Topical Instillation of N-Acetylcysteine and N-Acetylcysteine Amide Impedes Age-Related Lens Opacity in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture
2.3. Measurement of ROS in MLECs and HLECs
2.4. Cell Survival Assay
2.5. Real-Time Reverse Transcriptase-Quantitative Polymerase Chain Reaction (RT-qPCR)
2.6. Animals
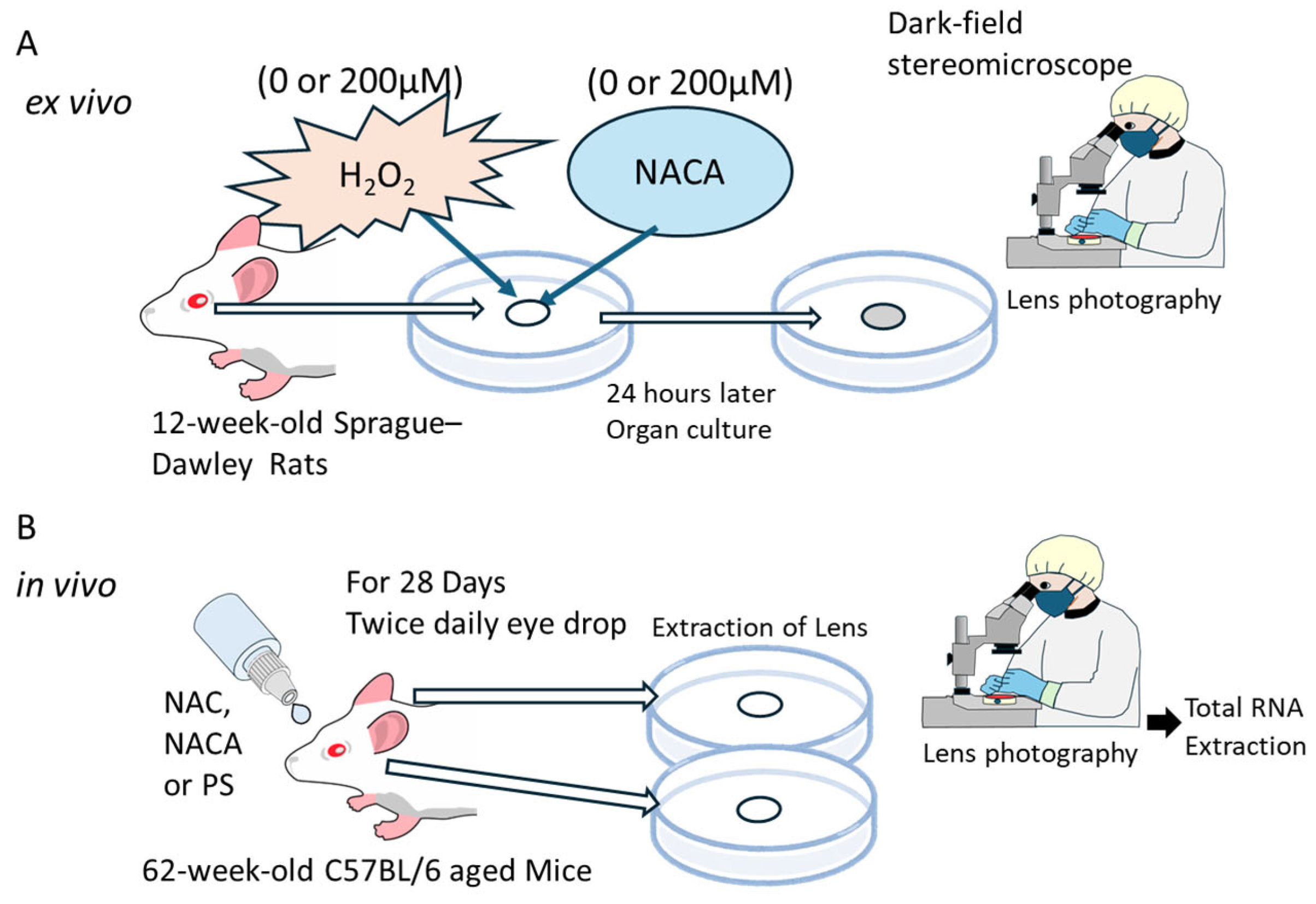
2.7. Ex Vivo Lens Organ Culture and Imaging
2.8. In Vivo Experiments on the Suppression of Aging-Related Cataracts by Topical Administration of NAC and NACA
2.9. Statistical Analysis
2.10. Application of Artificial Intelligence in Writing Assistance
3. Results
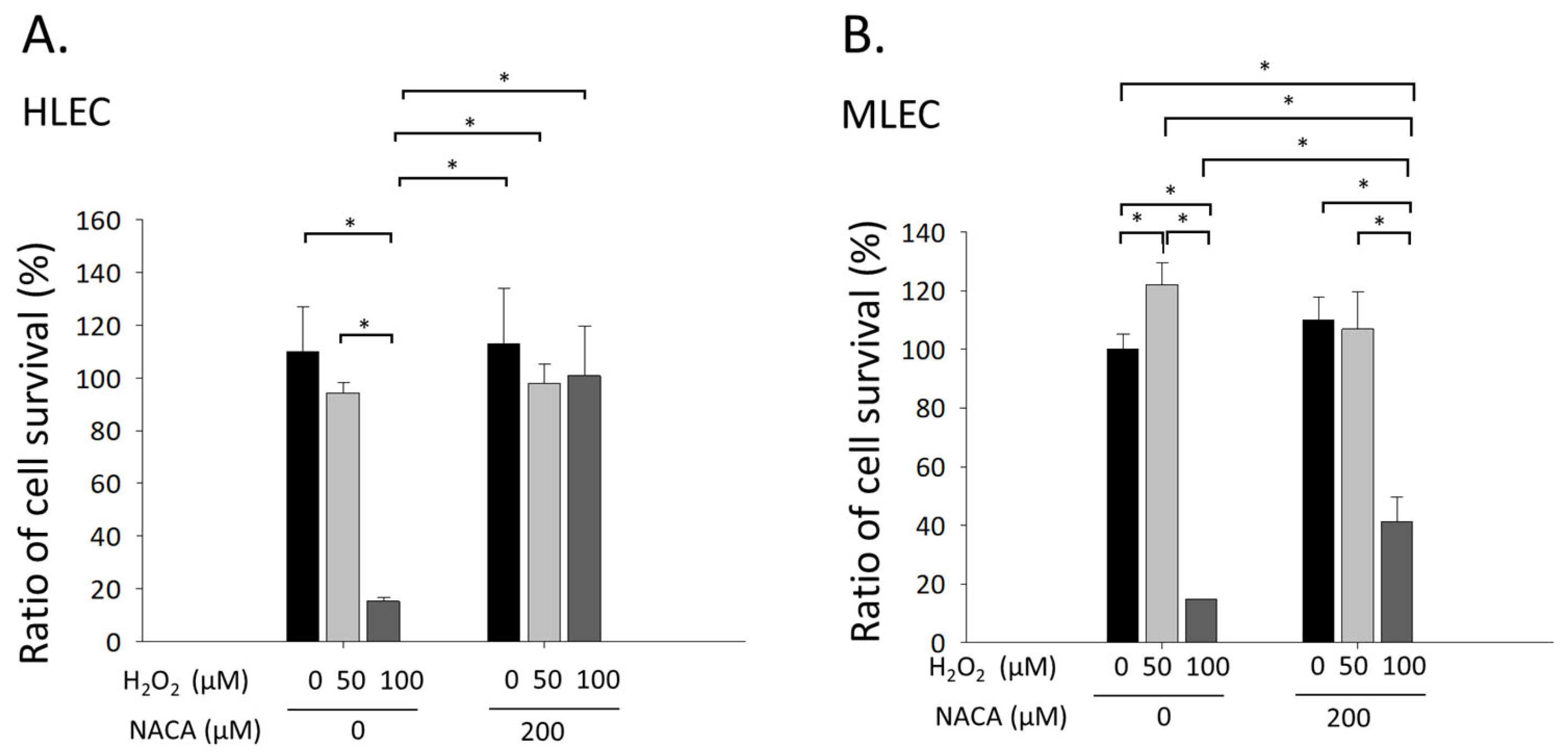
3.1. Effects of NACA on Cellular Viability in MLECs and HLECs Exposed to H2O2-Mediated Oxidative Stress
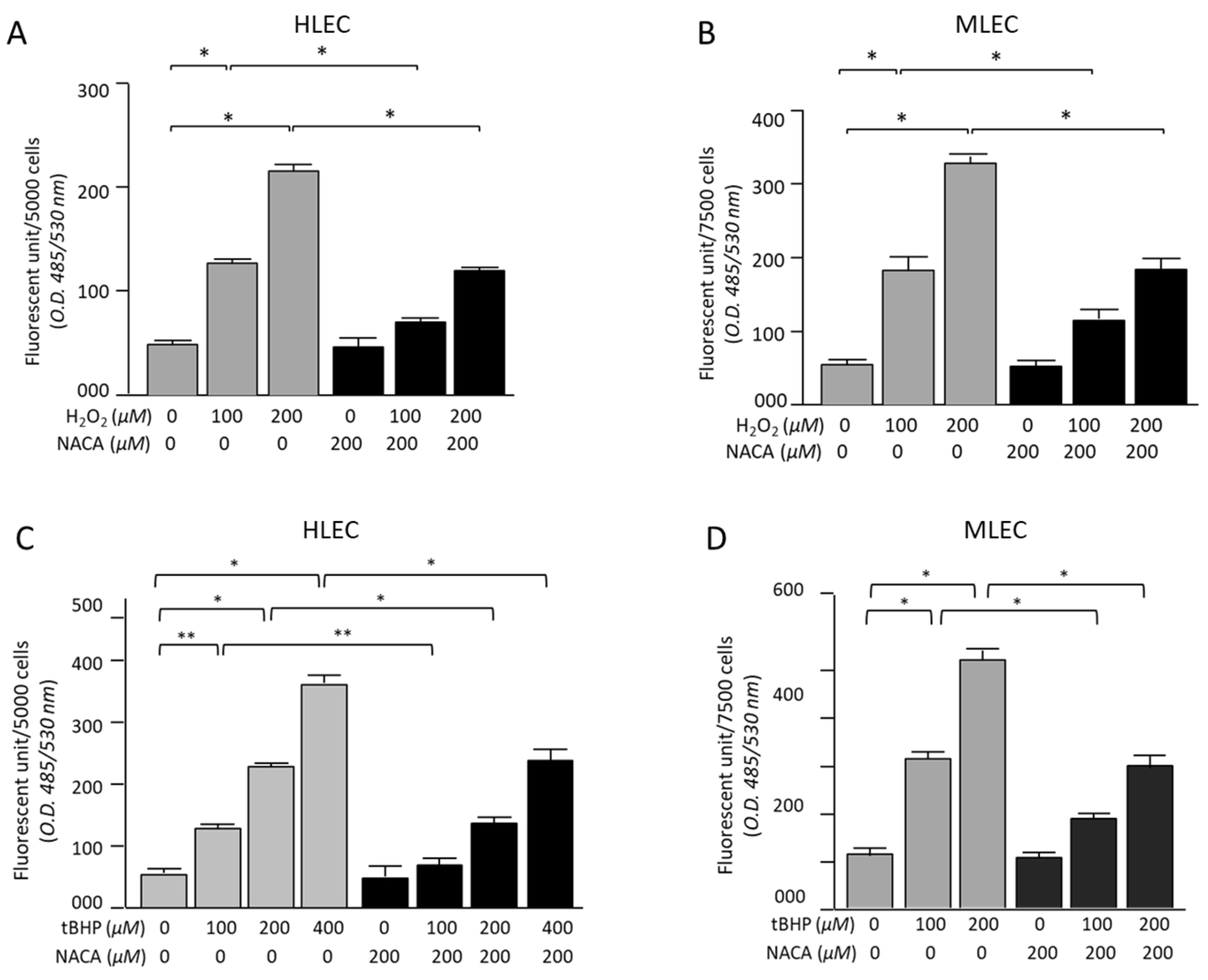
3.2. Effects of NACA on ROS Levels in HLECs and MLECs Under H2O2- or tBHP-Induced Oxidative Stress
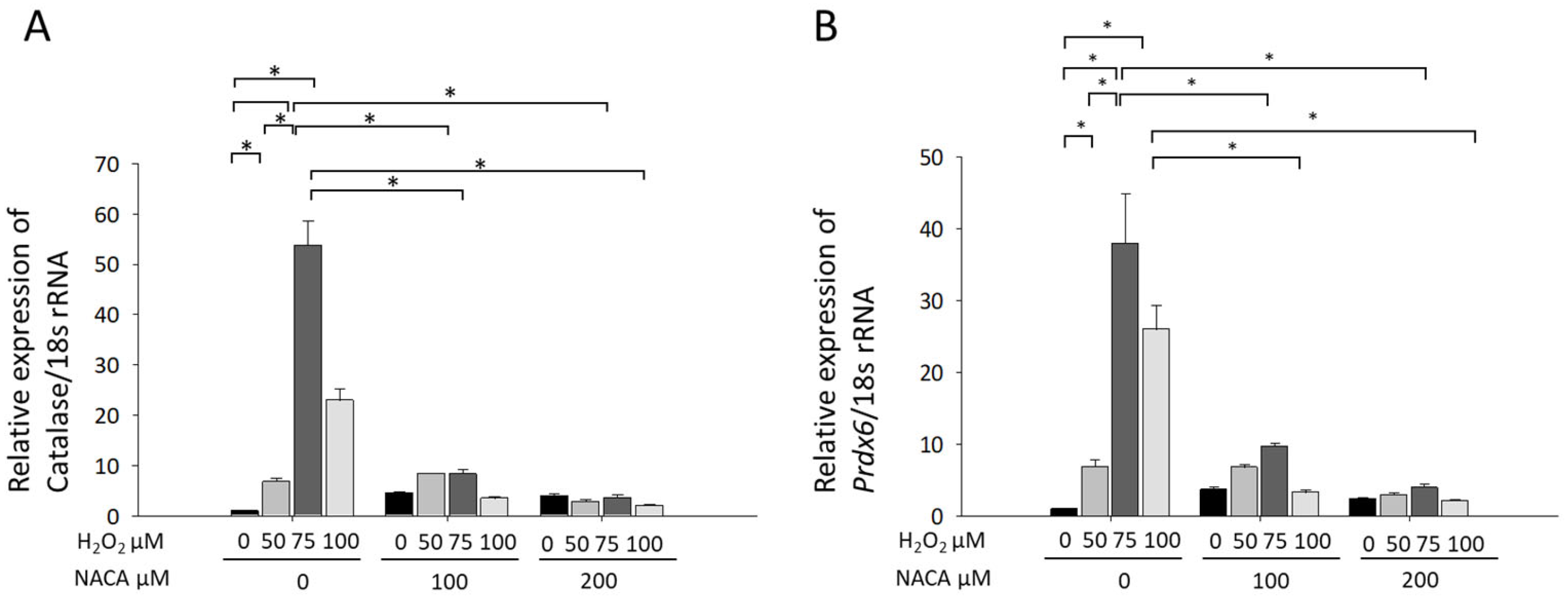
3.3. Effects of NACA on Prdx6 and Catalase mRNA Expression Under H2O2-Induced Oxidative Stress
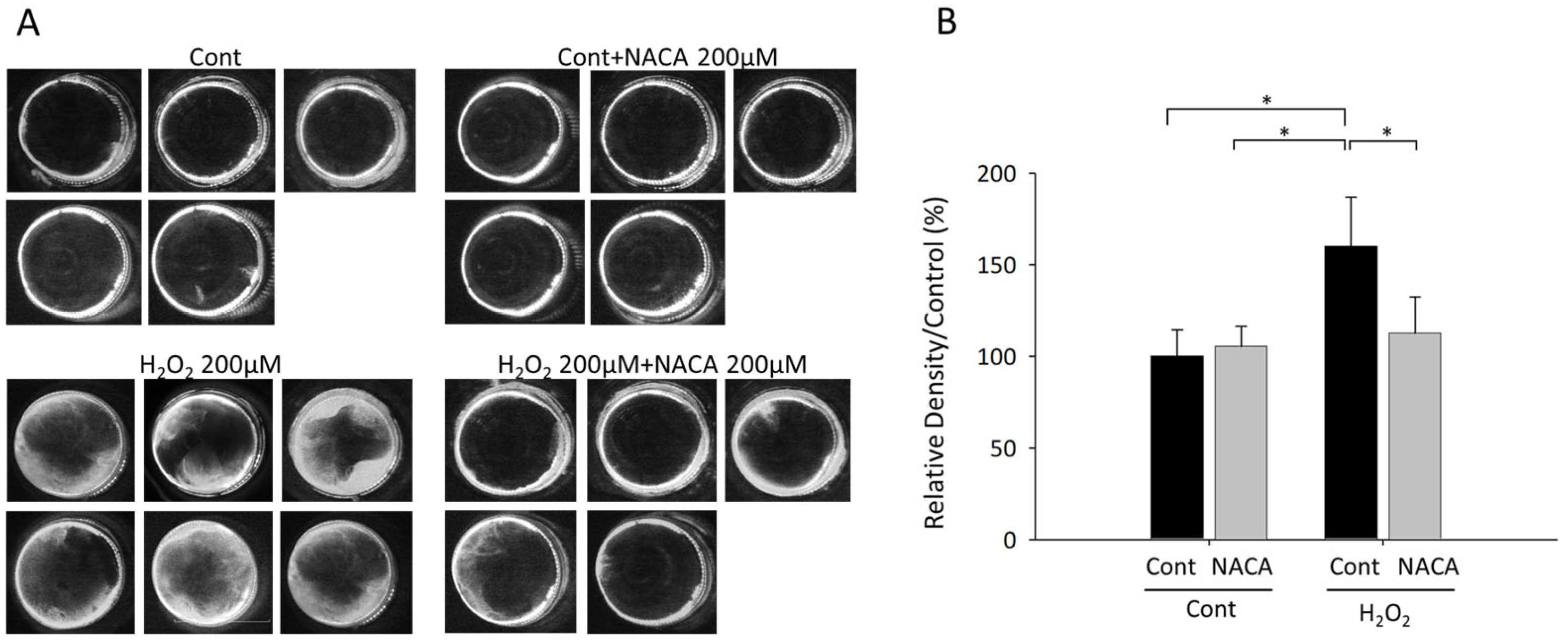
3.4. Effects of NACA on Lens Opacity Induced by H2O2 in Ex Vivo Organ Cultures
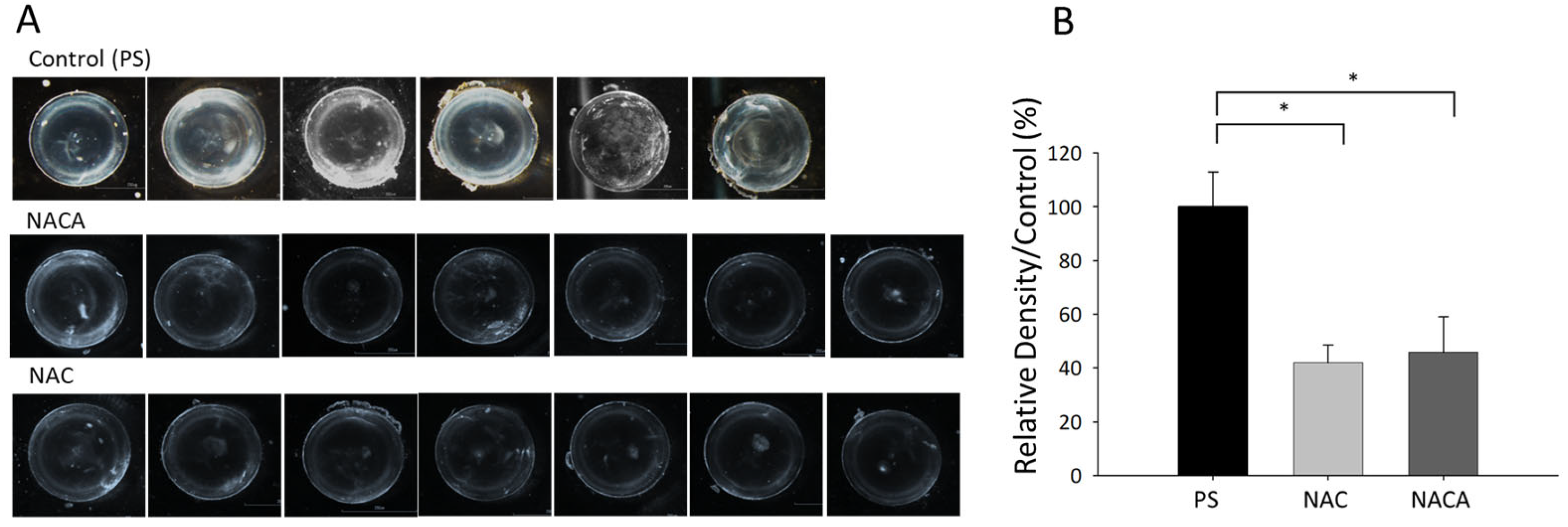
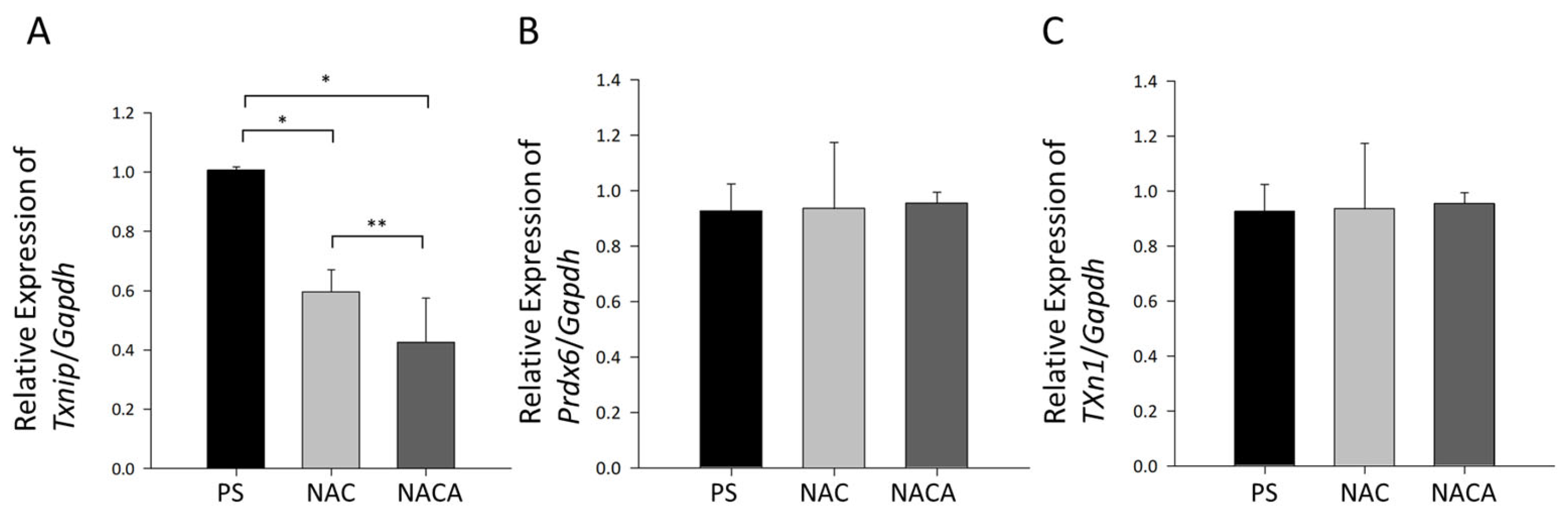
3.5. Effects of NAC and NACA Eye Drops on Age-Related Cataract and Oxidative Stress in Aged Mice In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | analysis of variance |
| BSO | L-buthionine-(S,R)-sulfoximine |
| DMEM | Dulbecco’s modified Eagle’s medium |
| FBS | fetal bovine serum |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
| GSH | glutathione |
| H2-DCF-DA | dichlorodihydrofluorescein diacetate |
| H2O2 | hydrogen peroxide |
| HLEC | human lens epithelial cell |
| LEC | lens epithelial cell |
| MLEC | mouse lens epithelial cell |
| NAC | N-acetylcysteine |
| NACA | N-acetylcysteine amide |
| NACET | N-acetyl-L-cysteine ethyl ester |
| NLRP3 | nucleotide-binding oligomerization domain-like receptor family pyrin domain containing 3 |
| PRDX6 | peroxiredoxin 6 |
| PS | physiological saline |
| PSC | posterior subcapsular cataract |
| ROS | reactive oxygen species |
| RT-qPCR | real-time reverse transcriptase-quantitative PCR |
| SD | standard deviation |
| tBHP | tert-butyl hydroperoxide |
| TRX | thioredoxin |
| TXN1 | thioredoxin-1 |
| TXNIP | thioredoxin-interacting protein |
References
- Bourne, R.R.; Stevens, G.A.; White, R.A.; Smith, J.L.; Flaxman, S.R.; Price, H.; Jonas, J.B.; Keeffe, J.; Leasher, J.; Naidoo, K.; et al. Causes of vision loss worldwide, 1990–2010: A systematic analysis. Lancet Glob. Health 2013, 1, e339–e349. [Google Scholar] [CrossRef]
- Stevens, G.A.; White, R.A.; Flaxman, S.R.; Price, H.; Jonas, J.B.; Keeffe, J.; Leasher, J.; Naidoo, K.; Pesudovs, K.; Resnikoff, S.; et al. Global prevalence of vision impairment and blindness: Magnitude and temporal trends, 1990-2010. Ophthalmology 2013, 120, 2377–2384. [Google Scholar] [CrossRef] [PubMed]
- Lou, M.F. Redox regulation in the lens. Prog. Retin. Eye Res. 2003, 22, 657–682. [Google Scholar] [CrossRef] [PubMed]
- Spector, A. Oxidative stress-induced cataract: Mechanism of action. FASEB J. 1995, 9, 1173–1182. [Google Scholar] [CrossRef]
- Truscott, R.J. Age-related nuclear cataract-oxidation is the key. Exp. Eye Res. 2005, 80, 709–725. [Google Scholar] [CrossRef]
- Beckman, K.B.; Ames, B.N. The free radical theory of aging matures. Physiol. Rev. 1998, 78, 547–581. [Google Scholar] [CrossRef]
- Dai, D.F.; Chiao, Y.A.; Marcinek, D.J.; Szeto, H.H.; Rabinovitch, P.S. Mitochondrial oxidative stress in aging and healthspan. Longev. Heal. 2014, 3, 6. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Spector, A. Review: Oxidative stress and disease. J. Ocul. Pharmacol. Ther. 2000, 16, 193–201. [Google Scholar] [CrossRef]
- Spector, A.; Wang, G.M.; Wang, R.R.; Li, W.C.; Kleiman, N.J. A brief photochemically induced oxidative insult causes irreversible lens damage and cataract. II. Mechanism of action. Exp. Eye Res. 1995, 60, 483–493. [Google Scholar] [CrossRef]
- Taylor, A.; Nowell, T. Oxidative stress and antioxidant function in relation to risk for cataract. Adv. Pharmacol. 1997, 38, 515–536. [Google Scholar] [PubMed]
- D’Autreaux, B.; Toledano, M.B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007, 8, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef]
- Zucker, S.N.; Fink, E.E.; Bagati, A.; Mannava, S.; Bianchi-Smiraglia, A.; Bogner, P.N.; Wawrzyniak, J.A.; Foley, C.; Leonova, K.I.; Grimm, M.J.; et al. Nrf2 amplifies oxidative stress via induction of Klf9. Mol. Cell 2014, 53, 916–928. [Google Scholar] [CrossRef]
- Chhunchha, B.; Kubo, E.; Singh, D.P. Obligatory Role of AMPK Activation and Antioxidant Defense Pathway in the Regulatory Effects of Metformin on Cellular Protection and Prevention of Lens Opacity. Cells 2022, 11, 3021. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T. Epigenetic Control of NRF2-Directed Cellular Antioxidant Status in Dictating Life-Death Decisions. Mol. Cell 2017, 68, 5–7. [Google Scholar] [CrossRef]
- Chhunchha, B.; Kumar, R.; Kubo, E.; Thakur, P.; Singh, D.P. Prdx6 Regulates Nlrp3 Inflammasome Activation-Driven Inflammatory Response in Lens Epithelial Cells. Int. J. Mol. Sci. 2023, 24, 16276. [Google Scholar] [CrossRef]
- Zou, Y.; Cui, B.; Liang, P.; Tian, X.; Ma, Y.; Zhao, S. Inhibition of NLRP3 Protects Human Lens Epithelial Cells against Oxidative Stress-Induced Apoptosis by NF-kappaB Signaling. Ophthalmic Res. 2020, 63, 174–181. [Google Scholar] [CrossRef]
- Marneros, A.G. Increased VEGF-A promotes multiple distinct aging diseases of the eye through shared pathomechanisms. EMBO Mol. Med. 2016, 8, 208–231. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, C.; Yang, P.; Chao, R.; Yue, Z.; Li, C.; Guo, J.; Li, M. Eldecalcitol Inhibits LPS-Induced NLRP3 Inflammasome-Dependent Pyroptosis in Human Gingival Fibroblasts by Activating the Nrf2/HO-1 Signaling Pathway. Drug Des. Devel Ther. 2020, 14, 4901–4913. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, G.; Ding, Q.; Zheng, F.; Shi, X.; Lin, Z.; Liang, Y. The ROS/TXNIP/NLRP3 pathway mediates LPS-induced microglial inflammatory response. Cytokine 2024, 181, 156677. [Google Scholar] [CrossRef] [PubMed]
- El-Azab, M.F.; Baldowski, B.R.; Mysona, B.A.; Shanab, A.Y.; Mohamed, I.N.; Abdelsaid, M.A.; Matragoon, S.; Bollinger, K.E.; Saul, A.; El-Remessy, A.B. Deletion of thioredoxin-interacting protein preserves retinal neuronal function by preventing inflammation and vascular injury. Br. J. Pharmacol. 2014, 171, 1299–1313. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, I.N.; Hafez, S.S.; Fairaq, A.; Ergul, A.; Imig, J.D.; El-Remessy, A.B. Thioredoxin-interacting protein is required for endothelial NLRP3 inflammasome activation and cell death in a rat model of high-fat diet. Diabetologia 2014, 57, 413–423. [Google Scholar] [CrossRef]
- Wang, W.; Wang, C.; Ding, X.Q.; Pan, Y.; Gu, T.T.; Wang, M.X.; Liu, Y.L.; Wang, F.M.; Wang, S.J.; Kong, L.D. Quercetin and allopurinol reduce liver thioredoxin-interacting protein to alleviate inflammation and lipid accumulation in diabetic rats. Br. J. Pharmacol. 2013, 169, 1352–1371. [Google Scholar] [CrossRef]
- Singh, D.P.; Ohguro, N.; Chylack, L.T., Jr.; Shinohara, T. Lens epithelium-derived growth factor: Increased resistance to thermal and oxidative stresses. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1444–1451. [Google Scholar]
- Fatma, N.; Kubo, E.; Sharma, P.; Beier, D.R.; Singh, D.P. Impaired homeostasis and phenotypic abnormalities in Prdx6-/-mice lens epithelial cells by reactive oxygen species: Increased expression and activation of TGFbeta. Cell Death Differ. 2005, 12, 734–750. [Google Scholar] [CrossRef]
- Chhunchha, B.; Kubo, E.; Kompella, U.B.; Singh, D.P. Engineered Sumoylation-Deficient Prdx6 Mutant Protein-Loaded Nanoparticles Provide Increased Cellular Defense and Prevent Lens Opacity. Antioxidants 2021, 10, 1245. [Google Scholar] [CrossRef]
- Dickerson, J.E., Jr.; Hellberg, M.; Lou, M.F. Evaluation of AL-05712 and AL-05741 as thioltransferase mimics for the prevention and recovery of pre-cataractous changes in lens. J. Ocul. Pharmacol. Ther. 1998, 14, 437–445. [Google Scholar] [CrossRef]
- Marchetti, M.A.; Pizarro, G.O.; Sagher, D.; Deamicis, C.; Brot, N.; Hejtmancik, J.F.; Weissbach, H.; Kantorow, M. Methionine sulfoxide reductases B1, B2, and B3 are present in the human lens and confer oxidative stress resistance to lens cells. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2107–2112. [Google Scholar] [CrossRef]
- Chhunchha, B.; Kubo, E.; Singh, D.P. Switching of Redox Signaling by Prdx6 Expression Decides Cellular Fate by Hormetic Phenomena Involving Nrf2 and Reactive Oxygen Species. Cells 2022, 11, 1266. [Google Scholar] [CrossRef]
- Kubo, E.; Hasanova, N.; Tanaka, Y.; Fatma, N.; Takamura, Y.; Singh, D.P.; Akagi, Y. Protein expression profiling of lens epithelial cells from Prdx6-depleted mice and their vulnerability to UV radiation exposure. Am. J. Physiol. Cell Physiol. 2010, 298, C342–C354. [Google Scholar] [CrossRef] [PubMed]
- Pfaff, A.; Chernatynskaya, A.; Vineyard, H.; Ercal, N. Thiol antioxidants protect human lens epithelial (HLE B-3) cells against tert-butyl hydroperoxide-induced oxidative damage and cytotoxicity. Biochem. Biophys. Rep. 2022, 29, 101213. [Google Scholar] [CrossRef] [PubMed]
- Martis, R.M.; Grey, A.C.; Wu, H.; Wall, G.M.; Donaldson, P.J.; Lim, J.C. N-Acetylcysteine amide (NACA) and diNACA inhibit H(2)O(2)-induced cataract formation ex vivo in pig and rat lenses. Exp. Eye Res. 2023, 234, 109610. [Google Scholar] [CrossRef] [PubMed]
- Savion, N.; Dahamshi, S.; Morein, M.; Kotev-Emeth, S. S-Allylmercapro-N-Acetylcysteine Attenuates the Oxidation-Induced Lens Opacification and Retinal Pigment Epithelial Cell Death In Vitro. Antioxidants 2019, 8, 25. [Google Scholar] [CrossRef]
- Sunitha, K.; Hemshekhar, M.; Thushara, R.M.; Santhosh, M.S.; Yariswamy, M.; Kemparaju, K.; Girish, K.S. N-Acetylcysteine amide: A derivative to fulfill the promises of N-Acetylcysteine. Free Radic. Res. 2013, 47, 357–367. [Google Scholar] [CrossRef]
- Kubo, E.; Chhunchha, B.; Singh, P.; Sasaki, H.; Singh, D.P. Sulforaphane reactivates cellular antioxidant defense by inducing Nrf2/ARE/Prdx6 activity during aging and oxidative stress. Sci. Rep. 2017, 7, 14130. [Google Scholar] [CrossRef]
- Kubo, E.; Fatma, N.; Akagi, Y.; Beier, D.R.; Singh, S.P.; Singh, D.P. TAT-mediated PRDX6 protein transduction protects against eye lens epithelial cell death and delays lens opacity. Am. J. Physiol. Cell Physiol. 2008, 294, C842–C855. [Google Scholar] [CrossRef]
- Wahlig, S.; Lovatt, M.; Mehta, J.S. Functional role of peroxiredoxin 6 in the eye. Free Radic. Biol. Med. 2018, 126, 210–220. [Google Scholar] [CrossRef]
- Kim, S.Y.; Jo, H.Y.; Kim, M.H.; Cha, Y.Y.; Choi, S.W.; Shim, J.H.; Kim, T.J.; Lee, K.Y. H2O2-dependent hyperoxidation of peroxiredoxin 6 (Prdx6) plays a role in cellular toxicity via up-regulation of iPLA2 activity. J. Biol. Chem. 2008, 283, 33563–33568. [Google Scholar] [CrossRef]
- Rohrdanz, E.; Schmuck, G.; Ohler, S.; Kahl, R. The influence of oxidative stress on catalase and MnSOD gene transcription in astrocytes. Brain Res. 2001, 900, 128–136. [Google Scholar] [CrossRef]
- Wang, P.; Liu, X.C.; Yan, H.; Li, M.Y. Hyperoxia-induced lens damage in rabbit: Protective effects of N-acetylcysteine. Mol. Vis. 2009, 15, 2945–2952. [Google Scholar] [PubMed]
- Lou, M.F. Thiol regulation in the lens. J. Ocul. Pharmacol. Ther. 2000, 16, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Aldini, G.; Altomare, A.; Baron, G.; Vistoli, G.; Carini, M.; Borsani, L.; Sergio, F. N-Acetylcysteine as an antioxidant and disulphide breaking agent: The reasons why. Free Radic. Res. 2018, 52, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Nagy, P. Kinetics and mechanisms of thiol-disulfide exchange covering direct substitution and thiol oxidation-mediated pathways. Antioxid. Redox Signal 2013, 18, 1623–1641. [Google Scholar] [CrossRef]
- Hernebring, M.; Adelof, J.; Wiseman, J.; Petersen, A.; Zetterberg, M. H2O2-induced cataract as a model of age-related cataract: Lessons learned from overexpressing the proteasome activator PA28alphabeta in mouse eye lens. Exp. Eye Res. 2021, 203, 108395. [Google Scholar] [CrossRef]
- Carey, J.W.; Pinarci, E.Y.; Penugonda, S.; Karacal, H.; Ercal, N. In vivo inhibition of l-buthionine-(S,R)-sulfoximine-induced cataracts by a novel antioxidant, N-acetylcysteine amide. Free Radic. Biol. Med. 2011, 50, 722–729. [Google Scholar] [CrossRef]
- Maddirala, Y.; Tobwala, S.; Karacal, H.; Ercal, N. Prevention and reversal of selenite-induced cataracts by N-acetylcysteine amide in Wistar rats. BMC Ophthalmol. 2017, 17, 54. [Google Scholar] [CrossRef]
- Pendergrass, W.; Penn, P.; Possin, D.; Wolf, N. Accumulation of DNA, nuclear and mitochondrial debris, and ROS at sites of age-related cortical cataract in mice. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4661–4670. [Google Scholar] [CrossRef]
- Pendergrass, W.; Zitnik, G.; Urfer, S.R.; Wolf, N. Age-related retention of fiber cell nuclei and nuclear fragments in the lens cortices of multiple species. Mol. Vis. 2011, 17, 2672–2684. [Google Scholar]
- Wolf, N.; Penn, P.; Pendergrass, W.; Van Remmen, H.; Bartke, A.; Rabinovitch, P.; Martin, G.M. Age-related cataract progression in five mouse models for anti-oxidant protection or hormonal influence. Exp. Eye Res. 2005, 81, 276–285. [Google Scholar] [CrossRef]
- Zhou, R.; Tardivel, A.; Thorens, B.; Choi, I.; Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010, 11, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Holmgren, A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014, 66, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yao, W.; Xu, J.; Qiu, Y.; Cao, F.; Li, S.; Yang, S.; Yang, H.; Wu, Z.; Hou, Y. The anti-inflammatory effects of acetaminophen and N-acetylcysteine through suppression of the NLRP3 inflammasome pathway in LPS-challenged piglet mononuclear phagocytes. Innate Immun. 2015, 21, 587–597. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; Lu, X.; Zhao, S. N-acetylcysteine alleviates ocular surface damage in STZ-induced diabetic mice by inhibiting the ROS/NLRP3/Caspase-1/IL-1beta signaling pathway. Exp. Eye Res. 2021, 209, 108654. [Google Scholar] [CrossRef]
- Li, H.; Xiao, L.; He, H.; Zeng, H.; Liu, J.; Jiang, C.; Mei, G.; Yu, J.; Chen, H.; Yao, P.; et al. Quercetin Attenuates Atherosclerotic Inflammation by Inhibiting Galectin-3-NLRP3 Signaling Pathway. Mol. Nutr. Food Res. 2021, 65, e2000746. [Google Scholar] [CrossRef]
- Tosi, G.M.; Giustarini, D.; Franci, L.; Minetti, A.; Imperatore, F.; Caldi, E.; Fiorenzani, P.; Aloisi, A.M.; Sparatore, A.; Rossi, R.; et al. Superior Properties of N-Acetylcysteine Ethyl Ester over N-Acetyl Cysteine to Prevent Retinal Pigment Epithelial Cells Oxidative Damage. Int. J. Mol. Sci. 2021, 22, 600. [Google Scholar] [CrossRef]
- Lian, L.; Le, Z.; Wang, Z.; Chen, Y.A.; Jiao, X.; Qi, H.; Hejtmancik, J.F.; Ma, X.; Zheng, Q.; Ren, Y. SIRT1 Inhibits High Glucose-Induced TXNIP/NLRP3 Inflammasome Activation and Cataract Formation. Investig. Ophthalmol. Vis. Sci. 2023, 64, 16. [Google Scholar] [CrossRef]
- Cao, X.; Jiang, Z.; Bu, X.; Li, Q.; Tian, Y.; Xu, Z.; Zhang, B.; Yuan, X. MicroRNA-204-5p Attenuates Oxidative Stress, Apoptosis and Inflammation by Targeting TXNIP in Diabetic Cataract. Biochem. Genet. 2024. [Google Scholar] [CrossRef]
- Li, Y.; Deng, W.; Wu, J.; He, Q.; Yang, G.; Luo, X.; Jia, Y.; Duan, Y.; Zhou, L.; Liu, D. TXNIP Exacerbates the Senescence and Aging-Related Dysfunction of beta Cells by Inducing Cell Cycle Arrest Through p38-p16/p21-CDK-Rb Pathway. Antioxid. Redox Signal 2023, 38, 480–495. [Google Scholar] [CrossRef]
- Deng, J.; Pan, T.; Liu, Z.; McCarthy, C.; Vicencio, J.M.; Cao, L.; Alfano, G.; Suwaidan, A.A.; Yin, M.; Beatson, R.; et al. The role of TXNIP in cancer: A fine balance between redox, metabolic, and immunological tumor control. Br. J. Cancer 2023, 129, 1877–1892. [Google Scholar] [CrossRef]
- Pan, M.; Zhang, F.; Qu, K.; Liu, C.; Zhang, J. TXNIP: A Double-Edged Sword in Disease and Therapeutic Outlook. Oxid. Med. Cell Longev. 2022, 2022, 7805115. [Google Scholar] [CrossRef] [PubMed]
- Palmquist, B.M.; Philipson, B.; Barr, P.O. Nuclear cataract and myopia during hyperbaric oxygen therapy. Br. J. Ophthalmol. 1984, 68, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Fledelius, H.C.; Jansen, E.C.; Thorn, J. Refractive change during hyperbaric oxygen therapy. A clinical trial including ultrasound oculometry. Acta Ophthalmol. Scand. 2002, 80, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Holekamp, N.M.; Shui, Y.B.; Beebe, D.C. Vitrectomy surgery increases oxygen exposure to the lens: A possible mechanism for nuclear cataract formation. Am. J. Ophthalmol. 2005, 139, 302–310. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishida, H.; Sasaki, Y.; Shibata, T.; Sasaki, H.; Chhunchha, B.; Singh, D.P.; Kubo, E. Topical Instillation of N-Acetylcysteine and N-Acetylcysteine Amide Impedes Age-Related Lens Opacity in Mice. Biomolecules 2025, 15, 442. https://doi.org/10.3390/biom15030442
Ishida H, Sasaki Y, Shibata T, Sasaki H, Chhunchha B, Singh DP, Kubo E. Topical Instillation of N-Acetylcysteine and N-Acetylcysteine Amide Impedes Age-Related Lens Opacity in Mice. Biomolecules. 2025; 15(3):442. https://doi.org/10.3390/biom15030442
Chicago/Turabian StyleIshida, Hidetoshi, Yu Sasaki, Teppei Shibata, Hiroshi Sasaki, Bhavana Chhunchha, Dhirendra P. Singh, and Eri Kubo. 2025. "Topical Instillation of N-Acetylcysteine and N-Acetylcysteine Amide Impedes Age-Related Lens Opacity in Mice" Biomolecules 15, no. 3: 442. https://doi.org/10.3390/biom15030442
APA StyleIshida, H., Sasaki, Y., Shibata, T., Sasaki, H., Chhunchha, B., Singh, D. P., & Kubo, E. (2025). Topical Instillation of N-Acetylcysteine and N-Acetylcysteine Amide Impedes Age-Related Lens Opacity in Mice. Biomolecules, 15(3), 442. https://doi.org/10.3390/biom15030442








