Role of γ-Aminobutyric Acid (GABA) as an Inhibitory Neurotransmitter in Diabetes Management: Mechanisms and Therapeutic Implications
Abstract
1. Introduction
2. Search and Data Collection Strategy
3. Overview and Biosynthesis
3.1. GABA in the Nervous System
3.2. GABA in the Endocrine System
4. Role of GABA in Diabetes Management
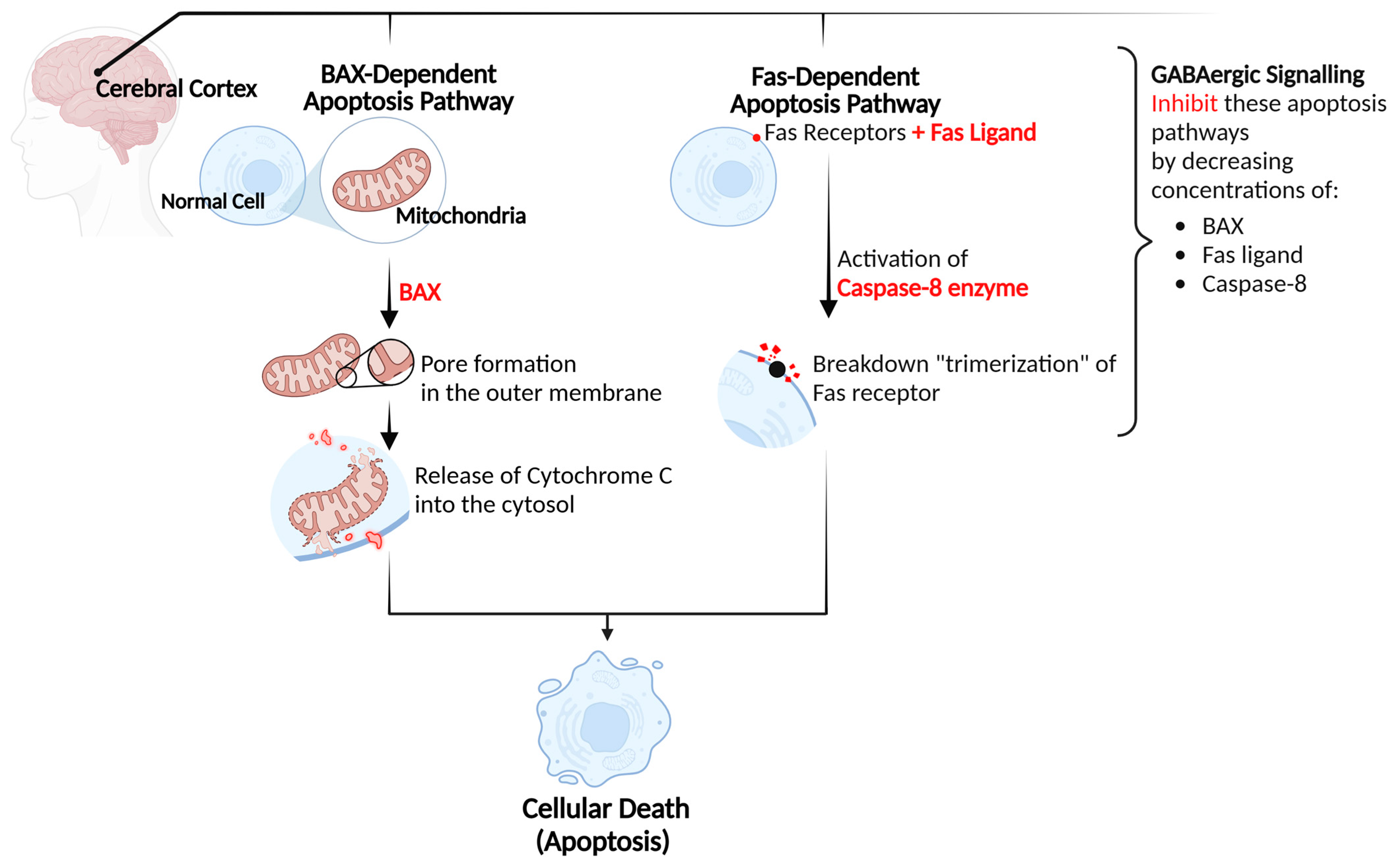
4.1. Neurological Protection
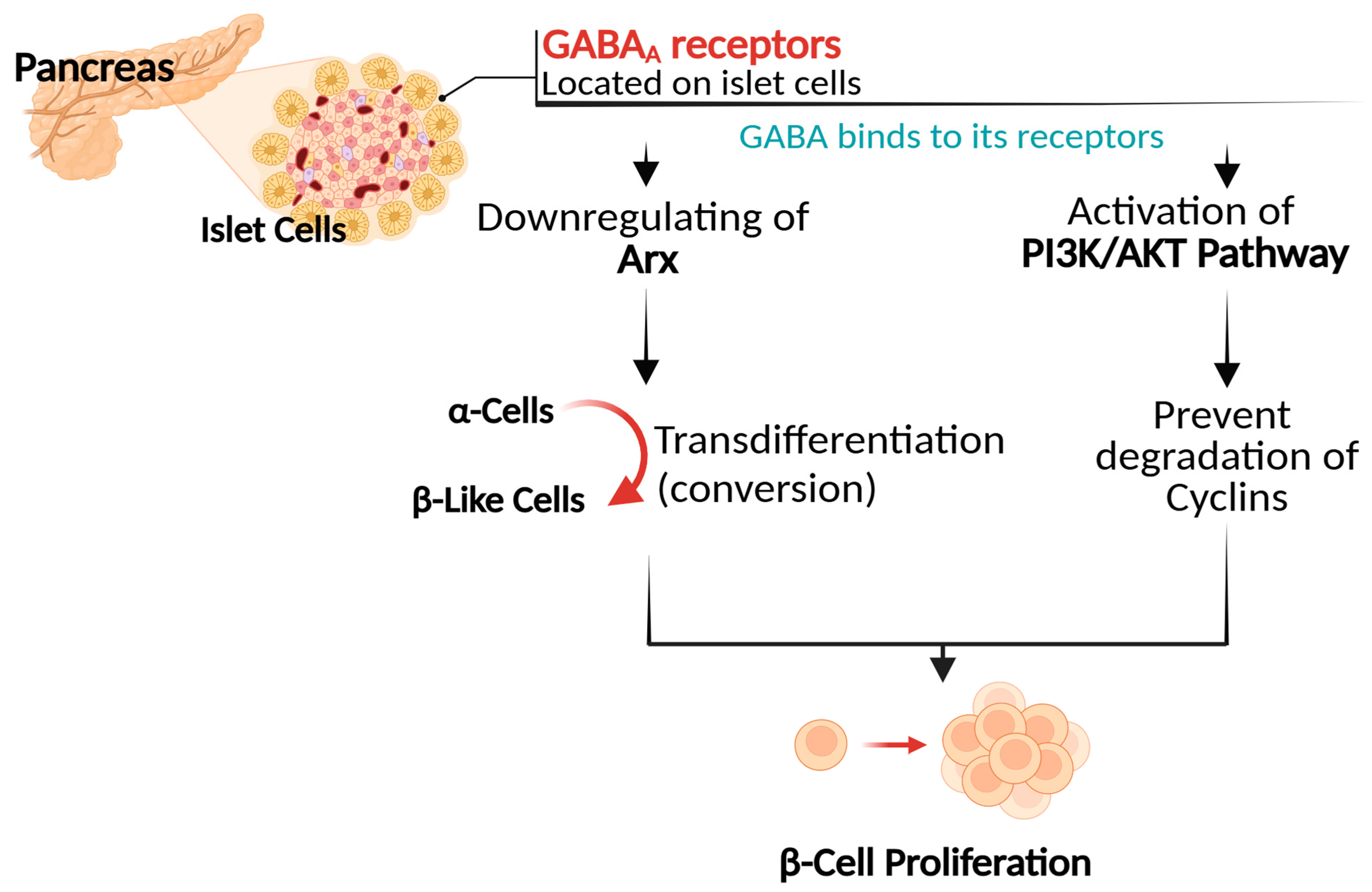
4.2. Promotion of Pancreatic β Cell Proliferation
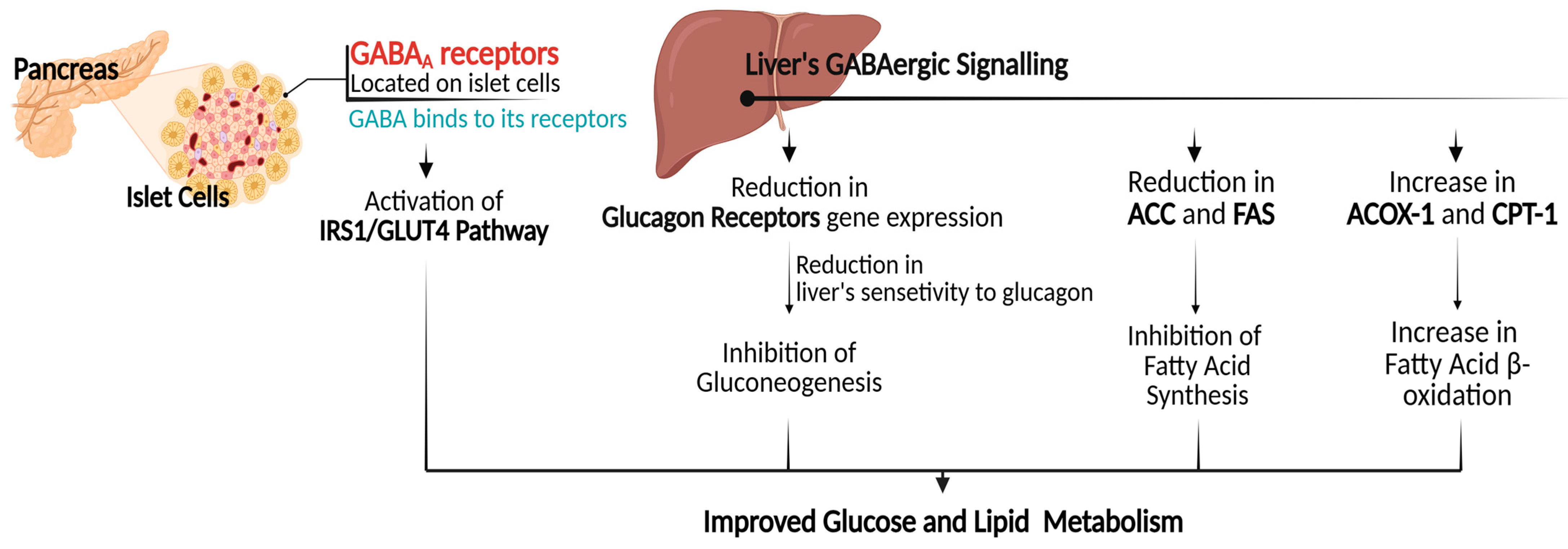
4.3. Gene Expression Regulation in Glucose and Lipid Metabolism
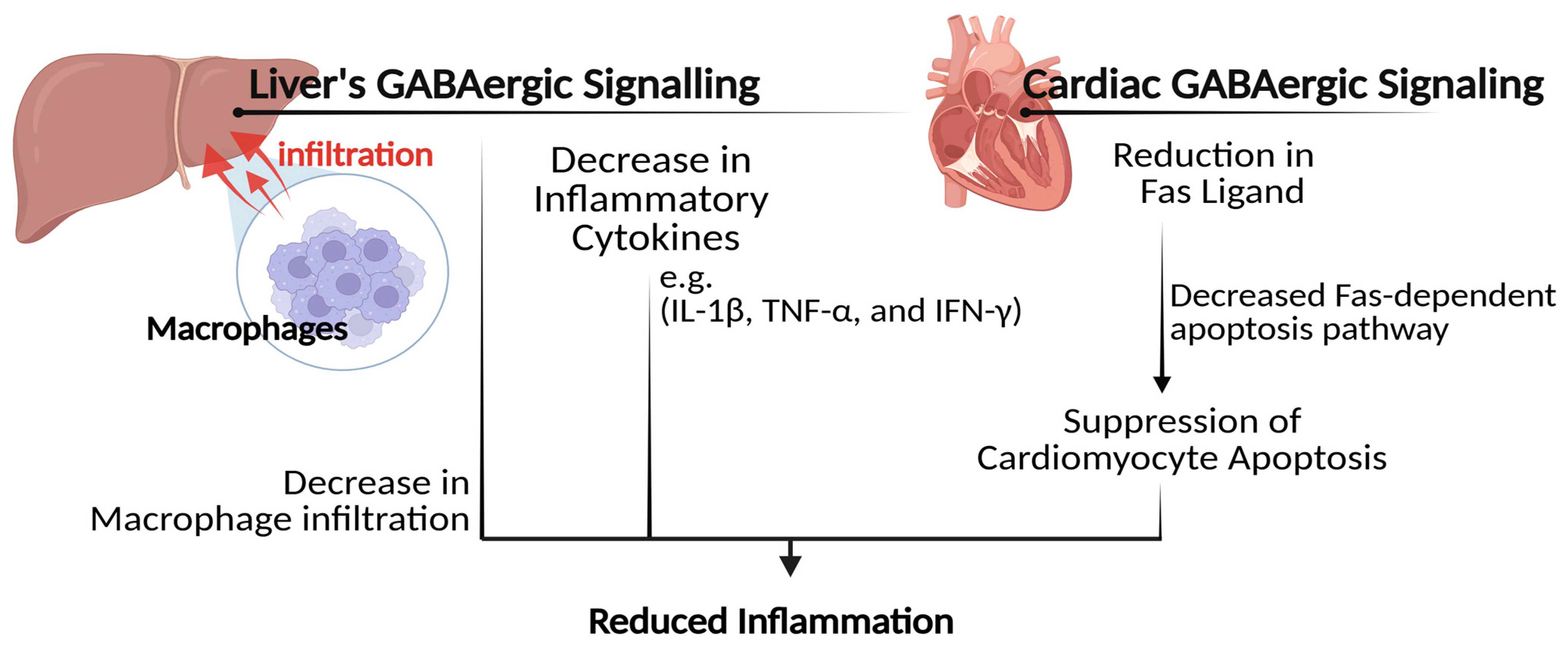
4.4. Inflammation Relief and Immune Response Modulation
4.5. Improvements in Gut Microbiota

5. Sources and Potential Food Applications
| Food Category | Food Item | GABA Content (mg/100 g) | Recommended Daily Intake * |
|---|---|---|---|
| Vegetables | Spinach | 232.10–381.00 | 100–200 (mg day−1) |
| Tomato | 219.86–404.89 | ||
| Sweet potato, cucumber, collard, and eggplant | <34.00 each | ||
| Fruits | Lychee | 170.00–350.00 | |
| Grape | 58.93–109.83 | ||
| Kiwifruit, Jujube fruit, and Mulberry fruit | <34.00 each | ||
| Cocoa Beans | 101.20 | ||
| Legumes | Black soybean | 61.00 | |
| Lupin | 46.00 | ||
| Pseudo-Cereals | Tartary buckwheat | 42.60–112.5 | |
| Quinoa | 7.00–66.10 | ||
| Cereals | Barley | 54.00 | |
| Brown rice | 27.00 | ||
| Wheat and corn | ≤15.50 each | ||
| Red rice, black rice, and millet | <10.00 each | ||
| Edible Mushrooms | Oyster mushroom | 32.15–57.73 | |
| Shiitake mushroom | 17.00–35.00 | ||
| White mushroom | 18.00–20.00 | ||
| Tea | White tea | 3.49–207.00 | |
| Oolong tea, green tea, and black tea | 0.24–97.00 each |
6. Safety Considerations
7. Future Insights
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tallan, H.H.; Moore, S.; Stein, W.H. Studies on the Free Amino Acids and Related Compounds in the Tissues of the Cat. CABI Databases 1954, 211, 927–939. [Google Scholar] [CrossRef]
- Briel, G.; Gylfe, E.; Hellman, B.; Neuhoff, V. Microdetermination of Free Amino Acids in Pancreatic Islets Isolated from Obese-Hyperglycemic Mice. Acta Physiol. Scand. 1972, 84, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Taniguchi, H.; Shimada, C.; Kurosawa, F. High Concentration of Γ-Aminobutyric Acid (Gaba) in the Langerhans’ Islets of the Pancreas. Proc. Jpn. Acad. 1975, 51, 760–762. [Google Scholar] [CrossRef]
- Okada, Y.; Taniguchi, H.; Schimada, C. High Concentration of Gaba and High Glutamate Decarboxylase Activity in Rat Pancreatic Islets and Human Insulinoma. Science 1976, 194, 620–622. [Google Scholar] [CrossRef] [PubMed]
- Hagan, D.W.; Ferreira, S.M.; Santos, G.J.; Phelps, E.A. The Role of Gaba in Islet Function. Front. Endocrinol. 2022, 13, 972115. [Google Scholar] [CrossRef]
- Korol, S.V.; Jin, Z.; Jin, Y.; Bhandage, A.K.; Tengholm, A.; Gandasi, N.R.; Barg, S.; Espes, D.; Carlsson, P.-O.; Laver, D. Functional Characterization of Native, High-Affinity Gabaa Receptors in Human Pancreatic Β Cells. EBioMedicine 2018, 30, 273–282. [Google Scholar] [CrossRef]
- Adeghate, E.; Ponery, A. Gaba in the Endocrine Pancreas: Cellular Localization and Function in Normal and Diabetic Rats. Tissue Cell 2002, 34, 1–6. [Google Scholar] [CrossRef]
- Röder, P.V.; Wu, B.; Liu, Y.; Han, W. Pancreatic Regulation of Glucose Homeostasis. Exp. Mol. Med. 2016, 48, e219. [Google Scholar] [CrossRef]
- Aloke, C.; Egwu, C.O.; Aja, P.M.; Obasi, N.A.; Chukwu, J.; Akumadu, B.O.; Ogbu, P.N.; Achilonu, I. Current Advances in the Management of Diabetes Mellitus. Biomedicines 2022, 10, 2436. [Google Scholar] [CrossRef]
- Galindo, R.J.; Trujillo, J.M.; Wang, C.C.L.; McCoy, R.G. Advances in the Management of Type 2 Diabetes in Adults. BMJ Med. 2023, 2, e000372. [Google Scholar] [CrossRef]
- Ferreira, P.L.; Morais, C.; Pimenta, R.; Ribeiro, I.; Amorim, I.; Alves, S.M.; Santiago, L. Knowledge About Type 2 Diabetes: Its Impact for Future Management. Public Health Front. 2024, 12, 1328001. [Google Scholar] [CrossRef] [PubMed]
- Menge, B.A.; Schrader, H.; Ritter, P.R.; Ellrichmann, M.; Uhl, W.; Schmidt, W.E.; Meier, J.J. Selective Amino Acid Deficiency in Patients with Impaired Glucose Tolerance and Type 2 Diabetes. Regul. Pept. 2010, 160, 75–80. [Google Scholar] [CrossRef]
- Mick, G.J.; McCormick, K.L. The Role of Gaba in Type 1 Diabetes. Front. Endocrinol. 2024, 15, 1453396. [Google Scholar] [CrossRef] [PubMed]
- Khachatryan, N.; Balagyozyan, R.; Balagyozyan, V.; Hekimyan, G.; Grigoryan, G.; Mardanyan, S.; Antonyan, A. Antidiabetic Effect of a Gaba-Supporting Mixture in a Streptozotocin-Induced Diabetes Model in Rats. Rom. J. Diabetes Nutr. Metab. Dis. 2024, 31, 371–378. [Google Scholar]
- Rastegar, S.; Sayyad-Amin, P. Gaba and Oxidative Stress and the Regulation of Antioxidants. In Gaba in Plants; Wiley: Hoboken, NJ, USA, 2025; pp. 225–241. [Google Scholar]
- Rashmi, D.; Zanan, R.; John, S.; Khandagale, K.; Nadaf, A. Γ-Aminobutyric Acid (Gaba): Biosynthesis, Role, Commercial Production, and Applications. Stud. Nat. Prod. Chem. 2018, 57, 413–452. [Google Scholar]
- Jewett, B.E.; Sharma, S. Physiology, GABA. In StatPearls [Internet]; StatPearls Publishing: Treasure Island FL, USA, 2025; p. 30020683. [Google Scholar] [PubMed]
- Ochoa-de la Paz, L.D.; Gulias-Cañizo, R.; Ruíz-Leyja, E.D.; Sánchez-Castillo, H.; Parodí, J. The Role of Gaba Neurotransmitter in the Human Central Nervous System, Physiology, and Pathophysiology. Rev. Mex. Neurocienc. 2021, 22, 67–76. [Google Scholar] [CrossRef]
- Sideraki, A.; Drigas, A. Gaba and Executive Functions in Asd. Sci. Electron. Arch. 2024, 17, 1–14. [Google Scholar] [CrossRef]
- Zhang, B.; Vogelzang, A.; Miyajima, M.; Sugiura, Y.; Wu, Y.; Chamoto, K.; Nakano, R.; Hatae, R.; Menzies, R.J.; Sonomura, K. B Cell-Derived Gaba Elicits Il-10+ Macrophages to Limit Anti-Tumour Immunity. Nature 2021, 599, 471–476. [Google Scholar] [CrossRef]
- Gilon, P.; Bertrand, G.; Loubatieres-Mariani, M.; Remacle, C.; Henquin, J.-C. The Influence of Γ-Aminobutyric Acid on Hormone Release by the Mouse and Rat Endocrine Pancreas. Endocrinology 1991, 129, 2521–2529. [Google Scholar] [CrossRef]
- Wendt, A.; Birnir, B.; Buschard, K.; Gromada, J.; Salehi, A.; Sewing, S.; Rorsman, P.; Braun, M. Glucose Inhibition of Glucagon Secretion from Rat A-Cells Is Mediated by Gaba Released from Neighboring Β-Cells. Diabetes 2004, 53, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Espes, D.; Liljebäck, H.; Hill, H.; Elksnis, A.; Caballero-Corbalan, J.; Birnir, B.; Carlsson, P.-O. Gaba Induces a Hormonal Counter-Regulatory Response in Subjects with Long-Standing Type 1 Diabetes. BMJ Open Diabetes Res. Care 2021, 9, e002442. [Google Scholar] [CrossRef]
- Martin, A.; Mick, G.J.; Choat, H.M.; Lunsford, A.A.; Tse, H.M.; McGwin, G.G., Jr.; McCormick, K.L. A Randomized Trial of Oral Gamma Aminobutyric Acid (Gaba) or the Combination of Gaba with Glutamic Acid Decarboxylase (Gad) on Pancreatic Islet Endocrine Function in Children with Newly Diagnosed Type 1 Diabetes. Nat. Commun. 2022, 13, 7928. [Google Scholar] [CrossRef]
- Cherng, S.-H.; Huang, C.-Y.; Kuo, W.-W.; Lai, S.-E.; Tseng, C.-Y.; Lin, Y.-M.; Tsai, F.-J.; Wang, H.-F. Gaba Tea Prevents Cardiac Fibrosis by Attenuating Tnf-Alpha and Fas/Fasl-Mediated Apoptosis in Streptozotocin-Induced Diabetic Rats. Food Chem. Toxicol. 2014, 65, 90–96. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Kuo, W.-W.; Wang, H.-F.; Lin, C.-J.; Lin, Y.-M.; Chen, J.-L.; Kuo, C.-H.; Chen, P.-K.; Lin, J.-Y. Gaba Tea Ameliorates Cerebral Cortex Apoptosis and Autophagy in Streptozotocin-Induced Diabetic Rats. J. Funct. Foods 2014, 6, 534–544. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, H.; Zhang, C.; Lu, Y.; Zhu, X.; Lu, Z. Γ-Aminobutyric Acid-Rich Yogurt Fermented by Streptococcus Salivarius Subsp. Thermophiles Fmb5 Apprars to Have Anti-Diabetic Effect on Streptozotocin-Induced Diabetic Mice. J. Funct. Foods 2016, 20, 267–275. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Zhu, X.; Lu, Z.; Lu, Y. Effect of Γ-Aminobutyric Acid-Rich Yogurt on Insulin Sensitivity in a Mouse Model of Type 2 Diabetes Mellitus. J. Dairy Sci. 2020, 103, 7719–7729. [Google Scholar] [CrossRef] [PubMed]
- Chaiyasut, C.; Woraharn, S.; Sivamaruthi, B.S.; Lailerd, N.; Kesika, P.; Peerajan, S. Lactobacillus Fermentum Hp3–Mediated Fermented Hericium Erinaceus Juice as a Health Promoting Food Supplement to Manage Diabetes Mellitus. J. Evid. Based Integr. Med. 2018, 23, 2515690X18765699. [Google Scholar] [CrossRef]
- Abdelazez, A.; Alshehry, G.; Algarni, E.; Al Jumayi, H.; Abdel-Motaal, H.; Meng, X.-C. Postbiotic Gamma-Aminobutyric Acid and Camel Milk Intervention as Innovative Trends against Hyperglycemia and Hyperlipidemia in Streptozotocin-Induced C57bl/6j Diabetic Mice. Front. Microbiol. 2022, 13, 943930. [Google Scholar] [CrossRef]
- Shang, W.; Si, X.; Zhou, Z.; Strappe, P.; Blanchard, C. Wheat Bran with Enriched Gamma-Aminobutyric Acid Attenuates Glucose Intolerance and Hyperinsulinemia Induced by a High-Fat Diet. Food Funct. 2018, 9, 2820–2828. [Google Scholar] [CrossRef]
- Zhang, A.; Jiang, X.; Ge, Y.; Xu, Q.; Li, Z.; Tang, H.; Cao, D.; Zhang, D. The Effects of Gaba-Rich Adzuki Beans on Glycolipid Metabolism, as Well as Intestinal Flora, in Type 2 Diabetic Mice. Front. Nutr. 2022, 9, 849529. [Google Scholar] [CrossRef]
- Chen, Q.; Gao, Y.; Yang, F.; Deng, H.; Wang, Y.; Yuan, L. Angiotensin-Converting Enzyme 2 Improves Hepatic Insulin Resistance by Regulating Gabaergic Signaling in the Liver. J. Biol. Chem. 2022, 298, 102603. [Google Scholar] [CrossRef]
- Sarnobat, D.; Moffett, R.C.; Flatt, P.R.; Irwin, N.; Tarasov, A.I. Gaba and Insulin but Not Nicotinamide Augment A-to Β-Cell Transdifferentiation in Insulin-Deficient Diabetic Mice. Biochem. Pharmacol. 2022, 199, 115019. [Google Scholar] [CrossRef]
- Ben-Othman, N.; Vieira, A.; Courtney, M.; Record, F.; Gjernes, E.; Avolio, F.; Hadzic, B.; Druelle, N.; Napolitano, T.; Navarro-Sanz, S. Long-Term Gaba Administration Induces Alpha Cell-Mediated Beta-Like Cell Neogenesis. Cell 2017, 168, 73–85.e11. [Google Scholar] [CrossRef] [PubMed]
- Rezazadeh, H.; Sharifi, M.R.; Sharifi, M.; Soltani, N. Gamma-Aminobutyric Acid Attenuates Insulin Resistance in Type 2 Diabetic Patients and Reduces the Risk of Insulin Resistance in Their Offspring. Biomed. Pharmacother. 2021, 138, 111440. [Google Scholar] [CrossRef] [PubMed]
- Hosseini Dastgerdi, A.; Sharifi, M.; Soltani, N. Gaba Administration Improves Liver Function and Insulin Resistance in Offspring of Type 2 Diabetic Rats. Sci. Rep. 2021, 11, 23155. [Google Scholar] [CrossRef] [PubMed]
- Sohrabipour, S.; Sharifi, M.R.; Talebi, A.; Sharifi, M.; Soltani, N. Gaba Dramatically Improves Glucose Tolerance in Streptozotocin-Induced Diabetic Rats Fed with High-Fat Diet. Eur. J. Pharmacol. 2018, 826, 75–84. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zhang, Y.; Wang, D.; Tao, K.; Zhang, S.; Wei, L.; Chen, Q. Rapamycin/Gaba Combination Treatment Ameliorates Diabetes in Nod Mice. Mol. Immunol. 2016, 73, 130–137. [Google Scholar] [CrossRef]
- Wang, S.; Luo, Y.; Feng, A.; Li, T.; Yang, X.; Nofech-Mozes, R.; Yu, M.; Wang, C.; Li, Z.; Yi, F. Ethanol Induced Impairment of Glucose Metabolism Involves Alterations of Gabaergic Signaling in Pancreatic Β-Cells. Toxicology 2014, 326, 44–52. [Google Scholar] [CrossRef]
- Larsson, M.; Lietzau, G.; Nathanson, D.; Östenson, C.-G.; Mallard, C.; Johansson, M.E.; Nyström, T.; Patrone, C.; Darsalia, V. Diabetes Negatively Affects Cortical and Striatal Gabaergic Neurons: An Effect That Is Partially Counteracted by Exendin-4. Biosci. Rep. 2016, 36, e00421. [Google Scholar] [CrossRef]
- Zheng, H.; Zheng, Y.; Zhao, L.; Chen, M.; Bai, G.; Hu, Y.; Hu, W.; Yan, Z.; Gao, H. Cognitive Decline in Type 2 Diabetic Db/Db Mice May Be Associated with Brain Region-Specific Metabolic Disorders. Biochim. Biophys. Acta 2017, 1863, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, J.; Zhao, S.; Huang, L. Diabetic Encephalopathy Causes the Imbalance of Neural Activities between Hippocampal Glutamatergic Neurons and Gabaergic Neurons in Mice. Brain Res. 2020, 1742, 146863. [Google Scholar] [CrossRef]
- Ramsey, D.J.; Ripps, H.; Qian, H. Streptozotocin-Induced Diabetes Modulates Gaba Receptor Activity of Rat Retinal Neurons. Exp. Eye Res. 2007, 85, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Sherin, A.; Peeyush, K.; Naijil, G.; Chinthu, R.; Paulose, C. Hypoglycemia Induced Behavioural Deficit and Decreased Gaba Receptor, Creb Expression in the Cerebellum of Streptozoticin Induced Diabetic Rats. Brain Res. Bul. 2010, 83, 360–366. [Google Scholar] [CrossRef]
- Wu, S.; Wang, L.; Fang, Y.; Huang, H.; You, X.; Wu, J. Advances in Encapsulation and Delivery Strategies for Islet Transplantation. Adv. Healthc. Mater. 2021, 10, 2100965. [Google Scholar] [CrossRef]
- Du, H.; Xie, C.; Yuan, Y.; Luo, Y.; Cao, J.; Li, Z.; Yuan, J.; Li, W. Efficacy and Safety of Stem Cell Therapy for Diabetic Kidney Disease: A Systematic Review and Meta-Analysis. medRxiv 2024, 24316903. [Google Scholar]
- Mahajan, P.V.; Morey, A.; Subramanian, S.; Bandre, A.; Ware, H. Management of Type 1 Diabetes Mellitus with Autologous Mesenchymal Stem Cells. CHRISMED J. Health Res. 2019, 6, 64–66. [Google Scholar] [CrossRef]
- Morales, N.B.; de Plata, C.A. Role of Akt/Mtorc1 Pathway in Pancreatic Β-Cell Proliferation. Colomb. Med. 2012, 43, 235. [Google Scholar] [CrossRef]
- Camaya, I.; Donnelly, S.; O’Brien, B. Targeting the Pi3k/Akt Signaling Pathway in Pancreatic Β-Cells to Enhance Their Survival and Function: An Emerging Therapeutic Strategy for Type 1 Diabetes. J. Diabetes 2022, 14, 247–260. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, Q.; Zhou, Z.; Ikeda, Y. Pdx1, Neurogenin-3, and Mafa: Critical Transcription Regulators for Beta Cell Development and Regeneration. Stem Cell Res. Ther. 2017, 8, 1–7. [Google Scholar] [CrossRef]
- Khan, A.; Pessin, J. Insulin Regulation of Glucose Uptake: A Complex Interplay of Intracellular Signalling Pathways. Diabetologia 2002, 45, 1475–1483. [Google Scholar] [PubMed]
- van Gerwen, J.; Shun-Shion, A.S.; Fazakerley, D.J. Insulin Signalling and Glut4 Trafficking in Insulin Resistance. Biochem. Soc. Trans. 2023, 51, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.; Tang, X.; Bai, X.; Xiong, H. Fgf19 Promotes the Proliferation and Insulin Secretion from Human Pancreatic Β Cells Via the Irs1/Glut4 Pathway. Exp. Clin. Endocrinol. Diabetes 2024, 132, 152–161. [Google Scholar] [CrossRef]
- Boden, G.; Chen, X.; Stein, T.P. Gluconeogenesis in Moderately and Severely Hyperglycemic Patients with Type 2 Diabetes Mellitus. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E23–E30. [Google Scholar] [CrossRef]
- Winther-Sørensen, M.; Holst, J.J.; Albrechtsen, N.J.W. The Feedback Cycles between Glucose, Amino Acids and Lipids and Alpha Cell Secretion and Their Role in Metabolic Fatty Liver Disease. Curr. Opin. Lipidol. 2023, 34, 27–31. [Google Scholar] [CrossRef]
- Berndt, J.; Kovacs, P.; Ruschke, K.; Klöting, N.; Fasshauer, M.; Schön, M.R.; Körner, A.; Stumvoll, M.; Blüher, M. Fatty Acid Synthase Gene Expression in Human Adipose Tissue: Association with Obesity and Type 2 Diabetes. Diabetologia 2007, 50, 1472–1480. [Google Scholar] [CrossRef] [PubMed]
- Groeger, M.; Matsuo, K.; Heidary Arash, E.; Pereira, A.; Le Guillou, D.; Pino, C.; Telles-Silva, K.A.; Maher, J.J.; Hsiao, E.C.; Willenbring, H. Modeling and Therapeutic Targeting of Inflammation-Induced Hepatic Insulin Resistance Using Human Ipsc-Derived Hepatocytes and Macrophages. Nat. Commun. 2023, 14, 3902. [Google Scholar] [CrossRef]
- Abdullah, Z.; Knolle, P.A. Liver Macrophages in Healthy and Diseased Liver. Pflüg. Arch.-Eur. J. Physiol. 2017, 469, 553–560. [Google Scholar] [CrossRef]
- Sica, A.; Invernizzi, P.; Mantovani, A. Macrophage Plasticity and Polarization in Liver Homeostasis and Pathology. Hepatology 2014, 59, 2034–2042. [Google Scholar] [CrossRef]
- Cnop, M.; Welsh, N.; Jonas, J.-C.; Jorns, A.; Lenzen, S.; Eizirik, D.L. Mechanisms of Pancreatic Β-Cell Death in Type 1 and Type 2 Diabetes: Many Differences, Few Similarities. Diabetes 2005, 54, S97–S107. [Google Scholar] [CrossRef]
- von Herrath, M.G.; Oldstone, M.B. Interferon-Γ Is Essential for Destruction of Β Cells and Development of Insulin-Dependent Diabetes Mellitus. J. Exp. Med. 1997, 185, 531–540. [Google Scholar] [CrossRef]
- Senn, J.J.; Klover, P.J.; Nowak, I.A.; Mooney, R.A. Interleukin-6 Induces Cellular Insulin Resistance in Hepatocytes. Diabetes 2002, 51, 3391–3399. [Google Scholar] [CrossRef] [PubMed]
- Kurauti, M.A.; Costa-Júnior, J.M.; Ferreira, S.M.; Santos, G.J.; Sponton, C.H.; Carneiro, E.M.; Telles, G.D.; Chacon-Mikahil, M.P.; Cavaglieri, C.R.; Rezende, L.F. Interleukin-6 Increases the Expression and Activity of Insulin-Degrading Enzyme. Sci. Rep. 2017, 7, 46750. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, H.-l.; Lan, H.-y. Tgf-Β Signaling in Diabetic Nephropathy: An Update. Diabet. Nephrop. 2022, 2, 7–16. [Google Scholar] [CrossRef]
- Novianti, Y.; Nur’aeny, N. Exploring Interleukin-10 Levels in Diabetes Patients with and without Oral Diseases: A Systematic Review. J. Inflamm. Res. 2024, 17, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Bhandage, A.K.; Jin, Z.; Korol, S.V.; Shen, Q.; Pei, Y.; Deng, Q.; Espes, D.; Carlsson, P.-O.; Kamali-Moghaddam, M.; Birnir, B. Gaba Regulates Release of Inflammatory Cytokines from Peripheral Blood Mononuclear Cells and Cd4+ T Cells and Is Immunosuppressive in Type 1 Diabetes. EBioMedicine 2018, 30, 283–294. [Google Scholar] [CrossRef]
- Braga, J.D.; Thongngam, M.; Kumrungsee, T. Gamma-Aminobutyric Acid as a Potential Postbiotic Mediator in the Gut–Brain Axis. NPJ Sci. Food. 2024, 8, 16. [Google Scholar] [CrossRef]
- Bielka, W.; Przezak, A.; Pawlik, A. The Role of the Gut Microbiota in the Pathogenesis of Diabetes. Int. J. Mol. Sci. 2022, 23, 480. [Google Scholar] [CrossRef]
- Patterson, E.; Ryan, P.M.; Cryan, J.F.; Dinan, T.G.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Gut Microbiota, Obesity and Diabetes. Postgrad. Med. J. 2016, 92, 286–300. [Google Scholar] [CrossRef]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of Gut Microbiota in Type 2 Diabetes Pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef]
- Aljutaily, T.; Barakat, H.; Moustafa, M.M.A.; Rehan, M. Incorporation of Sukkari Date in Probiotic-Enriched Fermented Camel Milk Improves the Nutritional, Physicochemical, and Organoleptical Characteristics. Fermentation 2022, 8, 5. [Google Scholar] [CrossRef]
- Marques, T.M.; Patterson, E.; Wall, R.; O’Sullivan, O.; Fitzgerald, G.F.; Cotter, P.D.; Dinan, T.G.; Cryan, J.F.; Ross, R.P.; Stanton, C. Influence of Gaba and Gaba-Producing Lactobacillus Brevis Dpc 6108 on the Development of Diabetes in a Streptozotocin Rat Model. Benef. Microbes 2016, 7, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, J.; Wang, H.; Huang, Y.; Qi, M.; Liao, S.; Bin, P.; Yin, Y. Effects of Gaba Supplementation on Intestinal Siga Secretion and Gut Microbiota in the Healthy and Etec-Infected Weanling Piglets. Mediat. Inflamm. 2020, 2020, 7368483. [Google Scholar] [CrossRef]
- Wilkins, L.J.; Monga, M.; Miller, A.W. Defining Dysbiosis for a Cluster of Chronic Diseases. Sci. Rep. 2019, 9, 12918. [Google Scholar] [CrossRef]
- Hou, D.; Tang, J.; Feng, Q.; Niu, Z.; Shen, Q.; Wang, L.; Zhou, S. Gamma-Aminobutyric Acid (Gaba): A Comprehensive Review of Dietary Sources, Enrichment Technologies, Processing Effects, Health Benefits, and Its Applications. Crit. Rev. Food Sci. Nutr. 2024, 64, 8852–8874. [Google Scholar] [CrossRef] [PubMed]
- Icer, M.A.; Sarikaya, B.; Kocyigit, E.; Atabilen, B.; Çelik, M.N.; Capasso, R.; Ağagündüz, D.; Budán, F. Contributions of Gamma-Aminobutyric Acid (Gaba) Produced by Lactic Acid Bacteria on Food Quality and Human Health: Current Applications and Future Prospects. Foods 2024, 13, 2437. [Google Scholar] [CrossRef]
- Alharbi, Y.M.; Sakr, S.S.; Albarrak, S.M.; Almundarij, T.I.; Barakat, H.; Hassan, M.F. Antioxidative, Antidiabetic, and Hypolipidemic Properties of Probiotic-Enriched Fermented Camel Milk Combined with Salvia Officinalis Leaves Hydroalcoholic Extract in Streptozotocin-Induced Diabetes in Rats. Antioxidants 2022, 11, 668. [Google Scholar] [CrossRef]
- Kim, S.; Jo, K.; Hong, K.-B.; Han, S.H.; Suh, H.J. Gaba and L-Theanine Mixture Decreases Sleep Latency and Improves Nrem Sleep. Pharm. Biol. 2019, 57, 64–72. [Google Scholar] [CrossRef]
- Ruggiero, M. Effects of a Topical Composition of Gaba and Microbial Chondroitin Sulfate on Mental Calmness and Brain Function. Preprint 2024. [Google Scholar] [CrossRef]
- Guimarães, A.P.; Seidel, H.; Pires, L.V.d.M.; Trindade, C.O.; Baleeiro, R.d.S.; Souza, P.M.d.; Silva, F.G.D.e.; Coelho, D.B.; Becker, L.K.; Oliveira, E.C.d. Gaba Supplementation, Increased Heart-Rate Variability, Emotional Response, Sleep Efficiency and Reduced Depression in Sedentary Overweight Women Undergoing Physical Exercise: Placebo-Controlled, Randomized Clinical Trial. J. Diet. Suppl. 2024, 21, 512–526. [Google Scholar] [CrossRef]
- Amsterdam, V.J.; Brunt, T.; McMaster, M.; Van den Brink, W. Neurotoxicity Due to Repeated Comas Following Excessive Use of Gamma-Hydroxybutyric Acid. In Neuropathology of Drug Addictions and Substance Misuse; Elsevier: Amsterdam, The Netherlands, 2016; pp. 453–459. [Google Scholar]
- Bäckström, T.; Das, R.; Bixo, M. Positive Gabaa Receptor Modulating Steroids and Their Antagonists: Implications for Clinical Treatments. J. Neuroendocrinol. 2022, 34, e13013. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Byun, J.-I.; Shin, W.C. Efficacy and Safety of Low-Dose Gamma-Aminobutyric Acid from Unpolished Rice Germ as a Health Functional Food for Promoting Sleep: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Clin. Neurol. 2022, 18, 478. [Google Scholar] [CrossRef] [PubMed]
- West, B. Dietary Gamma-Aminobutyric Acid (Gaba) Health Benefits: A Mini-Review. OSF Prepr. 2023. [Google Scholar] [CrossRef]
| Study Design | Subjects Characteristics | GABA Treatment | Main Outcomes | Reference |
|---|---|---|---|---|
| Clinical trial (11-day duration) | Adult male individuals with long-standing T1D (n = 6; ave age 24.8 years; disease duration 14.7 years) | Oral administration of GABA tablet (Remygen) at escalating doses (200, 600, and 1200 mg) as a single daily dose while fasting, for three consecutive days. |
| Espes et al. [23] |
| One-year randomized, double-blind, placebo-controlled trial | Children with newly diagnosed T1D; (n = 97; age 4–18 years) | Oral administration of GABA capsules or placebo at 1.5 g/day, divided into two daily doses (morning and evening) + injections of GAD or placebo (20 μg/dose) twice a day. |
| Martin et al. [24] |
| Animal trial | Streptozotocin-induced male Wistar rats (n = 12) | Oral administration of GABA tea extract at two doses, low and high (4.55 and 45.5 mg/kg BW), in 0.2 mL distilled water daily for six weeks. |
| Cherng et al. [25] |
| Animal trial | Streptozotocin-induced male Wistar rats (n = 9) | Intragastrical administration of GABA tea extract at two doses, low and high (3.01 and 30.1 µg/rat per day) for six weeks. |
| Huang et al. [26] |
| Animal trial | Streptozotocin-induced male C57BL/6 mice (n = 6) | Oral administration of GABA-rich yogurt fermented by Streptococcus thermophilus at three doses: (1, 2 and 4 g/L) for six weeks. |
| Chen et al. [27] |
| Animal trial | HFD-fed + streptozotocin-induced male C57BL/6 mice (n = 8) | Oral administration of GABA-rich yogurt fermented by Streptococcus thermophilus at three doses: (0.5, 1, and 2 g/L) daily for 12 weeks. |
| Li et al. [28] |
| Animal trial | Streptozotocin-induced male Wistar rats (n = 10) | Oral administration of fermented Hericium erinaceus Juice (GABA dose: 1.27 g/kg) daily for 12 weeks (pre- and post-treatment). |
| Chaiyasut et al. [29] |
| Animal trial | Streptozotocin-induced C57BL/6 mice (n = 8) | Intragastrical administration of two Lactobacillus brevis strains at 250 μL × 105 CFU/mL/day (GABA conc. 0.05–1.98 g/L) for 4 weeks. |
| Abdelazez et al. [30] |
| Animal trial | HFD-fed male Sprague–Dawley rats (n = 5) | 15% of GABA-enriched wheat bran incorporated into the daily HFD for 8 weeks. |
| Shang et al. [31] |
| Animal trial | HFD-fed + streptozotocin-induced male C57BL/6 mice (n = 8) | GABA-rich sprouted adzuki beans were incorporated into the daily HFD at 15, 25, and 35 g/100 g for 6 weeks. |
| Zhang et al. [32] |
| Animal trial | HFD–induced hyperinsulinemia in male C57BL/6 mice (n = 5) | Oral administration of GABA at 6 mg/mL in drinking water for 4 weeks. |
| Chen et al. [33] |
| Animal trial | Streptozotocin-induced C57BL/6 mice (n = 6) | Intraperitoneal injection of GABA at 10 mg/kg per day for 10 days. |
| Sarnobat et al. [34] |
| Animal trial | Streptozotocin-induced wild-type mice (n = 6) | Intraperitoneal injection of GABA at 250 mg/kg daily for 6 months. |
| Ben-Othman et al. [35] |
| Animal trial | HFD-fed + streptozotocin-induced male and female Wistar rats (n = 8) | Intraperitoneal injection of GABA at 1.5 g/kg per day during mating, pregnancy, and breastfeeding. |
| Rezazadeh et al. [36] |
| Animal trial | HFD-fed + streptozotocin-induced male and female Wistar rats (n = 7) | Intraperitoneal injection of GABA at 1.5 g/kg per day + HFD feeding. |
| Hosseini Dastgerdi et al. [37] |
| Animal trial | HFD-fed + streptozotocin-induced male Wistar rats (n = 10) | Intraperitoneal injection of GABA at 1.5 g/kg BW per day for three months. |
| Sohrabipour et al. [38] |
| Animal trial | NOD mice (n = 21–26) | Intragastrical administration of GABA at 1 mmol/kg (twice a day) and/or Rapamycin at 1 mg/kg (once a day) for 12 weeks. |
| He et al. [39] |
| Experimental in vitro study | Ethanol-induced rat pancreatic β-cells (INS-1 cells) | Exposure to GABA at 100 μM for 2 h. |
| Wang et al. [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barakat, H.; Aljutaily, T. Role of γ-Aminobutyric Acid (GABA) as an Inhibitory Neurotransmitter in Diabetes Management: Mechanisms and Therapeutic Implications. Biomolecules 2025, 15, 399. https://doi.org/10.3390/biom15030399
Barakat H, Aljutaily T. Role of γ-Aminobutyric Acid (GABA) as an Inhibitory Neurotransmitter in Diabetes Management: Mechanisms and Therapeutic Implications. Biomolecules. 2025; 15(3):399. https://doi.org/10.3390/biom15030399
Chicago/Turabian StyleBarakat, Hassan, and Thamer Aljutaily. 2025. "Role of γ-Aminobutyric Acid (GABA) as an Inhibitory Neurotransmitter in Diabetes Management: Mechanisms and Therapeutic Implications" Biomolecules 15, no. 3: 399. https://doi.org/10.3390/biom15030399
APA StyleBarakat, H., & Aljutaily, T. (2025). Role of γ-Aminobutyric Acid (GABA) as an Inhibitory Neurotransmitter in Diabetes Management: Mechanisms and Therapeutic Implications. Biomolecules, 15(3), 399. https://doi.org/10.3390/biom15030399









