Regulatory Mechanism of DHCR7 Gene Expression by Estrogen in Chicken Granulosa Cells of Pre-Hierarchical Follicles
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sample Collection
2.2. Culture and Treatment of Granulosa Cells
2.3. Bioinformatics Analysis
2.4. Plasmid Construction
2.5. Real-Time Quantitative PCR (RT-qPCR)
2.6. Protein Extraction and Western Blotting
2.7. Dual-Luciferase Reporter Gene Analysis
2.8. CUT&RUN-qPCR
2.9. MeRIP-RT-qPCR
2.10. Statistical Analysis
3. Results
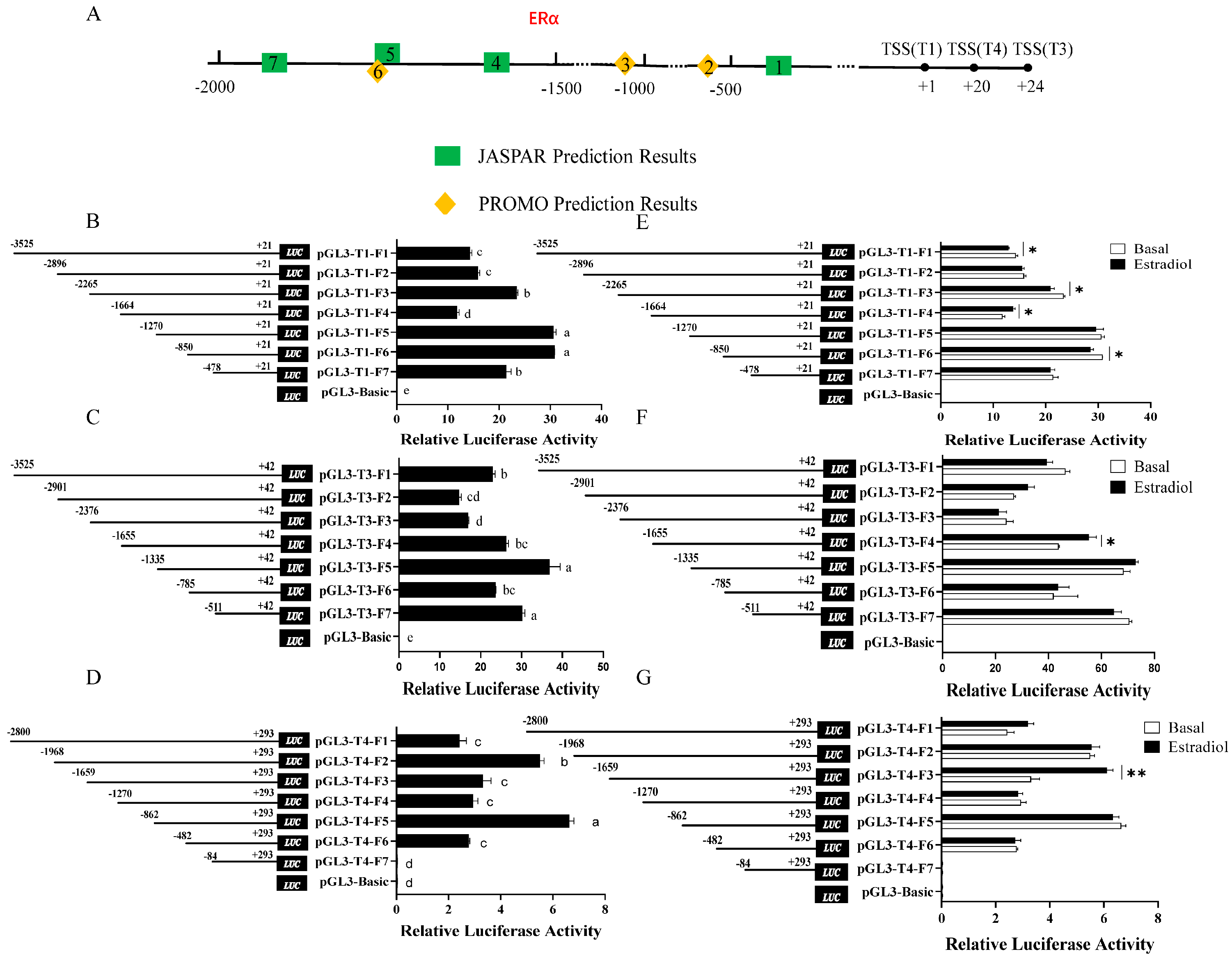
3.1. Critical Regulatory Segments of the T1, T3, and T4 Promoters and Regions Responsive to Estrogen Stimulation of DHCR7 in Chicken Pre-GCs
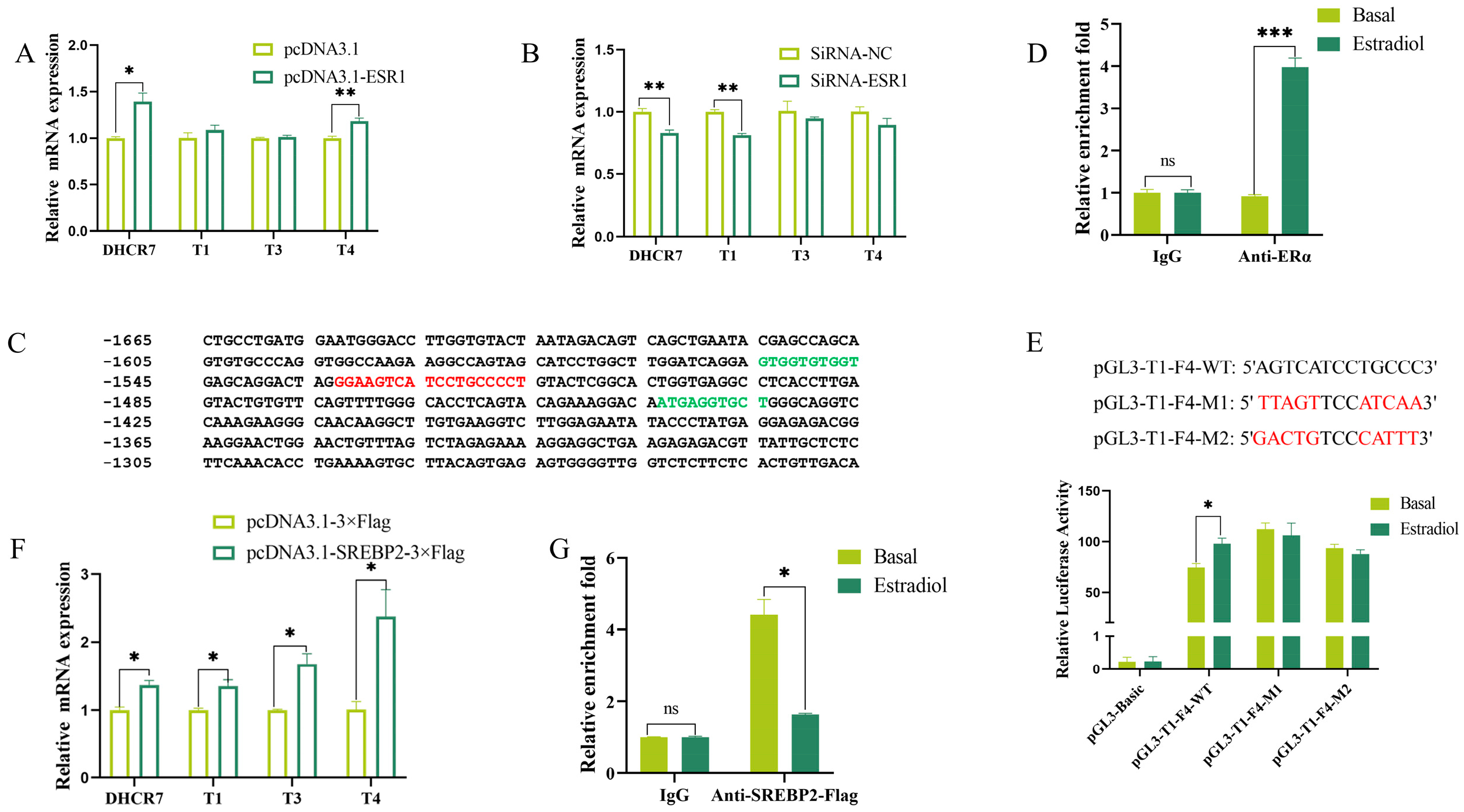
3.2. ER and SREBP2 Promote the Expression of DHCR7 by Binding to the Promoter Regions of T1, T3, and T4 in Chicken Pre-GCs
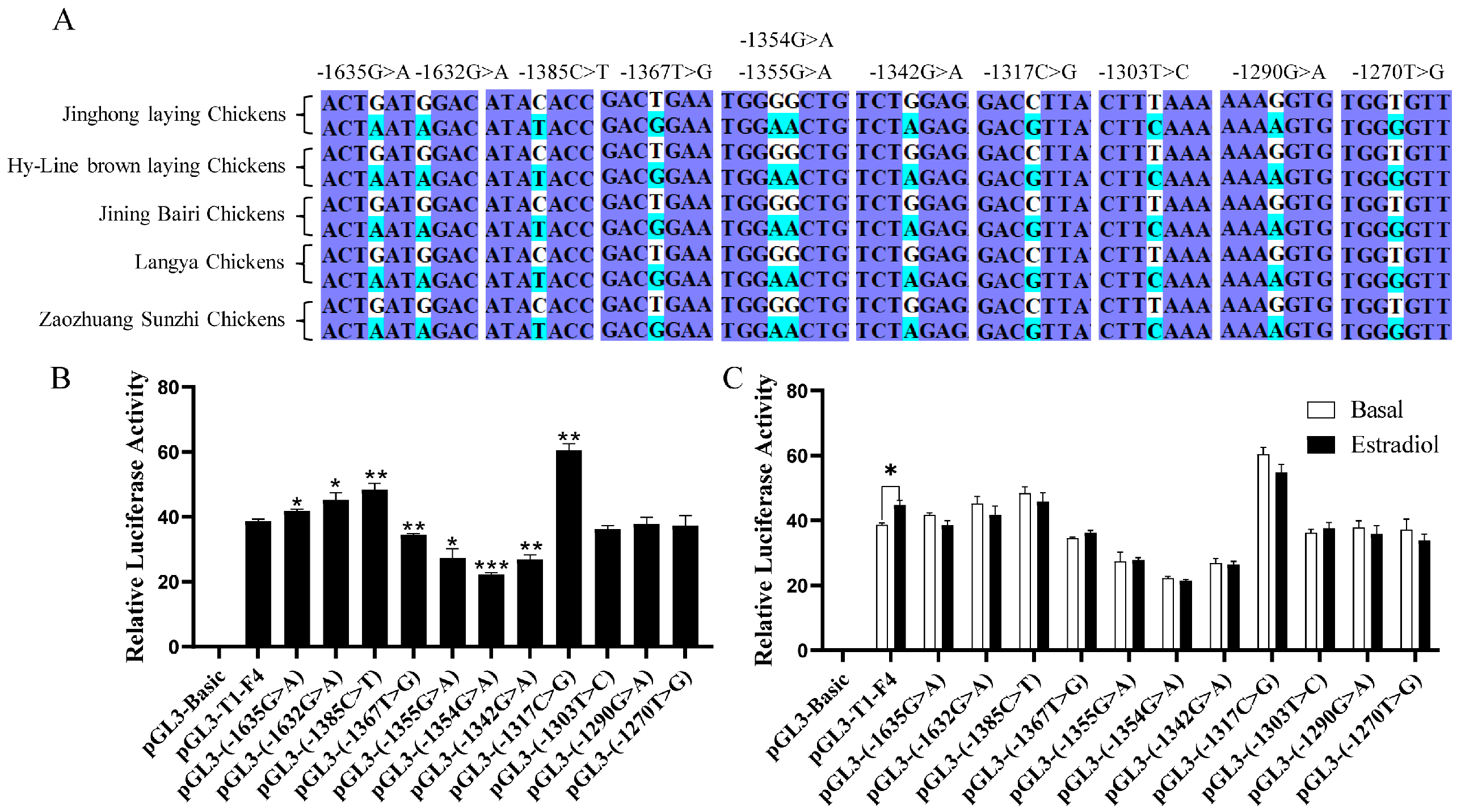
3.3. Genetic Polymorphism of DHCR7 and Its Response to Estrogen in Chicken Pre-GCs
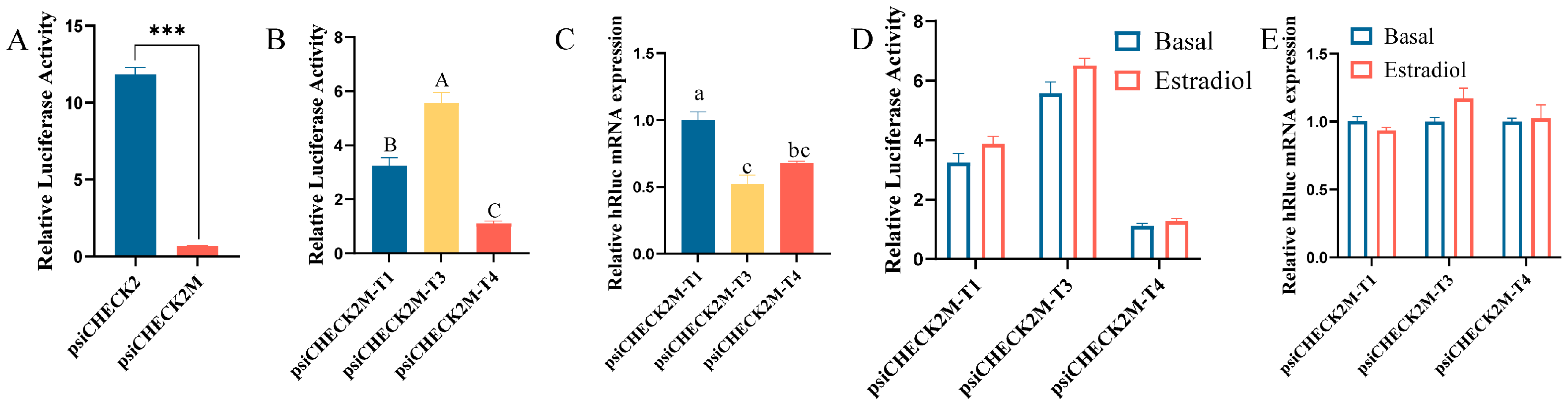
3.4. Effects of 5′UTRs on DHCR7 Expression in Chicken Pre-GCs
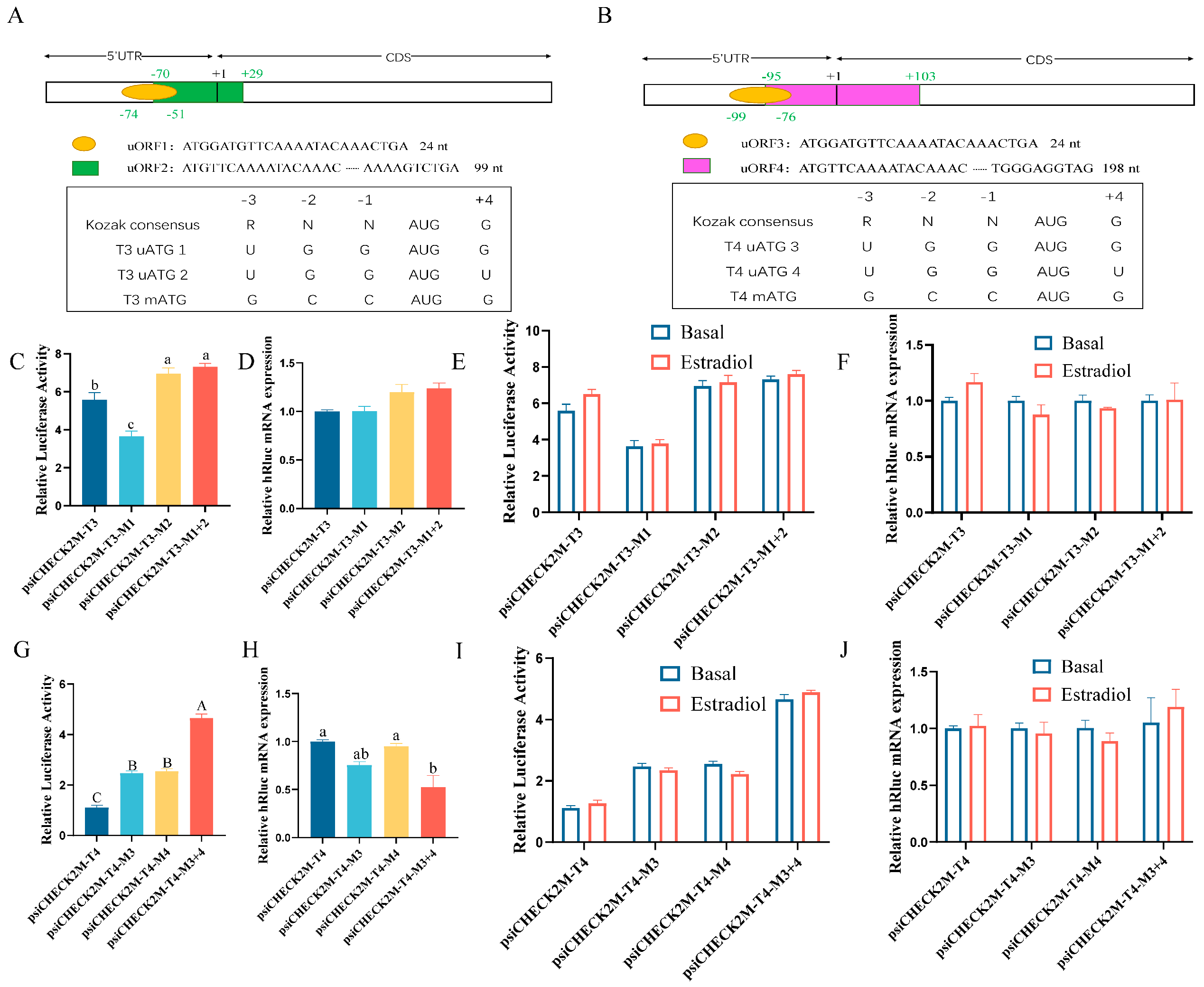
3.5. Effect of uORFs in 5′UTR on DHCR7 Expression in Chicken Pre-GCs
3.6. Effect of m6A Modification in 5′UTR on DHCR7 Expression in Chicken Pre-GCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DHCR7 | 7-Dehydrocholesterol reductase |
| T1, T3 and T4 | Three DHCR7 transcript variants |
| SREBP2 | Sterol-regulatory element binding protein 2 |
| uORFs | Upstream open reading frames |
| m6A | N6-methyladenosine modification |
| Pre-GCs | Granulosa cells of pre-hierarchical follicles |
| 5′UTR | 5′ untranslated region |
| mORF | Main open reading frame |
| PBS | Phosphate-buffered saline |
| siRNAs | Small interfering RNA |
| ESR1 | Estrogen receptor 1 |
| ESR2 | Estrogen receptor 2 |
| ERα | Estrogen receptor α |
| ERβ | Estrogen receptor β |
| hRluc | Humanized Renilla luciferase |
| PVDF | Polyvinylidene fluoride |
| SEM | Standard error of the mean |
| ER | Estrogen receptor |
| TSS | Transcription start site |
| ERE | Estrogen response element |
| SNP | Single nucleotide polymorphism |
| CDS | Coding sequence |
| Post-GCs | Granulosa cells of hierarchical follicles |
| SCAP | SREBP cleavage-activating protein |
References
- Duan, Y.; Gong, K.; Xu, S.; Zhang, F.; Meng, X.; Han, J. Regulation of Cholesterol Homeostasis in Health and Diseases: From Mechanisms to Targeted Therapeutics. Signal Transduct. Target. Ther. 2022, 7, 265. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.L. Ovarian Follicle Selection and Granulosa Cell Differentiation. Poult. Sci. 2014, 94, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhong, C.; Sun, Y.; Kang, L.; Jiang, Y. Identification of Genes Involved in Chicken Follicle Selection by ONT Sequencing on Granulosa Cells. Front. Genet. 2023, 13, 1090603. [Google Scholar] [CrossRef]
- Shefer, S.; Salen, G.; Honda, A.; Batta, A.K.; Nguyen, L.B.; Tint, G.S.; Ioannou, Y.A.; Desnick, R. Regulation of Rat Hepatic 3β-Hydroxysterol Δ7-Reductase: Substrate Specificity, Competitive and Non-Competitive Inhibition, and Phosphorylation/Dephosphorylation. J. Lipid Res. 1998, 39, 2471–2476. [Google Scholar] [CrossRef]
- Eleftheriou, A.; Ong, K.K.; Hughes, I.A.; Petry, C.J. Leptin and IGF-1 in Infancy Are Associated With Variants in DHCR7 and CYP2R1 That Relate With Type 1 Diabetes and 25OHD. J. Clin. Endocrinol. Metab. 2023, 108, E1394–E1402. [Google Scholar] [CrossRef] [PubMed]
- Fliesler, S.J.; Xu, L. Oxysterols and Retinal Degeneration in a Rat Model of Smith-Lemli-Opitz Syndrome: Implications for an Improved Therapeutic Intervention. Molecules 2018, 23, 2720. [Google Scholar] [CrossRef]
- Horton, J.D.; Shah, N.A.; Warrington, J.A.; Anderson, N.N.; Park, S.W.; Brown, M.S.; Goldstein, J.L. Combined Analysis of Oligonucleotide Microarray Data from Transgenic and Knockout Mice Identifies Direct SREBP Target Genes. Proc. Natl. Acad. Sci. USA 2003, 100, 12027–12032. [Google Scholar] [CrossRef]
- Prabhu, A.V.; Luu, W.; Sharpe, L.J.; Brown, A.J. Cholesterol-Mediated Degradation of 7-Dehydrocholesterol Reductase Switches the Balance from Cholesterol to Vitamin D Synthesis. J. Biol. Chem. 2016, 291, 8363–8376. [Google Scholar] [CrossRef]
- Dewailly, D.; Robin, G.; Peigne, M.; Decanter, C.; Pigny, P.; Catteau-Jonard, S. Interactions between Androgens, FSH, Anti-Mullerian Hormone and Estradiol during Folliculogenesis in the Human Normal and Polycystic Ovary. Hum. Reprod. Update 2016, 22, 709–724. [Google Scholar] [CrossRef]
- Guo, M.; Li, Y.; Chen, Y.; Guo, X.; Yuan, Z.; Jiang, Y. Genome-Wide Mapping of Estrogen Receptor α Binding Sites by ChIP-Seq to Identify Genes Related to Sexual Maturity in Hens. Gene 2018, 642, 32–42. [Google Scholar] [CrossRef]
- Liu, T.; Huang, Y.; Lin, H. Estrogen Disorders: Interpreting the Abnormal Regulation of Aromatase in Granulosa Cells (Review). Int. J. Mol. Med. 2021, 47, 73. [Google Scholar] [CrossRef]
- Young, S.K.; Wek, R.C. Upstream Open Reading Frames Differentially Regulate Genespecific Translation in the Integrated Stress Response. J. Biol. Chem. 2016, 291, 16927–16935. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Liang, Y.; Yu, Q.; Hu, L.; Li, H.; Zhang, Z.; Xu, X. Analysis of Human Upstream Open Reading Frames and Impact on Gene Expression. Hum. Genet. 2015, 134, 605–612. [Google Scholar] [CrossRef]
- Zaccara, S.; Ries, R.J.; Jaffrey, S.R. Reading, Writing and Erasing MRNA Methylation. Nat. Rev. Mol. Cell Biol. 2019, 20, 608–624. [Google Scholar] [CrossRef]
- Su, X.; Lu, R.; Qu, Y.; Mu, D. Methyltransferase-like 3 Mediated RNA M6A Modifications in the Reproductive System: Potentials for Diagnosis and Therapy. J. Cell. Mol. Med. 2024, 28, e18128. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Ma, Z.; Wang, Z.; Li, H.; Wang, Y.; Tian, Y.; Li, D.; Liu, X. Evolution, Expression Profile, Regulatory Mechanism, and Functional Verification of EBP-Like Gene in Cholesterol Biosynthetic Process in Chickens (Gallus Gallus). Front. Genet. 2021, 11, 587546. [Google Scholar] [CrossRef] [PubMed]
- Regassa, A.; Kim, K.K. Transcriptome Analysis of Hen Preadipocytes Treated with an Adipogenic Cocktail (DMIOA) with or without 20(S)-Hydroxylcholesterol. BMC Genom. 2015, 16, 91. [Google Scholar] [CrossRef]
- Hu, L.; Li, D.; Wei, Q.; Kang, L.; Sun, Y.; Jiang, Y. Characterization of a Novel IGFBP-2 Transcript in the Ovarian Granulosa Cells of Chicken Follicles: MRNA Expression, Function and Effect of Reproductive Hormones and IGF1. Poult. Sci. 2024, 103, 104501. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2-ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Prabhu, A.V.; Sharpe, L.J.; Brown, A.J. The Sterol-Based Transcriptional Control of Human 7-Dehydrocholesterol Reductase (DHCR7): Evidence of a Cooperative Regulatory Program in Cholesterol Synthesis. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2014, 1841, 1431–1439. [Google Scholar] [CrossRef]
- Onagbesan, O.; Bruggeman, V.; Decuypere, E. Intra-Ovarian Growth Factors Regulating Ovarian Function in Avian Species: A Review. Anim. Reprod. Sci. 2009, 111, 121–140. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Fang, C.; Li, J.; Mo, C.; Wang, Y.; Li, J. Transcriptomic Diversification of Granulosa Cells during Follicular Development in Chicken. Sci. Rep. 2019, 9, 5462. [Google Scholar] [CrossRef]
- Bahadur, U.; Ganjam, G.K.; Vasudevan, N.; Kondaiah, P. Estrogen Regulation of Chicken Riboflavin Carrier Protein Gene Is Mediated by ERE Half Sites without Direct Binding of Estrogen Receptor. Mol. Cell. Endocrinol. 2005, 231, 1–11. [Google Scholar] [CrossRef]
- Klinge, C.M. Estrogen Receptor Interaction with Estrogen Response Elements. Nucleic Acids Res. 2001, 29, 2905–2919. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, R.; Nguyen, D.; Rocha, W.; White, J.H.; Mader, S. Diversity in the Mechanisms of Gene Regulation by Estrogen Receptors. BioEssays 2002, 24, 244–254. [Google Scholar] [CrossRef]
- Duan, R.; Ginsburg, E.; Vonderhaar, B.K. Estrogen Stimulates Transcription from the Human Prolactin Distal Promoter through AP1 and Estrogen Responsive Elements in T47D Human Breast Cancer Cells. Mol. Cell. Endocrinol. 2008, 281, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Brüning, J.C.; Lingohr, P.; Gillette, J.; Hanstein, B.; Avci, H.; Krone, W.; Müller-Wieland, D.; Kotzka, J. Estrogen Receptor-α and Sp1 Interact in the Induction of the Low Density Lipoprotein-Receptor. J. Steroid Biochem. Mol. Biol. 2003, 86, 113–121. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Rawson, R.B.; Brown, M.S. Mutant Mammalian Cells as Tools to Delineate the Sterol Regulatory Element-Binding Protein Pathway for Feedback Regulation of Lipid Synthesis. Arch. Biochem. Biophys. 2002, 397, 139–148. [Google Scholar] [CrossRef]
- Yang, T.; Espenshade, P.J.; Wright, M.E.; Yabe, D.; Gong, Y.; Aebersold, R.; Goldstein, J.L.; Brown, M.S. Crucial Step in Cholesterol Homeostasis: Sterols Promote Binding of SCAP to INSIG-1, a Membrane Protein That Facilitates Retention of SREBPs in ER. Cell 2002, 110, 489–500. [Google Scholar] [CrossRef]
- Guo, Z.; Cao, H.; Zhao, J.; Bai, S.; Peng, W.; Li, J.; Sun, L.; Chen, L.; Lin, Z.; Shi, C. A Natural UORF Variant Confers Phosphorus Acquisition Diversity in Soybean. Nat. Commun. 2022, 13, 3796. [Google Scholar] [CrossRef]
- Yang, S.Y.; Lu, W.C.; Ko, S.S.; Sun, C.M.; Hung, J.C.; Chiou, T.J. Upstream Open Reading Frame and Phosphate-Regulated Expression of Rice OsNLA1 Controls Phosphate Transport and Reproduction. Plant Physiol. 2020, 182, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Scholz, A.; Rappl, P.; Böffinger, N.; Mota, A.C.; Brüne, B.; Schmid, T. Translation of TNFAIP2 Is Tightly Controlled by Upstream Open Reading Frames. Cell. Mol. Life Sci. 2020, 77, 2017–2027. [Google Scholar] [CrossRef] [PubMed]
- Filatova, A.Y.; Vasilyeva, T.A.; Marakhonov, A.V.; Sukhanova, N.V.; Voskresenskaya, A.A.; Zinchenko, R.A.; Skoblov, M.Y. Upstream ORF Frameshift Variants in the PAX6 5ʹUTR Cause Congenital Aniridia. Hum. Mutat. 2021, 42, 1053–1065. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.D.; Patil, D.P.; Zhou, J.; Zinoviev, A.; Skabkin, M.A.; Elemento, O.; Pestova, T.V.; Qian, S.-B.; Jaffrey, S.R. 5′ UTR m6A Promotes Cap-Independent Translation. Cell 2015, 163, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Dahl, J.A.; Niu, Y.; Fedorcsak, P.; Huang, C.M.; Li, C.J.; Vågbø, C.B.; Shi, Y.; Wang, W.L.; Song, S.H. ALKBH5 Is a Mammalian RNA Demethylase That Impacts RNA Metabolism and Mouse Fertility. Mol. Cell. 2013, 49, 18–29. [Google Scholar] [CrossRef]
- Zeng, Y.; Luo, Y.; Zhao, K.; Liu, S.; Wu, K.; Wu, Y.; Du, K.; Pan, W.; Dai, Y.; Liu, Y.; et al. m6A-Mediated Induction of 7-Dehydrocholesterol Reductase Stimulates Cholesterol Synthesis and cAMP Signaling to Promote Bladder Cancer Metastasis. Cancer Res. 2024, 84, 3402–3418. [Google Scholar] [CrossRef]
- Li, K.; Huang, W.; Wang, Z.; Nie, Q. m6A Demethylase FTO Regulate CTNNB1 to Promote Adipogenesis of Chicken Preadipocyte. J. Anim. Sci. Biotechnol. 2022, 13, 147. [Google Scholar] [CrossRef]
- Xue, X.; Li, C.; Chen, S.; Zheng, Y.; Zhang, F.; Xu, Y. 17β-estradiol Promotes the Progression of Temporomandibular Joint Osteoarthritis by Regulating the FTO/IGF2BP1/m6A-NLRC5 Axis. Immun. Inflamm. Dis. 2024, 12, e1361. [Google Scholar] [CrossRef]
- Nomura, A.; Iwasaki, Y.; Saito, M.; Aoki, Y.; Yamamori, E.; Ozaki, N.; Tachikawa, K.; Mutsuga, N.; Morishita, M.; Yoshida, M.; et al. Involvement of Upstream Open Reading Frames in Regulation of Rat V1b Vasopressin Receptor Expression. Am. J. Physiol.-Endocrinol. Metab. 2001, 280, 780–787. [Google Scholar] [CrossRef]
- Li, Z.; Fu, Y.; Shen, J.; Liang, J. Upstream Open Reading Frame Mediated Translation of Wnk8 Is Required for Aba Response in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 10683. [Google Scholar] [CrossRef]
- Bronson, M.W.; Hillenmeyer, S.; Park, R.W.; Brodsky, A.S. Estrogen Coordinates Translation and Transcription, Revealing a Role for NRSF in Human Breast Cancer Cells. Mol. Endocrinol. 2010, 24, 1120–1135. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barbosa, C.; Peixeiro, I.; Romão, L. Gene Expression Regulation by Upstream Open Reading Frames and Human Disease. PLoS Genet. 2013, 9, e1003529. [Google Scholar] [CrossRef] [PubMed]
- Fard, S.S.; Holz, M.K. Regulation of MRNA Translation by Estrogen Receptor in Breast Cancer. Steroids 2023, 200, 109316. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Hu, L.; Wei, Q.; Kang, L.; Sun, Y.; Jiang, Y. Regulatory Mechanism of DHCR7 Gene Expression by Estrogen in Chicken Granulosa Cells of Pre-Hierarchical Follicles. Biomolecules 2025, 15, 668. https://doi.org/10.3390/biom15050668
Li D, Hu L, Wei Q, Kang L, Sun Y, Jiang Y. Regulatory Mechanism of DHCR7 Gene Expression by Estrogen in Chicken Granulosa Cells of Pre-Hierarchical Follicles. Biomolecules. 2025; 15(5):668. https://doi.org/10.3390/biom15050668
Chicago/Turabian StyleLi, Dandan, Longxiao Hu, Qingqing Wei, Li Kang, Yi Sun, and Yunliang Jiang. 2025. "Regulatory Mechanism of DHCR7 Gene Expression by Estrogen in Chicken Granulosa Cells of Pre-Hierarchical Follicles" Biomolecules 15, no. 5: 668. https://doi.org/10.3390/biom15050668
APA StyleLi, D., Hu, L., Wei, Q., Kang, L., Sun, Y., & Jiang, Y. (2025). Regulatory Mechanism of DHCR7 Gene Expression by Estrogen in Chicken Granulosa Cells of Pre-Hierarchical Follicles. Biomolecules, 15(5), 668. https://doi.org/10.3390/biom15050668






