. Biomolecules 2020, 10, 591. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, Z.; Wu, W.K.K. TIP30: A Novel Tumor-Suppressor Gene. Oncol. Res. 2015, 22, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Grund, A.; Szaroszyk, M.; Korf-Klingebiel, M.; Malek Mohammadi, M.; Trogisch, F.A.; Schrameck, U.; Gigina, A.; Tiedje, C.; Gaestel, M.; Kraft, T.; et al. TIP30 counteracts cardiac hypertrophy and failure by inhibiting translational elongation. EMBO Mol. Med. 2019, 11, e10018. [Google Scholar] [CrossRef] [PubMed]
- Sasikumar, A.N.; Perez, W.B.; Kinzy, T.G. The many roles of the eukaryotic elongation factor 1 complex. Wiley Interdiscip. Rev. RNA 2012, 3, 543–555. [Google Scholar] [CrossRef]
- Blackwood, E.A.; Hofmann, C.; Santo Domingo, M.; Bilal, A.S.; Sarakki, A.; Stauffer, W.; Arrieta, A.; Thuerauf, D.J.; Kolkhorst, F.W.; Müller, O.J.; et al. ATF6 Regulates Cardiac Hypertrophy by Transcriptional Induction of the mTORC1 Activator, Rheb. Circ. Res. 2019, 124, 79–93. [Google Scholar] [CrossRef]
- Yan, Y.; Tang, R.; Li, B.; Cheng, L.; Ye, S.; Yang, T.; Han, Y.-C.; Liu, C.; Dong, Y.; Qu, L.-H.; et al. The cardiac translational landscape reveals that micropeptides are new players involved in cardiomyocyte hypertrophy. Mol. Ther. 2021, 29, 2253–2267. [Google Scholar] [CrossRef]
- Calvo, S.E.; Pagliarini, D.J.; Mootha, V.K. Upstream open reading frames cause widespread reduction of protein expression and are polymorphic among humans. Proc. Natl. Acad. Sci. USA 2009, 106, 7507–7512. [Google Scholar] [CrossRef]
- Leppek, K.; Das, R.; Barna, M. Functional 5′ UTR mRNA structures in eukaryotic translation regulation and how to find them. Nat. Rev. Mol. Cell Biol. 2018, 19, 158–174. [Google Scholar] [CrossRef]
- Hedaya, O.M.; Venkata Subbaiah, K.C.; Jiang, F.; Xie, L.H.; Wu, J.; Khor, E.-S.; Zhu, M.; Mathews, D.H.; Proschel, C.; Yao, P. Secondary structures that regulate mRNA translation provide insights for ASO-mediated modulation of cardiac hypertrophy. Nat. Commun. 2023, 14, 6166. [Google Scholar] [CrossRef]
- Whitcomb, J.; Gharibeh, L.; Nemer, M. From embryogenesis to adulthood: Critical role for GATA factors in heart development and function. IUBMB Life 2020, 72, 53–67. [Google Scholar] [CrossRef]
- Pikkarainen, S.; Tokola, H.; Kerkelä, R.; Ruskoaho, H. GATA transcription factors in the developing and adult heart. Cardiovasc. Res. 2004, 63, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.R.; Geballe, A.P. Upstream Open Reading Frames as Regulators of mRNA Translation. Mol. Cell Biol. 2000, 20, 8635–8642. [Google Scholar] [CrossRef] [PubMed]
- Saadat, S.; Noureddini, M.; Mahjoubin-Tehran, M.; Nazemi, S.; Shojaie, L.; Aschner, M.; Maleki, B.; Abbasi-kolli, M.; Rajabi Moghadam, H.; Alani, B.; et al. Pivotal Role of TGF-β/Smad Signaling in Cardiac Fibrosis: Non-coding RNAs as Effectual Players. Front. Cardiovasc. Med. 2021, 7, 588347. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.; Molkentin, J.D. Myofibroblasts: Trust your heart and let fate decide. J. Mol. Cell Cardiol. 2014, 70, 9–18. [Google Scholar] [CrossRef]
- Travers, J.G.; Kamal, F.A.; Robbins, J.; Yutzey, K.E.; Blaxall, B.C. Cardiac Fibrosis: The Fibroblast Awakens. Circ. Res. 2016, 118, 1021–1040. [Google Scholar] [CrossRef]
- Yao, P.; Fox, P.L. Aminoacyl-tRNA synthetases in medicine and disease. EMBO Mol. Med. 2013, 5, 332–343. [Google Scholar] [CrossRef]
- Arif, A.; Yao, P.; Terenzi, F.; Jia, J.; Ray, P.S.; Fox, P.L. The GAIT translational control system. Wiley Interdiscip. Rev. RNA 2018, 9, e1441. [Google Scholar] [CrossRef]
- Mendes, M.I.; Gutierrez Salazar, M.; Guerrero, K.; Thiffault, I.; Salomons, G.S.; Gauquelin, L.; Tran, L.T.; Forget, D.; Gauthier, M.-S.; Waisfisz, Q.; et al. Bi-allelic Mutations in EPRS, Encoding the Glutamyl-Prolyl-Aminoacyl-tRNA Synthetase, Cause a Hypomyelinating Leukodystrophy. Am. J. Hum. Genet. 2018, 102, 676–684. [Google Scholar] [CrossRef]
- Wu, J.; Subbaiah, K.C.V.; Xie, L.H.; Jiang, F.; Khor, E.-S.; Mickelsen, D.; Myers, J.R.; Tang, W.H.W.; Yao, P. Glutamyl-Prolyl-tRNA Synthetase Regulates Proline-Rich Pro-Fibrotic Protein Synthesis During Cardiac Fibrosis. Circ. Res. 2020, 127, 827–846. [Google Scholar] [CrossRef]
- McKenna, W.J.; Maron, B.J.; Thiene, G. Classification, Epidemiology, and Global Burden of Cardiomyopathies. Circ. Res. 2017, 121, 722–730. [Google Scholar] [CrossRef]
- McNally, E.M.; Mestroni, L. Dilated Cardiomyopathy: Genetic Determinants and Mechanisms. Circ. Res. 2017, 121, 731–748. [Google Scholar] [CrossRef] [PubMed]
- Gay, D.M.; Lund, A.H.; Jansson, M.D. Translational control through ribosome heterogeneity and functional specialization. Trends Biochem. Sci. 2022, 47, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Huo, Y.; He, X.; Yao, L.; Zhang, H.; Cui, Y.; Xiao, H.; Xie, W.; Zhang, D.; Wang, Y.; et al. A male germ-cell-specific ribosome controls male fertility. Nature 2022, 612, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Yang, L.; Shi, R.; Qi, Y.; Zhang, X.; Qi, H. Proteostasis regulated by testis-specific ribosomal protein RPL39L maintains mouse spermatogenesis. iScience 2021, 24, 103396. [Google Scholar] [CrossRef]
- Shi, Z.; Fujii, K.; Kovary, K.M.; Genuth, N.R.; Röst, H.L.; Teruel, M.N.; Barna, M. Heterogeneous Ribosomes Preferentially Translate Distinct Subpools of mRNAs Genome-wide. Mol. Cell. 2017, 67, 71–83.e7. [Google Scholar] [CrossRef]
- Gerst, J.E. Pimp My Ribosome: Ribosomal Protein Paralogs Specify Translational Control. Trends Genet. 2018, 34, 832–845. [Google Scholar] [CrossRef]
- Ferretti, M.B.; Karbstein, K. Does functional specialization of ribosomes really exist? RNA 2019, 25, 521–538. [Google Scholar] [CrossRef]
- Meskauskas, A.; Dinman, J.D. Ribosomal protein L3: Gatekeeper to the A-site. Mol Cell. 2007, 25, 877–888. [Google Scholar] [CrossRef]
- Litviňuková, M.; Talavera-López, C.; Maatz, H.; Reichart, D.; Worth, C.L.; Lindberg, E.L.; Kanda, M.; Polanski, K.; Heinig, M.; Lee, M.; et al. Cells of the adult human heart. Nature 2020, 588, 466–472. [Google Scholar] [CrossRef]
- Gupta, V.; Warner, J.R. Ribosome-omics of the human ribosome. RNA 2014, 20, 1004–1013. [Google Scholar] [CrossRef]
- Van Raay, T.J.; Connors, T.D.; Klinger, K.W.; Landes, G.M.; Burn, T.C. A novel ribosomal protein L3-like gene (RPL3L) maps to the autosomal dominant polycystic kidney disease gene region. Genomics 1996, 37, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Nannapaneni, H.; Ghaleb, S.; Arya, S.; Gajula, V.; Taylor, M.B.; Das, B.B. Further Evidence of Autosomal Recessive Inheritance of RPL3L Pathogenic Variants with Rapidly Progressive Neonatal Dilated Cardiomyopathy. J. Cardiovasc. Dev. Dis. 2022, 9, 65. [Google Scholar] [CrossRef]
- Shiraishi, C.; Matsumoto, A.; Ichihara, K.; Yamamoto, T.; Yokoyama, T.; Mizoo, T.; Hatano, A.; Matsumoto, M.; Tanaka, Y.; Matsuura-Suzuki, E.; et al. RPL3L-containing ribosomes determine translation elongation dynamics required for cardiac function. Nat. Commun. 2023, 14, 2131. [Google Scholar] [CrossRef]
- Ikeuchi, K.; Izawa, T.; Inada, T. Recent Progress on the Molecular Mechanism of Quality Controls Induced by Ribosome Stalling. Front. Genet. 2018, 9, 743. [Google Scholar] [CrossRef]
- Milenkovic, I.; Santos Vieira, H.G.; Lucas, M.C.; Ruiz-Orera, J.; Patone, G.; Kesteven, S.; Wu, J.; Feneley, M.; Espadas, G.; Sabidó, E.; et al. Dynamic interplay between RPL3- and RPL3L-containing ribosomes modulates mitochondrial activity in the mammalian heart. Nucleic Acids Res. 2023, 51, 5301–5324. [Google Scholar] [CrossRef] [PubMed]
- Grimes, K.M.; Prasad, V.; Huo, J.; Kuwabara, Y.; Vanhoutte, D.; Baldwin, T.A.; Bowers, S.L.K.; Johansen, A.K.Z.; Sargent, M.A.; Lin, S.-C.J.; et al. Rpl3l gene deletion in mice reduces heart weight over time. Front. Physiol. 2023, 14, 1054169. [Google Scholar] [CrossRef] [PubMed]
- Wolozin, B.; Ivanov, P. Stress granules and neurodegeneration. Nat. Rev. Neurosci. 2019, 20, 649–666. [Google Scholar] [CrossRef]
- Protter, D.S.W.; Parker, R. Principles and Properties of Stress Granules. Trends Cell Biol. 2016, 26, 668–679. [Google Scholar] [CrossRef]
- Lennermann, D.; Backs, J.; van den Hoogenhof, M.M.G. New Insights in RBM20 Cardiomyopathy. Curr. Heart Fail. Rep. 2020, 17, 234–246. [Google Scholar] [CrossRef]
- Schneider, J.W.; Oommen, S.; Qureshi, M.Y.; Goetsch, S.C.; Pease, D.R.; Sundsbak, R.S.; Guo, W.; Sun, M.; Sun, H.; Kuroyanagi, H.; et al. Dysregulated ribonucleoprotein granules promote cardiomyopathy in RBM20 gene-edited pigs. Nat. Med. 2020, 26, 1788. [Google Scholar] [CrossRef]
- Arrell, D.K. Delineating RBM20 regulation of alternative splicing in dilated cardiomyopathy. Circ. Cardiovasc. Genet. 2014, 7, 732–733. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eschenhagen, T.; Bolli, R.; Braun, T.; Field, L.J.; Fleischmann, B.K.; Frisén, J.; Giacca, M.; Hare, J.M.; Houser, S.; Lee, R.T.; et al. Cardiomyocyte Regeneration: A Consensus Statement. Circulation 2017, 136, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Geuens, T.; Bouhy, D.; Timmerman, V. The hnRNP family: Insights into their role in health and disease. Hum. Genet. 2016, 135, 851–867. [Google Scholar] [CrossRef] [PubMed]
- Backlund, M.; Stein, F.; Rettel, M.; Schwarzl, T.; Perez-Perri, J.I.; Brosig, A.; Zhou, Y.; Neu-Yilik, G.; Hentze, M.W.; Kulozik, A.E. Plasticity of nuclear and cytoplasmic stress responses of RNA-binding proteins. Nucleic Acids Res. 2020, 48, 4725–4740. [Google Scholar] [CrossRef]
- Martino, F.; Varadarajan, N.M.; Perestrelo, A.R.; Hejret, V.; Durikova, H.; Vukic, D.; Horvath, V.; Cavalieri, F.; Caruso, F.; Albihlal, W.S.; et al. The mechanical regulation of RNA binding protein hnRNPC in the failing heart. Sci. Transl. Med. 2022, 14, eabo5715. [Google Scholar] [CrossRef]
- Taegtmeyer, H.; Wilson, C.R.; Razeghi, P.; Sharma, S. Metabolic energetics and genetics in the heart. Ann. N. Y. Acad. Sci. 2005, 1047, 208–218. [Google Scholar] [CrossRef]
- Bae, J.; Salamon, R.J.; Brandt, E.B.; Paltzer, W.G.; Zhang, Z.; Britt, E.C.; Hacker, T.A.; Fan, J.; Mahmoud, A.I. Malonate Promotes Adult Cardiomyocyte Proliferation and Heart Regeneration. Circulation 2021, 143, 1973–1986. [Google Scholar] [CrossRef]
- Wu, J.; Subbaiah, K.C.V.; Hedaya, O.; Chen, S.; Munger, J.; Tang, W.H.W.; Yan, C.; Yao, P. FAM210A regulates mitochondrial translation and maintains cardiac mitochondrial homeostasis. Cardiovasc. Res. 2023, 119, 2441–2457. [Google Scholar] [CrossRef]
- Vind, A.C.; Genzor, A.V.; Bekker-Jensen, S. Ribosomal stress-surveillance: Three pathways is a magic number. Nucleic Acids Res. 2020, 48, 10648–10661. [Google Scholar] [CrossRef]
- Gao, F.; Liang, T.; Lu, Y.W.; Pu, L.; Fu, X.; Dong, X.; Hong, T.; Zhang, F.; Liu, N.; Zhou, Y.; et al. Reduced Mitochondrial Protein Translation Promotes Cardiomyocyte Proliferation and Heart Regeneration. Circulation 2023, 148, 1887–1906. [Google Scholar] [CrossRef]
- Gao, F.; Liang, T.; Lu, Y.W.; Fu, X.; Dong, X.; Pu, L.; Hong, T.; Zhou, Y.; Zhang, Y.; Liu, N.; et al. A defect in mitochondrial protein translation influences mitonuclear communication in the heart. Nat. Commun. 2023, 14, 1595. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.M.Y.; McMurray, J.J.V.; Lorenzo-Almorós, A.; Kristensen, S.L.; Sattar, N.; Jhund, P.S.; Petrie, M.C. Diabetic cardiomyopathy. Heart. 2019, 105, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Hill, M.A.; Sowers, J.R. Diabetic Cardiomyopathy: An Update of Mechanisms Contributing to This Clinical Entity. Circ. Res. 2018, 122, 624–638. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.G. Mitochondrial dysfunction in diabetic cardiomyopathy. Biochim. Biophys. Acta 2011, 1813, 1351–1359. [Google Scholar] [CrossRef]
- Saliminejad, K.; Khorram Khorshid, H.R.; Soleymani Fard, S.; Ghaffari, S.H. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J. Cell Physiol. 2019, 234, 5451–5465. [Google Scholar] [CrossRef]
- Zhang, X.; Zuo, X.; Yang, B.; Li, Z.; Xue, Y.; Zhou, Y.; Huang, J.; Zhao, X.; Zhou, J.; Yan, Y.; et al. MicroRNA directly enhances mitochondrial translation during muscle differentiation. Cell 2014, 158, 607–619. [Google Scholar] [CrossRef]
- Jakubik, D.; Fitas, A.; Eyileten, C.; Jarosz-Popek, J.; Nowak, A.; Czajka, P.; Wicik, Z.; Sourij, H.; Siller-Matula, J.M.; De Rosa, S.; et al. MicroRNAs and long non-coding RNAs in the pathophysiological processes of diabetic cardiomyopathy: Emerging biomarkers and potential therapeutics. Cardiovasc. Diabetol. 2021, 20, 55. [Google Scholar] [CrossRef]
- Pant, T.; Dhanasekaran, A.; Fang, J.; Bai, X.; Bosnjak, Z.J.; Liang, M.; Ge, Z.-D. Current status and strategies of long noncoding RNA research for diabetic cardiomyopathy. BMC Cardiovasc. Disord. 2018, 18, 197. [Google Scholar] [CrossRef]
- Tatekoshi, Y.; Tanno, M.; Kouzu, H.; Abe, K.; Miki, T.; Kuno, A.; Yano, T.; Ishikawa, S.; Ohwada, W.; Sato, T.; et al. Translational regulation by miR-301b upregulates AMP deaminase in diabetic hearts. J. Mol. Cell Cardiol. 2018, 119, 138–146. [Google Scholar] [CrossRef]
- Xu, L.; Chen, W.; Ma, M.; Chen, A.; Tang, C.; Zhang, C.; Cai, L. Microarray profiling analysis identifies the mechanism of miR-200b-3p/mRNA-CD36 affecting diabetic cardiomyopathy via peroxisome proliferator activated receptor-γ signaling pathway. J. Cell Biochem. 2019, 120, 5193–5206. [Google Scholar] [CrossRef]
- Zhan, J.; Jin, K.; Xie, R.; Fan, J.; Tang, Y.; Chen, C.; Li, H.; Wang, D.W. AGO2 Protects Against Diabetic Cardiomyopathy by Activating Mitochondrial Gene Translation. Circulation 2024, 149, 1102–1120. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Fan, J.; Zhao, Y.; Zhang, X.; Dai, B.; Zhan, J.; Yin, Z.; Nie, X.; Fu, X.-D.; Chen, C.; et al. Nuclear miR-320 Mediates Diabetes-Induced Cardiac Dysfunction by Activating Transcription of Fatty Acid Metabolic Genes to Cause Lipotoxicity in the Heart. Circ. Res. 2019, 125, 1106–1120. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wang, Q.; Yang, Y.; Near, D.; Wang, H.; Colon, M.; Nguyen, C.; Slattery, C.; Keepers, B.; Farber, G.; et al. Translational landscape of direct cardiac reprogramming reveals a role of Ybx1 in repressing cardiac fate acquisition. Nat. Cardiovasc. Res. 2023, 2, 1060–1077. [Google Scholar] [CrossRef]
- Wu, X.; Cao, Y.; Nie, J.; Liu, H.; Lu, S.; Hu, X.; Zhu, J.; Zhao, X.; Chen, J.; Chen, X.; et al. Genetic and pharmacological inhibition of Rheb1-mTORC1 signaling exerts cardioprotection against adverse cardiac remodeling in mice. Am. J. Pathol. 2013, 182, 2005–2014. [Google Scholar] [CrossRef]
- Jiang, S.-L.; Mo, J.-L.; Peng, J.; Lei, L.; Yin, J.-Y.; Zhou, H.-H.; Liu, Z.-Q.; Hong, W.-X. Targeting translation regulators improves cancer therapy. Genomics 2021, 113, 1247–1256. [Google Scholar] [CrossRef]
- Pallet, N.; Legendre, C. Adverse events associated with mTOR inhibitors. Expert Opin. Drug Saf. 2013, 12, 177–186. [Google Scholar] [CrossRef]
- Tardif, J.-C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef]
- Liang, X.-H.; Sun, H.; Shen, W.; Wang, S.; Yao, J.; Migawa, M.T.; Bui, H.-H.; Damle, S.S.; Riney, S.; Graham, M.J.; et al. Antisense oligonucleotides targeting translation inhibitory elements in 5′ UTRs can selectively increase protein levels. Nucleic Acids Res. 2017, 45, 9528–9546. [Google Scholar] [CrossRef]
- Radke, M.H.; Badillo-Lisakowski, V.; Britto-Borges, T.; Kubli, D.A.; Jüttner, R.; Parakkat, P.; Carballo, J.L.; Hüttemeister, J.; Liss, M.; Hansen, A.; et al. Therapeutic inhibition of RBM20 improves diastolic function in a murine heart failure model and human engineered heart tissue. Sci. Transl. Med. 2021, 13, eabe8952. [Google Scholar] [CrossRef]
- Lauffer, M.C.; van Roon-Mom, W.; Aartsma-Rus, A. Possibilities and limitations of antisense oligonucleotide therapies for the treatment of monogenic disorders. Commun. Med. 2024, 4, 6. [Google Scholar] [CrossRef]

| Protein | Function | Related Disease Process | References |
|---|---|---|---|
| PABPC1 | Translational initiation and recruitment of the 40S ribosomal subunit | Cardiomyocyte Hypertrophy | [30] |
| mTORC1 | Promoting interactions between translational components during initiation and regulation of elongation through downstream effectors | Cardiomyocyte Hypertrophy | [35,36,37] |
| IRE1α | Coordination of the assembly of the translational initiation complex (through interactions with eIF3 and eIF4G) | Cardiomyocyte Hypertrophy | [29] |
| TIP30 | Inhibition of elongation factor 1A1 (eEF1A1) to slow translational elongation and protein synthesis | Cardiomyocyte Hypertrophy | [44] |
| KIF5B | Spatial positioning of translational machinery in response to phenylephrine-induced hypertrophy | Cardiomyocyte Hypertrophy | [28] |
| FLCN | Inhibition of the protein synthesis pathway (mTOR) during hypertrophic growth | Cardiomyocyte Hypertrophy | [17] |
| YBX1 | RNA- and DNA-binding protein involved in transcription, translation, and mRNA stability | Cardiomyocyte Hypertrophy and Fibroblast–Cardiomyocyte Reprogramming | [41,104] |
| RBM20 | RNA-binding protein and splicing factor with a role in stress granule formation | Dilated Cardiomyopathy | [80,81,82] |
| RPL3L | Paralog of RPL3. Component of the 60S ribosomal subunit. Regulates ribosomal collisions and interactions between ribosomes and mitochondria | Dilated Cardiomyopathy | [74,76,77] |
| MRPS5 | Regulation of mitochondrial mRNA translation; component of the small subunit of the mitochondrial ribosome | Myocardial Infarction | [91,92] |
| FAM210A | Anchoring of TUFM for upregulation of mitochondrial protein expression | Myocardial Infarction | [60,89] |
| hnRNPC | Regulation of splicing. In the diseased heart: Regulation of localized translation at Z-discs | Myocardial Infarction | [86] |
| AGO2 | Recruitment of translation factor (TUFM) to promote mitochondrial protein translation | Diabetic Cardiomyopathy | [102] |
| EPRS | Synthesis of aminoacyl tRNAs and regulation of pro-fibrotic protein synthesis | Fibrosis | [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsoy, S.; Liu, J. Regulation of Protein Synthesis at the Translational Level: Novel Findings in Cardiovascular Biology. Biomolecules 2025, 15, 692. https://doi.org/10.3390/biom15050692
Tsoy S, Liu J. Regulation of Protein Synthesis at the Translational Level: Novel Findings in Cardiovascular Biology. Biomolecules. 2025; 15(5):692. https://doi.org/10.3390/biom15050692
Chicago/Turabian StyleTsoy, Sergey, and Jiandong Liu. 2025. "Regulation of Protein Synthesis at the Translational Level: Novel Findings in Cardiovascular Biology" Biomolecules 15, no. 5: 692. https://doi.org/10.3390/biom15050692
APA StyleTsoy, S., & Liu, J. (2025). Regulation of Protein Synthesis at the Translational Level: Novel Findings in Cardiovascular Biology. Biomolecules, 15(5), 692. https://doi.org/10.3390/biom15050692







