A Lipidomic Approach to Studying the Downregulation of Free Fatty Acids by Cytosolic Phospholipase A2 Inhibitors
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. GIVA cPLA2 Inhibitors
2.3. Cell Culture and Treatment with GIVA cPLA2 Inhibitors
2.4. MTT Assay
2.5. Sample Preparation for Analysis
2.6. Instrumentation
2.7. Method Validation
3. Results
3.1. Selection of GIVA cPLA2 Inhibitors
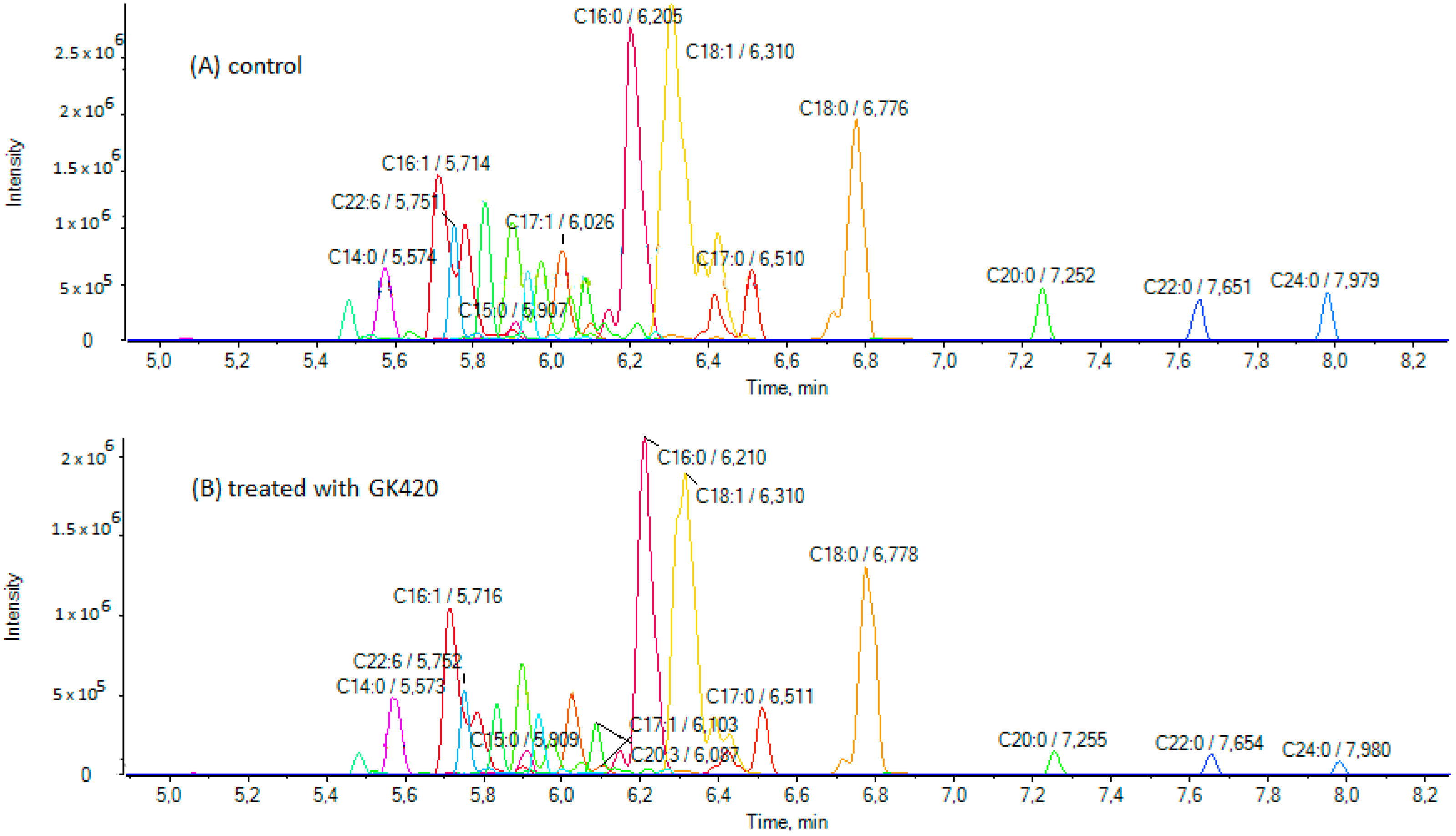
3.2. Treatment of SH-SY5Y Cells with GIVA cPLA2 Inhibitors and Determination of FFAs by LC-HRMS
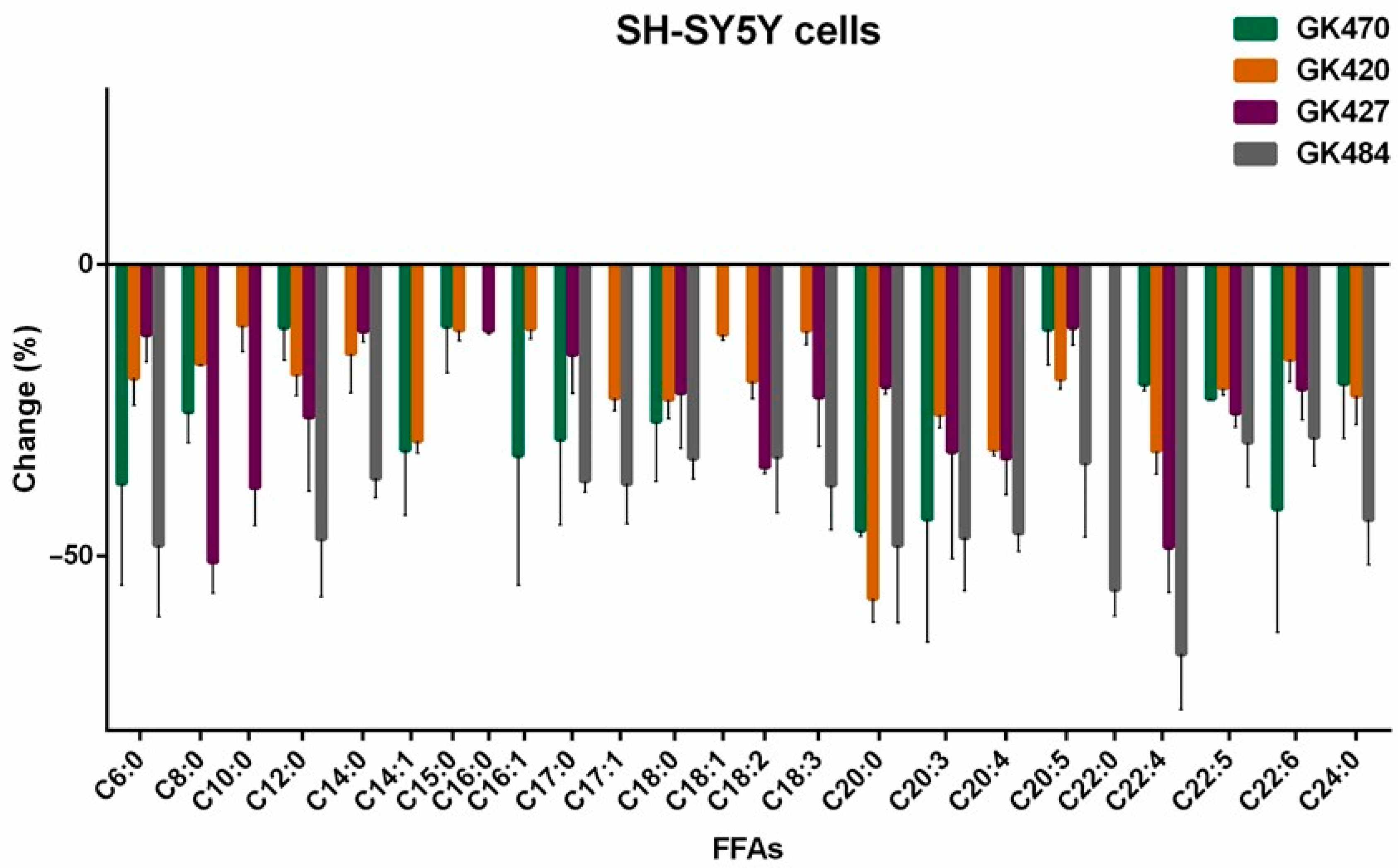
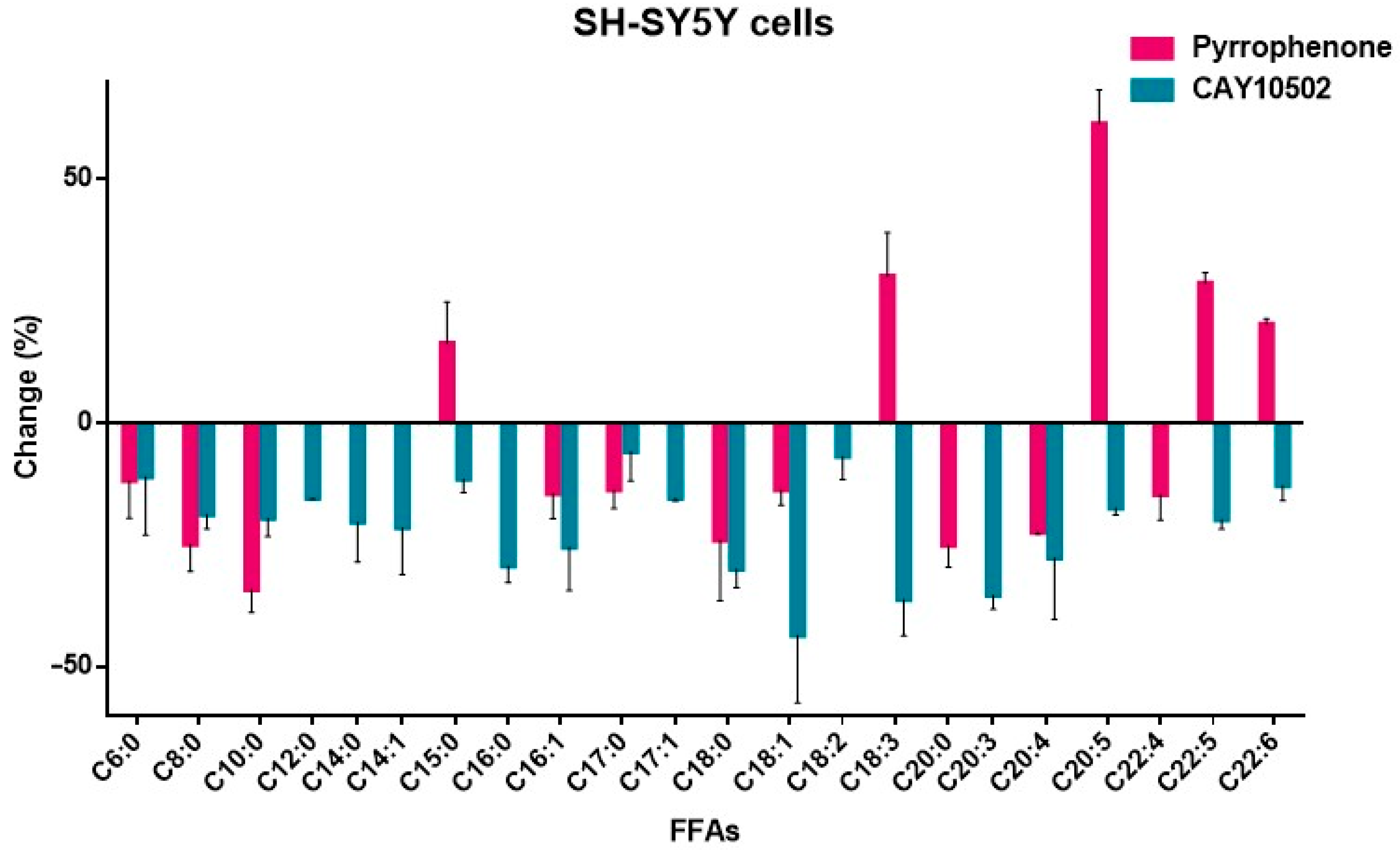
3.3. Effects of GIVA cPLA2 Inhibitors on Intracellular FFA Levels in SH-SY5Y Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, S.A.; Ilies, M.A. Phospholipases A2. In Metalloenzymes; Elsevier: Amsterdam, The Netherlands, 2024; pp. 101–136. ISBN 9780128239742. [Google Scholar]
- Khan, S.A.; Ilies, M.A. The phospholipase A2 superfamily: Structure, isozymes, catalysis, physiologic and pathologic roles. Int. J. Mol. Sci. 2023, 24, 1353. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Sato, H.; Taketomi, Y. Updating phospholipase A2 biology. Biomolecules 2020, 10, 1457. [Google Scholar] [CrossRef]
- Leslie, C.C. Cytosolic phospholipase A₂: Physiological function and role in disease. J. Lipid Res. 2015, 56, 1386–1402. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Wang, S.; Liu, R.; Zhang, D.; Zhang, J.; Qi, X.; Li, Z.; Miao, M.; Cai, X.; Su, G. Research progress of cPLA2 in cardiovascular diseases. Mol. Med. Rep. 2025, 31, 103. [Google Scholar] [CrossRef] [PubMed]
- Funk, C.D. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science 2001, 294, 1871–1875. [Google Scholar] [CrossRef]
- Leskovac, A. Natural inhibitors of phospholipase A2: Current knowledge and therapeutic approaches. In Phospholipases in Physiology and Pathology; Elsevier: Amsterdam, The Netherlands, 2023; pp. 67–77. ISBN 9780323956871. [Google Scholar]
- Arockiasamy, P.; Srinivasan, S.; Pugalendhi, M.A.; Josephinol, S.; Murugan, K.K. Analyzing the interaction of synthetic inhibitors with phospholipases through in silico methods. In Phospholipases in Physiology and Pathology; Elsevier: Amsterdam, The Netherlands, 2023; pp. 243–254. ISBN 9780323956871. [Google Scholar]
- Sales, T.A.; Marcussi, S.; Ramalho, T.C. Current anti-inflammatory therapies and the potential of secretory phospholipase A2 inhibitors in the design of new anti-inflammatory drugs: A review of 2012–2018. Cur. Med. Chem. 2020, 27, 477–497. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Sun, J.; Zhang, W.; Guo, Z.; Ma, Q. Arachidonic acid metabolism in health and disease. MedComm 2023, 4, e363. [Google Scholar] [CrossRef]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism pathways of arachidonic acids: Mechanisms and potential therapeutic targets. Sig. Transduct. Target Ther. 2021, 6, 94. [Google Scholar] [CrossRef]
- Qin, J.; Ye, L.; Wen, X.; Zhang, X.; Di, Y.; Chen, Z.; Wang, Z. Fatty acids in cancer chemoresistance. Cancer Lett. 2023, 572, 216352. [Google Scholar] [CrossRef]
- Koundouros, N.; Poulogiannis, G. Reprogramming of fatty acid metabolism in cancer. Br. J. Cancer 2020, 122, 4–22. [Google Scholar] [CrossRef]
- Tong, B.; Ba, Y.; Li, Z.; Yang, C.; Su, K.; Qi, H.; Zhang, D.; Liu, X.; Wu, Y.; Chen, Y.; et al. Targeting dysregulated lipid metabolism for the treatment of Alzheimer’s disease and Parkinson’s disease: Current advancements and future prospects. Neurobiol. Dis. 2024, 196, 106505. [Google Scholar] [CrossRef] [PubMed]
- Lohitaksha, K.; Kumari, D.; Shukla, M.; Byagari, L.; Ashireddygari, V.R.; Tammineni, P.; Reddanna, P.; Gorla, M. Eicosanoid signaling in neuroinflammation associated with Alzheimer’s disease. Eur. J. Pharmacol. 2024, 976, 176694. [Google Scholar] [CrossRef] [PubMed]
- Ugalde, C.L.; Lawson, V.A.; Finkelstein, D.I.; Hill, A.F. The role of lipids in α-synuclein misfolding and neurotoxicity. J. Biol. Chem. 2019, 294, 9016–9028. [Google Scholar] [CrossRef]
- Ramirez, J.; Pancoe, S.X.; Rhoades, E.; Petersson, E.J. The effects of lipids on α-synuclein aggregation in vitro. Biomolecules 2023, 13, 1476. [Google Scholar] [CrossRef]
- Fecchio, C.; Palazzi, L.; Polverino De Laureto, P. α-Synuclein and polyunsaturated fatty acids: Molecular basis of the interaction and implication in neurodegeneration. Molecules 2018, 23, 1531. [Google Scholar] [CrossRef] [PubMed]
- Iljina, M.; Tosatto, L.; Choi, M.L.; Sang, J.C.; Ye, Y.; Hughes, C.D.; Bryant, C.E.; Gandhi, S.; Klenerman, D. Arachidonic acid mediates the formation of abundant alpha-helical multimers of alpha-synuclein. Sci. Rep. 2016, 6, 33928. [Google Scholar] [CrossRef]
- De Franceschi, G.; Frare, E.; Pivato, M.; Relini, A.; Penco, A.; Greggio, E.; Bubacco, L.; Fontana, A.; De Laureto, P.P. Structural and morphological characterization of aggregated species of α-synuclein induced by docosahexaenoic acid. J. Biol. Chem. 2011, 286, 22262–22274. [Google Scholar] [CrossRef]
- Xylaki, M.; Boumpoureka, I.; Kokotou, M.G.; Marras, T.; Papadimitriou, G.; Kloukina, I.; Magrioti, V.; Kokotos, G.; Vekrellis, K.; Emmanouilidou, E. Changes in the cellular fatty acid profile drive the proteasomal degradation of α-synuclein and enhance neuronal survival. FASEB J. 2020, 34, 15123–15145. [Google Scholar] [CrossRef] [PubMed]
- Ashcroft, F.J.; Bourboula, A.; Mahammad, N.; Barbayianni, E.; Feuerherm, A.J.; Nguyen, T.T.; Hayashi, D.; Kokotou, M.G.; Alevizopoulos, K.; Dennis, E.A.; et al. Next generation thiazolyl ketone inhibitors of cytosolic phospholipase A2 α for targeted cancer therapy. Nat. Commun. 2025, 16, 164. [Google Scholar] [CrossRef]
- Koeberle, A. Target identification and lead discovery by functional lipidomics. Future Med. Chem. 2016, 8, 2169–2171. [Google Scholar] [CrossRef]
- Han, X. Lipidomics for studying metabolism. Nat. Rev. Endocrinol. 2016, 12, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Vekrellis, K.; Xilouri, M.; Emmanouilidou, E.; Stefanis, L. Inducible over-expression of wild type alpha-synuclein in human neuronal cells leads to caspase-dependent non-apoptotic death. J. Neurochem. 2009, 109, 1348–1362. [Google Scholar] [CrossRef]
- Kokotou, M.G.; Mantzourani, C.; Kokotos, G. Development of a liquid chromatography–high resolution mass spectrometry method for the determination of free fatty acids in milk. Molecules 2020, 25, 1548. [Google Scholar] [CrossRef] [PubMed]
- Kokotos, G.; Feuerherm, A.J.; Barbayianni, E.; Shah, I.; Sæther, M.; Magrioti, V.; Nguyen, T.; Constantinou-Kokotou, V.; Dennis, E.A.; Johansen, B. Inhibition of group IVA cytosolic phospholipase A2 by thiazolyl ketones in vitro, ex vivo, and in vivo. J. Med. Chem. 2014, 57, 7523–7535. [Google Scholar] [CrossRef]
- Psarra, A.; Kokotou, M.G.; Galiatsatou, G.; Mouchlis, V.D.; Dennis, E.A.; Kokotos, G. Highly potent 2-oxoester inhibitors of cytosolic phospholipase A2 (GIVA cPLA2). ACS Omega 2018, 3, 8843–8853. [Google Scholar] [CrossRef] [PubMed]
- Seno, K.; Okuno, T.; Nishi, K.; Murakami, Y.; Watanabe, F.; Matsuura, T.; Wada, M.; Fujii, Y.; Yamada, M.; Ogawa, T.; et al. Pyrrolidine inhibitors of human cytosolic phospholipase A2. J. Med. Chem. 2000, 43, 1041–1044. [Google Scholar] [CrossRef]
- Mouchlis, V.D.; Armando, A.; Dennis, E.A. Substrate-specific inhibition constants for phospholipase A2 acting on unique phospholipid substrates in mixed micelles and membranes using lipidomics. J. Med. Chem. 2019, 62, 1999–2007. [Google Scholar] [CrossRef]
- Ludwig, J.; Bovens, S.; Brauch, C.; Elfringhoff, A.S.; Lehr, M. Design and synthesis of 1-indol-1-yl-propan-2-ones as inhibitors of human cytosolic phospholipase A2α. J. Med. Chem. 2006, 49, 2611–2620. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Yun, B.; Lee, H.; Ewing, H.; Gelb, M.H.; Leslie, C.C. Off-target effect of the cPLA2α inhibitor pyrrophenone: Ιnhibition of calcium release from the endoplasmic reticulum. Biochem. Biophys. Res. Commun. 2016, 479, 61–66. [Google Scholar] [CrossRef]
- Yuan, J.; Zhu, C.; Hong, Y.; Sun, Z.; Fang, X.; Wu, B.; Li, S. The role of cPLA2 in methylglyoxal-induced cell apoptosis of HUVECs. Toxicol. Appl. Pharmacol. 2017, 323, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.L.; Foley, M.A.; Chen, L.; Behnke, M.L.; Lovering, F.E.; Kirincich, S.J.; Wang, W.; Shim, J.; Tam, S.; Shen, M.W.H.; et al. Discovery of Ecopladib, an indole inhibitor of cytosolic phospholipase A2α. J. Med. Chem. 2007, 50, 1380–1400. [Google Scholar] [CrossRef] [PubMed]
- A Study to Determine the Safety & Efficacy of ZPL-5,212,372 in Healthy Subjects and in Subjects with Atopic Dermatitis. Identifier: NCT02795832. Available online: https://clinicaltrials.gov/study/NCT02795832?term=NCT02795832&rank=1 (accessed on 1 February 2025).
- Ortner, V.K.; Johansen, B.; Kilov, K.; Castillo Mondragón, A.; Duvold, T.; Kihl, J.; Ashcroft, F.J.; Feuerherm, A.J.; Pind Laugesen, C.; Marcker Espersen, M.L.; et al. The Copenhagen Actinic Keratosis Study (COAKS). A decentralised clinical trial to evaluate tolerability, safety and efficacy of daily field-directed topical treatment with cytosolic phospholipase A2 inhibitor, AVX001, in participants with actinic keratosis: Protocol for a randomised controlled phase I/IIa trial. BMJ Open 2022, 12, e061012. [Google Scholar] [CrossRef] [PubMed]
- Kanai, S.; Ishihara, K.; Kawashita, E.; Tomoo, T.; Nagahira, K.; Hayashi, Y.; Akiba, S. ASB14780, an orally active inhibitor of group IVA phospholipase A2, is a pharmacotherapeutic candidate for nonalcoholic fatty liver disease. J. Pharmacol. Exp. Ther. 2016, 356, 604–614. [Google Scholar] [CrossRef]
- Mouchlis, V.D.; Chen, Y.; McCammon, J.A.; Dennis, E.A. Membrane allostery and unique hydrophobic sites promote enzyme substrate specificity. J. Am. Chem. Soc. 2018, 140, 3285–3291. [Google Scholar] [CrossRef]
- Hayashi, D.; Mouchlis, V.D.; Dennis, E.A. Omega-3 versus omega-6 fatty acid availability is controlled by hydrophobic site geometries of phospholipase A2s. J. Lipid Res. 2021, 62, 100113. [Google Scholar] [CrossRef]
- Monge, P.; Garrido, A.; Rubio, J.M.; Magrioti, V.; Kokotos, G.; Balboa, M.A.; Balsinde, J. The contribution of cytosolic group IVA and calcium-independent group VIA phospholipase A2s to adrenic acid mobilization in murine macrophages. Biomolecules 2020, 10, 542. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, H.; Ma, X.; Zhu, D.; Zhao, L.; Xiao, W. Adrenic acid: A promising biomarker and therapeutic target. Int. J. Mol. Med. 2024, 55, 20. [Google Scholar] [CrossRef]
- Quehenberger, O.; Armando, A.M.; Cedeno, T.H.; Loomba, R.; Sanyal, A.J.; Dennis, E.A. Novel eicosanoid signature in plasma provides diagnostic for metabolic dysfunction-associated steatotic liver disease. J. Lipid Res. 2024, 65, 100647. [Google Scholar] [CrossRef]
- Burke, J.E.; Babakhani, A.; Gorfe, A.A.; Kokotos, G.; Li, S.; Woods, V.L., Jr.; McCammon, J.A.; Dennis, E.A. Location of inhibitors bound to group IVA phospholipase A2 determined by molecular dynamics and deuterium exchange mass spectrometry. J. Am. Chem. Soc. 2009, 131, 8083–8091. [Google Scholar] [CrossRef]
- Ping, P.; Li, J.; Lei, H.; Xu, X. Fatty acid metabolism: A new therapeutic target for cervical cancer. Front. Oncol. 2023, 13, 1111778. [Google Scholar] [CrossRef] [PubMed]
- Tu, K.; Ma, T.; Zhou, R.; Xu, L.; Fang, Y.; Zhang, C. Association between dietary fatty acid patterns and colorectal cancer risk: A large-scale case-control study in China. Nutrients 2022, 14, 4375. [Google Scholar] [CrossRef] [PubMed]
- Koundouros, N.; Karali, E.; Tripp, A.; Valle, A.; Inglese, P.; Perry, N.J.S.; Magee, D.J.; Anjomani Virmouni, S.; Elder, G.A.; Tyson, A.L.; et al. Metabolic fingerprinting links oncogenic PIK3CA with enhanced arachidonic acid-derived eicosanoids. Cell 2020, 181, 1596–1611. [Google Scholar] [CrossRef] [PubMed]
- Mantzourani, C.; Batsika, C.S.; Kokotou, M.G.; Kokotos, G. Free fatty acid profiling of Greek yogurt by liquid chromatography-high resolution mass spectrometry (LC-HRMS) analysis. Food Res. Int. 2022, 160, 111751. [Google Scholar] [CrossRef]

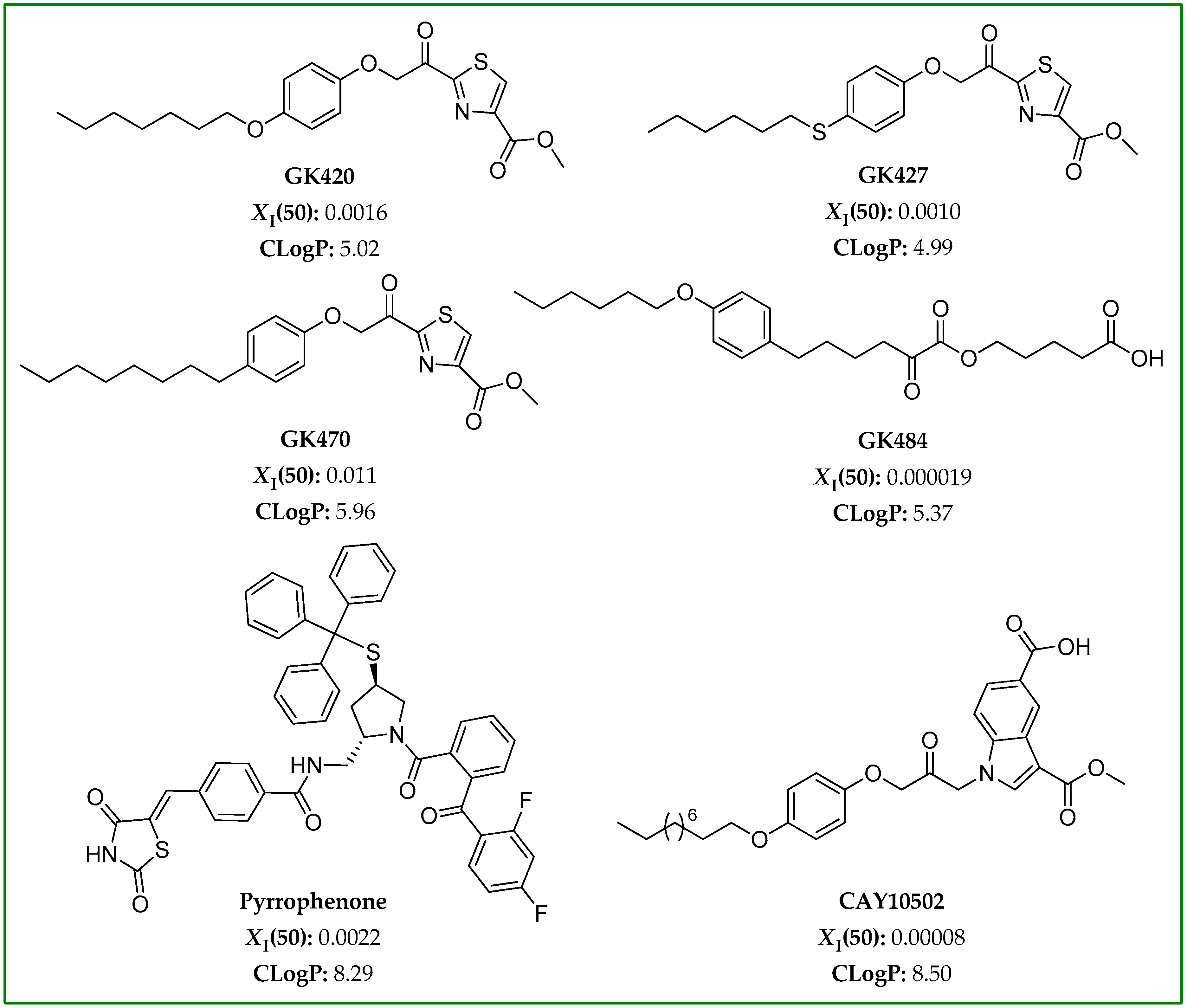

| Fatty Acid | GK470 | GK420 | GK427 | GK484 | ||||
|---|---|---|---|---|---|---|---|---|
| Change (%) | SD | Change (%) | SD | Change (%) | SD | Change (%) | SD | |
| Caproic acid (C6:0) | −37.56 | 17.54 | −19.52 | 4.71 | −12.07 | 4.71 | −48.19 | 12.22 |
| Caprylic acid (C8:0) | −25.28 | 5.29 | −17.08 | 0.31 | −51.06 | 5.30 | <10 | - |
| Capric acid (C10:0) | <10 | - | −10.48 | 4.46 | −38.29 | 6.47 | <10 | - |
| Lauric acid (C12:0) | −10.82 | 5.66 | −18.84 | 3.71 | −26.19 | 12.74 | −47.09 | 9.91 |
| Myristic acid (C14:0) | <10 | - | −15.30 | 6.76 | −11.51 | 1.84 | −36.71 | 3.32 |
| Myristoleic acid (C14:1) | −31.81 | 11.23 | −30.28 | 2.13 | <10 | - | <10 | - |
| Pentadecanoic acid (C15:0) | −10.62 | 7.95 | −11.30 | 1.84 | <10 | - | <10 | - |
| Palmitic acid (C16:0) | <10 | - | <10 | - | −11.38 | 0.58 | <10 | - |
| 9-Palmitoleic acid (C16:1) | −32.79 | 22.29 | −11.08 | 1.77 | <10 | - | <10 | - |
| Margaric acid (C17:0) | −29.97 | 14.75 | <10 | - | −15.50 | 6.66 | −37.15 | 1.93 |
| 10-Z-Heptadecenoic acid (C17:1) | <10 | - | −22.93 | 2.23 | <10 | - | −37.65 | 6.88 |
| Stearic acid (C18:0) | −26.90 | 10.39 | −23.20 | 3.32 | −22.06 | 9.46 | −33.28 | 3.54 |
| Oleic acid (C18:1) | <10 | - | −11.99 | 0.99 | <10 | - | <10 | - |
| Linoleic acid (C18:2) | <10 | - | −20.02 | 3.09 | −34.79 | 1.08 | −32.99 | 9.62 |
| Total Linolenic acid (C18:3) | <10 | - | −11.36 | 2.38 | −22.79 | 8.43 | −37.87 | 7.65 |
| Arachidic acid (C20:0) | −45.61 | 1.07 | −57.30 | 4.09 | −20.97 | 1.28 | −48.17 | 13.31 |
| Bishomo-γ-linolenic acid (C20:3) | −43.70 | 21.05 | −25.76 | 2.23 | −32.13 | 18.37 | −46.85 | 9.09 |
| Arachidonic acid (C20:4) | <10 | - | −31.76 | 0.98 | −33.21 | 6.34 | −46.09 | 3.19 |
| EPA (C20:5) | −11.12 | 6.19 | −19.67 | 1.76 | −10.83 | 3.03 | −34.08 | 12.73 |
| Behenic acid (C22:0) | <10 | - | <10 | - | <10 | - | −55.75 | 4.54 |
| Adrenic acid (C22:4) | −20.54 | 1.21 | −32.01 | 3.97 | −48.52 | 7.81 | −66.85 | 9.54 |
| Docosapentaenoic acid (C22:5) | −23.03 | 0.13 | −21.21 | 1.26 | −25.52 | 2.40 | −30.52 | 7.70 |
| DHA (C22:6) | −41.95 | 21.14 | −16.31 | 3.80 | −21.39 | 5.26 | −29.67 | 4.83 |
| Lignoceric acid (C24:0) | −20.48 | 9.46 | −22.50 | 4.97 | <10 | - | −43.77 | 7.76 |
| Fatty Acid | Pyrrophenone | CAY10502 | ||
|---|---|---|---|---|
| Change (%) | SD | Change (%) | SD | |
| Caproic acid (C6:0) | −11.84 | 7.62 | −11.04 | 11.93 |
| Caprylic acid (C8:0) | −24.86 | 5.59 | −18.75 | 2.92 |
| Capric acid (C10:0) | −34.16 | 4.72 | −19.51 | 3.77 |
| Lauric acid (C12:0) | <10 | - | −15.41 | 0.18 |
| Myristic acid (C14:0) | <10 | - | −20.33 | 8.11 |
| Myristoleic acid (C14:1) | <10 | - | −21.47 | 9.54 |
| Pentadecanoic acid (C15:0) | +16.34 | 8.47 | −11.52 | 2.76 |
| Palmitic acid (C16:0) | <10 | - | −29.31 | 3.39 |
| 9-Palmitoleic acid (C16:1) | −14.47 | 5.11 | −25.44 | 8.89 |
| Margaric acid (C17:0) | −13.71 | 3.81 | −5.83 | 5.97 |
| 10-Z-Heptadecenoic acid (C17:1) | <10 | - | −15.41 | 0.59 |
| Stearic acid (C18:0) | −24.01 | 12.39 | −29.92 | 3.78 |
| Oleic acid (C18:1) | −13.69 | 3.14 | −43.49 | 13.93 |
| Linoleic acid (C18:2) | <10 | - | −6.86 | 4.69 |
| Total Linolenic acid (C18:3) | +30.12 | 8.93 | −36.16 | 7.48 |
| Arachidic acid (C20:0) | −25.12 | 4.43 | <10 | - |
| Bishomo-γ-linolenic acid (C20:3) | <10 | - | −35.30 | 2.91 |
| Arachidonic acid (C20:4) | −22.39 | 0.38 | −27.69 | 12.56 |
| EPA (C20:5) | +61.42 | 6.92 | −17.42 | 1.42 |
| Behenic acid (C22:0) | <10 | - | <10 | - |
| Adrenic acid (C22:4) | −14.72 | 5.19 | <10 | - |
| Docosapentaenoic acid (C22:5) | +28.69 | 2.15 | −19.76 | 1.95 |
| DHA (C22:6) | +20.29 | 1.13 | −12.80 | 3.15 |
| Lignoceric acid (C24:0) | <10 | - | <10 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bourboula, A.; Mantzourani, C.; Chalatsa, I.; Machalia, C.; Emmanouilidou, E.; Kokotou, M.G.; Kokotos, G. A Lipidomic Approach to Studying the Downregulation of Free Fatty Acids by Cytosolic Phospholipase A2 Inhibitors. Biomolecules 2025, 15, 626. https://doi.org/10.3390/biom15050626
Bourboula A, Mantzourani C, Chalatsa I, Machalia C, Emmanouilidou E, Kokotou MG, Kokotos G. A Lipidomic Approach to Studying the Downregulation of Free Fatty Acids by Cytosolic Phospholipase A2 Inhibitors. Biomolecules. 2025; 15(5):626. https://doi.org/10.3390/biom15050626
Chicago/Turabian StyleBourboula, Asimina, Christiana Mantzourani, Ioanna Chalatsa, Christina Machalia, Evangelia Emmanouilidou, Maroula G. Kokotou, and George Kokotos. 2025. "A Lipidomic Approach to Studying the Downregulation of Free Fatty Acids by Cytosolic Phospholipase A2 Inhibitors" Biomolecules 15, no. 5: 626. https://doi.org/10.3390/biom15050626
APA StyleBourboula, A., Mantzourani, C., Chalatsa, I., Machalia, C., Emmanouilidou, E., Kokotou, M. G., & Kokotos, G. (2025). A Lipidomic Approach to Studying the Downregulation of Free Fatty Acids by Cytosolic Phospholipase A2 Inhibitors. Biomolecules, 15(5), 626. https://doi.org/10.3390/biom15050626











