Effects of Tofacitinib Therapy on Circulating Tumour-Associated Antigens and Their Relationship with Clinical, Laboratory and Vascular Parameters in Rheumatoid Arthritis
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients and Study Design
2.2. Clinical Assessment
2.3. Assessment of Vascular Physiology by Ultrasound
2.4. Laboratory Measurements and Disease Activity
2.5. Tumour-Associated Antigens and Other Biomarkers
2.6. Statistical Analysis
3. Results
3.1. Characteristics of Patients
3.2. Effects of Tofacitinib Therapy on Disease Characteristics and Vascular Pathophysiology
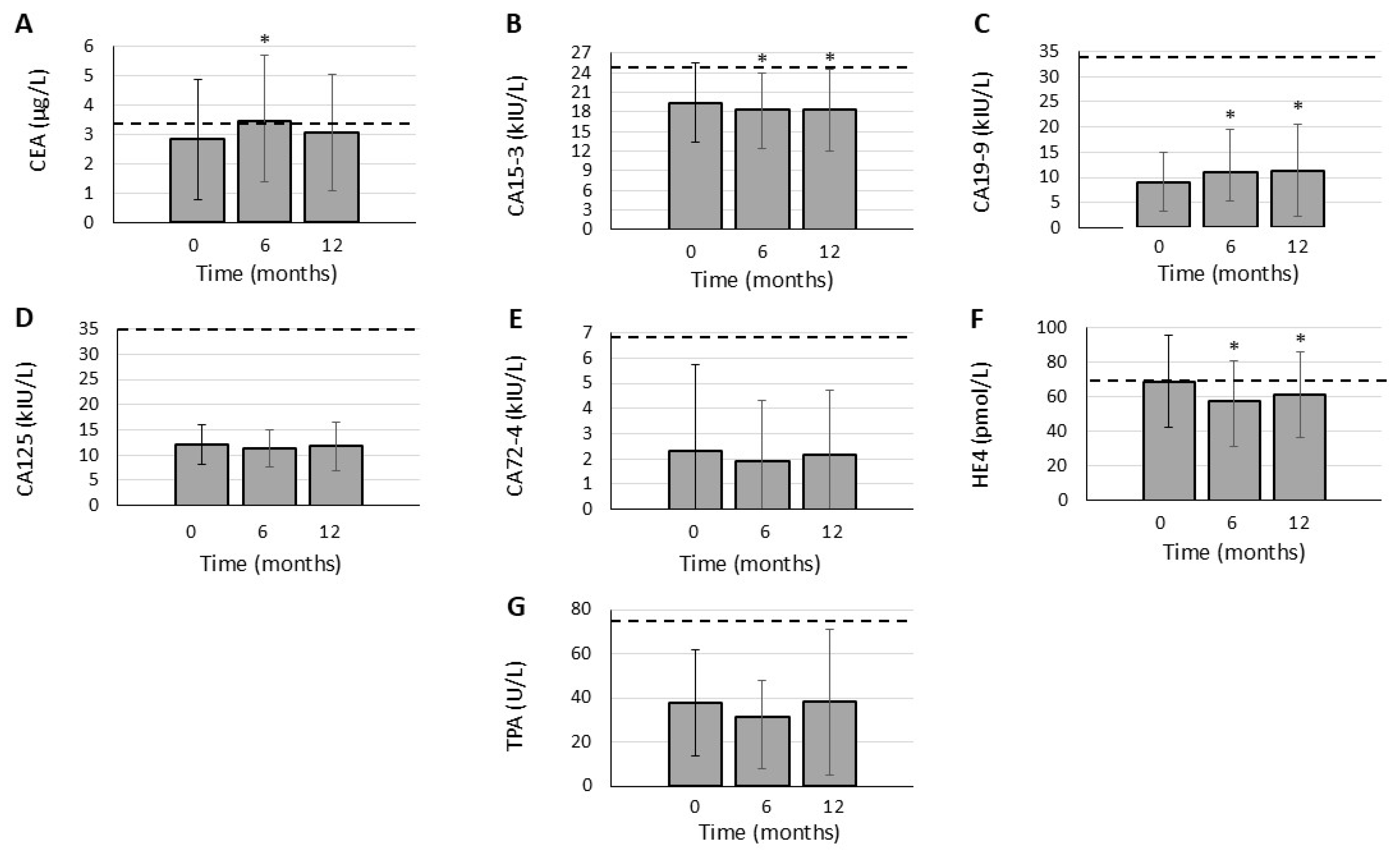
3.3. Effects of JAK Inhibition on TAA Levels
3.4. Correlations of TAA Levels with Other Parameters in RA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Shea, J.J. Targeting the Jak/STAT pathway for immunosuppression. Ann. Rheum. Dis. 2004, 63 (Suppl. S2), ii67–ii71. [Google Scholar] [CrossRef] [PubMed]
- Villarino, A.V.; Kanno, Y.; Ferdinand, J.R.; O’Shea, J.J. Mechanisms of Jak/STAT signaling in immunity and disease. J. Immunol. 2015, 194, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Winthrop, K.L. The emerging safety profile of JAK inhibitors in rheumatic disease. Nat. Rev. Rheumatol. 2017, 13, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Riese, R.J.; Krishnaswami, S.; Kremer, J. Inhibition of JAK kinases in patients with rheumatoid arthritis: Scientific rationale and clinical outcomes. Best Pr. Res. Clin. Rheumatol. 2010, 24, 513–526. [Google Scholar] [CrossRef]
- Smolen, J.S.; Landewe, R.B.M.; Bijlsma, J.W.J.; Burmester, G.R.; Dougados, M.; Kerschbaumer, A.; McInnes, I.B.; Sepriano, A.; van Vollenhoven, R.F.; de Wit, M.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann. Rheum. Dis. 2020, 79, 685–699. [Google Scholar] [CrossRef]
- Kerekes, G.; Szekanecz, Z.; Der, H.; Sandor, Z.; Lakos, G.; Muszbek, L.; Csipo, I.; Sipka, S.; Seres, I.; Paragh, G.; et al. Endothelial dysfunction and atherosclerosis in rheumatoid arthritis: A multiparametric analysis using imaging techniques and laboratory markers of inflammation and autoimmunity. J. Rheumatol. 2008, 35, 398–406. [Google Scholar]
- Agca, R.; Heslinga, S.C.; Rollefstad, S.; Heslinga, M.; McInnes, I.B.; Peters, M.J.; Kvien, T.K.; Dougados, M.; Radner, H.; Atzeni, F.; et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann. Rheum. Dis. 2017, 76, 17–28. [Google Scholar] [CrossRef]
- Nurmohamed, M.T.; Heslinga, M.; Kitas, G.D. Cardiovascular comorbidity in rheumatic diseases. Nat. Rev. Rheumatol. 2015, 11, 693–704. [Google Scholar] [CrossRef]
- Bodnar, N.; Kerekes, G.; Seres, I.; Paragh, G.; Kappelmayer, J.; Nemethne, Z.G.; Szegedi, G.; Shoenfeld, Y.; Sipka, S.; Soltesz, P.; et al. Assessment of subclinical vascular disease associated with ankylosing spondylitis. J. Rheumatol. 2011, 38, 723–729. [Google Scholar] [CrossRef]
- Nikiphorou, E.; de Lusignan, S.; Mallen, C.D.; Khavandi, K.; Bedarida, G.; Buckley, C.D.; Galloway, J.; Raza, K. Cardiovascular risk factors and outcomes in early rheumatoid arthritis: A population-based study. Heart 2020, 106, 1566–1572. [Google Scholar] [CrossRef]
- Kerekes, G.; Soltesz, P.; Nurmohamed, M.T.; Gonzalez-Gay, M.A.; Turiel, M.; Vegh, E.; Shoenfeld, Y.; McInnes, I.; Szekanecz, Z. Validated methods for assessment of subclinical atherosclerosis in rheumatology. Nat. Rev. Rheumatol. 2012, 8, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Soos, B.; Hamar, A.; Pusztai, A.; Czokolyova, M.; Vegh, E.; Szamosi, S.; Petho, Z.; Gulyas, K.; Kerekes, G.; Szanto, S.; et al. Effects of tofacitinib therapy on arginine and methionine metabolites in association with vascular pathophysiology in rheumatoid arthritis: A metabolomic approach. Front. Med. 2022, 9, 1011734. [Google Scholar] [CrossRef] [PubMed]
- Vegh, E.; Kerekes, G.; Pusztai, A.; Hamar, A.; Szamosi, S.; Vancsa, A.; Bodoki, L.; Pogacsas, L.; Balazs, F.; Hodosi, K.; et al. Effects of 1-year anti-TNF-alpha therapy on vascular function in rheumatoid arthritis and ankylosing spondylitis. Rheumatol. Int. 2020, 40, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Blanken, A.B.; Agca, R.; van Sijl, A.M.; Voskuyl, A.E.; Boellaard, R.; Smulders, Y.M.; van der Laken, C.J.; Nurmohamed, M.T. Arterial wall inflammation in rheumatoid arthritis is reduced by anti-inflammatory treatment. Semin. Arthritis Rheum. 2021, 51, 457–463. [Google Scholar] [CrossRef]
- Szekanecz, Z.; Kerekes, G.; Soltesz, P. Vascular effects of biologic agents in RA and spondyloarthropathies. Nat. Rev. Rheumatol. 2009, 5, 677–684. [Google Scholar] [CrossRef]
- Szekanecz, E.; Sandor, Z.; Antal-Szalmas, P.; Soos, L.; Lakos, G.; Besenyei, T.; Szentpetery, A.; Simkovics, E.; Szanto, J.; Kiss, E.; et al. Increased production of the soluble tumor-associated antigens CA19-9, CA125, and CA15-3 in rheumatoid arthritis: Potential adhesion molecules in synovial inflammation? Ann. N. Y. Acad. Sci. 2007, 1108, 359–371. [Google Scholar] [CrossRef]
- Szekanecz, E.; Andras, C.; Sandor, Z.; Antal-Szalmas, P.; Szanto, J.; Tamasi, L.; Kiss, E.; Szekanecz, Z. Malignancies and soluble tumor antigens in rheumatic diseases. Autoimmun. Rev. 2006, 6, 42–47. [Google Scholar] [CrossRef]
- Szekanecz, E.; Szucs, G.; Szekanecz, Z.; Tarr, T.; Antal-Szalmas, P.; Szamosi, S.; Szanto, J.; Kiss, E. Tumor-associated antigens in systemic sclerosis and systemic lupus erythematosus: Associations with organ manifestations, immunolaboratory markers and disease activity indices. J. Autoimmun. 2008, 31, 372–376. [Google Scholar] [CrossRef]
- Szekanecz, Z.; Haines, G.K.; Harlow, L.A.; Shah, M.R.; Fong, T.W.; Fu, R.; Lin, S.J.; Koch, A.E. Increased synovial expression of the adhesion molecules CD66a, CD66b, and CD31 in rheumatoid and osteoarthritis. Clin. Immunol. Immunopathol. 1995, 76, 180–186. [Google Scholar] [CrossRef]
- Lance, M.P. CEA as a cell adhesion molecule. Gastroenterology 1990, 99, 277–278. [Google Scholar] [CrossRef]
- Torsteinsdottir, I.; Arvidson, N.G.; Hallgren, R.; Hakansson, L. Enhanced expression of integrins and CD66b on peripheral blood neutrophils and eosinophils in patients with rheumatoid arthritis, and the effect of glucocorticoids. Scand. J. Immunol. 1999, 50, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, S.; Morato, E.; Bazzo, M.; Neves, F.; Fialho, S.; Castro, G.; Zimmermann, A.; Pereira, I. Tumor markers are elevated in patients with rheumatoid arthritis and do not indicate presence of cancer. Int. J. Rheum. Dis. 2012, 15, 179–182. [Google Scholar] [CrossRef]
- Guo, L.; Wang, J.; Li, J.; Yao, J.; Zhao, H. Biomarkers of rheumatoid arthritis-associated interstitial lung disease: A systematic review and meta-analysis. Front. Immunol. 2024, 15, 1455346. [Google Scholar] [CrossRef]
- Koch, M.C.; Pereira, I.A.; Nobre, L.F.; Neves, F.S. Computed tomography of pulmonary changes in rheumatoid arthritis: Carcinoembryonic antigen (CEA) as a marker of airway disease. Rheumatol. Int. 2016, 36, 531–539. [Google Scholar] [CrossRef]
- Kruit, A.; Gerritsen, W.B.; Pot, N.; Grutters, J.C.; van den Bosch, J.M.; Ruven, H.J. CA 15-3 as an alternative marker for KL-6 in fibrotic lung diseases. Sarcoidosis Vasc. Diffus. Lung Dis. 2010, 27, 138–146. [Google Scholar]
- Colmenares, V.; Hedman, A.; Hesslow, A.; Wahlin, B.; Sodergren, A. Cohort study of serological biomarkers for interstitial lung disease in patients with rheumatoid arthritis. Scand. J. Rheumatol. 2024, 53, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.C.; Choi, K.H.; Jacob, J.; Song, J.W. Prognostic role of blood KL-6 in rheumatoid arthritis-associated interstitial lung disease. PLoS ONE 2020, 15, e0229997. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zheng, X.J.; Ji, Y.L.; Liang, Z.A.; Liang, B.M. Tumour markers in rheumatoid arthritis-associated interstitial lung disease. Clin. Exp. Rheumatol. 2016, 34, 587–591. [Google Scholar]
- Sargin, G.; Kose, R.; Senturk, T. Tumor-Associated Antigens in Rheumatoid Arthritis Interstitial Lung Disease or Malignancy? Arch. Rheumatol. 2018, 33, 431–437. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, X.; Liang, J.; Peng, L.; Qin, J.; Zhou, F.; Liu, T.; Dai, L. Mucin 1 aggravates synovitis and joint damage of rheumatoid arthritis by regulating inflammation and aggression of fibroblast-like synoviocytes. Bone Jt. Res. 2022, 11, 639–651. [Google Scholar] [CrossRef]
- Fabris, C.; Falleti, E.; Pirisi, M.; Soardo, G.; Toniutto, P.; Vitulli, D.; Bortolotti, N.; Gonano, F.; Bartoli, E. Non-specific increase of serum carbohydrate antigen 19-9 in patients with liver disease associated with increased circulating levels of adhesion molecules. Clin. Chim. Acta 1995, 243, 25–33. [Google Scholar] [CrossRef] [PubMed]
- St Hill, C.A. Interactions between endothelial selectins and cancer cells regulate metastasis. Front. Biosci. 2011, 16, 3233–3251. [Google Scholar] [CrossRef]
- Szekanecz, Z.; Szegedi, G.; Koch, A.E. Cellular adhesion molecules in rheumatoid arthritis: Regulation by cytokines and possible clinical importance. J. Investig. Med. 1996, 44, 124–135. [Google Scholar]
- Isozaki, T.; Amin, M.A.; Ruth, J.H.; Campbell, P.L.; Tsou, P.S.; Ha, C.M.; Stinson, W.A.; Domino, S.E.; Koch, A.E. Fucosyltransferase 1 mediates angiogenesis in rheumatoid arthritis. Arthritis Rheumatol. 2014, 66, 2047–2058. [Google Scholar] [CrossRef]
- Shimomura, C.; Eguchi, K.; Kawakami, A.; Migita, K.; Nakao, H.; Otsubo, T.; Ueki, Y.; Tezuka, H.; Yamashita, S.; Matsunaga, M.; et al. Elevation of a tumor associated antigen CA 19-9 levels in patients with rheumatic diseases. J. Rheumatol. 1989, 16, 1410–1415. [Google Scholar] [PubMed]
- Zheng, M.; Lou, A.; Zhang, H.; Zhu, S.; Yang, M.; Lai, W. Serum KL-6, CA19-9, CA125 and CEA are Diagnostic Biomarkers for Rheumatoid Arthritis-Associated Interstitial Lung Disease in the Chinese Population. Rheumatol. Ther. 2021, 8, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Tan, L.; Ling, Y.; Huang, F.; Ma, W. Association between Rheumatoid Arthritis Disease Activity and Risk of Ovarian Malignancy in Middle-Aged and Elderly Women. Biomed. Res. Int. 2022, 2022, 1062703. [Google Scholar] [CrossRef]
- Tsavaris, N.; Mavragani, C.P.; Pikazis, D. Rheumatoid arthritis: Correlation between rheumatoid factor levels and CA-125 tumour marker elevation. Ann. Rheum. Dis. 2007, 66, 980. [Google Scholar] [CrossRef]
- Mariampillai, A.I.; Cruz, J.P.D.; Suh, J.; Sivapiragasam, A.; Nevins, K.; Hindenburg, A.A. Cancer Antigen 72-4 for the Monitoring of Advanced Tumors of the Gastrointestinal Tract, Lung, Breast and Ovaries. Anticancer. Res. 2017, 37, 3649–3656. [Google Scholar]
- Bai, X.; Sun, M.; He, Y.; Liu, R.; Cui, L.; Wang, C.; Wan, F.; Wang, M.; Li, X.; Li, H.; et al. Serum CA72-4 is specifically elevated in gout patients and predicts flares. Rheumatology 2020, 59, 2872–2880. [Google Scholar] [CrossRef]
- Nagy, B., Jr.; Bhattoa, H.P.; Steiber, Z.; Csoban, M.; Szilasi, M.; Mehes, G.; Muller, M.; Lazar, J.; Kappelmayer, J.; Antal-Szalmas, P. Serum human epididymis protein 4 (HE4) as a tumor marker in men with lung cancer. Clin. Chem. Lab. Med. 2014, 52, 1639–1648. [Google Scholar] [CrossRef] [PubMed]
- Nagy, B., Jr.; Nagy, B.; Fila, L.; Clarke, L.A.; Gonczy, F.; Bede, O.; Nagy, D.; Ujhelyi, R.; Szabo, A.; Anghelyi, A.; et al. Human Epididymis Protein 4: A Novel Serum Inflammatory Biomarker in Cystic Fibrosis. Chest 2016, 150, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Chen, J.; Di, C.; Zhan, M.; Bao, H.; Xia, C.; Fan, C.; Liu, Y. Serum Human Epididymis Protein 4 as a Novel Biomarker in Identifying Patients with Interstitial Lung Disease in Rheumatoid Arthritis. Front. Med. 2021, 8, 755268. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Xu, S.; Wang, Y.; Nian, X.; Shan, X.; Jiang, T.; Qiu, M. Human epididymis protein 4 as a new diagnostic biomarker for rheumatoid arthritis-associated interstitial lung disease. Clin. Exp. Rheumatol. 2022, 40, 2167–2174. [Google Scholar] [CrossRef]
- Meng, K.; Tian, M.; Gui, X.; Xie, M.; Gao, Y.; Shi, S.; Zhao, T.; Xiao, Y.; Cai, H.; Ding, J. Human epididymis protein 4 is associated with severity and poor prognosis of connective tissue disease-associated interstitial lung disease with usual interstitial pneumonia pattern. Int. Immunopharmacol. 2022, 108, 108704. [Google Scholar] [CrossRef]
- Sugiyama, Y.; Kawaguchi, Y. Tissue polypeptide antigen (TPA). Nihon Rinsho 2005, 63 (Suppl. S8), 705–707. [Google Scholar]
- Orbach, H.; Zandman-Goddard, G.; Amital, H.; Barak, V.; Szekanecz, Z.; Szucs, G.; Danko, K.; Nagy, E.; Csepany, T.; Carvalho, J.F.; et al. Novel biomarkers in autoimmune diseases: Prolactin, ferritin, vitamin D, and TPA levels in autoimmune diseases. Ann. N. Y. Acad. Sci. 2007, 1109, 385–400. [Google Scholar] [CrossRef]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., 3rd; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann. Rheum. Dis. 2010, 69, 1580–1588. [Google Scholar] [CrossRef]
- Kerekes, G.; Czokolyova, M.; Hamar, A.; Pusztai, A.; Tajti, G.; Katko, M.; Vegh, E.; Petho, Z.; Bodnar, N.; Horvath, A.; et al. Effects of 1-year tofacitinib therapy on angiogenic biomarkers in rheumatoid arthritis. Rheumatology 2023, 62, SI304–SI312. [Google Scholar] [CrossRef]
- Hamar, A.; Hascsi, Z.; Pusztai, A.; Czokolyova, M.; Vegh, E.; Petho, Z.; Gulyas, K.; Soos, B.; Kerekes, G.; Szekanecz, E.; et al. Prospective, simultaneous assessment of joint and vascular inflammation by PET/CT in tofacitinib-treated patients with rheumatoid arthritis: Associations with vascular and bone status. RMD Open 2021, 7, e001804. [Google Scholar] [CrossRef]
| Tofacitinib-Treated Patients | |
|---|---|
| Number of patients (n) | 26 |
| Female:male ratio | 23:3 |
| Age (years), mean ± SD (range) | 51.9 ± 9.7 (27–69) |
| BMI (kg/m2), mean ± SD (range) | 30.3 ± 7.4 (20.8–51.4) |
| Positive CV history, n (%) | 6 (23.1) |
| Positive history of hypertension, n (%) | 13 (50.0) |
| Positive history of diabetes mellitus, n (%) | 2 (7.7) |
| Smoking (current), n (%) | 7 (26.9) |
| Disease duration (years), mean ± SD (range) | 7.5 ± 4.8 (1–21) |
| RF positivity, n (%) | 22 (84.6) |
| Anti-CCP positivity, n (%) | 22 (84.6) |
| DAS28 (baseline), mean ± SD | 5.12 ± 0.82 |
| TAA | Baseline | 6 Months | 12 Months |
|---|---|---|---|
| CEA | 8 | 13 | 11 |
| CA15-3 | 7 | 3 | 3 |
| CA19-9 | 0 | 0 | 0 |
| CA125 | 0 | 0 | 0 |
| CA72-4 | 2 | 1 | 1 |
| HE4 | 7 | 5 | 7 |
| TPA | 2 | 1 | 2 |
| Dependent Variable | Independent Variable | Univariable Regression Analysis | Multivariable Regression Analysis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β | p | B | 95% CI | β | p | B | 95% CI | ||
| TAAs as Dependent Variables | |||||||||
| CEA-0 | TNF-0 | 0.487 | 0.012 | 0.057 | 0.014–0.100 | ||||
| CEA-6 | RF-0 | 0.391 | 0.048 | 0.004 | 0–0.008 | ||||
| IMT-6 | 0.520 | 0.006 | 10.384 | 3.197–17.570 | 0.520 | 0.006 | 10.384 | 3.197–17.570 | |
| CA153-0 | DAS28-0 | 0.442 | 0.023 | 4.911 | 1.222–7.888 | ||||
| IL6-0 | 0.565 | 0.003 | 0.075 | 0.029–0.121 | 0.565 | 0.003 | 0.075 | 0.029–0.121 | |
| PECAM1-0 | 0.486 | 0.012 | 0 | ||||||
| CA153-6 | DAS28-6 | 0.530 | 0.005 | 4.584 | 1.493–7.676 | ||||
| IL6-0 | 0.631 | 0.001 | 0.075 | 0.036–0.113 | |||||
| PECAM1-6 | 0.593 | 0.001 | 0 | 0.593 | 0.001 | 0 | |||
| CA153-12 | DAS28-6 | 0.484 | 0.012 | 4.826 | 1.146–8.503 | ||||
| IL6-0 | 0.655 | <0.001 | 0.089 | 0.046–0.133 | |||||
| PECAM1-12 | 0.647 | <0.001 | 0 | 0–0.001 | 0.647 | <0.001 | 0 | 0–0.001 | |
| CA199-12 | RF-0 | 0.418 | 0.034 | 0.017 | 0.001–0.033 | ||||
| CA125-6 | IMT-6 | 0.466 | 0.017 | 15.477 | 3.082–27.871 | ||||
| CA125-12 | PECAM1-12 | 0.463 | 0.017 | 0 | |||||
| IMT-12 | 0.414 | 0.035 | 17.753 | 1.330–34.177 | 0.442 | 0.001 | 18.937 | 4.874–32.999 | |
| CA724-0 | DAS28-0 | 0.425 | 0.030 | 1.937 | 0.199–3.675 | ||||
| IL6-0 | 0.449 | 0.021 | 0.034 | 0.005–0.062 | 0.430 | 0.041 | 0.032 | 0.006–0.059 | |
| HE4-0 | RF-0 | 0.403 | 0.041 | 0.049 | 0.002–0.096 | ||||
| TNF-0 | 0.489 | 0.011 | 0.743 | 0.185–1.302 | 0.480 | 0.006 | 0.730 | 0.228–1.232 | |
| IL8-0 | 0.429 | 0.029 | 0.392 | 0.044–0.739 | 0.419 | 0.015 | 0.383 | 0.081–0.684 | |
| HE4-6 | IL8-0 | 0.426 | 0.030 | 0.334 | 0.035–0.634 | ||||
| IMT-0 | 0.530 | 0.005 | 109.8 | 35.7–183.8 | 0.530 | 0.005 | 109.8 | 35.7–183.8 | |
| IMT-6 | 0.412 | 0.037 | 79.973 | 5.353–154.6 | |||||
| HE4-12 | RF-0 | 0.463 | 0.017 | 0.052 | 0.010–0.093 | ||||
| RF-6 | 0.456 | 0.019 | 0.061 | 0.011–0.112 | |||||
| RF-12 | 0.402 | 0.042 | 0.056 | 0.002–0.110 | |||||
| IL8-0 | 0.541 | 0.004 | 0.453 | 0.156–0.750 | 0.441 | 0.006 | 0.370 | 0.116–0.624 | |
| IMT-0 | 0.583 | 0.002 | 129.054 | 53.35–204.7 | 0.494 | 0.003 | 109.391 | 42.389–176.4 | |
| IMT-12 | 0.452 | 0.020 | 76.543 | 12.948–140.1 | |||||
| TPA-6 | IMT-6 | 0.441 | 0.037 | 60.853 | 4.016–117.69 | ||||
| TPA-12 | HAQ-12 | 0.401 | 0.042 | 19.747 | 0.752–38.741 | ||||
| ESR-0 | 0.394 | 0.047 | 0.613 | 0.010–1.215 | |||||
| ESR-12 | 0.501 | 0.009 | 0.918 | 0.250–1.587 | 0.435 | 0.010 | 0.797 | 0.207–1.387 | |
| RF-0 | 0.464 | 0.017 | 0.700 | 0.014–0.127 | |||||
| PWV-12 | 0.423 | 0.031 | 8.560 | 0.843–16.278 | |||||
| TAAs as Independent Variables | |||||||||
| IMT-6 | HE4-6 | 0.112 | 0.037 | 0.002 | 0–0.004 | ||||
| IMT-12 | CEA-6 | 0.430 | 0.028 | 0.028 | 0.003–0.053 | ||||
| CA153-6 | 0.430 | 0.028 | 0.011 | 0.001–0.021 | 0.433 | 0.013 | 0.011 | 0.003–0.020 | |
| HE4-6 | 0.461 | 0.018 | 0.003 | 0.001–0.005 | 0.464 | 0.009 | 0.003 | 0.001–0.005 | |
| HE4-12 | 0.452 | 0.020 | 0.003 | 0–0.005 | |||||
| PWV-12 | CEA-6 | 0.405 | 0.040 | 0.301 | 0.015–0.587 | ||||
| TPA-6 | 0.486 | 0.012 | 0.049 | 0.012–0.086 | 0.486 | 0.012 | 0.049 | 0.012–0.086 | |
| TPA-12 | 0.423 | 0.031 | 0.009 | 0.002–0.040 | |||||
| (A) | ||||
|---|---|---|---|---|
| Dependent Variable | Effect | F | p | Partial η2 |
| TAAs as Dependent Variables | ||||
| CEA (0-6-12) | disease duration | 4.188 | 0.037 | 0.149 |
| CA19-9 (0-6-12) | RF-0 | 3.426 | 0.041 | 0.125 |
| TNF-0 | 12.414 | <0.001 | 0.519 | |
| CA72-4 (0-6-12) | DAS28-0 | 6.392 | 0.012 | 0.210 |
| IL6-0 | 14.273 | 0.001 | 0.373 | |
| PECAM1-0 | 9.119 | <0.001 | 0.275 | |
| TAAs as Independent Variables | ||||
| IMT (0-6-12) | CA125-0 | 3.520 | 0.046 | 0.234 |
| (B) | ||||
| Dependent Variable | Effect | F | p | Partialη2 |
| TAAs as Dependent Variables | ||||
| CEA (0-6-12) | DAS28 (0-6-12) | 39.124 | <0.001 | 0.765 |
| HAQ (0-6-12) | 5.568 | 0.010 | 0.317 | |
| CRP (0-6-12) | 9.240 | 0.001 | 0.435 | |
| ESR (0-6-12) | 12.539 | <0.001 | 0.511 | |
| IL-6 (0-6-12) | 4.351 | 0.024 | 0.266 | |
| VEGF (0-6-12) | 6.350 | 0.006 | 0.346 | |
| CA15-3 (0-6-12) | CRP (0-6-12) | 8.348 | 0.002 | 0.410 |
| ESR (0-6-12) | 10.364 | 0.001 | 0.463 | |
| IL-6 (0-6-12) | 3.794 | 0.037 | 0.240 | |
| VEGF (0-6-12) | 6.165 | 0.007 | 0.399 | |
| CA19-9 (0-6-12) | DAS28 (0-6-12) | 10.029 | 0.001 | 0.455 |
| HAQ (0-6-12) | 4.532 | 0.021 | 0.274 | |
| CRP (0-6-12) | 8.348 | 0.002 | 0.410 | |
| ESR (0-6-12) | 11.804 | <0.001 | 0.496 | |
| IL-6 (0-6-12) | 5.091 | 0.014 | 0.298 | |
| VEGF (0-6-12) | 6.645 | 0.005 | 0.356 | |
| CA72-4 (0-6-12) | DAS28 (0-6-12) | 4.122 | 0.029 | 0.256 |
| CRP (0-6-12) | 6.885 | 0.004 | 0.365 | |
| ESR (0-6-12) | 10.561 | 0.001 | 0.468 | |
| IL-6 (0-6-12) | 4.606 | 0.020 | 0.277 | |
| VEGF (0-6-12) | 6.155 | 0.007 | 0.399 | |
| HE4 (0-6-12) | DAS28 (0-6-12) | 5.882 | 0.008 | 0.329 |
| HAQ (0-6-12) | 7.288 | 0.003 | 0.378 | |
| VEGF (0-6-12) | 4.511 | 0.022 | 0.273 | |
| IMT (0-6-12) | 7.586 | 0.003 | 0.378 | |
| TAA | DD | DAS28 | CRP | ESR | HAQ | RF | TNF-α | IL-6 | IL-8 | VEGF | PECAM-1 | IMT | PWV |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CEA | + | + | + | + | + | + | + | + | + | + | + | ||
| CA15-3 | + | + | + | + | + | + | + | ||||||
| CA19-9 | + | + | + | + | + | + | + | + | |||||
| CA125 | + | + | |||||||||||
| CA72-4 | + | + | + | + | + | + | |||||||
| HE4 | + | + | + | + | + | + | + | ||||||
| TPA | + | + | + | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sebestyén, E.; Csige, D.; Antal-Szalmás, P.; Horváth, Á.; Végh, E.; Soós, B.; Pethő, Z.; Bodnár, N.; Hamar, A.; Bodoki, L.; et al. Effects of Tofacitinib Therapy on Circulating Tumour-Associated Antigens and Their Relationship with Clinical, Laboratory and Vascular Parameters in Rheumatoid Arthritis. Biomolecules 2025, 15, 648. https://doi.org/10.3390/biom15050648
Sebestyén E, Csige D, Antal-Szalmás P, Horváth Á, Végh E, Soós B, Pethő Z, Bodnár N, Hamar A, Bodoki L, et al. Effects of Tofacitinib Therapy on Circulating Tumour-Associated Antigens and Their Relationship with Clinical, Laboratory and Vascular Parameters in Rheumatoid Arthritis. Biomolecules. 2025; 15(5):648. https://doi.org/10.3390/biom15050648
Chicago/Turabian StyleSebestyén, Enikő, Dóra Csige, Péter Antal-Szalmás, Ágnes Horváth, Edit Végh, Boglárka Soós, Zsófia Pethő, Nóra Bodnár, Attila Hamar, Levente Bodoki, and et al. 2025. "Effects of Tofacitinib Therapy on Circulating Tumour-Associated Antigens and Their Relationship with Clinical, Laboratory and Vascular Parameters in Rheumatoid Arthritis" Biomolecules 15, no. 5: 648. https://doi.org/10.3390/biom15050648
APA StyleSebestyén, E., Csige, D., Antal-Szalmás, P., Horváth, Á., Végh, E., Soós, B., Pethő, Z., Bodnár, N., Hamar, A., Bodoki, L., Kacsándi, D., Földesi, R., Kalina, E., Nagy, G., Kerekes, G., Nagy, B., Jr., Hodosi, K., Szamosi, S., Árkosy, P., ... Szekanecz, É. (2025). Effects of Tofacitinib Therapy on Circulating Tumour-Associated Antigens and Their Relationship with Clinical, Laboratory and Vascular Parameters in Rheumatoid Arthritis. Biomolecules, 15(5), 648. https://doi.org/10.3390/biom15050648







