Lycium barbarum Glycopeptide Promotes Testosterone Synthesis and Glucose Metabolism in Leydig Cells of the Testis
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of LbGp
2.2. Animals and Treatment
2.3. Primary Leydig Cell Isolation
2.4. Western Blots
2.5. Testosterone Measurements
2.6. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.7. Immunofluorescence
2.8. Adenosine 5′-Triphosphate (ATP) Measurement
2.9. Cell Proliferation Assays
2.10. EdU Incorporation Assay
2.11. Statistical Analysis
3. Results
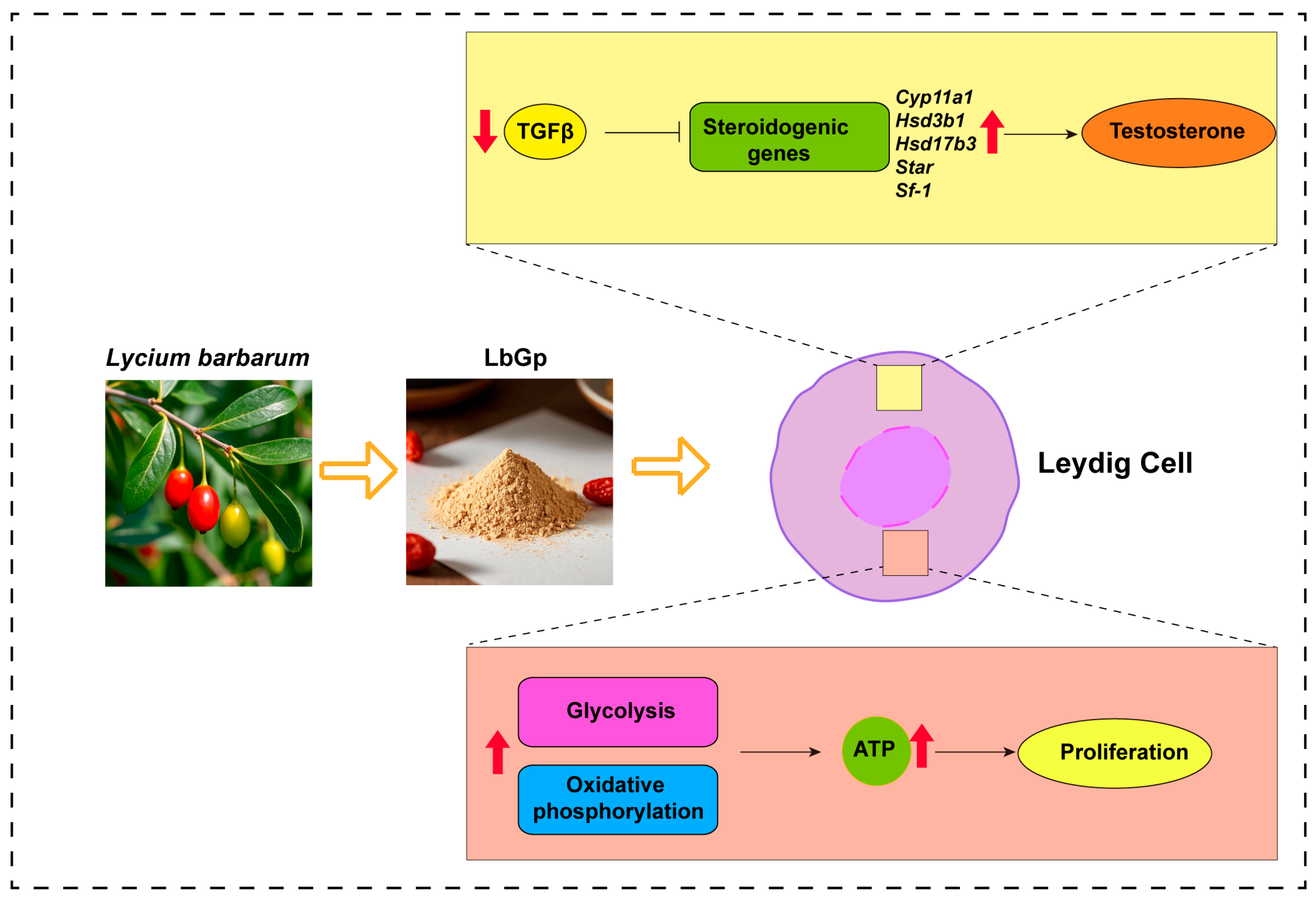
3.1. LbGp Enhances Leydig Cell Proliferation and Testosterone Synthesis
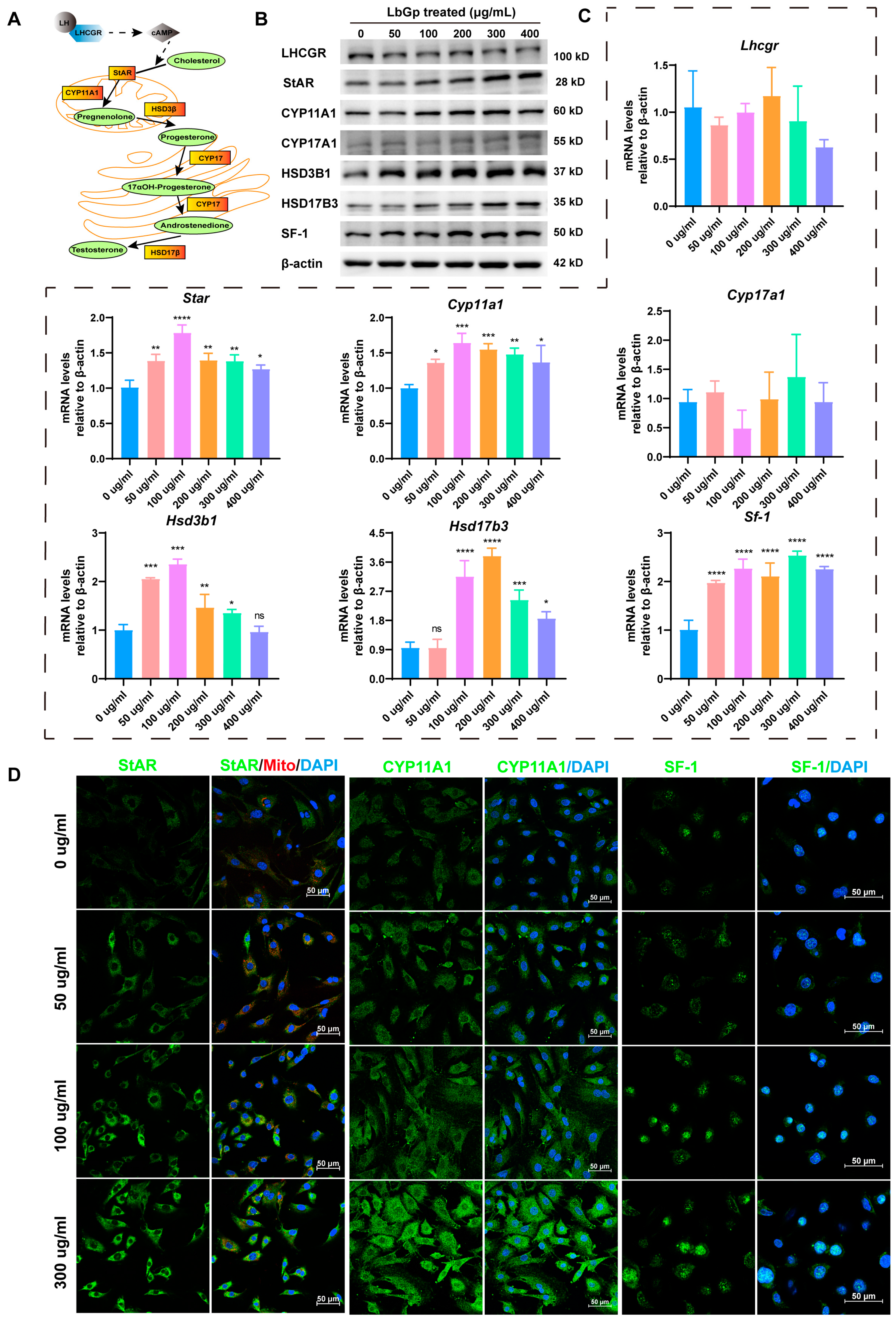
3.2. LbGp Up-Regulates the Expression of Steroidogenic Genes
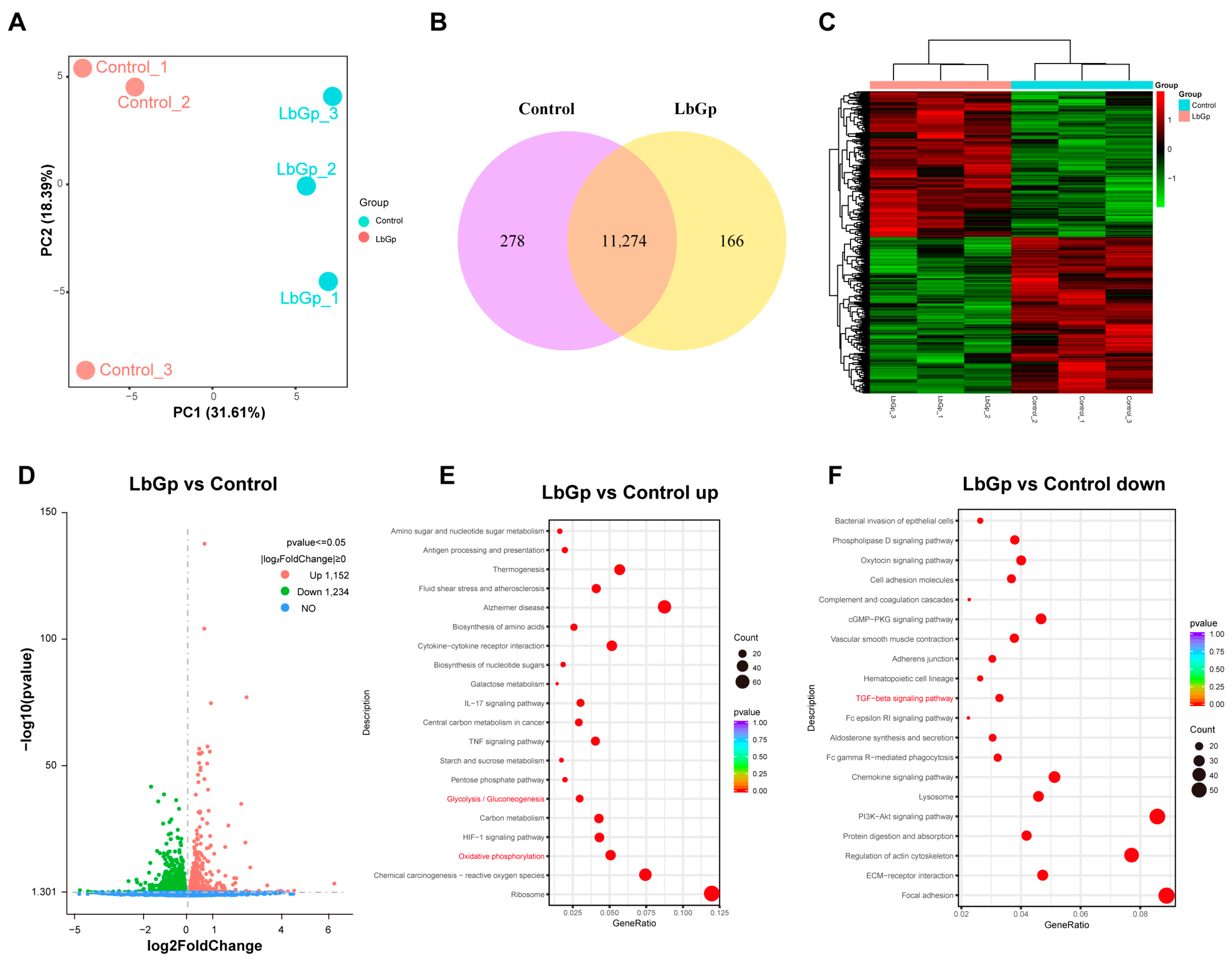
3.3. Profiling Gene Expression of Leydig Cells Treated with LbGp
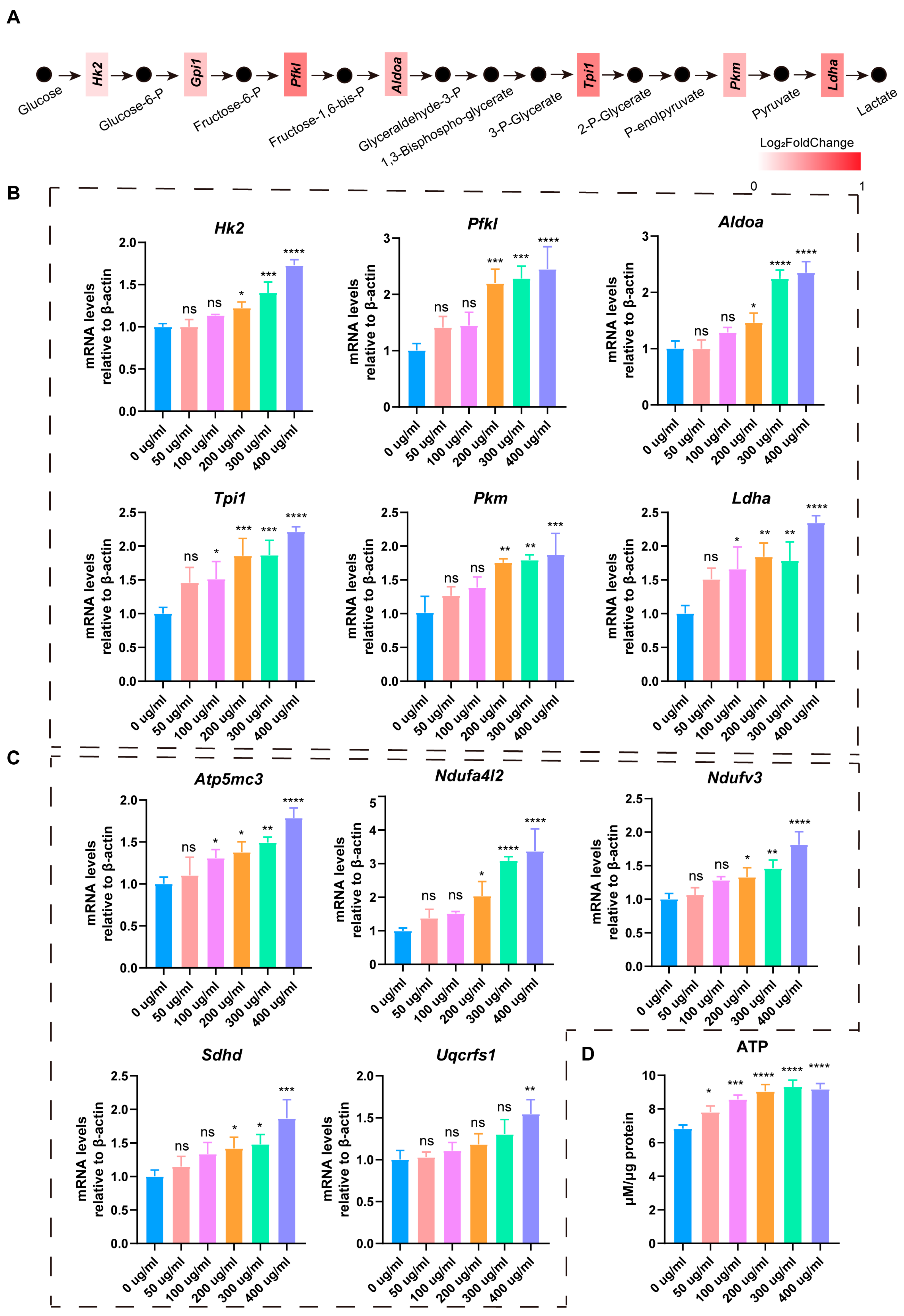
3.4. LbGp Promotes Glucose Metabolism and ATP Synthesis in Leydig Cells
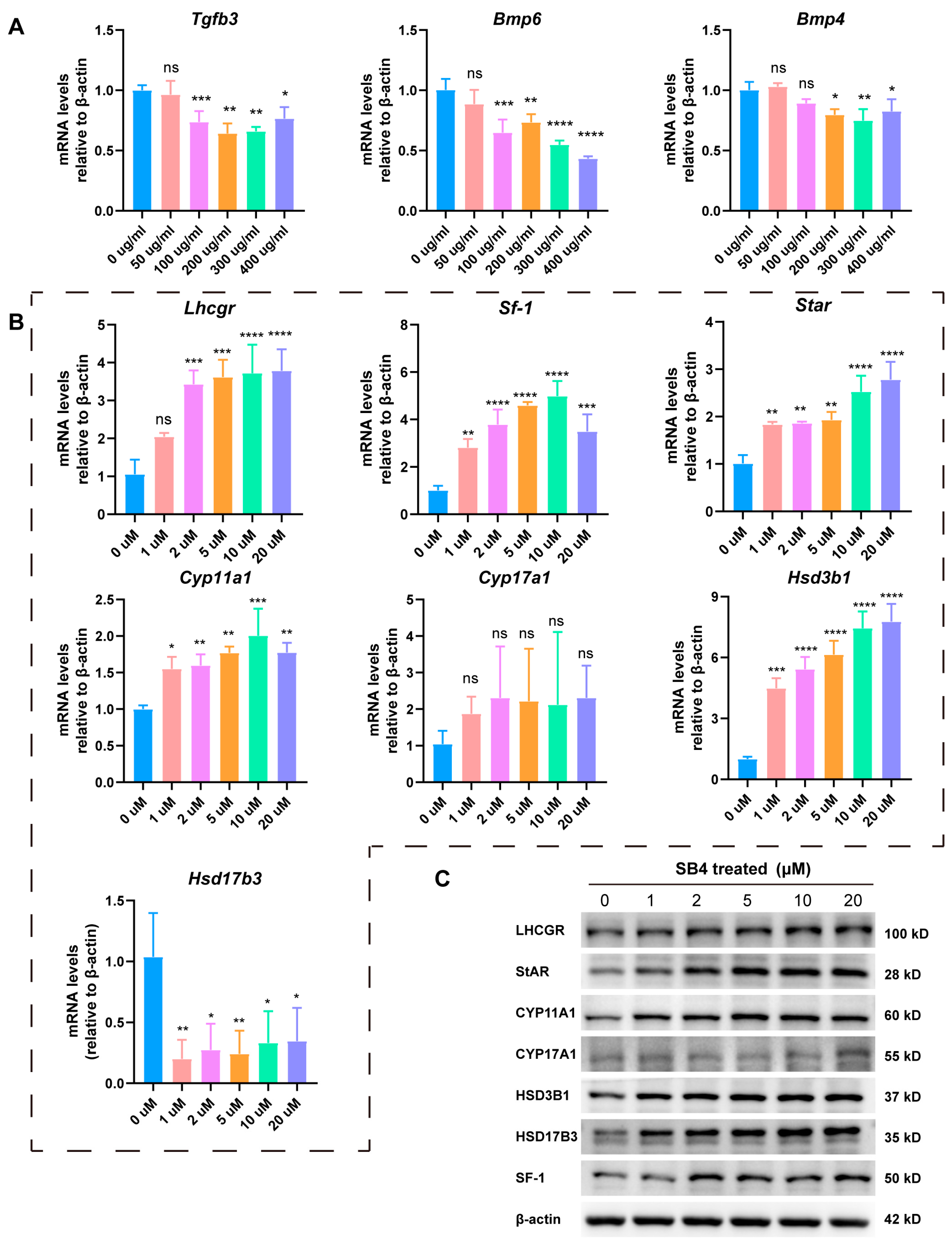
3.5. LbGp Promotes the Expression of Steroidogenic Genes by Inhibiting the TGF-β Signaling Pathway
3.6. LbGp Increases the Expression of Steroidogenic Genes In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LbGp | Lycium barbarum glycopeptide |
| LCs | Leydig cells |
| RNA seq | RNA sequencing |
| ATP | Adenosine 5′-triphosphate |
| TD | Testosterone deficiency |
| LBP | Lycium barbarum polysaccharide |
| FBS | Fetal bovine serum |
| TBST | Tris buffered saline with Tween 20 |
| ECL | Enhanced chemiluminescence |
| qRT-PCR | Quantitative real-time polymerase chain reaction |
| CCK8 | Counting Kit-8 |
| SB4 | SB431542 |
| DHT | Dihydrotestosterone |
| StAR | Steroidogenic acute regulatory |
| CYP11A1 | Cytochrome P450 family 11 subfamily A member 1 |
| HSD3B1 | Hydroxy-delta-5-steroid dehydrogenase, 3β-and steroid delta-isomerase 1 |
| HSD17B3 | Hydroxysteroid 17β dehydrogenase 3 |
| SF-1 | Steroidogenic factor 1 |
| CYP17A1 | Cytochrome P450 family 17 subfamily A member 1 |
| LHCGR | Luteinizing hormone receptor |
| DEGs | Differentially expressed genes |
| PCA | Principal component analysis |
References
- Neaves, W.B. Leydig cells. Contraception 1975, 11, 571–606. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Wu, J.; Liu, B.; Jiang, Y.; Chen, W.; Li, J.; He, Q.; He, Z. The roles and mechanisms of Leydig cells and myoid cells in regulating spermatogenesis. Cell. Mol. Life Sci. 2019, 76, 2681–2695. [Google Scholar] [CrossRef]
- Awoniyi, C.A.; Santulli, R.; Sprando, R.L.; Ewing, L.L.; Zirkin, B.R. Restoration of advanced spermatogenic cells in the experimentally regressed rat testis: Quantitative relationship to testosterone concentration within the testis. Endocrinology 1989, 124, 1217–1223. [Google Scholar] [CrossRef]
- Fujii, T. Roles of age and androgen in the regulation of sex accessory organs. Adv. Sex. Horm. Res. 1977, 3, 103–137. [Google Scholar]
- Hackett, G.; Kirby, M.; Edwards, D.; Jones, T.H.; Rees, J.; Muneer, A. UK policy statements on testosterone deficiency. Int. J. Clin. Pract. 2017, 71, e12901. [Google Scholar] [CrossRef]
- Bhasin, S.; Brito, J.P.; Cunningham, G.R.; Hayes, F.J.; Hodis, H.N.; Matsumoto, A.M.; Snyder, P.J.; Swerdloff, R.S.; Wu, F.C.; Yialamas, M.A. Testosterone Therapy in Men with Hypogonadism: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2018, 103, 1715–1744. [Google Scholar] [CrossRef] [PubMed]
- Hackett, G.; Kirby, M.; Rees, R.W.; Jones, T.H.; Muneer, A.; Livingston, M.; Ossei-Gerning, N.; David, J.; Foster, J.; Kalra, P.A.; et al. The British Society for Sexual Medicine Guidelines on Male Adult Testosterone Deficiency, with Statements for Practice. World J. Mens. Health 2023, 41, 508–537. [Google Scholar] [CrossRef] [PubMed]
- Zarotsky, V.; Huang, M.Y.; Carman, W.; Morgentaler, A.; Singhal, P.K.; Coffin, D.; Jones, T.H. Systematic literature review of the risk factors, comorbidities, and consequences of hypogonadism in men. Andrology 2014, 2, 819–834. [Google Scholar] [CrossRef]
- Ding, E.L.; Song, Y.; Malik, V.S.; Liu, S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: A systematic review and meta-analysis. Jama 2006, 295, 1288–1299. [Google Scholar] [CrossRef]
- Corona, G.; Vena, W.; Pizzocaro, A.; Vignozzi, L.; Sforza, A.; Maggi, M. Testosterone therapy in diabetes and pre-diabetes. Andrology 2023, 11, 204–214. [Google Scholar] [CrossRef]
- Lincoff, A.M.; Bhasin, S.; Flevaris, P.; Mitchell, L.M.; Basaria, S.; Boden, W.E.; Cunningham, G.R.; Granger, C.B.; Khera, M.; Thompson, I.M., Jr.; et al. Cardiovascular Safety of Testosterone-Replacement Therapy. N. Engl. J. Med. 2023, 389, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Khoo, T.K. Adverse events associated with testosterone administration. N. Engl. J. Med. 2010, 363, 1865–1866, author reply 1866–1867. [Google Scholar] [CrossRef]
- Vigen, R.; O’Donnell, C.I.; Barón, A.E.; Grunwald, G.K.; Maddox, T.M.; Bradley, S.M.; Barqawi, A.; Woning, G.; Wierman, M.E.; Plomondon, M.E.; et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. Jama 2013, 310, 1829–1836. [Google Scholar] [CrossRef]
- Tsametis, C.P.; Isidori, A.M. Testosterone replacement therapy: For whom, when and how? Metabolism 2018, 86, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Shehab, N.G.; Omolaoye, T.S.; Du Plessis, S.S.; Rawat, S.S.; Naidoo, N.; Abushawish, K.Y.; Ahmed, A.; Alaa, B.; Ihsan, H.; Abdelhalim, M.; et al. Phytochemical Evaluation of Lepidium meyenii, Trigonella foenum-graecum, Spirulina platensis, and Tribulus arabica, and Their Potential Effect on Monosodium Glutamate Induced Male Reproductive Dysfunction in Adult Wistar Rats. Antioxidants 2024, 13, 939. [Google Scholar] [CrossRef]
- Steels, E.; Rao, A.; Vitetta, L. Physiological aspects of male libido enhanced by standardized Trigonella foenum-graecum extract and mineral formulation. Phytother. Res. 2011, 25, 1294–1300. [Google Scholar] [CrossRef]
- Wang, J.; Bao, B.; Meng, F.; Deng, S.; Feng, J.; Dai, H.; Xu, H.; Zhao, Q.; Li, H.; Wang, B. In vitro and in vivo investigation of the therapeutic mechanism of Lycium Chinense and Cuscutae Semen on oligoasthenozoospermia. Andrologia 2021, 53, e14014. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Lin, Y.; Tian, G.; Ji, G. Isolation, purification and physico-chemical properties of immunoactive constituents from the fruit of Lycium barbarum L. Yao Xue Xue Bao 1998, 33, 512–516. [Google Scholar]
- Jiang, Z.; Zeng, Z.; He, H.; Li, M.; Lan, Y.; Hui, J.; Bie, P.; Chen, Y.; Liu, H.; Fan, H.; et al. Lycium barbarum glycopeptide alleviates neuroinflammation in spinal cord injury via modulating docosahexaenoic acid to inhibiting MAPKs/NF-kB and pyroptosis pathways. J. Transl. Med. 2023, 21, 770. [Google Scholar] [CrossRef]
- Dai, Y.; Guo, J.; Zhang, B.; Chen, J.; Ou, H.; He, R.R.; So, K.F.; Zhang, L. Lycium barbarum (Wolfberry) glycopeptide prevents stress-induced anxiety disorders by regulating oxidative stress and ferroptosis in the medial prefrontal cortex. Phytomedicine 2023, 116, 154864. [Google Scholar] [CrossRef]
- Lin, X.M.; Wang, M.; Xiao, X.; Shi, Y.L.; Zheng, Y.S.; Huang, Z.H.; Cheng, Y.T.; Huang, R.T.; Huang, F.; Li, K.; et al. Wolfberry (Lycium barbarum) glycopeptide attenuates dopaminergic neurons loss by inhibiting lipid peroxidation in Parkinson’s disease. Phytomedicine 2025, 136, 156275. [Google Scholar] [CrossRef]
- Yao, J.; Hui, J.W.; Chen, Y.J.; Luo, D.Y.; Yan, J.S.; Zhang, Y.F.; Lan, Y.X.; Yan, X.R.; Wang, Z.H.; Fan, H.; et al. Lycium barbarum glycopeptide targets PER2 to inhibit lipogenesis in glioblastoma by downregulating SREBP1c. Cancer Gene Ther. 2023, 30, 1084–1093. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.W.; Park, J.W.; Lee, K.W.; Jeong, H.C.; Choi, J.B.; Choi, S.W.; Bae, W.J.; Cho, H.J.; Ha, U.S.; Hong, S.H.; et al. Administration of Goji (Lycium chinense Mill.) Extracts Improves Erectile Function in Old Aged Rat Model. World J. Mens. Health 2017, 35, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.J.; Zheng, J.; Wu, J.; Qiao, H.Q.; Chang, Q.; Niu, Y.; Sun, T.; Li, Y.X.; Yu, J.Q. Protective effects of Lycium barbarum polysaccharide on male sexual dysfunction and fertility impairments by activating hypothalamic pituitary gonadal axis in streptozotocin-induced type-1 diabetic male mice. Endocr. J. 2017, 64, 907–922. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Z.; Shi, H.; Liu, Q.; Chang, Y.; Feng, W.; Zhu, S.; Sun, S. Effects of Lycium barbarum glycopeptide on renal and testicular injury induced by di(2-ethylhexyl) phthalate. Cell Stress. Chaperones 2022, 27, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Tang, Y.; Li, H.; Mei, J.; Cao, Z.; Xia, H.; Huang, R.; Yang, Y.; Huang, Y. Isolation of Primary Leydig Cells from Murine Testis. Bio Protoc. 2021, 11, e4223. [Google Scholar] [CrossRef]
- Galano, M.; Li, Y.; Li, L.; Sottas, C.; Papadopoulos, V. Role of Constitutive STAR in Leydig Cells. Int. J. Mol. Sci. 2021, 22, 2021. [Google Scholar] [CrossRef]
- Qin, F.; Zhang, J.; Zan, L.; Guo, W.; Wang, J.; Chen, L.; Cao, Y.; Shen, O.; Tong, J. Inhibitory effect of melatonin on testosterone synthesis is mediated via GATA-4/SF-1 transcription factors. Reprod. Biomed. Online 2015, 31, 638–646. [Google Scholar] [CrossRef]
- Park, E.; Song, C.H.; Park, J.I.; Ahn, R.S.; Choi, H.S.; Ko, C.; Lee, K. Transforming growth factor-β1 signaling represses testicular steroidogenesis through cross-talk with orphan nuclear receptor Nur77. PLoS ONE 2014, 9, e104812. [Google Scholar] [CrossRef]
- Yang, Q.; Ma, B.; Qiao, H.; Ma, H.; Dong, Y.; Cao, L.; Ma, J.; Li, Z. TGFB1 represses the expression of SF1 and LRH1 to inhibit E(2) production in rat LCs. Reproduction 2017, 153, 621–629. [Google Scholar] [CrossRef]
- Ma, B.; Qiao, H.; Guo, Y.; Wei, J.; Yang, Q.; Feng, X.; Li, Z. TGF-β1 induced activations of Smad2 and miRNAs inhibit SF-1- and LRH-1-dependent CYP19 expression in rat Leydig cells†. Biol. Reprod. 2023, 108, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.F.; You, Z.Q.; Gao, H.Y.; Zhou, G.L.; Chen, Y.X.; Yu, J.; Xuan, Y.X. Protective effect of Lycium barbarum polysaccharides against doxorubicin-induced testicular toxicity in rats. Phytother. Res. 2012, 26, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.J.; Zheng, J.; Han, X.X.; Jiang, Y.P.; Li, Z.M.; Wu, J.; Chang, Q.; Niu, Y.; Sun, T.; Li, Y.X.; et al. Lycium barbarum polysaccharide attenuates diabetic testicular dysfunction via inhibition of the PI3K/Akt pathway-mediated abnormal autophagy in male mice. Cell Tissue Res. 2018, 374, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, X.; Li, X.; Wang, L.; Zhang, H.; He, Y.; Wang, J.; Zhao, Y.; Zhang, B.; Xu, Y. Interference RNA-based silencing of endogenous SMAD4 in porcine granulosa cells resulted in decreased FSH-mediated granulosa cells proliferation and steroidogenesis. Reproduction 2011, 141, 643–651. [Google Scholar] [CrossRef]
- Yan, L.; Qu, X.; Yu, J.; Robinson, R.S.; Woad, K.J.; Shi, Z. Transforming growth factor-β1 disrupts angiogenesis during the follicular-luteal transition through the Smad-serpin family E member 1 (SERPINE1)/serpin family B member 5 (SERPINB5) signalling pathway in the cow. Reprod. Fertil. Dev. 2021, 33, 643–654. [Google Scholar] [CrossRef]
- Mattar, D.; Samir, M.; Laird, M.; Knight, P.G. Modulatory effects of TGF-β1 and BMP6 on thecal angiogenesis and steroidogenesis in the bovine ovary. Reproduction 2020, 159, 397–408. [Google Scholar] [CrossRef]
- Galvão, A.; Wolodko, K.; Rebordão, M.R.; Skarzynski, D.; Ferreira-Dias, G. TGFB1 modulates in vitro secretory activity and viability of equine luteal cells. Cytokine 2018, 110, 316–327. [Google Scholar] [CrossRef]
- Wang, Y.; Bilandzic, M.; Ooi, G.T.; Findlay, J.K.; Stenvers, K.L. Endogenous inhibins regulate steroidogenesis in mouse TM3 Leydig cells by altering SMAD2 signalling. Mol. Cell. Endocrinol. 2016, 436, 68–77. [Google Scholar] [CrossRef]
- Cao, L.; Wu, J.; Qu, X.; Sheng, J.; Cui, M.; Liu, S.; Huang, X.; Xiang, Y.; Li, B.; Zhang, X.; et al. Glycometabolic rearrangements--aerobic glycolysis in pancreatic cancer: Causes, characteristics and clinical applications. J. Exp. Clin. Cancer Res. 2020, 39, 267. [Google Scholar] [CrossRef]
- Cai, K.; Chen, S.; Zhu, C.; Li, L.; Yu, C.; He, Z.; Sun, C. FOXD1 facilitates pancreatic cancer cell proliferation, invasion, and metastasis by regulating GLUT1-mediated aerobic glycolysis. Cell Death Dis. 2022, 13, 765. [Google Scholar] [CrossRef]
- Enzo, E.; Santinon, G.; Pocaterra, A.; Aragona, M.; Bresolin, S.; Forcato, M.; Grifoni, D.; Pession, A.; Zanconato, F.; Guzzo, G.; et al. Aerobic glycolysis tunes YAP/TAZ transcriptional activity. Embo J. 2015, 34, 1349–1370. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, J.; Peng, T.; Hu, J.; So, K.F.; Zhang, H.; Chen, G.; Zhang, Y.-W. Lycium barbarum Glycopeptide Promotes Testosterone Synthesis and Glucose Metabolism in Leydig Cells of the Testis. Biomolecules 2025, 15, 425. https://doi.org/10.3390/biom15030425
Liang J, Peng T, Hu J, So KF, Zhang H, Chen G, Zhang Y-W. Lycium barbarum Glycopeptide Promotes Testosterone Synthesis and Glucose Metabolism in Leydig Cells of the Testis. Biomolecules. 2025; 15(3):425. https://doi.org/10.3390/biom15030425
Chicago/Turabian StyleLiang, Jinlian, Tianchan Peng, Jinrong Hu, Kwok Fai So, Hongyi Zhang, Guobin Chen, and Yuan-Wei Zhang. 2025. "Lycium barbarum Glycopeptide Promotes Testosterone Synthesis and Glucose Metabolism in Leydig Cells of the Testis" Biomolecules 15, no. 3: 425. https://doi.org/10.3390/biom15030425
APA StyleLiang, J., Peng, T., Hu, J., So, K. F., Zhang, H., Chen, G., & Zhang, Y.-W. (2025). Lycium barbarum Glycopeptide Promotes Testosterone Synthesis and Glucose Metabolism in Leydig Cells of the Testis. Biomolecules, 15(3), 425. https://doi.org/10.3390/biom15030425






