Low Fluoride Regulates Macrophage Polarization Through Mitochondrial Autophagy Mediated by PINK1/Parkin Axis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials, Reagents and Cell Culture
2.1.1. Material Reagents
2.1.2. Cell Culture and Induction
2.2. Study Location and Population Information
2.3. Mass Cytometry for the Detection of Monocytes and Related Markers in Blood
2.4. Establishment of a Rat Fluorosis Model
2.5. Quantitative Determination of Fluoride Content in Serum, Urine and Water
2.6. Diagnostic Criteria for Dental Fluorosis
2.7. Preparation of Spleen Cell Suspension
2.8. H&E Histological Staining
2.9. Ultrastructural Observation
2.10. Immunofluorescence Staining
2.11. Flow Cytometry Analysis of Macrophage Phenotype
2.12. Western Blot
2.13. ELISA
2.14. Mitochondrial Membrane Potential (MMP) Detection
2.15. Statistical Analysis
3. Results
3.1. Population Epidemiological Survey
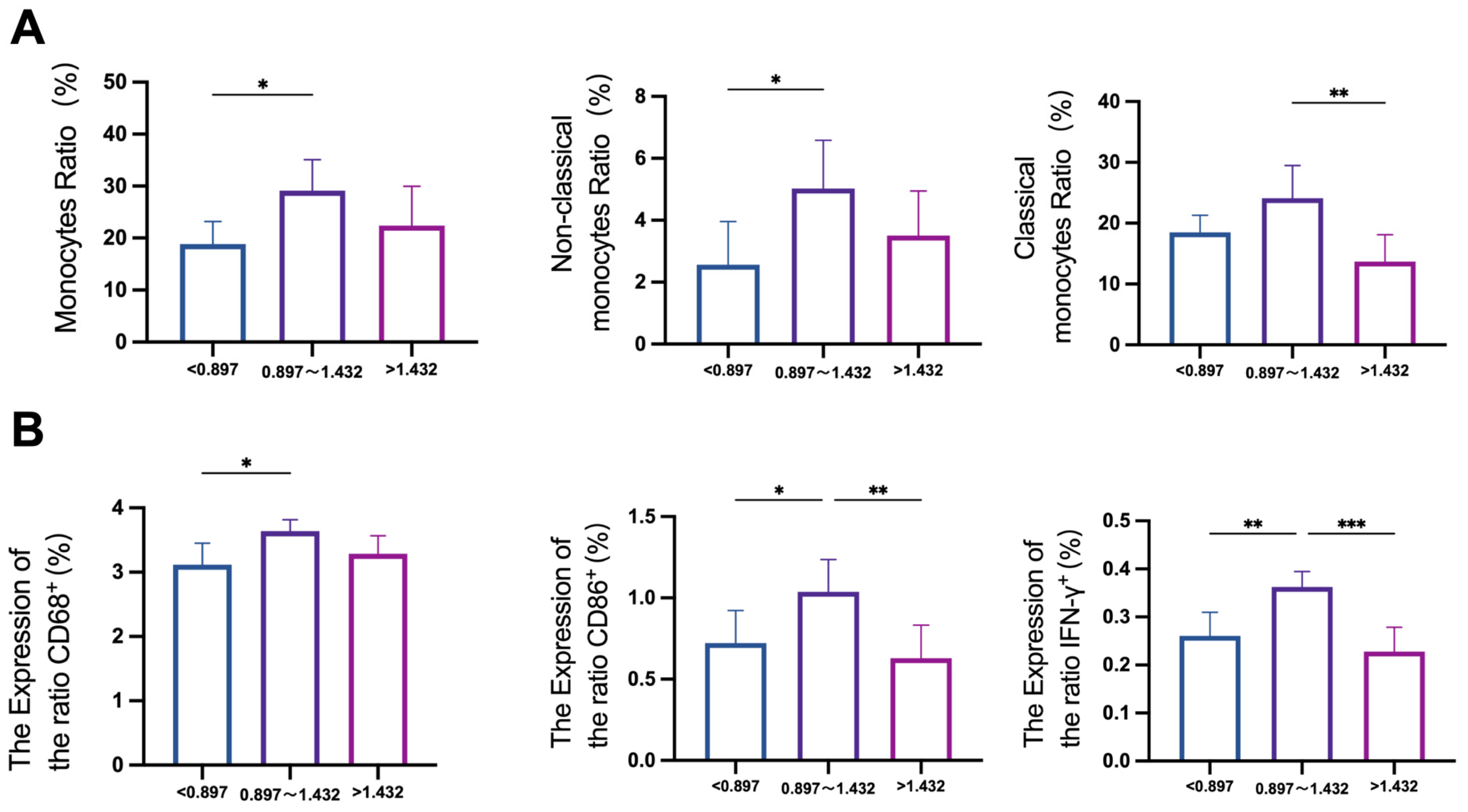
3.2. Effects of Fluoride on Monocyte Subsets in Human Blood
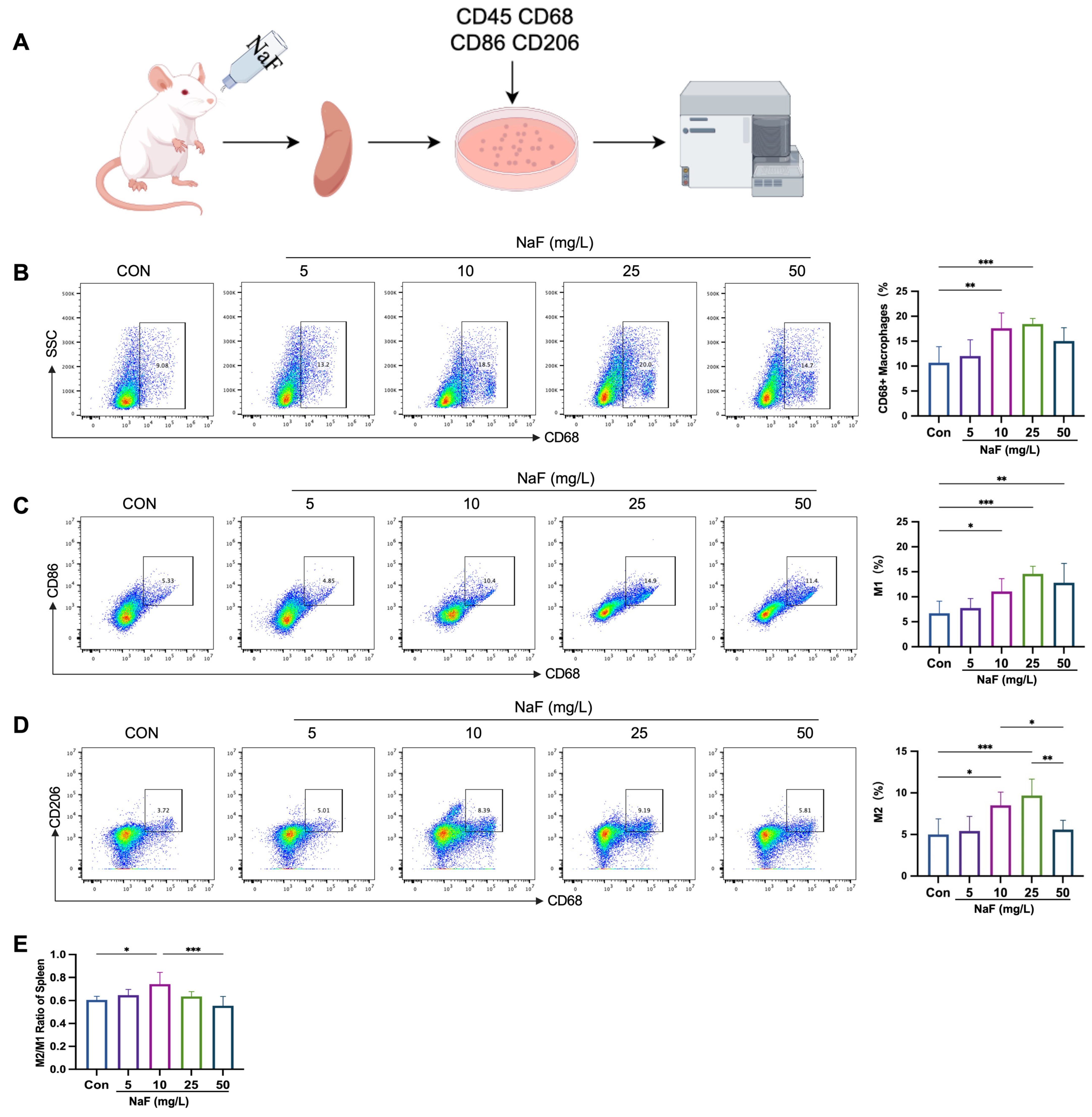
3.3. Verification of the Rat Fluorosis Model
3.4. Fluoride Causes Spleen Damage and Induces Inflammatory Responses
3.5. Low Fluoride Increases the M2/M1 Ratio in the Spleen
3.6. Fluoride Activates the Mitochondrial Autophagy Pathway in the Spleen
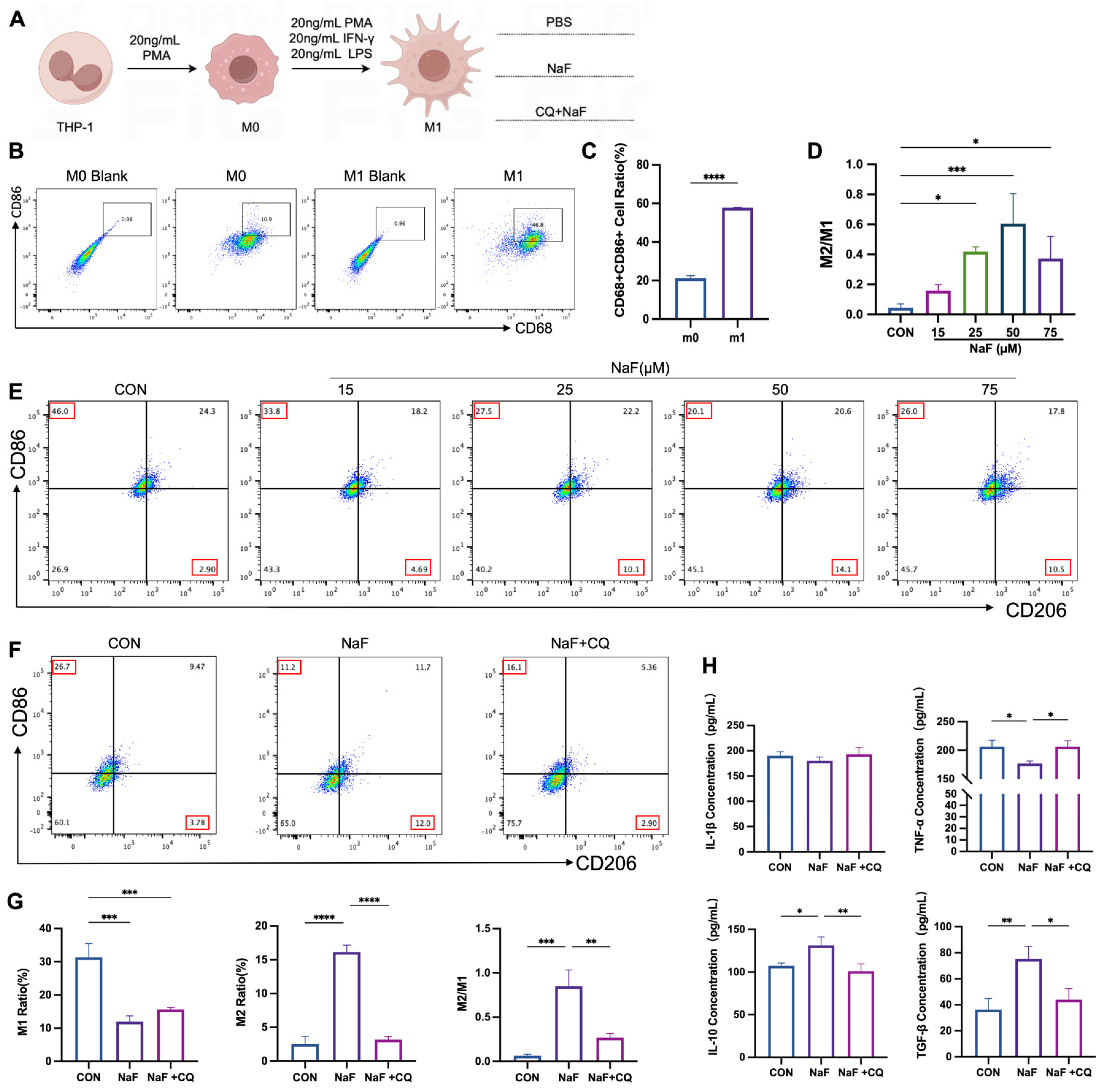
3.7. Mitochondrial Autophagy Mediates Fluoride-Induced Alterations in Macrophage M2/M1 Balance
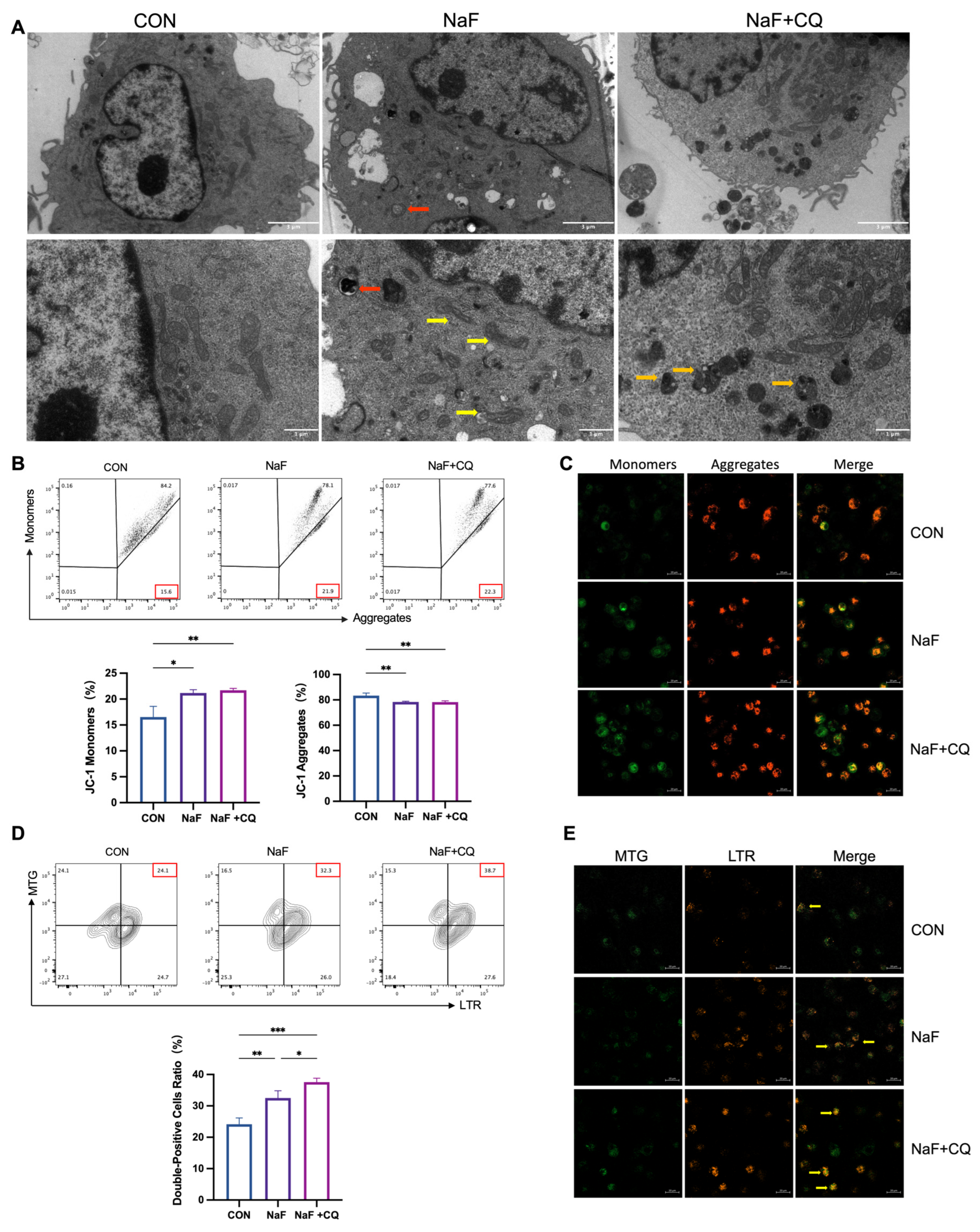
3.8. Fluoride Causes Mitochondrial Damage in Macrophages
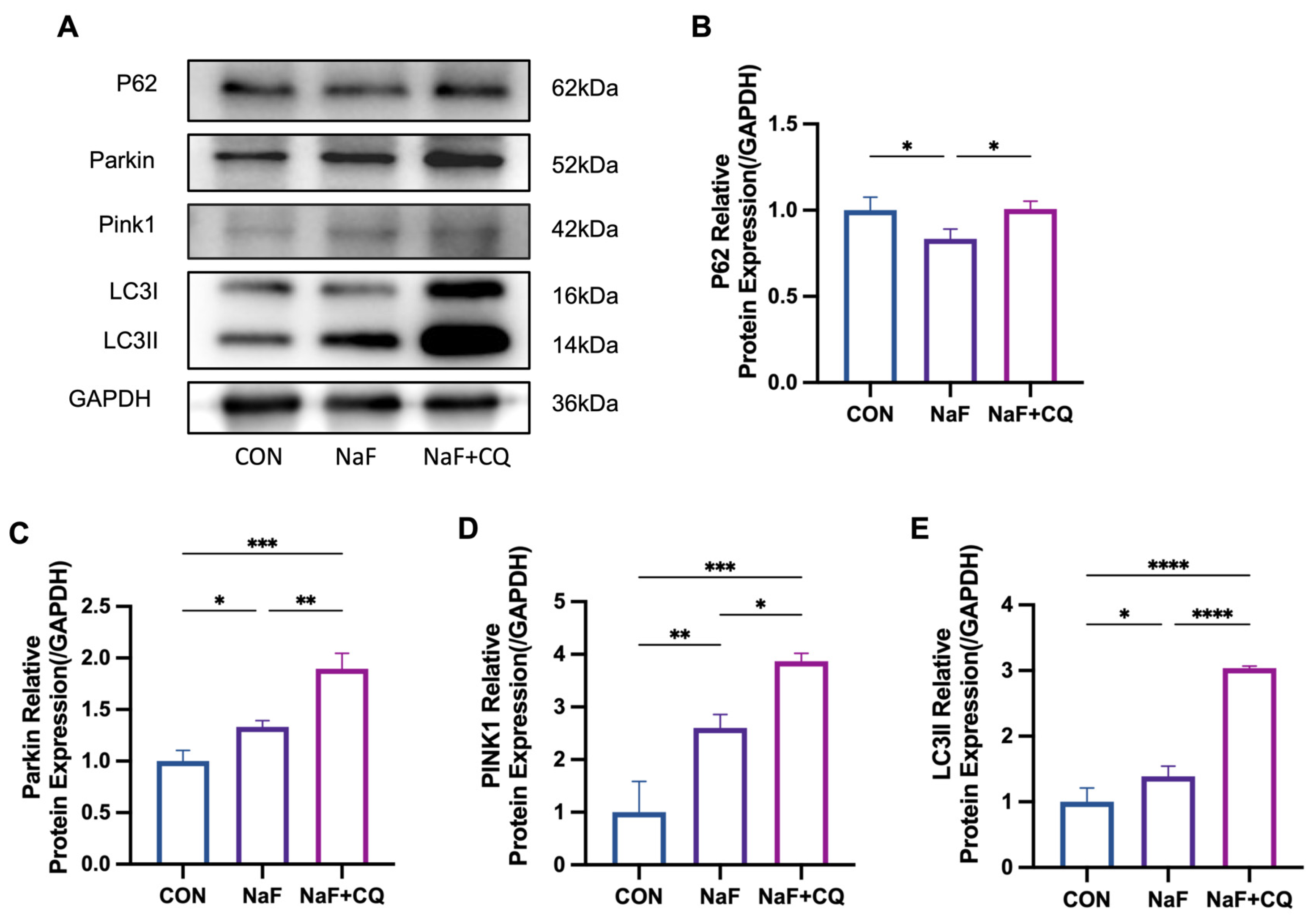
3.9. Fluoride Activates the PINK1/Parkin-Mediated Mitophagy Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Solanki, Y.S.; Agarwal, M.; Gupta, A.B.; Gupta, S.; Shukla, P. Fluoride occurrences, health problems, detection, and remediation methods for drinking water: A comprehensive review. Sci. Total Environ. 2022, 807, 150601. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Huang, T.; Zhang, T.; Li, Q.; Yan, C.; Wang, Q.; Chen, X.; Zhou, J.; Sun, Y.; Bo, W.; et al. A Bayesian benchmark concentration analysis for urinary fluoride and intelligence in adults in Guizhou, China. Sci. Total Environ. 2024, 925, 171326. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.A.; Yousefi, M.; Yaseri, M.; Jalilzadeh, M.; Mahvi, A.H. Skeletal fluorosis in relation to drinking water in rural areas of West Azerbaijan, Iran. Sci. Rep. 2017, 7, 17300. [Google Scholar] [CrossRef]
- Abtahi, M.; Dobaradaran, S.; Jorfi, S.; Koolivand, A.; Khaloo, S.S.; Spitz, J.; Saeedi, H.; Golchinpour, N.; Saeedi, R. Age-sex specific disability-adjusted life years (DALYs) attributable to elevated levels of fluoride in drinking water: A national and subnational study in Iran, 2017. Water Res. 2019, 157, 94–105. [Google Scholar] [CrossRef]
- Ling, Y.; Podgorski, J.; Sadiq, M.; Rasheed, H.; Eqani, S.; Berg, M. Monitoring and prediction of high fluoride concentrations in groundwater in Pakistan. Sci. Total Environ. 2022, 839, 156058. [Google Scholar] [CrossRef] [PubMed]
- Yadav, K.K.; Kumar, S.; Pham, Q.B.; Gupta, N.; Rezania, S.; Kamyab, H.; Yadav, S.; Vymazal, J.; Kumar, V.; Tri, D.Q.; et al. Fluoride contamination, health problems and remediation methods in Asian groundwater: A comprehensive review. Ecotoxicol. Environ. Saf. 2019, 182, 109362. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, D.; Wei, W. Necessity to Pay Attention to the Effects of Low Fluoride on Human Health: An Overview of Skeletal and Non-skeletal Damages in Epidemiologic Investigations and Laboratory Studies. Biol. Trace Elem. Res. 2023, 201, 1627–1638. [Google Scholar] [CrossRef]
- Lin, X.; Fu, B.; Xiong, Y.; Xu, S.; Liu, J.; Zaky, M.Y.; Qiu, D.; Wu, H. Folic acid Ameliorates the Declining Quality of Sodium Fluoride-Exposed Mouse Oocytes through the Sirt1/Sod2 Pathway. Aging Dis. 2022, 13, 1471–1487. [Google Scholar] [CrossRef]
- Chen, L.; Kuang, P.; Liu, H.; Wei, Q.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; et al. Sodium Fluoride (NaF) Induces Inflammatory Responses Via Activating MAPKs/NF-κB Signaling Pathway and Reducing Anti-inflammatory Cytokine Expression in the Mouse Liver. Biol. Trace Elem. Res. 2019, 189, 157–171. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Yi, J.; Li, Y.; Yang, B.; Shang, P.; Mehmood, K.; Bilal, R.M.; Zhang, H.; Chang, Y.F.; et al. The potential risks of chronic fluoride exposure on nephrotoxic via altering glucolipid metabolism and activating autophagy and apoptosis in ducks. Toxicology 2021, 461, 152906. [Google Scholar] [CrossRef]
- Dec, K.; Łukomska, A.; Skonieczna-Żydecka, K.; Jakubczyk, K.; Tarnowski, M.; Lubkowska, A.; Baranowska-Bosiacka, I.; Styburski, D.; Skórka-Majewicz, M.; Maciejewska, D.; et al. Chronic Exposure to Fluoride Affects GSH Level and NOX4 Expression in Rat Model of This Element of Neurotoxicity. Biomolecules 2020, 10, 422. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Hao, X.; Nie, Q.; Feng, C.; Wang, H.; Sun, Z.; Niu, R.; Wang, J. Effects of fluoride on the ultrastructure and expression of Type I collagen in rat hard tissue. Chemosphere 2015, 128, 36–41. [Google Scholar] [CrossRef]
- Jiang, S.; Liang, C.; Gao, Y.; Liu, Y.; Han, Y.; Wang, J.; Zhang, J. Fluoride exposure arrests the acrosome formation during spermatogenesis via down-regulated Zpbp1, Spaca1 and Dpy19l2 expression in rat testes. Chemosphere 2019, 226, 874–882. [Google Scholar] [CrossRef]
- Qiao, Y.; Cui, Y.; Tan, Y.; Zhuang, C.; Li, X.; Yong, Y.; Zhang, X.; Ren, X.; Cai, M.; Yang, J.; et al. Fluoride induces immunotoxicity by regulating riboflavin transport and metabolism partly through IL-17A in the spleen. J. Hazard. Mater. 2024, 476, 135085. [Google Scholar] [CrossRef]
- Liu, Z.; Liang, Q.; Ren, Y.; Guo, C.; Ge, X.; Wang, L.; Cheng, Q.; Luo, P.; Zhang, Y.; Han, X. Immunosenescence: Molecular mechanisms and diseases. Signal Transduct. Target. Ther. 2023, 8, 200. [Google Scholar] [CrossRef]
- Lucas, T.; Waisman, A.; Ranjan, R.; Roes, J.; Krieg, T.; Müller, W.; Roers, A.; Eming, S.A. Differential roles of macrophages in diverse phases of skin repair. J. Immunol. 2010, 184, 3964–3977. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Novak, M.L.; Koh, T.J. Phenotypic transitions of macrophages orchestrate tissue repair. Am. J. Pathol. 2013, 183, 1352–1363. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Taylor, P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005, 5, 953–964. [Google Scholar] [CrossRef]
- Stein, M.; Keshav, S.; Harris, N.; Gordon, S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: A marker of alternative immunologic macrophage activation. J. Exp. Med. 1992, 176, 287–292. [Google Scholar] [CrossRef]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Teshome, S.; Zealiyas, K.; Abubeker, A.; Tadesse, F.; Balakrishna, J.; Weigel, C.; Abebe, T.; Ahmed, E.H.; Baiocchi, R.A. Detection and Quantification of the Epstein-Barr Virus in Lymphoma Patients from Ethiopia: Molecular and Serological Approaches. Microorganisms 2023, 11, 2606. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Pamer, E.G. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 2011, 11, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef]
- Sindrilaru, A.; Peters, T.; Wieschalka, S.; Baican, C.; Baican, A.; Peter, H.; Hainzl, A.; Schatz, S.; Qi, Y.; Schlecht, A.; et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J. Clin. Investig. 2011, 121, 985–997. [Google Scholar] [CrossRef]
- Bi, J.; Mo, C.; Li, S.; Zeng, J.; Chai, Y.; Yao, M.; Liu, Z.; Yuan, P.; Ni, J.; Xu, S. High concentrations of NaF aggravate periodontitis by promoting M1 polarization in macrophages. Int. Immunopharmacol. 2024, 140, 112830. [Google Scholar] [CrossRef]
- Du, C.; Xiao, P.; Gao, S.; Chen, S.; Chen, B.; Huang, W.; Zhao, C. High Fluoride Ingestion Impairs Bone Fracture Healing by Attenuating M2 Macrophage Differentiation. Front. Bioeng. Biotechnol. 2022, 10, 791433. [Google Scholar] [CrossRef]
- Gutowska, I.; Baranowska-Bosiacka, I.; Baśkiewicz, M.; Milo, B.; Siennicka, A.; Marchlewicz, M.; Wiszniewska, B.; Machaliński, B.; Stachowska, E. Fluoride as a pro-inflammatory factor and inhibitor of ATP bioavailability in differentiated human THP1 monocytic cells. Toxicol. Lett. 2010, 196, 74–79. [Google Scholar] [CrossRef]
- Gutowska, I.; Baranowska-Bosiacka, I.; Goschorska, M.; Kolasa, A.; Łukomska, A.; Jakubczyk, K.; Dec, K.; Chlubek, D. Fluoride as a factor initiating and potentiating inflammation in THP1 differentiated monocytes/macrophages. Toxicol Vitr. 2015, 29, 1661–1668. [Google Scholar] [CrossRef]
- Harbauer, A.B.; Zahedi, R.P.; Sickmann, A.; Pfanner, N.; Meisinger, C. The protein import machinery of mitochondria-a regulatory hub in metabolism, stress, and disease. Cell Metab. 2014, 19, 357–372. [Google Scholar] [CrossRef]
- Li, J.; Yang, D.; Li, Z.; Zhao, M.; Wang, D.; Sun, Z.; Wen, P.; Dai, Y.; Gou, F.; Ji, Y.; et al. PINK1/Parkin-mediated mitophagy in neurodegenerative diseases. Ageing Res. Rev. 2023, 84, 101817. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Gao, Y.; He, Y.; Han, Y.; Manthari, R.K.; Tikka, C.; Chen, C.; Wang, J.; Zhang, J. Fluoride induced mitochondrial impairment and PINK1-mediated mitophagy in Leydig cells of mice: In vivo and in vitro studies. Environ. Pollut. 2020, 256, 113438. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, J.; Zhang, J.; Sun, Z.; Niu, R.; Manthari, R.K.; Ommati, M.M.; Wang, S.; Wang, J. Fluoride exposure induces mitochondrial damage and mitophagy via activation of the IL-17A pathway in hepatocytes. Sci. Total Environ. 2022, 804, 150184. [Google Scholar] [CrossRef]
- Xiao, Z.; Liu, M.; Yang, F.; Liu, G.; Liu, J.; Zhao, W.; Ma, S.; Duan, Z. Programmed cell death and lipid metabolism of macrophages in NAFLD. Front. Immunol. 2023, 14, 1118449. [Google Scholar] [CrossRef] [PubMed]
- U.S. Public Health Service Recommendation for Fluoride Concentration in Drinking Water for the Prevention of Dental Caries. Public Health Rep. 2015, 130, 318–331. [CrossRef]
- Biswas, A.; Prathibha, C. Nanocomposite of Ceria and Trititanate Nanotubes as an Efficient Defluoridating Material for Real-Time Groundwater: Synthesis, Regeneration, and Leached Metal Risk Assessment. ACS Omega 2021, 6, 31751–31764. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, Y.; Ye, Y.; Xia, Y.; Shang, L.; Ding, Z.; Wang, P. Comprehensive evaluation of the effectiveness of endemic disease prevention and control in Jiangsu Province, China, 2013–2022. Front. Public Health 2023, 11, 1271765. [Google Scholar] [CrossRef]
- Kheradpisheh, Z.; Mirzaei, M.; Mahvi, A.H.; Mokhtari, M.; Azizi, R.; Fallahzadeh, H.; Ehrampoush, M.H. Impact of Drinking Water Fluoride on Human Thyroid Hormones: A Case—Control Study. Sci. Rep. 2018, 8, 2674. [Google Scholar] [CrossRef]
- Zhou, G.; Yang, L.; Luo, C.; Liu, H.; Li, P.; Cui, Y.; Liu, L.; Yu, X.; Zeng, Q.; Chen, J.; et al. Low-to-moderate fluoride exposure, relative mitochondrial DNA levels, and dental fluorosis in Chinese children. Environ. Int. 2019, 127, 70–77. [Google Scholar] [CrossRef]
- Wang, F.; Li, Y.; Tang, D.; Zhao, J.; Yang, B.; Zhang, C.; Su, M.; He, Z.; Zhu, X.; Ming, D.; et al. Epidemiological analysis of drinking water-type fluorosis areas and the impact of fluorosis on children’s health in the past 40 years in China. Environ. Geochem. Health 2023, 45, 9925–9940. [Google Scholar] [CrossRef]
- Mohd Nor, N.A.; Chadwick, B.L.; Farnell, D.J.J.; Chestnutt, I.G. The impact of a reduction in fluoride concentration in the Malaysian water supply on the prevalence of fluorosis and dental caries. Community Dent. Oral Epidemiol. 2018, 46, 492–499. [Google Scholar] [CrossRef] [PubMed]
- WS/T 212-2001; Determination of Fluoride in Serum—Ion Selective Electrode Method. Disease Control and Prevention Administration: Beijing, China, 2001. Available online: https://www.ndcpa.gov.cn (accessed on 29 April 2025). (In Chinese)
- WS/T 89-2015; Determination of Fluoride in Urine—Ion Selective Electrode Method. Disease Control and Prevention Administration: Beijing, China, 2015. Available online: https://www.ndcpa.gov.cn (accessed on 29 April 2025). (In Chinese)
- Dean, H.T. Endemic fluorosis and its relation to dental caries, 1938. Public Health Rep. 2006, 121 (Suppl. S1), 213–219, discussion 212. [Google Scholar] [PubMed]
- Kratofil, R.M.; Kubes, P.; Deniset, J.F. Monocyte Conversion During Inflammation and Injury. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 35–42. [Google Scholar] [CrossRef]
- Death, C.; Coulson, G.; Kierdorf, U.; Kierdorf, H.; Morris, W.K.; Hufschmid, J. Dental fluorosis and skeletal fluoride content as biomarkers of excess fluoride exposure in marsupials. Sci. Total Environ. 2015, 533, 528–541. [Google Scholar] [CrossRef]
- Bingol, B.; Sheng, M. Mechanisms of mitophagy: PINK1, Parkin, USP30 and beyond. Free. Radic. Biol. Med. 2016, 100, 210–222. [Google Scholar] [CrossRef]
- Zhu, S.; Liu, B.; Fu, G.; Yang, L.; Wei, D.; Zhang, L.; Zhang, Q.; Gao, Y.; Sun, D.; Wei, W. PKC-θ is an important driver of fluoride-induced immune imbalance of regulatory T cells/effector T cells. Sci. Total Environ. 2024, 934, 173081. [Google Scholar] [CrossRef]
- Bagbi, Y.; Yomgam, P.; Libang, E.; Boruah, B.; Kaur, J.; Jayanthi, S.; Kumar, S.; Dhania, N.K. Waste bamboo-derived magnetically separable bamboo-activated carbon: From characterization to effective remediation of fluoride (F(-)) ions from water. RSC Adv. 2024, 14, 24952–24968. [Google Scholar] [CrossRef]
- WS/T 10023—2024; Safety Guideline for Urinary Fluoride in Populations. Disease Control and Prevention Administration: Beijing, China, 2024. Available online: https://www.ndcpa.gov.cn (accessed on 8 April 2025). (In Chinese)
- Food and Drug Administration. Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers; Food and Drug Administration: Rockville, MD, USA, 2005; p. 10.
- Younos, I.; Donkor, M.; Hoke, T.; Dafferner, A.; Samson, H.; Westphal, S.; Talmadge, J. Tumor- and organ-dependent infiltration by myeloid-derived suppressor cells. Int. Immunopharmacol. 2011, 11, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Kuang, P.; Cui, H.; Yu, L. Sodium fluoride suppresses spleen development through MAPK/ERK signaling pathway in mice. Ecotoxicol. Environ. Saf. 2022, 241, 113764. [Google Scholar] [CrossRef]
- Wei, W.; Wang, Q.; Pang, S.; Tan, S.; Sun, J.; Li, M.; Wang, S.; Sun, D. Excessive fluoride exposure induces thymocyte apoptosis and impairs cell division: Roles of the PERK and IRE1 pathways. Toxicol. Lett. 2020, 328, 35–44. [Google Scholar] [CrossRef]
- Wei, W.; Pang, S.; Fu, X.; Tan, S.; Wang, Q.; Wang, S.; Sun, D. The role of PERK and IRE1 signaling pathways in excessive fluoride mediated impairment of lymphocytes in rats’ spleen in vivo and in vitro. Chemosphere 2019, 223, 1–11. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, E.M.; Spiller, K.L. Pro-inflammatory polarization primes Macrophages to transition into a distinct M2-like phenotype in response to IL-4. J. Leukoc. Biol. 2022, 111, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, D.; Liu, M.; Ji, Q.; Zhang, B.; Mei, Q.; Cheng, Y.; Zhou, S. Tregs biomimetic nanoparticle to reprogram inflammatory and redox microenvironment in infarct tissue to treat myocardial ischemia reperfusion injury in mice. J. Nanobiotechnol. 2022, 20, 251. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.H.; Hsu, H.Y.; Chiou, H.C.; Tsai, M.L.; You, H.L.; Lin, Y.C.; Liao, W.T.; Lin, Y.C. Arsenic Induces M2 Macrophage Polarization and Shifts M1/M2 Cytokine Production via Mitophagy. Int. J. Mol. Sci. 2022, 23, 3879. [Google Scholar] [CrossRef]
- Tsai, M.L.; Tsai, Y.G.; Lin, Y.C.; Hsu, Y.L.; Chen, Y.T.; Tsai, M.K.; Liao, W.T.; Lin, Y.C.; Hung, C.H. IL-25 Induced ROS-Mediated M2 Macrophage Polarization via AMPK-Associated Mitophagy. Int. J. Mol. Sci. 2021, 23, 3. [Google Scholar] [CrossRef]
- Song, C.; Zhang, A.; Zhang, M.; Song, Y.; Huangfu, H.; Jin, S.; Sun, Y.; Zhang, C.; Shi, D.; Wang, J.; et al. Nrf2/PINK1-mediated mitophagy induction alleviates sodium fluoride-induced hepatic injury by improving mitochondrial function, oxidative stress, and inflammation. Ecotoxicol. Environ. Saf. 2023, 252, 114646. [Google Scholar] [CrossRef]
- Yan, X.; Wang, L.; Yang, X.; Qiu, Y.; Tian, X.; Lv, Y.; Tian, F.; Song, G.; Wang, T. Fluoride induces apoptosis in H9c2 cardiomyocytes via the mitochondrial pathway. Chemosphere 2017, 182, 159–165. [Google Scholar] [CrossRef]
- Ge, J.; Li, H.; Yang, J.Q.; Yue, Y.; Lu, S.Y.; Nie, H.Y.; Zhang, T.; Sun, P.M.; Yan, H.F.; Sun, H.W.; et al. Autophagy in hepatic macrophages can be regulator and potential therapeutic target of liver diseases: A review. Medicine 2023, 102, e33698. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, S.; Zhou, S.; Qiu, J.; Shi, C.; Liu, R.; Zhou, H.; Lu, L. Hyperglycemia aggravates acute liver injury by promoting liver-resident macrophage NLRP3 inflammasome activation via the inhibition of AMPK/mTOR-mediated autophagy induction. Immunol. Cell Biol. 2020, 98, 54–66. [Google Scholar] [CrossRef]
- Ohashi, K.; Parker, J.L.; Ouchi, N.; Higuchi, A.; Vita, J.A.; Gokce, N.; Pedersen, A.A.; Kalthoff, C.; Tullin, S.; Sams, A.; et al. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J. Biol. Chem. 2010, 285, 6153–6160. [Google Scholar] [CrossRef]
- Mironov, A.S.; Gusarov, I.; Rafikov, R.; Lopez, L.E.; Shatalin, K.; Kreneva, R.A.; Perumov, D.A.; Nudler, E. Sensing small molecules by nascent RNA: A mechanism to control transcription in bacteria. Cell 2002, 111, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.K.; Xu, X.; Wu, M.; Li, X.; Hoque, M.; Li, G.H.Y.; Lian, Q.; Long, K.; Zhou, T.; Piao, H.; et al. MDM2 induces pro-inflammatory and glycolytic responses in M1 macrophages by integrating iNOS-nitric oxide and HIF-1α pathways in mice. Nat. Commun. 2024, 15, 8624. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.M.; Wang, Q.M.; Huang, B.Y.; Mai, C.T.; Wang, C.L.; Wang, T.T.; Zhang, X.J. Dioscin ameliorates murine ulcerative colitis by regulating macrophage polarization. Pharmacol. Res. 2021, 172, 105796. [Google Scholar] [CrossRef] [PubMed]


| Variable | Sample | Urinary Fluoride Concentration (mg/L) c | t/F | p |
|---|---|---|---|---|
| Total | 249 | 1.19 (0.79, 1.58) | ||
| Age(years) b | 58.14 ± 10.76 | |||
| Sex a | −0.55 | 0.43 | ||
| Male | 101 (40.6%) | 1.08 (0.82, 1.58) | ||
| Female | 148 (59.4%) | 1.23 (0.78, 1.58) | ||
| BMI(kg/m2) b | 24.23 ± 3.35 | |||
| Educational level a | 1.36 | 0.26 | ||
| Primary school | 171 (69%) | 1.26 (0.82, 1.62) | ||
| Junior high school | 64 (26%) | 1.00 (0.74, 1.42) | ||
| Senior high school | 13 (5%) | 1.10 (0.72, 1.57) | ||
| Annual household income a | 0.57 | 0.57 | ||
| <1.2 w | 90 (36%) | 1.15 (0.81, 1.53) | ||
| 1.2–2 w | 59 (24%) | 1.23 (0.82, 1.58) | ||
| >2 w | 100 (40%) | 1.19 (0.79, 1.61) | ||
| Smoke a | 0.61 | 0.43 | ||
| YES | 71 (29%) | 1.18 (0.82, 1.56) | ||
| NO | 178 (71%) | 1.20 (0.78, 1.58) | ||
| Drink a | 3.25 | 0.07 | ||
| YES | 74 (30%) | 1.03 (0.82, 1.50) | ||
| NO | 175 (70%) | 1.24 (0.77, 1.59) | ||
| Colds(every year) a | −1.97 | 0.07 | ||
| 0–4 times | 236 (95%) | 1.18 (0.77, 1.58) | ||
| More than 4 times | 13 (5%) | 1.47 (0.96, 1.72) | ||
| Variable | Urinary Fluoride Concentration (mg/L) | F/χ2 | p | ||
|---|---|---|---|---|---|
| <0.897 | >0.897–≤1.432 | >1.432 | |||
| Urinary fluoride level a | 0.65 (0.50, 0.79) | 1.19 (0.99, 1.33) | 1.77 (1.58, 2.15) | 378.93 | 0.00 |
| Age(years) a | 56.89 ± 10.49 | 57.98 ± 11.53 | 59.55 ± 10.18 | 1.29 | 0.28 |
| BMI (kg/m2) a | 24.06 ± 3.45 | 25.02 ± 3.45 | 23.64 ± 3.03 | 3.45 | 0.03 |
| Fasting Blood Glucose (mmol/L) a | 6.61 ± 2.34 | 6.38 ± 2.56 | 6.34 ± 1.44 | 0.37 | 0.69 |
| Systolic Blood Pressure(mmHg) a | 138.4 ± 23.39 | 140.86 ± 23.54 | 137.57 ± 26.20 | 0.39 | 0.68 |
| Diastolic Blood Pressure (mmHg) a | 85.05 ± 12.31 | 87.68 ± 12.48 | 85.47 ± 12.83 | 0.99 | 0.37 |
| Sex b | 0.15 | 0.94 | |||
| Male | 35 (42.20%) | 33 (39.80%) | 33 (39.80%) | ||
| Female | 48 (57.80%) | 50 (60.20%) | 50 (60.20%) | ||
| Educational levela b | |||||
| Primary school | 55 (66.30%) | 53 (64.60%) | 63 (75.90%) | −0.08 | 0.21 |
| Junior high school | 24 (28.90%) | 24 (29.30%) | 16 (19.30%) | ||
| Senior high school | 4 (4.80%) | 5 (6.10%) | 4 (4.80%) | ||
| Annual household income b | |||||
| <1.2 w | 30 (36.10%) | 34 (41.00%) | 26 (31.30%) | 0.04 | 0.54 |
| 1.2–2 w | 17 (20.50%) | 24 (28.90%) | 18 (21.70%) | ||
| >2 w | 36 (43.40%) | 25 (30.10%) | 39 (39.00%) | ||
| Smoke b | 0.16 | 0.92 | |||
| YES | 25 (30.10%) | 23 (27.70%) | 23 (27.70%) | ||
| NO | 58 (69.90%) | 60 (72.30%) | 60 (72.30%) | ||
| Alcohol b | 1.19 | 0.55 | |||
| YES | 26 (31.30%) | 27 (32.50%) | 21 (25.30%) | ||
| NO | 57 (68.70%) | 56 (67.50%) | 62 (74.70%) | ||
| Colds(every year) b | |||||
| 0–4 times | 82 (98.80%) | 78 (94.00%) | 76 (91.60%) | 4.37 | 0.04 |
| more than 4 times | 1 (1.20%) | 5 (6.00%) | 7 (8.40%) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, F.; Zhou, J.; Liu, B.; Zhao, L.; Lv, C.; Zhang, Q.; Yuan, L.; Sun, D.; Wei, W. Low Fluoride Regulates Macrophage Polarization Through Mitochondrial Autophagy Mediated by PINK1/Parkin Axis. Biomolecules 2025, 15, 647. https://doi.org/10.3390/biom15050647
Xie F, Zhou J, Liu B, Zhao L, Lv C, Zhang Q, Yuan L, Sun D, Wei W. Low Fluoride Regulates Macrophage Polarization Through Mitochondrial Autophagy Mediated by PINK1/Parkin Axis. Biomolecules. 2025; 15(5):647. https://doi.org/10.3390/biom15050647
Chicago/Turabian StyleXie, Fengyu, Jing Zhou, Bingshu Liu, Lijun Zhao, Cunqi Lv, Qiong Zhang, Lin Yuan, Dianjun Sun, and Wei Wei. 2025. "Low Fluoride Regulates Macrophage Polarization Through Mitochondrial Autophagy Mediated by PINK1/Parkin Axis" Biomolecules 15, no. 5: 647. https://doi.org/10.3390/biom15050647
APA StyleXie, F., Zhou, J., Liu, B., Zhao, L., Lv, C., Zhang, Q., Yuan, L., Sun, D., & Wei, W. (2025). Low Fluoride Regulates Macrophage Polarization Through Mitochondrial Autophagy Mediated by PINK1/Parkin Axis. Biomolecules, 15(5), 647. https://doi.org/10.3390/biom15050647







