Abstract
Cholesterol sulfate (CS) is a naturally occurring cholesterol derivative that is widely distributed across various tissues and body fluids. In humans, its biosynthesis is primarily mediated by the sulfotransferase (SULT) 2B1b (SULT2B1b). Over the years, CS has been found to play critical roles in various physiological processes, including epidermal cell adhesion, sperm capacitation, platelet adhesion, coagulation, glucolipid metabolism, bone metabolism, gut microbiota metabolism, neurosteroid biosynthesis, T-cell receptor signaling, and immune cell migration. In this review, we first introduce the endogenous regulation of CS biosynthesis and metabolism. We then highlight current advances in the understanding of the physiological roles of CS. Finally, we delve into the implications of CS in various diseases, with a particular focus on its mechanism of action and potential therapeutic applications. A comprehensive understanding of CS’s physiological function, biosynthesis regulation, and role as a disease modifier offers novel insights that could pave the way for innovative therapeutic strategies targeting a wide range of conditions.
1. Introduction
Steroids are generally conjugated with other molecules, such as amino acids, proteins, and sugars, to prolong their biological time in the organism and facilitate the transport of steroids to target sites [1]. In contrast to hundreds of synthetic steroid conjugates, only two types of steroid conjugates occur in living organisms, namely, those conjugated either by sulfotransferases (SULTs) to form sulfates or by uridine diphosphate-glycosyltransferases (UGTs) to form glucuronides. The hydrophilicity of sulfates and glucuronides facilitates the excretion of conjugated steroids in urine and bile. In contrast to glucuronidation, which mainly controls the levels and biological activity of unconjugated hormonal steroids, steroid sulfates possess their own biological activities that differ from those of the respective unconjugated steroids. For example, although both steroid sulfates and their unconjugated steroids can bind to neurotransmitter receptors, their binding sites are different, and their effects on ligand-gated ion channels are often opposite (positive vs. negative) [2]. Moreover, the concentration of many sulfated steroids in blood is usually several-fold higher than that of their unconjugated counterparts, such as DHEA and estrone [3].
Cholesterol sulfate is among the most abundant steroid sulfates in circulation, and its concentration in human blood (platelets and red blood cells) ranges from 1.3 to 2.6 μg/mL [4]. In addition to being a predominant circulating sterol sulfate, CS has also emerged as a significant lipid constituent in a variety of biological fluids and tissues, such as seminal plasma, urine, bile, skin, adrenal glands, the uterine endometrium, and intestinal, kidney, and liver tissue [5]. As a hydrophilic excretion form of cholesterol, CS not only modulates cholesterol homeostasis by targeting cholesterol synthesis and esterification pathways but also has various physiologic functions through interactions with extracellular matrix proteins or cellular adhesive receptors. In addition to having the most investigated physiologic roles in epidermal keratinocyte differentiation and biological membrane homeostasis, CS also acts as a key player in glucolipid metabolism, the immune response, gut microbiota metabolism, and neurosteroid synthesis [5]. It is now evident that CS is associated with various diseases, ranging from diabetes and ulcerative colitis to cancer, bone metabolic disorders, skin diseases, and Alzheimer’s disease. In this review, we aim to elucidate this interesting molecule and focus mainly on the biological pathways influencing human health and disease.
2. CS Biosynthesis and Metabolism
CS is synthesized in a variety of tissues, such as the epidermis, red blood cells, and platelets. The production rate of CS has not been precisely determined, but it is estimated to be approximately 45 mg/day, comparable to that of dehydroepiandrosterone (DHEA) sulfate in the bloodstream [4]. The biosynthesis of CS involves two main steps: the activation of inorganic sulfate and the sulfonation of cholesterol mediated by sulfotransferases (SULTs) (Figure 1). Dietary-derived sulfate, an essential micronutrient, undergoes intestinal absorption through specific sulfate transporters. Following cellular uptake, sulfate activation initiates through ATP sulfurylase (ATPS) activity within the bifunctional 3′-phosphoadenosine 5′-phosphosulfate (PAPS) synthase complex [6]. This enzymatic reaction transfers the adenosine monophosphate (AMP) moiety from ATP to sulfate, generating adenosine-5′-phosphosulfate (APS), characterized by a high-energy phosphoric–sulfuric acid anhydride bond crucial for sulfate activation [7]. Subsequently, the APS kinase (APSK) domain of PAPS synthase phosphorylates APS at the 3′-position to yield the universal sulfate donor PAPS. The final sulfonation step is mediated by sulfotransferases (SULTs) that transfer the activated sulfate group from PAPS to the 3β-hydroxyl group of cholesterol, ultimately producing CS [8].
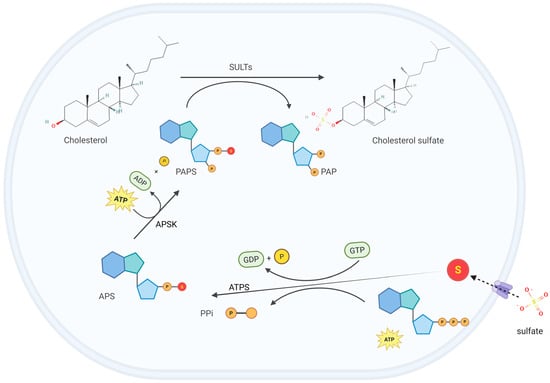
Figure 1.
Cholesterol sulfate (CS) biosynthesis. Dietary-derived sulfate is transported into cells through specific transporters. Once inside the cell, the adenosine monophosphate (AMP) moiety of adenosine triphosphate (ATP) is transferred to sulfate by the ATP sulfurylase (ATPS) activity within the bifunctional 3′-phosphoadenosine 5′-phosphosulfate (PAPS) synthase complex, generating adenosine-5′-phosphosulfate (APS). Subsequently, the APS kinase (APSK) domain of PAPS synthase phosphorylates APS at the 3′-position to generate PAPS, which contains the active sulfate group. Finally, sulfotransferases (SULTs) catalyze the transfer of the active sulfate group from PAPS to the hydroxyl group of cholesterol, producing CS. This figure was created in Biorender. Yu, X. (2025) https://BioRender.com/itac2bb (accessed on 10 March 2025).
SULTs are widely distributed in human tissues, such as the skin, liver, lungs, brain, prostate, placenta, mammary glands, platelets, and kidneys. SULTs are located mainly in the cytoplasm and the Golgi membrane [9]. Cytoplasmic SULTs are involved in the metabolism of endogenous substances (such as hormones and neurotransmitters) and exogenous substances (such as drugs), whereas membrane-bound SULTs mainly participate in the sulfation of tyrosine residues [10]. The human SULT gene family mainly includes SULT1, SULT2, SULT4, and SULT6, among which SULT2 is involved primarily in the sulfation of hydroxysteroids. The human SULT2 family comprises two subfamilies, SULT2A1 and SULT2B1. SULT2A1 preferentially uses DHEA as a substrate and is, therefore, also referred to as DHEA sulfotransferase [11]. The SULT2B1 subfamily consists of SULT2B1a and SULT2B1b, which are produced from the same gene, SULT2B1, on chromosome 19q13.3 through alternative splicing. Typically, SULT2B1b is expressed at higher levels than SULT2B1a. Although both SULT2B1a and SULT2B1b can sulfate DHEA, SULT2B1b preferentially catalyzes the sulfation of cholesterol to form CS, whereas SULT2B1a tends to sulfate pregnenolone [12,13].
CS can be excreted through pathways such as skin shedding, urine, and feces. Both rodents and humans exhibit high levels of CS in urine, intestinal tissues, and feces, with CS in feces constituting 2~20% of total cholesterol [4]. Intracellular CS can be hydrolyzed by the steroid sulfatase gene (STS)-encoded steroid sulfatases (SSases), which are located on the endoplasmic reticulum or Golgi membranes, into free cholesterol. The liberated cholesterol can then bind with steroidogenic acute regulatory protein (StAR), increasing the half-life of the StAR protein. This process promotes cholesterol transport into mitochondria and enhances the synthesis of steroids and high-density lipoprotein (HDL) [14]. The human STS gene is located on Xp22.3-Xpter and is expressed in various tissues, including the placenta, mammary glands, skin, lungs, ovaries, adrenal glands, and brain. STS is associated with high levels of estrogen and androgen within tumors, thereby contributing to the growth of steroid hormone-dependent cancers, such as breast cancer, ovarian cancer, prostate cancer, and endometrial cancer [15].
3. Physiological Functions
CS has diverse physiological functions in the human body. It participates in epidermal differentiation and barrier function formation, modulates T-cell receptor signaling, regulates glucose and lipid metabolism, exerts neuroprotective effects, maintains cell membrane stability, and is involved in platelet adhesion and coagulation processes.
3.1. CS Regulates Keratinocyte Differentiation and Skin Development
The epidermis is composed of the innermost basal layer, spinous layer, granular layer, and outermost stratum corneum, each of which is characterized by a particular type of keratinocyte [16]. After initiating differentiation in the basal layer, keratinocytes progressively move upward toward the surface of the epidermis during the differentiation process, forming a characteristic hierarchical structure. The protective function of the skin is primarily maintained by the stratum corneum, which is composed of multiple layers of lipid matrix interspersed with keratinocytes. The lipids in the stratum corneum mainly include equal amounts of ceramides, free fatty acids, and cholesterol. This balanced lipid composition is crucial for the barrier function of the stratum corneum. CS is distributed in a gradient manner across different epidermal layers, accounting for 1%, 5%, and 1% of the total lipids in the basal layer, granular layer, and stratum corneum, respectively [17]. Compared with the nonpolar and hydrophobic lipid matrix, the acidic sulfate groups of CS impart strong polarity and hydrogen bonding capacity, thereby affecting the fundamental structure of the lipid matrix. Computer modeling of the 3D structure of the lipid layers in the stratum corneum revealed that these layers are very rigid, with each lipid layer containing only one or two water molecules [18]. The incorporation of the polar molecule CS into the lipid layers can loosen the lipid packing and increase lipid hydration, significantly increasing the permeability of the stratum corneum [19].
In addition to serving as a component in the lipid matrix in the stratum corneum, CS also regulates keratinocyte differentiation through multiple mechanisms, maintaining the homeostasis of the epidermal barrier. (1) CS regulates the transcription of genes encoding cornified envelope proteins, such as transglutaminase 1 (TGM1) and involucrin, by coordinating with the AP-1 transcription factor to increase gene expression [20]. (2) CS is more effective than phosphatidylserine and phorbol esters in activating the η isoform of protein kinase C (PKCη), which catalyzes the phosphorylation of epidermal structural proteins and increases the formation of the cornified envelope. PKCη can also phosphorylate and activate AP-1, thereby increasing the transcription of differentiation-associated proteins, such as TG-1 and involucrin [21,22]. (3) During keratinocyte differentiation, the production of keratins stops after the cells move from the basal layer to the granular layer and instead begin to produce various barrier-forming proteins, such as filaggrin and loricrin. CS can induce filaggrin expression either by inducing the expression of Retinoic acid-related orphan receptor alpha (RORα) or serving as a ligand for RORα [23]. Additionally, CS is closely related to normal skin desquamation [17]. An increase in CS enhances cell membrane stability and intercellular cohesion in the stratum corneum, leading to disruption in the desquamation process and skin lesions characterized by dryness and scaling [24,25].
3.2. CS, as a Ligand, Plays Fundamental Roles in Signaling Pathways
RORα is a widely distributed transcription factor that regulates gene expression and is involved in various physiological functions, such as carbohydrate and lipid metabolism, cell cycle regulation, development, and tumorigenesis [26]. Although both CS and cholesterol can serve as endogenous ligands for RORα and participate in RORα-associated molecular signaling pathways, the sulfate group in the CS molecule forms more hydrogen bonds with the ligand-binding domain of RORα than the 3-hydroxyl group in the cholesterol molecule does, thereby enhancing molecular interactions [27]. Consequently, CS has a much greater affinity for RORα than does cholesterol.
CS plays a significant role in the substrate specificity of phosphoinositide 3-kinase (PI3K) [28]. PI3K can be activated by insulin, the platelet-derived growth factor (PDGF), and other factors. Activated PI3K phosphorylates phosphatidylinositol 4,5-bisphosphate (PI-4,5-P2) to generate the second messenger phosphatidylinositol 3,4,5-trisphosphate (PI-3,4,5-P3), which recruits signaling proteins from the cytoplasm to the cell membrane, thereby activating a series of signaling pathways that regulate physiological functions, such as carbohydrate and lipid metabolism, protein synthesis, and cell proliferation. In addition to PI-4,5-P2, PI3K can also phosphorylate phosphatidylinositol (PI) and phosphatidylinositol monophosphate (PI-4-P) [29]. CS enhances the substrate selectivity of PI3K for PI-4,5-P2, thereby maintaining the normal signaling pathway of the second messenger PI-3,4,5-P3 [28].
3.3. CS Maintains Biological Membrane Homeostasis
CS is an important component in various types of biological membranes, such as those in red blood cells, platelets, and sperm, where it acts as a stabilizer (Figure 2). The hydrophilicity of the sulfate group and hydrophobic side chain of cholesterol enable CS to have the ability to stabilize biological membranes and inhibit membrane fusion. CS interacts with phospholipids in a manner similar to that of cholesterol, but the intermembrane exchange rate of CS is significantly faster than that of cholesterol [30], which allows for the sorting of hydrocarbons in the lipid bilayer and stabilizes the membrane structure.
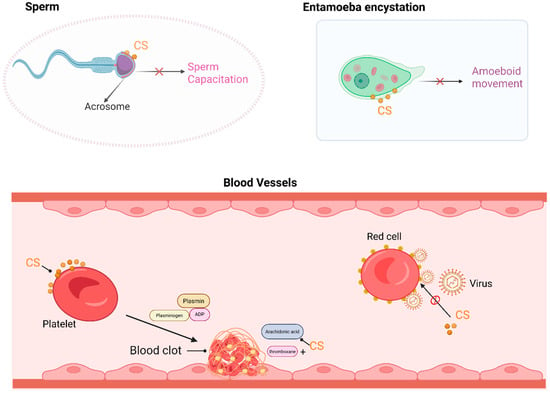
Figure 2.
CS functions as a component in biological membranes. In the sperm plasma membrane, primarily in the acrosomal region, the hydrolysis of CS can lead to membrane destabilization and trigger motility events. In Entamoeba encystation, CS dose-dependently induces and maintains encysting cells as spherical maturing cysts, with almost no phagocytotic activity. In platelets, CS binds to the membrane and enhances ADP (Adenosine diphosphate)- and thrombin-induced platelet aggregation and serotonin secretion. CS is also a potent inhibitor of Sendai virus fusion to erythrocyte membranes. This figure was created in Biorender. Yu, X. (2025) https://BioRender.com/oaeglkg (accessed on 10 March 2025).
In the bloodstream, physiological concentrations of CS can increase the stability of the red blood cell membrane, protecting the cells from osmotic hemolysis [4]. The exposure of red blood cells to high temperatures (51 °C) induces membrane rupture and hemolysis, whereas preincubation of the cells with CS can partially protect them against rupture [31]. Enveloped animal viruses, such as Sendai virus, require fusion between the viral envelope and the cytoplasmic membrane to enter cells [31,32]. CS, rather than cholesterol, can inhibit fusion between the virus and red blood cells, thereby suppressing Sendai virus-induced hemolysis [31]. CS is also a major component in platelets, and the concentration of CS in normal rat or human platelets ranges from 164 to 512 pmol/mL, accounting for approximately 1% of the total cell content [33]. The sulfate groups and steroid ring structure of CS increase its affinity for platelets. CS can be directly integrated into the platelet membrane without altering the platelet cholesterol composition to increase thrombin-induced production of thromboxane B2, which is an end product of arachidonic acid metabolism, leading to an increase in arachidonic acid-induced platelet aggregation and serotonin secretion [34].
CS exists at relatively high concentrations in the male reproductive tract, where it is absorbed by the sperm and is primarily localized in the acrosomal membrane [35]. CS hydrolysis in the sperm membrane may lead to membrane destabilization and trigger sperm motility, which are essential for sperm capacitation. During sperm maturation in the epididymis, CS acts as a membrane stabilizer and an effective inhibitor of the acrosomal protease. CS has a significant effect on sperm quality, with higher levels of CS in the semen of oligospermic or infertile patients, making it a critical predictor of semen quality [36]. In the female reproductive tract, CS is hydrolyzed by steroid sulfatase, which can reverse the inhibitory effects of CS on sperm function, leading to the acrosome reaction and a series of events that culminate in sperm capacitation and fertilization [37,38,39].
Entamoeba histolytica is a kind of amoeboid protozoan parasite, and its inhabitation in the human gastrointestinal tract causes amoebiasis, a global public health issue. Entamoeba infection and transmission occur mainly through the oral ingestion of its cysts [40]. CS is found in amoebic cysts, where it decreases membrane permeability and maintains the spherical shape of the membrane, attenuating the phagocytic activity of amoebic cysts and partially disrupting the transmission of Entamoeba histolytica [41]. Therefore, CS may offer a new strategy for the prevention and treatment of parasitic diseases, but the specific molecular mechanisms underlying this effect require further investigation.
3.4. CS Modulates Inflammatory and Immune Responses
Inflammation is a critical part of an immune system’s response to harmful stimuli, involving various inflammatory mediators and cell types [42]. 5-lipoxygenase (5-LO) is the key enzyme responsible for generating leukotrienes and other soluble inflammatory mediators [43]. Upon Ca2+ influx into the cell, 5-LO binds to the cell membrane and converts arachidonic acid into biologically active leukotrienes, which induce severe inflammation in asthma and bronchitis [44]. CS, as a component of the cell membrane, can directly interact with 5-LO, weakening its interactions with the membrane and thereby reducing leukotriene synthesis and release, ultimately inhibiting the inflammatory response [45]. CS has been shown to suppress IL-17 production in T cells, directly affecting osteoclastogenesis and inhibiting the inflammatory response in rheumatoid arthritis [46]. In addition, CS can inhibit proinflammatory macrophage polarization by increasing nicotinamide adenine dinucleotide phosphate (NADPH) levels and activating the AMP-activated protein kinase (AMPK)-cAMP response element-binding protein (CREB) signaling pathway, alleviating ischemic stroke in a mouse model [47].
The T-cell surface antigen receptor (TCR) is a transmembrane complex composed of antigen-binding subunits (TCRαβ) and CD3 signaling subunits (CD3ζζ, CD3δε, and CD3γε). The TCR is essential for T-cell activation, regulation, and function [48]. Upon binding to peptide antigens presented by major histocompatibility complex (MHC) molecules, the TCR is activated via tyrosine phosphorylation of the CD3 subunits, initiating a cascade of molecular events, including adapter protein phosphorylation, signaling complex formation, intracellular calcium flux, immunological synapse formation, cytokine secretion, and cell proliferation. Cholesterol can directly bind to the TCRβ transmembrane (TCRβ-TM) region to enhance TCR affinity for peptide–MHC ligands [49]. However, CS binds to the TCRβ-TM region with an affinity 300-fold greater than that of cholesterol [50], competitively disrupting TCR–ligand complex formation and inhibiting TCR signaling. CS can also suppress T-cell activation by inhibiting the tyrosine phosphorylation of the CD3 subunit. Furthermore, CS can induce dysfunctional T-cell microvilli, which are crucial for TCR signal transduction and T-cell function [51,52]. Additionally, CS acts as an intrinsic mediator in T-cell signaling, regulating thymocyte development. CS-deficient mice exhibit increased sensitivity to self-antigens, whereas CS injection into the thymus can inhibit thymic selection [53].
Dedicator of cytokinesis protein 2 (DOCK2) can activate the small G protein Rac by mediating the guanine nucleotide exchange, thereby stimulating lymphocyte activation, differentiation, trafficking, and homing [54]. CS, rather than cholesterol or other cholesterol derivatives, directly binds to the catalytic DHR2 domain of DOCK2, inhibiting its guanine-exchange activity and greatly suppressing DOCK2-mediated Rac activation and lymphocyte migration [55]. The ability of CS to suppress immune cell migration is exemplified in the tear film, where CS mediates immune evasion in the eyes [55]. Mice with a specific knockout of the Sult2b1 gene develop severe UV- or antigen-induced ocular surface inflammation, which can be reversed by the administration of CS-containing drops [55]. Moreover, the macrophage-inducible C-type lectin receptor (Mincle) is an innate immune receptor involved in allergic skin inflammation [56]. CS serves as an endogenous ligand for Mincle to modulate allergic skin inflammation [56], although the underlying molecular mechanisms remain unclear.
3.5. CS Regulates Glucose and Lipid Metabolism
CS orchestrates systemic glucose homeostasis through dual regulatory mechanisms. On one hand, CS inhibits hepatic gluconeogenesis by modulating the activity of hepatocyte nuclear factor 4α (HNF4α), a liver-specific transcription factor that governs the expression of key gluconeogenic genes, including PEPCK (Phosphoenolpyruvate carboxykinase) and G6Pase (glucose-6-phosphatase G-6-pase). The activity of HNF4α is positively correlated with its acetylation level. CS can inhibit HNF4α acetylation and interfere with its nuclear transport, thereby suppressing hepatic gluconeogenesis [57,58]. Genetic evidence from Sult2b1 knockout models confirms this regulatory axis. Sult2b1 knockdown decreases hepatic CS synthesis and subsequently inhibits the expression of the deacetylase sirtuin 1. This leads to an increase in HNF4α acetylation and a subsequent enhancement of HNF4α’s gluconeogenic activity [58]. On the other hand, CS enhances glucose-stimulated insulin secretion in pancreatic β-cells by optimizing mitochondrial bioenergetics. Insulin secretion relies on mitochondrial function and ATP production within β-cells. CS maintains the integrity of the mitochondrial membrane structure and enhances the efficiency of oxidative phosphorylation in β-cells, thereby increasing ATP production and promoting insulin secretion [6].
CS is a key regulator of lipid metabolism, exerting its effects through multiple mechanisms. CS acts as an endogenous ligand for RORα, which in turn activates AMPK. This activation leads to the suppression of hepatic liver X receptor α (LXRα), thereby inhibiting the expression of lipogenic genes. This cascade of events significantly reduces hepatic lipid accumulation [59]. Furthermore, CS attenuates cholesterol biosynthesis by inhibiting 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, which is the rate-limiting enzyme in cholesterol synthesis. It also blocks cholesterol esterification by inhibiting lecithin/cholesterol acyltransferase (LCAT), further reducing cholesterol levels [60]. In addition, CS serves as a major substrate for the synthesis of pregnenolone sulfate in adrenal glands or gonads, where the highly active mitochondrial cytochrome P450 cholesterol side chain cleavage enzyme (P450 SCC) converts CS to pregnenolone sulfate [61], which is a crucial step in steroid hormone production.
3.6. CS Alters Gut Microbiota-Derived Metabolites
CS is closely related to the gut microbiota and host intestinal metabolism. Compared with native starch (NS), dietary resistant starch (RS) is less digestible by intestinal enzymes but can be efficiently utilized by the gut microbiota [62]. Moreover, RS can significantly alter the gut microbiota composition, increasing the abundance of Bacteroides, Akkermansia, and Bifidobacteria, while decreasing the abundance of Firmicutes. These changes in microbial composition lead to alterations in host metabolic components, such as CS levels. For example, alterations in the gut microbiota in obese individuals have been associated with significantly elevated CS levels in both plasma and feces [63]. Recent studies have shown that the implantation of Bacteroides thetaiotaomicron into the cecum of germ-free mice increases the host’s CS levels [64,65]. Mechanistically, the genome of Bacteroides thetaiotaomicron encodes steroid SULT, APS kinase, and AS. Once sulfate is transported into bacterial cells, AS catalyzes the conversion of ATP and sulfate to APS, which is then phosphorylated by APS kinase to produce PAPS. Finally, SULTs catalyze the transfer of the sulfate group from PAPS to cholesterol to form CS [65]. Furthermore, CS affects host cell responses, reducing leukocyte migration in a dose-dependent manner, potentially exerting a negative effect on immune cell migration [65,66]. However, the mechanisms by which CS influences the composition and gene expression of the gut microbiota are not fully understood.
3.7. CS Regulates Neurosteroid Synthesis and Affects Nervous System Function
CS is also found in brain tissue, with the cerebellum having the highest CS content [67]. The brain contains many steroid hormones and sulfotransferase enzymes, such as P450SCC, P450-17 hydroxylase, P450 aromatase, 3β-hydroxysteroid dehydrogenase, sulfotransferases, sulfatases, and 5α-reductase [68,69]. Therefore, the brain is capable of synthesizing various steroid hormones, and their derivatives are known as neurosteroids, including pregnenolone (PREG) and PREG sulfate (PREGS), dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS), progesterone, 3α,5α-tetrahydroprogesterone (THP), 3α,5α-tetrahydrodeoxycorticosterone (THDOC), estrogen, and testosterone [70]. CS serves as a substrate for the synthesis of PREGS and DHEAS, directly regulating their intracellular synthesis levels [71]. PREGS and DHEAS play roles in sedation, hypnosis, anxiolysis, and anticonvulsant effects by antagonizing γ-aminobutyric acid (GABA) A receptors [72], which are widely distributed throughout the brain and are the primary inhibitory neurotransmitter receptors in the nervous system. Additionally, DHEA, DHEAS, and PREGS can excite N-methyl-D-aspartate (NMDA) receptors, which are the principal excitatory neurotransmitter receptors involved in memory and learning processes [73]. They increase neuronal excitability and synaptic plasticity, promote wakefulness, and enhance memory and cognitive function [74,75]. Integrative research based on proteomics, metabolomics, and genome-wide association studies (GWASs) has revealed that the levels of CS in individuals with schizophrenia differ significantly from those in healthy controls [76].
4. CS and Diseases
Current research has focused on the implications of CS in diseases such as X-linked ichthyosis, diabetes, Alzheimer’s disease, ulcerative colitis, bone metabolic diseases, and cancer (Figure 3), and advancements have been made in understanding the pathophysiological effects and therapeutic applications of CS.
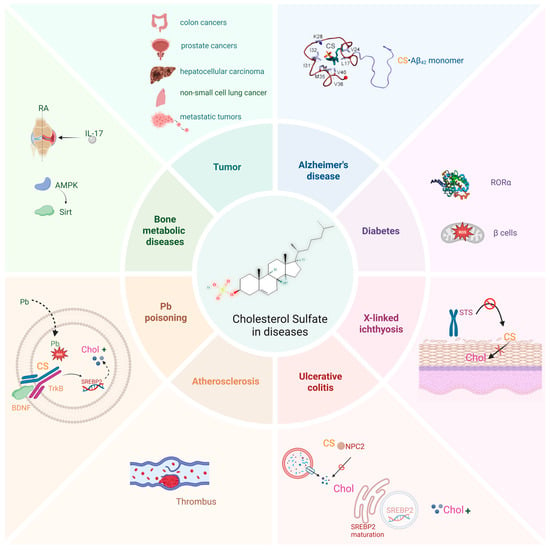
Figure 3.
CS is implicated in various diseases. CS plays an important biological role in various diseases, such as different types of cancers, Alzheimer’s disease, Atherosclerosis, diabetes, X-linked ichthyosis, and ulcerative colitis. This figure was created in Biorender. Yu, X. (2025) https://BioRender.com/ls81p2c (accessed on 10 March 2025).
4.1. X-Linked Ichthyosis
The protective function of the skin is primarily maintained by the stratum corneum, which consists of multiple layers of lipid matrices and embedded keratinocytes. CS is desulfated into cholesterol by SSase, a process essential for maintaining the homeostasis of stratum corneum lipids. SSase is encoded by the STS gene, which is located on the human X chromosome. Mutations in the STS gene lead to reduced SSase activity, which impairs the desulfation of CS into cholesterol. This results in an elevated CS/cholesterol ratio, causing the development of recessive X-linked ichthyosis (RXLI) [77].
Excess CS can also inhibit hydroxymethylglutaryl-CoA (HMG-CoA) reductase [60], a rate-limiting enzyme for cholesterol synthesis, resulting in an approximately 50% reduction in cholesterol content in RXLI patients. Recent studies analyzing skin samples from patients with RXLI have revealed a decreased expression of transglutaminase 1 enzyme (TGase 1), an enzyme crucial for the formation of the cornified envelope (CE) and the cornified lipid envelope (CLE). Supraphysiological levels of CS severely interfere with involucrin cross-linking and ω-hydroxyceramide esterification by TGase 1 [20]. Further transcriptomic analysis of STS-deficient keratinocytes reveals decreased CS production, along with the dysregulation of epidermal differentiation and lipid metabolism pathways. Notably, there was a marked downregulation in aldehyde dehydrogenase (ALDH) isoforms (ALDH1A1 and ALDH3A1) and the oxytocin receptor (OXTR) [78]. ALDH1A1 and ALDH3A1 are known to play roles in maintaining corneal transparency [79], while OXTR downregulation associates with neurobehavioral abnormalities via impaired oxytocin signaling [80]. Consistent with these findings, RXLI has long been associated with an increased risk of corneal opacities and autism-like features [81]. These findings not only elucidate the pathophysiological cascade from STS mutations to RXLI multisystem manifestations but also establish CS as a critical metabolic regulator interfacing epidermal differentiation, neuroendocrine signaling, and ocular homeostasis.
4.2. Diabetes
Type 2 diabetes (T2D) is characterized by the progressive deterioration of pancreatic β-cell function and a decrease in β-cell mass. Recent studies have indicated that improvements in metabolic status at the early stages of diabetes can maintain or even reverse β-cell function [82,83]. CS regulates cholesterol homeostasis by targeting key enzymes in the cholesterol synthesis pathway, such as 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) and lecithin-cholesterol acyltransferase [60]. Cholesterol metabolism is closely related to pancreatic β-cell function, as the accumulation of cholesterol within cells impairs β-cell function [84]. CS mitigates diabetes in streptozotocin-induced diabetic mice by increasing β-cell mass and function [6]. Additionally, CS regulates β-cell mitochondrial efficiency, reduces the production of reactive oxygen species (ROS), enhances β-cell antioxidant capacity, and decreases apoptosis [6].
CS is a natural ligand for RORα and can regulate various metabolic pathways through RORα [27]. RORα plays a role in controlling blood glucose homeostasis and the development of diabetes by modulating gluconeogenesis [85] and is also closely associated with lipogenesis, insulin production, and insulin sensitivity [86,87]. As a natural ligand of RORα, CS holds significant potential in influencing the pathogenesis and progression of diabetes.
4.3. Alzheimer’s Disease
Alzheimer’s disease (AD), a progressive neurodegenerative disorder characterized by cognitive decline and neuronal loss, represents the most prevalent form of dementia worldwide. Epidemiological studies project a dramatic increase in the disease burden, with dementia prevalence expected to double in Europe and triple globally by 2050 [88]. In China, a population-based cohort study (n = 626,276) revealed an AD prevalence of 3.48% and an annual incidence rate of 7.90 per 1000 person-years among older adults [89]. The pathological hallmarks of AD include the extracellular deposition of abnormal β-amyloid protein (Aβ) plaques, resulting from abnormal protein aggregation, and the intracellular accumulation of hyperphosphorylated tau protein (pTau), forming neurofibrillary tangles (NFTs) [90]. Notably, emerging evidence implicates astrocyte–neuron metabolic coupling in AD pathogenesis. Astrocytic SULT2B1b catalyzes the conversion of cholesterol into CS, which is subsequently transported to the mature neuron via the ATP-binding cassette transporter-2 (ABCA2) [91]. CS plays dual pathological roles in AD progression, not only promoting Aβ plaque formation but also facilitating tau hyperphosphorylation and NFT assembly [92,93] (Figure 4).
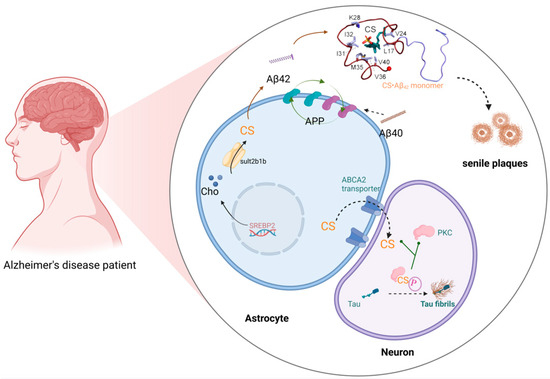
Figure 4.
CS in Alzheimer’s disease. CS binds to amyloid precursor protein (APP) and promotes the cleavage of APP into aggregation-prone Aβ42, fostering the formation of Aβ plaques. Sult2b1b in the astrocyte cytosol converts cholesterol into CS, both of which are provided by the astrocyte to the mature neuron via the ATP-binding cassette transporter-2 (ABCA2), which does not synthesize cholesterol. Once in the neuron, CS binds protein kinase C (PKC), causing it to hyperphosphorylate tau protein fibrils, which aggregate into toxic neurofibrillary tangles. This figure was created in Biorender. Yu, X. (2025) https://BioRender.com/7dts6k3 (accessed on 10 March 2025).
Molecular dynamics simulations demonstrate that CS exhibits remarkable nucleation-enhancing capacity, accelerating Aβ-42 fibril formation with a 260-fold greater efficiency than cholesterol [94]. Aβ is generated through the sequential proteolysis of amyloid precursor protein (APP) by various proteases, including α-, β-, γ-, η-, and δ-secretases, resulting mainly in the Aβ1-40 and Aβ1-42 forms [95]. APP is a cholesterol-sensitive protein expressed selectively in astrocytes and neurons [96]. Cholesterol and its metabolites can bind to APP, promoting its cleavage into aggregation-prone Aβ1-42, which then associates with cholesterol (in a 1:1 stoichiometry) to form neurotoxic plaques [97]. Compared with cholesterol, CS binds more tightly to Aβ peptides, and when CS accumulates in cell membrane plaques at approximately 50 times the normal level, it significantly accelerates the aggregation of Aβ into toxic, insoluble plaques in the brain, further inducing tau protein aggregation [92]. Biochemical assays show CS differentially activates PKC isoforms, inducing 12.0-, 6.0-, 5.0-, 1.5-, and 1.2-fold increases in PKCε, η, α, δ, and ζ activities, respectively [93,98]. Of particular relevance, PKCα and PKCδ directly phosphorylate tau protein [99]. Thus, CS exacerbates tau protein hyperphosphorylation and tau fibril formation through PKC activation. Therefore, research targeting CS and its rate-limiting enzyme SULT2B1b may provide insights into the metabolic processes and molecular mechanisms underlying AD, potentially identifying new strategies for reversing AD symptoms in the future (Figure 4). Furthermore, CS is a substrate for the synthesis of neuroactive steroids, such as PREGS and DHEAS, which can inhibit GABA_A receptor activation, leading to mood disorders such as depression, anxiety, and epilepsy [100,101].
4.4. Ulcerative Colitis
Ulcerative colitis (UC) is a chronic, nonspecific inflammatory bowel disease (IBD) characterized by periods of remission and relapse, involving persistent and confluent inflammatory responses in the mucosa and submucosa of the colon and rectum. It is a lifelong condition with an increasing incidence rate each year [102]. The pathophysiology of UC is complex [103]. Following damage to the intestinal epithelial barrier, macrophages and antigen-presenting cells are activated, leading to the maturation of infiltrating monocytes into macrophages that produce various proinflammatory cytokines, such as TNF, IL-12, IL-23, and IL-6. These cytokines increase the levels of chemokines that attract neutrophils, thereby promoting the development of intestinal inflammation [104]. Nonspecific anti-inflammatory drugs, such as 5-aminosalicylic acid derivatives, corticosteroids, and immunosuppressants, aim to suppress epithelial barrier damage through anti-inflammatory and immunosuppressive effects [103,105]. Recent studies have shown that CS accelerates mucosal healing in UC by promoting cholesterol synthesis in colon epithelial cells [106]. In UC patients, the expression of SULT2B1 and genes involved in cholesterol biosynthesis is upregulated, and the levels of CS and cholesterol are significantly elevated [106]. Mechanistically, its proinflammatory factors induce the expression of SULT2B1, which increases CS synthesis. CS can bind to the Niemann–Pick disease type C2 (NPC2) protein, disrupting cholesterol transport in lysosomes [107,108]. This activates sterol regulatory element-binding protein 2 (SREBP2), which promotes cholesterol biosynthesis and increases intracellular cholesterol levels, ultimately aiding in the repair of damaged intestinal epithelial cells [106]. Additionally, CS has been shown to limit neutrophil recruitment during mucosal damage by inhibiting DOCK2-mediated Rac activation, reducing excessive immune cell accumulation, preventing excessive intestinal inflammation, and accelerating tissue repair [109]. Dietary supplementation with CS can alleviate dextran sulfate sodium (DSS)-induced colitis, providing a novel strategy for UC treatment.
4.5. Bone Metabolic Diseases
Bone tissue constantly undergoes remodeling to repair damage and maintain balance through the actions of bone cells, including osteoclast-mediated bone resorption and osteoblast-mediated bone formation [110]. CS has been suggested to play a beneficial role in bone metabolism [111]. In vitro experiments have demonstrated that CS reduces the expression of TRAP (tartrate-resistant acid phosphatase), cathepsin K, and calcitonin receptor in a dose-dependent manner [112]. These factors are critical for osteoclast differentiation and bone resorption. CS has also been reported to activate the AMPK-Sirt1 axis in a RORα-independent manner, leading to the inhibition of NFATc1 activity and subsequent suppression of osteoclast differentiation [113]. In inflammation-induced bone destruction mouse models, CS has been shown to increase femoral bone density, trabecular bone number, bone surface area, and bone volume, while significantly reducing trabecular separation [114]. Rheumatoid arthritis (RA) is a chronic inflammatory arthritis characterized by progressive joint destruction [115]. CD4+ T (Th17) cells, which produce IL-17, play crucial roles in RA onset and progression [116]. CS, a natural ligand for RORα, can inhibit the proliferation of Th17 cells and reduce IL-17 production by modulating transcriptional sites in immature T cells. This effect is associated with a decreased expression of glycolytic molecules and p53 [46]. Collectively, these results suggest that CS may serve as a therapeutic strategy in diseases associated with abnormal bone metabolism.
4.6. Cancer
CS inhibits T lymphocyte immune function through various mechanisms, facilitating immune evasion by tumor cells (Figure 5). (1) CS suppresses DOCK2-mediated RAC activation and lymphocyte migration. DOCK2, an essential RAC activator for lymphocyte migration and activation, loses its ability to activate Rac upon binding with CS, thereby impeding lymphocyte migration and activation, resulting in severe immune deficiency [117]. Certain tumors, such as colorectal cancer and prostate cancer, produce large amounts of CS, creating a microenvironment that excludes T lymphocytes and resists immunotherapy. Clinical studies have shown a negative correlation between CS levels and the extent of CD8+ T-cell infiltration in human colorectal cancer tissues [118]. In hepatocellular carcinoma, CS inhibits the DOCK2 activity in T cells, promoting CD8+ T-cell exhaustion [119]. High expression of the CS-synthesizing enzyme SULT2B1 in colorectal cancer is associated with poor prognosis [120]. In mouse models, cancer cells that produce CS exhibit resistance to immune checkpoint blockade and antigen-specific T-cell-mediated tumor eradication [118]. (2) CS competitively binds to the TCR. The transmembrane domain of TCR can bind to both cholesterol and CS, but the affinity of CS for the site is 300-fold greater than that of cholesterol. CS binding inhibits TCR dimerization and thereby prevents the formation of the MHC: TCR complex, impairing the infiltration of effector T cells into tumor tissues and thereby leading to tumor immune evasion [117]. (3) CS protects cancer cells from cytotoxic T-cell attacks by modulating the tumor necrosis factor (TNF) receptor signaling pathway. Normally, upon binding with the ligand TNFα, TNF receptor 1 (TNFR1) recruits intracellular adaptor proteins with death domains, such as Fas-associated death domain protein (FADD) and TNFR1-associated death domain protein (TRADD), forming a “death-inducing signaling complex” that initiates caspase cascades (caspase-8, caspase-10, and caspase-3), leading to cytoskeletal proteolysis and apoptosis [121]. When the cellular CS concentration increases, TNFα binding instead mediates the recruitment of the inhibitor of nuclear factor-κB (IκB)-kinase (IKK) complex to TNFR1, thus allowing the activation of the pro-survival nuclear factor-κB (NF-κB) response and promoting cell survival [122]. Thus, CS acts as a critical signal shifting cells from apoptosis to survival. The exact mechanism of this shift is not fully understood but may involve changes in the lipid raft microdomains of cholesterol and sphingolipids, which are crucial for proper TNFR1 function [123].
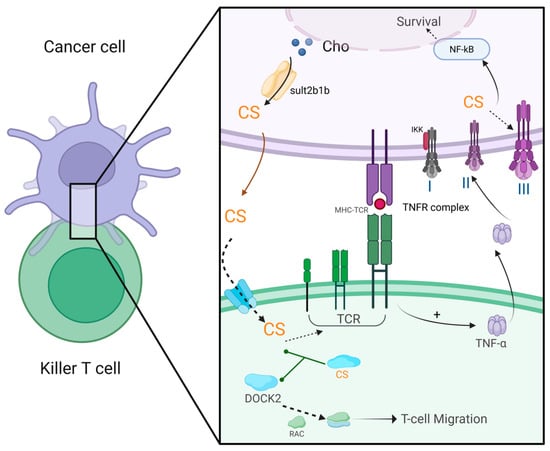
Figure 5.
CS in cancer biology. In cancer cells, Sult2b1b synthesizes CS, which is exported to the extracellular space via transporters. Upon entering mature killer T cells, CS binds to DOCK-2, blocking its ability to activate Ras-related C3 botulinum toxin substrate GTPase (RAC). This inhibition traps RAC in an inactive GDP-bound state, impairing T-cell migration and tumor infiltration. Simultaneously, CS binds the transmembrane domain of the TCR, preventing its dimerization and disrupting the formation of functional TCR-MHC complexes, thereby suppressing antigen recognition and cytokine release (e.g., TNFα). Within the cancer cell, elevated CS levels redirect TNFα signaling the following: instead of promoting apoptosis via FADD/caspase-8 activation (Complex II), TNFα binding stabilizes a pro-survival TNFR complex (Complex III). The pro-survival pathway is initiated by the activation of IKK, which phosphorylates IκB, causing release of NF-κB and exposing its nuclear-localization signaling peptide. As a result, NF-κB translocates to the nucleus and alters gene expression to inhibit apoptosis and promote survival. This figure was created in Biorender. Yu, X. (2025) https://BioRender.com/2bm0ov0 (accessed on 10 March 2025).
In addition, CS promotes cancer cell metastasis. Malignant cells shed from the primary tumor are remodeled to survive in the circulation and colonize in distant organs. Once inoculated, the cells must adapt to the available reservoir of new extracellular metabolites and survive under immune surveillance [124]. Metabolomic analysis revealed that CS is highly upregulated in spontaneous lung metastases compared with paired primary tumors [125]. CS, together with cholesterol, phospholipids, and ceramides, is an essential component that maintains the cytoplasmic membrane homeostasis. CS can alter the activity of serine proteases of the coagulation cascade [34], potentially facilitating platelet interactions with circulating cells, including the dissemination of tumor cells in the blood. CS also accelerates the proteolytic hydrolytic activity of matrix metalloproteinase-7 against selective substrates in the extracellular matrix, which may contribute to tumor cell transfer, and may modulate protein kinase C, which is involved in cellular differentiation and oncogenesis [126]. The presence of CS in lung lesions might also be caused by its uptake from the extracellular environment, as well as its production by cancer cells [125]. For the latter hypothesis, a fraction of the cells within the primary tumors may increase the expression of SULT2B1b, which promotes the production of CS, thus supporting the hematogenous spread of metastatic cancer cells to the lungs.
4.7. Atherosclerosis
Atherosclerosis is increasingly being reinterpreted as a systemic disorder of sulfate metabolism driven by CS deficiency, challenging traditional views that have long centered on lipid accumulation or inflammation [127]. CS, an amphipathic molecule, enhances cholesterol solubility and facilitates its transport. It also collaborates with sulfated glycosaminoglycans (sGAGs) to maintain the negative charge of the endothelial glycocalyx, which is critical for forming structured water layers that reduce erythrocyte adhesion and ensure proper capillary flow [128]. Atherosclerotic plaques are now proposed to act as compensatory reservoirs, recruiting macrophages and platelets to oxidize LDL and homocysteine for sulfate generation and CS synthesis, albeit at the cost of oxidative damage [129].
Environmental factors play a significant role in modulating sulfate availability. Sunlight exposure, for instance, enhances CS synthesis via endothelial nitric oxide synthase (eNOS)-mediated pathways, while toxins, such as aluminum and glyphosate, disrupt cytochrome P450 enzymes, thereby impairing sulfate metabolism [130]. Dietary enrichment with sulfur-containing compounds, such as those found in garlic, and reduced exposure to toxins may help restore sulfate homeostasis. Conversely, conventional anti-inflammatory therapies, which often suppress compensatory pathways, may inadvertently exacerbate CS deficiency [131]. This emerging paradigm positions CS deficiency as a unifying mechanism underlying atherosclerosis. It highlights the need for in vivo validation to translate these insights into effective therapeutic strategies, potentially offering a novel approach to managing this complex disease.
4.8. Lead Poisoning
Lead (Pb) is a harmful heavy metal widely present in the environment and industry that causes dysfunction in multiple organs, particularly affecting the brains of infants, who are relatively susceptible to lead-induced neurotoxicity [132,133]. In the brain, astrocyte cells (CTX) are the primary source of cholesterol production, supplying 98% of the brain’s cholesterol. This not only ensures the structural stability of these cells but also supports neuronal development [134]. However, exposure to Pb disrupts this delicate balance, leading to a decrease in intracellular cholesterol levels and an increase in cell apoptosis [135]. The brain-derived neurotrophic factor (BDNF) is a crucial neurotrophic factor that plays a vital role in neurogenesis, neuronal survival, synaptic plasticity, and memory formation [136,137]. Recent research has shown that pretreating cells with CS can effectively counteract Pb-induced cholesterol loss and cell apoptosis in CTX cells by activating the BDNF signaling pathway [138]. This activation of the BDNF pathway promotes the cleavage of SREBP2 in Schwann cells, which in turn supports the survival of dorsal root ganglia (DRG) neurons by orchestrating cholesterol metabolism [139]. Furthermore, CS treatment has been found to stabilize intracellular Pb levels, indicating that CS does not promote Pb excretion but instead activates the BDNF/TrkB signaling pathway. This activation mediates downstream cholesterol metabolism, compensating for cholesterol loss induced by Pb exposure [140]. However, further research, particularly in vivo studies, is needed to better understand these mechanisms and their potential therapeutic applications.
5. CS Quantification Strategies
The comprehensive analysis of CS remains methodologically challenging despite its critical role in metabolism and cellular signaling. Recently, Sanchez et al. performed an in-depth comparative analysis of the analytical strategies for cholesterol and oxysterol sulfates, revealing critical bottlenecks in current methodologies [5]. Current approaches typically involve liquid–liquid extraction (LLE) combined with solid-phase extraction (SPE) for sample purification, with detection achieved through liquid chromatography-tandem mass spectrometry (LC-MS/MS) in negative ion mode [5]. However, the accuracy of CS quantification is intrinsically linked to standardized experimental workflows, including sample collection, storage, extraction, fractionation, separation, detection, and quantification steps. First, sample pretreatment strategies are crucial in the discovery and validation of lipid-based markers. The choice of sample collection tubes, freeze–thaw cycles, and storage conditions can significantly affect the stability of samples and the recovery of plasma lipids [141,142]. For example, plasma oxysterol levels collected with K2-EDTA and citrate collection tubes differed from those in serum samples, supporting the use of EDTA-collection tubes [143]. In cases where serum samples were used, the addition of an antioxidant like butylated hydroxytoluene was suggested to increase the stability of oxysterols [143]. Second, the extraction of steroid-related compounds is typically conducted using LLE protocols, followed by SPE cartridges. LLE protocols are popular due to their simplicity, cost, and efficiency. However, the choice of solvent system can greatly impact the extraction performance, particularly for less abundant lipids. For oxysterol sulfates, extraction by protein precipitation with acetonitrile-ZnSO4, followed by C18 SPE fractionation, resulted in complete recovery [144]. Finally, the accuracy of quantification is affected by the detection sensitivity. In mass spectrometry, oxysterols are usually detected in the positive ion mode, while the presence of the sulfate group facilitates the detection of oxysterol sulfates in the negative ion mode [145]. Targeted detection methods like multiple reaction monitoring (MRM) are often used due to their increased sensitivity and specificity [145]. In chromatographic separation, the hydroxy group position affects the hydrophobicity of oxysterols, influencing chromatographic separation under reverse-phase conditions. This also affects the extraction efficiency of oxysterol sulfates from biological matrices. Despite methodological advancements, reported physiological CS concentrations remain orders of magnitude below biologically active thresholds in in vitro systems (μM range) [144,146]. This discrepancy underscores the urgent need for standardized protocols and ultrasensitive detection platforms.
6. Conclusions and Perspectives
Advances in analytical techniques, such as LC-MS and selected ion monitoring, have significantly enhanced our ability to determine CS in various biological materials and elucidate its physiologic roles. CS functions as a regulatory molecule involved in a wide range of biological processes, including keratinocyte differentiation, glucolipid metabolism, bone metabolism, the inflammatory response, the gut microbiota, and neurosteroid synthesis. It has also demonstrated therapeutic efficacy against ulcerative colitis and osteoclast differentiation. One of the most exciting recent findings has been the discovery of immune regulatory functions of CS, which inhibit T lymphocyte activity. This finding suggests that CS could serve as a therapeutic agent for alleviating T-cell-mediated hypersensitivity and as a potential target for treating autoimmune diseases [52]. However, these effects are mostly based on rodent studies. Owing to considerable differences in the biology of cholesterol between humans and commonly used laboratory animals, particularly regarding sulfate synthesis and sulfotransferase specificity, data from rodent models should be critically scrutinized. Indeed, the production of steroid sulfate is almost negligible in rodents compared to primates [147].
CS also exhibits significant pathological damage in certain diseases. For instance, mutations in the STS gene lead to reduced activity of SSase, preventing the effective desulfation of CS into cholesterol. This results in the abnormal accumulation in the stratum corneum [78]. Excess CS disrupts the balance of stratum corneum lipid metabolism by enhancing intercellular adhesion, inhibiting HMG-CoA reductase (which reduces cholesterol synthesis), and interfering with the function of TGase 1. These disruptions lead to dry skin and scaly lesions [20]. In cancer, CS weakens T-cell migration by inhibiting the DOCK2/RAC pathway, hinders MHC antigen presentation by competitively binding to the TCR, and modulates TNFR signaling pathways (activating NF-κB rather than the apoptotic pathway). These actions promote tumor immune evasion and cell survival [94]. Additionally, CS accelerates the degradation of the extracellular matrix and hematogenous metastasis of tumors by enhancing the activity of matrix metalloproteinase-7 and the coagulation cascade [148]. These mechanisms highlight the pleiotropic pathological effects of CS and provide key insights for targeted therapeutic interventions.
CS is biosynthesized by the sulfonation of cholesterol through the sulfotransferase SULT2B1b. Thus, deciphering the regulation of SULT2B1 expression, such as SULT2B1 transcription and tissue distribution, may enable us to understand the physiologic significance of CS. In addition to being detected in the skin, SULT2B1b has also been extensively detected in the brain, lower intestine, lung, tonsil, platelets, testes, and ovaries [149]. Further studies are needed to explore the definitive biological basis for the expression in each specific tissue. Although primarily responsible for the sulfonation of cholesterol, SULT2B1b has also been shown to sulfate oxysterols, including oxygenated derivatives of cholesterol (e.g., 7-ketocholesterol, 24- or 25-hydroxycholesterol, and epoxycholesterol) and hydroxysteroids (e.g., DHEA, androstenediol, and PREG) [93]. Therefore, continued efforts need to be made in the system regulation of the sulfation of cholesterol and other oxysterols, particularly the implications of these sulfated molecules and their homeostasis.
Author Contributions
X.Y., S.L., Y.S., T.L., J.L., J.W., and Z.S.: conceptualization. X.Y., S.L., Y.S., and Z.S.: writing original draft and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82270846) and the Sichuan Science and Technology Program (2025ZNSFSC1549, 2024YFFK0206, and 2023YFS0255).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Vitku, J.; Hampl, R. Steroid Conjugates and Their Physiological Role. Physiol. Res. 2023, 72, S317–S322. [Google Scholar] [CrossRef]
- Gunal, S.; Hardman, R.; Kopriva, S.; Mueller, J.W. Sulfation pathways from red to green. J. Biol. Chem. 2019, 294, 12293–12312. [Google Scholar] [CrossRef] [PubMed]
- Bjerregaard-Olesen, C.; Ghisari, M.; Kjeldsen, L.S.; Wielsoe, M.; Bonefeld-Jorgensen, E.C. Estrone sulfate and dehydroepiandrosterone sulfate: Transactivation of the estrogen and androgen receptor. Steroids 2016, 105, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Strott, C.A.; Higashi, Y. Cholesterol sulfate in human physiology: What’s it all about? J. Lipid Res. 2003, 44, 1268–1278. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, L.D.; Pontini, L.; Marinozzi, M.; Sanchez-Aranguren, L.C.; Reis, A.; Dias, I.H.K. Cholesterol and oxysterol sulfates: Pathophysiological roles and analytical challenges. Br. J. Pharmacol. 2021, 178, 3327–3341. [Google Scholar] [CrossRef]
- Zhang, X.; Deng, D.; Cui, D.; Liu, Y.; He, S.; Zhang, H.; Xie, Y.; Yu, X.; Yang, S.; Chen, Y.; et al. Cholesterol Sulfate Exerts Protective Effect on Pancreatic beta-Cells by Regulating beta-Cell Mass and Insulin Secretion. Front. Pharmacol. 2022, 13, 840406. [Google Scholar]
- Leyh, T.S. The physical biochemistry and molecular genetics of sulfate activation. Crit. Rev. Biochem. Mol. Biol. 1993, 28, 515–542. [Google Scholar] [CrossRef]
- Mueller, J.W.; Gilligan, L.C.; Idkowiak, J.; Arlt, W.; Foster, P.A. The Regulation of Steroid Action by Sulfation and Desulfation. Endocr. Rev. 2015, 36, 526–563. [Google Scholar] [CrossRef]
- Suiko, M.; Kurogi, K.; Hashiguchi, T.; Sakakibara, Y.; Liu, M.C. Updated perspectives on the cytosolic sulfotransferases (SULTs) and SULT-mediated sulfation. Biosci. Biotechnol. Biochem. 2017, 81, 63–72. [Google Scholar] [CrossRef]
- Nowell, S.; Falany, C.N. Pharmacogenetics of human cytosolic sulfotransferases. Oncogene 2006, 25, 1673–1678. [Google Scholar] [CrossRef]
- Lindsay, J.; Wang, L.L.; Li, Y.; Zhou, S.F. Structure, function and polymorphism of human cytosolic sulfotransferases. Curr. Drug Metab. 2008, 9, 99–105. [Google Scholar] [PubMed]
- Javitt, N.B.; Lee, Y.C.; Shimizu, C.; Fuda, H.; Strott, C.A. Cholesterol and hydroxycholesterol sulfotransferases: Identification, distinction from dehydroepiandrosterone sulfotransferase, and differential tissue expression. Endocrinology 2001, 142, 2978–2984. [Google Scholar] [CrossRef] [PubMed]
- Her, C.; Wood, T.C.; Eichler, E.E.; Mohrenweiser, H.W.; Ramagli, L.S.; Siciliano, M.J.; Weinshilboum, R.M. Human hydroxysteroid sulfotransferase SULT2B1: Two enzymes encoded by a single chromosome 19 gene. Genomics 1998, 53, 284–295. [Google Scholar] [CrossRef]
- Sugawara, T.; Nomura, E.; Hoshi, N. Cholesterol sulphate affects production of steroid hormones by reducing steroidogenic acute regulatory protein level in adrenocortical cells. J. Endocrinol. 2007, 195, 451–458. [Google Scholar] [CrossRef]
- Rizner, T.L. The Important Roles of Steroid Sulfatase and Sulfotransferases in Gynecological Diseases. Front. Pharmacol. 2016, 7, 30. [Google Scholar] [CrossRef]
- Polito, M.P.; Marini, G.; Palamenghi, M.; Enzo, E. Decoding the Human Epidermal Complexity at Single-Cell Resolution. Int. J. Mol. Sci. 2023, 24, 8544. [Google Scholar] [CrossRef] [PubMed]
- Rearick, J.I.; Jetten, A.M. Accumulation of cholesterol 3-sulfate during in vitro squamous differentiation of rabbit tracheal epithelial cells and its regulation by retinoids. J. Biol. Chem. 1986, 261, 13898–13904. [Google Scholar] [CrossRef] [PubMed]
- Sjovall, P.; Gregoire, S.; Wargniez, W.; Skedung, L.; Luengo, G.S. 3D Molecular Imaging of Stratum Corneum by Mass Spectrometry Suggests Distinct Distribution of Cholesteryl Esters Compared to Other Skin Lipids. Int. J. Mol. Sci. 2022, 23, 13799. [Google Scholar] [CrossRef]
- Fandrei, F.; Engberg, O.; Opalka, L.; Jancalkova, P.; Pullmannova, P.; Steinhart, M.; Kovacik, A.; Vavrova, K.; Huster, D. Cholesterol sulfate fluidizes the sterol fraction of the stratum corneum lipid phase and increases its permeability. J. Lipid Res. 2022, 63, 100177. [Google Scholar] [CrossRef]
- Nemes, Z.; Demeny, M.; Marekov, L.N.; Fesus, L.; Steinert, P.M. Cholesterol 3-sulfate interferes with cornified envelope assembly by diverting transglutaminase 1 activity from the formation of cross-links and esters to the hydrolysis of glutamine. J. Biol. Chem. 2000, 275, 2636–2646. [Google Scholar] [CrossRef]
- Takahashi, H.; Asano, K.; Manabe, A.; Kinouchi, M.; Ishida-Yamamoto, A.; Iizuka, H. The alpha and eta isoforms of protein kinase C stimulate transcription of human involucrin gene. J. Investig. Dermatol. 1998, 110, 218–223. [Google Scholar] [CrossRef]
- Kuroki, T.; Ikuta, T.; Kashiwagi, M.; Kawabe, S.; Ohba, M.; Huh, N.; Mizuno, K.; Ohno, S.; Yamada, E.; Chida, K. Cholesterol sulfate, an activator of protein kinase C mediating squamous cell differentiation: A review. Mutat. Res. 2000, 462, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Hanyu, O.; Nakae, H.; Miida, T.; Higashi, Y.; Fuda, H.; Endo, M.; Kohjitani, A.; Sone, H.; Strott, C.A. Cholesterol sulfate induces expression of the skin barrier protein filaggrin in normal human epidermal keratinocytes through induction of RORalpha. Biochem. Biophys. Res. Commun. 2012, 428, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Rearick, J.I.; Hesterberg, T.W.; Jetten, A.M. Human bronchial epithelial cells synthesize cholesterol sulfate during squamous differentiation in vitro. J. Cell. Physiol. 1987, 133, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.L.; Rutherford, S.L.; Ponec, M.; Hincenbergs, M.; Placzek, D.R.; Elias, P.M. Density-dependent variations in the lipid content and metabolism of cultured human keratinocytes. J. Investig. Dermatol. 1988, 91, 86–91. [Google Scholar] [CrossRef]
- Nematisouldaragh, D.; Kirshenbaum, E.; Uzonna, M.; Kirshenbaum, L.; Rabinovich-Nikitin, I. The Role of Retinoic-Acid-Related Orphan Receptor (RORs) in Cellular Homeostasis. Int. J. Mol. Sci. 2024, 25, 11340. [Google Scholar] [CrossRef]
- Kallen, J.; Schlaeppi, J.M.; Bitsch, F.; Delhon, I.; Fournier, B. Crystal structure of the human RORα ligand binding domain in complex with cholesterol sulfate at 2.2 Å. J. Biol. Chem. 2004, 279, 14033–14038. [Google Scholar] [CrossRef]
- Woscholski, R.; Kodaki, T.; Palmer, R.H.; Waterfield, M.D.; Parker, P.J. Modulation of the substrate specificity of the mammalian phosphatidylinositol 3-kinase by cholesterol sulfate and sulfatide. Biochemistry 1995, 34, 11489–11493. [Google Scholar] [CrossRef]
- Su, W.Y.; Tian, L.Y.; Guo, L.P.; Huang, L.Q.; Gao, W.Y. PI3K signaling-regulated metabolic reprogramming: From mechanism to application. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188952. [Google Scholar] [CrossRef]
- Reis, A.; Dias, I.H.K. Oxysterol sulfates in fluids, cells and tissues: How much do we know about their clinical significance, biological relevance and biophysical implications? Essays Biochem. 2024, 68, 401–410. [Google Scholar]
- Cheetham, J.J.; Epand, R.M.; Andrews, M.; Flanagan, T.D. Cholesterol sulfate inhibits the fusion of Sendai virus to biological and model membranes. J. Biol. Chem. 1990, 265, 12404–12409. [Google Scholar] [CrossRef] [PubMed]
- Cheetham, J.J.; Nir, S.; Johnson, E.; Flanagan, T.D.; Epand, R.M. The effects of membrane physical properties on the fusion of Sendai virus with human erythrocyte ghosts and liposomes. Analysis of kinetics and extent of fusion. J. Biol. Chem. 1994, 269, 5467–5472. [Google Scholar] [CrossRef] [PubMed]
- Blache, D.; Becchi, M.; Davignon, J. Occurrence and biological effects of cholesteryl sulfate on blood platelets. Biochim. Biophys. Acta 1995, 1259, 291–296. [Google Scholar] [CrossRef]
- Merten, M.; Dong, J.F.; Lopez, J.A.; Thiagarajan, P. Cholesterol sulfate: A new adhesive molecule for platelets. Circulation 2001, 103, 2032–2034. [Google Scholar] [CrossRef]
- Lopalco, P.; Vitale, R.; Cho, Y.S.; Totaro, P.; Corcelli, A.; Lobasso, S. Alteration of Cholesterol Sulfate/Seminolipid Ratio in Semen Lipid Profile of Men With Oligoasthenozoospermia. Front. Physiol. 2019, 10, 1344. [Google Scholar] [CrossRef]
- Di Nisio, A.; De Toni, L.; Sabovic, I.; Vignoli, A. Lipidomic Profile of Human Sperm Membrane Identifies a Clustering of Lipids Associated with Semen Quality and Function. Int. J. Mol. Sci. 2023, 25, 297. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.A. Capacitation mechanisms, and the role of capacitation as seen in eutherian mammals. Reprod. Fertil. Dev. 1996, 8, 581–594. [Google Scholar] [CrossRef]
- Osheroff, J.E.; Visconti, P.E.; Valenzuela, J.P.; Travis, A.J.; Alvarez, J.; Kopf, G.S. Regulation of human sperm capacitation by a cholesterol efflux-stimulated signal transduction pathway leading to protein kinase A-mediated up-regulation of protein tyrosine phosphorylation. Mol. Hum. Reprod. 1999, 5, 1017–1026. [Google Scholar] [CrossRef]
- Garolla, A.; Sabovic, I.; Tescari, S.; De Toni, L.; Menegazzo, M.; Cosci, I.; De Filippis, V.; Giarola, M.; Foresta, C. Impaired sperm function in infertile men relies on the membrane sterol pattern. Andrology 2018, 6, 325–334. [Google Scholar] [CrossRef]
- Guillen, N. Pathogenicity and virulence of Entamoeba histolytica, the agent of amoebiasis. Virulence 2023, 14, 2158656. [Google Scholar] [CrossRef]
- Mi-Ichi, F.; Tsugawa, H.; Arita, M.; Yoshida, H. Pleiotropic Roles of Cholesteryl Sulfate during Entamoeba Encystation: Involvement in Cell Rounding and Development of Membrane Impermeability. mSphere 2022, 7, e0029922. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Boyce, J.A.; Barrett, N.A. Cysteinyl Leukotrienes in Allergic Inflammation. Annu. Rev. Pathol. 2025, 20, 115–141. [Google Scholar] [CrossRef]
- Chen, F.; Ghosh, A.; Lin, J.; Zhang, C.; Pan, Y.; Thakur, A.; Singh, K.; Hong, H.; Tang, S. 5-lipoxygenase pathway and its downstream cysteinyl leukotrienes as potential therapeutic targets for Alzheimer’s disease. Brain Behav. Immun. 2020, 88, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Antoniu, S.A. Targeting 5-lipoxygenase-activating protein in asthma and chronic obstructive pulmonary disease. Expert. Opin. Ther. Targets 2014, 18, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrov, D.A.; Zagryagskaya, A.N.; Pushkareva, M.A.; Bachschmid, M.; Peters-Golden, M.; Werz, O.; Steinhilber, D.; Sud’ina, G.F. Cholesterol and its anionic derivatives inhibit 5-lipoxygenase activation in polymorphonuclear leukocytes and MonoMac6 cells. FEBS J. 2006, 273, 548–557. [Google Scholar] [CrossRef]
- Park, J.S.; Moon, S.J.; Lim, M.A.; Byun, J.K.; Hwang, S.H.; Yang, S.; Kim, E.K.; Lee, H.; Kim, S.M.; Lee, J.; et al. Retinoic Acid Receptor-Related Receptor Alpha Ameliorates Autoimmune Arthritis via Inhibiting of Th17 Cells and Osteoclastogenesis. Front. Immunol. 2019, 10, 2270. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, H.; Wang, Y.; Yao, Y.; Yang, C.; Meng, J.; Tan, X.; Nie, Y.; Xue, L.; Xu, B.; et al. Sult2b1 deficiency exacerbates ischemic stroke by promoting pro-inflammatory macrophage polarization in mice. Theranostics 2021, 11, 10074–10090. [Google Scholar] [CrossRef]
- Weng, N.P. Numbers and odds: TCR repertoire size and its age changes impacting on T cell functions. Semin. Immunol. 2023, 69, 101810. [Google Scholar] [CrossRef]
- Pathan-Chhatbar, S.; Drechsler, C.; Richter, K.; Morath, A.; Wu, W.; OuYang, B.; Xu, C.; Schamel, W.W. Direct Regulation of the T Cell Antigen Receptor’s Activity by Cholesterol. Front. Cell Dev. Biol. 2020, 8, 615996. [Google Scholar] [CrossRef]
- Wu, H.; Cao, R.; Wei, S.; Pathan-Chhatbar, S.; Wen, M.; Wu, B.; Schamel, W.W.; Wang, S.; OuYang, B. Cholesterol Binds in a Reversed Orientation to TCRbeta-TM in Which Its OH Group is Localized to the Center of the Lipid Bilayer. J. Mol. Biol. 2021, 433, 167328. [Google Scholar] [CrossRef]
- Cai, E.; Marchuk, K.; Beemiller, P.; Beppler, C.; Rubashkin, M.G.; Weaver, V.M.; Gérard, A.; Liu, T.L.; Chen, B.C.; Betzig, E.; et al. Visualizing dynamic microvillar search and stabilization during ligand detection by T cells. Science 2017, 356, aal3118. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-S.; Chung, I.-J.; Kim, H.-R.; Jun, C.-D. The Immunosuppressive Potential of Cholesterol Sulfate Through T Cell Microvilli Disruption. Immune Netw. 2023, 23, e29. [Google Scholar] [CrossRef]
- Wang, F.; Beck-Garcia, K.; Zorzin, C.; Schamel, W.W.; Davis, M.M. Inhibition of T cell receptor signaling by cholesterol sulfate, a naturally occurring derivative of membrane cholesterol. Nat. Immunol. 2016, 17, 844–850. [Google Scholar] [CrossRef]
- Kunimura, K.; Uruno, T.; Fukui, Y. DOCK family proteins: Key players in immune surveillance mechanisms. Int. Immunol. 2020, 32, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Uruno, T.; Sugiura, Y.; Tatsuguchi, T.; Yamamura, K.; Ushijima, M.; Hattori, Y.; Kukimoto-Niino, M.; Mishima-Tsumagari, C.; Watanabe, M.; et al. Cholesterol sulfate is a DOCK2 inhibitor that mediates tissue-specific immune evasion in the eye. Sci. Signal. 2018, 11, eaao4874. [Google Scholar] [CrossRef]
- Kostarnoy, A.V.; Gancheva, P.G.; Lepenies, B.; Tukhvatulin, A.I.; Dzharullaeva, A.S.; Polyakov, N.B.; Grumov, D.A.; Egorova, D.A.; Kulibin, A.Y.; Bobrov, M.A.; et al. Receptor Mincle promotes skin allergies and is capable of recognizing cholesterol sulfate. Proc. Natl. Acad. Sci. USA 2017, 114, E2758–E2765. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.H.; Shi, X.J.; Zhu, J.J.; Guan, X.D.; Garbacz, W.G.; Huang, Y.X.; Gao, L.; Yan, J.; Xu, M.S.; Ren, S.R.; et al. Regulation of Cholesterol Sulfotransferase SULT2B1b by Hepatocyte Nuclear Factor 4α Constitutes a Negative Feedback Control of Hepatic Gluconeogenesis. Mol. Cell Biol. 2018, 38, e00654-17. [Google Scholar] [CrossRef]
- Shi, X.J.; Cheng, Q.Q.; Xu, L.Y.; Yan, J.; Jiang, M.X.; He, J.H.; Xu, M.S.; Stefanovic-Racic, M.; Sipula, I.; O’Doherty, R.M.; et al. Cholesterol Sulfate and Cholesterol Sulfotransferase Inhibit Gluconeogenesis by Targeting Hepatocyte Nuclear Factor 4α. Mol. Cell Biol. 2014, 34, 485–497. [Google Scholar] [CrossRef]
- Kim, E.J.; Yoon, Y.S.; Hong, S.; Son, H.Y.; Na, T.Y.; Lee, M.H.; Kang, H.J.; Park, J.; Cho, W.J.; Kim, S.G.; et al. Retinoic acid receptor-related orphan receptor alpha-induced activation of adenosine monophosphate-activated protein kinase results in attenuation of hepatic steatosis. Hepatology 2012, 55, 1379–1388. [Google Scholar] [CrossRef]
- Williams, M.L.; Hughes-Fulford, M.; Elias, P.M. Inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity and sterol synthesis by cholesterol sulfate in cultured fibroblasts. Biochim. Biophys. Acta 1985, 845, 349–357. [Google Scholar] [CrossRef]
- Neunzig, J.; Bernhardt, R. Effect of sulfonated steroids on steroidogenic cytochrome P450-dependent steroid hydroxylases. J. Steroid Biochem. Mol. Biol. 2018, 179, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Koay, Y.C.; Wali, J.A.; Luk, A.W.S.; Macia, L.; Cogger, V.C.; Pulpitel, T.J.; Wahl, D.; Solon-Biet, S.M.; Holmes, A.; Simpson, S.J.; et al. Ingestion of resistant starch by mice markedly increases microbiome-derived metabolites. FASEB J. 2019, 33, 8033–8042. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Huang, X.; Liang, X.; Su, M.; Lai, K.P.; Chen, J. Integrated omics analysis reveals the alteration of gut microbe-metabolites in obese adults. Brief. Bioinform. 2021, 22, bbaa165. [Google Scholar] [CrossRef] [PubMed]
- Le, H.H.; Lee, M.T.; Besler, K.R.; Comrie, J.M.C.; Johnson, E.L. Characterization of interactions of dietary cholesterol with the murine and human gut microbiome. Nat. Microbiol. 2022, 7, 1390–1403. [Google Scholar] [CrossRef]
- Yao, L.; D’Agostino, G.D.; Park, J.; Hang, S.; Adhikari, A.A.; Zhang, Y.; Li, W.; Avila-Pacheco, J.; Bae, S.; Clish, C.B.; et al. A biosynthetic pathway for the selective sulfonation of steroidal metabolites by human gut bacteria. Nat. Microbiol. 2022, 7, 1404–1418. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, H.; Wang, Z.; Pan, Q.; Zhao, X.; Lu, B. Interactions between dietary cholesterol and intestinal flora and their effects on host health. Crit. Rev. Food Sci. Nutr. 2025, 65, 494–506. [Google Scholar] [CrossRef]
- Ivanisevic, J.; Epstein, A.A.; Kurczy, M.E.; Benton, P.H.; Uritboonthai, W.; Fox, H.S.; Boska, M.D.; Gendelman, H.E.; Siuzdak, G. Brain region mapping using global metabolomics. Chem. Biol. 2014, 21, 1575–1584. [Google Scholar] [CrossRef]
- Biagini, G.; Marinelli, C.; Panuccio, G.; Puia, G.; Avoli, M. Glia-Neuron Interactions: Neurosteroids and Epileptogenesis. In Jasper’s Basic Mechanisms of the Epilepsies, 4th ed.; Noebels, J.L., Avoli, M., Rogawski, M.A., Olsen, R.W., Delgado-Escueta, A.V., Eds.; Oxford University Press: Bethesda, MD, USA, 2012. [Google Scholar]
- Biagini, G.; Rustichelli, C.; Curia, G.; Vinet, J.; Lucchi, C.; Pugnaghi, M.; Meletti, S. Neurosteroids and epileptogenesis. J. Neuroendocrinol. 2013, 25, 980–990. [Google Scholar] [CrossRef]
- De Nicola, A.F.; Garay, L.I.; Meyer, M.; Guennoun, R.; Sitruk-Ware, R.; Schumacher, M.; Gonzalez Deniselle, M.C. Neurosteroidogenesis and progesterone anti-inflammatory/neuroprotective effects. J. Neuroendocrinol. 2018, 30, e12502. [Google Scholar] [CrossRef]
- Prah, J.; Winters, A.; Chaudhari, K.; Hersh, J.; Liu, R.; Yang, S.H. Cholesterol sulfate alters astrocyte metabolism and provides protection against oxidative stress. Brain Res. 2019, 1723, 146378. [Google Scholar] [CrossRef]
- Pierce, S.R.; Germann, A.L.; Steinbach, J.H.; Akk, G. The Sulfated Steroids Pregnenolone Sulfate and Dehydroepiandrosterone Sulfate Inhibit the alpha1beta3gamma2L GABA(A) Receptor by Stabilizing a Novel Nonconducting State. Mol. Pharmacol. 2022, 101, 68–77. [Google Scholar] [CrossRef]
- Clark, B.J.; Klinge, C.M. Structure-function of DHEA binding proteins. Vitam. Horm. 2023, 123, 587–617. [Google Scholar]
- Traish, A.M.; Kang, H.P.; Saad, F.; Guay, A.T. Dehydroepiandrosterone (DHEA)—A precursor steroid or an active hormone in human physiology. J. Sex. Med. 2011, 8, 2960–2982; quiz 2983. [Google Scholar] [CrossRef] [PubMed]
- Vitku, J.; Hill, M.; Kolatorova, L.; Kubala Havrdova, E.; Kancheva, R. Steroid Sulfation in Neurodegenerative Diseases. Front. Mol. Biosci. 2022, 9, 839887. [Google Scholar] [CrossRef] [PubMed]
- Kopylov, A.T.; Stepanov, A.A.; Butkova, T.V.; Malsagova, K.A.; Zakharova, N.V.; Kostyuk, G.P.; Elmuratov, A.U.; Kaysheva, A.L. Consolidation of metabolomic, proteomic, and GWAS data in connective model of schizophrenia. Sci. Rep. 2023, 13, 2139. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Liang, C.; Li, P.; Xiao, H. Revisiting X-linked congenital ichthyosis. Int. J. Dermatol. 2024, 64, 51–61. [Google Scholar] [CrossRef]
- McGeoghan, F.; Camera, E.; Maiellaro, M.; Menon, M.; Huang, M.; Dewan, P.; Ziaj, S.; Caley, M.P.; Donaldson, M.; Enright, A.J.; et al. RNA sequencing and lipidomics uncovers novel pathomechanisms in recessive X-linked ichthyosis. Front. Mol. Biosci. 2023, 10, 1176802. [Google Scholar] [CrossRef]
- Lassen, N.; Bateman, J.B.; Estey, T.; Kuszak, J.R.; Nees, D.W.; Piatigorsky, J.; Duester, G.; Day, B.J.; Huang, J.; Hines, L.M.; et al. Multiple and additive functions of ALDH3A1 and ALDH1A1-Cataract phenotype and ocular oxidative damage in Aldh3a1(-/-)/Aldh1a1(-/-) knock-out mice. J. Biol. Chem. 2007, 282, 25668–25676. [Google Scholar] [CrossRef]
- Jacob, S.; Brune, C.W.; Carter, C.S.; Leventhal, B.L.; Lord, C.; Cook, E.H. Association of the oxytocin receptor gene (OXTR) in Caucasian children and adolescents with autism. Neurosci. Lett. 2007, 417, 6–9. [Google Scholar] [CrossRef]
- Wren, G.H.; Davies, W. X-linked ichthyosis: New insights into a multi-system disorder. Skin. Health Dis. 2022, 2, e179. [Google Scholar] [CrossRef]
- Cui, D.; Feng, X.; Lei, S.; Zhang, H.; Hu, W.; Yang, S.; Yu, X.; Su, Z. Pancreatic beta-cell failure, clinical implications, and therapeutic strategies in type 2 diabetes. Chin. Med. J. 2024, 137, 791–805. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, S.; Zhou, R.; Zhang, X.; Yang, S.; Deng, D.; Zhang, C.; Yu, X.; Chen, Y.; Su, Z. Nuclear Factor-Y in Mouse Pancreatic beta-Cells Plays a Crucial Role in Glucose Homeostasis by Regulating beta-Cell Mass and Insulin Secretion. Diabetes 2021, 70, 1703–1716. [Google Scholar] [CrossRef]
- Perego, C.; Da Dalt, L.; Pirillo, A.; Galli, A.; Catapano, A.L.; Norata, G.D. Cholesterol metabolism, pancreatic beta-cell function and diabetes. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 2149–2156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, S.; Chen, J.; Su, Z. Unraveling the Regulation of Hepatic Gluconeogenesis. Front. Endocrinol. 2018, 9, 802. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Y.; Zhang, J.; Liu, Y.; Zhang, Y.; Su, Z. Retinoic acid receptor-related orphan receptor alpha stimulates adipose tissue inflammation by modulating endoplasmic reticulum stress. J. Biol. Chem. 2017, 292, 13959–13969. [Google Scholar] [CrossRef]
- Kuang, J.; Hou, X.; Zhang, J.; Chen, Y.; Su, Z. Identification of insulin as a novel retinoic acid receptor-related orphan receptor alpha target gene. FEBS Lett. 2014, 588, 1071–1079. [Google Scholar] [CrossRef]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chetelat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- Ji, Q.Q.; Chen, J.Q.; Li, Y.F.; Tao, E.X.; Zhan, Y.Q. Incidence and prevalence of Alzheimer’s disease in China: A systematic review and meta-analysis. Eur. J. Epidemiol. 2024, 39, 701–714. [Google Scholar] [CrossRef]
- Karran, E.; De Strooper, B. The amyloid hypothesis in Alzheimer disease: New insights from new therapeutics. Nat. Rev. Drug Discov. 2022, 21, 306–318. [Google Scholar] [CrossRef]
- Ahmad, F.; Sun, Q.; Patel, D.; Stommel, J.M. Cholesterol Metabolism: A Potential Therapeutic Target in Glioblastoma. Cancers 2019, 11, 146. [Google Scholar] [CrossRef]
- Elbassal, E.A.; Liu, H.; Morris, C.; Wojcikiewicz, E.P.; Du, D. Effects of Charged Cholesterol Derivatives on Abeta40 Amyloid Formation. J. Phys. Chem. B 2016, 120, 59–68. [Google Scholar] [CrossRef]
- Cook, I.; Leyh, T.S. Sulfotransferase 2B1b, Sterol Sulfonation, and Disease. Pharmacol. Rev. 2023, 75, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Cook, I.; Leyh, T.S. Sterol-activated amyloid beta fibril formation. J. Biol. Chem. 2023, 299, 105445. [Google Scholar] [CrossRef]
- Mary, A.; Eysert, F.; Checler, F.; Chami, M. Mitophagy in Alzheimer’s disease: Molecular defects and therapeutic approaches. Mol. Psychiatry 2023, 28, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Maulik, M.; Westaway, D.; Jhamandas, J.H.; Kar, S. Role of cholesterol in APP metabolism and its significance in Alzheimer’s disease pathogenesis. Mol. Neurobiol. 2013, 47, 37–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shou, Y.; Pan, J.; Du, Y.; Liu, C.; Wang, H. The relationship between cholesterol level and Alzheimer’s disease-associated APP proteolysis/Abeta metabolism. Nutr. Neurosci. 2019, 22, 453–463. [Google Scholar] [CrossRef]
- Denning, M.F.; Kazanietz, M.G.; Blumberg, P.M.; Yuspa, S.H. Cholesterol sulfate activates multiple protein kinase C isoenzymes and induces granular cell differentiation in cultured murine keratinocytes. Cell Growth Differ. 1995, 6, 1619–1626. [Google Scholar]
- Tanaka, T.; Tsujio, I.; Nishikawa, T.; Shinosaki, K.; Kudo, T.; Takeda, M. Significance of tau phosphorylation and protein kinase regulation in the pathogenesis of Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2000, 14 (Suppl. S1), S18–S24. [Google Scholar] [CrossRef]
- Froger, N. New therapeutic avenues for neurosteroids in psychiatric diseases. Biol. Aujourdhui 2019, 213, 131–140. [Google Scholar] [CrossRef]
- Monnet, F.P.; Maurice, T. The sigma1 protein as a target for the non-genomic effects of neuro(active)steroids: Molecular, physiological, and behavioral aspects. J. Pharmacol. Sci. 2006, 100, 93–118. [Google Scholar] [CrossRef]
- de Souza, H.S.P.; Fiocchi, C.; Iliopoulos, D. The IBD interactome: An integrated view of aetiology, pathogenesis and therapy. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Zhang, M.; Li, X.; Zhang, Q.; Yang, J.; Liu, G. Roles of macrophages on ulcerative colitis and colitis-associated colorectal cancer. Front. Immunol. 2023, 14, 1103617. [Google Scholar] [CrossRef] [PubMed]
- Harbord, M.; Eliakim, R.; Bettenworth, D.; Karmiris, K.; Katsanos, K.; Kopylov, U.; Kucharzik, T.; Molnar, T.; Raine, T.; Sebastian, S.; et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 2: Current Management. J. Crohn’s Colitis 2017, 11, 769–784. [Google Scholar] [CrossRef]
- Xu, D.; Ma, R.; Ju, Y.; Song, X.; Niu, B.; Hong, W.; Wang, R.; Yang, Q.; Zhao, Z.; Zhang, Y.; et al. Cholesterol sulfate alleviates ulcerative colitis by promoting cholesterol biosynthesis in colonic epithelial cells. Nat. Commun. 2022, 13, 4428. [Google Scholar] [CrossRef]
- Infante, R.E.; Radhakrishnan, A.; Abi-Mosleh, L.; Kinch, L.N.; Wang, M.L.; Grishin, N.V.; Goldstein, J.L.; Brown, M.S. Purified NPC1 protein: II. Localization of sterol binding to a 240-amino acid soluble luminal loop. J. Biol. Chem. 2008, 283, 1064–1075. [Google Scholar] [CrossRef]
- Xu, S.; Benoff, B.; Liou, H.L.; Lobel, P.; Stock, A.M. Structural basis of sterol binding by NPC2, a lysosomal protein deficient in Niemann-Pick type C2 disease. J. Biol. Chem. 2007, 282, 23525–23531. [Google Scholar] [CrossRef] [PubMed]
- Morino, K.; Kunimura, K.; Sugiura, Y.; Izumi, Y.; Matsubara, K.; Akiyoshi, S.; Maeda, R.; Hirotani, K.; Sakata, D.; Mizuno, S.; et al. Cholesterol sulfate limits neutrophil recruitment and gut inflammation during mucosal injury. Front. Immunol. 2023, 14, 1131146. [Google Scholar] [CrossRef]
- Minoia, A.; Dalle Carbonare, L.; Schwamborn, J.C.; Bolognin, S.; Valenti, M.T. Bone Tissue and the Nervous System: What Do They Have in Common? Cells 2022, 12, 51. [Google Scholar] [CrossRef]
- Hodgkinson, T.; Tsimbouri, P.M.; Llopis-Hernandez, V. The use of nanovibration to discover specific and potent bioactive metabolites that stimulate osteogenic differentiation in mesenchymal stem cells. Sci. Adv. 2021, 7, eabb7921. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, J.; Lee, G.R.; Kwon, M.; Lee, H.I.; Kim, N.; Kim, H.J.; Lee, M.O. Cholesterol sulfate inhibits osteoclast differentiation and survival by regulating the AMPK-Sirt1-NF-κB pathway. J. Cell. Physiol. 2023, 238, 2063–2075. [Google Scholar] [CrossRef] [PubMed]
- van de Kamp, J.M.; Bokenkamp, A.; Smith, D.E.C.; Wamelink, M.M.C.; Jansen, E.E.W.; Struys, E.A.; Waisfisz, Q.; Verkleij, M.; Hartmann, M.F.; Wang, R.; et al. Biallelic variants in the SLC13A1 sulfate transporter gene cause hyposulfatemia with a mild spondylo-epi-metaphyseal dysplasia. Clin. Genet. 2023, 103, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.W.; Park, J.Y. Host modulation therapy for improving the osseointegration of dental implants under bone healing-suppressed conditions: A preclinical rodent-model experiment. J. Periodontal Implant. Sci. 2024, 54, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.W.; Kim, H.R.; Kim, B.M.; Cho, M.L.; Lee, S.H. Th17 cytokines regulate osteoclastogenesis in rheumatoid arthritis. Am. J. Pathol. 2015, 185, 3011–3024. [Google Scholar] [CrossRef]
- Weaver, C.T.; Harrington, L.E.; Mangan, P.R.; Gavrieli, M.; Murphy, K.M. Th17: An effector CD4 T cell lineage with regulatory T cell ties. Immunity 2006, 24, 677–688. [Google Scholar] [CrossRef]
- Tatsuguchi, T.; Uruno, T.; Sugiura, Y.; Oisaki, K.; Takaya, D.; Sakata, D.; Izumi, Y.; Togo, T.; Hattori, Y.; Kunimura, K.; et al. Pharmacological intervention of cholesterol sulfate-mediated T cell exclusion promotes antitumor immunity. Biochem. Biophys. Res. Commun. 2022, 609, 183–188. [Google Scholar] [CrossRef]
- Tatsuguchi, T.; Uruno, T.; Sugiura, Y.; Sakata, D.; Izumi, Y.; Sakurai, T.; Hattori, Y.; Oki, E.; Kubota, N.; Nishimoto, K.; et al. Cancer-derived cholesterol sulfate is a key mediator to prevent tumor infiltration by effector T cells. Int. Immunol. 2022, 34, 277–289. [Google Scholar] [CrossRef]
- Wang, S.; Wang, R.; Xu, N.; Wei, X.Y.; Yang, Y.J.; Lian, Z.X.; Cen, B.N.; Shen, C.C.; Li, W.Y.; Wang, J.G.; et al. SULT2B1-CS-DOCK2 axis regulates effector T-cell exhaustion in HCC microenvironment. Hepatology 2023, 78, 1064–1078. [Google Scholar] [CrossRef]
- Hu, L.; Yang, G.Z.; Zhang, Y.; Feng, D.; Zhai, Y.X.; Gong, H.; Qi, C.Y.; Fu, H.; Ye, M.M.; Cai, Q.P.; et al. Overexpression of SULT2B1b is an independent prognostic indicator and promotes cell growth and invasion in colorectal carcinoma. Lab. Investig. 2015, 95, 1005–1018. [Google Scholar] [CrossRef]
- Micheau, O.; Tschopp, J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 2003, 114, 181–190. [Google Scholar] [CrossRef]
- Matsuda, T.; Shimada, M.; Sato, A.; Akase, T.; Yoshinari, K.; Nagata, K.; Yamazoe, Y. Tumor Necrosis Factor-Alpha-Nuclear Factor-Kappa B-Signaling Enhances St2b2 Expression during 12-Tetradecanoylphorbol-13-acetate-Induced Epidermal Hyperplasia. Biol. Pharm. Bull. 2011, 34, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Legler, D.F.; Micheau, O.; Doucey, M.A.; Tschopp, J.; Bron, C. Recruitment of TNF receptor 1 to lipid rafts is essential for TNFα-mediated NF-κB activation. Immunity 2003, 18, 655–664. [Google Scholar] [PubMed]
- Yao, W.; Guan, Y. Ginsenosides in cancer: A focus on the regulation of cell metabolism. Biomed. Pharmacother. 2022, 156, 113756. [Google Scholar] [CrossRef]
- Johnson, C.H.; Santidrian, A.F.; LeBoeuf, S.E.; Kurczy, M.E.; Rattray, N.J.W.; Rattray, Z.; Warth, B.; Ritland, M.; Hoang, L.T.; Loriot, C.; et al. Metabolomics guided pathway analysis reveals link between cancer metastasis, cholesterol sulfate, and phospholipids. Cancer Metab. 2017, 5, 9. [Google Scholar] [CrossRef]
- Yamamoto, K.; Miyazaki, K.; Higashi, S. Cholesterol sulfate alters substrate preference of matrix metalloproteinase-7 and promotes degradations of pericellular laminin-332 and fibronectin. J. Biol. Chem. 2010, 285, 28862–28873. [Google Scholar]
- Seneff, S.; Davidson, R.M.; Lauritzen, A.; Samsel, A.; Wainwright, G. A novel hypothesis for atherosclerosis as a cholesterol sulfate deficiency syndrome. Theor. Biol. Med. Model. 2015, 12, 9. [Google Scholar]
- Rohani, M.; Pollack, G.H. Flow through horizontal tubes submerged in water in the absence of a pressure gradient: Mechanistic considerations. Langmuir 2013, 29, 6556–6561. [Google Scholar] [CrossRef] [PubMed]
- McCully, K.S. Vascular pathology of homocysteinemia: Implications for the pathogenesis of arteriosclerosis. Am. J. Pathol. 1969, 56, 111–128. [Google Scholar]
- Samsel, A.; Seneff, S. Glyphosate, pathways to modern diseases II: Celiac sprue and gluten intolerance. Interdiscip. Toxicol. 2013, 6, 159–184. [Google Scholar] [CrossRef]
- Lonn, E.; Yusuf, S.; Arnold, M.J.; Sheridan, P.; Pogue, J.; Micks, M.; McQueen, M.J.; Probstfield, J.; Fodor, G.; Held, C.; et al. Homocysteine lowering with folic acid and B vitamins in vascular disease. N. Engl. J. Med. 2006, 354, 1567–1577. [Google Scholar]
- Mayans, L. Lead Poisoning in Children. Am. Fam. Physician 2019, 100, 24–30. [Google Scholar]
- Richetti, S.K.; Rosemberg, D.B.; Ventura-Lima, J.; Monserrat, J.M.; Bogo, M.R.; Bonan, C.D. Acetylcholinesterase activity and antioxidant capacity of zebrafish brain is altered by heavy metal exposure. Neurotoxicology 2011, 32, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Ferris, H.A.; Perry, R.J.; Moreira, G.V.; Shulman, G.I.; Horton, J.D.; Kahn, C.R. Loss of astrocyte cholesterol synthesis disrupts neuronal function and alters whole-body metabolism. Proc. Natl. Acad. Sci. USA 2017, 114, 1189–1194. [Google Scholar] [CrossRef]
- Wang, S.S.; Lu, A.X.; Li, W.H.; Zhang, H.; Hu, C.P.; Liu, J.X.; Pan, H.; Wu, M.Q.; Xu, X.; Yan, C.H.; et al. Effects of food-borne cholesterol supplementation on lead-induced neurodevelopmental impairments of rats based on BDNF signaling pathway and cholesterol metabolism. Ecotox Environ. Safe 2023, 259, 115026. [Google Scholar] [CrossRef] [PubMed]
- Carito, V.; Ceccanti, M.; Ferraguti, G.; Coccurello, R.; Ciafre, S.; Tirassa, P.; Fiore, M. NGF and BDNF Alterations by Prenatal Alcohol Exposure. Curr. Neuropharmacol. 2019, 17, 308–317. [Google Scholar] [CrossRef]
- Malavika, L.; Mitra, P.; Goyal, T.; Sharma, S.; Purohit, P.; Sharma, P. Association of blood lead levels with neurobehavior and BDNF expression in school going children. J. Trace Elem. Med. Biol. 2021, 66, 126749. [Google Scholar]
- Wang, S.S.; Xu, X.; Lu, A.X.; Li, W.H.; Liu, J.X.; Liu, C.; Yan, C.H. Amelioration of cholesterol sulfate for lead-induced CTX cell apoptosis based on BDNF signaling pathway mediated cholesterol metabolism. Ecotox Environ. Safe 2022, 248, 114307. [Google Scholar] [CrossRef]
- Follis, R.M.; Tep, C.; Genaro-Mattos, T.C.; Kim, M.L.; Ryu, J.C.; Morrison, V.E.; Chan, J.R.; Porter, N.; Carter, B.D.; Yoon, S.O. Metabolic Control of Sensory Neuron Survival by the p75 Neurotrophin Receptor in Schwann Cells. J. Neurosci. 2021, 41, 8710–8724. [Google Scholar] [CrossRef]
- Do, H.T.; Bruelle, C.; Pham, D.D.; Jauhiainen, M.; Eriksson, O.; Korhonen, L.T.; Lindholm, D. Nerve growth factor (NGF) and pro-NGF increase low-density lipoprotein (LDL) receptors in neuronal cells partly by different mechanisms: Role of LDL in neurite outgrowth. J. Neurochem. 2016, 136, 306–315. [Google Scholar] [CrossRef]
- Lee, D.Y.; Kind, T.; Yoon, Y.R.; Fiehn, O.; Liu, K.H. Comparative evaluation of extraction methods for simultaneous mass-spectrometric analysis of complex lipids and primary metabolites from human blood plasma. Anal. Bioanal. Chem. 2014, 406, 7275–7286. [Google Scholar] [CrossRef]
- Sarafian, M.H.; Gaudin, M.; Lewis, M.R.; Martin, F.P.; Holmes, E.; Nicholson, J.K.; Dumas, M.E. Objective Set of Criteria for Optimization of Sample Preparation Procedures for Ultra-High Throughput Untargeted Blood Plasma Lipid Profiling by Ultra Performance Liquid Chromatography-Mass Spectrometry. Anal. Chem. 2014, 86, 5766–5774. [Google Scholar] [CrossRef] [PubMed]
- Reinicke, M.; Schröter, J.; Müller-Klieser, D.; Helmschrodt, C.; Ceglarek, U. Free oxysterols and bile acids including conjugates—Simultaneous quantification in human plasma and cerebrospinal fluid by liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2018, 1037, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Guijo, A.; Oji, V.; Hartmann, M.F.; Schuppe, H.C.; Traupe, H.; Wudy, S.A. High levels of oxysterol sulfates in serum of patients with steroid sulfatase deficiency. J. Lipid Res. 2015, 56, 403–412. [Google Scholar] [CrossRef]
- Dias, I.H.K.; Ferreira, R.; Gruber, F.; Vitorino, R.; Rivas-Urbina, A.; Sanchez-Quesada, J.L.; Silva, J.V.; Fardilha, M.; de Freitas, V.; Reis, A. Sulfate-based lipids: Analysis of healthy human fluids and cell extracts. Chem. Phys. Lipids 2019, 221, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.Y.; Shen, S.W.; Ma, Y.J.; Kim, J.K.; Rodriguez-Agudo, D.; Heuman, D.M.; Hylemon, P.B.; Pandak, W.M.; Ren, S.L. 25-Hydroxycholesterol-3-sulfate attenuates inflammatory response via PPARγ signaling in human THP-1 macrophages. Am. J. Physiol-Endoc M. 2012, 302, E788–E799. [Google Scholar] [CrossRef]
- Schuler, G. Steroid sulfates in domestic mammals and laboratory rodents. Domest. Anim. Endocrinol. 2021, 76, 106622. [Google Scholar] [CrossRef]
- Yamamoto, K.; Miyazaki, K.; Higashi, S. Pericellular proteolysis by matrix metalloproteinase-7 is differentially modulated by cholesterol sulfate, sulfatide, and cardiolipin. FEBS J. 2014, 281, 3346–3356. [Google Scholar] [CrossRef]
- Geese, W.J.; Raftogianis, R.B. Biochemical characterization and tissue distribution of human SULT2B1. Biochem. Biophys. Res. Commun. 2001, 288, 280–289. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).