Molecular Insights into Oxidative-Stress-Mediated Cardiomyopathy and Potential Therapeutic Strategies
Abstract
1. Introduction
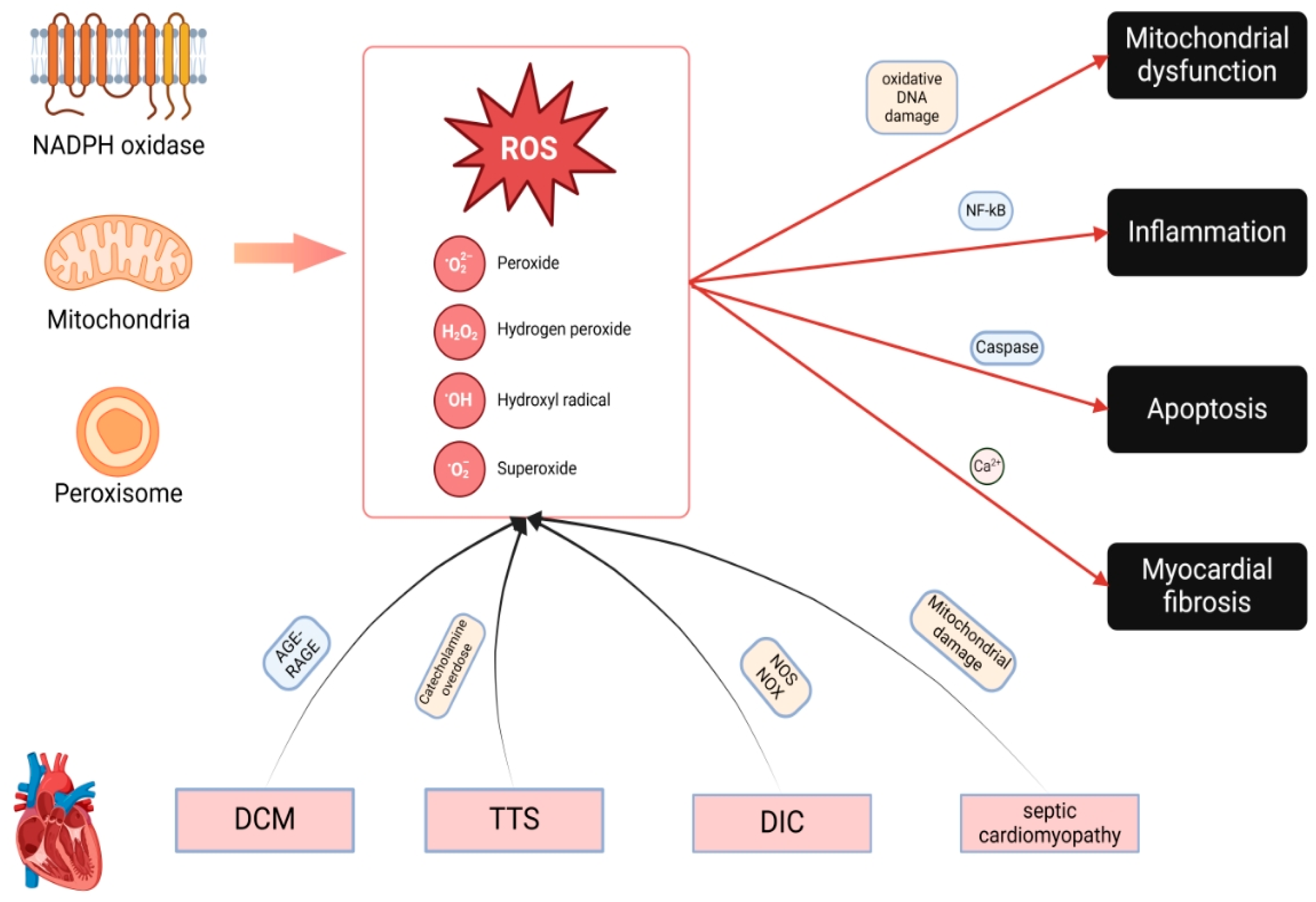
2. Sources and Biomarkers of OS in Cardiomyopathies
2.1. ROS-Generating Systems Contributing to Cardiomyopathy
2.1.1. Mechanisms of ROS Generation in Cardiomyocytes
2.1.2. Loss of Antioxidant Defense
2.1.3. Synergy of ROS Sources
2.2. Biomarkers of OS in Cardiomyopathy
2.2.1. Lipid Peroxidation Markers
2.2.2. Protein Oxidation Markers
2.2.3. DNA Damage Markers
2.2.4. Emerging Redox Biomarkers and Their Clinical Significance
2.3. Pathophysiological Effects of ROS on Cardiomyocyte Function
3. OS in Specific Cardiomyopathy Subtypes
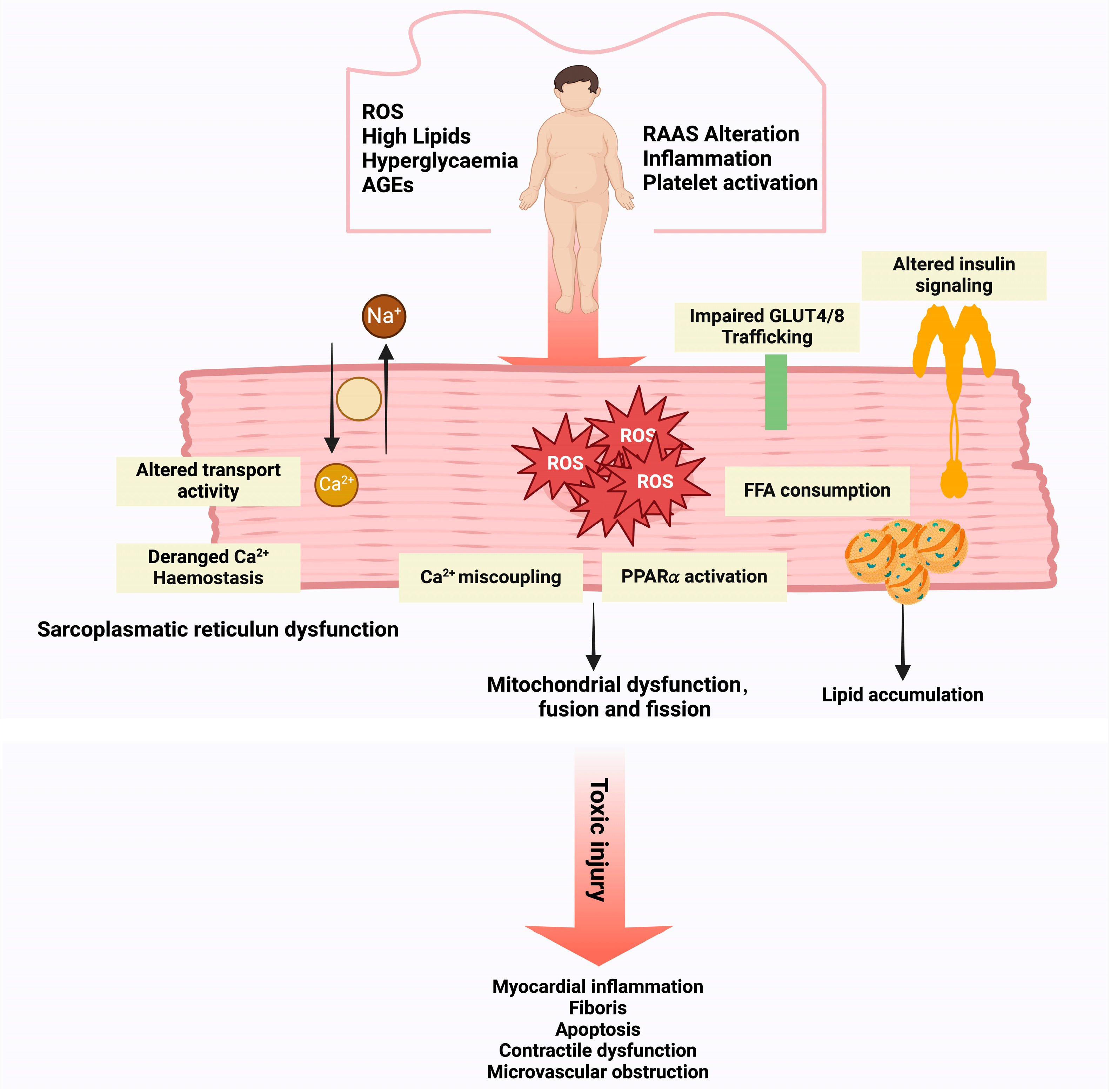
3.1. OS in DCM: Molecular Mechanisms and Pathophysiological Insights
3.1.1. OS and DCM
3.1.2. Pathophysiologic Effects of Nox on DCM
3.1.3. OS and Diastolic Dysfunction in DCM
3.1.4. Immune-Cell-Derived OS in DCM
3.2. OS and TTS
3.3. OS and DIC
3.4. OS and Septic Cardiomyopathy
3.5. Other OS-Associated Cardiac Disorders
4. Targeting OS: Therapeutic Strategies and Future Directions
4.1. Ferroptosis and OS in Cardiomyopathies
4.2. Antioxidant-Based Therapeutic Strategies
4.3. Iron Chelation and Redox Homeostasis
4.4. The Role and Mechanism of Ferroptosis in AD
4.5. Iron-Chelator-Related Targets and Drugs
4.6. Novel Drug Targets and Emerging Therapies
4.7. Mechanisms of Antioxidant Treatment Failure
5. Outlook and Future Perspective
6. Conclusions
7. Limitations
Author Contributions
Funding
Conflicts of Interest
References
- Maron, B.J.; Towbin, J.A.; Thiene, G.; Antzelevitch, C.; Corrado, D.; Arnett, D.; Moss, A.J.; Seidman, C.E.; Young, J.B. Contemporary definitions and classification of the cardiomyopathies: An American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 2006, 113, 1807–1816. [Google Scholar] [CrossRef]
- Elliott, P.; Andersson, B.; Arbustini, E.; Bilinska, Z.; Cecchi, F.; Charron, P.; Dubourg, O.; Kühl, U.; Maisch, B.; McKenna, W.J.; et al. Classification of the cardiomyopathies: A position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2008, 29, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Pan, B.; Lv, X.; Chen, C.; Li, K.; Wang, Y.; Liu, J. Ferroptosis: Roles and molecular mechanisms in diabetic cardiomyopathy. Front. Endocrinol. 2023, 14, 1140644. [Google Scholar] [CrossRef] [PubMed]
- Wallace, K.B.; Sardão, V.A.; Oliveira, P.J. Mitochondrial Determinants of Doxorubicin-Induced Cardiomyopathy. Circ. Res. 2020, 126, 926–941. [Google Scholar] [CrossRef]
- Liu, S.; Chong, W. Roles of LncRNAs in Regulating Mitochondrial Dysfunction in Septic Cardiomyopathy. Front. Immunol. 2021, 12, 802085. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Liu, C.; Zhu, P.; Li, Y. Novel Insights Into Molecular Mechanism of Mitochondria in Diabetic Cardiomyopathy. Front. Physiol. 2020, 11, 609157. [Google Scholar] [CrossRef]
- Xiao, M.; Guo, W.; Zhang, C.; Zhu, Y.; Li, Z.; Shao, C.; Jiang, J.; Yang, Z.; Zhang, J.; Lin, L. Jian Pi Sheng Sui Gao (JPSSG) alleviation of skeletal myoblast cell apoptosis, oxidative stress, and mitochondrial dysfunction to improve cancer-related fatigue in an AMPK-SIRT1- and HIF-1-dependent manner. Ann. Transl. Med. 2023, 11, 156. [Google Scholar] [CrossRef]
- Wei, C.; Shi, M.; Dong, S.; Li, Z.; Zhao, B.; Liu, D.; Li, G.; Cen, J.; Yu, L.; Liang, X.; et al. SIRT5-related lysine demalonylation of GSTP1 contributes to cardiomyocyte pyroptosis suppression in diabetic cardiomyopathy. Int. J. Biol. Sci. 2024, 20, 585–605. [Google Scholar] [CrossRef]
- Korovesis, D.; Rubio-Tomás, T.; Tavernarakis, N. Oxidative Stress in Age-Related Neurodegenerative Diseases: An Overview of Recent Tools and Findings. Antioxidants 2023, 12, 131. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Niculescu, A.G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef]
- Zhou, T.; Prather, E.R.; Garrison, D.E.; Zuo, L. Interplay between ROS and Antioxidants during Ischemia-Reperfusion Injuries in Cardiac and Skeletal Muscle. Int. J. Mol. Sci. 2018, 19, 417. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Budde, H.; Hassoun, R.; Mügge, A.; Kovács, Á.; Hamdani, N. Current Understanding of Molecular Pathophysiology of Heart Failure With Preserved Ejection Fraction. Front. Physiol. 2022, 13, 928232. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Wang, Z.; Tang, H.; Jia, Z.; Ji, X.; Yang, X.; Jiang, W. Bardoxolone Methyl Ameliorates Myocardial Ischemia/Reperfusion Injury by Activating the Nrf2/HO-1 Signaling Pathway. Cardiovasc. Ther. 2023, 2023, 5693732. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef]
- Guo, W.; Liu, Y.; Ji, X.; Guo, S.; Xie, F.; Chen, Y.; Zhou, K.; Zhang, H.; Peng, F.; Wu, D.; et al. Mutational signature of mtDNA confers mechanistic insight into oxidative metabolism remodeling in colorectal cancer. Theranostics 2023, 13, 324–338. [Google Scholar] [CrossRef]
- Holmström, K.M.; Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 411–421. [Google Scholar] [CrossRef]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, oxidants, and aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Bocsan, I.C.; Măgureanu, D.C.; Pop, R.M.; Levai, A.M.; Macovei, Ș.O.; Pătrașca, I.M.; Chedea, V.S.; Buzoianu, A.D. Antioxidant and Anti-Inflammatory Actions of Polyphenols from Red and White Grape Pomace in Ischemic Heart Diseases. Biomedicines 2022, 10, 2337. [Google Scholar] [CrossRef]
- Dewanjee, S.; Vallamkondu, J.; Kalra, R.S.; John, A.; Reddy, P.H.; Kandimalla, R. Autophagy in the diabetic heart: A potential pharmacotherapeutic target in diabetic cardiomyopathy. Ageing Res. Rev. 2021, 68, 101338. [Google Scholar] [CrossRef]
- Nan, W.; Yin, J.; Hao, W.; Meng, H.; Wu, J.; Yin, X.; Wu, H. Cardamonin protects against diabetic cardiomyopathy by activating macrophage NRF2 signaling through molecular interaction with KEAP1. Food Funct. 2024, 15, 11083–11095. [Google Scholar] [CrossRef]
- Yuan Hsieh, D.J.; Islam, M.N.; Kuo, W.W.; Shibu, M.A.; Lai, C.H.; Lin, P.Y.; Lin, S.Z.; Chen, M.Y.; Huang, C.Y. A combination of isoliquiritigenin with Artemisia argyi and Ohwia caudata water extracts attenuates oxidative stress, inflammation, and apoptosis by modulating Nrf2/Ho-1 signaling pathways in SD rats with doxorubicin-induced acute cardiotoxicity. Environ. Toxicol. 2023, 38, 3026–3042. [Google Scholar] [CrossRef]
- Zhang, W.; Qian, S.; Kang, P.; Shi, C. Curcumin Attenuates Ferroptosis-Induced Myocardial Injury in Diabetic Cardiomyopathy through the Nrf2 Pathway. Cardiovasc. Ther. 2022, 2022, 3159717. [Google Scholar] [CrossRef]
- Fukai, T.; Ushio-Fukai, M. Cross-Talk between NADPH Oxidase and Mitochondria: Role in ROS Signaling and Angiogenesis. Cells 2020, 9, 1849. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Mondragón, R.; Lozhkin, A.; Vendrov, A.E.; Runge, M.S.; Isom, L.L.; Madamanchi, N.R. NADPH Oxidases and Oxidative Stress in the Pathogenesis of Atrial Fibrillation. Antioxidants 2023, 12, 1833. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, N.; Yi, D.; Xiao, Y.; Li, X.; Shao, B.; Wu, Z.; Bai, J.; Shi, X.; Wu, C.; et al. ROS-mediated ferroptosis and pyroptosis in cardiomyocytes: An update. Life Sci. 2025, 370, 123565. [Google Scholar] [CrossRef]
- Zhang, C.; Chang, X.; Zhao, D.; He, Y.; Dong, G.; Gao, L. Decoding interaction between mitochondria and endoplasmic reticulum in ischemic myocardial injury: Targeting natural medicines. Front. Pharmacol. 2025, 16, 1536773. [Google Scholar] [CrossRef]
- Liu, S.; Chen, L.; Shang, Y. CEACAM5 exacerbates asthma by inducing ferroptosis and autophagy in airway epithelial cells through the JAK/STAT6-dependent pathway. Redox Rep. 2025, 30, 2444755. [Google Scholar] [CrossRef]
- Annaz, H.; Cacciola, F.; Kounnoun, A.; Bouayad, N.; Rharrabe, K. Impact of essential oils on enzymes activity, reserve products, biomarkers, and gene expression of Tribolium castaneum. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2025, 293, 110167. [Google Scholar] [CrossRef]
- Shen, C.; Zhang, Q. Forsythiaside A alleviates myocardial injury in streptozotocin-induced diabetes via endoplasmic reticulum stress-NLRP3 inflammasome pathway. Int. Immunopharmacol. 2025, 147, 113956. [Google Scholar] [CrossRef]
- Tan, X.; Chen, Y.F.; Zou, S.Y.; Wang, W.J.; Zhang, N.N.; Sun, Z.Y.; Xian, W.; Li, X.R.; Tang, B.; Wang, H.J.; et al. ALDH2 attenuates ischemia and reperfusion injury through regulation of mitochondrial fusion and fission by PI3K/AKT/mTOR pathway in diabetic cardiomyopathy. Free Radic. Biol. Med. 2023, 195, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Farromeque Vásquez, S.C.; Arbeláez, L.G.; Rojano, B.; Schinella, G.; Maiztegui, B.; Francini, F. Isoespintanol Isolated from Oxandra cf. xylopioides (Annonaceae) Leaves Ameliorates Pancreatic Dysfunction and Improves Insulin Sensitivity in Murine Model of Fructose-Induced Prediabetes. Plants 2025, 14, 745. [Google Scholar] [CrossRef] [PubMed]
- Momot, K.; Krauz, K.; Czarzasta, K.; Tomaszewski, J.; Dobruch, J.; Żera, T.; Zarębiński, M.; Cudnoch-Jędrzejewska, A.; Wojciechowska, M. Post-myocardial infarction heart failure and long-term high-fat diet: Cardiac endoplasmic reticulum stress and unfolded protein response in Sprague Dawley rat model. PLoS ONE 2024, 19, e0308833. [Google Scholar] [CrossRef]
- Bhattarai, U.; Xu, R.; He, X.; Pan, L.; Niu, Z.; Wang, D.; Zeng, H.; Chen, J.X.; Clemmer, J.S.; Chen, Y. High selenium diet attenuates pressure overload-induced cardiopulmonary oxidative stress, inflammation, and heart failure. Redox Biol. 2024, 76, 103325. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Sengar, A.S.; Lye, A.; Kumar, P.; Mukherjee, S.; Kumar, D.; Das, P.; Chatterjee, S.; Stewart, A.; Maity, B. FNDC5/irisin mitigates the cardiotoxic impacts of cancer chemotherapeutics by modulating ROS-dependent and -independent mechanisms. Redox Biol. 2025, 80, 103527. [Google Scholar] [CrossRef]
- Dag, Y.; Yildirim, S.; Sengul, E.; Aykurt, F.; Gok, M.; Cinar, A. Therapeutic role of melatonin on acrylamide-induced neurotoxicity via reducing ER stress, inflammation, and apoptosis in a rat model. BMC Pharmacol. Toxicol. 2025, 26, 57. [Google Scholar] [CrossRef]
- Karakus, S.; Dogan, H.O.; Özkaraca, M. The protective effect of Cloprostenol on ischemia/reperfusion injury in rat ovary: Histopathologic and immunohistochemically evaluation: An experimental study. Transpl. Immunol. 2024, 86, 102108. [Google Scholar] [CrossRef]
- Abo El Gheit, R.E.; Soliman, N.A.; Nagla, S.A.; El-Sayed, R.M.; Badawi, G.A.; Emam, M.N.; Abdel Ghafar, M.T.; Ibrahim, M.A.A.; Elswaidy, N.R.M.; Radwan, D.A.; et al. Melatonin epigenetic potential on testicular functions and fertility profile in varicocele rat model is mediated by silent information regulator 1. Br. J. Pharmacol. 2022, 179, 3363–3381. [Google Scholar] [CrossRef]
- Tanwar, S.S.; Dwivedi, S.; Khan, S.; Sharma, S. Cardiomyopathies and a brief insight into DOX-induced cardiomyopathy. Egypt. Heart J. 2025, 77, 29. [Google Scholar] [CrossRef]
- Michel, L.Y.M.; Esfahani, H.; De Mulder, D.; Verdoy, R.; Ambroise, J.; Roelants, V.; Bouchard, B.; Fabian, N.; Savary, J.; Dewulf, J.P.; et al. An NRF2/β3-Adrenoreceptor Axis Drives a Sustained Antioxidant and Metabolic Rewiring Through the Pentose-Phosphate Pathway to Alleviate Cardiac Stress. Circulation 2025, 151, 1312–1328. [Google Scholar] [CrossRef] [PubMed]
- Ran, Y.; Guo, Z.; Zhang, L.; Li, H.; Zhang, X.; Guan, X.; Cui, X.; Chen, H.; Cheng, M. Mitochondria-derived peptides: Promising microproteins in cardiovascular diseases (Review). Mol. Med. Rep. 2025, 31, 127. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Han, C.; Shi, Z.; Guan, X.; Cheng, L.; Wang, L.; Zou, W.; Liu, J. Umbilical mesenchymal stem cells mitigate T-cell compartments shift and Th17/Treg imbalance in acute ischemic stroke via mitochondrial transfer. Stem Cell Res. Ther. 2025, 16, 134. [Google Scholar] [CrossRef]
- Shah, A.S.; Sadayappan, S.; Urbina, E.M. Lipids: A Potential Molecular Pathway Towards Diastolic Dysfunction in Youth-Onset Type 2 Diabetes. Curr. Atheroscler. Rep. 2022, 24, 109–117. [Google Scholar] [CrossRef]
- Ren, J.; Wu, N.N.; Wang, S.; Sowers, J.R.; Zhang, Y. Obesity cardiomyopathy: Evidence, mechanisms, and therapeutic implications. Physiol. Rev. 2021, 101, 1745–1807. [Google Scholar] [CrossRef]
- Sánchez Milán, J.A.; Fernández-Rhodes, M.; Guo, X.; Mulet, M.; Ngan, S.C.; Iyappan, R.; Katoueezadeh, M.; Sze, S.K.; Serra, A.; Gallart-Palau, X. Trioxidized cysteine in the aging proteome mimics the structural dynamics and interactome of phosphorylated serine. Aging Cell 2024, 23, e14062. [Google Scholar] [CrossRef]
- Tai, P.; Chen, X.; Jia, G.; Chen, G.; Gong, L.; Cheng, Y.; Li, Z.; Wang, H.; Chen, A.; Zhang, G.; et al. WGX50 mitigates doxorubicin-induced cardiotoxicity through inhibition of mitochondrial ROS and ferroptosis. J. Transl. Med. 2023, 21, 823. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, S.; Terentyeva, R.; Martin, B.; Perger, F.; Li, J.; Stepanov, A.; Bonilla, I.M.; Knollmann, B.C.; Radwański, P.B.; Györke, S.; et al. Increased RyR2 activity is exacerbated by calcium leak-induced mitochondrial ROS. Basic Res. Cardiol. 2020, 115, 38. [Google Scholar] [CrossRef]
- Loescher, C.M.; Hobbach, A.J.; Linke, W.A. Titin (TTN): From molecule to modifications, mechanics, and medical significance. Cardiovasc. Res. 2022, 118, 2903–2918. [Google Scholar] [CrossRef]
- Dillmann, W.H. Diabetic Cardiomyopathy. Circ. Res. 2019, 124, 1160–1162. [Google Scholar] [CrossRef]
- Park, J.J. Epidemiology, Pathophysiology, Diagnosis and Treatment of Heart Failure in Diabetes. Diabetes Metab. J. 2021, 45, 146–157. [Google Scholar] [CrossRef]
- Ritchie, R.H.; Abel, E.D. Basic Mechanisms of Diabetic Heart Disease. Circ. Res. 2020, 126, 1501–1525. [Google Scholar] [CrossRef]
- Jankauskas, S.S.; Kansakar, U.; Varzideh, F.; Wilson, S.; Mone, P.; Lombardi, A.; Gambardella, J.; Santulli, G. Heart failure in diabetes. Metabolism 2021, 125, 154910. [Google Scholar] [CrossRef] [PubMed]
- Dubois-Deruy, E.; Peugnet, V.; Turkieh, A.; Pinet, F. Oxidative Stress in Cardiovascular Diseases. Antioxidants 2020, 9, 864. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; DeMarco, V.G.; Sowers, J.R. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat. Rev. Endocrinol. 2016, 12, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, X.; Tang, J.; Liu, C.; Zhang, Y.; Cai, C.; Du, Q. Sleep restriction exacerbates cardiac dysfunction in diabetic mice by causing cardiomyocyte death and fibrosis through mitochondrial damage. Cell Death Discov. 2024, 10, 446. [Google Scholar] [CrossRef]
- Godoy, J.A.; Rios, J.A.; Picón-Pagès, P.; Herrera-Fernández, V.; Swaby, B.; Crepin, G.; Vicente, R.; Fernández-Fernández, J.M.; Muñoz, F.J. Mitostasis, Calcium and Free Radicals in Health, Aging and Neurodegeneration. Biomolecules 2021, 11, 1012. [Google Scholar] [CrossRef]
- Yi, C.H.; Vakifahmetoglu-Norberg, H.; Yuan, J. Integration of apoptosis and metabolism. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 375–387. [Google Scholar] [CrossRef]
- Chen, Y.; Hua, Y.; Li, X.; Arslan, I.M.; Zhang, W.; Meng, G. Distinct Types of Cell Death and the Implication in Diabetic Cardiomyopathy. Front. Pharmacol. 2020, 11, 42. [Google Scholar] [CrossRef]
- Jia, G.; Whaley-Connell, A.; Sowers, J.R. Diabetic cardiomyopathy: A hyperglycaemia- and insulin-resistance-induced heart disease. Diabetologia 2018, 61, 21–28. [Google Scholar] [CrossRef]
- Vermot, A.; Petit-Härtlein, I.; Smith, S.M.E.; Fieschi, F. NADPH Oxidases (NOX): An Overview from Discovery, Molecular Mechanisms to Physiology and Pathology. Antioxidants 2021, 10, 890. [Google Scholar] [CrossRef] [PubMed]
- Smolka, C.; Schlösser, D.; Koentges, C.; Tarkhnishvili, A.; Gorka, O.; Pfeifer, D.; Bemtgen, X.; Asmussen, A.; Groß, O.; Diehl, P.; et al. Cardiomyocyte-specific miR-100 overexpression preserves heart function under pressure overload in mice and diminishes fatty acid uptake as well as ROS production by direct suppression of Nox4 and CD36. FASEB J. 2021, 35, e21956. [Google Scholar] [CrossRef]
- Visnagri, A.; Oexner, R.R.; Kmiotek-Wasylewska, K.; Zhang, M.; Zoccarato, A.; Shah, A.M. Nicotinamide Adenosine Dinucleotide Phosphate Oxidase-Mediated Signaling in Cardiac Remodeling. Antioxid. Redox Signal. 2023, 38, 371–387. [Google Scholar] [CrossRef] [PubMed]
- Alaeddine, L.M.; Harb, F.; Hamza, M.; Dia, B.; Mogharbil, N.; Azar, N.S.; Noureldein, M.H.; El Khoury, M.; Sabra, R.; Eid, A.A. Pharmacological regulation of cytochrome P450 metabolites of arachidonic acid attenuates cardiac injury in diabetic rats. Transl. Res. 2021, 235, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Hua, N.; Fu, X.; Pan, Y.; Li, B.; Li, X. Metformin Regulates the Expression of SK2 and SK3 in the Atria of Rats With Type 2 Diabetes Mellitus Through the NOX4/p38MAPK Signaling Pathway. J. Cardiovasc. Pharmacol. 2018, 72, 205–213. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, S.; Wang, X.; Liu, J.; Li, Y.; Wang, W.; Wang, S.; Wang, F. ShengMai-San Attenuates Cardiac Remodeling in Diabetic Rats by Inhibiting NOX-Mediated Oxidative Stress. Diabetes Metab. Syndr. Obes. 2021, 14, 647–657. [Google Scholar] [CrossRef]
- Tan, Y.Y.; Chen, L.X.; Fang, L.; Zhang, Q. Cardioprotective effects of polydatin against myocardial injury in diabetic rats via inhibition of NADPH oxidase and NF-κB activities. BMC Complement. Med. Ther. 2020, 20, 378. [Google Scholar] [CrossRef]
- Hussain, S.; Khan, A.W.; Akhmedov, A.; Suades, R.; Costantino, S.; Paneni, F.; Caidahl, K.; Mohammed, S.A.; Hage, C.; Gkolfos, C.; et al. Hyperglycemia Induces Myocardial Dysfunction via Epigenetic Regulation of JunD. Circ. Res. 2020, 127, 1261–1273. [Google Scholar] [CrossRef]
- Fan, L.; Xiao, Q.; Zhang, L.; Wang, X.; Huang, Q.; Li, S.; Zhao, X.; Li, Z. CAPE-pNO(2) attenuates diabetic cardiomyopathy through the NOX4/NF-κB pathway in STZ-induced diabetic mice. Biomed. Pharmacother. 2018, 108, 1640–1650. [Google Scholar] [CrossRef]
- Maalouf, R.M.; Eid, A.A.; Gorin, Y.C.; Block, K.; Escobar, G.P.; Bailey, S.; Abboud, H.E. Nox4-derived reactive oxygen species mediate cardiomyocyte injury in early type 1 diabetes. Am. J. Physiol. Cell Physiol. 2012, 302, C597–C604. [Google Scholar] [CrossRef]
- Liu, R.; Duan, T.; Yu, L.; Tang, Y.; Liu, S.; Wang, C.; Fang, W.J. Acid sphingomyelinase promotes diabetic cardiomyopathy via NADPH oxidase 4 mediated apoptosis. Cardiovasc. Diabetol. 2023, 22, 25. [Google Scholar] [CrossRef]
- Yang, X.; An, N.; Zhong, C.; Guan, M.; Jiang, Y.; Li, X.; Zhang, H.; Wang, L.; Ruan, Y.; Gao, Y.; et al. Enhanced cardiomyocyte reactive oxygen species signaling promotes ibrutinib-induced atrial fibrillation. Redox Biol. 2020, 30, 101432. [Google Scholar] [CrossRef] [PubMed]
- Olejnik, A.; Banaszkiewicz, M.; Krzywonos-Zawadzka, A.; Bil-Lula, I. The Klotho protein supports redox balance and metabolic functions of cardiomyocytes during ischemia/reperfusion injury. Cardiol. J. 2022, 29, 836–849. [Google Scholar] [CrossRef]
- Kayvanpour, E.; Sedaghat-Hamedani, F.; Li, D.T.; Miersch, T.; Weis, T.; Hoefer, I.; Frey, N.; Meder, B. Prognostic Value of Circulating Fibrosis Biomarkers in Dilated Cardiomyopathy (DCM): Insights into Clinical Outcomes. Biomolecules 2024, 14, 1137. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Dong, S.; Li, T.; Yang, F.; Yu, X.; Wu, J.; Zhong, X.; Zhao, Y.; Wang, L.; Xu, C.; et al. Exogenous Hydrogen Sulfide Attenuates Cardiac Fibrosis Through Reactive Oxygen Species Signal Pathways in Experimental Diabetes Mellitus Models. Cell. Physiol. Biochem. 2015, 36, 917–929. [Google Scholar] [CrossRef]
- Arozal, W.; Watanabe, K.; Veeraveedu, P.T.; Ma, M.; Thandavarayan, R.A.; Suzuki, K.; Tachikawa, H.; Kodama, M.; Aizawa, Y. Effects of angiotensin receptor blocker on oxidative stress and cardio-renal function in streptozotocin-induced diabetic rats. Biol. Pharm. Bull. 2009, 32, 1411–1416. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maeda, Y.; Inoguchi, T.; Takei, R.; Hendarto, H.; Ide, M.; Inoue, T.; Kobayashi, K.; Urata, H.; Nishiyama, A.; Takayanagi, R. Chymase inhibition prevents myocardial fibrosis through the attenuation of NOX4-associated oxidative stress in diabetic hamsters. J. Diabetes Investig. 2012, 3, 354–361. [Google Scholar] [CrossRef]
- Thandavarayan, R.A.; Watanabe, K.; Ma, M.; Gurusamy, N.; Veeraveedu, P.T.; Konishi, T.; Zhang, S.; Muslin, A.J.; Kodama, M.; Aizawa, Y. Dominant-negative p38alpha mitogen-activated protein kinase prevents cardiac apoptosis and remodeling after streptozotocin-induced diabetes mellitus. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H911–H919. [Google Scholar] [CrossRef]
- You, Q.; Wu, Z.; Wu, B.; Liu, C.; Huang, R.; Yang, L.; Guo, R.; Wu, K.; Chen, J. Naringin protects cardiomyocytes against hyperglycemia-induced injuries in vitro and in vivo. J. Endocrinol. 2016, 230, 197–214. [Google Scholar] [CrossRef]
- Kakoki, M.; Bahnson, E.M.; Hagaman, J.R.; Siletzky, R.M.; Grant, R.; Kayashima, Y.; Li, F.; Lee, E.Y.; Sun, M.T.; Taylor, J.M.; et al. Engulfment and cell motility protein 1 potentiates diabetic cardiomyopathy via Rac-dependent and Rac-independent ROS production. JCI Insight 2019, 4, e127660. [Google Scholar] [CrossRef]
- Rosenkranz, S. TGF-beta1 and angiotensin networking in cardiac remodeling. Cardiovasc. Res. 2004, 63, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Feng, J.; Yang, N.; Guo, Y.; Chen, C.; Qin, Q. Ginsenoside Rg3 attenuates angiotensin II-induced myocardial hypertrophy through repressing NLRP3 inflammasome and oxidative stress via modulating SIRT1/NF-κB pathway. Int. Immunopharmacol. 2021, 98, 107841. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Zhao, Q.; Xia, H.; Lyu, S.; Luo, J.; Fu, K.; Chen, R.; Yuan, W. Intracellular and extracellular Cyclophilin a promote cardiac fibrosis through TGF-β signaling in response to angiotensin II. Biochem. Pharmacol. 2024, 225, 116271. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.; Taimor, G.; Piper, H.M.; Schlüter, K.D. Redox-sensitive intermediates mediate angiotensin II-induced p38 MAP kinase activation, AP-1 binding activity, and TGF-beta expression in adult ventricular cardiomyocytes. FASEB J. 2001, 15, 2291–2293. [Google Scholar] [CrossRef]
- Rani, N.; Bharti, S.; Bhatia, J.; Nag, T.C.; Ray, R.; Arya, D.S. Chrysin, a PPAR-γ agonist improves myocardial injury in diabetic rats through inhibiting AGE-RAGE mediated oxidative stress and inflammation. Chem.-Biol. Interact. 2016, 250, 59–67. [Google Scholar] [CrossRef]
- Lozhkin, A.; Vendrov, A.E.; Ramos-Mondragón, R.; Canugovi, C.; Stevenson, M.D.; Herron, T.J.; Hummel, S.L.; Figueroa, C.A.; Bowles, D.E.; Isom, L.L.; et al. Mitochondrial oxidative stress contributes to diastolic dysfunction through impaired mitochondrial dynamics. Redox Biol. 2022, 57, 102474. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, J.; Liu, X.; Li, H.; Chang, C.; Gao, P.; Du, F.; Zhang, R. Tcap deficiency impedes striated muscle function and heart regeneration with elevated ROS and autophagy. Biochim. Biophys. Acta. Mol. Basis Dis. 2024, 1870, 167485. [Google Scholar] [CrossRef]
- Abudureyimu, M.; Luo, X.; Jiang, L.; Jin, X.; Pan, C.; Yu, W.; Ge, J.; Zhang, Y.; Ren, J. FBXL4 protects against HFpEF through Drp1-Mediated regulation of mitochondrial dynamics and the downstream SERCA2a. Redox Biol. 2024, 70, 103081. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, Z.; Zheng, C.; Wintergerst, K.A.; Keller, B.B.; Cai, L. Mechanisms of diabetic cardiomyopathy and potential therapeutic strategies: Preclinical and clinical evidence. Nat. Rev. Cardiol. 2020, 17, 585–607. [Google Scholar] [CrossRef]
- Saad, H.; Soliman, H.A.; Mahmoud, B.; Moneim, A.A.; Zaky, M.Y. The Pathogenic Role of Oxidative Stress, Cytokine Expression, and Impaired Hematological Indices in Diabetic Cardiovascular Diseases. Inflammation 2023, 46, 146–160. [Google Scholar] [CrossRef]
- Mlejnek, P.; Dolezel, P.; Kriegova, E.; Pastvova, N. N-acetylcysteine Can Induce Massive Oxidative Stress, Resulting in Cell Death with Apoptotic Features in Human Leukemia Cells. Int. J. Mol. Sci. 2021, 22, 12635. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Jaggers, R.M.; Gopalkrishna, S.; Dahdah, A.; Murphy, A.J.; Hanssen, N.M.J.; Nagareddy, P.R. Oxidative Stress in Neutrophils: Implications for Diabetic Cardiovascular Complications. Antioxid. Redox Signal. 2022, 36, 652–666. [Google Scholar] [CrossRef] [PubMed]
- Smolgovsky, S.; Bayer, A.L.; Kaur, K.; Sanders, E.; Aronovitz, M.; Filipp, M.E.; Thorp, E.B.; Schiattarella, G.G.; Hill, J.A.; Blanton, R.M.; et al. Impaired T cell IRE1α/XBP1 signaling directs inflammation in experimental heart failure with preserved ejection fraction. J. Clin. Investig. 2023, 133, e171874. [Google Scholar] [CrossRef] [PubMed]
- Teuber, J.P.; Essandoh, K.; Hummel, S.L.; Madamanchi, N.R.; Brody, M.J. NADPH Oxidases in Diastolic Dysfunction and Heart Failure with Preserved Ejection Fraction. Antioxidants 2022, 11, 1822. [Google Scholar] [CrossRef]
- Wenzl, F.A.; Ambrosini, S.; Mohammed, S.A.; Kraler, S.; Lüscher, T.F.; Costantino, S.; Paneni, F. Inflammation in Metabolic Cardiomyopathy. Front. Cardiovasc. Med. 2021, 8, 742178. [Google Scholar] [CrossRef]
- Hurst, R.T.; Prasad, A.; Askew, J.W., 3rd; Sengupta, P.P.; Tajik, A.J. Takotsubo cardiomyopathy: A unique cardiomyopathy with variable ventricular morphology. JACC. Cardiovasc. Imaging 2010, 3, 641–649. [Google Scholar] [CrossRef]
- Medeiros, K.; O’Connor, M.J.; Baicu, C.F.; Fitzgibbons, T.P.; Shaw, P.; Tighe, D.A.; Zile, M.R.; Aurigemma, G.P. Systolic and diastolic mechanics in stress cardiomyopathy. Circulation 2014, 129, 1659–1667. [Google Scholar] [CrossRef]
- Singh, T.; Khan, H.; Gamble, D.T.; Scally, C.; Newby, D.E.; Dawson, D. Takotsubo Syndrome: Pathophysiology, Emerging Concepts, and Clinical Implications. Circulation 2022, 145, 1002–1019. [Google Scholar] [CrossRef]
- Medina de Chazal, H.; Del Buono, M.G.; Keyser-Marcus, L.; Ma, L.; Moeller, F.G.; Berrocal, D.; Abbate, A. Stress Cardiomyopathy Diagnosis and Treatment: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 1955–1971. [Google Scholar] [CrossRef]
- Lyon, A.R.; Bossone, E.; Schneider, B.; Sechtem, U.; Citro, R.; Underwood, S.R.; Sheppard, M.N.; Figtree, G.A.; Parodi, G.; Akashi, Y.J.; et al. Current state of knowledge on Takotsubo syndrome: A Position Statement from the Taskforce on Takotsubo Syndrome of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2016, 18, 8–27. [Google Scholar] [CrossRef]
- Ghadri, J.R.; Wittstein, I.S.; Prasad, A.; Sharkey, S.; Dote, K.; Akashi, Y.J.; Cammann, V.L.; Crea, F.; Galiuto, L.; Desmet, W.; et al. International Expert Consensus Document on Takotsubo Syndrome (Part I): Clinical Characteristics, Diagnostic Criteria, and Pathophysiology. Eur. Heart J. 2018, 39, 2032–2046. [Google Scholar] [CrossRef] [PubMed]
- Ghadri, J.R.; Wittstein, I.S.; Prasad, A.; Sharkey, S.; Dote, K.; Akashi, Y.J.; Cammann, V.L.; Crea, F.; Galiuto, L.; Desmet, W.; et al. International Expert Consensus Document on Takotsubo Syndrome (Part II): Diagnostic Workup, Outcome, and Management. Eur. Heart J. 2018, 39, 2047–2062. [Google Scholar] [CrossRef] [PubMed]
- Ekenbäck, C.; Persson, J.; Tornvall, P.; Forsberg, L.; Spaak, J. Sympathetic nerve activity and response to physiological stress in Takotsubo syndrome. Clin. Auton. Res. 2024, 35, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.; Pasini, M.; Yun, S.; Gill, J.; Koirala, B. Genetic association between post-traumatic stress disorder and cardiovascular disease: A scoping review. J. Psychiatr. Res. 2024, 178, 331–348. [Google Scholar] [CrossRef]
- Ong, G.J.; Nguyen, T.H.; Kucia, A.; Liu, S.F.; Surikow, S.Y.; Girolamo, O.; Chong, C.R.; Chirkov, Y.Y.; Schenck-Gustafsson, K.; Frenneaux, M.P.; et al. Takotsubo Syndrome: Finally Emerging From the Shadows? Heart Lung Circ. 2021, 30, 36–44. [Google Scholar] [CrossRef]
- San-Millan, I.; Sparagna, G.C.; Chapman, H.L.; Warkins, V.L.; Chatfield, K.C.; Shuff, S.R.; Martinez, J.L.; Brooks, G.A. Chronic Lactate Exposure Decreases Mitochondrial Function by Inhibition of Fatty Acid Uptake and Cardiolipin Alterations in Neonatal Rat Cardiomyocytes. Front. Nutr. 2022, 9, 809485. [Google Scholar] [CrossRef]
- Thai, P.N.; Ren, L.; Xu, W.; Overton, J.; Timofeyev, V.; Nader, C.E.; Haddad, M.; Yang, J.; Gomes, A.V.; Hammock, B.D.; et al. Chronic Diclofenac Exposure Increases Mitochondrial Oxidative Stress, Inflammatory Mediators, and Cardiac Dysfunction. Cardiovasc. Drugs Ther. 2023, 37, 25–37. [Google Scholar] [CrossRef]
- Oda, S.; Kobayashi, S.; Nanno, T.; Ishiguchi, H.; Myoren, T.; Murakami, W.; Mochizuki, M.; Oda, T.; Okuda, S.; Yamada, J. Relationship between myocardial oxidative stress and cardiac sympathetic hyperactivity in patients with takotsubo cardiomyopathy. Bull. Yamaguchi Med. Sch. 2016, 63, 5–16. [Google Scholar]
- Nef, H.M.; Möllmann, H.; Troidl, C.; Kostin, S.; Böttger, T.; Voss, S.; Hilpert, P.; Krause, N.; Weber, M.; Rolf, A.; et al. Expression profiling of cardiac genes in Tako-Tsubo cardiomyopathy: Insight into a new cardiac entity. J. Mol. Cell. Cardiol. 2008, 44, 395–404. [Google Scholar] [CrossRef]
- Kobayashi, A.; Kang, M.I.; Watai, Y.; Tong, K.I.; Shibata, T.; Uchida, K.; Yamamoto, M. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol. Cell. Biol. 2006, 26, 221–229. [Google Scholar] [CrossRef]
- Yu, C.; Xiao, J.H. The Keap1-Nrf2 System: A Mediator between Oxidative Stress and Aging. Oxidative Med. Cell. Longev. 2021, 2021, 6635460. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Huang, Y.; Zhang, X.; Niu, Y.; Duan, Y.; Kan, H.; Zhang, R. Involvements of Nrf2 and oxidative stress in the ozone-elicited exacerbation in an allergic rhinitis model. Ecotoxicol. Environ. Saf. 2023, 255, 114822. [Google Scholar] [CrossRef] [PubMed]
- Münzel, T.; Templin, C.; Cammann, V.L.; Hahad, O. Takotsubo Syndrome: Impact of endothelial dysfunction and oxidative stress. Free Radic. Biol. Med. 2021, 169, 216–223. [Google Scholar] [CrossRef]
- Sha, W.; Zhao, B.; Wei, H.; Yang, Y.; Yin, H.; Gao, J.; Zhao, W.; Kong, W.; Ge, G.; Lei, T. Astragalus polysaccharide ameliorates vascular endothelial dysfunction by stimulating macrophage M2 polarization via potentiating Nrf2/HO-1 signaling pathway. Phytomedicine 2023, 112, 154667. [Google Scholar] [CrossRef] [PubMed]
- Vendrov, A.E.; Xiao, H.; Lozhkin, A.; Hayami, T.; Hu, G.; Brody, M.J.; Sadoshima, J.; Zhang, Y.Y.; Runge, M.S.; Madamanchi, N.R. Cardiomyocyte NOX4 regulates resident macrophage-mediated inflammation and diastolic dysfunction in stress cardiomyopathy. Redox Biol. 2023, 67, 102937. [Google Scholar] [CrossRef]
- Wenzel, P.; Knorr, M.; Kossmann, S.; Stratmann, J.; Hausding, M.; Schuhmacher, S.; Karbach, S.H.; Schwenk, M.; Yogev, N.; Schulz, E.; et al. Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation 2011, 124, 1370–1381. [Google Scholar] [CrossRef]
- Ueyama, T.; Kawabe, T.; Hano, T.; Tsuruo, Y.; Ueda, K.; Ichinose, M.; Kimura, H.; Yoshida, K. Upregulation of heme oxygenase-1 in an animal model of Takotsubo cardiomyopathy. Circ. J. 2009, 73, 1141–1146. [Google Scholar] [CrossRef]
- Wang, L.; He, C. Nrf2-mediated anti-inflammatory polarization of macrophages as therapeutic targets for osteoarthritis. Front. Immunol. 2022, 13, 967193. [Google Scholar] [CrossRef]
- Mao, S.; Luo, X.; Li, Y.; He, C.; Huang, F.; Su, C. Role of PI3K/AKT/mTOR Pathway Associated Oxidative Stress and Cardiac Dysfunction in Takotsubo Syndrome. Curr. Neurovascular Res. 2020, 17, 35–43. [Google Scholar] [CrossRef]
- Ago, T.; Kuroda, J.; Pain, J.; Fu, C.; Li, H.; Sadoshima, J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ. Res. 2010, 106, 1253–1264. [Google Scholar] [CrossRef]
- Zhang, Z.; Jin, S.; Teng, X.; Duan, X.; Chen, Y.; Wu, Y. Hydrogen sulfide attenuates cardiac injury in takotsubo cardiomyopathy by alleviating oxidative stress. Nitric Oxide 2017, 67, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Willis, B.C.; Salazar-Cantú, A.; Silva-Platas, C.; Fernández-Sada, E.; Villegas, C.A.; Rios-Argaiz, E.; González-Serrano, P.; Sánchez, L.A.; Guerrero-Beltrán, C.E.; García, N.; et al. Impaired oxidative metabolism and calcium mishandling underlie cardiac dysfunction in a rat model of post-acute isoproterenol-induced cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H467–H477. [Google Scholar] [CrossRef] [PubMed]
- Thuny, F.; Naidoo, J.; Neilan, T.G. Cardiovascular complications of immune checkpoint inhibitors for cancer. Eur. Heart J. 2022, 43, 4458–4468. [Google Scholar] [CrossRef] [PubMed]
- Raisi-Estabragh, Z.; Kobo, O.; Freeman, P.; Petersen, S.E.; Kolman, L.; Miller, R.J.H.; Roguin, A.; Van Spall, H.G.C.; Vuong, J.; Yang, E.H.; et al. Temporal trends in disease-specific causes of cardiovascular mortality amongst patients with cancer in the USA between 1999 and 2019. Eur. Heart J. Qual. Care Clin. Outcomes 2022, 9, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shi, S.; Dai, Y. Research progress of therapeutic drugs for doxorubicin-induced cardiomyopathy. Biomed. Pharmacother. 2022, 156, 113903. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.; Bawa-Khalfe, T.; Lu, L.S.; Lyu, Y.L.; Liu, L.F.; Yeh, E.T. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat. Med. 2012, 18, 1639–1642. [Google Scholar] [CrossRef]
- Ichikawa, Y.; Ghanefar, M.; Bayeva, M.; Wu, R.; Khechaduri, A.; Naga Prasad, S.V.; Mutharasan, R.K.; Naik, T.J.; Ardehali, H. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J. Clin. Investig. 2014, 124, 617–630. [Google Scholar] [CrossRef]
- Zuo, S.; Kong, D.; Wang, C.; Liu, J.; Wang, Y.; Wan, Q.; Yan, S.; Zhang, J.; Tang, J.; Zhang, Q.; et al. CRTH2 promotes endoplasmic reticulum stress-induced cardiomyocyte apoptosis through m-calpain. EMBO Mol. Med. 2018, 10, e8237. [Google Scholar] [CrossRef]
- Szponar, J.; Ciechanski, E.; Ciechanska, M.; Dudka, J.; Mandziuk, S. Evolution of Theories on Doxorubicin-Induced Late Cardiotoxicity-Role of Topoisomerase. Int. J. Mol. Sci. 2024, 25, 13567. [Google Scholar] [CrossRef]
- Shaikh, F.; Dupuis, L.L.; Alexander, S.; Gupta, A.; Mertens, L.; Nathan, P.C. Cardioprotection and Second Malignant Neoplasms Associated With Dexrazoxane in Children Receiving Anthracycline Chemotherapy: A Systematic Review and Meta-Analysis. J. Natl. Cancer Inst. 2016, 108, djv357. [Google Scholar] [CrossRef]
- Friedman, D.L.; Whitton, J.; Leisenring, W.; Mertens, A.C.; Hammond, S.; Stovall, M.; Donaldson, S.S.; Meadows, A.T.; Robison, L.L.; Neglia, J.P. Subsequent neoplasms in 5-year survivors of childhood cancer: The Childhood Cancer Survivor Study. J. Natl. Cancer Inst. 2010, 102, 1083–1095. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Ye, C.; Zhu, Y.; Zhang, T.; Gu, J.; Pan, J.; Wang, F.; Wu, F.; Huang, K.; Xu, K.; et al. Oxidative Injury in Ischemic Stroke: A Focus on NADPH Oxidase 4. Oxidative Med. Cell. Longev. 2022, 2022, 1148874. [Google Scholar] [CrossRef] [PubMed]
- Priya, L.B.; Baskaran, R.; Huang, C.Y.; Padma, V.V. Neferine ameliorates cardiomyoblast apoptosis induced by doxorubicin: Possible role in modulating NADPH oxidase/ROS-mediated NFκB redox signaling cascade. Sci. Rep. 2017, 7, 12283. [Google Scholar] [CrossRef]
- Paclet, M.H.; Laurans, S.; Dupré-Crochet, S. Regulation of Neutrophil NADPH Oxidase, NOX2: A Crucial Effector in Neutrophil Phenotype and Function. Front. Cell Dev. Biol. 2022, 10, 945749. [Google Scholar] [CrossRef] [PubMed]
- Yousefian, M.; Hosseinzadeh, H.; Hayes, A.W.; Hadizadeh, F.; Karimi, G. The Protective Effect of Natural Compounds on Doxorubicin-Induced Cardiotoxicity via Nicotinamide Adenine Dinucleotide Phosphate Oxidase Inhibition. J. Pharm. Pharmacol. 2022, 74, 351–359. [Google Scholar] [CrossRef]
- Brandes, R.P.; Weissmann, N.; Schröder, K. Nox family NADPH oxidases: Molecular mechanisms of activation. Free Radic. Biol. Med. 2014, 76, 208–226. [Google Scholar] [CrossRef]
- Cheng, D.; Chen, L.; Tu, W.; Wang, H.; Wang, Q.; Meng, L.; Li, Z.; Yu, Q. Protective effects of valsartan administration on doxorubicin-induced myocardial injury in rats and the role of oxidative stress and NOX2/NOX4 signaling. Mol. Med. Rep. 2020, 22, 4151–4162. [Google Scholar] [CrossRef]
- Zeng, C.; Duan, F.; Hu, J.; Luo, B.; Huang, B.; Lou, X.; Sun, X.; Li, H.; Zhang, X.; Yin, S.; et al. NLRP3 inflammasome-mediated pyroptosis contributes to the pathogenesis of non-ischemic dilated cardiomyopathy. Redox Biol. 2020, 34, 101523. [Google Scholar] [CrossRef]
- Cheng, D.; Tu, W.; Chen, L.; Wang, H.; Wang, Q.; Liu, H.; Zhu, N.; Fang, W.; Yu, Q. MSCs enhances the protective effects of valsartan on attenuating the doxorubicin-induced myocardial injury via AngII/NOX/ROS/MAPK signaling pathway. Aging 2021, 13, 22556–22570. [Google Scholar] [CrossRef]
- Lin, J.; Fang, L.; Li, H.; Li, Z.; Lyu, L.; Wang, H.; Xiao, J. Astragaloside IV alleviates doxorubicin induced cardiomyopathy by inhibiting NADPH oxidase derived oxidative stress. Eur. J. Pharmacol. 2019, 859, 172490. [Google Scholar] [CrossRef]
- Hoang, H.M.; Johnson, H.E.; Heo, J. Rac-dependent feedforward autoactivation of NOX2 leads to oxidative burst. J. Biol. Chem. 2021, 297, 100982. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, Y.; Zheng, D.; Wei, M.; Xu, H.; Peng, T. Rac1 signalling mediates doxorubicin-induced cardiotoxicity through both reactive oxygen species-dependent and -independent pathways. Cardiovasc. Res. 2013, 97, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hu, C.; Kong, C.Y.; Song, P.; Wu, H.M.; Xu, S.C.; Yuan, Y.P.; Deng, W.; Ma, Z.G.; Tang, Q.Z. FNDC5 alleviates oxidative stress and cardiomyocyte apoptosis in doxorubicin-induced cardiotoxicity via activating AKT. Cell Death Differ. 2020, 27, 540–555. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Hollenberg, S.M.; Singer, M. Pathophysiology of sepsis-induced cardiomyopathy. Nat. Rev. Cardiol. 2021, 18, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xu, Y.; Fang, Y.; Wang, C.; Xue, Y.; Wang, F.; Cheng, J.; Ren, H.; Wang, J.; Guo, W.; et al. Pathogenetic mechanisms of septic cardiomyopathy. J. Cell. Physiol. 2022, 237, 49–58. [Google Scholar] [CrossRef]
- Zhu, X.X.; Wang, X.; Jiao, S.Y.; Liu, Y.; Shi, L.; Xu, Q.; Wang, J.J.; Chen, Y.E.; Zhang, Q.; Song, Y.T.; et al. Cardiomyocyte peroxisome proliferator-activated receptor α prevents septic cardiomyopathy via improving mitochondrial function. Acta Pharmacol. Sin. 2023, 44, 2184–2200. [Google Scholar] [CrossRef]
- Keshani, M.; Alikiaii, B.; Babaei, Z.; Askari, G.; Heidari, Z.; Sharma, M.; Bagherniya, M. The effects of L-carnitine supplementation on inflammation, oxidative stress, and clinical outcomes in critically Ill patients with sepsis: A randomized, double-blind, controlled trial. Nutr. J. 2024, 23, 31. [Google Scholar] [CrossRef]
- She, H.; Tan, L.; Du, Y.; Zhou, Y.; Guo, N.; Zhang, J.; Du, Y.; Wang, Y.; Wu, Z.; Ma, C.; et al. VDAC2 malonylation participates in sepsis-induced myocardial dysfunction via mitochondrial-related ferroptosis. Int. J. Biol. Sci. 2023, 19, 3143–3158. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Z. Sepsis-induced myocardial dysfunction: The role of mitochondrial dysfunction. Inflamm. Res. 2021, 70, 379–387. [Google Scholar] [CrossRef]
- Durak, A.; Turan, B. Liraglutide provides cardioprotection through the recovery of mitochondrial dysfunction and oxidative stress in aging hearts. J. Physiol. Biochem. 2023, 79, 297–311. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, N.; Hou, Y.; Li, Y.; Yin, C.; Yang, E.; Cao, H.; Hu, G.; Xue, J.; Yang, J.; et al. L-Arginine-Loaded Gold Nanocages Ameliorate Myocardial Ischemia/Reperfusion Injury by Promoting Nitric Oxide Production and Maintaining Mitochondrial Function. Adv. Sci. 2023, 10, e2302123. [Google Scholar] [CrossRef] [PubMed]
- Mason, F.E.; Pronto, J.R.D.; Alhussini, K.; Maack, C.; Voigt, N. Cellular and mitochondrial mechanisms of atrial fibrillation. Basic Res. Cardiol. 2020, 115, 72. [Google Scholar] [CrossRef] [PubMed]
- Zou, R.; Tao, J.; Qiu, J.; Lu, H.; Wu, J.; Zhu, H.; Li, R.; Mui, D.; Toan, S.; Chang, X.; et al. DNA-PKcs promotes sepsis-induced multiple organ failure by triggering mitochondrial dysfunction. J. Adv. Res. 2022, 41, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Youle, R.J.; van der Bliek, A.M. Mitochondrial fission, fusion, and stress. Science 2012, 337, 1062–1065. [Google Scholar] [CrossRef]
- Welty-Wolf, K.E.; Simonson, S.G.; Huang, Y.C.; Fracica, P.J.; Patterson, J.W.; Piantadosi, C.A. Ultrastructural changes in skeletal muscle mitochondria in gram-negative sepsis. Shock 1996, 5, 378–384. [Google Scholar] [CrossRef]
- Khadour, F.H.; Panas, D.; Ferdinandy, P.; Schulze, C.; Csont, T.; Lalu, M.M.; Wildhirt, S.M.; Schulz, R. Enhanced NO and superoxide generation in dysfunctional hearts from endotoxemic rats. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H1108–H1115. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Ben-Shaul, V.; Lomnitski, L.; Nyska, A.; Zurovsky, Y.; Bergman, M.; Grossman, S. The effect of natural antioxidants, NAO and apocynin, on oxidative stress in the rat heart following LPS challenge. Toxicol. Lett. 2001, 123, 1–10. [Google Scholar] [CrossRef]
- Brealey, D.; Brand, M.; Hargreaves, I.; Heales, S.; Land, J.; Smolenski, R.; Davies, N.A.; Cooper, C.E.; Singer, M. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 2002, 360, 219–223. [Google Scholar] [CrossRef]
- Soriano, F.G.; Nogueira, A.C.; Caldini, E.G.; Lins, M.H.; Teixeira, A.C.; Cappi, S.B.; Lotufo, P.A.; Bernik, M.M.; Zsengellér, Z.; Chen, M.; et al. Potential role of poly(adenosine 5′-diphosphate-ribose) polymerase activation in the pathogenesis of myocardial contractile dysfunction associated with human septic shock. Crit. Care Med. 2006, 34, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Zang, Q.; Maass, D.L.; Tsai, S.J.; Horton, J.W. Cardiac mitochondrial damage and inflammation responses in sepsis. Surg. Infect. 2007, 8, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Celes, M.R.; Torres-Dueñas, D.; Prado, C.M.; Campos, E.C.; Moreira, J.E.; Cunha, F.Q.; Rossi, M.A. Increased sarcolemmal permeability as an early event in experimental septic cardiomyopathy: A potential role for oxidative damage to lipids and proteins. Shock 2010, 33, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hu, B.C.; Chen, C.Q.; Gong, S.J.; Yu, Y.H.; Dai, H.W.; Yan, J. Role of mitochondrial damage during cardiac apoptosis in septic rats. Chin. Med. J. 2013, 126, 1860–1866. [Google Scholar] [CrossRef]
- Nakagawa, Y. Initiation of apoptotic signal by the peroxidation of cardiolipin of mitochondria. Ann. N. Y. Acad. Sci. 2004, 1011, 177–184. [Google Scholar] [CrossRef]
- Seth, R.B.; Sun, L.; Ea, C.K.; Chen, Z.J. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell 2005, 122, 669–682. [Google Scholar] [CrossRef]
- Itoh, S.; Lemay, S.; Osawa, M.; Che, W.; Duan, Y.; Tompkins, A.; Brookes, P.S.; Sheu, S.S.; Abe, J. Mitochondrial Dok-4 recruits Src kinase and regulates NF-kappaB activation in endothelial cells. J. Biol. Chem. 2005, 280, 26383–26396. [Google Scholar] [CrossRef]
- Maass, D.L.; White, J.; Sanders, B.; Horton, J.W. Role of cytosolic vs. mitochondrial Ca2+ accumulation in burn injury-related myocardial inflammation and function. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H744–H751. [Google Scholar] [CrossRef]
- Ranjbarvaziri, S.; Kooiker, K.B.; Ellenberger, M.; Fajardo, G.; Zhao, M.; Vander Roest, A.S.; Woldeyes, R.A.; Koyano, T.T.; Fong, R.; Ma, N.; et al. Altered Cardiac Energetics and Mitochondrial Dysfunction in Hypertrophic Cardiomyopathy. Circulation 2021, 144, 1714–1731. [Google Scholar] [CrossRef]
- Ramachandra, C.J.A.; Kp, M.M.J.; Chua, J.; Hernandez-Resendiz, S.; Liehn, E.A.; Knöll, R.; Gan, L.M.; Michaëlsson, E.; Jonsson, M.K.B.; Ryden-Markinhuhta, K.; et al. Inhibiting cardiac myeloperoxidase alleviates the relaxation defect in hypertrophic cardiomyocytes. Cardiovasc. Res. 2022, 118, 517–530. [Google Scholar] [CrossRef]
- Yan, Q.; Liu, S.; Sun, Y.; Chen, C.; Yang, S.; Lin, M.; Long, J.; Yao, J.; Lin, Y.; Yi, F.; et al. Targeting oxidative stress as a preventive and therapeutic approach for cardiovascular disease. J. Transl. Med. 2023, 21, 519. [Google Scholar] [CrossRef] [PubMed]
- Ramachandra, C.J.A.; Hernandez-Resendiz, S.; Crespo-Avilan, G.E.; Lin, Y.H.; Hausenloy, D.J. Mitochondria in acute myocardial infarction and cardioprotection. EBioMedicine 2020, 57, 102884. [Google Scholar] [CrossRef]
- Bezna, M.C.; Danoiu, S.; Bezna, M.; Voisneanu, I.A.; Genunche-Dumitrescu, A.; Istratoaie, O. The Importance of Oxidative Stress Biomarkers and the Need of Antioxidant Therapy in the Control of Cardiac Arrhythmias. Eur. Cardiol. 2023, 18, e32. [Google Scholar] [CrossRef]
- Hegyi, B.; Pölönen, R.P.; Hellgren, K.T.; Ko, C.Y.; Ginsburg, K.S.; Bossuyt, J.; Mercola, M.; Bers, D.M. Cardiomyocyte Na(+) and Ca(2+) mishandling drives vicious cycle involving CaMKII, ROS, and ryanodine receptors. Basic Res. Cardiol. 2021, 116, 58. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.L.; Fu, Y.; Wu, C.W.; Zhang, Y.; Ren, H.; Zhou, S.S. Signaling Pathways Related to Oxidative Stress in Diabetic Cardiomyopathy. Front. Endocrinol. 2022, 13, 907757. [Google Scholar] [CrossRef]
- Dai, L.; Wang, Q. Targeting ferroptosis: Opportunities and challenges of mesenchymal stem cell therapy for type 1 diabetes mellitus. Stem Cell Res. Ther. 2025, 16, 47. [Google Scholar] [CrossRef]
- Qing, J.; Zhang, L.; Fan, R.; Zhi, H.; Li, C.; Li, Y.; Wu, J.; Han, C.; Li, Y. GPX4 expression changes in proximal tubule cells highlight the role of ferroptosis in IgAN. Sci. Rep. 2025, 15, 3886. [Google Scholar] [CrossRef] [PubMed]
- She, G.; Hai, X.X.; Jia, L.Y.; Zhang, Y.J.; Ren, Y.J.; Pang, Z.D.; Wu, L.H.; Han, M.Z.; Zhang, Y.; Li, J.J.; et al. Hippo pathway activation mediates cardiomyocyte ferroptosis to promote dilated cardiomyopathy through downregulating NFS1. Redox Biol. 2025, 82, 103597. [Google Scholar] [CrossRef]
- Lin, L.; Ling, X.; Chen, T.; Zhou, Q.; Huang, J.; Huang, L.; Lin, X.; Lin, L. Inhibition of Hippocampal Neuronal Ferroptosis by Liproxstatin-1 Improves Learning and Memory Function in Aged Mice with Perioperative Neurocognitive Dysfunction. J. Inflamm. Res. 2025, 18, 2991–3007. [Google Scholar] [CrossRef]
- Xie, Y.; Hou, W.; Song, X.; Yu, Y.; Huang, J.; Sun, X.; Kang, R.; Tang, D. Ferroptosis: Process and function. Cell Death Differ. 2016, 23, 369–379. [Google Scholar] [CrossRef]
- Onuma, K.; Watanabe, K.; Isayama, K.; Ogi, S.; Tokunaga, Y.; Mizukami, Y. Bardoxolone methyl prevents metabolic dysfunction-associated steatohepatitis by inhibiting macrophage infiltration. Br. J. Pharmacol. 2024, 181, 2545–2565. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Díaz, I.Y.; González-Trujano, M.E.; Martínez-Vargas, D.; Moreno-Pérez, G.F.; Hernandez-Leon, A.; Narváez-González, H.F.; Ventura-Martínez, R.; Pellicer, F.; López-Muñoz, F.J. Pharmacological interactions of sulforaphane and gabapentin in a murine fibromyalgia-like pain model. Biomed. Pharmacother. 2025, 184, 117929. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xiong, R.; Jin, S.; Li, Y.; Dong, T.; Wang, W.; Song, X.; Guan, C. MitoQ alleviates H(2)O(2)-induced mitochondrial dysfunction in keratinocytes through the Nrf2/PINK1 pathway. Biochem. Pharmacol. 2025, 234, 116811. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, J.; Hu, H.; Gong, M.; Wu, M.; Ye, B.; Hu, H.; Du, Z.; Liu, A.; Huang, S.; et al. The resveratrol attenuates reactive oxygen species mediated DNA damage in cardiac malformations caused by 4-tert-octylphenol. Toxicol. Appl. Pharmacol. 2025, 498, 117284. [Google Scholar] [CrossRef]
- Sun, J.; Shen, H.; Dong, J.; Zhang, J.; Yue, T.; Zhang, R. Melanin-Deferoxamine Nanoparticles Targeting Ferroptosis Mitigate Acute Kidney Injury via RONS Scavenging and Iron Ion Chelation. ACS Appl. Mater. Interfaces 2025, 17, 282–296. [Google Scholar] [CrossRef]
- Murillo Ortiz, B.O.; Ramírez Emiliano, J.; Romero Vázquez, M.J.; Amador Medina, L.F.; Martínez Garza, S.; Ramos Rodríguez, E.M. Impact of iron chelation with deferasirox on telomere length and oxidative stress in hemodialysis patients: A randomized study. Nefrologia 2025, 45, 68–76. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J. The Importance and Essentiality of Natural and Synthetic Chelators in Medicine: Increased Prospects for the Effective Treatment of Iron Overload and Iron Deficiency. Int. J. Mol. Sci. 2024, 25, 4654. [Google Scholar] [CrossRef]
- Sridhar, A.; DeSantiago, J.; Chen, H.; Pavel, M.A.; Ly, O.; Owais, A.; Barney, M.; Jousma, J.; Nukala, S.B.; Abdelhady, K.; et al. Modulation of NOX2 causes obesity-mediated atrial fibrillation. J. Clin. Investig. 2024, 134, e175447. [Google Scholar] [CrossRef]
- Bayoumi, A.A.; Ahmad, E.A.; Ibrahim, I.; Mahmoud, M.F.; Elbatreek, M.H. Inhibition of both NOX and TNF-α exerts substantial renoprotective effects in renal ischemia reperfusion injury rat model. Eur. J. Pharmacol. 2024, 970, 176507. [Google Scholar] [CrossRef]
- Zheng, H.; Ou, J.; Han, H.; Lu, Q.; Shen, Y. SS-31@Fer-1 Alleviates ferroptosis in hypoxia/reoxygenation cardiomyocytes via mitochondrial targeting. Biomed. Pharmacother. 2025, 183, 117832. [Google Scholar] [CrossRef]
- Ni, R.; Ji, X.Y.; Cao, T.; Liu, X.W.; Wang, C.; Lu, C.; Peng, A.; Zhang, Z.X.; Fan, G.C.; Zhang, J.; et al. Nicotinamide mononucleotide protects septic hearts in mice via preventing cyclophilin F modification and lysosomal dysfunction. Acta Pharmacol. Sin. 2024, 46, 976–988. [Google Scholar] [CrossRef]
- Li, O.; An, K.; Wang, H.; Li, X.; Wang, Y.; Huang, L.; Du, Y.; Qin, N.; Dong, J.; Wei, J.; et al. Targeting YBX1-m5C mediates RNF115 mRNA circularisation and translation to enhance vulnerability of ferroptosis in hepatocellular carcinoma. Clin. Transl. Med. 2025, 15, e70270. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.W.; Tang, H.; Chen, X.X.; Li, X.X.; Xu, H.H.; Chen, M.H.; Ba, H.J.; Lin, Q.; Dai, J.X.; Cai, J.Y.; et al. Urolithin B Attenuates Cerebral Ischemia-reperfusion Injury by Modulating Nrf2-regulated Anti-oxidation in Rats. Neuroscience 2024, 538, 46–58. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, B.; Lin, Q.; Zhu, X.; Lv, Y.; Bai, X.; Weng, X.; Du, J.; Li, M.; Zhu, Y.; et al. Spermine delivered by ZIF90 nanoparticles alleviates atherosclerosis by targeted inhibition of macrophage ferroptosis in plaque. J. Nanobiotechnol. 2025, 23, 165. [Google Scholar] [CrossRef]
- Li, D.; Ma, Q. Ubiquitin-specific protease: An emerging key player in cardiomyopathy. Cell Commun. Signal. 2025, 23, 143. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chao, D.; Dong, Q.; Zhang, X.; Zhang, K.; Ju, Z. Bimetallic NiCu-MOF Protects DOX-Induced Myocardial Injury and Cardiac Dysfunction by Suppressing Ferroptosis and Inflammation. Adv. Healthc. Mater. 2025, 14, e2405175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Qin, Y.; Wang, H.; Wang, J. Vagus nerve stimulation alleviates myocardial injury following hepatic ischemia-reperfusion in rats by inhibiting ferroptosis via the activation of the SLC7A11/GPX4 axis. Eur. J. Med. Res. 2025, 30, 162. [Google Scholar] [CrossRef]
- Mu, X.; Feng, L.; Wang, Q.; Li, H.; Zhou, H.; Yi, W.; Sun, Y. Decreased gut microbiome-derived indole-3-propionic acid mediates the exacerbation of myocardial ischemia/reperfusion injury following depression via the brain-gut-heart axis. Redox Biol. 2025, 81, 103580. [Google Scholar] [CrossRef]
- Wang, T.; Li, Z.; Lei, J.; Zhang, Y.; Tong, Y.; Guan, X.; Wang, S. RGD peptide-functionalized micelles loaded with crocetin ameliorate doxorubicin-induced cardiotoxicity. Int. J. Pharm. X 2025, 9, 100326. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, C.; Yin, D.; Dou, K. Ferroptosis: Mechanism and role in diabetes-related cardiovascular diseases. Cardiovasc. Diabetol. 2025, 24, 60. [Google Scholar] [CrossRef]
- Xiang, H.; Lyu, Q.; Chen, S.; Ouyang, J.; Xiao, D.; Liu, Q.; Long, H.; Zheng, X.; Yang, X.; Lu, H. PACS2/CPT1A/DHODH signaling promotes cardiomyocyte ferroptosis in diabetic cardiomyopathy. Cardiovasc. Diabetol. 2024, 23, 432. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, S.; Tian, J.; Yang, F.; Chen, H.; Bai, S.; Kang, J.; Pang, K.; Huang, J.; Dong, M.; et al. Impaired Iron-Sulfur Cluster Synthesis Induces Mitochondrial PARthanatos in Diabetic Cardiomyopathy. Adv. Sci. 2025, 12, e2406695. [Google Scholar] [CrossRef]
- Jin, E.J.; Jo, Y.; Wei, S.; Rizzo, M.; Ryu, D.; Gariani, K. Ferroptosis and iron metabolism in diabetes: Pathogenesis, associated complications, and therapeutic implications. Front. Endocrinol. 2024, 15, 1447148. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, S.; Guan, B.; Yan, X.; Huang, C.; Du, Y.; Yang, F.; Zhang, N.; Li, Y.; Lu, J.; et al. MAP4K4 exacerbates cardiac microvascular injury in diabetes by facilitating S-nitrosylation modification of Drp1. Cardiovasc. Diabetol. 2024, 23, 164. [Google Scholar] [CrossRef]
- Wu, M.; Chen, M.; Zhao, Y.; Zhang, X.; Ding, X.; Yuan, J.; Shi, J.; Yu, W.; Zhu, H. Neutrophil Hitchhiking-Mediated Delivery of ROS-Scavenging Biomimetic Nanoparticles for Enhanced Treatment of Atherosclerosis. Small Methods 2025, 20, e2402019. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.M. Mitochondrial Dysfunction in Cardiovascular Diseases. Int. J. Mol. Sci. 2025, 26, 1917. [Google Scholar] [CrossRef]
- Luo, Y.; Melhem, S.; Feelisch, M.; Chatre, L.; Morton, N.M.; Dolga, A.M.; van Goor, H. Thiosulphate sulfurtransferase: Biological roles and therapeutic potential. Redox Biol. 2025, 82, 103595. [Google Scholar] [CrossRef]
- Liang, B.; Huang, X.; Li, Z.; Huang, Y.; Deng, Y.; Chen, X.; Zhong, Y.; Yang, X.; Feng, Y.; Bai, R.; et al. Polystyrene nanoplastics trigger ferroptosis in Nrf2-deficient gut via ether phospholipid accumulation. Environ. Int. 2025, 197, 109367. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, F.; Yan, J.; Liang, L.; Chang, F.; Dong, M.; Diao, J.; Wu, H. Ecdysterone Alleviates Atherosclerosis by Inhibiting NCF2 and Inhibiting Ferroptosis Mediated by the PI3K/Akt/Nrf2 Pathway. J. Cell. Mol. Med. 2025, 29, e70446. [Google Scholar] [CrossRef]
- Yang, G.; Dong, C.; Wu, Z.; Wu, P.; Yang, C.; Li, L.; Zhang, J.; Wu, X. Single-cell RNA sequencing-guided engineering of mitochondrial therapies for intervertebral disc degeneration by regulating mtDNA/SPARC-STING signaling. Bioact. Mater. 2025, 48, 564–582. [Google Scholar] [CrossRef]
- Prananda, A.T.; Halim, P.; Syahputra, R.A. Targeting miRNA with flavonoids: Unlocking novel pathways in cardiovascular disease management. Front. Pharmacol. 2025, 16, 1532986. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Lai, H.; Yang, X.; Huang, Y.; Shi, Y.; Ke, L.; Chen, L.; Chen, M.; Chen, H.; Wang, Q. Unveiling an indole derivative YM818 as a novel tyrosinase inhibitor with anti-melanogenic and anti-melanin transfer effects. Int. J. Biol. Macromol. 2025, 306, 141557. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Song, Y.; Hua, Y.; Li, K.; Li, S.; Wang, Y. Molecular Mechanism of Aerobic Exercise Ameliorating Myocardial Mitochondrial Injury in Mice with Heart Failure. Int. J. Mol. Sci. 2025, 26, 2136. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Deng, L.; Xu, T.; Xu, L.; Xu, Z.; Lai, S.; Ai, Y.; Wang, Y.; Yan, G.; Zhu, L. Getah virus triggers ROS-mediated autophagy in mouse Leydig cells. Front. Microbiol. 2024, 15, 1519694. [Google Scholar] [CrossRef]
- Yu, W.; Ding, J.; Chen, J.; Jiang, Y.; Zhao, J.; Liu, J.; Zhou, J.; Liu, J. Magnesium Ion-Doped Mesoporous Bioactive Glasses Loaded with Gallic Acid Against Myocardial Ischemia/Reperfusion Injury by Affecting the Biological Functions of Multiple Cells. Int. J. Nanomed. 2024, 19, 347–366. [Google Scholar] [CrossRef]
- Liu, M.; Dudley, S.C., Jr. Beyond Ion Homeostasis: Hypomagnesemia, Transient Receptor Potential Melastatin Channel 7, Mitochondrial Function, and Inflammation. Nutrients 2023, 15, 3920. [Google Scholar] [CrossRef]
- Ma, D.; Zhang, J.; Zhang, Y.; Zhang, X.; Han, X.; Song, T.; Zhang, Y.; Chu, L. Inhibition of myocardial hypertrophy by magnesium isoglycyrrhizinate through the TLR4/NF-κB signaling pathway in mice. Int Immunopharmacol. 2018, 55, 237–244. [Google Scholar] [CrossRef]
- Likitsatian, T.; Koonyosying, P.; Paradee, N.; Roytrakul, S.; Ge, H.; Pourzand, C.; Srichairatanakool, S. Camellia Tea Saponin Ameliorates 5-Fluorouracil-Induced Damage of HaCaT Cells by Regulating Ferroptosis and Inflammation. Nutrients 2025, 17, 764. [Google Scholar] [CrossRef]
- Loi, M.; Valenti, F.; Medici, G.; Mottolese, N.; Candini, G.; Bove, A.M.; Trebbi, F.; Pincigher, L.; Fato, R.; Bergamini, C.; et al. Beneficial Antioxidant Effects of Coenzyme Q10 in In Vitro and In Vivo Models of CDKL5 Deficiency Disorder. Int. J. Mol. Sci. 2025, 26, 2204. [Google Scholar] [CrossRef]
- Shi, X.; Xia, X.; Xiao, Y.; Zhang, Y.; Gong, Y.; Chen, Y.; Shi, C.; Wang, W.; Liu, J.; Huang, J.; et al. Increased melanin induces aberrant keratinocyte - melanocyte - basal - fibroblast cell communication and fibrogenesis by inducing iron overload and ferroptosis resistance in keloids. Cell Commun. Signal. 2025, 23, 141. [Google Scholar] [CrossRef]
- Cao, Y.; Li, J.; Chen, Y.; Wang, Y.; Liu, Z.; Huang, L.; Liu, B.; Feng, Y.; Yao, S.; Zhou, L.; et al. Monounsaturated fatty acids promote cancer radioresistance by inhibiting ferroptosis through ACSL3. Cell Death Dis. 2025, 16, 184. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, H.; Yang, J.; Zhao, B.; Lei, Y.; Li, H.; Yang, K.; Liu, B.; Diao, Y. Sodium Iodate-Induced Ferroptosis in Photoreceptor-Derived 661W Cells Through the Depletion of GSH. Int. J. Mol. Sci. 2025, 26, 2334. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Pain, J.; Singh, P.; Dancis, A.; Pain, D. Mitochondrial glutaredoxin Grx5 functions as a central hub for cellular iron-sulfur cluster assembly. J. Biol. Chem. 2025, 301, 108391. [Google Scholar] [CrossRef] [PubMed]
- He, J.; He, M.; Yang, P.; Shangguan, J.; Jiang, L.; Liu, Z. Activation of SIRT1 by Hydroxysafflor Yellow A Attenuates Chronic Unpredictable Mild Stress-Induced Microglia Activation and Iron Death in Depressed Rats. Brain Behav. 2025, 15, e70385. [Google Scholar] [CrossRef]
- Lee, G.A.; Hsu, J.B.; Chang, Y.W.; Hsieh, L.C.; Li, Y.T.; Wu, Y.C.; Chu, C.Y.; Chiang, Y.H.; Guo, W.Y.; Wu, C.C.; et al. IL-19 as a promising theranostic target to reprogram the glioblastoma immunosuppressive microenvironment. J. Biomed. Sci. 2025, 32, 34. [Google Scholar] [CrossRef]
- He, Y.; Lin, Y.; Song, J.; Song, M.; Nie, X.; Sun, H.; Xu, C.; Han, Z.; Cai, J. From mechanisms to medicine: Ferroptosis as a Therapeutic target in liver disorders. Cell Commun. Signal. 2025, 23, 125. [Google Scholar] [CrossRef]
- Yu, L.M.; Dong, X.; Xue, X.D.; Xu, S.; Zhang, X.; Xu, Y.L.; Wang, Z.S.; Wang, Y.; Gao, H.; Liang, Y.X.; et al. Melatonin attenuates diabetic cardiomyopathy and reduces myocardial vulnerability to ischemia-reperfusion injury by improving mitochondrial quality control: Role of SIRT6. J. Pineal Res. 2021, 70, e12698. [Google Scholar] [CrossRef]
- Zhang, S.; Tian, W.; Duan, X.; Zhang, Q.; Cao, L.; Liu, C.; Li, G.; Wang, Z.; Zhang, J.; Li, J.; et al. Melatonin attenuates diabetic cardiomyopathy by increasing autophagy of cardiomyocytes via regulation of VEGF-B/GRP78/PERK signaling pathway. Cardiovasc. Diabetol. 2024, 23, 19. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Tang, Y.; Huang, C. Melatonin alleviates doxorubicin-induced cardiotoxicity via inhibiting oxidative stress, pyroptosis and apoptosis by activating Sirt1/Nrf2 pathway. Biomed. Pharmacother. 2023, 162, 114591. [Google Scholar] [CrossRef]
- Taha, A.M.; Mahmoud, A.M.; Ghonaim, M.M.; Kamran, A.; AlSamhori, J.F.; AlBarakat, M.M.; Shrestha, A.B.; Jaiswal, V.; Reiter, R.J. Melatonin as a potential treatment for septic cardiomyopathy. Biomed. Pharmacother. 2023, 166, 115305. [Google Scholar] [CrossRef]
- Dugbartey, G.J.; Wonje, Q.L.; Alornyo, K.K.; Robertson, L.; Adams, I.; Boima, V.; Mensah, S.D. Combination Therapy of Alpha-Lipoic Acid, Gliclazide and Ramipril Protects Against Development of Diabetic Cardiomyopathy via Inhibition of TGF-β/Smad Pathway. Front. Pharmacol. 2022, 13, 850542. [Google Scholar] [CrossRef] [PubMed]
- Shati, A.A.; Zaki, M.S.A.; Alqahtani, Y.A.; Al-Qahtani, S.M.; Haidara, M.A.; Dawood, A.F.; AlMohanna, A.M.; El-Bidawy, M.H.; Alaa Eldeen, M.; Eid, R.A. Antioxidant Activity of Vitamin C against LPS-Induced Septic Cardiomyopathy by Down-Regulation of Oxidative Stress and Inflammation. Curr. Issues Mol. Biol. 2022, 44, 2387–2400. [Google Scholar] [CrossRef] [PubMed]
- Ojo, O.O.; Obaidu, I.M.; Obigade, O.C.; Olorunsogo, O.O. Quercetin and vitamin E ameliorate cardio-apoptotic risks in diabetic rats. Mol. Cell. Biochem. 2022, 477, 793–803. [Google Scholar] [CrossRef]
- Liu, C.; Lu, X.Z.; Shen, M.Z.; Xing, C.Y.; Ma, J.; Duan, Y.Y.; Yuan, L.J. N-Acetyl Cysteine improves the diabetic cardiac function: Possible role of fibrosis inhibition. BMC Cardiovasc. Disord. 2015, 15, 84. [Google Scholar] [CrossRef]
- Şehirli, A.; Aksoy, U.; Sibai, A.; Orhan, K.; Sayıner, S. Effects of N-acetyl-L-cysteine against apical periodontitis in rats with adriamycin-induced cardiomyopathy and nephropathy. Int. Endod. J. 2024, 57, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, X.; Yang, S.; Zhou, Z.; Tian, L.; Li, W.; Wei, J.; Abliz, Z.; Wang, Z. Integrated mass spectrometry imaging reveals spatial-metabolic alteration in diabetic cardiomyopathy and the intervention effects of ferulic acid. J. Pharm. Anal. 2023, 13, 1496–1509. [Google Scholar] [CrossRef]
- Ding, G.; Fu, M.; Qin, Q.; Lewis, W.; Kim, H.W.; Fukai, T.; Bacanamwo, M.; Chen, Y.E.; Schneider, M.D.; Mangelsdorf, D.J.; et al. Cardiac peroxisome proliferator-activated receptor gamma is essential in protecting cardiomyocytes from oxidative damage. Cardiovasc. Res. 2007, 76, 269–279. [Google Scholar] [CrossRef]
- Gu, J.; Yan, X.; Dai, X.; Wang, Y.; Lin, Q.; Xiao, J.; Zhou, S.; Zhang, J.; Wang, K.; Zeng, J.; et al. Metallothionein Preserves Akt2 Activity and Cardiac Function via Inhibiting TRB3 in Diabetic Hearts. Diabetes 2018, 67, 507–517. [Google Scholar] [CrossRef]
- Gu, J.; Cheng, Y.; Wu, H.; Kong, L.; Wang, S.; Xu, Z.; Zhang, Z.; Tan, Y.; Keller, B.B.; Zhou, H.; et al. Metallothionein Is Downstream of Nrf2 and Partially Mediates Sulforaphane Prevention of Diabetic Cardiomyopathy. Diabetes 2017, 66, 529–542. [Google Scholar] [CrossRef]
- Su, W.; Zhang, Y.; Zhang, Q.; Xu, J.; Zhan, L.; Zhu, Q.; Lian, Q.; Liu, H.; Xia, Z.Y.; Xia, Z.; et al. N-acetylcysteine attenuates myocardial dysfunction and postischemic injury by restoring caveolin-3/eNOS signaling in diabetic rats. Cardiovasc. Diabetol. 2016, 15, 146. [Google Scholar] [CrossRef]
- Ni, R.; Cao, T.; Xiong, S.; Ma, J.; Fan, G.C.; Lacefield, J.C.; Lu, Y.; Le Tissier, S.; Peng, T. Therapeutic inhibition of mitochondrial reactive oxygen species with mito-TEMPO reduces diabetic cardiomyopathy. Free Radic. Biol. Med. 2016, 90, 12–23. [Google Scholar] [CrossRef] [PubMed]
- López-Chillón, M.T.; Carazo-Díaz, C.; Prieto-Merino, D.; Zafrilla, P.; Moreno, D.A.; Villaño, D. Effects of long-term consumption of broccoli sprouts on inflammatory markers in overweight subjects. Clin. Nutr. 2019, 38, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Shi, M.; Zhang, X.; Liu, X.; Chen, J.; Zhang, R.; Wang, X.; Zhang, H. GLP-1R agonists ameliorate peripheral nerve dysfunction and inflammation via p38 MAPK/NF-κB signaling pathways in streptozotocin-induced diabetic rats. Int. J. Mol. Med. 2018, 41, 2977–2985. [Google Scholar] [CrossRef]
- Qi, B.; Zheng, Y.; Gao, W.; Qi, Z.; Gong, Y.; Liu, Y.; Wang, Y.; Cheng, X.; Ning, M.; Lang, Y.; et al. Alpha-lipoic acid impedes myocardial ischemia-reperfusion injury, myocardial apoptosis, and oxidative stress by regulating HMGB1 expression. Eur. J. Pharmacol. 2022, 933, 175295. [Google Scholar] [CrossRef] [PubMed]
- Tadokoro, T.; Ikeda, M.; Ide, T.; Deguchi, H.; Ikeda, S.; Okabe, K.; Ishikita, A.; Matsushima, S.; Koumura, T.; Yamada, K.I.; et al. Mitochondria-dependent ferroptosis plays a pivotal role in doxorubicin cardiotoxicity. JCI Insight 2020, 5, e132747. [Google Scholar] [CrossRef]
- Zeng, H.; Xu, J.; Wu, R.; Wang, X.; Jiang, Y.; Wang, Q.; Guo, J.; Xiao, F. FTO alleviated ferroptosis in septic cardiomyopathy via mediating the m6A modification of BACH1. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 167307. [Google Scholar] [CrossRef]
- Hasinoff, B.B.; Herman, E.H. Dexrazoxane: How it works in cardiac and tumor cells. Is it a prodrug or is it a drug? Cardiovasc. Toxicol. 2007, 7, 140–144. [Google Scholar] [CrossRef]
- Swain, S.M.; Whaley, F.S.; Gerber, M.C.; Weisberg, S.; York, M.; Spicer, D.; Jones, S.E.; Wadler, S.; Desai, A.; Vogel, C.; et al. Cardioprotection with dexrazoxane for doxorubicin-containing therapy in advanced breast cancer. J. Clin. Oncol. 1997, 15, 1318–1332. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Wang, H.; Han, D.; Xie, E.; Yang, X.; Wei, J.; Gu, S.; Gao, F.; Zhu, N.; Yin, X.; et al. Ferroptosis as a target for protection against cardiomyopathy. Proc. Natl. Acad. Sci. USA 2019, 116, 2672–2680. [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.; Xue, M.; Li, X.; Han, F.; Liu, X.; Xu, L.; Lu, Y.; Cheng, Y.; Li, T.; et al. SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc. Diabetol. 2019, 18, 15. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, Y.; Chen, R.; Shen, J.; Zhang, S.; Gu, Y.; Shi, J.; Meng, G. Dihydromyricetin Attenuates Diabetic Cardiomyopathy by Inhibiting Oxidative Stress, Inflammation and Necroptosis via Sirtuin 3 Activation. Antioxidants 2023, 12, 200. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Shi, Y.; Chen, X.; Sun, Z.; Luo, W.; Hu, X.; Jin, G.; You, S.; Qian, Y.; Wu, W.; et al. Isoliquiritigenin attenuates diabetic cardiomyopathy via inhibition of hyperglycemia-induced inflammatory response and oxidative stress. Phytomedicine 2020, 78, 153319. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Luo, W.; Qian, Y.; Zhu, W.; Qian, J.; Li, J.; Jin, Y.; Xu, X.; Liang, G. Luteolin protects against diabetic cardiomyopathy by inhibiting NF-κB-mediated inflammation and activating the Nrf2-mediated antioxidant responses. Phytomedicine 2019, 59, 152774. [Google Scholar] [CrossRef]
- Seo, K.; Yamamoto, Y.; Kirillova, A.; Kawana, M.; Yadav, S.; Huang, Y.; Wang, Q.; Lane, K.V.; Pruitt, B.L.; Perez, M.V.; et al. Improved Cardiac Performance and Decreased Arrhythmia in Hypertrophic Cardiomyopathy With Non-β-Blocking R-Enantiomer Carvedilol. Circulation 2023, 148, 1691–1704. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, W.; Zhou, H.; Wu, Q.; Duan, M.; Liu, C.; Wu, H.; Deng, W.; Shen, D.; Tang, Q. Ferritinophagy-mediated ferroptosis is involved in sepsis-induced cardiac injury. Free Radic. Biol. Med. 2020, 160, 303–318. [Google Scholar] [CrossRef]
- Li, F.J.; Hu, H.; Wu, L.; Luo, B.; Zhou, Y.; Ren, J.; Lin, J.; Reiter, R.J.; Wang, S.; Dong, M.; et al. Ablation of mitophagy receptor FUNDC1 accentuates septic cardiomyopathy through ACSL4-dependent regulation of ferroptosis and mitochondrial integrity. Free. Radic. Biol. Med. 2024, 225, 75–86. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.; Zhou, W.; Men, H.; Bao, T.; Sun, Y.; Wang, Q.; Tan, Y.; Keller, B.B.; Tong, Q.; et al. Ferroptosis is essential for diabetic cardiomyopathy and is prevented by sulforaphane via AMPK/NRF2 pathways. Acta Pharm. Sin. B 2022, 12, 708–722. [Google Scholar] [CrossRef]
- Song, Y.J.; Zhong, C.B.; Wu, W. Resveratrol and Diabetic Cardiomyopathy: Focusing on the Protective Signaling Mechanisms. Oxidative Med. Cell. Longev. 2020, 2020, 7051845. [Google Scholar] [CrossRef]
- Al Hroob, A.M.; Abukhalil, M.H.; Hussein, O.E.; Mahmoud, A.M. Pathophysiological mechanisms of diabetic cardiomyopathy and the therapeutic potential of epigallocatechin-3-gallate. Biomed. Pharmacother. 2019, 109, 2155–2172. [Google Scholar] [CrossRef]
- Abukhalil, M.H.; Althunibat, O.Y.; Aladaileh, S.H.; Al-Amarat, W.; Obeidat, H.M.; Al-Khawalde, A.A.A.; Hussein, O.E.; Alfwuaires, M.A.; Algefare, A.I.; Alanazi, K.M.; et al. Galangin attenuates diabetic cardiomyopathy through modulating oxidative stress, inflammation and apoptosis in rats. Biomed. Pharmacother. 2021, 138, 111410. [Google Scholar] [CrossRef]
- Bano, N.; Khan, S.; Ahamad, S.; Dar, N.J.; Alanazi, H.H.; Nazir, A.; Bhat, S.A. Microglial NOX2 as a therapeutic target in traumatic brain injury: Mechanisms, Consequences, and Potential for Neuroprotection. Ageing Res. Rev. 2025, 108, 102735. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, Z.; Jin, X.; Ji, M.; Huang, T.; Meng, P.; Xu, T.; Wang, Y.; Lin, Q.; Zhao, Y.; et al. NADPH oxidase 2 inhibitor GSK2795039 prevents against cardiac remodeling after MI through reducing oxidative stress and mitochondrial dysfunction. Eur. J. Pharmacol. 2025, 997, 177483. [Google Scholar] [CrossRef]
- Bucheli, O.T.M.; Rodrigues, D.; Ulbricht, C.; Hauser, A.E.; Eyer, K. Dynamic Activation of NADPH Oxidases in Immune Responses Modulates Differentiation, Function, and Lifespan of Plasma Cells. Eur. J. Immunol. 2025, 55, e202350975. [Google Scholar] [CrossRef] [PubMed]
- Jie, H.; Zhang, J.; Wu, S.; Yu, L.; Li, S.; Dong, B.; Yan, F. Interplay between energy metabolism and NADPH oxidase-mediated pathophysiology in cardiovascular diseases. Front. Pharmacol. 2024, 15, 1503824. [Google Scholar] [CrossRef]
- Pires Da Silva, J.; Casa de Vito, M.; Miyano, C.; Sucharov, C.C. Mitochondrial Dysfunction in Congenital Heart Disease. J. Cardiovasc. Dev. Dis. 2025, 12, 42. [Google Scholar] [CrossRef]
- Oropeza-Almazán, Y.; Blatter, L.A. Role of Mitochondrial ROS for Calcium Alternans in Atrial Myocytes. Biomolecules 2024, 14, 144. [Google Scholar] [CrossRef]
- Chen, B.; Daneshgar, N.; Lee, H.C.; Song, L.S.; Dai, D.F. Mitochondrial Oxidative Stress Mediates Bradyarrhythmia in Leigh Syndrome Mitochondrial Disease Mice. Antioxidants 2023, 12, 1001. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Nie, Y.; Wang, J. The emerging significance of mitochondrial targeted strategies in NAFLD treatment. Life Sci. 2023, 329, 121943. [Google Scholar] [CrossRef]
- Rudolph, T.E.; Roths, M.; Freestone, A.D.; Yap, S.Q.; Michael, A.; Rhoads, R.P.; White-Springer, S.H.; Baumgard, L.H.; Selsby, J.T. Biological sex impacts oxidative stress in skeletal muscle in a porcine heat stress model. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2024, 326, R578–R587. [Google Scholar] [CrossRef]
- Hou, K.; Liu, L.; Fang, Z.H.; Zong, W.X.; Sun, D.; Guo, Z.; Cao, L. The role of ferroptosis in cardio-oncology. Arch. Toxicol. 2024, 98, 709–734. [Google Scholar] [CrossRef]
- Liu, J.; Kang, R.; Tang, D. Signaling pathways and defense mechanisms of ferroptosis. FEBS J. 2022, 289, 7038–7050. [Google Scholar] [CrossRef] [PubMed]
- Cheung, E.C.; Vousden, K.H. The role of ROS in tumour development and progression. Nat. Rev. Cancer 2022, 22, 280–297. [Google Scholar] [CrossRef] [PubMed]
| Cardiomyopathy Type | Key Mechanisms of Damage | Representative Biomarkers | Therapeutic Targets/Strategies |
|---|---|---|---|
| Diabetic Cardiomyopathy (DCM) | Fibrosis (TGF-β/ERK), impaired Ca2+ handling, diastolic dysfunction | 4-HNE, 8-OHdG, MDA | Nrf2 activation, NOX4 inhibition, Mg2+ |
| Takotsubo Syndrome (TTS) | Transient contractile dysfunction, mitochondrial stress | HO-1, ROS, SOD | β-blockers, antioxidant enzymes |
| Doxorubicin-Induced Cardiomyopathy (DIC) | Ferroptosis, mitochondrial fission, pyroptosis | ROS, iron, 3-NT | Ferroptosis inhibitors, iron chelators |
| Septic Cardiomyopathy | Inflammation, mitochondrial dysfunction, PARP activation | 8-OHdG, nitric oxide, MDA | NOX inhibition, PARP inhibitors |
| Hypertrophic Cardiomyopathy (HCM) | Sarcomeric dysfunction, hypertrophy, fibrosis | Protein carbonyls, GSH/GSSG | Mito-targeted antioxidants, MYBPC3-related |
| Myocardial Infarction (MI) | Ischemia-reperfusion injury, inflammation, necrosis | MDA, LDH, MPO | Preconditioning, mitochondria protection |
| Therapeutic Strategy | Mechanism of Action | Representative Drugs | References |
|---|---|---|---|
| Ferroptosis Inhibition | Prevents lipid peroxidation, protects mitochondria | Ferrostatin-1 | [178] |
| Liproxstatin-1 | [179] | ||
| Zileuton | [180] | ||
| Antioxidant Therapy | Enhances endogenous antioxidant system, scavenges ROS | Bardoxolone methyl | [181] |
| Sulforaphane | [182] | ||
| MitoQ | [183] | ||
| NAC | [184] | ||
| Iron Chelation Therapy | Reduces free iron, decreases ROS production | Deferoxamine | [185] |
| Deferasirox | [186] | ||
| Quercetin | [187] | ||
| NOX Inhibition | Inhibits NADPH oxidase, reduces oxidative damage | Apocynin | [188] |
| GKT137831 | [189] | ||
| Mitochondrial Protection | Maintains mitochondrial function, reduces OS | SS31 | [190] |
| MitoTEMPO | [191] | ||
| Precision Therapy | Optimizes interventions based on biomarkers | 4-HNE | [192] |
| 8-OHDG | [193] | ||
| ox-LDL | [194] |
| Antioxidant | Mechanism | Target | References |
|---|---|---|---|
| Vitamin C | Reduction in inflammatory biomarkers and attenuation of cardiomyocyte damage. | OS | [232] |
| Vitamin E | Regulation of mitochondria-mediated apoptosis. | Cytochrome c | [233] |
| Zinc | Reduced cardiac morphological damage and fibrosis. | Metallothionein | [238] |
| SFN | Reduces cardiac OS, hypertrophy, and fibrosis. | Nrf2 | [235,239] |
| NAC | Reduced myocardial OS, reduced cardiac hypertrophy and fibrosis. | OS | [240] |
| mito-TEMPO | Reduces cardiac mitochondrial ROS production and OS; reduces cardiomyocyte apoptosis and cardiac hypertrophy. | Superoxide | [241] |
| Broccoli sprouts | Reduced plasma IL-6 and CRP levels. | Nrf2 | [242] |
| chrysin | Reduces myocardial OS, inflammation and apoptosis. | Nox4 | [85] |
| GKT137831 | Mild inhibition of Hi Glu/ThmG-induced ROS. | Nox4 | [243] |
| Melatonin | Inhibits OS and apoptosis, and enhances autophagy. | Nrf2 | [227,228,229,230] |
| ALA | Blocking apoptosis, oxidation, and inflammatory responses. | NF-κB | [244] |
| Quercetin | Regulation of mitochondria-mediated apoptosis. | Cytochrome c | [233] |
| MnTBAP | Prevention of superoxide-induced cardiac pathology in PPARγ knockout mice. | PPARγ | [237] |
| Fer-1 | Inhibition of ferroptosis and apoptosis. | BACH1 | [245,246] |
| DXZ | Cardioprotective, anti-inflammatory, and antioxidant. | free iron | [247,248] |
| MitoTEMPO | Prevents mitochondrial oxidative damage. | Mitochondria | [249] |
| Empagliflozin | Significantly ameliorated myocardial OS injury and myocardial fibrosis in diabetic mice. | Nrf2 | [250] |
| DHY | Suppressed OS, inflammation and necrosis. | SIRT3 | [251] |
| ISL | Attenuates cardiac hypertrophy, fibrosis, and apoptosis. | Nrf2 | [252] |
| Luteolin | Modulation of the inflammatory response. | Nrf2 | [253] |
| Carvedilol | Arrhythmia suppression. | RyR2 | [254] |
| SFN : Sulforaphane Fer-1: Ferrostatin-1 DXZ: Dexamethasone DHY: Dihydromyricetin ISL: Isoliquiritigenin | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, Z.; Liao, Y.; Zhang, Z.; Wan, Z.; Liang, S.; Guo, J. Molecular Insights into Oxidative-Stress-Mediated Cardiomyopathy and Potential Therapeutic Strategies. Biomolecules 2025, 15, 670. https://doi.org/10.3390/biom15050670
Xiong Z, Liao Y, Zhang Z, Wan Z, Liang S, Guo J. Molecular Insights into Oxidative-Stress-Mediated Cardiomyopathy and Potential Therapeutic Strategies. Biomolecules. 2025; 15(5):670. https://doi.org/10.3390/biom15050670
Chicago/Turabian StyleXiong, Zhenyu, Yuanpeng Liao, Zhaoshan Zhang, Zhengdong Wan, Sijia Liang, and Jiawei Guo. 2025. "Molecular Insights into Oxidative-Stress-Mediated Cardiomyopathy and Potential Therapeutic Strategies" Biomolecules 15, no. 5: 670. https://doi.org/10.3390/biom15050670
APA StyleXiong, Z., Liao, Y., Zhang, Z., Wan, Z., Liang, S., & Guo, J. (2025). Molecular Insights into Oxidative-Stress-Mediated Cardiomyopathy and Potential Therapeutic Strategies. Biomolecules, 15(5), 670. https://doi.org/10.3390/biom15050670







