Enzymatic Glycosylation of Ganoderma Terpenoid via Bacterial Glycosyltransferases and Glycoside Hydrolases
Abstract
1. Introduction
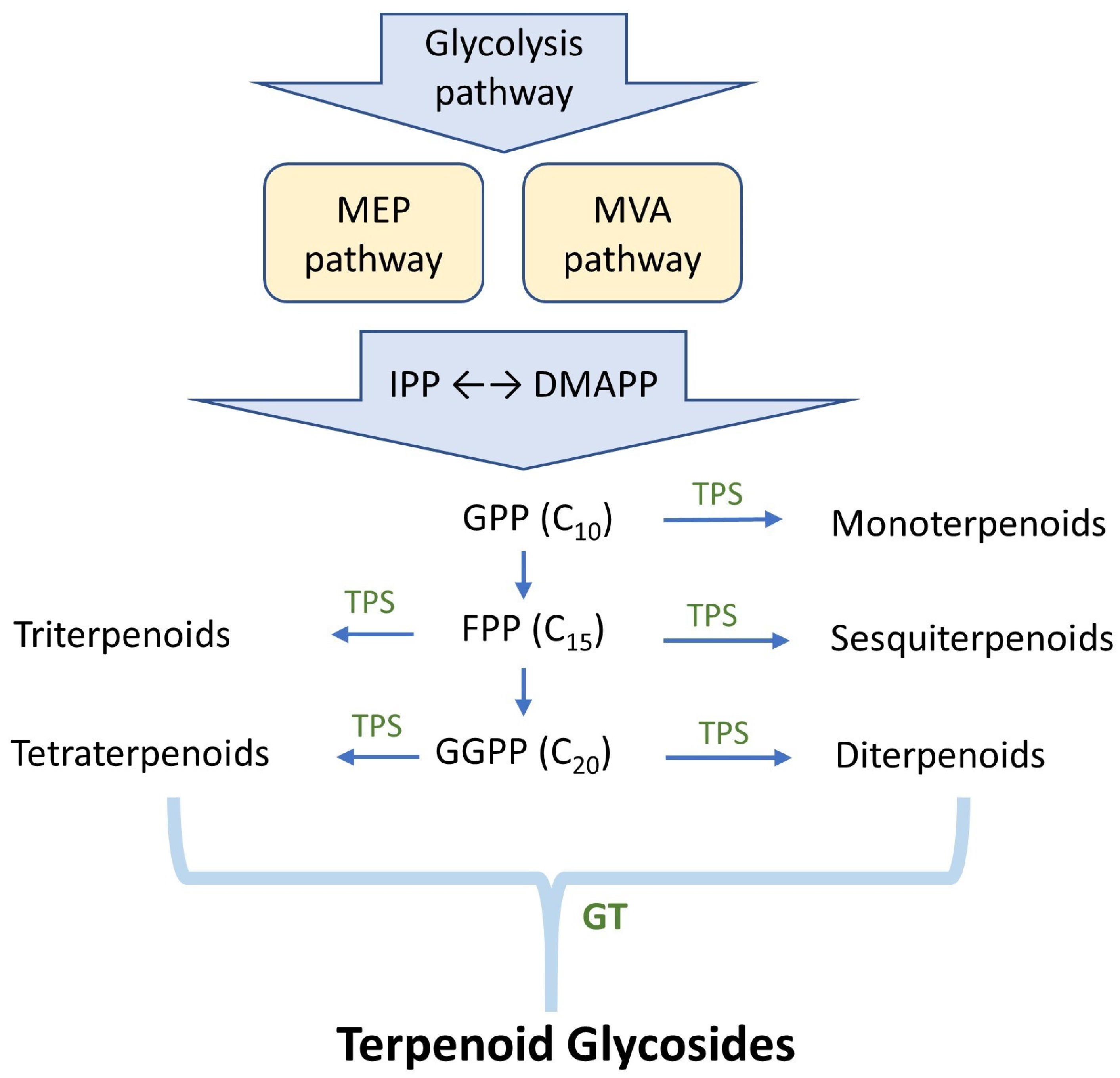
2. Strategies for Producing Novel Terpenoid Glycosides
3. Bacterial GT/GH Enzymes for New Ganoderma Terpenoid Glycosides
| Enzyme Type | Enzyme | Precursor | Product | Property of the New Glycosides | Illustration | References |
|---|---|---|---|---|---|---|
| Glycosyltransferase (GT) | BsUGT489 1,2, BsUGT398 1,2, BtBT_16345 1,3 | Ganoderic acid A (GAA) | GAA-15-O-β-glucoside | Improved solubility | Figure 4 | [67,68,69] |
| BsGT110 1,2 | GAA | GAA-26-O-β-glucoside | Improved solubility | Figure 4 | [70] | |
| BsUGT489 | Ganoderic acid G (GAG) | GAG-3-O-β-glucoside | Improved solubility | Figure 4 | [71] | |
| BsGT110 | GAG | GAG-26-O-β-glucoside | Improved solubility | Figure 4 | [71] | |
| Combination of BtBT_16345 and BsGT110 | GAA | GAA-15,26-O-β-diglucoside | Improved solubility | Figure 5 | [72] | |
| Glycoside hydrolase (GH) | Combination of BtBT_16345 and Toruzyme 4 | GAA | GAA-15-O-[α-glucopyranosyl-(1→4)-β-glucopyranoside] | Improved solubility | Figure 5 | [73] |
| DgAS 1,5 | GAA | Glucosyl-(2→26)-GAA anomers | Unique anomers | Figure 6 | [74] | |
| DgAS | GAG | Glucosyl-(2→26)-GAG anomers | Unique anomers | Figure 6 | [74] | |
| DgAS | Ganoderic acid F (GAF) | Glucosyl-(2→26)-GAF anomers | Unique anomers | Figure 6 | [74] |

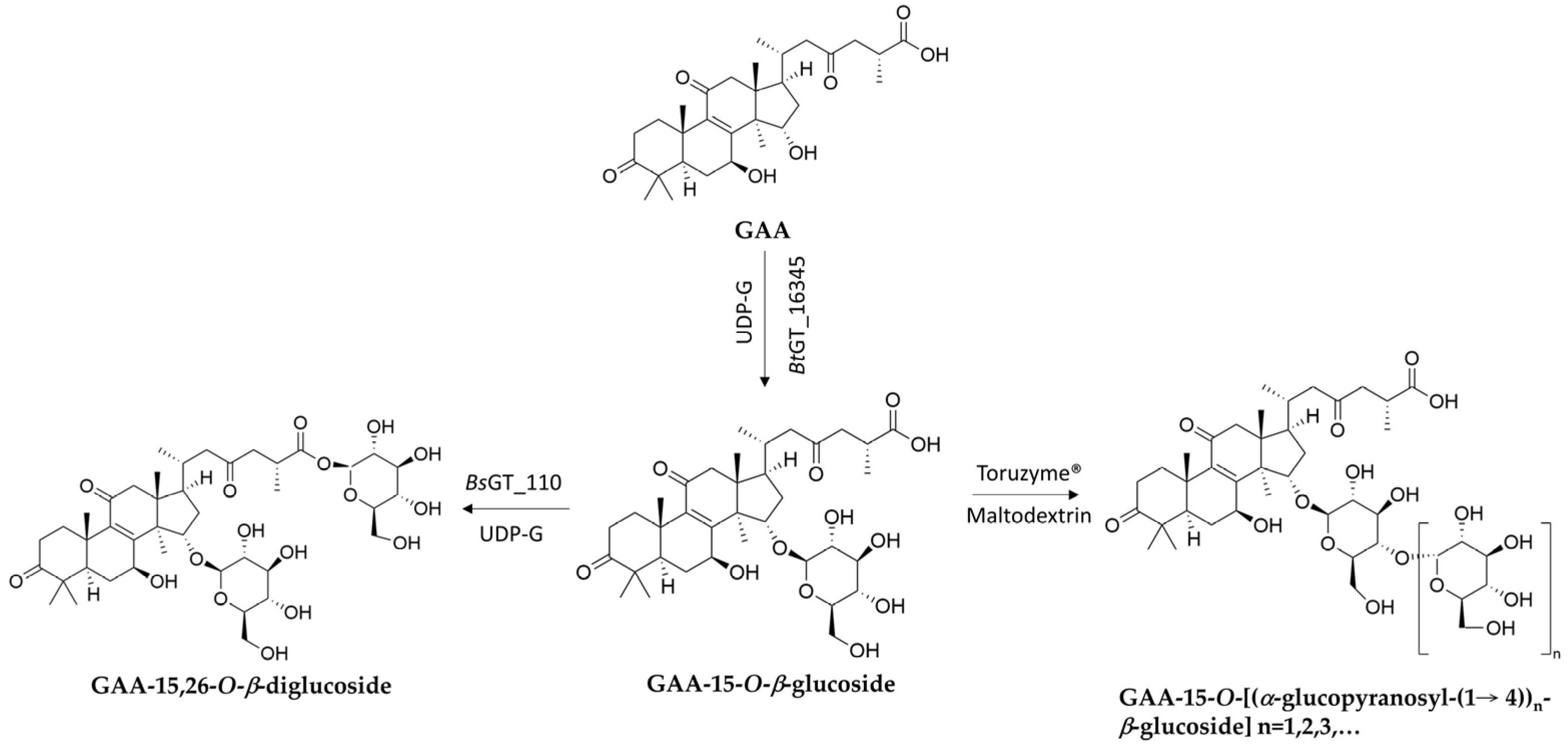
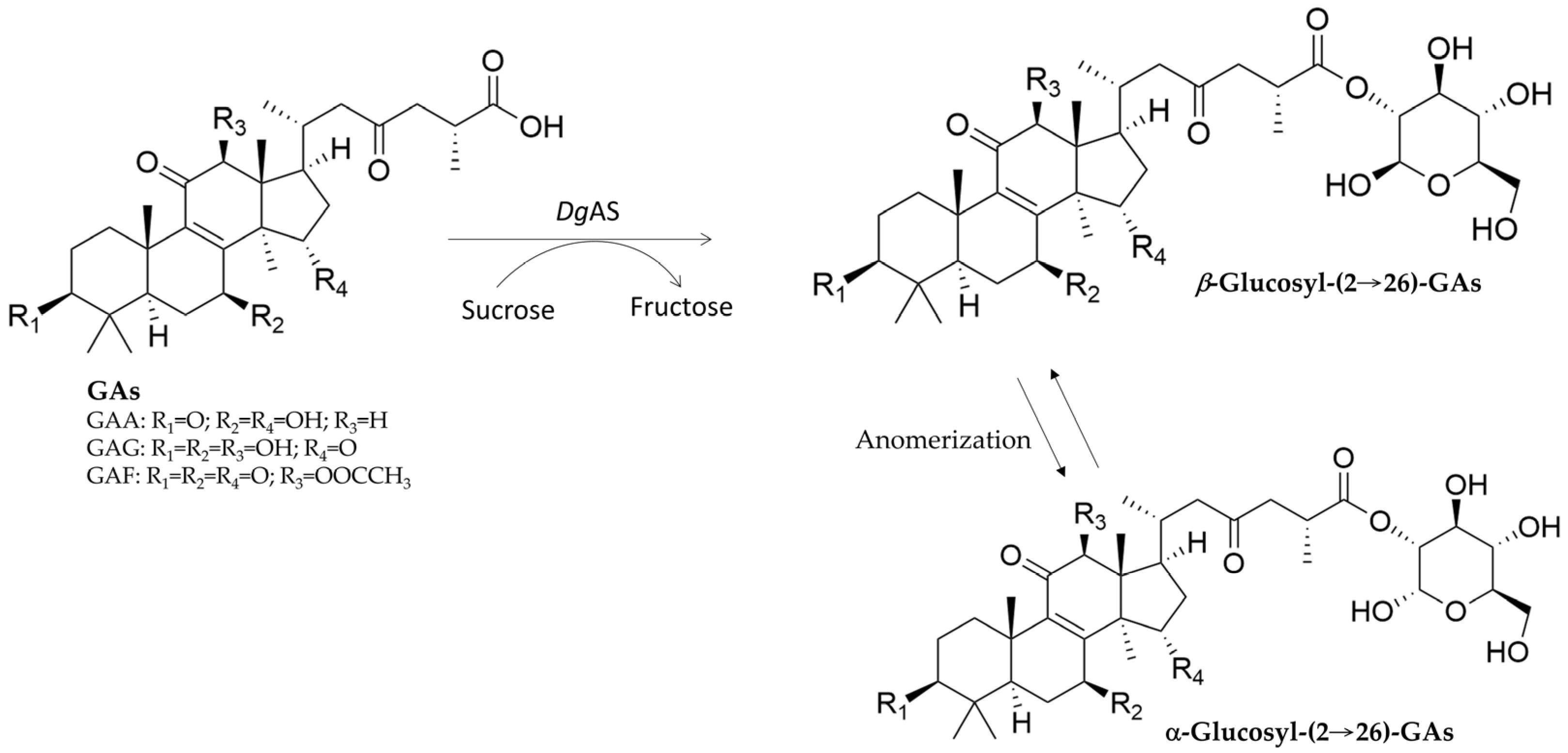
3.1. New Terpenoid Glycosides from GT-Catalyzed Biotransformation
3.2. New Terpenoid Glycosides from GH-Catalyzed Biotransformation
4. Bacterial GTs/GHs Applied to Other Natural Compound Derivatives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Badshah, S.L.; Faisal, S.; Muhammad, A.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Antiviral activities of flavonoids. Biomed. Pharmacother. 2021, 140, 111596. [Google Scholar] [CrossRef] [PubMed]
- Ekiert, H.M.; Szopa, A. Biological activities of natural products. Molecules 2020, 25, 5769. [Google Scholar] [CrossRef] [PubMed]
- Couillaud, J.; Leydet, L.; Duquesne, K.; Iacazio, G. The terpene mini-path, a new promising alternative for terpenoids bio-production. Genes 2021, 12, 1974. [Google Scholar] [CrossRef]
- Boncan, D.A.T.; Tsang, S.S.K.; Li, C.; Lee, I.H.T.; Lam, H.M.; Chan, T.F.; Hui, J.H.L. Terpenes and terpenoids in plants: Interactions with environment and insects. Int. J. Mol. Sci. 2020, 21, 7382. [Google Scholar] [CrossRef]
- de Souza, J.J.; Vieira, I.J.; Rodrigues-Filho, E.; Braz-Filho, R. Terpenoids from endophytic fungi. Molecules 2011, 16, 10604–10618. [Google Scholar] [CrossRef] [PubMed]
- Berrue, F.; McCulloch, M.W.; Kerr, R.G. Marine diterpene glycosides. Bioorganic Med. Chem. 2011, 19, 6702–6719. [Google Scholar] [CrossRef]
- Khan, H.; Khan, Z.; Amin, S.; Mabkhot, Y.N.; Mubarak, M.S.; Hadda, T.B.; Maione, F. Plant bioactive molecules bearing glycosides as lead compounds for the treatment of fungal infection: A review. Biomed. Pharmacother. 2017, 93, 498–509. [Google Scholar] [CrossRef]
- Mondol, M.A.M.; Shin, H.J.; Rahman, M.A.; Islam, M.T. Sea cucumber glycosides: Chemical structures, producing species and important biological properties. Mar. Drugs 2017, 15, 317. [Google Scholar] [CrossRef]
- Xiao, G.; Shao, X.; Zhu, D.; Yu, B. Chemical synthesis of marine saponins. Nat. Prod. Rep. 2019, 36, 769–787. [Google Scholar] [CrossRef]
- Song, Y.P.; Miao, F.P.; Liu, X.H.; Yin, X.L.; Ji, N.Y. Seven chromanoid norbisabolane derivatives from the marine-alga-endophytic fungus Trichoderma asperellum A-YMD-9-2. Fitoterapia 2019, 135, 107–113. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Tan, X.-M.; Wang, Y.-D.; Yang, J.; Zhang, Y.-G.; Sun, B.-D.; Gong, T.; Guo, L.-P.; Ding, G. Bioactive seco-Sativene Sesquiterpenoids from an Artemisia desertorum Endophytic Fungus, Cochliobolus sativus. J. Nat. Prod. 2020, 83, 1488–1494. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, D.; Cheng, Z.; Proksch, P.; Lin, W. Cytotoxic trichothecene-type sesquiterpenes from the sponge-derived fungus Stachybotrys chartarum with tyrosine kinase inhibition. RSC Adv. 2017, 7, 7259–7267. [Google Scholar] [CrossRef]
- Liu, S.; Haibo, W.; Mingzhi, S.; Ja, H.G.; Jongki, H.; and Jung, J.H. New metabolites from the sponge-derived fungus Aspergillus sydowii J05B-7F-4. Nat. Prod. Res. 2017, 31, 1682–1686. [Google Scholar] [CrossRef]
- Zhang, M.-Q.; Xu, K.-X.; Xue, Y.; Cao, F.; Yang, L.-J.; Hou, X.-M.; Wang, C.-Y.; Shao, C.-L. Sordarin diterpene glycosides with an unusual 1,3-dioxolan-4-one ring from the zoanthid-derived fungus Curvularia hawaiiensis TA26-15. J. Nat. Prod. 2019, 82, 2477–2482. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gao, Y.K.; Lei, F.H.; Tan, X.C.; Shen, L.Q.; Gao, C.H.; Yi, X.X.; Li, X.Y. A new glycosyl ester isolated from marine-derived Penicillium sp. Herbs 2019, 50, 2518–2523. [Google Scholar]
- Tang, G.-H.; Na, L.; Wei, L.; Min, W.; Yun-Yun, C.; Hai-Ying, Z.; and He, S.-Y. Mannosylxylarinolide, a new 3,4-seco-ergostane-type steroidal saponin featuring a β-d-mannose from the endophytic fungus Xylaria sp. J. Asian Nat. Prod. Res. 2020, 22, 397–403. [Google Scholar] [CrossRef]
- Deyrup, S.T.; Gloer, J.B.; O’Donnell, K.; Wicklow, D.T. Kolokosides A−D: Triterpenoid glycosides from a Hawaiian isolate of Xylaria sp. J. Nat. Prod. 2007, 70, 378–382. [Google Scholar] [CrossRef]
- Wang, Q.; Cao, R.; Zhang, Y.; Qi, P.; Wang, L.; Fang, S. Biosynthesis and regulation of terpenoids from basidiomycetes: Exploration of new research. AMB Express 2021, 11, 150. [Google Scholar] [CrossRef]
- Chen, F.; Tholl, D.; Bohlmann, J.; Pichersky, E. The family of terpene synthases in plants: A mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant J. 2011, 66, 212–229. [Google Scholar] [CrossRef]
- Zhao, J.N.; Wang, R.F.; Zhao, S.J.; Wang, Z.T. Advance in glycosyltransferases, the important bioparts for production of diversified ginsenosides. Chin. J. Nat. Med. 2020, 18, 643–658. [Google Scholar] [CrossRef]
- Feng, Y.; Yao, M.; Wang, Y.; Ding, M.; Zha, J.; Xiao, W.; Yuan, Y. Advances in engineering UDP-sugar supply for recombinant biosynthesis of glycosides in microbes. Biotechnol. Adv. 2020, 41, 107538. [Google Scholar] [CrossRef]
- Kim, B.G.; Yang, S.M.; Kim, S.Y.; Cha, M.N.; Ahn, J.H. Biosynthesis and production of glycosylated flavonoids in Escherichia coli: Current state and perspectives. Appl. Microbiol. Biotechnol. 2015, 99, 2979–2988. [Google Scholar] [CrossRef]
- Ren, J.; Barton, C.D.; Zhan, J. Engineered production of bioactive polyphenolic O-glycosides. Biotechnol. Adv. 2023, 65, 108146. [Google Scholar] [CrossRef] [PubMed]
- Overwin, H.; Wray, V.; Seeger, M.; Sepulveda-Boza, S.; Hofer, B. Flavanone and isoflavone glucosylation by non-Leloir glycosyltransferases. J. Biotechnol. 2016, 233, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Mrudulakumari Vasudevan, U.; Lee, E.Y. Flavonoids, terpenoids, and polyketide antibiotics: Role of glycosylation and biocatalytic tactics in engineering glycosylation. Biotechnol. Adv. 2020, 41, 107550. [Google Scholar] [CrossRef] [PubMed]
- Moulis, C.; Andre, I.; Remaud-Simeon, M. GH13 amylosucrases and GH70 branching sucrases, atypical enzymes in their respective families. Cell. Mol. Life Sci. 2016, 73, 2661–2679. [Google Scholar] [CrossRef]
- Nidetzky, B.; Gutmann, A.; Zhong, C. Leloir glycosyltransferases as biocatalysts for chemical production. ACS Catal. 2018, 8, 6283–6300. [Google Scholar] [CrossRef]
- Mestrom, L.; Przypis, M.; Kowalczykiewicz, D.; Pollender, A.; Kumpf, A.; Marsden, S.R.; Bento, I.; Jarzebski, A.B.; Szymanska, K.; Chrusciel, A.; et al. Leloir glycosyltransferases in applied biocatalysis: A multidisciplinary approach. Int. J. Mol. Sci. 2019, 20, 5263. [Google Scholar] [CrossRef]
- Tian, Y.; Xu, W.; Zhang, W.; Zhang, T.; Guang, C.; Mu, W. Amylosucrase as a transglucosylation tool: From molecular features to bioengineering applications. Biotechnol. Adv. 2018, 36, 1540–1552. [Google Scholar] [CrossRef]
- Herrera-Gonzalez, A.; Nunez-Lopez, G.; Morel, S.; Amaya-Delgado, L.; Sandoval, G.; Gschaedler, A.; Remaud-Simeon, M.; Arrizon, J. Functionalization of natural compounds by enzymatic fructosylation. Appl. Microbiol. Biotechnol. 2017, 101, 5223–5234. [Google Scholar] [CrossRef]
- Moulis, C.; Guieysse, D.; Morel, S.; Severac, E.; Remaud-Simeon, M. Natural and engineered transglycosylases: Green tools for the enzyme-based synthesis of glycoproducts. Curr. Opin. Chem. Biol. 2021, 61, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Slamova, K.; Kapesova, J.; Valentova, K. “Sweet flavonoids”: Glycosidase-catalyzed modifications. Int. J. Mol. Sci. 2018, 19, 2126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, Q.; Zhu, J.; Xia, G.; Zang, H. Synthesis, alpha-glucosidase inhibition and molecular docking studies of tyrosol derivatives. Nat. Prod. Res. 2021, 35, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Zhang, H.; Sun, X.; Zhao, H.; Wu, L.; Zhu, D.; Yang, G.; Shao, Y.; Zhang, X.; Mao, X.; et al. A comprehensive review of the structure elucidation and biological activity of triterpenoids from Ganoderma spp. Molecules 2014, 19, 17478–17535. [Google Scholar] [CrossRef]
- Zhao, W.Y.; Yi, J.; Chang, Y.B.; Sun, C.P.; Ma, X.C. Recent studies on terpenoids in Aspergillus fungi: Chemical diversity, biosynthesis, and bioactivity. Phytochemistry 2022, 193, 113011. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, Z.; Zhang, L.; Wang, J.; Wu, C. Glycosyltransferase GT1 family: Phylogenetic distribution, substrates coverage, and representative structural features. Comput. Structrucral Biotechnol. J. 2020, 18, 1383–1390. [Google Scholar] [CrossRef]
- Pichersky, E.; Raguso, R.A. Why do plants produce so many terpenoid compounds? New Phytol. Found. 2018, 220, 692–702. [Google Scholar] [CrossRef]
- Wright, G.D. Bacterial resistance to antibiotics: Enzymatic degradation and modification. Adv. Drug Deliv. Rev. 2005, 57, 1451–1470. [Google Scholar] [CrossRef]
- Khairullina, A.; Tsardakas Renhuldt, N.; Wiesenberger, G.; Bentzer, J.; Collinge, D.B.; Adam, G.; Bulow, L. Identification and functional characterisation of two oat UDP-glucosyltransferases involved in deoxynivalenol detoxification. Toxins 2022, 14, 446. [Google Scholar] [CrossRef]
- Della Gala, V.; Dato, L.; Wiesenberger, G.; Jæger, D.; Adam, G.; Hansen, J.; Welner, D.H. Plant-derived UDP-glycosyltransferases for glycosylation-mediated detoxification of deoxynivalenol: Enzyme discovery, characterization, and in vivo resistance assessment. Toxins 2025, 17, 153. [Google Scholar] [CrossRef]
- Yuan, S.; Sun, Y.; Chang, W.; Zhang, J.; Sang, J.; Zhao, J.; Song, M.; Qiao, Y.; Zhang, C.; Zhu, M.; et al. The silkworm (Bombyx mori) gut microbiota is involved in metabolic detoxification by glucosylation of plant toxins. Commun. Biol. 2023, 6, 790. [Google Scholar] [CrossRef]
- Cadamuro, R.D.; da Silveira Bastos, I.M.A.; Silva, I.T.; da Cruz, A.C.C.; Robl, D.; Sandjo, L.P.; Alves, S., Jr.; Lorenzo, J.M.; Rodriguez-Lazaro, D.; Treichel, H.; et al. Bioactive compounds from mangrove endophytic fungus and their uses for microorganism control. J. Fungi 2021, 7, 455. [Google Scholar] [CrossRef]
- Du, H.F.; Zhang, Y.H.; Zhang, M.; Liu, Q.A.; Zhu, H.J.; Cao, F. Marine fungal metabolites as a source of drug leads against aquatic pathogens. Appl. Microbiol. Biotechnol. 2022, 106, 3337–3350. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, S.K.; Dufosse, L.; Chhipa, H.; Saxena, S.; Mahajan, G.B.; Gupta, M.K. Fungal endophytes: A potential source of antibacterial compounds. J. Fungi 2022, 8, 164. [Google Scholar] [CrossRef]
- Hridoy, M.; Gorapi, M.Z.H.; Noor, S.; Chowdhury, N.S.; Rahman, M.M.; Muscari, I.; Masia, F.; Adorisio, S.; Delfino, D.V.; Mazid, M.A. Putative anticancer compounds from plant-derived endophytic aungi: A review. Molecules 2022, 27, 296. [Google Scholar] [CrossRef]
- Srinivasan, R.; Kannappan, A.; Shi, C.; Lin, X. Marine bacterial secondary metabolites: A treasure house for structurally unique and effective antimicrobial compounds. Mar. Drugs 2021, 19, 530. [Google Scholar] [CrossRef] [PubMed]
- Manganyi, M.C.; Ateba, C.N. Untapped potentials of endophytic fungi: A review of novel bioactive compounds with biological applications. Microorganisms 2020, 8, 1934. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Wu, Z.; Guo, H.; Liu, L.; Chen, S. A review of terpenes from marine-derived fungi: 2015–2019. Mar. Drugs 2020, 18, 321. [Google Scholar] [CrossRef]
- Yu, C.-H.; Hermosa, G.C.; Sun, A.-C.; Wu, C.-W.K.; Gao, M.-T.; Wu, C.; David Wang, H.-M. Monacolin-K loaded MIL-100(Fe) metal–organic framework induces ferroptosis on metastatic triple-negative breast cancer. Chem. Eng. J. 2024, 498, 154751. [Google Scholar] [CrossRef]
- Cheng, T.-H.; Lin, R.-H.; Cheng, Y.-S.; Shih, P.-K.; Show, P.L.; Chen, H.-Y.; Nakmee, P.S.; Chang, J.-J.; Huang, D.-M.; Wang, H.-M.D. A biomimetic micropillar wound dressing with flavone and polyphenol control release in vitro and in vivo. J. Taiwan Inst. Chem. Eng. 2024, 160, 105385. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, H.; Zhang, Z.; Verstrepen, K.J.; Wang, Q.; Dai, Z. Metabolic engineering of Yarrowia lipolytica for terpenoids production: Advances and perspectives. Crit. Rev. Biotechnol. 2022, 42, 618–633. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.J.; Wang, Y.Z.; Wang, L.R.; Shi, T.Q.; Sun, X.M.; Huang, H. Advanced strategies for the synthesis of terpenoids in Yarrowia lipolytica. J. Agricutural Food Chem. 2021, 69, 2367–2381. [Google Scholar] [CrossRef]
- Lin, P.C.; Pakrasi, H.B. Engineering cyanobacteria for production of terpenoids. Planta 2019, 249, 145–154. [Google Scholar] [CrossRef]
- Yang, Y.L.; Zhang, S.; Ma, K.; Xu, Y.; Tao, Q.; Chen, Y.; Chen, J.; Guo, S.; Ren, J.; Wang, W.; et al. Discovery and characterization of a new family of diterpene cyclases in bacteria and fungi. Angew. Chem. Int. Ed. Engl. 2017, 56, 4749–4752. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Minami, A.; Ozaki, T.; Wu, J.; Kawagishi, H.; Maruyama, J.I.; Oikawa, H. Efficient reconstitution of basidiomycota diterpene erinacine gene cluster in ascomycota host Aspergillus oryzae based on genomic DNA sequences. J. Am. Chem. Soc. 2019, 141, 15519–15523. [Google Scholar] [CrossRef]
- Knowles, S.L.; Raja, H.A.; Isawi, I.H.; Flores-Bocanegra, L.; Reggio, P.H.; Pearce, C.J.; Burdette, J.E.; Rokas, A.; Oberlies, N.H. Wheldone: Characterization of a unique scaffold from the coculture of Aspergillus fischeri and Xylaria flabelliformis. Org. Lett. 2020, 22, 1878–1882. [Google Scholar] [CrossRef] [PubMed]
- Adnani, N.; Chevrette, M.G.; Adibhatla, S.N.; Zhang, F.; Yu, Q.; Braun, D.R.; Nelson, J.; Simpkins, S.W.; McDonald, B.R.; Myers, C.L.; et al. Coculture of marine invertebrate-associated bacteria and interdisciplinary technologies enable biosynthesis and discovery of a new antibiotic, keyicin. ACS Chem. Biol. 2017, 12, 3093–3102. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, J.; Xie, Y. Improvement strategies for the oral bioavailability of poorly water-soluble flavonoids: An overview. Int. J. Pharm. 2019, 570, 118642. [Google Scholar] [CrossRef]
- Elshahawi, S.I.; Shaaban, K.A.; Kharel, M.K.; Thorson, J.S. A comprehensive review of glycosylated bacterial natural products. Chem. Soc. Rev. 2015, 44, 7591–7697. [Google Scholar] [CrossRef]
- Rivas, F.; Parra, A.; Martinez, A.; Garcia-Granados, A. Enzymatic glycosylation of terpenoids. Phytochem. Rev. 2013, 12, 327–339. [Google Scholar] [CrossRef]
- Singh, G.; Dhar, Y.V.; Asif, M.H.; Misra, P. Exploring the functional significance of sterol glycosyltransferase enzymes. Prog. Lipid Res. 2018, 69, 1–10. [Google Scholar] [CrossRef]
- Seo, D.H.; Yoo, S.H.; Choi, S.J.; Kim, Y.R.; Park, C.S. Versatile biotechnological applications of amylosucrase, a novel glucosyltransferase. Food Sci. Biotechnol. 2020, 29, 1–16. [Google Scholar] [CrossRef]
- Chang, T.S.; Wang, T.Y.; Chiang, C.M.; Lin, Y.J.; Chen, H.L.; Wu, Y.W.; Ting, H.J.; Wu, J.Y. Biotransformation of celastrol to a novel, well-soluble, low-toxic and anti-oxidative celastrol-29-O-beta-glucoside by Bacillus glycosyltransferases. J. Biosci. Bioeng. 2021, 131, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-Y.; Ding, H.-Y.; Wang, T.-Y.; Tsai, Y.-L.; Ting, H.-J.; Chang, T.-S. Improving aqueous solubility of natural antioxidant mangiferin through glycosylation by maltogenic amylase from Parageobacillus galactosidasius DSM 18751. Antioxidants 2021, 10, 1817. [Google Scholar] [CrossRef]
- Chang, T.S.; Wang, T.Y.; Yang, S.Y.; Kao, Y.H.; Wu, J.Y.; Chiang, C.M. Potential industrial production of a well-soluble, alkaline-stable, and anti-inflammatory isoflavone glucoside from 8-hydroxydaidzein glucosylated by recombinant amylosucrase of Deinococcus geothermalis. Molecules 2019, 24, 2236. [Google Scholar] [CrossRef]
- Chang, T.S.; Wu, J.Y.; Ding, H.Y.; Tayo, L.L.; Suratos, K.S.; Tsai, P.W.; Wang, T.Y.; Fong, Y.N.; Ting, H.J. Predictive production of a new highly soluble glucoside, corylin-7-O-beta-glucoside with potent anti-inflammatory and anti-melanoma activities. Appl. Biochem. Biotechnol. 2024, 197, 1174–1191. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.S.; Wang, T.Y.; Hsueh, T.Y.; Lee, Y.W.; Chuang, H.M.; Cai, W.X.; Wu, J.Y.; Chiang, C.M.; Wu, Y.W. A genome-centric approach reveals a novel glycosyltransferase from the GA A07 strain of Bacillus thuringiensis responsible for catalyzing 15-O-glycosylation of ganoderic acid A. Int. J. Mol. Sci. 2019, 20, 5192. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.S.; Wu, J.Y.; Wang, T.Y.; Wu, K.Y.; Chiang, C.M. Uridine diphosphate-dependent glycosyltransferases from Bacillus subtilis ATCC 6633 catalyze the 15-O-glycosylation of ganoderic acid A. Int. J. Mol. Sci. 2018, 19, 3469. [Google Scholar] [CrossRef]
- Chang, T.S.; Chiang, C.M.; Wang, T.Y.; Lee, C.H.; Lee, Y.W.; Wu, J.Y. New triterpenoid from novel triterpenoid 15-O-glycosylation on ganoderic acid A by intestinal bacteria of zebrafish. Molecules 2018, 23, 2345. [Google Scholar] [CrossRef]
- Chang, T.S.; Chiang, C.M.; Kao, Y.H.; Wu, J.Y.; Wu, Y.W.; Wang, T.Y. A new triterpenoid glucoside from a novel acidic glycosylation of ganoderic acid A via recombinant glycosyltransferase of Bacillus subtilis. Molecules 2019, 24, 3457. [Google Scholar] [CrossRef]
- Wu, J.Y.; Ding, H.Y.; Wang, T.Y.; Zhang, Y.R.; Chang, T.S. Glycosylation of ganoderic acid G by Bacillus glycosyltransferases. Int. J. Mol. Sci. 2021, 22, 9744. [Google Scholar] [CrossRef]
- Chang, T.-S.; Chiang, C.-M.; Wu, J.-Y.; Tsai, Y.-L.; Ting, H.-J. Production of a new triterpenoid disaccharide saponin from sequential glycosylation of ganoderic acid A by 2 Bacillus glycosyltransferases. Biosci. Biotechnol. Biochem. 2021, 85, 687–690. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-S.; Chiang, C.-M.; Wang, T.-Y.; Tsai, Y.-L.; Wu, Y.-W.; Ting, H.-J.; Wu, J.-Y. One-pot bi-enzymatic cascade synthesis of novel Ganoderma triterpenoid saponins. Catalysts 2021, 11, 580. [Google Scholar] [CrossRef]
- Wu, J.Y.; Ding, H.Y.; Luo, S.Y.; Wang, T.Y.; Tsai, Y.L.; Chang, T.S. Novel glycosylation by amylosucrase to produce glycoside anomers. Biology 2022, 11, 822. [Google Scholar] [CrossRef]
- Lombard, V.; Golaconda Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, D490–D495. [Google Scholar] [CrossRef]
- Tian, Y.; Xu, W.; Guang, C.; Zhang, W.; Mu, W. Glycosylation of flavonoids by sucrose- and starch-utilizing glycoside hydrolases: A practical approach to enhance glycodiversification. Crit. Rev. Food Scienes Nutr. 2023, 63, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.B.; Feng, B.; Huang, H.Z.; Qin, Y.J.; Wang, Y.Z.; Kang, L.P.; Zhao, Y.; Wang, X.N.; Cai, Y.; Tan, D.W.; et al. Enzymatic synthesis of alpha-glucosyl-timosaponin BII catalyzed by the extremely thermophilic enzyme: Toruzyme 3.0L. Carbohydr. Res. 2010, 345, 1752–1759. [Google Scholar] [CrossRef]
- Moon, S.S.; Lee, H.J.; Mathiyalagan, R.; Kim, Y.J.; Yang, D.U.; Lee, D.Y.; Min, J.W.; Jimenez, Z.; Yang, D.C. Synthesis of a novel alpha-glucosyl ginsenoside F1 by cyclodextrin glucanotransferase and its in vitro cosmetic applications. Biomolecules 2018, 8, 142. [Google Scholar] [CrossRef]
- Gonzalez-Alfonso, J.L.; Rodrigo-Frutos, D.; Belmonte-Reche, E.; Penalver, P.; Poveda, A.; Jimenez-Barbero, J.; Ballesteros, A.O.; Hirose, Y.; Polaina, J.; Morales, J.C.; et al. Enzymatic synthesis of a novel pterostilbene alpha-glucoside by the combination of cyclodextrin glucanotransferase and amyloglucosidase. Molecules 2018, 23, 1271. [Google Scholar] [CrossRef]
- Chang, T.S.; Wu, J.Y.; Ding, H.Y.; Lin, H.Y.; Wang, T.Y. Exploring gingerol glucosides with enhanced anti-inflammatory activity through a newly identified alpha-glucosidase (ArG) from Agrobacterium radiobacter DSM 30147. J. Biosci. Bioeng. 2024, 138, 218–224. [Google Scholar] [CrossRef]
- Wu, J.Y.; Wang, T.Y.; Ding, H.Y.; Zhang, Y.R.; Lin, S.Y.; Chang, T.S. Enzymatic synthesis of novel vitexin glucosides. Molecules 2021, 26, 6274. [Google Scholar] [CrossRef] [PubMed]
- Karlova, R.; Busscher, J.; Schempp, F.M.; Buchhaupt, M.; van Dijk, A.D.J.; Beekwilder, J. Detoxification of monoterpenes by a family of plant glycosyltransferases. Phytochemistry 2022, 203, 113371. [Google Scholar] [CrossRef] [PubMed]
- Poppenberger, B.; Berthiller, F.; Lucyshyn, D.; Sieberer, T.; Schuhmacher, R.; Krska, R.; Kuchler, K.; Glossl, J.; Luschnig, C.; Adam, G. Detoxification of the fusarium mycotoxin deoxynivalenol by a UDP-glucosyltransferase from Arabidopsis thaliana. J. Biol. Chem. 2003, 278, 47905–47914. [Google Scholar] [CrossRef]
- Lim, H.Y.; Ong, P.S.; Wang, L.; Goel, A.; Ding, L.; Li-Ann Wong, A.; Ho, P.C.; Sethi, G.; Xiang, X.; Goh, B.C. Celastrol in cancer therapy: Recent developments, challenges and prospects. Cancer Lett. 2021, 521, 252–267. [Google Scholar] [CrossRef]
- Li, M.; Xie, F.; Wang, L.; Zhu, G.; Qi, L.W.; Jiang, S. Celastrol: An update on its hepatoprotective properties and the linked molecular mechanisms. Front. Pharmacol. 2022, 13, 857956. [Google Scholar] [CrossRef]
- Chiang, C.-M.; Wang, T.-Y.; Yang, S.-Y.; Wu, J.-Y.; Chang, T.-S. Production of new isoflavone glucosides from glycosylation of 8-hydroxydaidzein by glycosyltransferase from Bacillus subtilis ATCC 6633. Catalysts 2018, 8, 387. [Google Scholar] [CrossRef]
- Chang, T.S.; Ding, H.Y.; Wu, J.Y.; Lin, H.Y.; Wang, T.Y. Glycosylation of 6-gingerol and unusual spontaneous deglucosylation of two novel intermediates to form 6-shogaol-4′-O-β-glucoside by bacterial glycosyltransferase. Appl. Enviromental Microbiol. 2024, 9, e0077924. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.-M.; Wang, T.-Y.; Wu, J.-Y.; Zhang, Y.-R.; Lin, S.-Y.; Chang, T.-S. Production of new isoflavone diglucosides from glycosylation of 8-hydroxydaidzein by Deinococcus geothermalis amylosucrase. Fermentation 2021, 7, 232. [Google Scholar] [CrossRef]
- Ding, H.-Y.; Wang, T.-Y.; Wu, J.-Y.; Tsai, Y.-L.; Chang, T.-S. Enzymatic synthesis of novel and highly soluble puerarin glucoside by Deinococcus geothermalis amylosucrase. Molecules 2022, 27, 4074. [Google Scholar] [CrossRef]



| Enzyme Type | Enzyme Name | Precursor | Product | Property of the New Glycosides | Illustration | References |
|---|---|---|---|---|---|---|
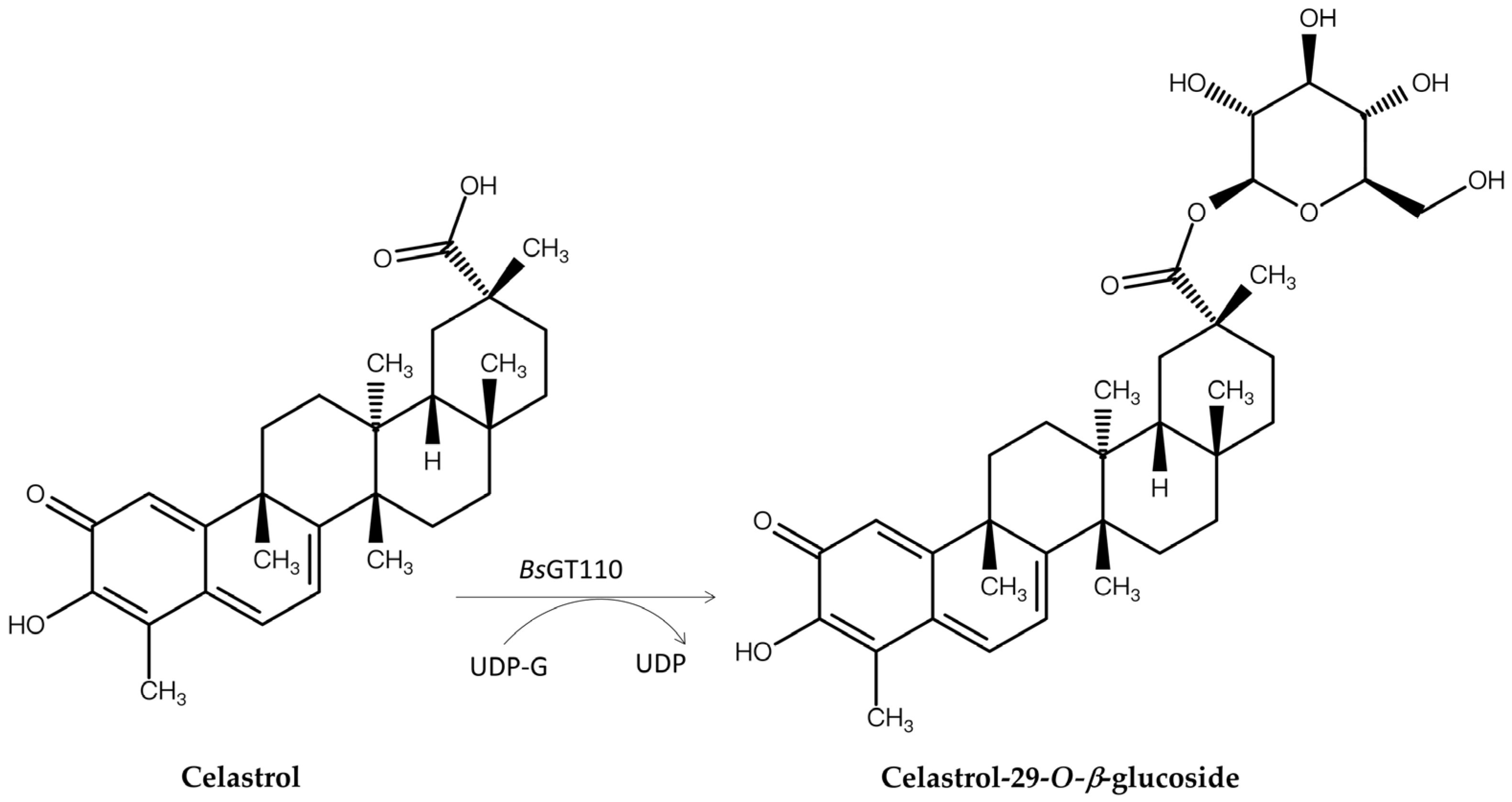
| GT | BsGT110 1,2 | Celastrol | Celastrol-29-O-β-glucoside | Detoxification | Figure 7 | [63] |
| BsGT110 | 8-Hydroxydaidzein (8-OHDe) | 8-OHDe-7-O-β-glucoside 8-OHDe-8-O-β-glucoside | Improved solubility and stability | Figure 8 | [86] | |
| BsUGT489 1,2 | 6-Gingerol | 6-Gingerol-5-O-β-glucoside 6-Gingerol-5,4′-O-β-diglucoside | Improved solubility and anti-inflammatory activity | Figure 9 | [87] | |
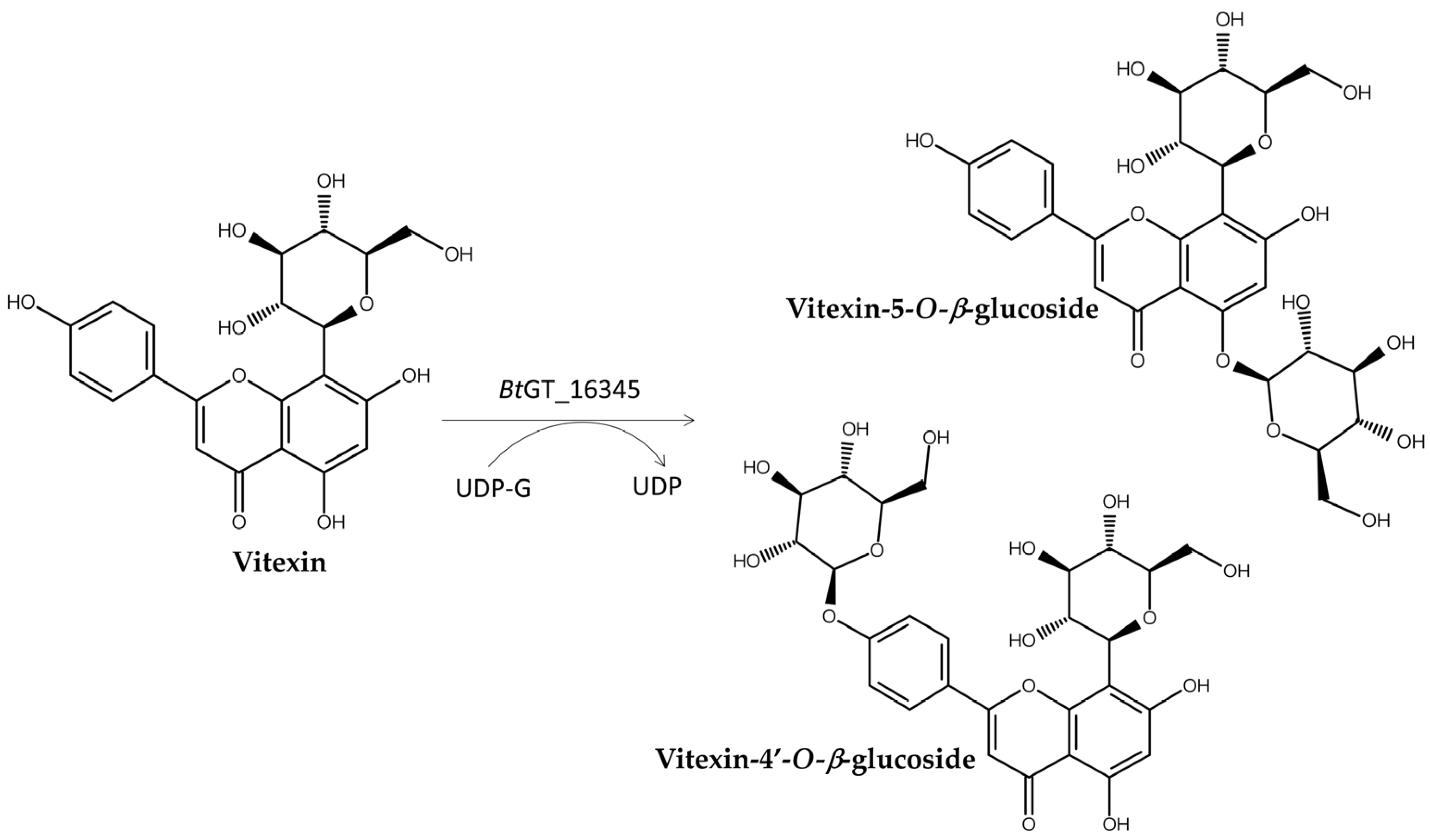
| BtBT_16345 1,3 | Vitexin | Vitexin-5-O-β-glucoside Vitexin-4′-O-β-glucoside | Improved solubility | Figure 10 | [81] | |
| GH | DgAS 1,4 | 8-OHDe | 8-OHDe-7-O-α-glucoside 8-OHDe-7,4′-O-α-diglucoside | Improved solubility and stability | Figure 8 | [65,88] |
| DgAS | Puerarin | Puerarin-4′-O-α-glucoside | Improved solubility | Figure 8 | [89] | |
| ArG 1,5 | 6-Gingerol | 6-Gingerol-5-O-α-glucoside | Improved stability and anti-inflammatory activity | Figure 9 | [80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, T.-S.; Wu, J.-Y.; Ding, H.-Y.; Wang, T.-Y. Enzymatic Glycosylation of Ganoderma Terpenoid via Bacterial Glycosyltransferases and Glycoside Hydrolases. Biomolecules 2025, 15, 655. https://doi.org/10.3390/biom15050655
Chang T-S, Wu J-Y, Ding H-Y, Wang T-Y. Enzymatic Glycosylation of Ganoderma Terpenoid via Bacterial Glycosyltransferases and Glycoside Hydrolases. Biomolecules. 2025; 15(5):655. https://doi.org/10.3390/biom15050655
Chicago/Turabian StyleChang, Te-Sheng, Jiumn-Yih Wu, Hsiou-Yu Ding, and Tzi-Yuan Wang. 2025. "Enzymatic Glycosylation of Ganoderma Terpenoid via Bacterial Glycosyltransferases and Glycoside Hydrolases" Biomolecules 15, no. 5: 655. https://doi.org/10.3390/biom15050655
APA StyleChang, T.-S., Wu, J.-Y., Ding, H.-Y., & Wang, T.-Y. (2025). Enzymatic Glycosylation of Ganoderma Terpenoid via Bacterial Glycosyltransferases and Glycoside Hydrolases. Biomolecules, 15(5), 655. https://doi.org/10.3390/biom15050655








