The Wheat Intrinsically Disordered Protein TdRL1 Negatively Regulates the Type One Protein Phosphatase TdPP1
Abstract
1. Introduction
2. Materials and Methods
2.1. Nicotiana benthamiana Growth Conditions and Agro-Infiltration Assays
2.2. Molecular Cloning
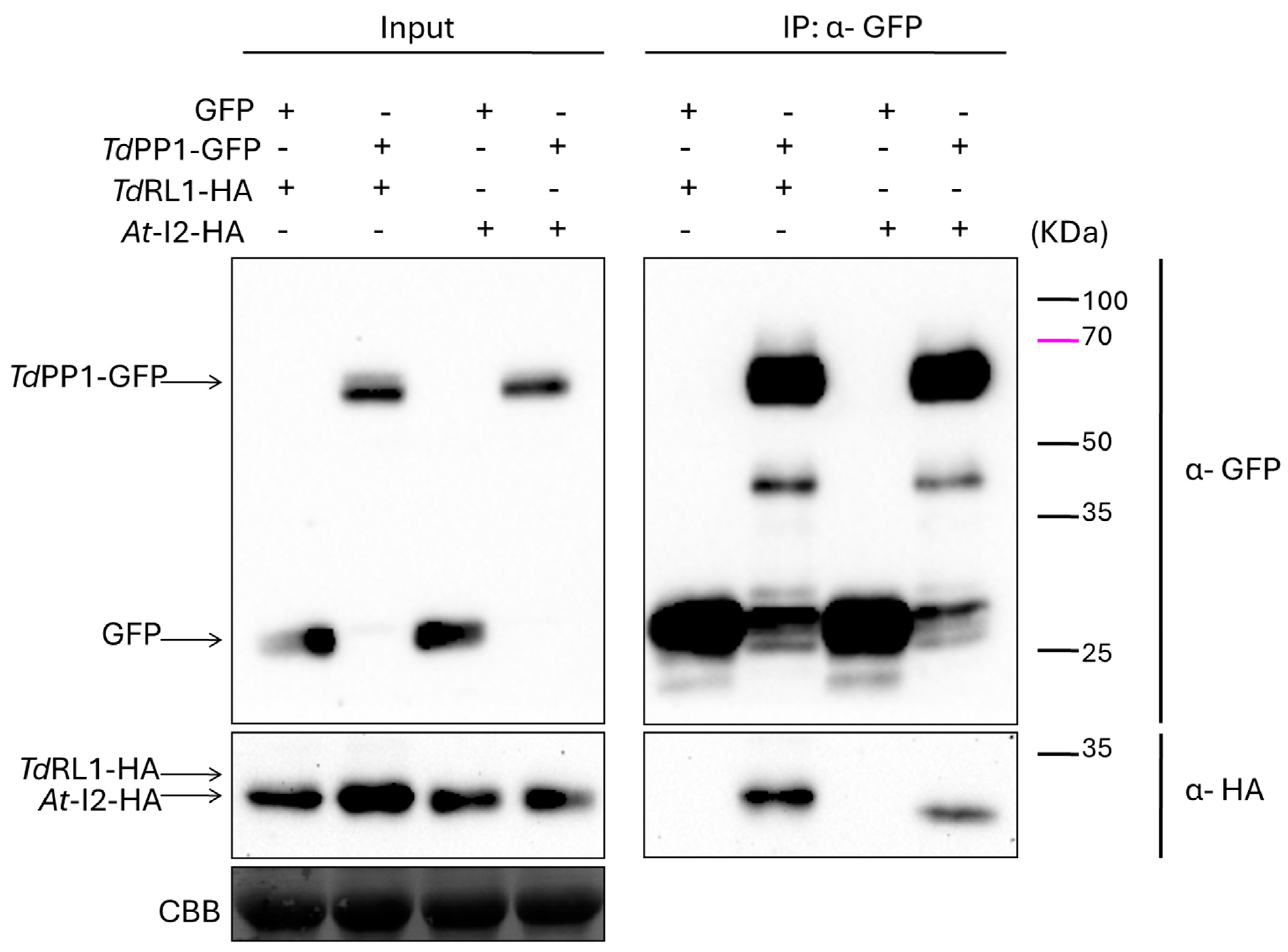
2.3. Co-Immunoprecipitation Assays
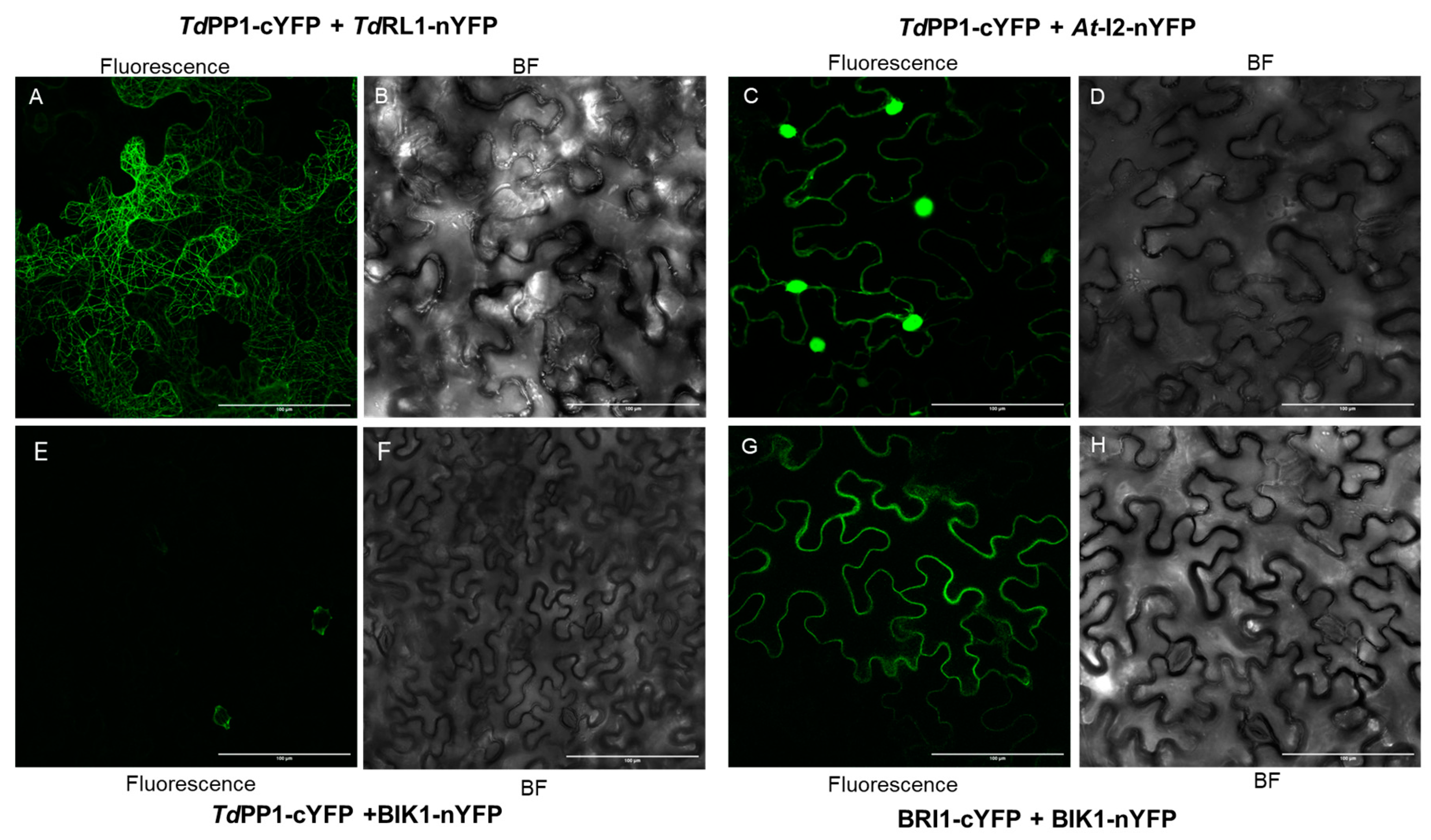
2.4. Bimolecular Fluorescence Complementation (BiFC)
2.5. Production and Purification of Recombinant Proteins
2.6. Phosphatase Assays
2.7. Statistical Analysis
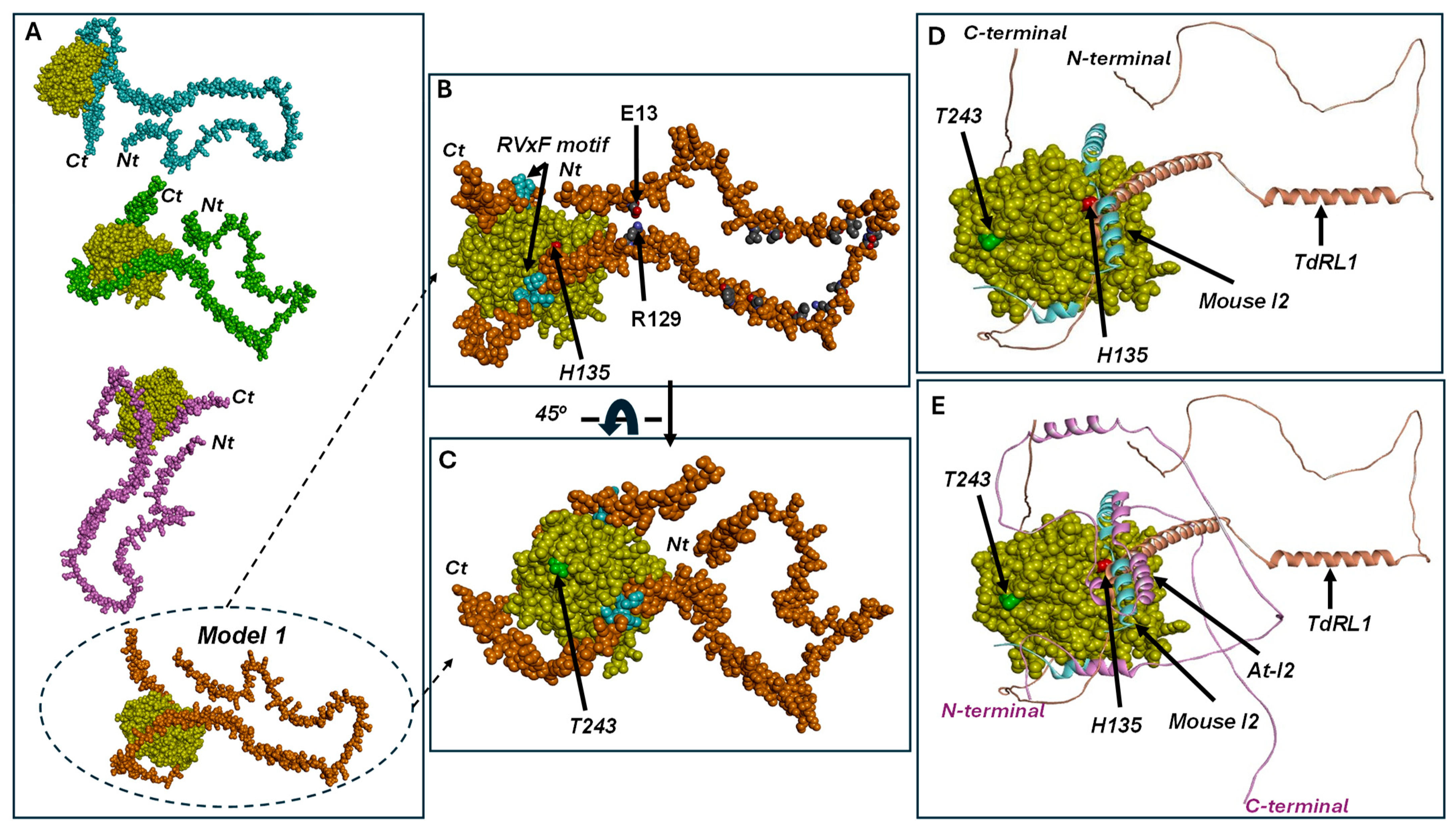
2.8. Homology Modeling and Complex Structure Optimization
3. Results
3.1. TdRL1 Interacts with TdPP1
3.2. TdPP1’s Activity Is Inhibited by TdRL1
3.3. Structural Study of At-I2 and TdRL1 Interactions with TdPP1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heroes, E.; Lesage, B.; Görnemann, J.; Beullens, M.; Van Meervelt, L.; Bollen, M. The PP1 Binding Code: A Molecular-Lego Strategy That Governs Specificity. FEBS J. 2013, 280, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Casamayor, A.; Ariño, J. Controlling Ser/Thr Protein Phosphatase PP1 Activity and Function through Interaction with Regulatory Subunits. Adv. Protein Chem. Struct. Biol. 2020, 122, 231–288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qin, Q.; Nan, X.; Guo, Z.; Liu, Y.; Jadoon, S.; Chen, Y.; Zhao, L.; Yan, L.; Hou, S. Role of Protein Phosphatase1 Regulatory Subunit3 in Mediating the Abscisic Acid Response. Plant Physiol. 2020, 184, 1317. [Google Scholar] [CrossRef] [PubMed]
- Takemiya, A.; Yamauchi, S.; Yano, T.; Ariyoshi, C.; Shimazaki, K.I. Identification of a Regulatory Subunit of Protein Phosphatase 1 Which Mediates Blue Light Signaling for Stomatal Opening. Plant Cell Physiol. 2013, 54, 24–35. [Google Scholar] [CrossRef]
- Franck, C.M.; Westermann, J.; Bürssner, S.; Lentz, R.; Lituiev, D.S.; Boisson-Dernier, A. The Protein Phosphatases ATUNIS1 and ATUNIS2 Regulate Cell Wall Integrity in Tip-Growing Cells. Plant Cell 2018, 30, 1906–1923. [Google Scholar] [CrossRef]
- Qin, Q.; Wang, W.; Guo, X.; Yue, J.; Huang, Y.; Xu, X.; Li, J.; Hou, S. Arabidopsis DELLA Protein Degradation Is Controlled by a Type-One Protein Phosphatase, TOPP4. PLoS Genet. 2014, 10, e1004464. [Google Scholar] [CrossRef]
- Yan, J.; Liu, Y.; Huang, X.; Li, L.; Hu, Z.; Zhang, J.; Qin, Q.; Yan, L.; He, K.; Wang, Y.; et al. An Unreported NB-LRR Protein SUT1 Is Required for the Autoimmune Response Mediated by Type One Protein Phosphatase 4 Mutation (Topp4-1) in Arabidopsis. Plant J. 2019, 100, 357–373. [Google Scholar] [CrossRef]
- Bradai, M.; Amorim-Silva, V.; Belgaroui, N.; Esteban Del Valle, A.; Chabouté, M.E.; Schmit, A.C.; Lozano-Duran, R.; Botella, M.A.; Hanin, M.; Ebel, C. Wheat Type One Protein Phosphatase Participates in the Brassinosteroid Control of Root Growth via Activation of Bes1. Int. J. Mol. Sci. 2021, 22, 10424. [Google Scholar] [CrossRef]
- Djemal, R.; Hanin, M.; Ebel, C.; Djemal, R.; Hanin, M.; Ebel, C. Control of Plant Responses to Salt Stress: Significance of Auxin and Brassinosteroids. In Making Plant Life Easier and Productive Under Salinity—Updates and Prospects; IntechOpen: London UK, 2023; ISBN 978-1-83768-877-7. [Google Scholar]
- Liao, Y.D.; Lin, K.H.; Chen, C.C.; Chiang, C.M. Oryza Sativa Protein Phosphatase 1a (OsPP1a) Involved in Salt Stress Tolerance in Transgenic Rice. Mol. Breed. 2016, 36, 22. [Google Scholar] [CrossRef]
- Wakula, P.; Beullens, M.; Ceulemans, H.; Stalmans, W.; Bollen, M. Degeneracy and Function of the Ubiquitous RVXF Motif That Mediates Binding to Protein Phosphatase-1. J. Biol. Chem. 2003, 278, 18817–18823. [Google Scholar] [CrossRef]
- Bollen, M.; Peti, W.; Ragusa, M.J.; Beullens, M. The Extended PP1 Toolkit: Designed to Create Specificity. Trends Biochem. Sci. 2010, 35, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Peti, W.; Nairn, A.C.; Page, R. Structural Basis for Protein Phosphatase 1 Regulation and Specificity. FEBS J. 2013, 280, 596–611. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, J.; Huang, H.-B.; Kwon, Y.G.; Greengard, P.; Nairn, A.C.; Kuriyan, J. Three-Dimensional Structure of the Catalytic Subunit of Protein Serine/Threonine Phosphatase-1. Nature 1995, 376, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Egloff, M.P.; Cohen, P.T.W.; Reinemer, P.; Barford, D. Crystal Structure of the Catalytic Subunit of Human Protein Phosphatase 1 and Its Complex with Tungstate. J. Mol. Biol. 1995, 254, 942–959. [Google Scholar] [CrossRef]
- McWhirter, C.; Lund, E.A.; Tanifum, E.A.; Feng, G.; Sheikh, Q.I.; Hengge, A.C.; Williams, N.H. Mechanistic Study of Protein Phosphatase-1 (PP1), a Catalytically Promiscuous Enzyme. J. Am. Chem. Soc. 2008, 130, 13673–13682. [Google Scholar] [CrossRef]
- Egloff, M.P.; Johnson, D.F.; Moorhead, G.; Cohen, P.T.W.; Cohen, P.; Barford, D. Structural Basis for the Recognition of Regulatory Subunits by the Catalytic Subunit of Protein Phosphatase 1. EMBO J. 1997, 16, 1876–1887. [Google Scholar] [CrossRef]
- Terrak, M.; Kerff, F.; Langseimo, K.; Tao, T.; Dominguez, R. Structural Basis of Protein Phosphatase 1 Regulation. Nature 2004, 429, 780–784. [Google Scholar] [CrossRef]
- Hendrickx, A.; Beullens, M.; Ceulemans, H.; Den Abt, T.; Van Eynde, A.; Nicolaescu, E.; Lesage, B.; Bollen, M. Docking Motif-Guided Mapping of the Interactome of Protein Phosphatase-1. Chem. Biol. 2009, 16, 365–371. [Google Scholar] [CrossRef]
- Lemaire, S.; Bollen, M. Protein Phosphatase-1: Dual Activity Regulation by Inhibitor-2. Biochem. Soc. Trans. 2020, 48, 2229–2240. [Google Scholar] [CrossRef]
- Templeton, G.W.; Nimick, M.; Morrice, N.; Campbell, D.; Goudreault, M.; Gingras, A.-C.; Takemiya, A.; Shimazaki, K.; Moorhead, G.B.G. Identification and Characterization of AtI-2, an Arabidopsis Homologue of an Ancient Protein Phosphatase 1 (PP1) Regulatory Subunit. Biochem. J. 2011, 435, 73–83. [Google Scholar] [CrossRef]
- Hou, Y.J.; Zhu, Y.; Wang, P.; Zhao, Y.; Xie, S.; Batelli, G.; Wang, B.; Duan, C.G.; Wang, X.; Xing, L.; et al. Type One Protein Phosphatase 1 and Its Regulatory Protein Inhibitor 2 Negatively Regulate ABA Signaling. PLoS Genet. 2016, 12, e1005835. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, D.; Abe, K.; Miyao, A.; Kojima, M.; Sakakibara, H.; Mizutani, M.; Morita, H.; Toda, Y.; Hobo, T.; Sato, Y.; et al. RSS1 Regulates the Cell Cycle and Maintains Meristematic Activity under Stress Conditions in Rice. Nat. Commun. 2011, 2, 278. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, D.; Morita, H.; Hattori, T.; Takeda, S. Molecular Characterization of the Rice Protein RSS1 Required for Meristematic Activity under Stressful Conditions. Plant Physiol. Biochem. 2012, 61, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Mahjoubi, H.; Ebel, C.; Hanin, M. Molecular and Functional Characterization of the Durum Wheat TdRL1, a Member of the Conserved Poaceae RSS1-like Family That Exhibits Features of Intrinsically Disordered Proteins and Confers Stress Tolerance in Yeast. Funct. Integr. Genom. 2015, 15, 717–728. [Google Scholar] [CrossRef]
- Mahjoubi, H.; Tamari, Y.; Takeda, S.; Bouchabké-Coussa, O.; Hanin, M.; Herzog, E.; Schmit, A.C.; Chabouté, M.E.; Ebel, C. The Wheat TdRL1 Is the Functional Homolog of the Rice RSS1 and Promotes Plant Salt Stress Tolerance. Plant Cell Rep. 2018, 37, 1625–1637. [Google Scholar] [CrossRef]
- Karimi, M.; Inzé, D.; Depicker, A. GATEWAYTM Vectors for Agrobacterium-Mediated Plant Transformation. Trends Plant Sci. 2002, 7, 193–195. [Google Scholar] [CrossRef]
- Bradai, M.; Mahjoubi, H.; Chini, A.; Chabouté, M.E.; Hanin, M.; Ebel, C. Genome Wide Identification of Wheat and Brachypodium Type One Protein Phosphatases and Functional Characterization of Durum Wheat TdPP1a. PLoS ONE 2018, 13, e0191272. [Google Scholar] [CrossRef]
- Nakagawa, T.; Kurose, T.; Hino, T.; Tanaka, K.; Kawamukai, M.; Niwa, Y.; Toyooka, K.; Matsuoka, K.; Jinbo, T.; Kimura, T. Development of Series of Gateway Binary Vectors, PGWBs, for Realizing Efficient Construction of Fusion Genes for Plant Transformation. J. Biosci. Bioeng. 2007, 104, 34–41. [Google Scholar] [CrossRef]
- Gehl, C.; Waadt, R.; Kudla, J.; Mendel, R.R.; Hänsch, R. New GATEWAY Vectors for High Throughput Analyses of Protein-Protein Interactions by Bimolecular Fluorescence Complementation. Mol. Plant 2009, 2, 1051–1058. [Google Scholar] [CrossRef]
- Amorim-Silva, V.; García-Moreno, Á.; Castillo, A.G.; Lakhssassi, N.; Del Valle, A.E.; Pérez-Sancho, J.; Li, Y.; Posé, D.; Pérez-Rodriguez, J.; Lin, J.; et al. TTL Proteins Scaffold Brassinosteroid Signaling Components at the Plasma Membrane to Optimize Signal Transduction in Arabidopsis. Plant Cell 2019, 31, 1807–1828. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, S.; Clemens, J.C.; Hakes, D.J.; Barford, D.; Dixon, J.E. Expression, Purification, Crystallization, and Biochemical Characterization of a Recombinant Protein Phosphatase. J. Biol. Chem. 1993, 268, 17754–17761. [Google Scholar] [CrossRef] [PubMed]
- SWISS-MODEL Interactive Workspace. Available online: https://swissmodel.expasy.org/interactive (accessed on 23 January 2025).
- RCSB PDB: Homepage. Available online: https://www.rcsb.org/ (accessed on 23 January 2025).
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Pierce, B.G.; Wiehe, K.; Hwang, H.; Kim, B.H.; Vreven, T.; Weng, Z. ZDOCK Server: Interactive Docking Prediction of Protein-Protein Complexes and Symmetric Multimers. Bioinformatics 2014, 30, 1771–1773. [Google Scholar] [CrossRef]
- Guex, N.; Peitsch, M.C.; Schwede, T. Automated Comparative Protein Structure Modeling with SWISS-MODEL and Swiss-PdbViewer: A Historical Perspective. Electrophoresis 2009, 30 (Suppl. S1), S162–S173. [Google Scholar] [CrossRef]
- Ebel, C.; Hanin, M. Maintenance of Meristem Activity under Stress: Is There an Interplay of RSS1-like Proteins with the RBR Pathway? Plant Biol. 2016, 18, 167–170. [Google Scholar] [CrossRef]
- Lin, W.; Lu, D.; Gao, X.; Jiang, S.; Ma, X.; Wang, Z.; Mengiste, T.; Shan, L. Inverse Modulation of Plant Immune and Brassinosteroid Signaling Pathways by the Receptor-like Cytoplasmic Kinase BIK1. Proc. Natl. Acad. Sci. USA 2013, 110, 12114–12119. [Google Scholar] [CrossRef]
- Ceulemans, H.; Bollen, M. Functional Diversity of Protein Phosphatase-1, a Cellular Economizer and Reset Button. Physiol. Rev. 2004, 84, 1–39. [Google Scholar] [CrossRef]
- Bielas, S.L.; Serneo, F.F.; Chechlacz, M.; Deerinck, T.J.; Perkins, G.A.; Allen, P.B.B.; Ellisman, M.H.; Gleeson, J.G. Spinophilin Facilitates Dephosphorylation of Doublecortin by PP1 to Mediate Microtubule Bundling at the Axonal Wrist. Cell 2007, 129, 579–591. [Google Scholar] [CrossRef]
- Guo, X.; Qin, Q.; Yan, J.; Niu, Y.; Huang, B.; Guan, L.; Li, Y.; Ren, D.; Li, J.; Hou, S. Type-One Protein Phosphatase4 Regulates Pavement Cell Interdigitation by Modulating PIN-FORMED1 Polarity and Trafficking in Arabidopsis. Plant Physiol. 2015, 167, 1058–1075. [Google Scholar] [CrossRef]
- Beullens, M.; Van Eynde, A.; Vulsteke, V.; Connor, J.; Shenolikar, S.; Stalmans, W.; Bollen, M. Molecular Determinants of Nuclear Protein Phosphatase-1 Regulation by NIPP-1. J. Biol. Chem. 1999, 274, 14053–14061. [Google Scholar] [CrossRef] [PubMed]
- Hurley, T.D.; Yang, J.; Zhang, L.; Goodwin, K.D.; Zou, Q.; Cortese, M.; Dunker, A.K.; DePaoli-Roach, A.A. Structural Basis for Regulation of Protein Phosphatase 1 by Inhibitor-2. J. Biol. Chem. 2007, 282, 28874–28883. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Z.; Brew, K.; Lee, E.Y.C. Mutational Analysis of the Catalytic Subunit of Muscle Protein Phosphatase-1. Biochemistry 1996, 35, 6276–6282. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amor, F.; Bradai, M.; Zaidi, I.; Amorim-Silva, V.; Miled, N.; Hanin, M.; Ebel, C. The Wheat Intrinsically Disordered Protein TdRL1 Negatively Regulates the Type One Protein Phosphatase TdPP1. Biomolecules 2025, 15, 631. https://doi.org/10.3390/biom15050631
Amor F, Bradai M, Zaidi I, Amorim-Silva V, Miled N, Hanin M, Ebel C. The Wheat Intrinsically Disordered Protein TdRL1 Negatively Regulates the Type One Protein Phosphatase TdPP1. Biomolecules. 2025; 15(5):631. https://doi.org/10.3390/biom15050631
Chicago/Turabian StyleAmor, Fatma, Mariem Bradai, Ikram Zaidi, Vitor Amorim-Silva, Nabil Miled, Moez Hanin, and Chantal Ebel. 2025. "The Wheat Intrinsically Disordered Protein TdRL1 Negatively Regulates the Type One Protein Phosphatase TdPP1" Biomolecules 15, no. 5: 631. https://doi.org/10.3390/biom15050631
APA StyleAmor, F., Bradai, M., Zaidi, I., Amorim-Silva, V., Miled, N., Hanin, M., & Ebel, C. (2025). The Wheat Intrinsically Disordered Protein TdRL1 Negatively Regulates the Type One Protein Phosphatase TdPP1. Biomolecules, 15(5), 631. https://doi.org/10.3390/biom15050631





