Evaluation of Biocompatible Materials for Enhanced Mesenchymal Stem Cell Expansion: Collagen-Coated Alginate Microcarriers and PLGA Nanofibers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Alginate Microcarriers Production
2.3. Polylactic-Co-Glycolic Acid (PLGA) Nanofibers Production
2.4. Collagen Coating and Disinfection Procedure
2.5. Alginate Microcarriers and PLGA Nanofiber Physicochemical and Structural Characterization
2.6. Antibiotic and Serum-Free Mesenchymal Stem Cell Primary Culture and Subculture
2.7. Flow Cytometry Porcine bmMSC Characterization
2.8. Mycoplasma Testing
2.9. Alginate Microcarriers and PLGA Nanofiber Growth Kinetics for Biological Characterization
2.10. Cell Number-Absorbance Standard Curve
2.11. Alginate Microcarriers and PLGA Nanofiber Scanning Electron Microscopy (SEM)
2.12. Biocompatibility Test
2.13. Statistical Analysis
3. Results
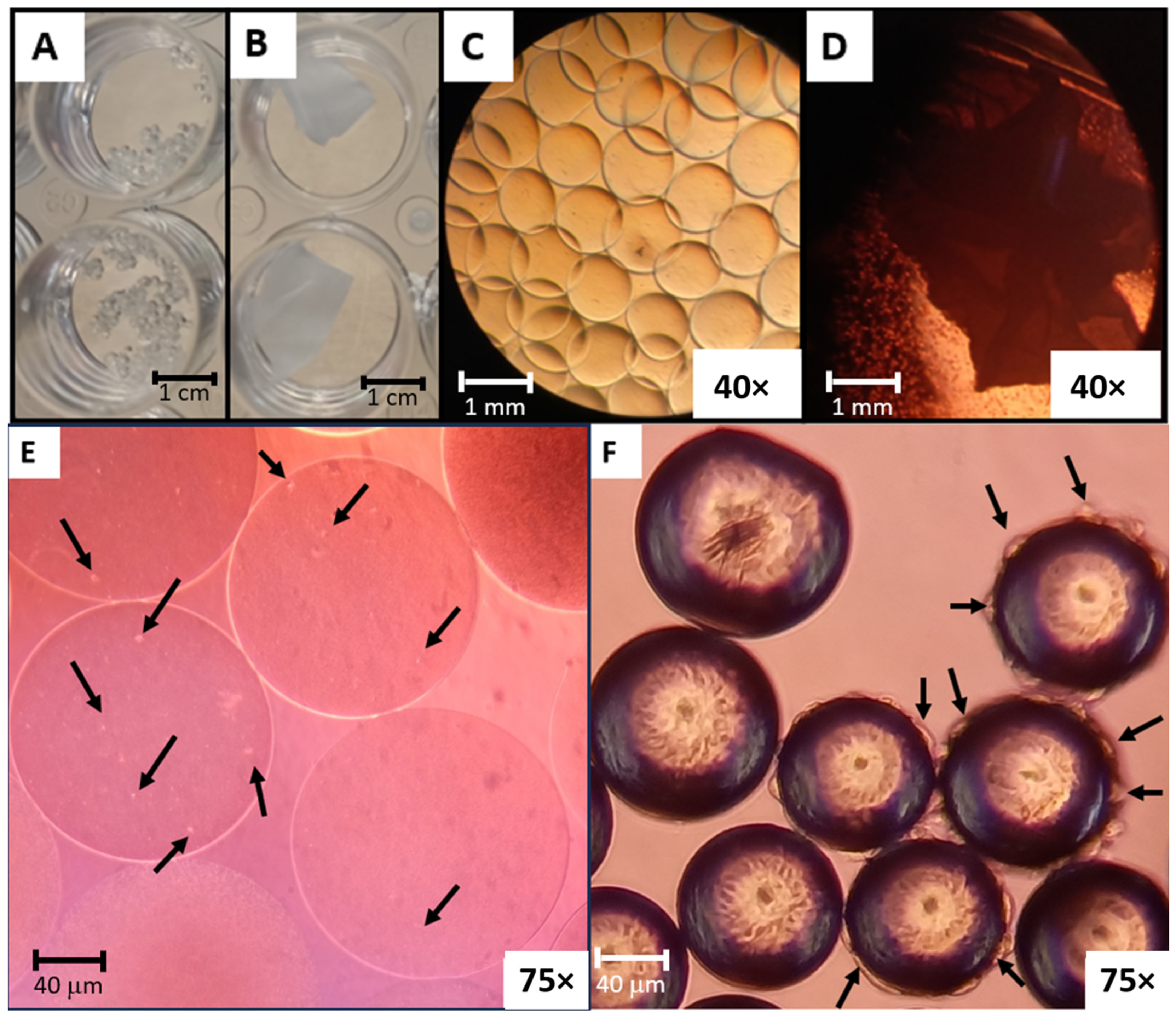
3.1. Alginate Microcarriers and Polylactic-Co-Glycolic Acid (PLGA) Nanofibers Production
3.2. Disinfected and Collagen-Coated Alginate Microcarriers and PLGA Nanofibers Production
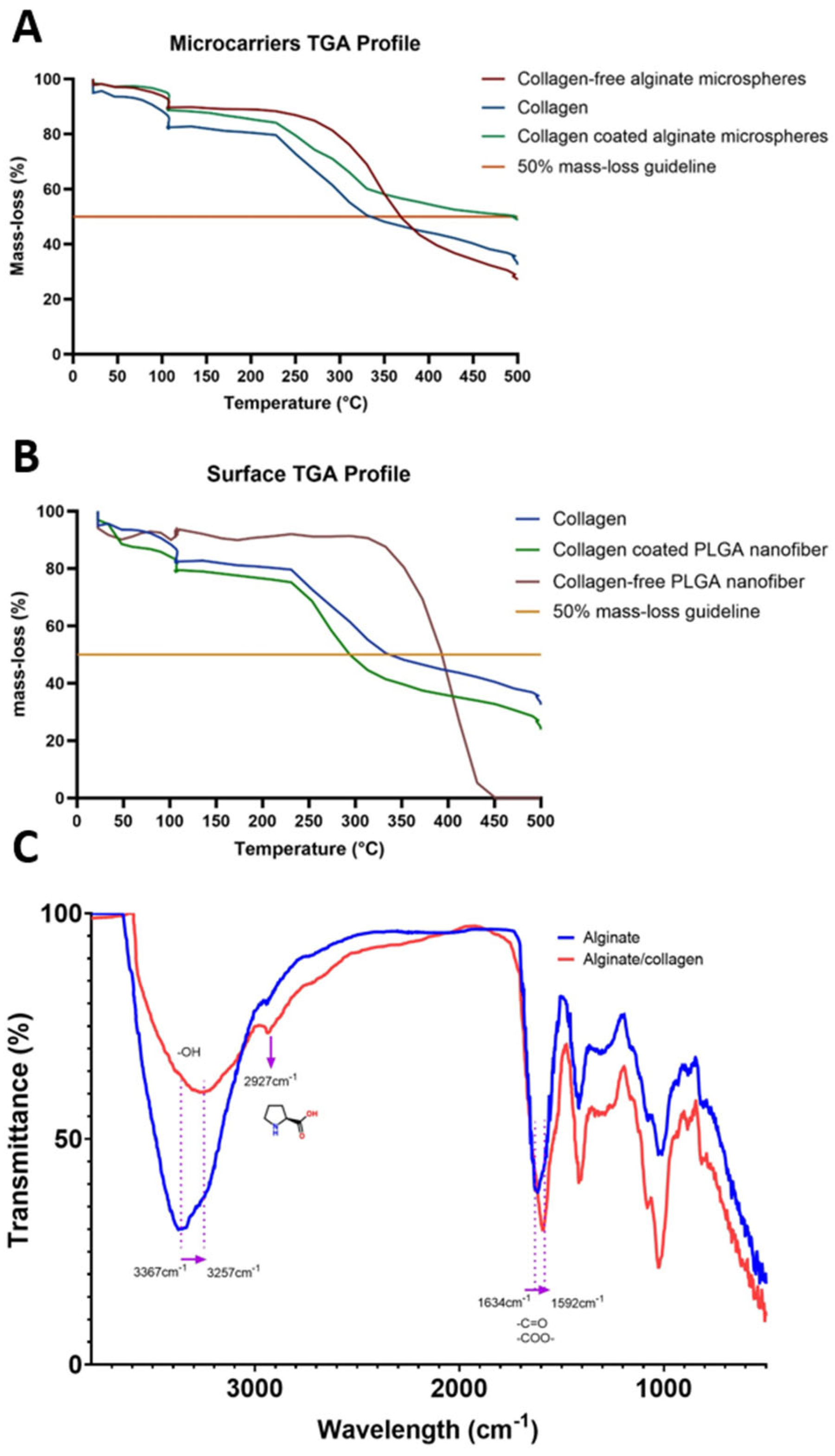
3.3. Alginate Microcarriers and PLGA Nanofibers Physicochemical and Structural Characterization
3.4. Antibiotic and Serum-Free Mesenchymal Stem Cell Primary Culture and Flow Cytometry Characterization
3.5. Alginate Microcarriers and PLGA Nanofiber Growth Kinetics
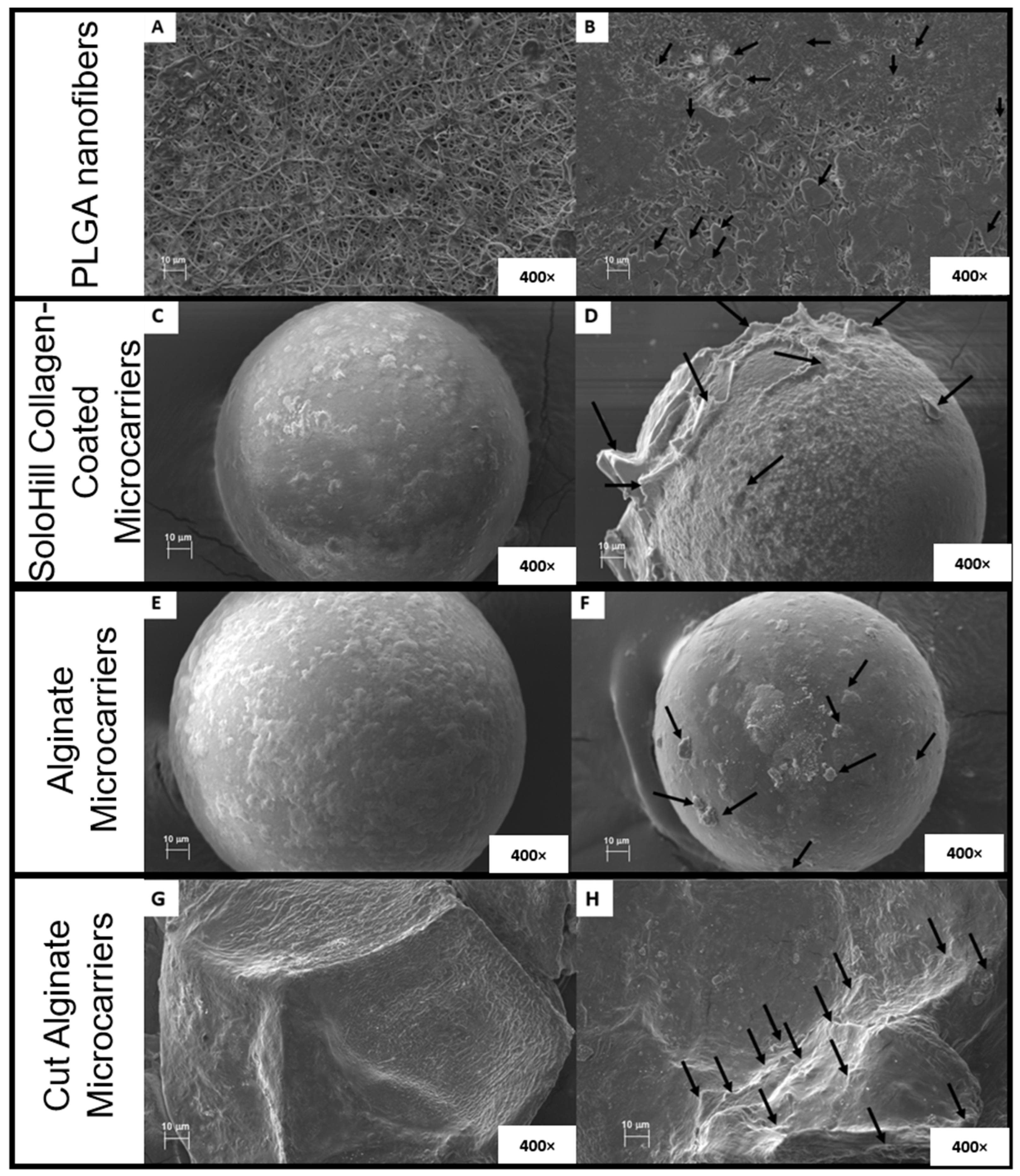
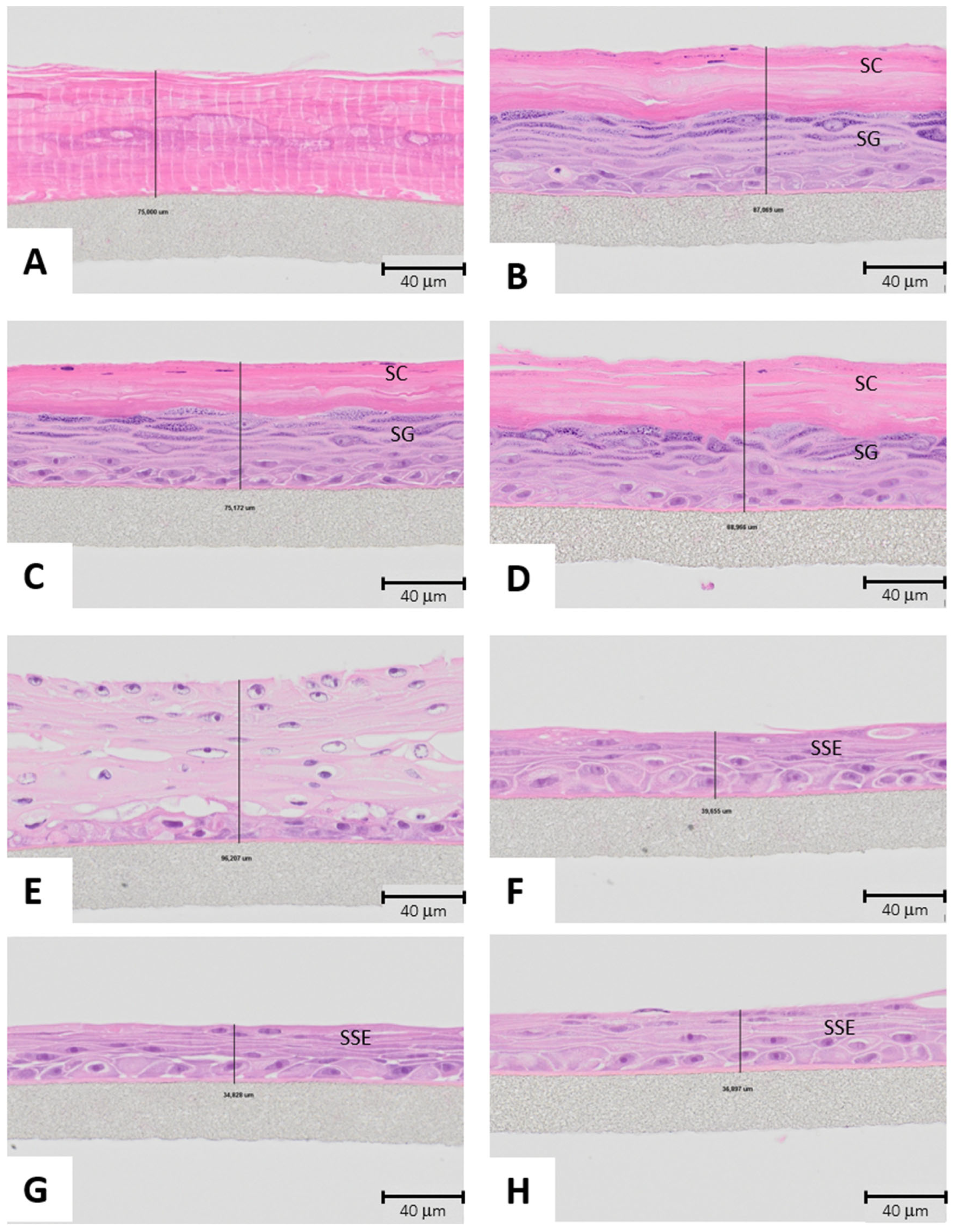
3.6. Morphology of Alginate Microcarriers and PLGA Nanofibers
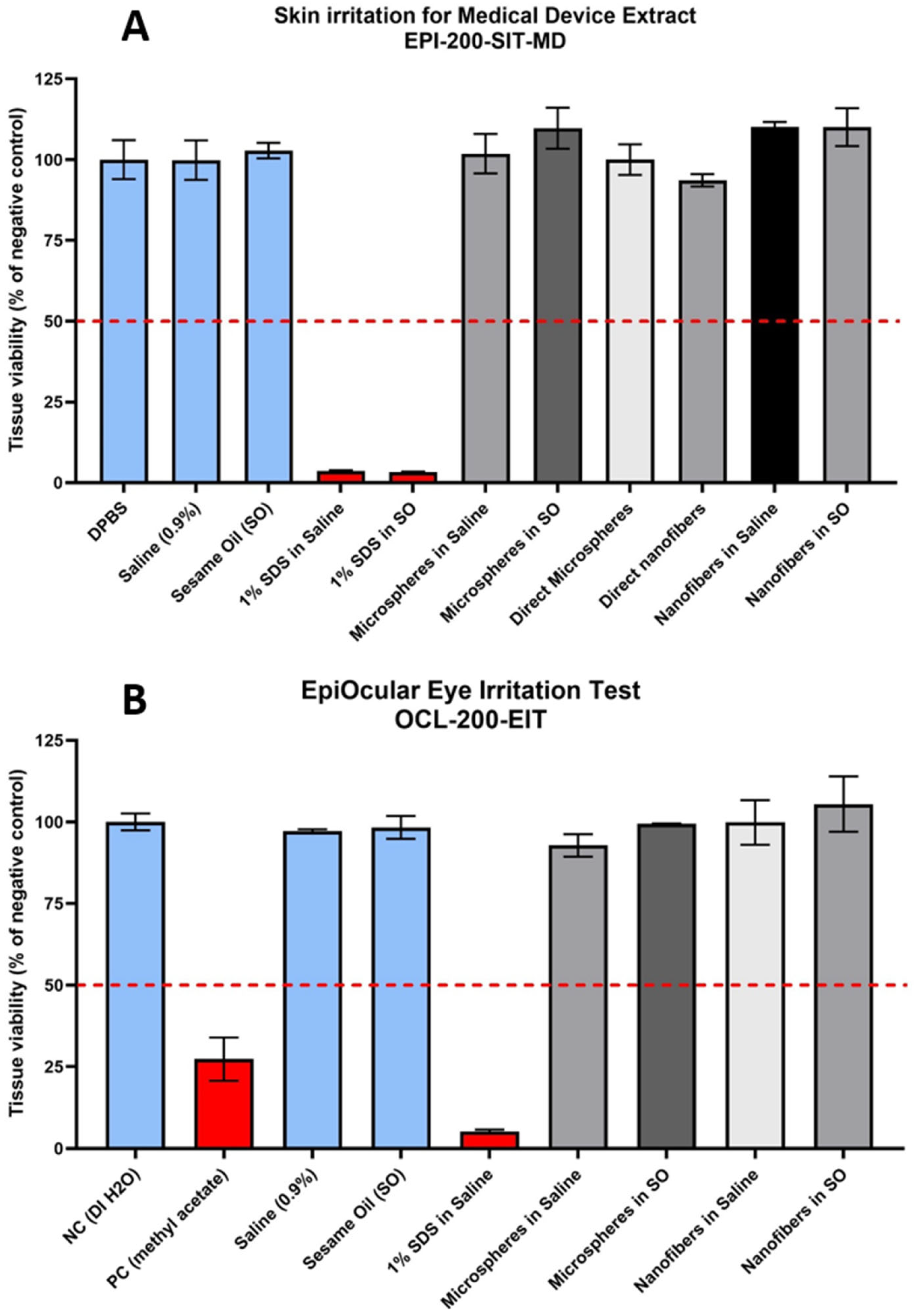
3.7. Biocompatibility Test
4. Discussion
4.1. Alginate Microcarriers and Polylactic-Co-Glycolic Acid (PLGA) Nanofibers Production
4.2. Disinfected and Collagen-Coated Alginate Microcarriers and PLGA Nanofibers Production
4.3. Alginate Microcarriers and PLGA Nanofibers Physicochemical and Structural Characterization
4.4. Antibiotic and Serum-Free Mesenchymal Stem Cell Primary Culture and Flow Cytometry Characterization
4.5. Alginate Microcarriers and PLGA Nanofiber Growth Kinetics
4.6. Morphology of Alginate Microcarriers and PLGA Nanofibers
4.7. Biocompatibility Test
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MSCs | Mesenchymal Stem Cells |
| bmMSCs | Bone marrow-derived Mesenchymal Stem Cells |
| PLGA | Polylactic-co-glycolic acid |
| SEM | Scanning Electron Microscopy |
| PBS | Phosphate Buffer Solution |
| SDS | Sodium Dodecyl Sulfate |
| TGA | Thermogravimetric Analysis |
| FT-IR | Fourier Transform Infrared Spectroscopy |
Appendix A

| Treatment | n | Mean | Grouping | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Collagen-coated alginate microspheres 96 h | 3 | 1,362,500 | A | |||||||||
| Collagen-coated alginate microspheres 84 h | 3 | 1,347,800 | A | |||||||||
| Collagen-coated alginate microspheres 72 h | 3 | 1,260,233 | A | B | ||||||||
| Collagen-coated alginate microspheres 60 h | 3 | 1,168,733 | B | C | ||||||||
| Collagen-coated alginate microspheres 48 h | 3 | 1,134,267 | C | D | ||||||||
| SoloHill collagen-coated microcarriers 84 h | 3 | 1,034,300 | D | E | ||||||||
| SoloHill collagen-coated microcarriers 96 h | 3 | 1,021,167 | E | |||||||||
| SoloHill collagen-coated microcarriers 72 h | 3 | 961,867 | E | F | ||||||||
| Collagen-coated alginate microspheres 36 h | 3 | 941,900 | E | F | ||||||||
| SoloHill collagen-coated microcarriers 60 h | 3 | 931,133 | E | F | ||||||||
| SoloHill collagen-coated microcarriers 48 h | 3 | 896,333 | F | |||||||||
| SoloHill collagen-coated microcarriers 36 h | 3 | 757,733 | G | |||||||||
| Collagen-coated alginate microspheres 24 h | 3 | 712,333 | G | |||||||||
| SoloHill collagen-coated microcarriers 24 h | 3 | 502,700 | H | |||||||||
| Collagen-coated alginate microspheres 12 h | 3 | 286,767 | I | |||||||||
| SoloHill collagen-coated microcarriers 12 h | 3 | 264,533 | I | |||||||||
| Collagen-coated alginate microspheres 0 h | 3 | 143,800 | J | |||||||||
| SoloHill collagen-coated microcarriers 0 h | 3 | 101,367 | J | |||||||||
| Collagen-free alginate microspheres 48 h | 3 | 63,167 | J | |||||||||
| Collagen-free alginate microspheres 0 h | 3 | 59,900 | J | |||||||||
| Collagen-free alginate microspheres 84 h | 3 | 59,500 | J | |||||||||
| Collagen-free alginate microspheres 96 h | 3 | 58,733 | J | |||||||||
| Collagen-free alginate microspheres 60 h | 3 | 57,967 | J | |||||||||
| Collagen-free alginate microspheres 36 h | 3 | 53,200 | J | |||||||||
| Collagen-free alginate microspheres 24 h | 3 | 52,133 | J | |||||||||
| Collagen-free alginate microspheres 72 h | 3 | 51,900 | J | |||||||||
| Collagen-free alginate microspheres 12 h | 3 | 47,067 | J | |||||||||
| Treatment | n | Mean | Grouping | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12-well cell culture plate 96 h | 3 | 945,167 | A | |||||||||
| Collagen-coated PLGA nanofibers 96 h | 3 | 932,633 | A | B | ||||||||
| 12-well cell culture plate 84 h | 3 | 879,133 | A | B | C | |||||||
| Collagen-coated PLGA nanofibers 84 h | 3 | 873,867 | A | B | C | |||||||
| 12-well cell culture plate 72 h | 3 | 824,833 | B | C | D | |||||||
| 12-well cell culture plate 60 h | 3 | 819,100 | C | D | ||||||||
| Collagen-coated PLGA nanofibers 60 h | 3 | 804,333 | C | D | ||||||||
| Collagen-coated PLGA nanofibers 72 h | 3 | 779,533 | C | D | ||||||||
| 12-well cell culture plate 48 h | 3 | 729,800 | D | |||||||||
| Collagen-coated PLGA nanofibers 48 h | 3 | 725,500 | D | E | ||||||||
| Collagen-coated PLGA nanofibers 36 h | 3 | 619,267 | E | F | ||||||||
| 12-well cell culture plate 36 h | 3 | 588,467 | F | |||||||||
| 12-well cell culture plate 24 h | 3 | 465,767 | G | |||||||||
| Collagen-coated PLGA nanofibers 24 h | 3 | 387,367 | G | |||||||||
| Collagen-coated PLGA nanofibers 12 h | 3 | 206,100 | H | |||||||||
| 12-well cell culture plate 12 h | 3 | 139,633 | H | I | ||||||||
| Collagen-free PLGA nanofibers 96 h | 3 | 65,233 | I | |||||||||
| Collagen-free PLGA nanofibers 48 h | 3 | 63,667 | I | |||||||||
| Collagen-free PLGA nanofibers 60 h | 3 | 61,833 | I | |||||||||
| 12-well cell culture plate Plate 0 h | 3 | 59,500 | I | |||||||||
| Collagen-coated PLGA nanofibers 0 h | 3 | 58,200 | I | |||||||||
| Collagen-free PLGA nanofibers 0h | 3 | 57,000 | I | |||||||||
| Collagen-free PLGA nanofibers 24 h | 3 | 55,333 | I | |||||||||
| Collagen-free PLGA nanofibers 72 h | 3 | 55,300 | I | |||||||||
| Collagen-free PLGA nanofibers 36 h | 3 | 52,167 | I | |||||||||
| Collagen-free PLGA nanofibers 12 h | 3 | 51,733 | I | |||||||||
References
- Cun, X.; Hosta-Rigau, L. Topography: A Biophysical Approach to Direct the Fate of Mesenchymal Stem Cells in Tissue Engineering Applications. Nanomaterials 2020, 10, 2070. [Google Scholar] [CrossRef] [PubMed]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Discher, D.E.; Péalult, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yuan, Q.; Xie, L. Mesenchymal Stem Cell-Based Immunomodulation: Properties and Clinical Application. Stem Cells Int. 2018, 2018, 3057624. [Google Scholar] [CrossRef] [PubMed]
- Najar, M.; Fayyad-Kazan, H.; Faour, W.H.; Merimi, M.; Sokal, E.M.; Lombard, C.A.; Fahmi, H. Immunological modulation following bone marrow-derived mesenchymal stromal cells and Th17 lymphocyte co-cultures. Inflamm. Res. 2019, 68, 203–213. [Google Scholar] [CrossRef]
- Wu, X.; Jiang, J.; Gu, Z.; Zhang, J.; Chen, Y.; Liu, X. Mesenchymal stromal cell therapies: Immunomodulatory properties and clinical progress. Stem Cell Res. Ther. 2020, 11, 345. [Google Scholar] [CrossRef]
- Sharma, X.; Thind, S.S.; Kaur, A. In vitro meat production system: Why and how? J. Food Sci. Technol. 2015, 52, 7599–7607. [Google Scholar] [CrossRef]
- Koh, B.; Sulaíman, N.; Fauzi, M.B.; Law, J.X.; Ng, M.H.; Idrus, R.B.H.; Yazid, M.D. Three dimensional microcarrier system in mesenchymal stem cell culture: A systematic review. Cell Biosci. 2020, 10, 75. [Google Scholar] [CrossRef]
- Jaime-Rodríguez, M.; Cadena-Hernández, A.L.; Rosales-Valencia, L.D.; Padilla-Sánchez, J.M.; Chavez-Santoscoy, R.A. Are genetic drift and stem cell adherence in laboratory culture issues for cultivated meat production? Front. Nutr. 2023, 10, 1189664. [Google Scholar] [CrossRef]
- Tsai, A.C.; Jeske, R.; Chen, X.; Yuan, X.; Li, Y. Influence of Microenvironment on Mesenchymal Stem Cell Therapeutic Potency: From Planar Culture to Microcarriers. Front. Bioeng. Biotechnol. 2020, 8, 2020. [Google Scholar] [CrossRef]
- Xie, X.; Chen, Y.; Wang, X.; Xu, X.; Shen, Y.; Khan, A.R.; Aldalbahi, A.; Fetz, A.E.; Bowlin, G.L.; El-Newehy, M.; et al. Electrospinning nanofiber scaffolds for soft and hard tissue regeneration. J. Mater. Sci. Technol. 2020, 59, 243–261. [Google Scholar] [CrossRef]
- Samadzadeh, S.; Mousazadeh, H.; Ghareghomi, S.; Dadashpour, M.; Babazadeh, M.; Zarghami, N. In vitro anticancer efficacy of Metformin-loaded PLGA nanofibers towards the post-surgical therapy of lung cancer. J. Drug Deliv. Sci. Technol. 2021, 61, 102318. [Google Scholar] [CrossRef]
- Cameau, E.; Glover, C.; Pedregal, A. Cost modelling comparison of adherent multi-trays with suspension and fixed-bed bioreactors for the manufacturing of gene therapy products. Cell Gene Ther. Insights 2019, 5, 1663–1674. [Google Scholar] [CrossRef]
- ISO 10993:2018; Biological Evaluation of Medical Devices. International Organization for Standardization: Geneva, Switzerland, 2018.
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, and Applications. Chem. Rev. 2019, 199, 5298–5415. [Google Scholar] [CrossRef]
- Yu, C.C.; Chen, Y.W.; Yeh, P.Y.; Hsiao, Y.S.; Lin, W.T.; Kuo, C.W.; Chueh, D.Y.; You, Y.W.; Shyue, J.J.; Chang, Y.C.; et al. Random and aligned electrospun PLGA nanofibers embedded in microfluidic chips for cancer cell isolation and integration with air foam technology for cell release. J. Nanobiotechnol. 2019, 17, 31. [Google Scholar] [CrossRef]
- Hong, Y.; Gao, C.; Xie, Y.; Gong, Y.; Shen, J. Collagen-coated polylactide microcarriers as chondrocyte microcarriers. Biomaterials 2005, 26, 6305–6313. [Google Scholar] [CrossRef]
- Young, L.; Sung, J.; Stacey, G.; Masters, J.R. Detection of Mycoplasma in cell cultures. Nat. Protoc. 2010, 5, 929–934. [Google Scholar] [CrossRef]
- Kandarova, H.; Willoughby, J.A.; De Jong, W.H.; Letasiova, S.; Milasova, T.; Bachelor, M.A.; Bridget, B.; Handa, Y.; De la Fonteyne, L.; Coleman, K.P. Pre-validation of an in vitro skin irritation test for medical devices using the reconstructed human tissue model EpiDermTM. Toxicol. In Vitr. 2018, 50, 407–417. [Google Scholar] [CrossRef]
- Pobis, P.; Kubalcova, J.; Milasova, T.; Kandarova, H. Development of Sensitive In Vitro Protocols for the Biocompatibility Testing of Medical Devices and Pharmaceuticals Intended for Contact with the Eyes: Acute Irritation and Photoxicity Assessment. Altern. Lab. Anim. 2024, 52, 261–275. [Google Scholar] [CrossRef]
- Sukhavattanakul, P.; Thanyacharoen, T.; Chysinuan, P.; Techasakul, S.; Ummartyotin, S. Influence of a Transparent and Edible Coating of Encapsulated Cannabidiol Nanoparticles on the Quality and Shelf Life of Strawberries. ACS Appl. Mater. Interfaces 2023, 15, 23834–23843. [Google Scholar] [CrossRef]
- Puscaselu, R.G.; Lobiuc, A.; Dimian, M.; Covasa, M. Alginate: From Food Industry to Biomedical Applications and Management of Metabolic Disorders. Polymers 2020, 12, 2417. [Google Scholar] [CrossRef] [PubMed]
- Martau, G.A.; Mihai, M.; Vodnar, D. The use of chitosan, alginate and pectin in the biomedical and food sector—Biocompatibility, bioadhesiveness, and biodegradability. Polymers 2019, 11, 1837. [Google Scholar] [CrossRef]
- Bhushani, J.A.; Anandharamakrishnan, C. Electrospinning and electrospraying techniques: Potential food Based applications. Trends Food Sci. Technol. 2014, 38, 21–33. [Google Scholar] [CrossRef]
- Harrington, R.E.; Guda, T.; Lambert, B.; Martin, J. Sterilization and Disinfection of Biomaterials for Medical Devices. In Biomaterials Science, 4th ed.; Wagner, W.R., Sakiyama-Elbert, S.E., Zhang, G., Yaszemski, M.J., Eds.; Academic Press: Cambridge, UK, 2020; pp. 1431–1446. [Google Scholar]
- Ng, E.X.; Wang, M.; Neo, S.H.; Tee, C.A.; Chen, C.H.; Van Vliet, K.J. Dissolvable Gelatin-Based Microcarriers Generated through Droplet Microfluidics for Expansion and Culture of Mesenchymal Stromal Cells. Biotechnol. J. 2021, 16, 2000048. [Google Scholar] [CrossRef] [PubMed]
- Long, E.G.; Buluk, M.; Gallagher, M.B.; Schneider, J.M.; Brown, J.L. Human mesenchymal stem cell morphology, migration, and differentiation on micro and nano-textured titanium. Bioact. Mater. 2019, 4, 249–255. [Google Scholar] [CrossRef]
- Burciaga-Montemayor, N.G.; Claudio-Rizo, J.A.; Cano-Salazar, L.F.; Martínez-Luévanos, A.; Vega-Sánchez, P. Hydrogel composites with application in the remotion of heavy metals present in wastewater. TIP Rev. Espec. Cienc. Químico Biológicas 2020, 23, 1–13. [Google Scholar]
- Lins, L.C.; Bugatti, V.; Livi, S.; Gorrasi, G. Ionic Liquid as Surfactant Agent of Hydrotalcite: Influence on the Final Properties of Polycaprolactone Matrix. Polymers 2018, 10, 44. [Google Scholar] [CrossRef]
- Jose, M.V.; Thomas, V.; Dean, D.R.; Nyairo, E. Fabrication and characterization of aligned nanofibrous PLGA/Collagen blends as bone tissue scaffolds. Polymer 2009, 50, 3778–3785. [Google Scholar] [CrossRef]
- Hou, F.; Zhu, Y.; Zou, Q.; Zhang, C.; Wang, H.; Liao, Y.; Wang, Q.; Yang, X.; Yang, Y. One-step preparation of multifunctional alginate microcarriers loaded with in situ-formed gold nanostars as a photothermal agent. Mater. Chem. Front. 2019, 3, 2018–2024. [Google Scholar] [CrossRef]
- Marangoni-Júnior, L.; Rodrigues, P.R.; da Silva, R.G.; Vieira, R.P.; Alves, R.M.V. Sustainable Packaging Films Composed of Sodium Alginate and Hydrolyzed Collagen: Preparation and Characterization. Food Bioprocess. Technol. 2021, 14, 2336–2346. [Google Scholar] [CrossRef]
- Derkach, S.R.; Voron’ko, N.G.; Sokolan, N.I.; Kolotova, D.S.; Kuchina, Y.A. Interactions between gelatin and sodium alginate: UV and FTIR studies. J. Dispers. Sci. Technol. 2019, 41, 2020. [Google Scholar] [CrossRef]
- Caroline, M.L.; Kandasamy, A.; Mohan, R.; Vasudevan, S. Growth and characterization of dichlorobis L-proline Zn (II): A semiorganic nonlinear optical single crystal. J. Cryst. Growth 2009, 311, 1161–1165. [Google Scholar] [CrossRef]
- Krane, S.M. The importance of proline residues in the structure, stability and susceptibility to proteolytic degradation of collagens. Amino Acids 2008, 35, 703–710. [Google Scholar] [CrossRef]
- Baker, M. Reproducibility: Respect your cells! Nature 2016, 537, 433–435. [Google Scholar] [CrossRef]
- Choi, K.H.; Lee, C.K. Pig Pluripotent Stem Cells as a Candidate for Biomedical Applications. J. Anim. Reprod. Biotechnol. 2019, 34, 139–147. [Google Scholar] [CrossRef]
- Noort, W.A.; Oerlemans, M.I.F.J.; Rozemuller, H.; Feyen, S.; Jaksani, S.; Stecher, D.; Naajkens, B.; Martens, A.C.; Bühring, H.J.; Doevendans, P.A.; et al. Human versus porcine mesenchymal stromal cells: Phenotype, differentiation potential, immunomodulation, and cardiac improvement after transplantation. J. Cell. Mol. Med. 2012, 16, 1827–1839. [Google Scholar] [CrossRef]
- McDaniel, S.; Antebi, B.; Pilia, M.; Hurtgen, B.J.; Belenkly, S.; Necsoiu, C.; Cancio, L.C.; Rathbone, C.R.; Batchinsky, A.I. Quantitative Assessment of Optimal Bone Marrow Site for the Isolation of Porcine Mesenchymal Stem Cells. Stem Cells Int. 2017, 2017, 1836960. [Google Scholar] [CrossRef]
- Yang, Z.; Vajta, G.; Xu, Y.; Luan, J.; Lin, M.; Liu, C.; Tian, J.; Dou, H.; Li, Y.; Liu, T.; et al. Production of pigs by hand-made cloning using mesenchymal stem cells and fibroblasts. Cell. Reprogram. 2016, 18, 256–263. [Google Scholar] [CrossRef]
- Maleki, M.; Ghanbarvand, F.; Behvarz, M.R.; Ejtemaei, M.; Ghadirkhomi, E. Comparison of Mesenchymal Stem Cell Markers in Multiple Human Adult Stem Cells. Int. J. Stem Cells 2014, 7, 118–126. [Google Scholar] [CrossRef]
- Grau-Vorster, M.; Laitinen, A.; Nystedt, J.; Vives, J. HLA-DR expression in clinical-grade bone marrow-derived multipotent mesenchymal stromal cells: A two-site study. Stem Cell Res. Ther. 2019, 10, 164. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Sloper-Cortenbach, I.; Marini, F.C.; Krouse, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells: The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- D’Apolito, D.; D’Aiello, L.; Pasqua, S.; Pecoraro, L.; Barbera, F.; Douradinha, B.; Di Martino, G.; Di Bartolo, C.; Conaldi, P.G. Strategy and validation of a consistent and reproducible nucleic acid technique for mycoplasma detection in advanced therapy medicinal products. Biologicals 2020, 64, 49–57. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J.; Harley, B.A.; Yannas, I.V.; Gibson, L.J. The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials 2005, 26, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Titushkin, I.; Cho, M. Regulation of mesenchymal stem cell adhesion and orientation in 3D collagen scaffold by electrical stimulus. Bioelectrochemistry 2006, 69, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Niwa, D.; Fujie, T.; Lang, T.; Goda, N.; Takeoka, S. Heterofunctional nanosheet controlling cell adhesion properties by collagen coating. J. Biomater. Appl. 2011, 27, 131–141. [Google Scholar] [CrossRef]
- Barcena, A.J.R.; Mishra, A.; Bolinas, D.K.M.; Martin, B.M.; Melancon, M.P. Integration of Electrospun Scaffolds and Biological Polymers for Enhancing the Delivery and Efficacy of Mesenchymal Stem/Stromal Cell Therapies. Front. Biosci. 2024, 29, 228. [Google Scholar] [CrossRef]
- Amiri, F.Z.; Pashandi, Z.; Lotfibakhshaiesh, N.; Mirzaei-Parsa, M.J.; Ghanbari, H.; Faridi-Majidi, R. Cell Attachment effects of collagen nanoparticles on crosslinked electrospun nanofibers. Int. J. Artif. Organs 2020, 44, 199–207. [Google Scholar] [CrossRef]
- Muraya, K.; Kawasaki, T.; Yamamoto, T.; Akutsu, H. Enhancement of Cellular Adhesion and Proliferation in Human Mesenchymal Stromal Cells by the Direct Addition of Recombinant Collagen I Peptide to the Culture Medium. Biores. Open Access 2019, 8, 210–218. [Google Scholar] [CrossRef]
- Li, K.; Clarkson, C.M.; Wang, L.; Liu, Y.; Lamm, M.; Pang, Z.; Zhou, Y.; Qian, J.; Tajvidi, M.; Gardner, D.J.; et al. Alignment of Cellulose Nanofibers: Harnessing Nanoscale Properties to Macroscale Benefits. ACS Nano 2021, 15, 3646–3673. [Google Scholar] [CrossRef]
- Yildiz, A.; Kara, A.A.; Acartürk, F. Peptide-protein based nanofibers in pharmaceutical and biomedical applications. Int. J. Biol. Macromol. 2020, 148, 1084–1097. [Google Scholar] [CrossRef]
- Kamble, P.; Sadarani, B.; Majumdar, A.; Bhullar, S. Nanofiber based drug delivery systems for skin: A promising therapeutic approach. J. Drug Deliv. Sci. Technol. 2017, 41, 124–133. [Google Scholar] [CrossRef]
- Via, A.G.; Frizziero, A.; Oliva, F. Biological properties of mesenchymal Stem Cells from different sources. Muscles Ligaments Tendons J. 2012, 2, 154–162. [Google Scholar] [PubMed]
- Zhou, S.; Huang, Y.; Chen, Y.; Liu, S.; Xu, M.; Jiang, T.; Song, Q.; Jiang, G.; Gu, X.; Gao, X.; et al. Engineering ApoE3-incorporated biomimetic nanoparticle for efficient vaccine delivery to dendritic cells via micropinocytosis to enhance cancer immunotherapy. Biomaterials 2020, 235, 119795. [Google Scholar] [CrossRef]
- Tsai, A.C.; Pacak, C.A. Bioprocessing of Human Mesenchymal Stem Cells: From Planar Culture to Microcarrier-Based Bioreactors. Bioengineering 2021, 8, 96. [Google Scholar] [CrossRef]
- Lofty, A.; Salama, M.; Zahran, F.; Jones, E.; Badawy, A.; Sobh, M. Characterization of Mesenchymal Stem Cells Derived from Rat Bone Marrow and Adipose Tissue: A Comparative Study. Int. J. Stem Cells 2014, 7, 135–142. [Google Scholar]
- Schwarz, C.; Leicht, U.; Rothe, C.; Drosse, I.; Luibl, V.; Röcken, M.; Schleker, M. Effects of different media on proliferation and differentiation capacity of canine, equine and porcine adipose derived stem cells. Res. Vet. Sci. 2012, 93, 457–462. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, K.; Yang, Y.; Deng, D.; Lyu, C.; Xu, H.; Liu, W.; Du, Y. Dispersible and Dissolvable Porous Microcarrier Tablets Enable Efficient Large-Scale Human Mesenchymal Stem Cell Expansion. Tissue Eng. Part C Methods 2020, 26, 263–275. [Google Scholar] [CrossRef]
- Couto, P.S.; Rotondi, M.C.; Berseney, A.; Hewitt, C.J.; Nienow, A.W.; Verter, F.; Rafiq, Q.A. Expansion of human mesenchymal stem/stromal cells (hMSCs) in bioreactors using microcarriers: Lessons learnt and what the future holds. Biotechnol. Adv. 2020, 45, 107636. [Google Scholar]
- Gu, Y.; Li, T.; Ding, Y.; Sun, L.; Tu, T.; Zhu, W.; Hu, J.; Sun, X. Changes in mesenchymal stem cells following long-term culture in vitro. Mol. Med. Rep. 2016, 13, 5207–5215. [Google Scholar] [CrossRef]
- Ciuffreda, M.C.; Malpasso, G.; Musaro, P.; Turco, V.; Gnecchi, M. Protocols for in vitro Differentiation of Human Mesenchymal Stem Cells into Osteogenic, Chondrogenic and Adipogenic Lineages. In Mesenchymal Stem Cells; Gnecchi, M., Ed.; Methods in Molecular Biology; Human Press: New York, NY, USA, 2016; Volume 1416. [Google Scholar]
- Zhang, X.; Morits, M.; Jonkergouw, C.; Ora, A.; Valle-Delgado, J.J.; Farooq, M.; Ajdary, R.; Huan, S.; Linder, M.; Rojas, O.; et al. Three-Dimensional Printed Cell Culture Model Based on Spherical Colloidal Lignin Particles and Cellulose Nanofibril-Alginate Hydrogel. Biomacromolecules 2020, 21, 1875–1885. [Google Scholar] [CrossRef]
- Song, W.; Liu, P.; Li, H.; Ding, S. Large-Scale Expansion of Porcine Adipose-Derived Stem Cells Based on Microcarriers System for Cultured Meat Production. Foods 2022, 11, 3364. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chi, X.; Meng, H.; Ma, M.; Wang, J.; Feng, Z.; Quan, Q.; Liu, G.; Wang, Y.; Xie, Y.; et al. Polylysine-decorated microporous microcarriers laden with adipose-derived stem cells promote nerve regeneration in vivo. Bioact. Mater. 2021, 6, 3987–3998. [Google Scholar] [PubMed]
- Ding, S.L.; Liu, X.; Zhao, X.Y.; Wang, K.T.; Xiong, W.; Gao, Z.L.; Sun, C.Y.; Jia, M.X.; Li, C.; Gu, Q.; et al. Microcarriers in application for cartilage tissue engineering: Recent progress and challenges. Bioact. Mater. 2022, 17, 81–108. [Google Scholar] [CrossRef] [PubMed]
- Yen, F.C.; Glusac, J.; Levi, S.; Zernov, A.; Baruch, L.; Davidovich-Pinhas, M.; Fishman, A.; Machluf, M. Cultured meat platform developed through the structuring of edible microcarrier-derived microtissues with oleogel-based fat substitute. Nat. Commun. 2023, 14, 2942. [Google Scholar] [CrossRef]


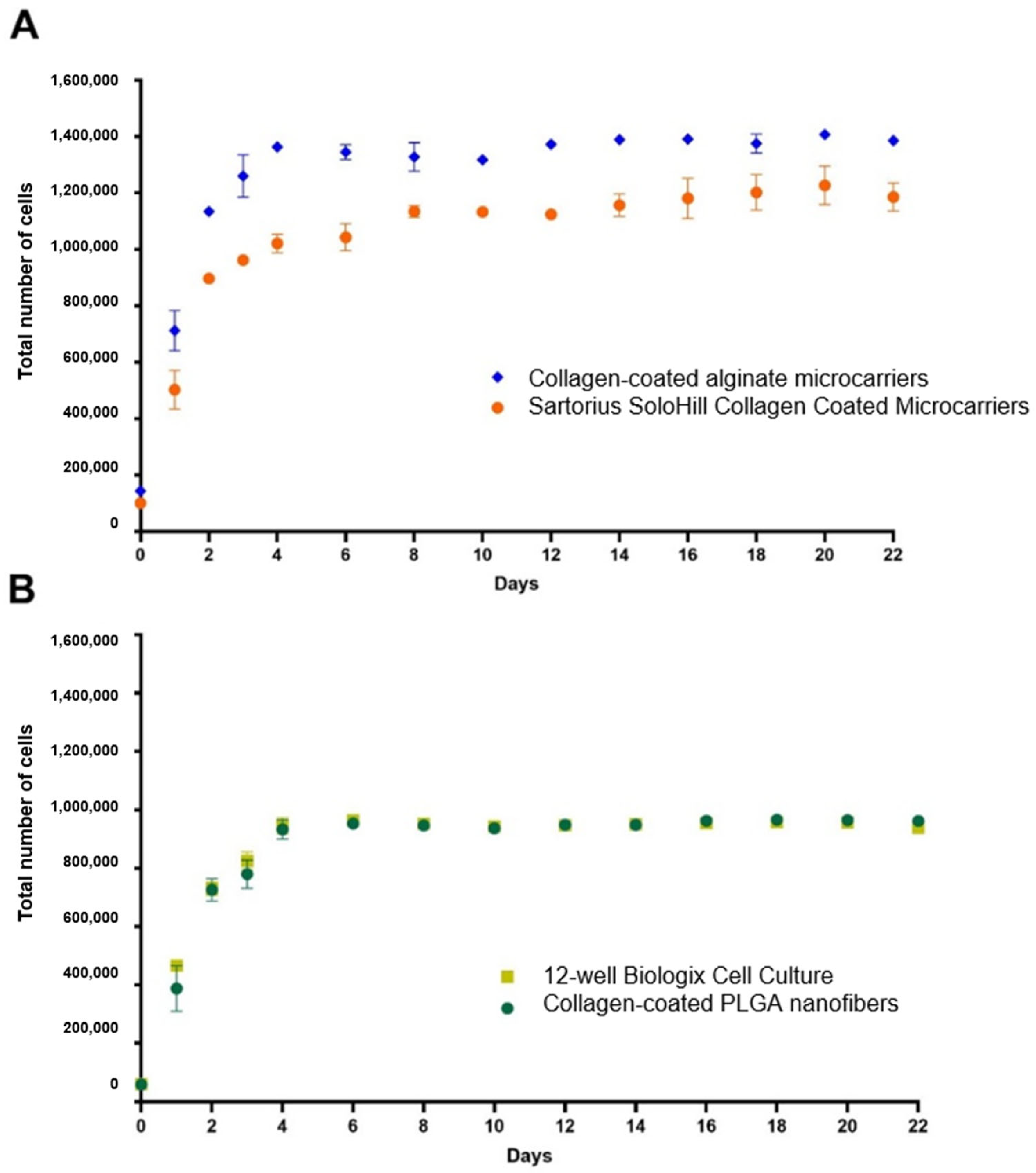
| Treatment | Total Cells (Mean ± Standard Deviation) |
|---|---|
| Collagen-coated alginate microcarriers | 1,362,500 ± 0.0194 A* |
| SoloHill collagen-coated microcarriers | 1,021,167 ± 0.0324 B* |
| 12-well cell culture plate | 945,167 ± 0.0287 C* |
| Collagen-coated PLGA nanofibers | 932,633 ± 0.0330 C* |
| Collagen-free PLGA nanofibers | 65,233 ± 0.0029 D* |
| Collagen-free alginate microcarriers | 58,733 ± 0.0027 D* |
| Treatment | Total Cells at Initial Time | Total Cells at 96-h Incubation | 96-h Cell Population Increase | % Relative Growth Efficiency |
|---|---|---|---|---|
| Collagen-coated alginate microcarriers | 200,000 | 1,362,500 | 6.81 | 144.15 |
| SoloHill collagen-coated microcarriers | 200,000 | 1,021,167 | 5.1 | 108.04 |
| Collagen-coated PLGA nanofibers | 200,000 | 932,633 | 4.7 | 98.67 |
| 12-well cell culture | 200,000 | 945,167 | 4.7 | 100 |
| Collagen-free PLGA nanofibers | 200,000 | 65,233 | 0.3 | 6.9 |
| Collagen-free alginate microcarriers | 200,000 | 58,733 | 0.3 | 6.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaime-Rodríguez, M.; Del Prado-Audelo, M.L.; Sosa-Hernández, N.A.; Anaya-Trejo, D.P.; Villarreal-Gómez, L.J.; Cabrera-Ramírez, Á.H.; Ruiz-Aguirre, J.A.; Núñez-Tapia, I.; Puskar, M.; Marques dos Reis, E.; et al. Evaluation of Biocompatible Materials for Enhanced Mesenchymal Stem Cell Expansion: Collagen-Coated Alginate Microcarriers and PLGA Nanofibers. Biomolecules 2025, 15, 345. https://doi.org/10.3390/biom15030345
Jaime-Rodríguez M, Del Prado-Audelo ML, Sosa-Hernández NA, Anaya-Trejo DP, Villarreal-Gómez LJ, Cabrera-Ramírez ÁH, Ruiz-Aguirre JA, Núñez-Tapia I, Puskar M, Marques dos Reis E, et al. Evaluation of Biocompatible Materials for Enhanced Mesenchymal Stem Cell Expansion: Collagen-Coated Alginate Microcarriers and PLGA Nanofibers. Biomolecules. 2025; 15(3):345. https://doi.org/10.3390/biom15030345
Chicago/Turabian StyleJaime-Rodríguez, Manuel, María Luisa Del Prado-Audelo, Norma Angélica Sosa-Hernández, Dulce Patricia Anaya-Trejo, Luis Jesús Villarreal-Gómez, Ángel Humberto Cabrera-Ramírez, Jesus Augusto Ruiz-Aguirre, Israel Núñez-Tapia, Marek Puskar, Emily Marques dos Reis, and et al. 2025. "Evaluation of Biocompatible Materials for Enhanced Mesenchymal Stem Cell Expansion: Collagen-Coated Alginate Microcarriers and PLGA Nanofibers" Biomolecules 15, no. 3: 345. https://doi.org/10.3390/biom15030345
APA StyleJaime-Rodríguez, M., Del Prado-Audelo, M. L., Sosa-Hernández, N. A., Anaya-Trejo, D. P., Villarreal-Gómez, L. J., Cabrera-Ramírez, Á. H., Ruiz-Aguirre, J. A., Núñez-Tapia, I., Puskar, M., Marques dos Reis, E., Letasiova, S., & Chávez-Santoscoy, R. A. (2025). Evaluation of Biocompatible Materials for Enhanced Mesenchymal Stem Cell Expansion: Collagen-Coated Alginate Microcarriers and PLGA Nanofibers. Biomolecules, 15(3), 345. https://doi.org/10.3390/biom15030345










