The Prognostic, Predictive and Clinicopathological Implications of KRT81/HNF1A- and GATA6-Based Transcriptional Subtyping in Pancreatic Cancer
Abstract
1. Background
2. Materials and Methods
2.1. Study Outline and Patient Selection
2.2. Tumor Samples and Immunohistochemistry
2.3. Statistical and In Silico Analyses
3. Results
3.1. Transcriptional Subtypes According to the Expression of KRT81/HNF1A or GATA6 Widely Overlap but May Change During Metastatic Progression
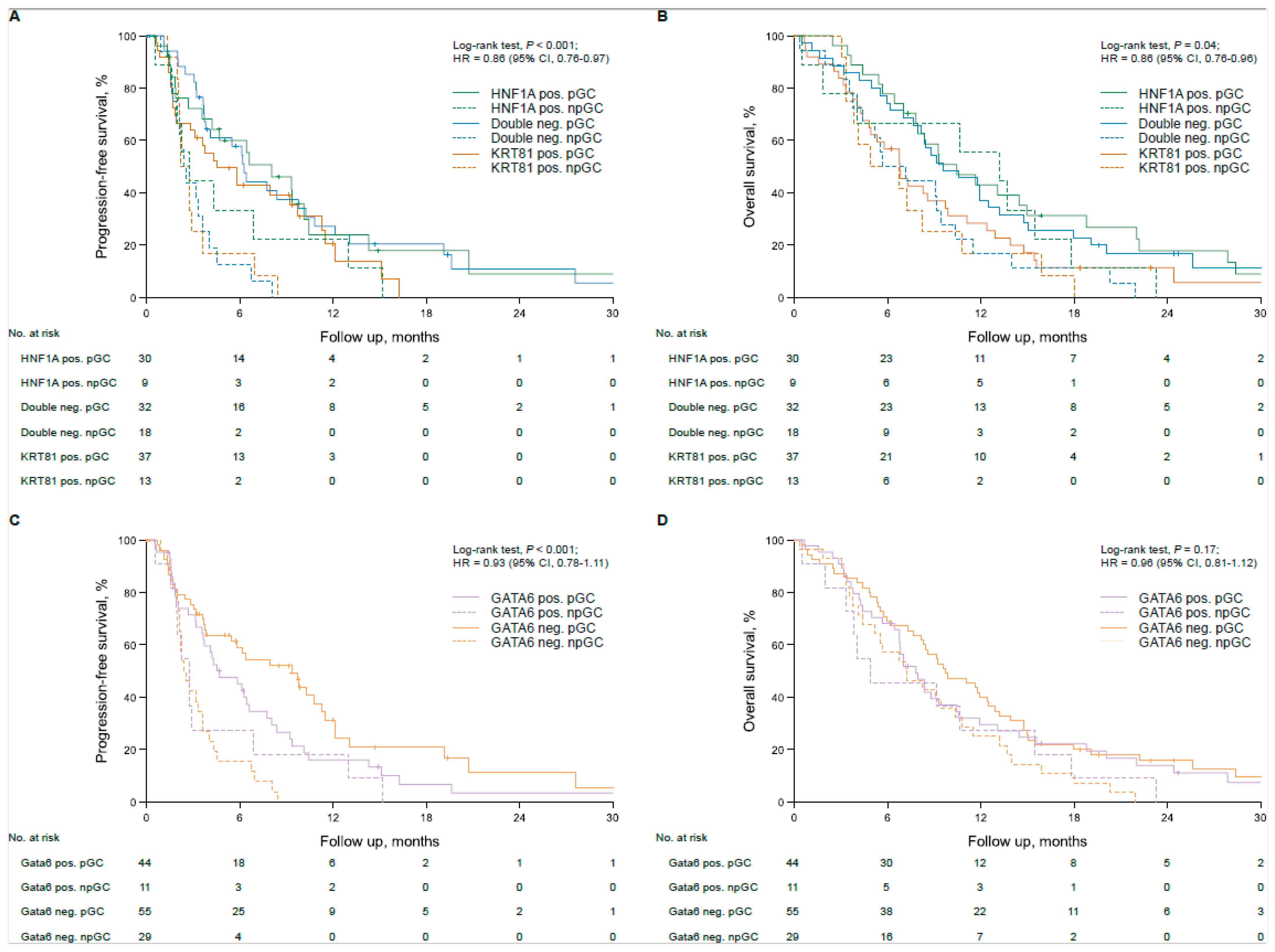
3.2. Transcriptional Subtypes Affect Patient Outcome Dependent on Palliative Therapy in Advanced PDAC Patients
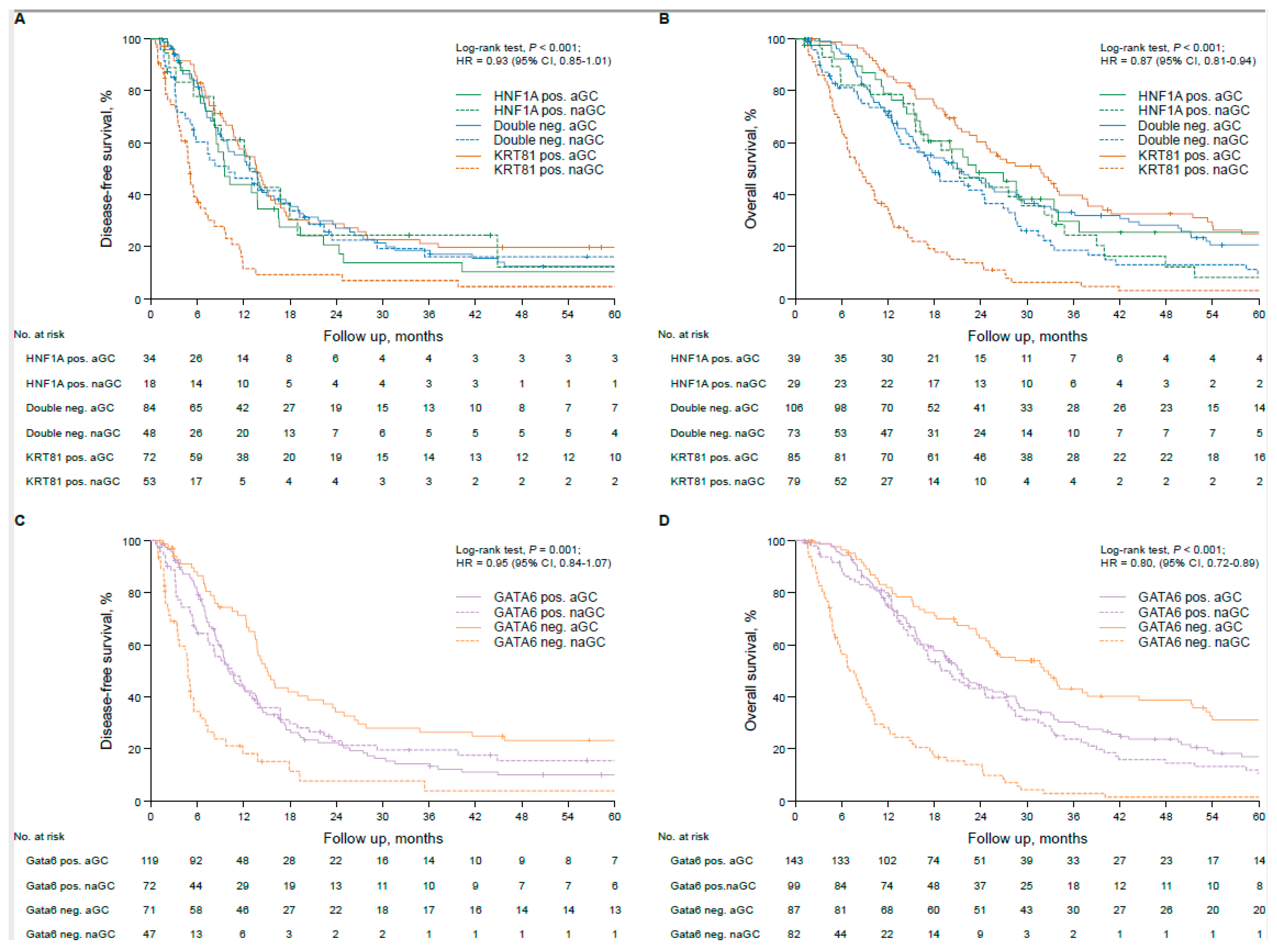
3.3. Transcriptional Subtypes Affect Patient Outcome Dependent on Adjuvant Therapy in Resected PDAC Patients
3.4. In Silico Validation and Potential Mechanistic Background
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| aPDAC | advanced pancreatic ductal adenocarcinoma |
| aGC | adjuvant gemcitabine |
| CTX | chemotherapy |
| DFS | disease-free survival |
| FFPE | formalin fixed paraffin embedded |
| FFX | folfirinox |
| HR | hazard ratio |
| ICGC | International Cancer Genome Consortium |
| IHC | immunohistochemistry |
| naGC | no adjuvant gemcitabine |
| OS | overall survival |
| PDAC | pancreatic ductal adenocarcinoma |
| PFS | progression-free survival |
| pGC | palliative gemcitabine-based chemotherapy |
| pnGC | palliative non-gemcitabine-based chemotherapy |
| rPDAC | resected pancreatic ductal adenocarcinoma |
| RCT | randomized controlled trial |
| RNAseq | RNA sequencing |
| TCGA | the cancer genome atlas |
References
- Park, W.; Chawla, A.; O’Reilly, E.M. Pancreatic cancer: A review. JAMA 2021, 326, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Guenther, M.; Boeck, S.; Heinemann, V.; Werner, J.; Engel, J.; Ormanns, S. The impact of adjuvant therapy on outcome in UICC stage I pancreatic cancer. Int. J. Cancer 2022, 151, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Tempero, M.A.; Malafa, M.P.; Al-Hawary, M.; Behrman, S.W.; Benson, A.B.; Cardin, D.B.; Chiorean, E.G.; Chung, V.; Czito, B.; Del Chiaro, M.; et al. Pancreatic adenocarcinoma, version 2. 2021, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 439–457. [Google Scholar]
- Collisson, E.A.; Bailey, P.; Chang, D.K.; Biankin, A.V. Molecular subtypes of pancreatic cancer. Nat. Rev. Gastroenterol Hepatol. 2019, 16, 207–220. [Google Scholar] [CrossRef]
- Noll, E.M.; Eisen, C.; Stenzinger, A.; Espinet, E.; Muckenhuber, A.; Klein, C.; Vogel, V.; Klaus, B.; Nadler, W.; Rösli, C.; et al. CYP3A5 mediates basal and acquired therapy resistance in different subtypes of pancreatic ductal adenocarcinoma. Nat. Med. 2016, 22, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, P.; Carrillo-de Santa Pau, E.; Cox, T.; Sainz, B.; Dusetti, N.; Greenhalf, W.; Rinaldi, L.; Costello, E.; Ghaneh, P.; Malats, N.; et al. GATA6 regulates EMT and tumour dissemination, and is a marker of response to adjuvant chemotherapy in pancreatic cancer. Gut 2017, 66, 1665. [Google Scholar] [CrossRef]
- Muckenhuber, A.; Berger, A.K.; Schlitter, A.M.; Steiger, K.; Konukiewitz, B.; Trumpp, A.; Eils, R.; Werner, J.; Friess, H.; Esposito, I.; et al. Pancreatic Ductal Adenocarcinoma Subtyping Using the Biomarkers Hepatocyte Nuclear Factor-1A and Cytokeratin-81 Correlates with Outcome and Treatment Response. Clin. Cancer Res. 2018, 24, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Haas, M.; Ormanns, S.; Baechmann, S.; Remold, A.; Kruger, S.; Westphalen, C.B.; Siveke, J.T.; Wenzel, P.; Schlitter, A.M.; Esposito, I.; et al. Extended RAS analysis and correlation with overall survival in advanced pancreatic cancer. Br. J. Cancer 2017, 116, 1462–1469. [Google Scholar] [CrossRef]
- Heinemann, V.; Vehling-Kaiser, U.; Waldschmidt, D.; Kettner, E.; Märten, A.; Winkelmann, C.; Klein, S.; Kojouharoff, G.; Gauler, T.C.; von Weikersthal, L.F.; et al. Gemcitabine plus erlotinib followed by capecitabine versus capecitabine plus erlotinib followed by gemcitabine in advanced pancreatic cancer: Final results of a randomised phase 3 trial of the ‘Arbeitsgemeinschaft Internistische Onkologie’(AIO-PK0104). Gut 2013, 62, 751–759. [Google Scholar] [CrossRef]
- Guenther, M.; Surendran, S.A.; Haas, M.; Heinemann, V.; von Bergwelt-Baildon, M.; Engel, J.; Werner, J.; Boeck, S.; Ormanns, S. TPX2 expression as a negative predictor of gemcitabine efficacy in pancreatic cancer. Br. J. Cancer 2023, 129, 175–182. [Google Scholar] [CrossRef]
- Kruger, S.F.; Lohneis, A.; Abendroth, A.; Berger, A.W.; Ettrich, T.J.; Waidmann, O.; Kapp, M.; Steiner, B.; Kumbrink, J.; Reischer, A.; et al. Prognosis and tumor biology of pancreatic cancer patients with isolated lung metastases: Translational results from the German multicenter AIO-YMO-PAK-0515 study. ESMO Open 2022, 7, 100388. [Google Scholar] [CrossRef]
- Zhang, J.; Bajari, R.; Andric, D.; Gerthoffert, F.; Lepsa, A.; Nahal-Bose, H.; Stein, L.D.; Ferretti, V. The international cancer genome consortium data portal. Nat. Biotechnol. 2019, 37, 367–369. [Google Scholar] [CrossRef] [PubMed]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lu, H.; Yan, A.; Yang, Y.; Meng, Q.; Sun, L.; Pang, H.; Li, C.; Dong, X.; Cai, L. ABCC3 as a marker for multidrug resistance in non-small cell lung cancer. Sci. Rep. 2013, 3, 3120. [Google Scholar] [CrossRef]
- Yu, X.; Liu, W.; Wang, Z.; Wang, H.; Liu, J.; Huang, C.; Zhao, T.; Wang, X.; Gao, S.; Ma, Y.; et al. CD73 induces gemcitabine resistance in pancreatic ductal adenocarcinoma: A promising target with non-canonical mechanisms. Cancer Lett. 2021, 519, 289–303. [Google Scholar] [CrossRef]
- O'Kane, G.M.; Grünwald, B.T.; Jang, G.H.; Masoomian, M.; Picardo, S.; Grant, R.C.; Denroche, R.E.; Zhang, A.; Wang, Y.; Lam, B.; et al. GATA6 Expression Distinguishes Classical and Basal-like Subtypes in Advanced Pancreatic Cancer. Clin. Cancer Res. 2020, 26, 4901–4910. [Google Scholar] [CrossRef]
- Duan, K.; Jang, G.H.; Grant, R.C.; Wilson, J.M.; Notta, F.; O'Kane, G.M.; Knox, J.J.; Gallinger, S.; Fischer, S. The value of GATA6 immunohistochemistry and computer-assisted diagnosis to predict clinical outcome in advanced pancreatic cancer. Sci. Rep. 2021, 11, 14951. [Google Scholar] [CrossRef]
- de Andrés, M.P.; Jackson, R.J.; Felipe, I.; Zagorac, S.; Pilarsky, C.; Schlitter, A.M.; de Villareal, J.M.; Jang, G.H.; Costello, E.; Gallinger, S.; et al. GATA4 and GATA6 loss-of-expression is associated with extinction of the classical programme and poor outcome in pancreatic ductal adenocarcinoma. Gut 2023, 72, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Beutel, A.K.; Schutte, L.; Scheible, J.; Roger, E.; Muller, M.; Perkhofer, L.; Kestler, A.M.T.U.; Kraus, J.M.; Kestler, H.A.; Barth, T.F.E.; et al. A Prospective Feasibility Trial to Challenge Patient-Derived Pancreatic Cancer Organoids in Predicting Treatment Response. Cancers 2021, 13, 2539. [Google Scholar] [CrossRef]
- Shoucair, S.; Baker, A.; Ghabi, E.; Lafaro, K.; He, J.; Yu, J. GATA6 and Keratin5 Expression Profile is a Reliable Biomarker for Prediction of Improved Survival with Adjuvant Chemotherapy after Surgical Resection in PDAC. HPB 2022, 24 (Suppl. S1), S75. [Google Scholar] [CrossRef]
- Heredia-Soto, V.; Gutiérrez-Sainz, L.; Ghanem, I.; Guerra, L.; Palacios, E.; de Uribe, M.; Trilla-Fuertes, L.; de Miguel, M.; Cejas, P.; Medina, L.; et al. The Relationship between the Expression of GATA4 and GATA6 with the Clinical Characteristics and Prognosis of Resectable Pancreatic Adenocarcinoma. Biomedicines 2023, 11, 252. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Yoo, J.E.; Hwang, H.K.; Kang, C.M.; Lee, C.K.; Kim, M.H.; Bang, S.; Park, Y.N. Combined tumor epithelial and stromal histopathology with keratin 81 expression predicts prognosis for pancreatic ductal adenocarcinoma. J. Hepatobiliary Pancreat. Sci. 2022, 29, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Xu, D.; Peng, J.; Luo, Z.; Chen, C.; Chen, Y.; Chen, H.; Zheng, M.; Yin, P.; Wang, Z. HNF1A inhibition induces the resistance of pancreatic cancer cells to gemcitabine by targeting ABCB1. EBioMedicine 2019, 44, 403–418. [Google Scholar] [CrossRef] [PubMed]
- Kaissis, G.; Ziegelmayer, S.; Lohöfer, F.; Steiger, K.; Algül, H.; Muckenhuber, A.; Yen, H.-Y.; Rummeny, E.; Friess, H.; Schmid, R.; et al. A machine learning algorithm predicts molecular subtypes in pancreatic ductal adenocarcinoma with differential response to gemcitabine-based versus FOLFIRINOX chemotherapy. PLoS ONE 2019, 14, e0218642. [Google Scholar] [CrossRef]
- Kaissis, G.A.; Jungmann, F.; Ziegelmayer, S.; Lohöfer, F.K.; Harder, F.N.; Schlitter, A.M.; Muckenhuber, A.; Steiger, K.; Schirren, R.; Friess, H.; et al. Multiparametric Modelling of Survival in Pancreatic Ductal Adenocarcinoma Using Clinical, Histomorphological, Genetic and Image-Derived Parameters. J. Clin. Med. 2020, 9, 1250. [Google Scholar] [CrossRef]
- Peng, L.; Li, Y.; Yao, S.; Gaedcke, J.; Baart, V.M.; Sier, C.F.M.; Neesse, A.; Ellenrieder, V.; Bohnenberger, H.; Fuchs, F.; et al. Urokinase-Type Plasminogen Activator Receptor (uPAR) Cooperates with Mutated KRAS in Regulating Cellular Plasticity and Gemcitabine Response in Pancreatic Adenocarcinomas. Cancers 2023, 15, 1587. [Google Scholar] [CrossRef]
- Singh, S.; Hasselluhn, M.C.; Neesse, A. A tangled tale of molecular subtypes in pancreatic cancer. Gut 2019, 68, 953–954. [Google Scholar] [CrossRef]
- Birnbaum, D.J.; Finetti, P.; Birnbaum, D.; Mamessier, E.; Bertucci, F. Validation and comparison of the molecular classifications of pancreatic carcinomas. Mol. Cancer 2017, 16, 168. [Google Scholar] [CrossRef]
- Bailey, P.; Chang, D.K.; Nones, K.; Johns, A.L.; Patch, A.-M.; Gingras, M.-C.; Miller, D.K.; Christ, A.N.; Bruxner, T.J.C.; Quinn, M.C.; et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016, 531, 47–52. [Google Scholar] [CrossRef]
- Maurer, C.; Holmstrom, S.R.; He, J.; Laise, P.; Su, T.; Ahmed, A.; Hibshoosh, H.; Chabot, J.A.; Oberstein, P.E.; Sepulveda, A.R.; et al. Experimental microdissection enables functional harmonisation of pancreatic cancer subtypes. Gut 2019, 68, 1034–1043. [Google Scholar] [CrossRef]
- Collisson, E.A.; Sadanandam, A.; Olson, P.; Gibb, W.J.; Truitt, M.; Gu, S.; Cooc, J.; Weinkle, J.; Kim, G.E.; Jakkula, L.; et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat. Med. 2011, 17, 500–503. [Google Scholar] [CrossRef]
- Hegewisch-Becker, S.; Aldaoud, A.; Wolf, T.; Krammer-Steiner, B.; Linde, H.; Scheiner-Sparna, R.; Hamm, D.; Jänicke, M.; Marschner, N. TPK-Group (Tumour Registry Pancreatic Cancer) Results from the prospective German TPK clinical cohort study: Treatment algorithms and survival of 1,174 patients with locally advanced, inoperable, or metastatic pancreatic ductal adenocarcinoma. Int. J. Cancer 2019, 144, 981–990. [Google Scholar] [CrossRef]
- Santucci, J.; Tacey, M.; Thomson, B.; Michael, M.; Wong, R.; Shapiro, J.; Jennens, R.; Clarke, K.; Pattison, S.; Burge, M.; et al. Impact of first-line FOLFIRINOX versus Gemcitabine/Nab-Paclitaxel chemotherapy on survival in advanced pancreatic cancer: Evidence from the prospective international multicentre PURPLE pancreatic cancer registry. Eur. J. Cancer 2022, 174, 102–112. [Google Scholar] [CrossRef]
- Riedl, J.M.; Posch, F.; Horvath, L.; Gantschnigg, A.; Renneberg, F.; Schwarzenbacher, E.; Moik, F.; Barth, D.A.; Rossmann, C.H.; Stotz, M.; et al. Gemcitabine/nab-Paclitaxel versus FOLFIRINOX for palliative first-line treatment of advanced pancreatic cancer: A propensity score analysis. Eur. J. Cancer 2021, 151, 3–13. [Google Scholar] [CrossRef]
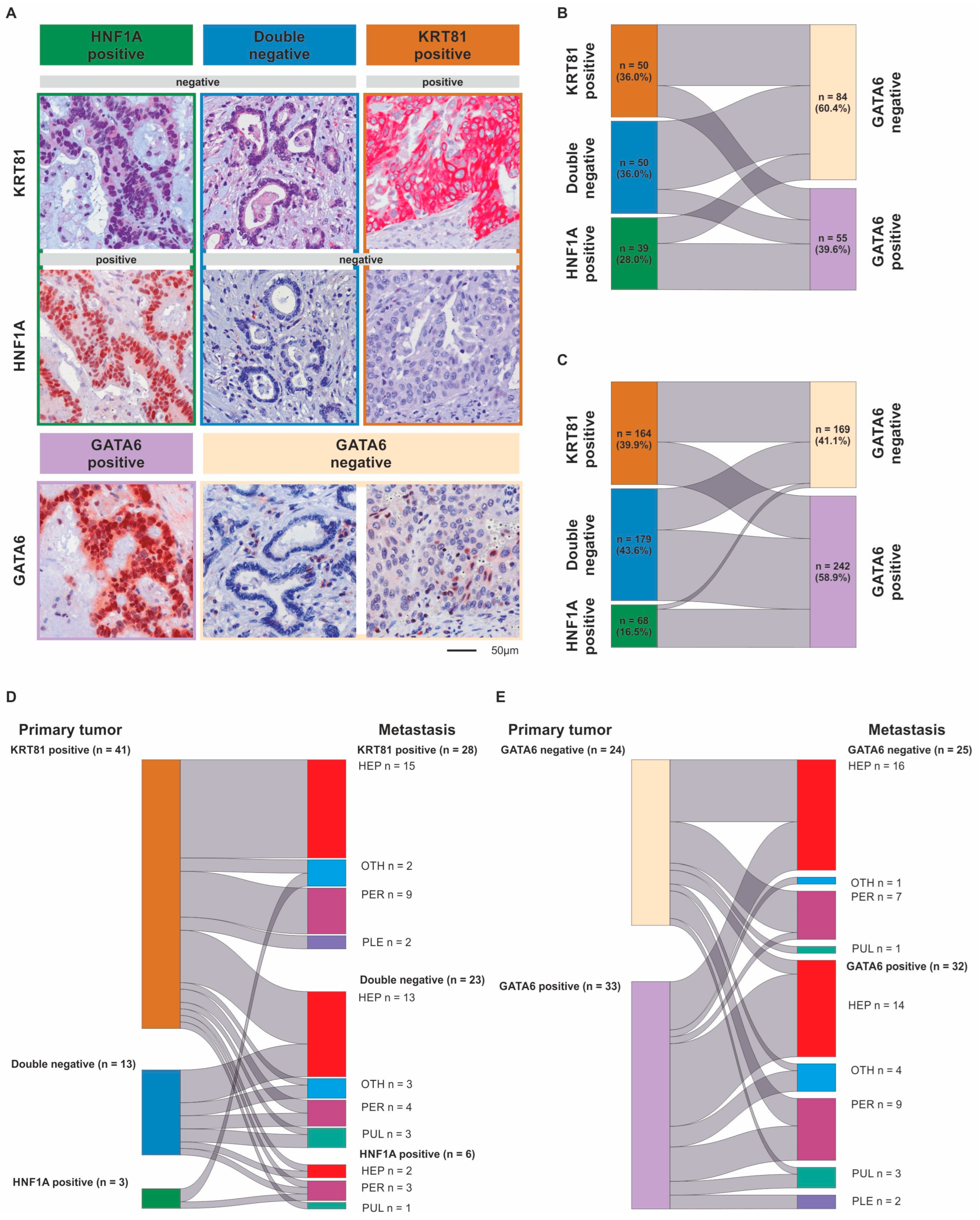
| Subtype, no (%) | Subtype, no (%) | ||||||
|---|---|---|---|---|---|---|---|
| KRT81 Pos. (n = 50) | Double Neg. (n = 50) | HNF1A Pos. (n = 39) | p-Value (χ2-Test) | GATA6 Neg. (n = 84) | GATA6 Pos. (n = 55) | p-Value (χ2-Test) | |
| sex | |||||||
| female | 30 (60.0) | 29 (58.0) | 20 (51.3) | 0.70 | 38 (45.2) | 22 (40.0) | 0.54 |
| male | 20 (40.0) | 21 (42.0) | 19 (48.7) | 46 (54.8) | 33 (60.0) | ||
| age group | |||||||
| ≤60 | 20 (40.0) | 19 (38.0) | 18 (46.2) | 0.73 | 33 (39.3) | 24 (43.6) | 0.61 |
| >60 | 30 (60.0) | 31 (62.0) | 21 (53.8) | 51 (60.7) | 31 (56.4) | ||
| treatment arm | |||||||
| non-gemcitabine based | 13 (26.0) | 18 (36.0) | 9 (23.1) | 0.35 | 29 (34.5) | 11 (20.0) | 0.06 |
| gemcitabine based | 37 (74.0) | 32 (64.0) | 30 (76.9) | 55 (65.5) | 44 (80.0) | ||
| KPS | |||||||
| ≤80 | 18 (36.0) | 19 (38.8) | 14 (35..9) | 0.95 | 32 (38.1) | 19 (35.2) | 0.73 |
| >80 | 32 (64.0) | 30 (61.2) | 25 (64.1) | 52 (61.9) | 35 (64.8) | ||
| grade group | |||||||
| G1-G2 | 17 (34.0) | 23 (46.0) | 21 (53.8) | 0.16 | 33 (39.3) | 28 (50.9) | 0.18 |
| G3-G4 | 33 (66.0) | 27 (54.0) | 18 (46.2) | 51 (60.7) | 27 (49.1) | ||
| stage at therapy start | |||||||
| locally advanced | 9 (18.0) | 8 (16.0) | 7 (17.9) | 0.96 | 15 (17.9) | 9 (16.4) | 0.82 |
| metastatic | 41 (82.0) | 42 (84.0) | 32 (82.1) | 69 (82.1) | 46 (83.6) | ||
| Subtype, no (%) | Subtype, no (%) | ||||||
|---|---|---|---|---|---|---|---|
| KRT81-Positive (n = 164) | Double-Negative (n = 179) | HNF1A-Positive (n = 68) | p-Value (χ2 ) | GATA6 Neg. (n = 169) | GATA6 Pos. (n = 242) | p-Value (χ2 ) | |
| sex | |||||||
| female | 72 (43.9) | 99 (55.3) | 29 (42.6) | 0.06 | 72 (42.6) | 128 (52.9) | 0.04 |
| male | 92 (56.1) | 80 (44.7) | 39 (57.4) | 97 (57.4) | 114 (47.1) | ||
| age group | |||||||
| ≤68 | 91 (55.5) | 93 (52.0) | 29 (42.6) | 0.20 | 73 (43.2) | 125 (51.7) | 0.09 |
| >68 | 73 (44.5) | 86 (48.0) | 39 (57.4) | 96 (56.8) | 117 (48.3) | ||
| treatment arm, no (%) | |||||||
| non-gemcitabinebased | 79 (48.2) | 73 (40.8) | 29 (42.6) | 0.38 | 82 (48.5) | 99 (40.9) | 0.13 |
| gemcitabinebased | 85 (51.8) | 106 (59.2) | 39 (57.4) | 87 (51.5) | 143 (59.1) | ||
| UICC stage (2017) | |||||||
| stage IA | 6 (3.7) | 12 (6.7) | 6 (8.8) | 0.37 | 8 (4.7) | 16 (6.6) | 0.92 |
| stage IB | 33 (20.1) | 32 (17.9) | 16 (23.5) | 33 (19.5) | 48 (19.8) | ||
| stage IIA | 16 (9.8) | 14 (7.8) | 5 (7.4) | 16 (9.5) | 19 (7.9) | ||
| stage IIB | 64 (39.0) | 54 (30.2) | 18 (26.5) | 56 (33.1) | 80 (33.1) | ||
| stage III | 25 (15.2) | 41 (22.9) | 16 (23.5) | 32 (18.9) | 50 (20.7) | ||
| stage IV | 20 (12.2) | 26 (14.5) | 7 (10.3) | 24 (14.2) | 29 (12.0) | ||
| pT (2017) | |||||||
| pT1a | 1 (0.6) | 0 (0.0) | 2 (2.9) | 0.06 | 0 (0.0) | 3 (1.2) | 0.36 |
| pT1b | 1 (0.6) | 4 (2.2) | 0 (0.0) | 2 (1.2) | 3 (1.2) | ||
| pT1c | 11 (6.7) | 26 (14.5) | 8 (11.8) | 14 (8.3) | 31 (12.8) | ||
| pT2 | 104 (63.4) | 99 (55.3) | 40 (58.8) | 106 (62.7) | 137 (56.6) | ||
| pT3 | 46 (28.0) | 46 (25.7) | 18 (26.5) | 46 (27.2) | 64 (26.4) | ||
| pT4 | 1 (0.6) | 4 (2.2) | 0 (0.0) | 1 (0.6) | 4 (1.7) | ||
| pN (2017) | |||||||
| pN0 | 62 (37.8) | 71 (39.7) | 31 (45.6) | 0.18 | 69 (40.8) | 95 (39.3) | 0.88 |
| pN1 | 68 (41.5) | 62 (34.6) | 17 (25.0) | 61 (36.1) | 86 (35.5) | ||
| pN2 | 34 (20.7) | 46 (25.7) | 20 (29.4) | 39 (23.1) | 61 (25.2) | ||
| R-status | |||||||
| 0 | 106 (64.6) | 121 (67.7) | 48 (70.6) | 0.66 | 108 (63.9) | 167 (69.0) | 0.28 |
| 1 | 58 (35.4) | 58 (32.4) | 20 (29.4) | 61 (36.1) | 75 (31.0) | ||
| grade group | |||||||
| G1-G2 | 45 (27.4) | 53 (29.6) | 23 (33.8) | 0.62 | 51 (30.2) | 70 (28.9) | 0.78 |
| G3-G4 | 119 (72.6) | 126 (70.4) | 45 (66.2) | 118 (69.8) | 172 (71.1) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guenther, M.; Surendran, S.A.; Steinke, L.M.; Liou, I.; Palm, M.A.; Heinemann, V.; Haas, M.; Boeck, S.; Ormanns, S. The Prognostic, Predictive and Clinicopathological Implications of KRT81/HNF1A- and GATA6-Based Transcriptional Subtyping in Pancreatic Cancer. Biomolecules 2025, 15, 426. https://doi.org/10.3390/biom15030426
Guenther M, Surendran SA, Steinke LM, Liou I, Palm MA, Heinemann V, Haas M, Boeck S, Ormanns S. The Prognostic, Predictive and Clinicopathological Implications of KRT81/HNF1A- and GATA6-Based Transcriptional Subtyping in Pancreatic Cancer. Biomolecules. 2025; 15(3):426. https://doi.org/10.3390/biom15030426
Chicago/Turabian StyleGuenther, Michael, Sai Agash Surendran, Lea Margareta Steinke, Iduna Liou, Melanie Alexandra Palm, Volker Heinemann, Michael Haas, Stefan Boeck, and Steffen Ormanns. 2025. "The Prognostic, Predictive and Clinicopathological Implications of KRT81/HNF1A- and GATA6-Based Transcriptional Subtyping in Pancreatic Cancer" Biomolecules 15, no. 3: 426. https://doi.org/10.3390/biom15030426
APA StyleGuenther, M., Surendran, S. A., Steinke, L. M., Liou, I., Palm, M. A., Heinemann, V., Haas, M., Boeck, S., & Ormanns, S. (2025). The Prognostic, Predictive and Clinicopathological Implications of KRT81/HNF1A- and GATA6-Based Transcriptional Subtyping in Pancreatic Cancer. Biomolecules, 15(3), 426. https://doi.org/10.3390/biom15030426







