Ionic Strength Investigation on the Interaction Between miR-155 and a PNA-Based Probe by Atomic Force Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Oligonucleotides
2.2. Tips and Substrate Functionalization
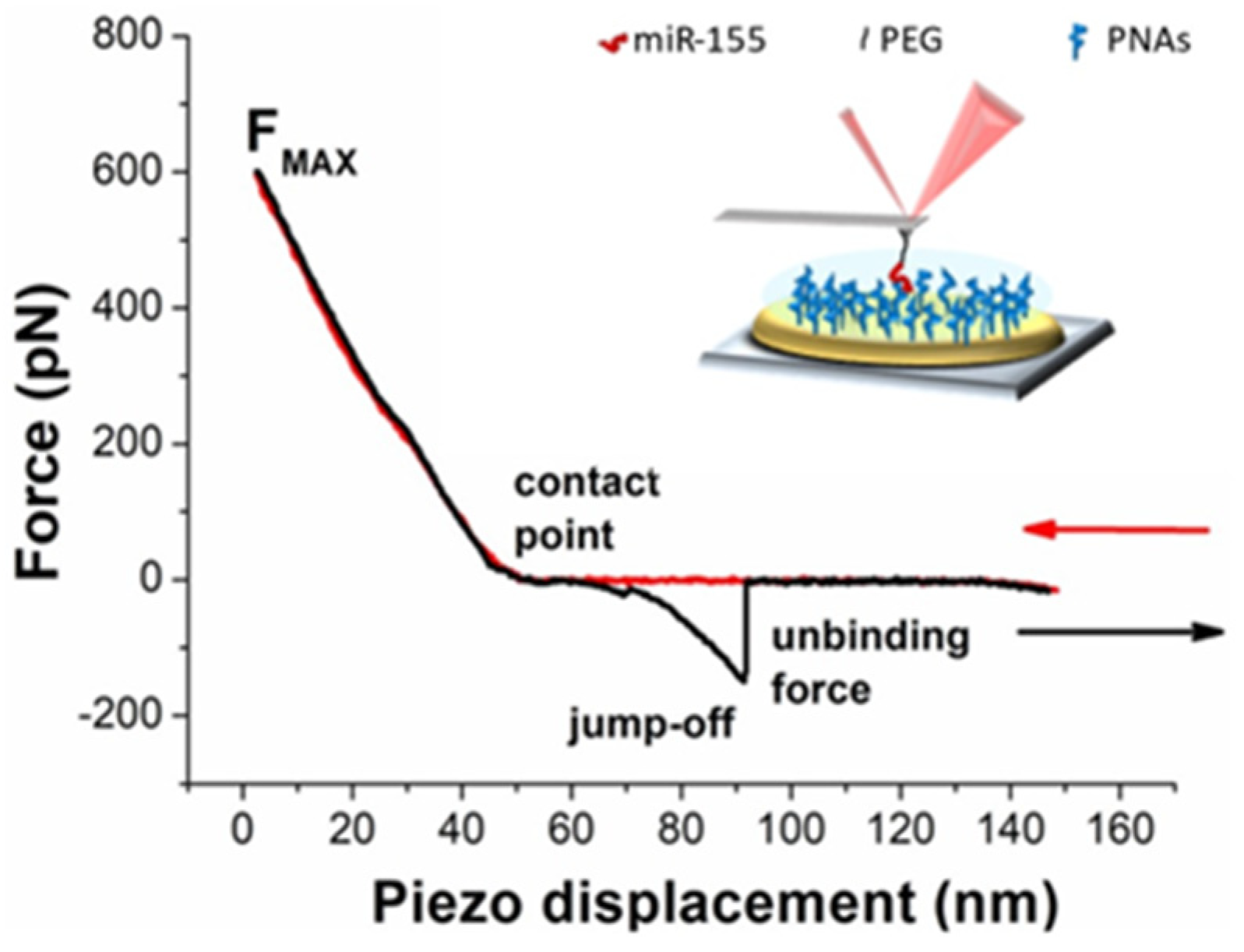
2.3. AFS Experiments
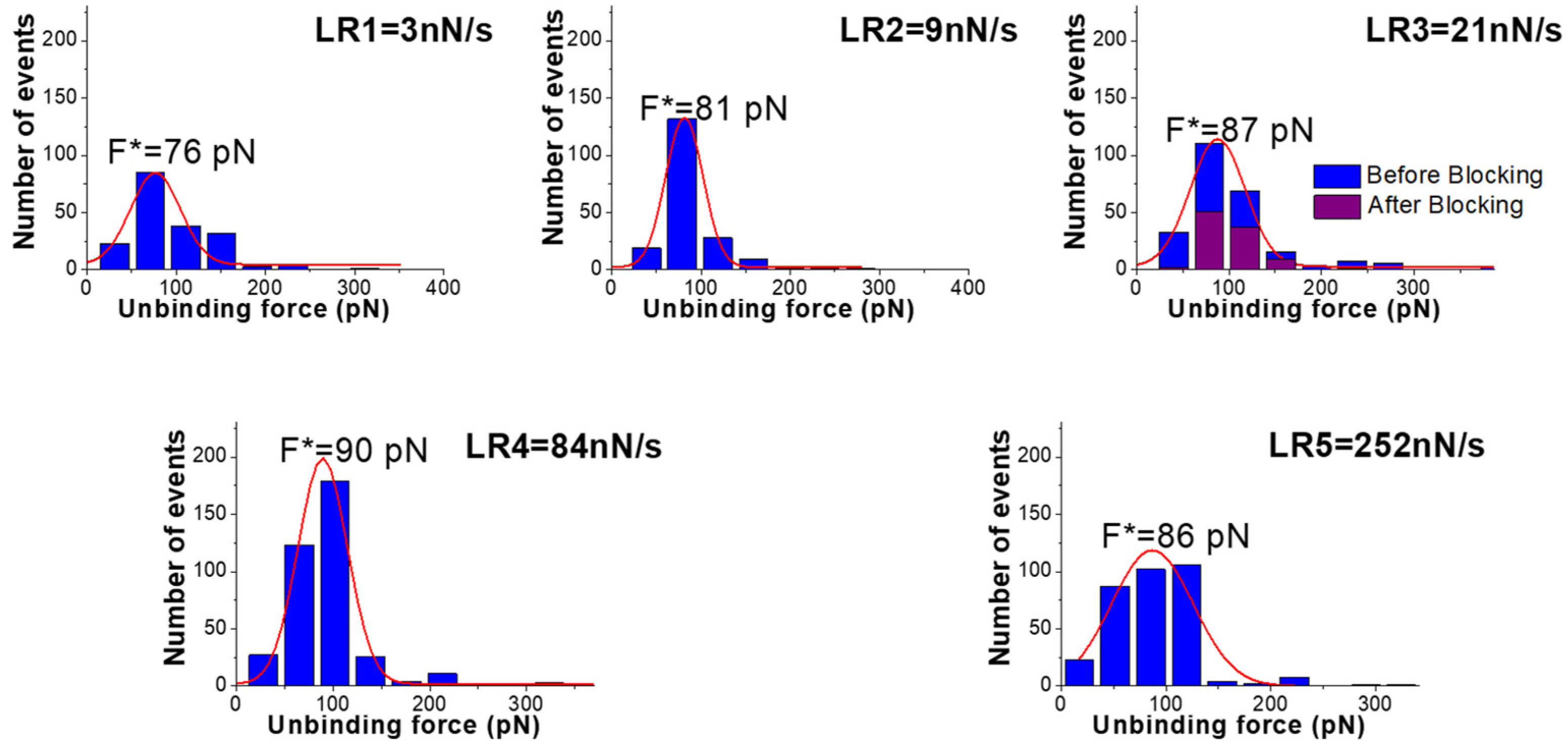
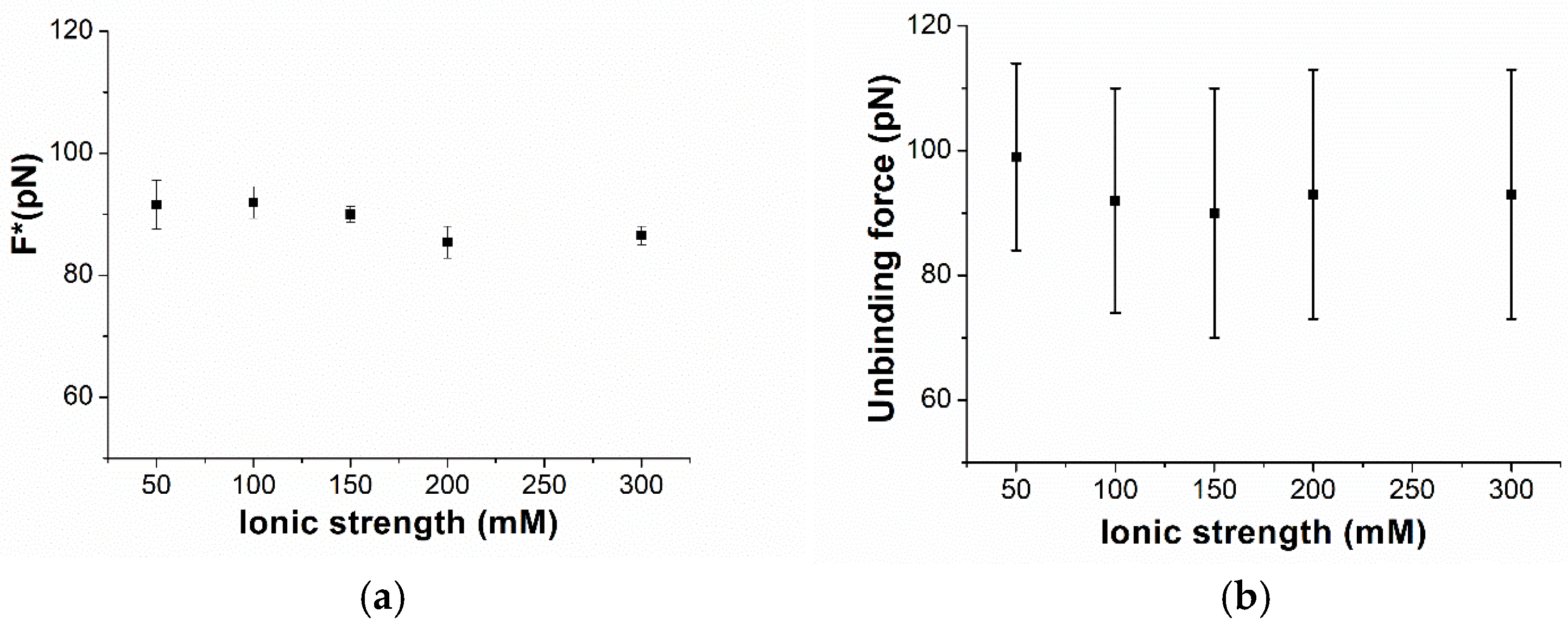
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ananthanawat, C.; Vilaivan, T.; Hoven, V.P.; Su, X. Comparison of DNA, Aminoethylglycyl PNA and Pyrrolidinyl PNA as Probes for Detection of DNA Hybridization Using Surface Plasmon Resonance Technique. Biosens. Bioelectron. 2010, 25, 1064–1069. [Google Scholar] [CrossRef]
- Saarbach, J.; Sabale, P.M.; Winssinger, N. Peptide Nucleic Acid (PNA) and Its Applications in Chemical Biology, Diagnostics, and Therapeutics. Curr. Opin. Chem. Biol. 2019, 52, 112–124. [Google Scholar] [CrossRef]
- Nielsen, P.E. PNA Technology. Mol. Biotechnol. 2004, 26, 233–248. [Google Scholar] [CrossRef]
- Nielsen, P.E. Applications of peptide nucleic acids. Curr. Opin. Biotechnol. 1999, 10, 71–75. [Google Scholar] [CrossRef]
- Gupta, A.; Mishra, A.; Puri, N. Peptide nucleic acids: Advanced tools for biomedical applications. J. Biotechnol. 2017, 259, 148–159. [Google Scholar] [CrossRef]
- Eriksson, M.; Nielsen, P.E. PNA-Nucleic Acid Complexes. Structure, Stability and Dynamics. Q. Rev. Biophys. 1996, 29, 369–394. [Google Scholar] [CrossRef]
- Yavari, N.; Goltermann, L.; Nielsen, P.E. Uptake, stability, and activity of antisense anti-acpP PNA-peptide conjugates in Escherichia coli and the role of SbmA. ACS Chem. Biol. 2021, 16, 471–479. [Google Scholar] [CrossRef]
- Schwarz, F.P.; Robinson, S.; Butler, J.M. Thermodynamic Comparison of PNA/DNA and DNA/DNA Hybridization Reactions at Ambient Temperature. Nucleic Acids Res. 1999, 27, 4792–4800. [Google Scholar] [CrossRef]
- Karkare, S.; Bhatnagar, D. Promising nucleic acid analogs and mimics: Characteristic features and applications of PNA, LNA, and morpholino. Appl. Microbiol. Biotechnol. 2006, 71, 575–586. [Google Scholar] [CrossRef]
- Nakatsuka, N.; Yang, K.A.; Abendroth, J.M.; Cheung, K.M.; Xu, X.; Yang, H.; Zhao, C.; Zhu, B.; Rim, Y.S.; Yang, Y.; et al. Aptamer-Field-Effect Transistors Overcome Debye Length Limitations for Small-Molecule Sensing. Science 2018, 362, 319–324. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Hammond, S.M. An Overview of MicroRNAs. Adv. Drug Deliv. Rev. 2015, 87, 3–14. [Google Scholar] [CrossRef]
- Suzuki, H.I. Roles of microRNAs in disease biology. JMA J. 2023, 6, 104–113. [Google Scholar]
- Chen, X.; Xie, D.; Zhao, Q.; You, Z.H. MicroRNAs and complex diseases: From experimental results to computational models. Brief. Bioinform. 2019, 20, 515–539. [Google Scholar] [CrossRef]
- Smolarz, B.; Durczyński, A.; Romanowicz, H.; Szyłło, K.; Hogendorf, P. miRNAs in cancer (review of literature). Int. J. Mol. Sci. 2022, 23, 2805. [Google Scholar] [CrossRef]
- Vannini, I.; Fanini, F.; Fabbri, M. Emerging roles of microRNAs in cancer. Curr. Opin. Genet. Dev. 2018, 48, 128–133. [Google Scholar] [CrossRef]
- Zhu, C.S.; Zhu, L.; Tan, D.A.; Qiu, X.Y.; Liu, C.Y.; Xie, S.S.; Zhu, L.Y. Avenues Toward microRNA Detection In Vitro: A Review of Technical Advances and Challenges. Comput. Struct. Biotechnol. J. 2019, 17, 904–916. [Google Scholar] [CrossRef]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical biosensors-sensor principles and architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef]
- Brydges, D.C.; Federbush, P. Debye screening. Commun. Math. Phys. 1980, 73, 197–246. [Google Scholar] [CrossRef]
- Tadmor, R.; Hernández-Zapata, E.; Chen, N.; Pincus, P.; Israelachvili, J.N. Debye length and double-layer forces in polyelectrolyte solutions. Macromolecules 2002, 35, 2380–2388. [Google Scholar] [CrossRef]
- Stern, E.; Wagner, R.; Sigworth, F.J.; Breaker, R.; Fahmy, T.M.; Reed, M.A. Importance of the Debye screening length on nanowire field effect transistor sensors. Nano Lett. 2007, 7, 3405–3409. [Google Scholar] [CrossRef] [PubMed]
- Kaisti, M.; Kerko, A.; Aarikka, E.; Saviranta, P.; Boeva, Z.; Soukka, T.; Lehmusvuori, A. Real-time wash-free detection of unlabeled PNA-DNA hybridization using discrete FET sensor. Sci. Rep. 2017, 7, 15734. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.Q.; Wanunu, M.; Wang, M.X.; McReynolds, L.; Wang, Y. Detection of miRNAs with a nanopore single-molecule counter. Expert Rev. Mol. Diagn. 2012, 12, 573–584. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mishra, S.; Lahiri, H.; Banerjee, S.; Mukhopadhyay, R. Molecularly Resolved Label-Free Sensing of Single Nucleobase Mismatches by Interfacial LNA Probes. Nucleic Acids Res. 2016, 44, 3739–3749. [Google Scholar] [CrossRef]
- Bizzarri, A.R.; Cannistraro, S. The Application of Atomic Force Spectroscopy to the Study of Biological Complexes Undergoing a Biorecognition Process. Chem. Soc. Rev. 2010, 39, 734–749. [Google Scholar] [CrossRef]
- Ritzefeld, M.; Walhorn, V.; Anselmetti, D.; Sewald, N. Analysis of DNA interactions using single-molecule force spectroscopy. Amino Acids 2013, 44, 1457–1475. [Google Scholar]
- Mattiske, S.; Suetani, R.J.; Neilsen, P.M.; Callen, D.F. The Oncogenic Role of MiR-155 in Breast Cancer. Cancer Epidemiol. Biomark. Prev. 2012, 21, 1236–1243. [Google Scholar] [CrossRef]
- MacFarlane, L.-A.; Murphy, P.R. MicroRNA: Biogenesis, Function and Role in Cancer. Curr. Genom. 2010, 11, 537–561. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, J.; Wang, X.; Shi, S.; Wang, W.; Chen, Z. Appraising MicroRNA-155 as a Noninvasive Diagnostic Biomarker for Cancer Detection: A Meta-Analysis. Medicine 2016, 95, e2450. [Google Scholar] [CrossRef]
- Vaghf, A.; Khansarinejad, B.; Ghaznavi-Rad, E.; Mondanizadeh, M. The Role of MicroRNAs in Diseases and Related Signaling Pathways. Mol. Biol. Rep. 2022, 49, 6789–6801. [Google Scholar] [CrossRef]
- Due, H.; Svendsen, P.; Bødker, J.S.; Schmitz, A.; Bøgsted, M.; Johnsen, H.E.; El-Galaly, T.C.; Roug, A.S.; Dybkær, K. MiR-155 as a Biomarker in B-Cell Malignancies. Biomed Res. Int. 2016, 2016, 9513037. [Google Scholar] [CrossRef] [PubMed]
- Lavecchia di Tocco, F.; Botti, V.; Cannistraro, S.; Bizzarri, A.R. Detection of MiR-155 Using Peptide Nucleic Acid at Physiological-like Conditions by Surface Plasmon Resonance and Bio-Field Effect Transistor. Biosensors 2024, 14, 79. [Google Scholar] [CrossRef]
- Lavecchia di Tocco, F.; Cannistraro, S.; Bizzarri, A.R. A PEG-Based Strategy to Improve Detection of Clinical MicroRNA 155 by Bio-Field Effect Transistor in High Ionic Strength Environment. Talanta 2025, 292, 127881. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Wang, X.; Zheng, L.; Weng, T.; Sun, L.; Chen, X.; Zhang, Y.; Zhao, B.; Wang, D. DNAzyme-Assisted the Detection of rps27l mRNA in Protein Nanopores. Anal. Chim. Acta 2025, 1344, 343711. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, Y.; Cao, D.; He, L.; Guo, Y.; Liu, Z.; Zhang, H. Sandwich-type graphene electrochemical sensor for nucleic acid detection of SARS-CoV-2. Acta Biochim. Biophys. Sin. 2025. [Google Scholar] [CrossRef]
- Xu, H.; Xiao, C.; Zhao, F.; Suo, Z.; Liu, Y.; Wei, M.; Jin, B. A novel amplification strategy based on target-induced multicomponent nuclease-mediated catalytic hairpin assembly for fluorescent DNA sensor. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 335, 125979. [Google Scholar] [CrossRef]
- Montazersaheb, S.; Hejazi, M.S.; Charoudeh, H.N. Potential of peptide nucleic acids in future therapeutic applications. Adv. Pharm. Bull. 2018, 8, 551. [Google Scholar] [CrossRef]
- MacLelland, V.; Kravitz, M.; Gupta, A. Therapeutic and diagnostic applications of antisense peptide nucleic acids. Mol. Ther. Nucleic Acids 2024, 35, 102086. [Google Scholar] [CrossRef]
- Botti, V.; Marrone, S.; Cannistraro, S.; Bizzarri, A.R. Interaction between MiR4749 and Human Serum Albumin as Revealed by Fluorescence, FRET, Atomic Force Spectroscopy and Computational Modelling. Int. J. Mol. Sci. 2022, 23, 1291. [Google Scholar] [CrossRef]
- Botti, V.; Cannistraro, S.; Bizzarri, A.R. Interaction of MiR-155 with Human Serum Albumin: An Atomic Force Spectroscopy, Fluorescence, FRET, and Computational Modelling Evidence. Int. J. Mol. Sci. 2022, 23, 10728. [Google Scholar] [CrossRef]
- Johnson, B.N.; Mutharasan, R. Regeneration of gold surfaces covered by adsorbed thiols and proteins using liquid-phase hydrogen peroxide-mediated UV-photooxidation. J. Phys. Chem. C 2013, 117, 1335–1341. [Google Scholar] [CrossRef]
- Hutter, J.L.; Bechhoefer, J. Calibration of Atomic-Force Microscope Tips. Rev. Sci. Instrum. 1993, 64, 1868–1873. [Google Scholar] [CrossRef]
- Friedsam, C.; Wehle, A.K.; Kühner, F.; Gaub, H.E. Dynamic Single-Molecule Force Spectroscopy: Bond Rupture Analysis with Variable Spacer Length. J. Phys. Condens. Matter 2003, 15, S1709–S1723. [Google Scholar] [CrossRef]
- Bizzarri, A.R.; Cannistraro, S. Free Energy Evaluation of the P53-Mdm2 Complex from Unbinding Work Measured by Dynamic Force Spectroscopy. Phys. Chem. Chem. Phys. 2011, 13, 2738–2743. [Google Scholar] [CrossRef]
- Botti, V.; Lavecchia di Tocco, F.; Cannistraro, S.; Bizzarri, A.R. Hybridization Kinetics of MiR-155 on Gold Surfaces as Investigated by Surface Plasmon Resonance and Atomic Force Spectroscopy. ACS Omega 2023, 8, 38941–38949. [Google Scholar] [CrossRef]
- Roychoudhury, A.; Dear, J.W.; Bachmann, T.T. Proximity sensitive detection of microRNAs using electrochemical impedance spectroscopy biosensors. Biosens. Bioelectron. 2022, 212, 114404. [Google Scholar] [CrossRef]
- Paimard, G.; Ghasali, E.; Baeza, M. Screen-printed electrodes: Fabrication, modification, and biosensing applications. Chemosensors 2023, 11, 113. [Google Scholar] [CrossRef]
- Hinterdorfer, P.; Baumgartner, W.; Gruber, H.J.; Schilcher, K.; Schindler, H. Detection and Localization of Individual Antibody-Antigen Recognition Events by Atomic Force Microscopy. Proc. Natl. Acad. Sci. USA 1996, 93, 3477. [Google Scholar] [CrossRef]
- Bizzarri, A.R.; Cannistraro, S. Atomic Force Spectroscopy in Biological Complex Formation: Strategies and Perspectives. J. Phys. Chem. B 2009, 113, 16449–16464. [Google Scholar] [CrossRef]
- Bell, G.I. Models for the Specific Adhesion of Cells to Cells. Science 1978, 200, 618–627. [Google Scholar] [CrossRef]
- Evans, E.; Ritchie, K. Dynamic Strength of Molecular Adhesion Bonds. Biophys. J. 1997, 72, 1541–1555. [Google Scholar] [CrossRef] [PubMed]
- Moreno, N.; Chevalier, M.; Ronzon, F.; Manin, C.; Dupuy, M.; Krell, T.; Rieu, J.P. Unbinding Forces of Single Pertussis Toxin–Antibody Complexes Measured by Atomic Force Spectroscopy Correlate with Their Dissociation Rates Determined by Surface Plasmon Resonance. J. Mol. Recognit. 2011, 24, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Horcas, I.; Fernández, R.; Gómez-Rodríguez, J.M.; Colchero, J.; Gómez-Herrero, J.; Baro, A.M. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atzei, D.; Lavecchia di Tocco, F.; Bizzarri, A.R. Ionic Strength Investigation on the Interaction Between miR-155 and a PNA-Based Probe by Atomic Force Spectroscopy. Biomolecules 2025, 15, 634. https://doi.org/10.3390/biom15050634
Atzei D, Lavecchia di Tocco F, Bizzarri AR. Ionic Strength Investigation on the Interaction Between miR-155 and a PNA-Based Probe by Atomic Force Spectroscopy. Biomolecules. 2025; 15(5):634. https://doi.org/10.3390/biom15050634
Chicago/Turabian StyleAtzei, Davide, Francesco Lavecchia di Tocco, and Anna Rita Bizzarri. 2025. "Ionic Strength Investigation on the Interaction Between miR-155 and a PNA-Based Probe by Atomic Force Spectroscopy" Biomolecules 15, no. 5: 634. https://doi.org/10.3390/biom15050634
APA StyleAtzei, D., Lavecchia di Tocco, F., & Bizzarri, A. R. (2025). Ionic Strength Investigation on the Interaction Between miR-155 and a PNA-Based Probe by Atomic Force Spectroscopy. Biomolecules, 15(5), 634. https://doi.org/10.3390/biom15050634






