Abstract
PHI-1, encoded by PPP1R14B, regulates cellular protein phosphatase-1 (PP1) signaling and has emerged as both a biomarker and therapeutic target. Initially identified as a phospholipase-neighboring gene (PNG), PHI-1 is now known for its phosphorylation-dependent inhibition of PP1 holoenzymes, with bi-directional roles depending on its expression levels. Under physiological conditions, PHI-1 selectively regulates PP1 activity to maintain cellular homeostasis, whereas its pathological upregulation promotes oncogenic pathways, stabilizes tumor-promoting proteins, and modulates immune responses. This article explores PHI-1’s emerging role as a pan-cancer biomarker in parallel with emphasizing its physiological functions in signaling networks, smooth muscle contraction, cytoskeletal dynamics, and selective proteostasis. The mechanistic insights highlight PHI-1’s potential in precision oncology, offering opportunities for developing diagnostics and therapies that target its conditional functions.
1. Introducing PHI-1/PPP1R14B: Its Historical Journey into Cellular PP1 Regulation
1.1. Cellular Phosphatase Signaling in Physiology and Pathology
Reciprocal phosphorylation–dephosphorylation cycles determine cellular phosphorylation levels. Protein phosphatases play a vital role in controlling the response rate, intensity, duration, and sensitivity to physiological stimuli and pathological stressors. Protein phosphatase-1 (PP1) is one of the most abundant Ser/Thr phosphatase in eukaryotic cells and the amino acid sequence is highly conserved between yeasts and mammals [1,2,3,4]. In general, the catalytic subunits of PP1—PP1α, PP1δ(β), PP1γ1, and PP1γ2—possess a broad substrate specificity [1,2,3,4]. The interaction with PP1 regulatory subunits, involving over 100 gene products, confers specific cellular actions to the PP1 catalytic subunits [1,2,3,4]. In parallel, there are endogenous PP1 inhibitor proteins that mediate cellular stimuli into each PP1 holoenzyme, a complex of the PP1 catalytic and regulatory subunits [1,4,5]. To fully unravel signaling pathways governing cellular phosphorylation homeostasis, we must characterize cellular functions of each PP1 inhibitor protein in both preserving the physiological balance and contributing to pathological processes [6].
1.2. The PPP1R14 Family
The vertebrate genome encodes four members of the PPP1R14 gene family: CPI-17 (PPP1R14A), PHI-1 (PPP1R14B), KEPI (PPP1R14C), and GBPI (PPP1R14D) (Figure 1A). Each gene product shares the PHIN domain, —a structural element essential for phosphorylation-dependent PP1 holoenzyme inhibition—and plays distinct roles in cellular regulation [7,8,9,10,11]. In addition, except for CPI-17, a putative PP1 binding motif, RVXF, is found at the N-terminal domains of PHI-1, GBPI, and KEPI, although it remains unknown whether the site docks at PP1 catalytic subunits. Each member is suggested to recognize a specific subset of PP1 holoenzymes, probably due to direct interaction with regulatory subunits of PP1 holoenzymes [12].
1.3. Discovery and Characterization of PHI-1/PPP1R14B
PHI-1, encoded by PPP1R14B and renamed by the HUGO Gene Nomenclature Committee (HGNC:9057), is a selective endogenous inhibitor of PP1 holoenzymes, playing a multifaceted role in cellular regulation [8,11]. Initially identified as the phospholipase-C neighboring gene (PNG) during the mapping of the MEN1 locus on chromosome 11q13, PHI-1 was later redefined as a structural and functional regulator unrelated to MEN1-associated tumorigenesis [13,14,15]. Its ubiquitous expression and evolutionary conservation across species underscore its significance as a housekeeping gene involved in cellular homeostasis, although only limited numbers of PHI-1 orthologs have been detected in avian species [13,14,16].
1.4. Structure of PHI-1/PPP1R14B
Figure 1B highlights PHI-1’s evolutionary conservation, including its PHIN domain and the key phosphorylation site at Thr57. The phosphorylation of PHI-1 at Thr57, which corresponds to CPI-17’s Thr38, is a key event converting PHI-1 into a potent inhibitor of PP1 holoenzymes, with an IC50 of 2–30 nM [8]. AlphaFold predictions suggest an N-terminal intrinsically disordered domain preceding the PHIN domain (Figure 1, red dotted line) [11]. Based on the studies on CPI-17, the direct docking of the phosphorylated-PHIN domain at the active site of PP1 as a pseudo substrate is pivotal to PP1 regulation [11].
1.5. Structure–Function Relationship of PHI-1
Studies on zebrafish have provided evidence of the functional divergence within the PPP1R14B gene family. CPI-17 paralogs are primarily expressed in smooth muscle and neurons while PHI-1 paralogs are found in skeletal muscle and neurons [17]. Experiments with chimeric proteins have revealed that the PHIN domain determines inhibitory potency, reinforcing its critical role in functional specificity [17]. CPI-17 paralogs successfully rescue developmental phenotypes in zebrafish embryos, unlike PHI-1 paralogs, highlighting their selective roles in regulating PP1 pathways [17]. In addition to the PHIN domain, the N-terminal unstructured domain of CPI-17 is indispensable for regulating the myosin phosphatase in smooth muscle. The N-terminal region is unique among PHI-1, CPI-17, GBPI, and KEPI (Figure 1A), possibly contributing to their diverse roles in regulating a specific subset of cellular PP1 holoenzymes. Interestingly, the unstructured N-terminal domain of PHI-1 seems to be classified into three groups, birds, mammals, and anamniotes (fish and amphibians). PHI-1 may play distinct roles in PP1 holoenzyme regulation across vertebrates.
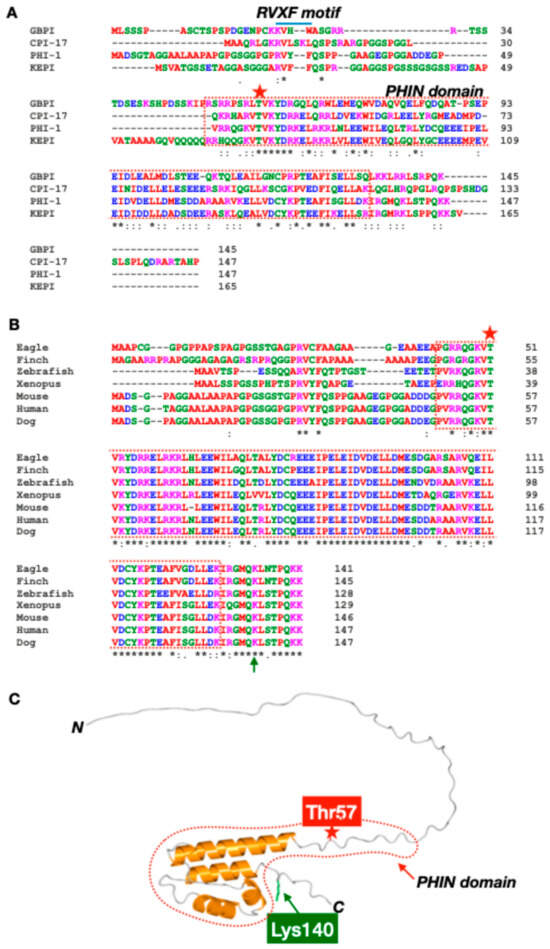
Figure 1.
Structural insights into PHI-1. Sequence alignment of PPP1R14 family (A) and PHI-1 (B): FASTA data of PHI-1 proteins were obtained from NCBI Protein Database and subjected to analysis with Clustal Omega ver. 1.2.4 [18] for the sequence alignment. Color of each residue is based on their chemical properties: red (A, V, F, P, M, I, L, W), blue (D, E), green (S, T, Y, H, C, N, G, Q), and magenta (R, K). * indicates conserved residues. (C) Predicted 3D Architecture of human PHI-1: The 3D structure of unphosphorylated PHI-1 was predicted using AlphaFold 3.0 [19]. File conversion from CIF to PDB was conducted using PDBx/mmCIF software (https://mmcif.pdbj.org/converter/index.php?l=en, accessed on 9 January 2025). The 3D image was visualized and annotated using Waals 3.0 software (Altif Laboratories Inc., Tokyo, Japan). Red stars indicate Thr57, the phosphorylation site necessary for the PP1 inhibition, and green arrows indicate Lys140, the lactylation site regulating protein stability. Red dotted lines indicate the PHIN domain, conserved in the PPP1R14 gene family.
2. Deciphering PHI-1: Mechanisms Orchestrating Cellular Homeostasis in Normal States Encompassing Cytoskeletal Dynamics and Selective Proteostasis
Accumulating lines of evidence suggest that PHI-1 actions extend to key processes such as smooth muscle contraction [20,21,22,23], apoptosis [24,25], cell motility [26,27,28], metabolic regulation [29], and cytoskeletal dynamics [26] (Figure 2).
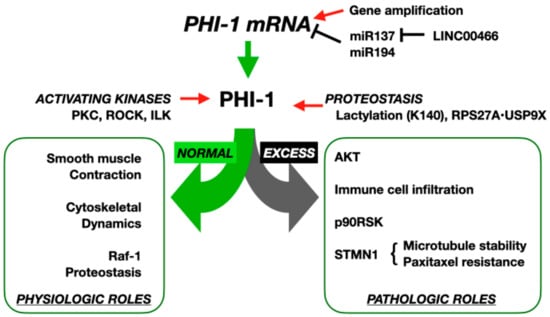
Figure 2.
Physiological and pathological functions of PHI-1/PPP1R14B. At physiological expression levels, PHI-1 is activated by kinases such as PKC, ROCK, and ILK, leading to inhibition of PP1 holoenzymes that regulate smooth muscle contraction, cytoskeletal dynamics, and Raf-1 proteostasis. In pathological states, gene amplification or miR137 reduction by LIC00466 elevates PHI-1 mRNA; plus, Lys140 lactylation or interaction with RPS27A/USP9X increases PHI-1 stability. These post-transcriptional and post-translational mechanisms result in excessive PHI-1, driving AKT activation, immune cell infiltration, p90RSK activation, and STMN1-mediated microtubule stabilization, contributing to paclitaxel resistance in cancer cells.
2.1. PHI-1’s Role in Regulating the Myosin Phosphatase and Smooth Muscle Contraction
PHI-1 was identified as a homolog of CPI-17, an endogenous regulator that selectively regulates the myosin phosphatase, a PP1 holoenzyme, and blood pressure [8,30,31]. The resemblance has driven extensive studies on PHI-1’s role in regulating smooth muscle contraction [20,21,22,23,32]. Recombinant PHI-1 thio-phosphorylated at Thr57 effectively inhibits endogenous myosin phosphatase activity, driving contraction in skinned smooth muscle fibers [20,22]. Among the kinases capable of phosphorylating Thr57, PKC, ROCK, and integrin-linked kinase (ILK) are prominent, with ILK demonstrating the highest specificity (Figure 2) [20,21,23]. In smooth muscle tissues such as the chicken gizzard and aorta, PHI-1 phosphorylation is induced by agonists like angiotensin II, thrombin, and a thromboxane analog, U-46619, in parallel with elevated myosin light chain phosphorylation [21,23]. This process is G-protein-mediated and inhibited by PKC or ROCK antagonists, highlighting the upstream regulation of PHI-1 by these kinases [21,22,23]. Upon stimulation, phosphorylated PHI-1 interacts with PP1, further supporting its role as a phosphorylation-dependent regulator of the myosin phosphatase (Figure 2) [22]. These findings demonstrate possible roles of PHI-1 as a phosphorylation-dependent regulator of the myosin phosphatase, linking its structural features and kinase-mediated activation to essential processes such as smooth muscle contraction and cytoskeletal regulation.
Although PHI-1 shares structural similarities with CPI-17, their functional profiles differ significantly. Unlike CPI-17, PHI-1 does not respond to phosphorylation by ZIPK/DAPK3, highlighting distinct activation pathways, although the sequence around the phosphorylation site is highly conserved (Figure 1A) [33]. Species-specific differences further illustrate the functional nuances of PHI-1. In chickens, where CPI-17 is absent, PHI-1 assumes a role of the myosin phosphatase inhibitor, partially compensating for the lack of CPI-17 [21,32]. PHI-1 is less effective in mediating the PKC-dependent Ca²⁺ sensitization of chicken aorta tissues, suggesting that functional limitations may occur due to a significantly slower rate of PHI-1 phosphorylation by PKC and with less potency compared to that of CPI-17 [32]. These differences clearly show unique roles of PHI-1 in PP1 regulatory pathways, reflecting its ability to adapt to diverse cellular and species-specific demands while complementing the functions of CPI-17.
2.2. PHI-1’s Tissue-Specific Expression and Subcellular Distribution
PHI-1 is ubiquitously expressed across tissues [8,13,34] whereas CPI-17 expression is mostly confined to smooth muscle tissues, particularly arteries [30]. The broader tissue distribution of PHI-1 suggests PHI-1’s involvement in additional physiological roles beyond smooth muscle regulation and pathological processes such as tumor progression [8,13]. The distinct roles of PHI-1 and CPI-17 are also supported by their cellular distributions, in which CPI-17 is found in the cytoplasm whereas PHI-1 is concentrated at juxtamembrane regions in intestinal smooth muscle cells [34]. The distinct phosphorylation pathways and broader distribution of PHI-1 point to its involvement in unique physiological processes, raising questions about its primary functions and evolutionary significance.
2.3. PHI-1’s Roles in Regulating Cell Migration and Cytoskeletal Reorganization
The ubiquitous expression pattern aligns with PHI-1’s classification as a housekeeping gene, essential for maintaining basal functions in normal cells, involving the regulation of cytoskeletal dynamics. Tountas et al. demonstrated that PHI-1 localizes to the trailing edge of migrating endothelial cells, in which PHI-1 is highly expressed [26]. The knockdown of PHI-1 impairs migration and enhances spreading by reducing retraction events, suggesting a key regulatory role in cytoskeletal dynamics and adhesion turnover. Because the retraction of the trailing edge of migrating cells is regulated by the myosin phosphatase [35], localized regulation by PHI-1 may drive normal cell migration (Figure 2). PHI-1’s coordination of multiple PP1 holoenzymes regulating cytoskeletal dynamics and adhesion turnover appears indispensable for efficient cell migration.
2.4. PHI-1’s Roles in Selective Proteostasis
Although knocking down PHI-1 in cancer cells reduced cell proliferation, HEK293 cells, whose PHI-1 expression levels are less than 50% of those in HeLa cells, exhibited the opposite response to siRNA-mediated knockdown [25]. PHI-1 knockdown led to a 7.2-fold increase in Raf-1 expression and a 21-fold increase in ERK1/2 phosphorylation under growth conditions, along with an elevated cell proliferation and a reduced apoptosis [25]. Treatment with tautomycin, a PP1 inhibitor, decreased Raf-1 levels, mimicking the effect of PHI-1. This indicates that PP1 activity modulates Raf-1 degradation [25]. The PP1–PHI-1 axis controls Raf-1 proteostasis as PHI-1-mediated PP1 inhibition affects Raf-1 protein turnover [25]. Interestingly, the regulation of proteostasis is specific to Raf-1, but not B-Raf or other kinases. The study highlighted PHI-1 as a selective regulator of Raf-1 proteostasis and downstream ERK1/2 signaling in non-cancer-derived cells characterized by a limited copy number (Figure 2) [25]. PHI-1 emerges as a multifaceted regulator of cellular PP1 pathways, orchestrating its roles in cytoskeletal dynamics, cell migration, and proteostasis to support normal cellular function and adapt to varying physiological and pathological conditions.
3. Rediscovering PHI-1/PPP1R14B: Bridging Molecular Mechanisms and Diagnostic/Prognostic Potential in Cancer
Following the exploration of PHI-1’s structural insights and its role in maintaining cellular homeostasis, we now shift focus to rediscovering PHI-1 in the context of cancer. Initially recognized for its regulatory function in normal cellular processes, accumulating lines of evidence have uncovered involvements of PHI-1 in multiple cancer types (Table 1). As discussed below, fluctuations in PHI-1 expression have been linked to advanced tumor stages, poor survival rates, and aggressive phenotypes. This section highlights how PHI-1 transitions from a precise regulator of cellular homeostasis to a potential oncogenic driver, setting the stage for understanding its complex role in cancer biology.
3.1. Mechanisms of Dysregulated PHI-1/PPP1R14B Expression in Cancer Cells
Most findings linking PHI-1 expression to cancer pathogenesis have emerged from bioinformatic analyses of large datasets, in parallel with genetic analysis techniques such as microarray assay (Table 1). As listed in Table 1, these analyses reveal consistent patterns of PHI-1 dysregulation across multiple cancer types, such as bladder urothelial carcinoma (BLCA), endometrial carcinoma (UCEC), prostate cancer (PRAD), glioblastoma multiforme (GBM), and triple-negative breast cancer (TNBC), indicating its broad relevance in cancer biology. PHI-1 dysregulation in cancer is driven by alterations at multiple levels—genetic, post-transcriptional, and post-translational—transforming it from a selective regulator to a broad-spectrum disruptor of PP1 signaling (Table 1).
3.1.1. Gene Amplification and mRNA Elevation
PHI-1 gene amplifications, as reported by Deng et al. [36], are observed in cancers such as BLCA, PRAD, and liver hepatocellular carcinoma (LIHC), leading to elevated PHI-1 expression. This upregulation contributes to enhanced oncogenic signaling, promoting proliferation and tumor progression. The frequent amplification of the PPP1R14B gene is associated with poor survival outcomes, as observed in bladder, endometrial, and prostate cancers [36]. These amplifications also correlate with increased mRNA expression and adverse clinical outcomes, suggesting a dose-dependent oncogenic role that may amplify PP1 signaling disruptions, thereby promoting tumor progression. Using the databases of ICGC and GEO, Mosquera-Ogueira et al. mined a novel three-gene expression signature (with SCGB2A1, KLF4, and PHI-1) that predicts short survival in a small subset of chronic lymphocytic leukemia patients (Table 1) [37]. This signature, including PHI-1, operates independently of traditional prognostic markers, providing new insights into the molecular basis of aggressive disease [37]. Worley et al. also found the PHI-1 mRNA to be among the gene products that are elevated in endometriosis-associated ovarian clear cell carcinoma compared with benign endometriosis (Table 1) [38]. In prostate cancer, PHI-1 mRNA is among the top upregulated genes identified via the Oncomine analysis, though it was not detected in patient plasma samples, limiting its utility as a non-invasive biomarker [39].
3.1.2. Post-Transcriptional Fluctuations
PHI-1 expression is further modulated post-transcriptionally. In glioblastoma (GBM) and lower-grade gliomas (LGG), Zhao et al. demonstrated that LINC00466 acts as a competitive endogenous RNA (ceRNA), suppressing miR-137 and thereby increasing PHI-1 expression, which is linked to temozolomide resistance [40]. In contrast, Huang et al. reported that miRNA-194 reduces PHI-1 expression, adding another layer of complexity. The presence of elevated miR-194 levels was identified as a prognostic biomarker for gastrointestinal cancers (gastric, colorectal, and liver cancers) through genome-wide association studies (Table 1) [41]. The upregulation of miR-194 in cancer cells significantly reduced the expression of the four target genes, including PHI-1, at both mRNA and protein levels, suggesting that miR194-induced PHI-1 downregulation occurs in proliferating gastrointestinal cancers [41]. This observation may relate to the discovery of PPP1R14B-AS, a lncRNA transcribed from the antisense strand of the PPP1R14B gene locus. This lncRNA enhances mitochondrial respiration in LIHC and LUAD [42], regulates breast cancer growth through ceRNA mechanisms [43], and contributes to a ferroptosis-related signature in uveal melanoma [44]. Notably, Yang et al. suggested that PPP1R14B-AS1 interferes with PHI-1 mRNA, emphasizing the need to differentiate their roles [42]. Although roles in PHI-1 signaling remains unclear, further investigations into PPP1R14B-AS1 may help clarify its distinct contribution compared to PHI-1 upregulation. This paradox in the findings of PHI-1 post-transcriptional regulation—where both its up- and downregulation contribute to cancer progression—suggests the presence of multiple PHI-1 targets within cancers and highlights the need for careful studies that consider the cancer type, genetic background, tumor microenvironment, and treatment conditions.
3.1.3. Post-Translational Modifications (PTMs)
Lysine lactylation (Kla) has emerged as a key focus in cancer biology because elevated glycolysis due to the Warburg effect leads to increased lactate production, which can enhance lysine lactylation [45]. Proteomic analysis for cellular lactylation, lactose analysis, has revealed that PHI-1 is lactylated at Lys140 within the C-terminal loop region (Figure 1, green) [29]. The Kla modification is elevated in cervical cancer cells with poor prognosis compared to normal tissues (Table 1) [29]. The K140R mutant PHI-1, a Kla-null version, has shown a significantly reduced ability to promote cervical cancer cell proliferation, migration, and invasion compared to the wild-type protein [29]. This reduced activity is due to the decreased protein stability of the Kla-null mutant [29]. In addition to Kla modification, Liao et al. demonstrated that deubiquitination via USP9X stabilizes PHI-1, correlating with aggressive tumor behavior and chemoresistance [28]. Ribosomal protein S27a (RPS27A) recruits the deubiquitinase USP9X to stabilize PHI-1, preventing its proteasome-mediated degradation, leading to the post-translational upregulation of PHI-1 and paclitaxicel resistance [28]. These findings highlight that post-translational modifications, such as lysine lactylation and deubiquitination, are critical for PHI-1 stability and function, directly influencing cancer cell proliferation, metastasis, and drug resistance, making them potential targets for therapeutic intervention.
The dysregulation of PHI-1 expression—whether through gene amplification, post-transcriptional modulation, or post-translational modifications—consistently correlates with aggressive tumor phenotypes and poor clinical outcomes. Understanding the causes of PHI-1 up- or downregulation, its underlying mechanisms in tumor progression, and its impact on therapy resistance could uncover novel clinical benefits, including potential biomarkers for prognosis and new targets for cancer treatment. Further studies are needed to elucidate the comprehensive interplay between PHI-1 and other oncogenic pathways.
3.2. Pathophysiological Role of PHI-1/PPP1R14B in Cancer Cells
Interestingly, many of PHI-1’s molecular functions in normal cells—such as regulating cytoskeletal dynamics, protein stability, and cell proliferation—are hijacked in cancer to support tumor growth and metastasis.
3.2.1. Proliferation and Survival
Miquel-Serra et al., in 2010, reported that the PPP1R14B transcript is downregulated in an established human melanoma cell line (MeWo) when a tumor suppressor, the V3 isoform of versican, is ectopically expressed (Table 1) [46]. The downregulation in PHI-1 mRNA reduces the cell proliferation rate and cell cycle progression, suggesting a role of PHI-1 in the phenotypic change induced by the V3 overexpression (Table 1) [46]. In cervical cancer, PHI-1 activates the Akt pathway to promote cell survival [24]. PHI-1 knockdown reduces proliferation and increases apoptosis in HeLa and HEC-1A cell lines, likely through a decrease in Akt phosphorylation levels, suggesting that PHI-1 upregulation potentially promotes proliferation and inhibits apoptosis by activating the Akt signaling pathway in cancer cells (Figure 2) [24]. In addition, in hepatocellular carcinoma (LIHC), PHI-1 stabilizes p90RSK, amplifying oncogenic signaling [47]. The knockdown of PHI-1 reduces proliferation and colony formation in hepatocellular carcinoma cell lines, supporting its role in promoting tumor growth. PHI-1 was co-precipitated with p90RSK, stabilizing its protein levels and enhancing phosphorylation at Ser363 and Ser380 [47]. The mutation of p90RSK phosphorylation sites (Ser363/380) blocks PHI1-mediated effects on cell viability and migration, supporting a model where the pathological expression of PHI-1 elevates p90RSK stability, amplifying the ERK signaling in cancer cells [47] (Figure 2). Thus, excess PHI-1 in cancer cells likely elicits aberrant Akt and ERK signaling through the pathological off-target inhibition of PP1 holoenzymes.
3.2.2. Migration and Invasion
The in vivo assay using the shRNA KD mouse model revealed that PHI-1 enhances tumor growth, lung metastasis, and paclitaxel resistance [28]. PHI-1, stabilized through the RPS27A/USP9X axis, selectively forms a complex with PP1α and PP1γ1 isoforms and regulates stathmin 1 (STMN1), a microtubule-destabilizing protein in TNBC [28]. The PHI-1-mediated regulation of STMN1 decreases α-tubulin acetylation and microtubule stability, enhancing TNBC cell cycle progression and resistance to paclitaxel (Figure 2) [28]. By promoting microtubule depolymerization, STMN1 drives cytoskeletal remodeling essential for migration, invasion, and mitotic progression. Its overexpression is often associated with increased tumor aggressiveness and poor clinical outcomes in various cancers. Thus, the RPS27A/USP9X-PHI1-STMN1 axis is a critical driver of TNBC progression and paclitaxel resistance. Consistently, PHI-1 knockdown reduces cell migration and invasion in vitro, whereas its overexpression promotes these aggressive behaviors. In liver cancer models, PHI-1 depletion suppresses tumor growth and metastasis in xenograft assays [47]. Similarly, PHI-1 knockdown induces an elongated shape and impairs the migration of HeLa cells [26]. These findings suggest that PHI-1 regulates cancer cell migration and invasion, primarily through the RPS27A/USP9X-PHI-1-STMN1 axis, though additional pathways may be involved depending on the cancer type. A deeper characterization of PHI-1’s role in metastasis and therapy resistance is essential for developing targeted cancer therapies.
3.2.3. Immune Modulation
PHI-1 is linked to immune cell infiltration patterns. In UCEC, He et al. [48] demonstrated that PHI-1 correlates with increased Th2 cell infiltration and reduced cytotoxic immune cell activity, suggesting an immunosuppressive tumor microenvironment. In kidney renal clear cell carcinoma (KIRC), PHI-1 expression is associated with CD8+ T cell infiltration and with the proliferation and migration of KIRC, implicating it in immune evasion strategies [27]. PHI-1 expression also positively correlates with the presence of myeloid-derived suppressor cells (MDSCs) in breast and liver cancers [36], suggesting that PHI-1 contributes to immune evasion mechanisms that may impact the efficacy of immunotherapies. A key unanswered question is how PHI-1 upregulation in cancer cells promotes immune evasion: by inducing immunosuppressive cytokines, altering metabolism, enhancing extracellular vesicle secretion, or modulating immune checkpoints. Further investigation is warranted to establish these pathways.
Despite its diverse functions, PHI-1 seems to consistently promote tumor aggressiveness. Its ability to regulate both intrinsic tumor behaviors and the tumor microenvironment positions it as a critical mediator in cancer pathophysiology. PHI-1’s dual roles—supportive in normal physiology but dysregulated in cancer—likely arise from multifaceted functions of cellular PP1 holoenzymes, illustrating how its regulatory mechanisms can be subverted, contributing to malignancy.
3.3. Clinical Relevance of PHI-1 in Cancer
PHI-1’s role as a cancer driver directly translates into its clinical implications, serving as both a biomarker for tumor aggressiveness and therapy resistance as well as a potential therapeutic target across multiple cancer types (Table 1).
3.3.1. Diagnostic Potential
PHI-1 distinguishes malignant from normal tissues in cancers such as prostate adenocarcinoma (PRAD) and ovarian clear cell carcinoma (OCCC), enhancing diagnostic accuracy [38,39]. In a study, PHI-1 upregulation was further confirmed through mRNA and protein analyses, highlighting its potential in tissue-based diagnostics [38].
3.3.2. Prognostic Significance
High PHI-1 expression correlates with poor survival in TNBC, UCEC, and glioblastoma (GBM), serving as a strong prognostic marker [28,40,48]. In UCEC, PHI-1 expression is significantly associated with an advanced tumor grade and stage, as well as with poor overall survival [48]. The same gene signature analysis in chronic lymphocytic leukemia (CLL) characterizes PHI-1’s prognostic significance independent of traditional markers [37]. To establish PHI-1 as a prognostic biomarker and a potential therapeutic target in tumor management, further investigation is needed to link its role in immune cell infiltration with tumor progression and patient survival.
3.3.3. Predictive Biomarker
PHI-1 predicts therapy resistance in TNBC (paclitaxel resistance) and GBM (temozolomide resistance), offering a tool for personalized treatment strategies [29,40]. Functional assays have demonstrated that PHI-1 knockdown restores drug sensitivity in resistant cell lines, suggesting a potential target for overcoming chemoresistance [29,40]. Expanding PHI-1 as a pan-therapeutic marker requires further investigation to determine whether its upregulation contributes to chemoresistance in other tumor types, thereby potentially broadening its relevance in cancer therapy. Furthermore, although there are currently no reports linking PHI-1 mutations to pathological conditions, the accumulation of clinical data may reveal such associations in the future.
The evidence clearly establishes PHI-1 as a key driver of tumor progression, therapy resistance, and immune evasion, highlighting its clinical versatility as a diagnostic, prognostic, and predictive biomarker. PHI-1 has the potential to fill gaps in current cancer biomarker panels, particularly in predicting therapy responses where traditional markers fall short. As research advances, fully harnessing PHI-1’s clinical potential could open new avenues for cancer diagnostics and targeted therapies.

Table 1.
List of tumors in which PHI-1 is up-/downregulated. Italics indicate tumors with downregulated PHI-1.
Table 1.
List of tumors in which PHI-1 is up-/downregulated. Italics indicate tumors with downregulated PHI-1.
| Tumor Name | Datasets | References |
|---|---|---|
| MeWo Melanoma (V3 Isoform of Versican) | OncoChip cDNA microarray (CNIO, Madrid, Spain) | [46] |
| Endometriosis-Associated Ovarian Clear Cell Carcinoma (OCCC) | Affymetrix Human Gene 1.1 ST Arrays via the GeneAtlas Fluidic Station | [38] |
| Chronic Lymphocytic Leukemia (CLL) | International Cancer Genome Consortium (ICGC, EGAD00010000875), GEO (GSE22762) | [37] |
| Prostate Cancer | Oncomine database | [39] |
| Bladder Urothelial Carcinoma (BLCA) | TIMER2.0 | [36] |
| Breast Invasive Carcinoma (BRCA) | TIMER2.0 | [36] |
| Cervical Squamous Cell Carcinoma and Endocervical Adenocarcinoma (CESC) | TIMER2.0, TCGA, GTEx, Human Protein Atlas (HPA), Kaplan–Meier Plotter | [24,36] |
| Cholangiocarcinoma (CHOL) | TIMER2.0 | [36] |
| Colon Adenocarcinoma (COAD) | TIMER2.0, CPTAC | [36] |
| Diffuse Large B-cell Lymphoma (DLBCL) | GTEx | [36] |
| Esophageal Carcinoma (ESCA) | TIMER2.0 | [36] |
| Glioblastoma Multiforme (GBM) | TIMER2.0, TCGA-GBM/LGG, GTEx database, qRT-PCR | [36,40] |
| Brain Lower-Grade Glioma (LGG) | GTEx, TCGA-GBM/LGG, qRT-PCR | [36,40] |
| Head and Neck Squamous Cell Carcinoma (HNSC) | TIMER2.0 | [36] |
| Kidney Chromophobe (KICH) | TIMER2.0 | [36] |
| Kidney Renal Clear Cell Carcinoma (KIRC) | TIMER2.0, TCGA, ICGC, GEO (GSE40435, GSE53757, GSM4630028), UALCAN, RT-qPCR, LinkedOmics, TISIDB, single-cell RNA-seq | [27,36] |
| Kidney Renal Papillary Cell Carcinoma (KIRP) | TIMER2.0 | [36] |
| Liver Hepatocellular Carcinoma (LIHC) | TIMER2.0, TCGA-LIHC (Liver Hepatocellular Carcinoma), Human Protein Atlas, Label-Free Quantitative Proteomics, In Vitro and In Vivo Experimental Data | [36,47] |
| Lung Adenocarcinoma (LUAD) | TIMER2.0, CPTAC | [36] |
| Lung Squamous Cell Carcinoma (LUSC) | TIMER2.0 | [36] |
| Ovarian Cancer (OV) | GTEx, CPTAC | [36] |
| Prostate Adenocarcinoma (PRAD) | TIMER2.0 | [36] |
| Rectum Adenocarcinoma (READ) | TIMER2.0 | [36] |
| Stomach Adenocarcinoma (STAD) | TIMER2.0 | [36] |
| Thyroid Carcinoma (THCA) | TIMER2.0 | [36] |
| Uterine Carcinosarcoma (UCS) | GTEx | [36] |
| Uterine Corpus Endometrial Carcinoma (UCEC) | TIMER2.0, CPTAC, TCGA, GEO (GSE17025), Human Protein Atlas (HPA), Clinical Samples, GTEx, Kaplan-Meier Plotter | [24,36,48] |
| Gastrointestinal Cancer (GIC) | TCGA, RNA-Seq data (GSE137070, GSE134308), Ago-HITS-CLIP-seq (GSE137071), CRISPR/Cas9 proliferation screening data (DepMap) | [41] |
| Triple-Negative Breast Cancer (TNBC) | FUSCC-TNBC cohort (Quantitative proteomics: n = 90 TNBC tissues, n = 72 adjacent normal tissues; RNA-seq: n = 360 TNBC tissues, n = 88 adjacent normal tissues), TCGA, METABRIC | [28] |
| Cervical Cancer | TCGA, CGCI, GSE44001, single-cell RNA-seq (GSE168652), proteomics (HeLa cells), TIMER2.0 (immune cell infiltration), lactylation-specific proteomics data | [29] |
4. Decoding PHI-1/PPP1R14B Paradigm: A Balancing Act Between Cellular Homeostasis and Tumor Progression
As illustrated in Figure 2, PHI-1 acts as a dual-faceted regulator of PP1 signaling, with critical roles in both physiological and pathological states. Under physiological conditions, PHI-1 functions as a selective inhibitor for a subset of cellular PP1 holoenzymes, precisely modulating cytoskeletal dynamics, the proteostasis of key signaling molecules like Raf-1, and likely other PP1-driven functions. This fine-tuned regulatory capacity is vital for maintaining cellular homeostasis and supporting fundamental processes such as cell motility and proliferation.
The transition from physiological to pathological states, however, is characterized by PHI-1 upregulation that may lead to the non-specific inhibition of cellular PP1 holoenzymes and disrupt regulatory circuits in cellular phosphorylation signaling. This dysregulation triggers oncogenic signaling pathways, stabilizes tumor-promoting proteins, and contributes to immune evasion, collectively driving tumor progression in multiple cancer types. Furthermore, the discussion in this article has been based entirely on the assumption that PHI-1 regulates PP1 signaling. However, it remains possible that PHI-1 influences other systems beyond PP1, particularly under pathological conditions where its expression is upregulated. To fully understand the physiological and pathological roles of PHI-1, it is essential to identify its physiological and pathological targets.
The dual nature of PHI-1, with it functioning as both a precise regulator in healthy cells and a driver of oncogenesis under pathological conditions, positions it as a promising diagnostic marker and therapeutic target. While its upregulation is associated with tumor aggressiveness and poor prognosis, its controlled inhibition is essential for normal cellular functions. Future research should focus on unraveling mechanisms governing this transition and developing strategies to selectively modulate PHI-1 activity. By addressing these challenges, we must be able to deepen our understanding of cellular PP1 signaling and pave the way for precision medicine approaches targeting PHI-1 in cancer and beyond.
Funding
This work was supported by JSPS Grants-in-Aid for Scientific Research (23K06339, 23H27393, and 19K06573).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The author declares no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PKC | Protein kinase C |
| ROCK | Rho-associated coiled-coil-containing protein kinase |
| ILK | Integrin-linked kinase |
| ZIPK/DAPK3 | Zipper-interacting protein kinase/Death-associated protein kinase 3 |
| USP9X | Ubiquitin specific peptidase 9 X-linked |
| AKT | AK strain transforming/protein kinase B |
| RSK | Ribosomal S6 kinase |
References
- Cohen, P.T. Protein phosphatase 1—Targeted in many directions. J. Cell Sci. 2002, 115, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Virshup, D.M.; Shenolikar, S. From Promiscuity to Precision: Protein Phosphatases Get a Makeover. Mol. Cell 2009, 33, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Brautigan, D.L. Protein Ser/Thr Phosphatases: The Ugly Ducklings of Cell Signaling. FEBS J. 2012, 280, 324–325. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Beullens, M.; Bollen, M.; Van Eynde, A. Functions and therapeutic potential of protein phosphatase 1: Insights from mouse genetics. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 16–30. [Google Scholar] [CrossRef]
- Peti, W.; Nairn, A.C.; Page, R. Structural basis for protein phosphatase 1 regulation and specificity. FEBS J. 2013, 280, 596–611. [Google Scholar] [CrossRef]
- Cao, X.; Lemaire, S.; Bollen, M. Protein phosphatase 1: Life-course regulation by SDS22 and Inhibitor-3. FEBS J. 2022, 289, 3072–3085. [Google Scholar] [CrossRef]
- Eto, M.; Senba, S.; Morita, F.; Yazawa, M. Molecular cloning of a novel phosphorylation-dependent inhibitory protein of protein phosphatase-1 (CPI17) in smooth muscle: Its specific localization in smooth muscle. FEBS Lett. 1997, 410, 356–360. [Google Scholar] [CrossRef]
- Eto, M.; Karginov, A.; Brautigan, D.L. A novel phosphoprotein inhibitor of protein type-1 phosphatase holoenzymes. Biochemistry 1999, 38, 16952–16957. [Google Scholar] [CrossRef]
- Liu, Q.R.; Zhang, P.W.; Zhen, Q.; Walther, D.; Wang, X.B.; Uhl, G.R. KEPI, a PKC-dependent protein phosphatase 1 inhibitor regulated by morphine. J. Biol. Chem. 2002, 277, 13312–13320. [Google Scholar] [CrossRef]
- Liu, Q.R.; Zhang, P.W.; Lin, Z.; Li, Q.F.; Woods, A.S.; Troncoso, J.; Uhl, G.R. GBPI, a novel gastrointestinal- and brain-specific PP1-inhibitory protein, is activated by PKC and inactivated by PKA. Biochem. J. 2004, 377, 171–181. [Google Scholar] [CrossRef]
- Eto, M.; Brautigan, D.L. Endogenous inhibitor proteins that connect Ser/Thr kinases and phosphatases in cell signaling. IUBMB Life 2012, 64, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Bollen, M.; Peti, W.; Ragusa, M.J.; Beullens, M. The extended PP1 toolkit: Designed to create specificity. Trends Biochem. Sci. 2011, 35, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Lagercrantz, J.; Carson, E.; Larsson, C.; Nordenskjold, M.; Weber, G. Isolation and characterization of a novel gene close to the human phosphoinositide-specific phospholipase C b3 gene on chromosomal resion 11q13. Genomics 1996, 31, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Lagercrantz, J.; Kendra, D.; Carson, E.; Nordenskjold, M.; Dumanski, J.P.; Weber, G.; Piehl, F. Sequence and expression of the mouse homologue to human phospholipase C b3 Neighboring gene. Biochem. Biophys. Res. Commun. 1996, 223, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, I.; Merregaert, J.; Van de Ven, W.J.; Kas, K.; Zhang, C.X.; Giraud, S.; Wautot, V.; Buisson, N.; De Witte, K.; Salandre, J.; et al. Construction of a 1.2-Mb sequence-ready contig of chromosome 11q13 encompassing the multiple endocrine neoplasia type 1 (MEN1) gene. The European Consortium on MEN1. Genomics 1997, 44, 94–100. [Google Scholar] [CrossRef]
- Eto, M.; Katsuki, S.; Ohashi, M.; Miyagawa, Y.; Tanaka, Y.; Takeya, K.; Kitazawa, T. Possible roles of N- and C-terminal unstructured tails of CPI-17 in regulating Ca2+ sensitization force of smooth muscle. J. Smooth Muscle Res. 2022, 58, 22–33. [Google Scholar] [CrossRef]
- Lang, I.; Virk, G.; Zheng, D.C.; Young, J.; Nguyen, M.J.; Amiri, R.; Fong, M.; Arata, A.; Chadaideh, K.S.; Walsh, S.; et al. The Evolution of Duplicated Genes of the Cpi-17/Phi-1 (ppp1r14) Family of Protein Phosphatase 1 Inhibitors in Teleosts. Int. J. Mol. Sci. 2020, 21, 5709. [Google Scholar] [CrossRef]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
- Deng, J.T.; Sutherland, C.; Brautigan, D.L.; Eto, M.; Walsh, M.P. Phosphorylation of the myosin phosphatase inhibitors, CPI-17 and PHI-1, by integrin-linked kinase. Biochem. J. 2002, 367, 517–524. [Google Scholar] [CrossRef]
- El-Touhky, A.; Given, A.M.; Cochard, A.; Brozovich, F.V. PHI-1 induced enhancement of myosin phosphorylation in chicken smooth muscle. FEBS Lett. 2005, 579, 4271–4277. [Google Scholar] [CrossRef] [PubMed]
- El-Toukhy, A.; Given, A.M.; Ogut, O.; Brozovich, F.V. PHI-1 interacts with the catalytic subunit of myosin light chain phosphatase to produce a Ca2+ independent increase in MLC(20) phosphorylation and force in avian smooth muscle. FEBS Lett. 2006, 580, 5779–5784. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Guo, Z.; Xie, Z.; Su, W.; Gong, M.C. Divergent kinase signaling mediates agonist-induced phosphorylation of phosphatase inhibitory proteins PHI-1 and CPI-17 in vascular smooth muscle cells. Am. J. Physiol. Cell 2006, 290, C892–C899. [Google Scholar] [CrossRef][Green Version]
- Xiang, N.; Chen, T.; Zhao, X.; Zhao, M. In vitro assessment of roles of PPP1R14B in cervical and endometrial cancer. Tissue Cell 2022, 77, 101845. [Google Scholar] [CrossRef]
- Kirkbride, J.A.; Nilsson, G.Y.; Kim, J.I.; Takeya, K.; Tanaka, Y.; Tokumitsu, H.; Suizu, F.; Eto, M. PHI-1, an Endogenous Inhibitor Protein for Protein Phosphatase-1 and a Pan-Cancer Marker, Regulates Raf-1 Proteostasis. Biomolecules 2023, 13, 1741. [Google Scholar] [CrossRef]
- Tountas, N.A.; Brautigan, D.L. Migration and retraction of endothelial and epithelial cells require PHI-1, a specific protein-phosphatase-1 inhibitor protein. J. Cell Sci. 2004, 117, 5905–5912. [Google Scholar] [CrossRef]
- Cheng, L.; Mi, J.; Zhang, J.; Huang, H.; Mo, Z. Upregulated PPP1R14B is connected to cancer progression and immune infiltration in kidney renal clear cell carcinoma. Clin. Transl. Oncol. 2023, 26, 119–135. [Google Scholar] [CrossRef]
- Liao, L.; Zhang, Y.L.; Deng, L.; Chen, C.; Ma, X.Y.; Andriani, L.; Yang, S.Y.; Hu, S.Y.; Zhang, F.L.; Shao, Z.M.; et al. Protein Phosphatase 1 Subunit PPP1R14B Stabilizes STMN1 to Promote Progression and Paclitaxel Resistance in Triple-Negative Breast Cancer. Cancer Res. 2023, 83, 471–484. [Google Scholar] [CrossRef]
- He, C.; Zhang, J.; Bai, X.; Lu, C.; Zhang, K. Lysine lactylation-based insight to understanding the characterization of cervical cancer. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 167356. [Google Scholar] [CrossRef]
- Eto, M.; Kitazawa, T. Deversity and plasticity in signaling pathways that regulate smooth muscle responsiveness: Paradigms and paradoxes for the myosin phosphatase, the master regulator of smooth muscle contraction. J. Smooth Muscle Res. 2017, 53, 1–19. [Google Scholar] [CrossRef]
- Yang, Q.; Fujii, W.; Kaji, N.; Kakuta, S.; Kada, K.; Kuwahara, M.; Tsubone, H.; Ozaki, H.; Hori, M. The essential role of phospho-T38 CPI-17 in the maintenance of physiological blood pressure using genetically modified mice. FASEB J. 2018, 32, 2095–2109. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, T.; Polzin, A.N.; Eto, M. CPI-17-deficient smooth muscle of chicken. J. Physiol. 2004, 557, 515–528. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, J.A.; Eto, M.; Borman, M.A.; Brautigan, D.L.; Haystead, T.A.J. Dual Ser and Thr phosphorylation of CPI-17, an inhibitor of myosin phosphatase, by MYPT-associated kinase. FEBS Lett. 2001, 493, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Tountas, N.A.; Mandell, J.W.; Everett, A.D.; Brautigan, D.L. Juxtamembrane localization of the protein phosphatase-1 inhibitor protein PHI-1 in smooth muscle cells. Histochem. Cell Biol. 2004, 121, 343–350. [Google Scholar] [CrossRef]
- Murata, K.; Hirano, K.; Villa-Moruzzi, E.; Hartshorne, D.J.; Brautigan, D.L. Differential localization of myosin and myosin phosphatase subunits in smooth muscle cells and migrating fibroblasts. Mol. Biol. Cell 1997, 8, 663–673. [Google Scholar] [CrossRef]
- Deng, M.; Peng, L.; Li, J.; Liu, X.; Xia, X.; Li, G. PPP1R14B Is a Prognostic and Immunological Biomarker in Pan-Cancer. Front. Genet. 2021, 12, 763561. [Google Scholar] [CrossRef]
- Mosquera Orgueira, A.; Antelo Rodríguez, B.; Díaz Arias, J.; Díaz Varela, N.; Bello López, J.L. A Three-Gene Expression Signature Identifies a Cluster of Patients with Short Survival in Chronic Lymphocytic Leukemia. J. Oncol. 2019, 2019, 9453539. [Google Scholar] [CrossRef]
- Worley, M.J., Jr.; Liu, S.; Hua, Y.; Kwok, J.S.; Samuel, A.; Hou, L.; Shoni, M.; Lu, S.; Sandberg, E.M.; Keryan, A.; et al. Molecular changes in endometriosis-associated ovarian clear cell carcinoma. Eur. J. Cancer 2015, 51, 1831–1842. [Google Scholar] [CrossRef]
- Wang, R.; Wu, Y.; Yu, J.; Yang, G.; Yi, H.; Xu, B. Plasma Messenger RNAs Identified Through Bioinformatics Analysis are Novel, Non-Invasive Prostate Cancer Biomarkers. Onco Targets Ther. 2020, 13, 541–548. [Google Scholar] [CrossRef]
- Zhao, M.; Shao, Y.; Xu, J.; Zhang, B.; Li, C.; Gong, J. LINC00466 Impacts Cell Proliferation, Metastasis and Sensitivity to Temozolomide of Glioma by Sponging miR-137 to Regulate PPP1R14B Expression. Onco Targets Ther. 2021, 14, 1147–1159. [Google Scholar] [CrossRef]
- Huang, P.; Xia, L.; Guo, Q.; Huang, C.; Wang, Z.; Huang, Y.; Qin, S.; Leng, W.; Li, D. Genome-wide association studies identify miRNA-194 as a prognostic biomarker for gastrointestinal cancer by targeting ATP6V1F, PPP1R14B, BTF3L4 and SLC7A5. Front. Oncol. 2022, 12, 1025594. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Miao, L.; Liao, W.; Liao, W. LncRNA PPP1R14B-AS1 Promotes Tumor Cell Proliferation and Migration via the Enhancement of Mitochondrial Respiration. Front. Genet. 2020, 11, 557614. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, L.; Guan, X.; Dong, Y.I.; Liu, T. Long noncoding RNA PPP1R14B-AS1 imitates microRNA-134-3p to facilitate breast cancer progression by upregulating LIM and SH3 protein 1. Oncol. Res. 2021, 29, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yu, S.; Zhao, B.; Bai, W.; Cui, Y.; Ni, J.; Lyu, Q.; Zhao, J. Development and Validation of a Novel Ferroptosis-Related LncRNA Signature for Predicting Prognosis and the Immune Landscape Features in Uveal Melanoma. Front. Immunol. 2022, 13, 922315. [Google Scholar] [CrossRef]
- Li, H.; Sun, L.; Gao, P.; Hu, H. Lactylation in cancer: Current understanding and challenges. Cancer Cell 2024, 42, 1803–1807. [Google Scholar] [CrossRef]
- Miquel-Serra, L.; Hernandez, D.; Docampo, M.J.; Bassols, A. Differential expression of endoglin in human melanoma cells expressing the V3 isoform of versican by microarray analysis. Mol. Med. Rep. 2010, 3, 1035–1039. [Google Scholar] [CrossRef]
- Zhou, N.; Guo, C.; Du, J.; Xu, Q.; Li, J.; Huang, D.; Zheng, X.; Tu, L. PPP1R14B-mediated phosphorylation enhances protein stability of RPS6KA1 to promote hepatocellular carcinoma tumorigenesis. Biochim. Biophys. Acta Mol. Cell Res. 2024, 1871, 119840. [Google Scholar] [CrossRef]
- He, K.; Wang, T.; Huang, X.; Yang, Z.; Wang, Z.; Zhang, S.; Sui, X.; Jiang, J.; Zhao, L. PPP1R14B is a diagnostic prognostic marker in patients with uterine corpus endometrial carcinoma. J. Cell. Mol. Med. 2023, 27, 846–863. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).