Therapeutic Effects of GLP-1 Receptor Agonists and DPP-4 Inhibitors in Neuropathic Pain: Mechanisms and Clinical Implications
Abstract
1. Introduction
2. Literature Search on GLP-1RA-Based Therapies for Neuropathic Pain
3. Methods
3.1. Literature Search Strategy
“Mechanisms” AND (“neuropathic pain” OR “pain management”)
3.2. Inclusion and Exclusion Criteria
3.3. Data Synthesis and Categorization
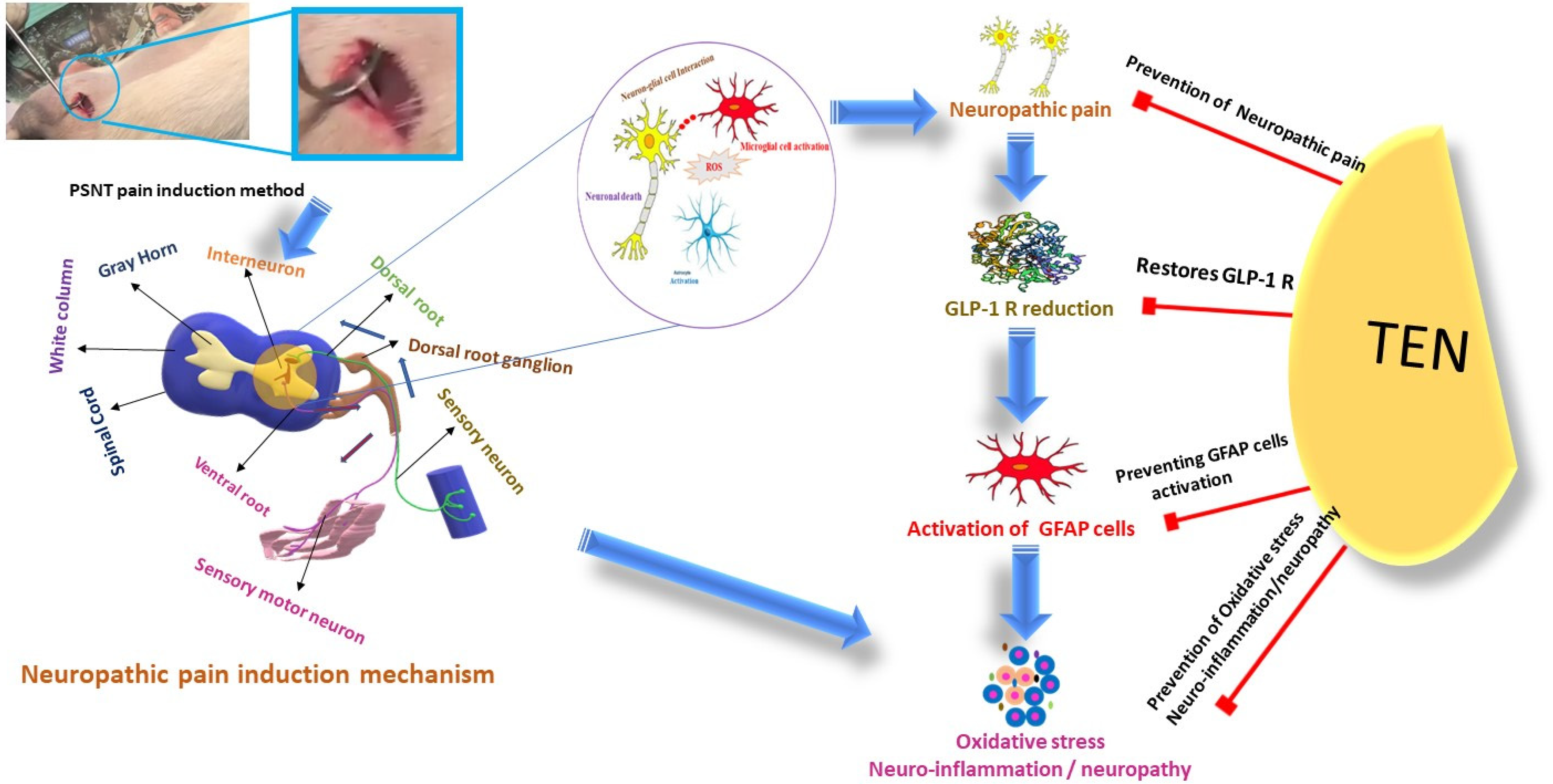
4. Pathophysiology of Chronic Pain
4.1. Neuroinflammation
4.2. Oxidative Stress
4.3. Mitochondrial Impairment
5. Physiological and Pathophysiological Roles of GLP-1 and DPP-4 in the Nervous System
6. Neuroprotective Benefits of GLP-1R Agonists and DPP-4 Inhibitors: Anti-Inflammatory and Antioxidant Mechanisms
6.1. DPP-4is
6.2. GLP-1RAs
6.3. Short-Acting GLP-1RAs
6.4. Long-Acting GLP-1RAs
6.5. Targeting Inflammation in Neuropathic Pain
6.6. Modulation of Oxidative Stress and Central Mechanisms in Neuropathic Pain: Molecular Pathways, Mitochondrial Protection, and Clinical Implications
7. Preclinical Evidence and Mechanisms of GLP-1RA-Based Therapies for Neuropathic Pain: Clinical Trials, Diabetic Neuropathy, and Combination Therapies
7.1. Synergy with Gabapentinoids and Opioids
7.2. Benefits in Diabetic Neuropathy
| Aspect | GLP-1 Receptor Agonists (GLP-1RAs) | DPP-4 Inhibitors (DPP-4is) |
|---|---|---|
| Mechanism of Action | Activation of GLP-1 receptor → modulates insulin, inflammation, oxidative stress | Inhibits degradation of GLP-1 → indirectly enhances GLP-1 signaling |
| Preclinical Findings | → Reduces pro-inflammatory cytokines (e.g., TNF-α, IL-1β) [25] → Lowers oxidative stress markers (e.g., MDA, 8-OHdG) [110] → Restores mitochondrial function [92] → Decreases glial cell activation and neuroinflammation [92] → Improves pain behaviors in diabetic neuropathy and nerve injury models [111] | → Reduces neuroinflammation via microglial and astrocyte inhibition [11,47] → Enhances neuronal survival [112] → Lowers ROS levels and improves mitochondrial integrity [31] → Shows antinociceptive effects in sciatic nerve injury models [11] |
| Clinical Findings | → Small-scale studies show reduced peripheral neuropathy symptoms [18]. → Improvements in quality-of-life scores in diabetic and osteoarthritis patients [113,114] → Ongoing trials suggest possible central modulation of pain pathways [10] | → Reduction in systemic inflammatory markers (e.g., CRP, IL-6) in T2DM patients [115] → Limited direct clinical data in neuropathic pain, but systemic anti-inflammatory effect is promising |
| CNS Penetration | → Crosses blood–brain barrier (e.g., liraglutide, semaglutide) → potential central effects [116] | → Limited CNS penetration; indirect CNS effects through peripheral GLP-1 elevation [117] |
| Key Molecular Targets | → GLP-1 receptor in CNS and peripheral nerves; AMPK, PGC-1α (mitochondrial pathways) [8] | → DPP-4 enzyme on neurons/glial cells; prolongs endogenous GLP-1 action [118] |
| Synergistic Potential | → Shown benefit in combination with gabapentinoids or opioids to reduce dosage needs [119,120] | → May enhance effects when co-administered with opioids; mitigates morphine tolerance via glial inhibition [12] |
| Therapeutic Implications | → Dual benefits: pain modulation and metabolic control; potential for CNS-focused drug development | → May be safer alternatives to NSAIDs or opioids; promising for diabetic patients with comorbid pain |
| Limitations/Challenges | → Debate on GLP-1R expression in spinal cord; need for targeted delivery for CNS | → Lack of clinical studies in neuropathic pain; lower potency compared to GLP-1RAs |
8. Future Directions and Challenges in Translating GLP-1RA-Based Therapies to Neuropathic Pain Treatment
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Colloca, L.; Ludman, T.; Bouhassira, D.; Baron, R.; Dickenson, A.H.; Yarnitsky, D.; Freeman, R.; Truini, A.; Attal, N.; Finnerup, N.B.; et al. Neuropathic pain. Nat. Rev. Dis. Primers 2017, 3, 17002. [Google Scholar] [CrossRef] [PubMed]
- Leoni, M.L.G.; Mercieri, M.; Viswanath, O.; Cascella, M.; Rekatsina, M.; Pasqualucci, A.; Caruso, A.; Varrassi, G. Neuropathic Pain: A Comprehensive Bibliometric Analysis of Research Trends, Contributions, and Future Directions. Curr. Pain Headache Rep. 2025, 29, 73. [Google Scholar] [CrossRef] [PubMed]
- Meacham, K.; Shepherd, A.; Mohapatra, D.P.; Haroutounian, S. Neuropathic Pain: Central vs. Peripheral Mechanisms. Curr. Pain Headache Rep. 2017, 21, 28. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.N.; Meyer, R.A. Mechanisms of neuropathic pain. Neuron 2006, 52, 77–92. [Google Scholar] [CrossRef]
- Nashtahosseini, Z.; Eslami, M.; Paraandavaji, E.; Haraj, A.; Dowlat, B.F.; Hosseinzadeh, E.; Oksenych, V.; Naderian, R. Cytokine Signaling in Diabetic Neuropathy: A Key Player in Peripheral Nerve Damage. Biomedicines 2025, 13, 589. [Google Scholar] [CrossRef]
- Gong, N.; Xiao, Q.; Zhu, B.; Zhang, C.Y.; Wang, Y.C.; Fan, H.; Ma, A.N.; Wang, Y.X. Activation of spinal glucagon-like peptide-1 receptors specifically suppresses pain hypersensitivity. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 5322–5334. [Google Scholar] [CrossRef]
- Shekunova, E.V.; Kashkin, V.A.; Muzhikyan, A.А.; Makarova, M.N.; Balabanyan, V.Y.; Makarov, V.G. Therapeutic efficacy of arginine-rich exenatide on diabetic neuropathy in rats. Eur. J. Pharmacol. 2020, 866, 172835. [Google Scholar] [CrossRef]
- Zheng, Z.; Zong, Y.; Ma, Y.; Tian, Y.; Pang, Y.; Zhang, C.; Gao, J. Glucagon-like peptide-1 receptor: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 234. [Google Scholar] [CrossRef]
- Gallwitz, B. Clinical Use of DPP-4 Inhibitors. Front. Endocrinol. 2019, 10, 389. [Google Scholar] [CrossRef]
- He, Y.; Xu, B.; Zhang, M.; Chen, D.; Wu, S.; Gao, J.; Liu, Y.; Zhang, Z.; Kuang, J.; Fang, Q. Advances in GLP-1 receptor agonists for pain treatment and their future potential. J. Headache Pain 2025, 26, 46. [Google Scholar] [CrossRef]
- Kuthati, Y.; Rao, V.N.; Busa, P.; Wong, C.S. Teneligliptin Exerts Antinociceptive Effects in Rat Model of Partial Sciatic Nerve Transection Induced Neuropathic Pain. Antioxidants 2021, 10, 1438. [Google Scholar] [CrossRef]
- Kuthati, Y.; Rao, V.N.; Huang, W.H.; Busa, P.; Wong, C.S. Teneligliptin Co-Infusion Alleviates Morphine Tolerance by Inhibition of Spinal Microglial Cell Activation in Streptozotocin-Induced Diabetic Rats. Antioxidants 2023, 12, 1478. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-O.; Kuthati, Y.; Huang, W.-H.; Wong, C.-S. Semaglutide Ameliorates Diabetic Neuropathic Pain by Inhibiting Neuroinflammation in the Spinal Cord. Cells 2024, 13, 1857. [Google Scholar] [CrossRef] [PubMed]
- Olukorode, J.O.; Orimoloye, D.A.; Nwachukwu, N.O.; Onwuzo, C.N.; Oloyede, P.O.; Fayemi, T.; Odunaike, O.S.; Ayobami-Ojo, P.S.; Divine, N.; Alo, D.J.; et al. Recent Advances and Therapeutic Benefits of Glucagon-Like Peptide-1 (GLP-1) Agonists in the Management of Type 2 Diabetes and Associated Metabolic Disorders. Cureus 2024, 16, e72080. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, M.; Wen, Z.; Lu, Z.; Cui, L.; Fu, C.; Xue, H.; Liu, Y.; Zhang, Y. GLP-1 Receptor Agonists: Beyond Their Pancreatic Effects. Front. Endocrinol. 2021, 12, 721135. [Google Scholar] [CrossRef]
- Alharbi, S.H. Anti-inflammatory role of glucagon-like peptide 1 receptor agonists and its clinical implications. Ther. Adv. Endocrinol. Metab. 2024, 15, 20420188231222367. [Google Scholar] [CrossRef]
- Daniels, S.; Karlsson, C.; Schrauwen, P.; Parker, V.E.R. Glucagon-like peptide-1 receptor agonism and end-organ protection. Trends Endocrinol. Metab. 2025, 36, 301–315. [Google Scholar] [CrossRef]
- Dhanapalaratnam, R.; Issar, T.; Poynten, A.M.; Milner, K.L.; Kwai, N.C.G.; Krishnan, A.V. Impact of glucagon-like peptide-1 receptor agonists on axonal function in diabetic peripheral neuropathy. J. Neurophysiol. 2025, 133, 14–21. [Google Scholar] [CrossRef]
- Halloum, W.; Dughem, Y.A.; Beier, D.; Pellesi, L. Glucagon-like peptide-1 (GLP-1) receptor agonists for headache and pain disorders: A systematic review. J. Headache Pain 2024, 25, 112. [Google Scholar] [CrossRef]
- Serrano Afonso, A.; Carnaval, T.; Videla Cés, S. Combination Therapy for Neuropathic Pain: A Review of Recent Evidence. J. Clin. Med. 2021, 10, 3533. [Google Scholar] [CrossRef]
- Lindborg, J.A.; Niemi, J.P.; Howarth, M.A.; Liu, K.W.; Moore, C.Z.; Mahajan, D.; Zigmond, R.E. Molecular and cellular identification of the immune response in peripheral ganglia following nerve injury. J. Neuroinflammation 2018, 15, 192. [Google Scholar] [CrossRef]
- Smith, J.A.; Das, A.; Ray, S.K.; Banik, N.L. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res. Bull. 2012, 87, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Breitinger, U.; Breitinger, H.G. Excitatory and inhibitory neuronal signaling in inflammatory and diabetic neuropathic pain. Mol. Med. 2023, 29, 53. [Google Scholar] [CrossRef]
- Houldsworth, A. Role of oxidative stress in neurodegenerative disorders: A review of reactive oxygen species and prevention by antioxidants. Brain Commun. 2024, 6, fcad356. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Men, X.; Liu, X.; Luo, J. Research progress on antioxidants and protein aggregation inhibitors in cataract prevention and therapy (Review). Mol. Med. Rep. 2025, 31, 22. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, C.; Naziroǧlu, M.; Rodríguez, A.B.; Pariente, J.A. Neuropathic Pain: Delving into the Oxidative Origin and the Possible Implication of Transient Receptor Potential Channels. Front. Physiol. 2018, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, N.; Papadopoulos, V. Role of Mitochondrial Dysfunction in Neuropathy. Int. J. Mol. Sci. 2025, 26, 3195. [Google Scholar] [CrossRef]
- Gaggini, M.; Sabatino, L.; Suman, A.F.; Chatzianagnostou, K.; Vassalle, C. Insights into the Roles of GLP-1, DPP-4, and SGLT2 at the Crossroads of Cardiovascular, Renal, and Metabolic Pathophysiology. Cells 2025, 14, 387. [Google Scholar] [CrossRef]
- Kopp, K.O.; Glotfelty, E.J.; Li, Y.; Greig, N.H. Glucagon-like peptide-1 (GLP-1) receptor agonists and neuroinflammation: Implications for neurodegenerative disease treatment. Pharmacol. Res. 2022, 186, 106550. [Google Scholar] [CrossRef]
- Deacon, C.F. Physiology and Pharmacology of DPP-4 in Glucose Homeostasis and the Treatment of Type 2 Diabetes. Front. Endocrinol. 2019, 10, 80. [Google Scholar] [CrossRef]
- Shao, S.; Xu, Q.; Yu, X.; Pan, R.; Chen, Y. Dipeptidyl peptidase 4 inhibitors and their potential immune modulatory functions. Pharmacol. Ther. 2020, 209, 107503. [Google Scholar] [CrossRef] [PubMed]
- Monti, G.; Gomes Moreira, D.; Richner, M.; Mutsaers, H.A.M.; Ferreira, N.; Jan, A. GLP-1 Receptor Agonists in Neurodegeneration: Neurovascular Unit in the Spotlight. Cells 2022, 11, 2023. [Google Scholar] [CrossRef] [PubMed]
- Zeng, N.; Cutts, E.J.; Lopez, C.B.; Kaur, S.; Duran, M.; Virkus, S.A.; Hardaway, J.A. Anatomical and Functional Characterization of Central Amygdala Glucagon-Like Peptide 1 Receptor Expressing Neurons. Front. Behav. Neurosci. 2021, 15, 724030. [Google Scholar] [CrossRef]
- Graham, D.L.; Durai, H.H.; Trammell, T.S.; Noble, B.L.; Mortlock, D.P.; Galli, A.; Stanwood, G.D. A novel mouse model of glucagon-like peptide-1 receptor expression: A look at the brain. J. Comp. Neurol. 2020, 528, 2445–2470. [Google Scholar] [CrossRef]
- Kanoski, S.E.; Fortin, S.M.; Arnold, M.; Grill, H.J.; Hayes, M.R. Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4. Endocrinology 2011, 152, 3103–3112. [Google Scholar] [CrossRef]
- Jensen, C.B.; Pyke, C.; Rasch, M.G.; Dahl, A.B.; Knudsen, L.B.; Secher, A. Characterization of the Glucagonlike Peptide-1 Receptor in Male Mouse Brain Using a Novel Antibody and In Situ Hybridization. Endocrinology 2017, 159, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Vahl, T.P.; Tauchi, M.; Durler, T.S.; Elfers, E.E.; Fernandes, T.M.; Bitner, R.D.; Ellis, K.S.; Woods, S.C.; Seeley, R.J.; Herman, J.P.; et al. Glucagon-Like Peptide-1 (GLP-1) Receptors Expressed on Nerve Terminals in the Portal Vein Mediate the Effects of Endogenous GLP-1 on Glucose Tolerance in Rats. Endocrinology 2007, 148, 4965–4973. [Google Scholar] [CrossRef]
- Timper, K.; Del Río-Martín, A.; Cremer, A.L.; Bremser, S.; Alber, J.; Giavalisco, P.; Varela, L.; Heilinger, C.; Nolte, H.; Trifunovic, A.; et al. GLP-1 Receptor Signaling in Astrocytes Regulates Fatty Acid Oxidation, Mitochondrial Integrity, and Function. Cell Metab. 2020, 31, 1189–1205.e1113. [Google Scholar] [CrossRef]
- Hunter, K.; Hölscher, C. Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neurosci. 2012, 13, 33. [Google Scholar] [CrossRef]
- Athauda, D.; Foltynie, T. The glucagon-like peptide 1 (GLP) receptor as a therapeutic target in Parkinson’s disease: Mechanisms of action. Drug Discov. Today 2016, 21, 802–818. [Google Scholar] [CrossRef]
- Baggio, L.L.; Drucker, D.J. Glucagon-like peptide-1 receptors in the brain: Controlling food intake and body weight. J. Clin. Invest. 2014, 124, 4223–4226. [Google Scholar] [CrossRef] [PubMed]
- Sisley, S.; Gutierrez-Aguilar, R.; Scott, M.; D’Alessio, D.A.; Sandoval, D.A.; Seeley, R.J. Neuronal GLP1R mediates liraglutide’s anorectic but not glucose-lowering effect. J. Clin. Invest. 2014, 124, 2456–2463. [Google Scholar] [CrossRef]
- Li, Y.; Chigurupati, S.; Holloway, H.W.; Mughal, M.; Tweedie, D.; Bruestle, D.A.; Mattson, M.P.; Wang, Y.; Harvey, B.K.; Ray, B.; et al. Exendin-4 Ameliorates Motor Neuron Degeneration in Cellular and Animal Models of Amyotrophic Lateral Sclerosis. PLoS ONE 2012, 7, e32008. [Google Scholar] [CrossRef]
- Chen, B.; Yu, X.; Horvath-Diano, C.; Ortuño, M.J.; Tschöp, M.H.; Jastreboff, A.M.; Schneeberger, M. GLP-1 programs the neurovascular landscape. Cell Metab. 2024, 36, 2173–2189. [Google Scholar] [CrossRef] [PubMed]
- Tong, G.; Peng, T.; Chen, Y.; Sha, L.; Dai, H.; Xiang, Y.; Zou, Z.; He, H.; Wang, S. Effects of GLP-1 Receptor Agonists on Biological Behavior of Colorectal Cancer Cells by Regulating PI3K/AKT/mTOR Signaling Pathway. Front. Pharmacol. 2022, 13, 901559. [Google Scholar] [CrossRef] [PubMed]
- Rosenstock, J.; Zinman, B. Dipeptidyl peptidase-4 inhibitors and the management of type 2 diabetes mellitus. Curr. Opin. Endocrinol. Diabetes Obes. 2007, 14, 98–107. [Google Scholar] [CrossRef]
- Király, K.; Kozsurek, M.; Lukácsi, E.; Barta, B.; Alpár, A.; Balázsa, T.; Fekete, C.; Szabon, J.; Helyes, Z.; Bölcskei, K.; et al. Glial cell type-specific changes in spinal dipeptidyl peptidase 4 expression and effects of its inhibitors in inflammatory and neuropatic pain. Sci. Rep. 2018, 8, 3490. [Google Scholar] [CrossRef]
- McKillop, A.M.; Stevenson, C.L.; Moran, B.M.; Abdel-Wahab, Y.H.A.; Flatt, P.R. Tissue expression of DPP-IV in obesity-diabetes and modulatory effects on peptide regulation of insulin secretion. Peptides 2018, 100, 165–172. [Google Scholar] [CrossRef]
- Deacon, C.F. Circulation and degradation of GIP and GLP-1. Horm. Metab. Res. 2004, 36, 761–765. [Google Scholar] [CrossRef]
- Craddy, P.; Palin, H.J.; Johnson, K.I. Comparative effectiveness of dipeptidylpeptidase-4 inhibitors in type 2 diabetes: A systematic review and mixed treatment comparison. Diabetes Ther. 2014, 5, 1–41. [Google Scholar] [CrossRef]
- Madsbad, S. Exenatide and liraglutide: Different approaches to develop GLP-1 receptor agonists (incretin mimetics)--preclinical and clinical results. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Gerich, J. DPP-4 inhibitors: What may be the clinical differentiators? Diabetes Res. Clin. Pr. 2010, 90, 131–140. [Google Scholar] [CrossRef]
- Stonehouse, A.; Walsh, B.; Cuddihy, R. Exenatide once-weekly clinical development: Safety and efficacy across a range of background therapies. Diabetes Technol. Ther. 2011, 13, 1063–1069. [Google Scholar] [CrossRef]
- Baggio, L.L.; Drucker, D.J. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007, 132, 2131–2157. [Google Scholar] [CrossRef]
- Seufert, J.; Gallwitz, B. The extra-pancreatic effects of GLP-1 receptor agonists: A focus on the cardiovascular, gastrointestinal and central nervous systems. Diabetes Obes. Metab. 2014, 16, 673–688. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, L.; Camilleri, M.; Acosta, A. Gastric Sensory and Motor Functions and Energy Intake in Health and Obesity-Therapeutic Implications. Nutrients 2021, 13, 1158. [Google Scholar] [CrossRef]
- Maselli, D.B.; Camilleri, M. Effects of GLP-1 and Its Analogs on Gastric Physiology in Diabetes Mellitus and Obesity. Adv. Exp. Med. Biol. 2021, 1307, 171–192. [Google Scholar] [CrossRef] [PubMed]
- Uccellatore, A.; Genovese, S.; Dicembrini, I.; Mannucci, E.; Ceriello, A. Comparison Review of Short-Acting and Long-Acting Glucagon-like Peptide-1 Receptor Agonists. Diabetes Ther. Res. Treat. Educ. Diabetes Relat. Disord. 2015, 6, 239–256. [Google Scholar] [CrossRef]
- Gilbert, M.P.; Pratley, R.E. GLP-1 Analogs and DPP-4 Inhibitors in Type 2 Diabetes Therapy: Review of Head-to-Head Clinical Trials. Front. Endocrinol. 2020, 11, 178. [Google Scholar] [CrossRef]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Meier, J.J. GLP-1 receptor agonists in the treatment of type 2 diabetes—State-of-the-art. Mol. Metab. 2021, 46, 101102. [Google Scholar] [CrossRef]
- Latif, W.; Lambrinos, K.J.; Patel, P.; Rodriguez, R. Compare and Contrast the Glucagon-Like Peptide-1 Receptor Agonists (GLP1RAs). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Liu, C.; Wu, T.; Ren, N. Glucagon-like peptide-1 receptor agonists for the management of diabetic peripheral neuropathy. Front. Endocrinol. 2023, 14, 1268619. [Google Scholar] [CrossRef] [PubMed]
- Catalisano, G.; Campione, G.M.; Spurio, G.; Galvano, A.N.; di Villalba, C.P.; Giarratano, A.; Alongi, A.; Ippolito, M.; Cortegiani, A. Neuropathic pain, antidepressant drugs, and inflammation: A narrative review. J. Anesth. Analg. Crit. Care 2024, 4, 67. [Google Scholar] [CrossRef]
- Meurot, C.; Martin, C.; Sudre, L.; Breton, J.; Bougault, C.; Rattenbach, R.; Bismuth, K.; Jacques, C.; Berenbaum, F. Liraglutide, a glucagon-like peptide 1 receptor agonist, exerts analgesic, anti-inflammatory and anti-degradative actions in osteoarthritis. Sci. Rep. 2022, 12, 1567. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Q.; Liu, S.; Zheng, H.; Ji, L.; Yi, N.; Bao, W.; Zhu, X.; Sun, W.; Liu, X.; et al. Glucagon-like peptide-1 receptor agonist attenuates diabetic neuropathic pain via inhibition of NOD-like receptor protein 3 inflammasome in brain microglia. Diabetes Res. Clin. Pract. 2022, 186, 109806. [Google Scholar] [CrossRef]
- Xu, M.; Wu, H.Y.; Liu, H.; Gong, N.; Wang, Y.R.; Wang, Y.X. Morroniside, a secoiridoid glycoside from Cornus officinalis, attenuates neuropathic pain by activation of spinal glucagon-like peptide-1 receptors. Br. J. Pharmacol. 2017, 174, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Feng, P.; Zhang, X.; Li, D.; Wang, R.; Ji, C.; Li, G.; Hölscher, C. The diabetes drug semaglutide reduces infarct size, inflammation, and apoptosis, and normalizes neurogenesis in a rat model of stroke. Neuropharmacology 2019, 158, 107748. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Ju, P.; Wang, W.; Wei, J.; Wang, W.; Zhao, M.; Ahmad, K.A.; Wang, Y.; Chen, J. Microglial Activation of GLP-1R Signaling in Neuropathic Pain Promotes Gene Expression Adaption Involved in Inflammatory Responses. Neural Plast. 2021, 2021, 9923537. [Google Scholar] [CrossRef]
- Hendarto, H.; Inoguchi, T.; Maeda, Y.; Ikeda, N.; Zheng, J.; Takei, R.; Yokomizo, H.; Hirata, E.; Sonoda, N.; Takayanagi, R. GLP-1 analog liraglutide protects against oxidative stress and albuminuria in streptozotocin-induced diabetic rats via protein kinase A-mediated inhibition of renal NAD(P)H oxidases. Metab. Clin. Exp. 2012, 61, 1422–1434. [Google Scholar] [CrossRef]
- Ma, L.; Peng, S.; Wei, J.; Zhao, M.; Ahmad, K.A.; Chen, J.; Wang, Y.X. Spinal microglial β-endorphin signaling mediates IL-10 and exenatide-induced inhibition of synaptic plasticity in neuropathic pain. CNS Neurosci. Ther. 2021, 27, 1157–1172. [Google Scholar] [CrossRef]
- Mokhtari, T.; Lu, M.; El-Kenawy, A.E.-M. Potential anxiolytic and antidepressant-like effects of luteolin in a chronic constriction injury rat model of neuropathic pain: Role of oxidative stress, neurotrophins, and inflammatory factors. Int. Immunopharmacol. 2023, 122, 110520. [Google Scholar] [CrossRef]
- Zhang, G.L.; Liu, Y.; Liu, Y.F.; Huang, X.T.; Tao, Y.; Chen, Z.H.; Lai, H.L. Teneligliptin mitigates diabetic cardiomyopathy by inhibiting activation of the NLRP3 inflammasome. World J. Diabetes 2024, 15, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Waseem, R.; Zehra, Z.; Aiman, A.; Bhardwaj, P.; Ansari, J.; Hassan, M.I.; Islam, A. Mitochondrial Dysfunction: Pathophysiology and Mitochondria-Targeted Drug Delivery Approaches. Pharmaceutics 2022, 14, 2657. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Xu, S.; Li, J.; Tong, N. The Effects of DPP-4 Inhibitors, GLP-1RAs, and SGLT-2/1 Inhibitors on Heart Failure Outcomes in Diabetic Patients With and Without Heart Failure History: Insights From CVOTs and Drug Mechanism. Front. Endocrinol. 2020, 11, 599355. [Google Scholar] [CrossRef]
- Mehdi, S.F.; Pusapati, S.; Anwar, M.S.; Lohana, D.; Kumar, P.; Nandula, S.A.; Nawaz, F.K.; Tracey, K.; Yang, H.; LeRoith, D.; et al. Glucagon-like peptide-1: A multi-faceted anti-inflammatory agent. Front. Immunol. 2023, 14, 1148209. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, P.E.; Abdelkader, N.F.; El Awdan, S.A.; El-Shabrawy, O.A.; Zaki, H.F. Liraglutide ameliorated peripheral neuropathy in diabetic rats: Involvement of oxidative stress, inflammation and extracellular matrix remodeling. J. Neurochem. 2018, 146, 173–185. [Google Scholar] [CrossRef]
- Noguchi, T.; Katoh, H.; Nomura, S.; Okada, K.; Watanabe, M. The GLP-1 receptor agonist exenatide improves recovery from spinal cord injury by inducing macrophage polarization toward the M2 phenotype. Front. Neurosci. 2024, 18, 1342944. [Google Scholar] [CrossRef]
- Lambadiari, V.; Thymis, J.; Kouretas, D.; Skaperda, Z.; Tekos, F.; Kousathana, F.; Kountouri, A.; Balampanis, K.; Parissis, J.; Andreadou, I.; et al. Effects of a 12-Month Treatment with Glucagon-like Peptide-1 Receptor Agonists, Sodium-Glucose Cotransporter-2 Inhibitors, and Their Combination on Oxidant and Antioxidant Biomarkers in Patients with Type 2 Diabetes. Antioxidants 2021, 10, 1379. [Google Scholar] [CrossRef]
- Zhang, T.J.; Qiu, Y.; Hua, Z. The Emerging Perspective of Morphine Tolerance: MicroRNAs. Pain Res. Manag. 2019, 2019, 9432965. [Google Scholar] [CrossRef]
- Satoh-Asahara, N.; Sasaki, Y.; Wada, H.; Tochiya, M.; Iguchi, A.; Nakagawachi, R.; Odori, S.; Kono, S.; Hasegawa, K.; Shimatsu, A. A dipeptidyl peptidase-4 inhibitor, sitagliptin, exerts anti-inflammatory effects in type 2 diabetic patients. Metab. Clin. Exp. 2013, 62, 347–351. [Google Scholar] [CrossRef]
- Xie, W.; Song, X.; Liu, Z. Impact of dipeptidyl-peptidase 4 inhibitors on cardiovascular diseases. Vasc. Pharmacol. 2018, 109, 17–26. [Google Scholar] [CrossRef]
- Feng, Y.; Shang, B.; Yang, Y.; Zhang, D.; Liu, C.; Qin, Z.; Zhou, Y.; Meng, J.; Liu, X. Impact of DPP-4 Inhibitors on Interleukin Levels in Type 2 Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2025, 110, 1195–1204. [Google Scholar] [CrossRef]
- Nesto, R. C-reactive protein, its role in inflammation, Type 2 diabetes and cardiovascular disease, and the effects of insulin-sensitizing treatment with thiazolidinediones. Diabet. Med. A J. Br. Diabet. Assoc. 2004, 21, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, A.; Jalali Kondori, B.; Ghasemi, M.; Bahari, Z. Potential Role of Oxidative Stress on the Pathophysiology of Neuropathic Pain in the Inflammatory Diseases. J. Adv. Med. Biomed. Res. 2023, 31, 514–524. [Google Scholar] [CrossRef]
- Teixeira-Santos, L.; Albino-Teixeira, A.; Pinho, D. Neuroinflammation, oxidative stress and their interplay in neuropathic pain: Focus on specialized pro-resolving mediators and NADPH oxidase inhibitors as potential therapeutic strategies. Pharmacol. Res. 2020, 162, 105280. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.S.; Jun, H.S. Effects of Glucagon-Like Peptide-1 on Oxidative Stress and Nrf2 Signaling. Int. J. Mol. Sci. 2017, 19, 26. [Google Scholar] [CrossRef]
- Kabahizi, A.; Wallace, B.; Lieu, L.; Chau, D.; Dong, Y.; Hwang, E.S.; Williams, K.W. Glucagon-like peptide-1 (GLP-1) signalling in the brain: From neural circuits and metabolism to therapeutics. Br. J. Pharmacol. 2022, 179, 600–624. [Google Scholar] [CrossRef]
- Lin, Q.; Li, K.; Chen, Y.; Xie, J.; Wu, C.; Cui, C.; Deng, B. Oxidative Stress in Diabetic Peripheral Neuropathy: Pathway and Mechanism-Based Treatment. Mol. Neurobiol. 2023, 60, 4574–4594. [Google Scholar] [CrossRef]
- Eftekharpour, E.; Fernyhough, P. Oxidative Stress and Mitochondrial Dysfunction Associated with Peripheral Neuropathy in Type 1 Diabetes. Antioxid. Redox Signal. 2022, 37, 578–596. [Google Scholar] [CrossRef]
- Ghosh, P.; Fontanella, R.A.; Scisciola, L.; Pesapane, A.; Taktaz, F.; Franzese, M.; Puocci, A.; Ceriello, A.; Prattichizzo, F.; Rizzo, M.R.; et al. Targeting redox imbalance in neurodegeneration: Characterizing the role of GLP-1 receptor agonists. Theranostics 2023, 13, 4872–4884. [Google Scholar] [CrossRef]
- Chidambaram, S.B.; Anand, N.; Varma, S.R.; Ramamurthy, S.; Vichitra, C.; Sharma, A.; Mahalakshmi, A.M.; Essa, M.M. Superoxide dismutase and neurological disorders. IBRO Neurosci. Rep. 2024, 16, 373–394. [Google Scholar] [CrossRef]
- Luna-Marco, C.; de Marañon, A.M.; Hermo-Argibay, A.; Rodriguez-Hernandez, Y.; Hermenejildo, J.; Fernandez-Reyes, M.; Apostolova, N.; Vila, J.; Sola, E.; Morillas, C.; et al. Effects of GLP-1 receptor agonists on mitochondrial function, inflammatory markers and leukocyte-endothelium interactions in type 2 diabetes. Redox Biol. 2023, 66, 102849. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Li, D.; Huang, C.; Zhang, G.; Wang, Z.; Liu, J.; Yu, H.; Song, B.; Zhang, N.; Li, B.; et al. Glucose control independent mechanisms involved in the cardiovascular benefits of glucagon-like peptide-1 receptor agonists. Biomed. Pharmacother. 2022, 153, 113517. [Google Scholar] [CrossRef]
- Zong, Y.; Li, H.; Liao, P.; Chen, L.; Pan, Y.; Zheng, Y.; Zhang, C.; Liu, D.; Zheng, M.; Gao, J. Mitochondrial dysfunction: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 124. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, X.; An, X.; Zhang, Y.; Sun, W.; Zhang, Y.; Duan, Y.; Kang, X.; Sun, Y.; Jiang, L.; et al. Axonal transport deficits in the pathogenesis of diabetic peripheral neuropathy. Front. Endocrinol. 2023, 14, 1136796. [Google Scholar] [CrossRef]
- Westermeier, F.; Fisman, E.Z. Glucagon-like peptide-1 receptor agonists (GLP-1RAs) and cardiometabolic protection: Historical development and future challenges. Cardiovasc. Diabetol. 2025, 24, 44. [Google Scholar] [CrossRef] [PubMed]
- Yip, J.M.X.; Chiang, G.S.H.; Lee, I.C.J.; Lehming-Teo, R.; Dai, K.; Dongol, L.; Wang, L.Y.-T.; Teo, D.; Seah, G.T.; Lehming, N. Mitochondria and the Repurposing of Diabetes Drugs for Off-Label Health Benefits. Int. J. Mol. Sci. 2025, 26, 364. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L. AMPK-Mediated Multi-Organ Protective Effects of GLP-1 Receptor Agonists. Health Metab. 2025, 2, 4. [Google Scholar] [CrossRef]
- Hong, C.T.; Chen, J.H.; Hu, C.J. Role of glucagon-like peptide-1 receptor agonists in Alzheimer’s disease and Parkinson’s disease. J. Biomed. Sci. 2024, 31, 102. [Google Scholar] [CrossRef]
- Go, E.J.; Hwang, S.-M.; Jo, H.; Rahman, M.M.; Park, J.; Lee, J.Y.; Jo, Y.Y.; Lee, B.-G.; Jung, Y.; Berta, T.; et al. GLP-1 and its derived peptides mediate pain relief through direct TRPV1 inhibition without affecting thermoregulation. Exp. Mol. Med. 2024, 56, 2449–2464. [Google Scholar] [CrossRef]
- Diz-Chaves, Y.; Mastoor, Z.; Spuch, C.; González-Matías, L.C.; Mallo, F. Anti-Inflammatory Effects of GLP-1 Receptor Activation in the Brain in Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 9583. [Google Scholar] [CrossRef]
- Gabery, S.; Salinas, C.G.; Paulsen, S.J.; Ahnfelt-Rønne, J.; Alanentalo, T.; Baquero, A.F.; Buckley, S.T.; Farkas, E.; Fekete, C.; Frederiksen, K.S.; et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight 2020, 5, e133429. [Google Scholar] [CrossRef] [PubMed]
- Cabou, C.; Burcelin, R. GLP-1, the gut-brain, and brain-periphery axes. Rev. Diabet. Stud. RDS 2011, 8, 418–431. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Sun, W.; Wang, H.; Chang, R.; Wang, J.; Song, C.; Zhang, S.; Ni, Q.; An, X. Comparison of the effectiveness and safety of GLP-1 receptor agonists for type 2 diabetes mellitus patients with overweight/obesity: A systematic review and network meta-analysis. Diabetes Res. Clin. Pract. 2025, 222, 111999. [Google Scholar] [CrossRef] [PubMed]
- Latremoliere, A.; Woolf, C.J. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J. Pain 2009, 10, 895–926. [Google Scholar] [CrossRef]
- Fujita, S.; Ushio, S.; Ozawa, N.; Masuguchi, K.; Kawashiri, T.; Oishi, R.; Egashira, N. Exenatide Facilitates Recovery from Oxaliplatin-Induced Peripheral Neuropathy in Rats. PLoS ONE 2015, 10, e0141921. [Google Scholar] [CrossRef]
- Gudbergsen, H.; Henriksen, M.; Wæhrens, E.E.; Overgaard, A.; Bliddal, H.; Christensen, R.; Boesen, M.P.; Knop, F.K.K.; Astrup, A.; Rasmussen, M.U.; et al. Effect of liraglutide on body weight and pain in patients with overweight and knee osteoarthritis: Protocol for a randomised, double-blind, placebo-controlled, parallel-group, single-centre trial. BMJ Open 2019, 9, e024065. [Google Scholar] [CrossRef]
- Collins, L.; Costello, R.A. Glucagon-Like Peptide-1 Receptor Agonists. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Qeadan, F.; McCunn, A.; Tingey, B. The association between glucose-dependent insulinotropic polypeptide and/or glucagon-like peptide-1 receptor agonist prescriptions and substance-related outcomes in patients with opioid and alcohol use disorders: A real-world data analysis. Addiction 2025, 120, 236–250. [Google Scholar] [CrossRef]
- Birnbaum, Y. Glucagon-Like Peptide-1 Receptor Agonists for Abdominal Aortic Aneurysm? Cardiovasc. Drugs Ther. 2025, 39, 13–14. [Google Scholar] [CrossRef]
- García-Casares, N.; González-González, G.; de la Cruz-Cosme, C.; Garzón-Maldonado, F.J.; de Rojas-Leal, C.; Ariza, M.J.; Narváez, M.; Barbancho, M.; García-Arnés, J.A.; Tinahones, F.J. Effects of GLP-1 receptor agonists on neurological complications of diabetes. Rev. Endocr. Metab. Disord. 2023, 24, 655–672. [Google Scholar] [CrossRef]
- Jiang, X.; Li, J.; Yao, X.; Ding, H.; Gu, A.; Zhou, Z. Neuroprotective effects of dipeptidyl peptidase 4 inhibitor on Alzheimer’s disease: A narrative review. Front. Pharmacol. 2024, 15, 1361651. [Google Scholar] [CrossRef]
- Meurot, C.; Jacques, C.; Martin, C.; Sudre, L.; Breton, J.; Rattenbach, R.; Bismuth, K.; Berenbaum, F. Targeting the GLP-1/GLP-1R axis to treat osteoarthritis: A new opportunity? J. Orthop. Transl. 2022, 32, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.C.; Lourenço, O.; Morgado, M. SGLT2i and GLP1RA effects in patients followed in a hospital diabetology consultation. Expert Rev. Clin. Pharmacol. 2024, 17, 1081–1088. [Google Scholar] [CrossRef]
- Baggio, L.L.; Varin, E.M.; Koehler, J.A.; Cao, X.; Lokhnygina, Y.; Stevens, S.R.; Holman, R.R.; Drucker, D.J. Plasma levels of DPP4 activity and sDPP4 are dissociated from inflammation in mice and humans. Nat. Commun. 2020, 11, 3766. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Wen, S.; Zhou, L. The Relationship Between the Blood-Brain-Barrier and the Central Effects of Glucagon-Like Peptide-1 Receptor Agonists and Sodium-Glucose Cotransporter-2 Inhibitors. Diabetes Metab. Syndr. Obes. Targets Ther. 2022, 15, 2583–2597. [Google Scholar] [CrossRef] [PubMed]
- Darsalia, V.; Johansen, O.E.; Lietzau, G.; Nyström, T.; Klein, T.; Patrone, C. Dipeptidyl Peptidase-4 Inhibitors for the Potential Treatment of Brain Disorders; A Mini-Review With Special Focus on Linagliptin and Stroke. Front. Neurol. 2019, 10, 493. [Google Scholar] [CrossRef]
- Al-Badri, G.; Leggio, G.M.; Musumeci, G.; Marzagalli, R.; Drago, F.; Castorina, A. Tackling dipeptidyl peptidase IV in neurological disorders. Neural Regen. Res. 2018, 13, 26–34. [Google Scholar] [CrossRef]
- Chuong, V.; Farokhnia, M.; Khom, S.; Pince, C.L.; Elvig, S.K.; Vlkolinsky, R.; Marchette, R.C.; Koob, G.F.; Roberto, M.; Vendruscolo, L.F.; et al. The glucagon-like peptide-1 (GLP-1) analogue semaglutide reduces alcohol drinking and modulates central GABA neurotransmission. JCI Insight 2023, 8, e170671. [Google Scholar] [CrossRef]
- Wang, W.; Volkow, N.D.; Wang, Q.; Berger, N.A.; Davis, P.B.; Kaelber, D.C.; Xu, R. Semaglutide and Opioid Overdose Risk in Patients with Type 2 Diabetes and Opioid Use Disorder. JAMA Netw. Open 2024, 7, e2435247. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuthati, Y.; Davuluri, V.N.G.; Wong, C.-S. Therapeutic Effects of GLP-1 Receptor Agonists and DPP-4 Inhibitors in Neuropathic Pain: Mechanisms and Clinical Implications. Biomolecules 2025, 15, 622. https://doi.org/10.3390/biom15050622
Kuthati Y, Davuluri VNG, Wong C-S. Therapeutic Effects of GLP-1 Receptor Agonists and DPP-4 Inhibitors in Neuropathic Pain: Mechanisms and Clinical Implications. Biomolecules. 2025; 15(5):622. https://doi.org/10.3390/biom15050622
Chicago/Turabian StyleKuthati, Yaswanth, Venkata Naga Goutham Davuluri, and Chih-Shung Wong. 2025. "Therapeutic Effects of GLP-1 Receptor Agonists and DPP-4 Inhibitors in Neuropathic Pain: Mechanisms and Clinical Implications" Biomolecules 15, no. 5: 622. https://doi.org/10.3390/biom15050622
APA StyleKuthati, Y., Davuluri, V. N. G., & Wong, C.-S. (2025). Therapeutic Effects of GLP-1 Receptor Agonists and DPP-4 Inhibitors in Neuropathic Pain: Mechanisms and Clinical Implications. Biomolecules, 15(5), 622. https://doi.org/10.3390/biom15050622







