Long-Term Alterations of Glucocorticoid Receptor Expression and CD4+ T Cells in Adolescent Rhesus Macaques Following Early-Life Adversity
Abstract
1. Introduction
2. Methods
2.1. Subjects and Housing
2.2. Collection of Blood and Hair
2.3. Hair Cortisol Assay
2.4. PBMC Culture and Stimulation
2.5. Quantitative RT-PCR
2.6. TNFa Assay
2.7. Immunophenotyping and Flow Cytometry
2.8. Data Analysis
3. Results
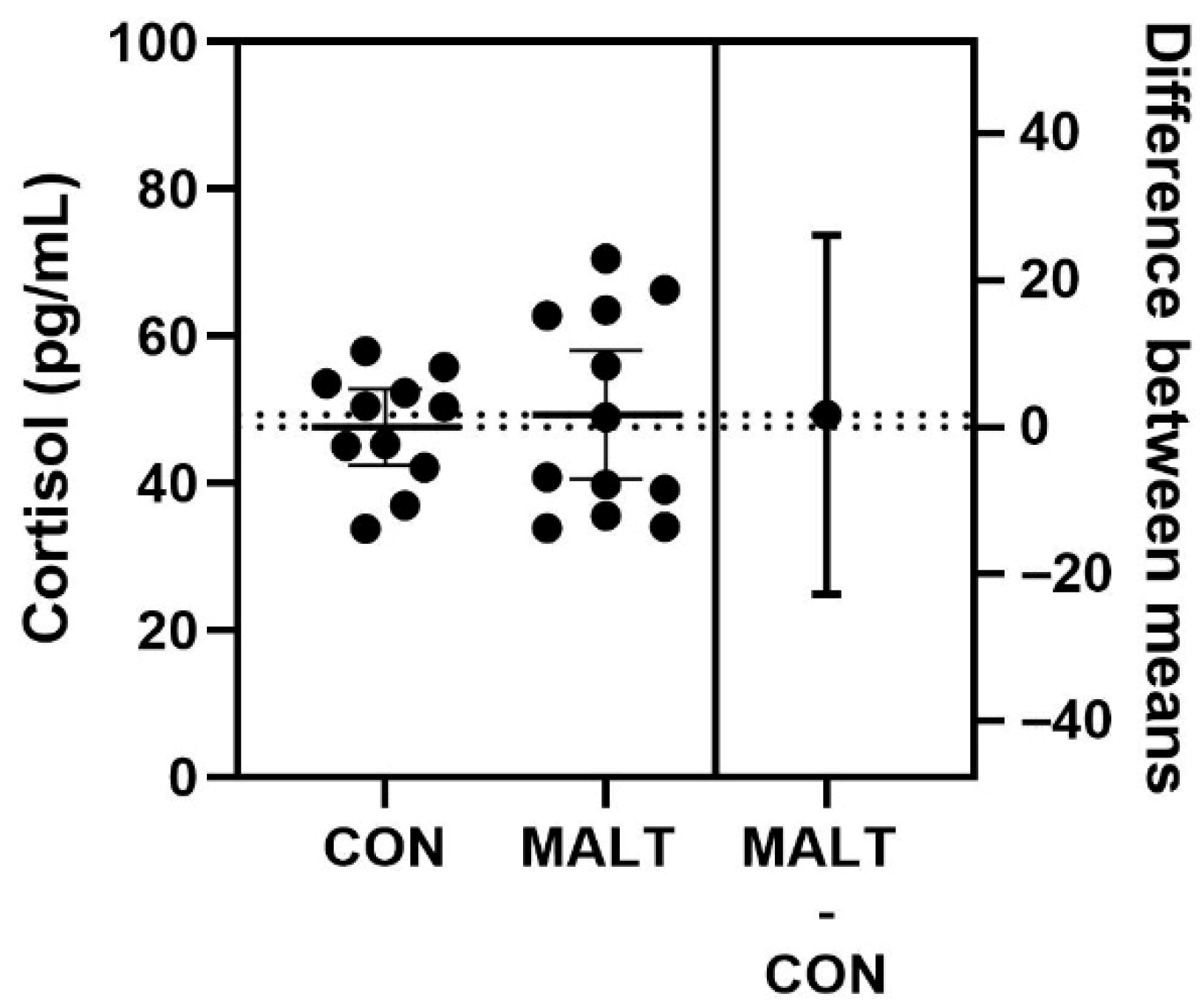
3.1. MALT Did Not Alter Hair Cortisol
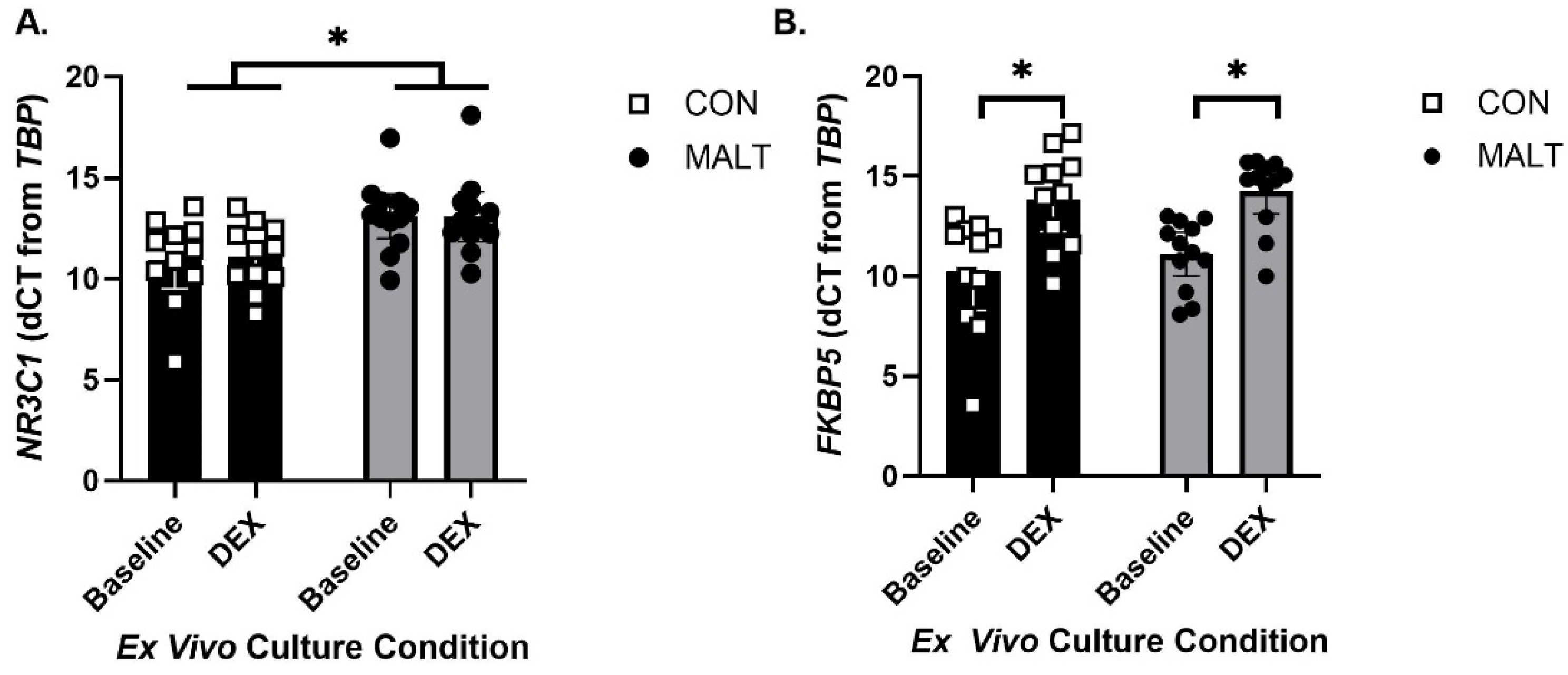
3.2. MALT Rearing Altered Expression of NR3C1 in PBMCs
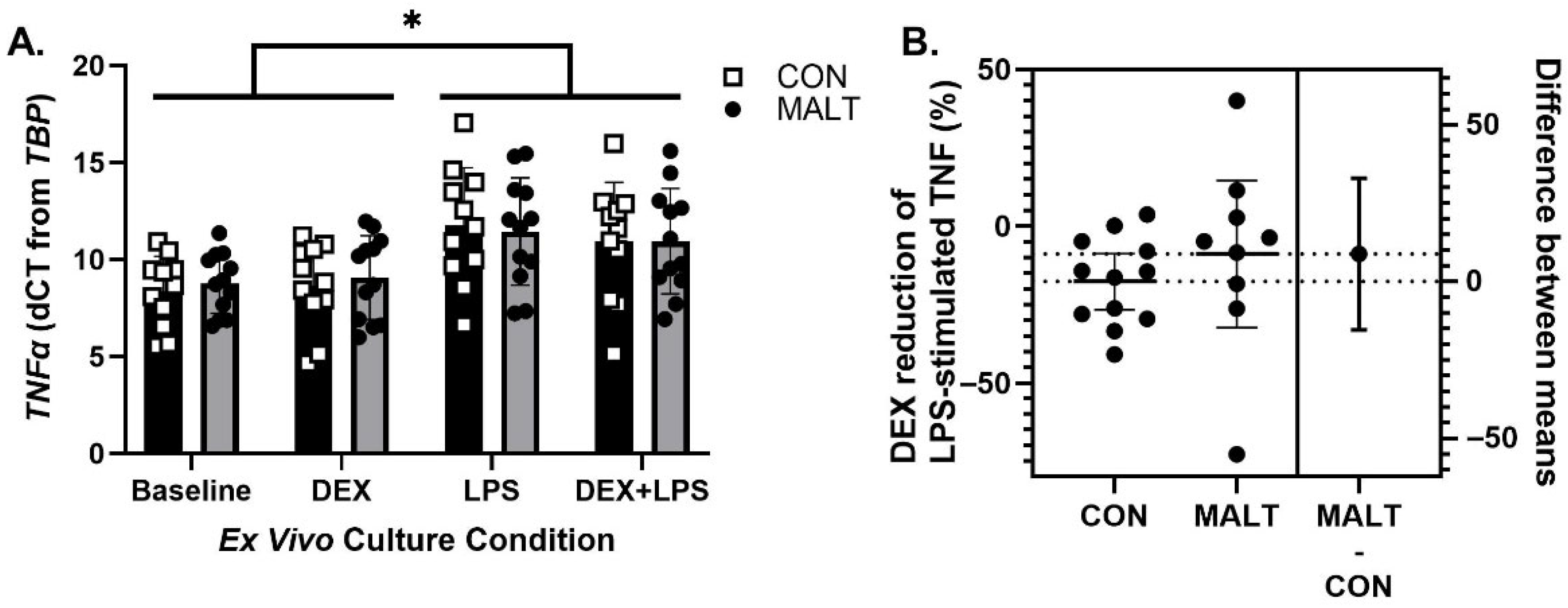
3.3. Ex Vivo TNFα Gene Expression and DEX Suppression of TNFα Protein Was Not Altered by MALT
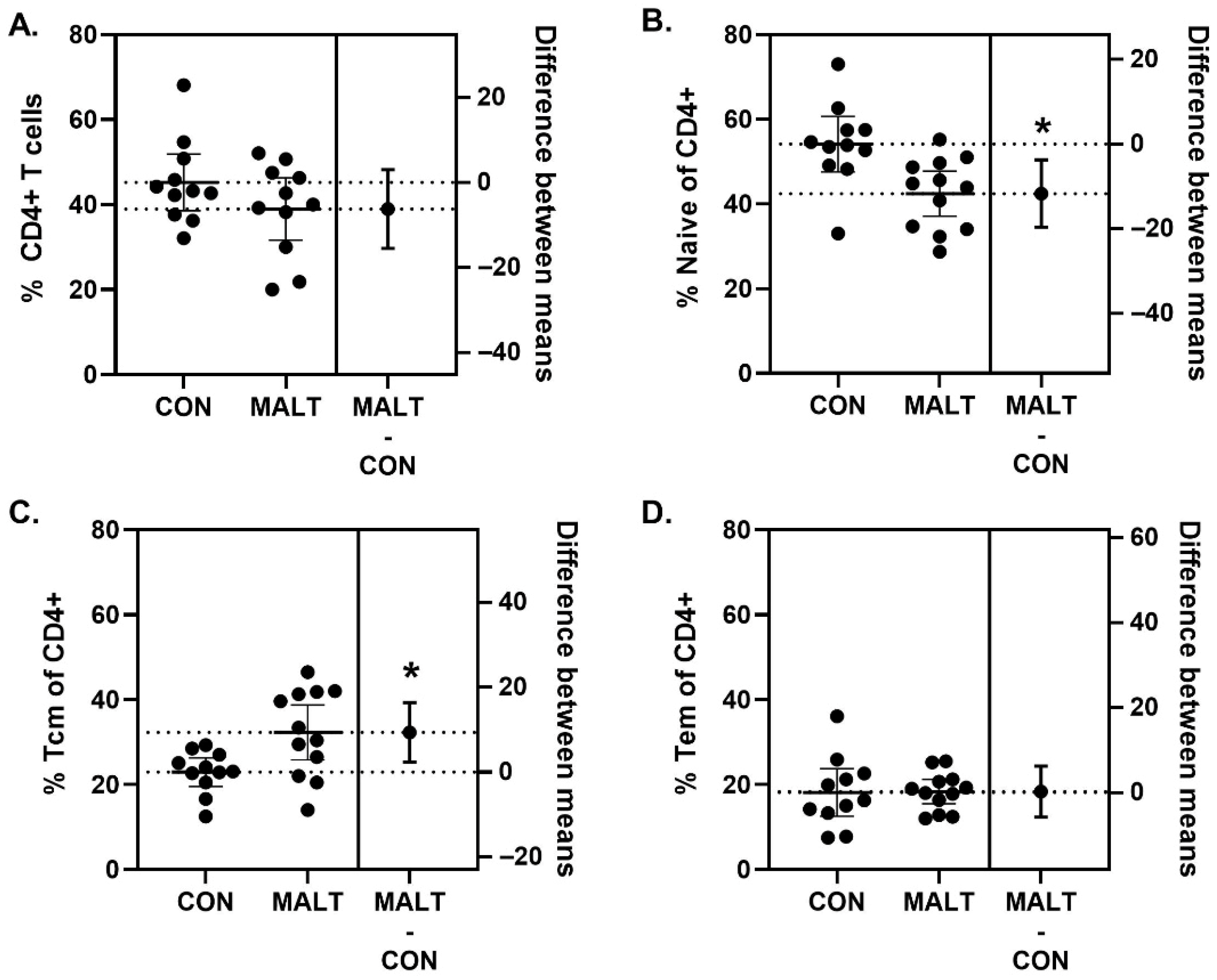
3.4. Immunophenotype of CD4+ T Cells Within PBMCs Was Altered by MALT Rearing
4. Discussion
5. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ELA | Early-life adversity |
| MALT | Maternal maltreatment |
| CON | Control |
| DEX | Dexamethasone |
| LPS | Lipopolysaccharide |
| GR | Glucocorticoid Receptor |
| NHP | Nonhuman Primate |
| CRF | Corticotropin-Releasing Factor |
| ACTH | Adrenocorticotropic Hormone |
| PBMC | Peripheral Blood Mononuclear Cell |
| HPA | Hypothalamic–Pituitary–Adrenal |
| ENPRC | Emory National Primate Research Center |
| CI | Confidence Interval |
| d | Cohen’s d |
| ICI | Intraclass Correlation Coefficient |
| Tem | Effector memory T cells |
| Tcm | Central memory T cells |
References
- Schwartz, K.A.; Preer, G.; McKeag, H.; Newton, A.W. Child maltreatment: A review of key literature in 2013. Curr. Opin. Pediatr. 2014, 26, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.; Embleton, L.; Mwangi, A.; Morantz, G.; Vreeman, R.; Ayaya, S.; Ayuku, D.; Braitstein, P. Physical and sexual abuse in orphaned compared to non-orphaned children in sub-Saharan Africa: A systematic review and meta-analysis. Child Abus. Negl. 2014, 38, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.M.; Parsekar, S.S.; Nair, S.N. An epidemiological overview of child sexual abuse. J. Fam. Med. Prim. Care 2014, 3, 430–435. [Google Scholar] [CrossRef]
- Norman, R.E.; Byambaa, M.; De, R.; Butchart, A.; Scott, J.; Vos, T. The long-term health consequences of child physical abuse, emotional abuse, and neglect: A systematic review and meta-analysis. PLoS Med. 2012, 9, e1001349. [Google Scholar] [CrossRef]
- Danese, A.; Tan, M. Childhood maltreatment and obesity: Systematic review and meta-analysis. Mol. Psychiatry 2014, 19, 544–554. [Google Scholar] [CrossRef]
- Children’s Bureau, US Department of Health and Human Services. Child Maltreatment 2018. 2020. Available online: https://acf.gov/cb/report/child-maltreatment-2018 (accessed on 24 March 2025).
- Elwenspoek, M.M.C.; Hengesch, X.; Leenen, F.A.D.; Schritz, A.; Sias, K.; Schaan, V.K.; Meriaux, S.B.; Schmitz, S.; Bonnemberger, F.; Schachinger, H.; et al. Proinflammatory T Cell Status Associated with Early Life Adversity. J. Immunol. 2017, 199, 4046–4055. [Google Scholar] [CrossRef]
- Kuhlman, K.R.; Tan, E.N.; Cole, S.W.; Rao, U. Differential immune profiles in the context of chronic stress among childhood adversity-exposed adolescents. Brain Behav. Immun. 2025, 127, 183–192. [Google Scholar] [CrossRef]
- Ross, A.J.; Russotti, J.; Cicchetti, D.; Handley, E.D. The Effects of Childhood Abuse on Emerging Adulthood Inflammation: Investigating Protective Characteristics. Child Maltreat 2025, 30, 649–660. [Google Scholar] [CrossRef]
- Tarullo, A.R.; Gunnar, M.R. Child maltreatment and the developing HPA axis. Horm. Behav. 2006, 50, 632–639. [Google Scholar] [CrossRef]
- McCormack, K.M.; Howell, B.R.; Higgins, M.; Bramlett, S.; Guzman, D.; Morin, E.L.; Villongco, C.; Liu, Y.; Meyer, J.; Sanchez, M.M. The developmental consequences of early adverse care on infant macaques: A cross-fostering study. Psychoneuroendocrinology 2022, 146, 105947. [Google Scholar] [CrossRef] [PubMed]
- McCormack, K.; Bramlett, S.; Morin, E.L.; Siebert, E.R.; Guzman, D.; Howell, B.; Sanchez, M.M. Long-Term Effects of Adverse Maternal Care on Hypothalamic-Pituitary-Adrenal (HPA) Axis Function of Juvenile and Adolescent Macaques. Biology 2025, 14, 204. [Google Scholar] [CrossRef]
- Bugental, D.B.; Martorell, G.A.; Barraza, V. The hormonal costs of subtle forms of infant maltreatment. Horm. Behav. 2003, 43, 237–244. [Google Scholar] [CrossRef]
- Kaufman, J.; Birmaher, B.; Perel, J.; Dahl, R.E.; Moreci, P.; Nelson, B.; Wells, W.; Ryan, N.D. The corticotropin-releasing hormone challenge in depressed abused, depressed nonabused, and normal control children. Biol. Psychiatry 1997, 42, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Hulme, P.A. Childhood sexual abuse, HPA axis regulation, and mental health: An integrative review. West. J. Nurs. Res. 2011, 33, 1069–1097. [Google Scholar] [CrossRef]
- Goldman-Mellor, S.; Hamer, M.; Steptoe, A. Early-life stress and recurrent psychological distress over the lifecourse predict divergent cortisol reactivity patterns in adulthood. Psychoneuroendocrinology 2012, 37, 1755–1768. [Google Scholar] [CrossRef]
- Rao, U.; Hammen, C.; Ortiz, L.R.; Chen, L.A.; Poland, R.E. Effects of early and recent adverse experiences on adrenal response to psychosocial stress in depressed adolescents. Biol. Psychiatry 2008, 64, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Rinne, T.; de Kloet, E.R.; Wouters, L.; Goekoop, J.G.; DeRijk, R.H.; van den Brink, W. Hyperresponsiveness of hypothalamic-pituitary-adrenal axis to combined dexamethasone/corticotropin-releasing hormone challenge in female borderline personality disorder subjects with a history of sustained childhood abuse. Biol. Psychiatry 2002, 52, 1102–1112. [Google Scholar] [CrossRef]
- Shea, A.; Walsh, C.; Macmillan, H.; Steiner, M. Child maltreatment and HPA axis dysregulation: Relationship to major depressive disorder and post traumatic stress disorder in females. Psychoneuroendocrinology 2005, 30, 162–178. [Google Scholar] [CrossRef]
- Peng, H.; Long, Y.; Li, J.; Guo, Y.; Wu, H.; Yang, Y.; Ding, Y.; He, J.; Ning, Y. Hypothalamic-pituitary-adrenal axis functioning and dysfunctional attitude in depressed patients with and without childhood neglect. BMC Psychiatry 2014, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Power, C.; Thomas, C.; Li, L.; Hertzman, C. Childhood psychosocial adversity and adult cortisol patterns. Br. J. Psychiatry 2012, 201, 199–206. [Google Scholar] [CrossRef]
- Kumari, M.; Head, J.; Bartley, M.; Stansfeld, S.; Kivimaki, M. Maternal separation in childhood and diurnal cortisol patterns in mid-life: Findings from the Whitehall II study. Psychol. Med. 2013, 43, 633–643. [Google Scholar] [CrossRef]
- Trickett, P.K.; Noll, J.G.; Susman, E.J.; Shenk, C.E.; Putnam, F.W. Attenuation of cortisol across development for victims of sexual abuse. Dev. Psychopathol. 2010, 22, 165–175. [Google Scholar] [CrossRef]
- Arnett, M.G.; Pan, M.S.; Doak, W.; Cyr, P.E.; Muglia, L.M.; Muglia, L.J. The role of glucocorticoid receptor-dependent activity in the amygdala central nucleus and reversibility of early-life stress programmed behavior. Transl. Psychiatry 2015, 5, e542. [Google Scholar] [CrossRef] [PubMed]
- Arabadzisz, D.; Diaz-Heijtz, R.; Knuesel, I.; Weber, E.; Pilloud, S.; Dettling, A.C.; Feldon, J.; Law, A.J.; Harrison, P.J.; Pryce, C.R. Primate early life stress leads to long-term mild hippocampal decreases in corticosteroid receptor expression. Biol. Psychiatry 2010, 67, 1106–1109. [Google Scholar] [CrossRef]
- Romens, S.E.; McDonald, J.; Svaren, J.; Pollak, S.D. Associations between early life stress and gene methylation in children. Child Dev. 2015, 86, 303–309. [Google Scholar] [CrossRef]
- Bockmuhl, Y.; Patchev, A.V.; Madejska, A.; Hoffmann, A.; Sousa, J.C.; Sousa, N.; Holsboer, F.; Almeida, O.F.; Spengler, D. Methylation at the CpG island shore region upregulates Nr3c1 promoter activity after early-life stress. Epigenetics Off. J. DNA Methylation Soc. 2015, 10, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Maestripieri, D.; Carroll, K.A. Child abuse and neglect: Usefulness of the animal data. Psychol. Bull. 1998, 123, 211–223. [Google Scholar] [CrossRef]
- Maestripieri, D. Parenting styles of abusive mothers in group-living rhesus macaques. Anim. Behav. 1998, 55, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Maestripieri, D. The biology of human parenting: Insights from nonhuman primates. Neurosci. Biobehav. Rev. 1999, 23, 411–422. [Google Scholar] [CrossRef]
- Maestripieri, D.; Tomaszycki, M.; Carroll, K.A. Consistency and change in the behavior of rhesus macaque abusive mothers with successive infants. Dev. Psychobiol. 1999, 34, 29–35. [Google Scholar] [CrossRef]
- Maestripieri, D. Early experience affects the intergenerational transmission of infant abuse in rhesus monkeys. Proc. Natl. Acad. Sci. USA 2005, 102, 9726–9729. [Google Scholar] [CrossRef]
- Sanchez, M.M.; McCormack, K.; Grand, A.P.; Fulks, R.; Graff, A.; Maestripieri, D. Effects of sex and early maternal abuse on adrenocorticotropin hormone and cortisol responses to the corticotropin-releasing hormone challenge during the first 3 years of life in group-living rhesus monkeys. Dev. Psychopathol. 2010, 22, 45–53. [Google Scholar] [CrossRef]
- Panagiotakopoulos, L.; Neigh, G.N. Development of the HPA axis: Where and when do sex differences manifest? Front. Neuroendocrinol. 2014, 35, 285–302. [Google Scholar] [CrossRef]
- Davies, T.H.; Ning, Y.M.; Sanchez, E.R. A new first step in activation of steroid receptors: Hormone-induced switching of FKBP51 and FKBP52 immunophilins. J. Biol. Chem. 2002, 277, 4597–4600. [Google Scholar] [CrossRef]
- Wochnik, G.M.; Ruegg, J.; Abel, G.A.; Schmidt, U.; Holsboer, F.; Rein, T. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J. Biol. Chem. 2005, 280, 4609–4616. [Google Scholar] [CrossRef]
- McNally, J.G.; Muller, W.G.; Walker, D.; Wolford, R.; Hager, G.L. The glucocorticoid receptor: Rapid exchange with regulatory sites in living cells. Science 2000, 287, 1262–1265. [Google Scholar] [CrossRef] [PubMed]
- Klengel, T.M.E.; Howell, B.R.; Bramlett, S.; Guzman, D.B.; Meyer, J.S.; Ressler, K.J.; Sanchez, M.M. Molecular and behavioral effects of infant maltreatment across generations in rhesus monkeys. bioRxiv 2019. [Google Scholar] [CrossRef]
- van der Doelen, R.H.; Calabrese, F.; Guidotti, G.; Geenen, B.; Riva, M.A.; Kozicz, T.; Homberg, J.R. Early life stress and serotonin transporter gene variation interact to affect the transcription of the glucocorticoid and mineralocorticoid receptors, and the co-chaperone FKBP5, in the adult rat brain. Front. Behav. Neurosci. 2014, 8, 355. [Google Scholar] [CrossRef] [PubMed]
- Criado-Marrero, M.; Smith, T.M.; Gould, L.A.; Kim, S.; Penny, H.J.; Sun, Z.; Gulick, D.; Dickey, C.A.; Blair, L.J. FKBP5 and early life stress affect the hippocampus by an age-dependent mechanism. Brain Behav. Immun. Health 2020, 9, 100143. [Google Scholar] [CrossRef]
- Tran, C.H.; Shannon Weickert, C.; Weickert, T.W.; Sinclair, D. Early Life Stress Alters Expression of Glucocorticoid Stress Response Genes and Trophic Factor Transcripts in the Rodent Basal Ganglia. Int. J. Mol. Sci. 2022, 23, 5333. [Google Scholar] [CrossRef]
- Burenkova, O.V.; Grigorenko, E.L. The role of epigenetic mechanisms in the long-term effects of early-life adversity and mother-infant relationship on physiology and behavior of offspring in laboratory rats and mice. Dev. Psychobiol. 2024, 66, e22479. [Google Scholar] [CrossRef] [PubMed]
- Rowson, S.; Bekhbat, M.; Kelly, S.; Hyer, M.M.; Dyer, S.; Weinshenker, D.; Neigh, G. Chronic adolescent stress alters GR-FKBP5 interactions in the hippocampus of adult female rats. Stress 2024, 27, 2312467. [Google Scholar] [CrossRef]
- Fagundes, C.P.; Glaser, R.; Kiecolt-Glaser, J.K. Stressful early life experiences and immune dysregulation across the lifespan. Brain Behav. Immun. 2013, 27, 8–12. [Google Scholar] [CrossRef]
- Burke, C.G.; Myers, J.R.; Boule, L.A.; Post, C.M.; Brookes, P.S.; Lawrence, B.P. Early life exposures shape the CD4(+) T cell transcriptome, influencing proliferation, differentiation, and mitochondrial dynamics later in life. Sci. Rep. 2019, 9, 11489. [Google Scholar] [CrossRef]
- Klopack, E.T.; Crimmins, E.M.; Cole, S.W.; Seeman, T.E.; Carroll, J.E. Social stressors associated with age-related T lymphocyte percentages in older US adults: Evidence from the US Health and Retirement Study. Proc. Natl. Acad. Sci. USA 2022, 119, e2202780119. [Google Scholar] [CrossRef]
- Reid, B.M.; Desjardins, C.; Thyagarajan, B.; Linden, M.A.; Gunnar, M. Early Life Stress Is Associated with Alterations in Lymphocyte Subsets Independent of Increased Inflammation in Adolescents. Biomolecules 2024, 14, 262. [Google Scholar] [CrossRef]
- Tung, J.; Barreiro, L.B.; Johnson, Z.P.; Hansen, K.D.; Michopoulos, V.; Toufexis, D.; Michelini, K.; Wilson, M.E.; Gilad, Y. Social environment is associated with gene regulatory variation in the rhesus macaque immune system. Proc. Natl. Acad. Sci. USA 2012, 109, 6490–6495. [Google Scholar] [CrossRef]
- Rosado, M.R.S.; Marzan-Rivera, N.; Watowich, M.M.; Valle, A.D.N.; Pantoja, P.; Pavez-Fox, M.A.; Siracusa, E.R.; Cooper, E.B.; Valle, J.E.N.; Phillips, D.; et al. Immune cell composition varies by age, sex and exposure to social adversity in free-ranging Rhesus Macaques. Geroscience 2024, 46, 2107–2122. [Google Scholar] [CrossRef]
- Coe, C.L.; Shirtcliff, E.A. Growth trajectory evident at birth affects age of first delivery in female monkeys. Pediatr. Res. 2004, 55, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.; Novak, M.; Hamel, A.; Rosenberg, K. Extraction and analysis of cortisol from human and monkey hair. J. Vis. Exp. 2014, e50882. [Google Scholar] [CrossRef] [PubMed]
- Cervasi, B.; Carnathan, D.G.; Sheehan, K.M.; Micci, L.; Paiardini, M.; Kurupati, R.; Tuyishime, S.; Zhou, X.Y.; Else, J.G.; Ratcliffe, S.J.; et al. Immunological and virological analyses of rhesus macaques immunized with chimpanzee adenoviruses expressing the simian immunodeficiency virus Gag/Tat fusion protein and challenged intrarectally with repeated low doses of SIVmac. J. Virol. 2013, 87, 9420–9430. [Google Scholar] [CrossRef]
- Sumpter, B.; Dunham, R.; Gordon, S.; Engram, J.; Hennessy, M.; Kinter, A.; Paiardini, M.; Cervasi, B.; Klatt, N.; McClure, H.; et al. Correlates of preserved CD4(+) T cell homeostasis during natural, nonpathogenic simian immunodeficiency virus infection of sooty mangabeys: Implications for AIDS pathogenesis. J. Immunol. 2007, 178, 1680–1691. [Google Scholar] [CrossRef]
- Xiang, L.; Marshall, G.D., Jr. Glucocorticoid receptor BclI polymorphism associates with immunomodulatory response to stress hormone in human peripheral blood mononuclear cells. Int. J. Immunogenet. 2013, 40, 222–229. [Google Scholar] [CrossRef]
- De Groote, D.; Zangerle, P.F.; Gevaert, Y.; Fassotte, M.F.; Beguin, Y.; Noizat-Pirenne, F.; Pirenne, J.; Gathy, R.; Lopez, M.; Dehart, I.; et al. Direct stimulation of cytokines (IL-1 beta, TNF-alpha, IL-6, IL-2, IFN-gamma and GM-CSF) in whole blood. I. Comparison with isolated PBMC stimulation. Cytokine 1992, 4, 239–248. [Google Scholar] [CrossRef]
- Mavropoulos, A.; Smyk, D.; Rigopoulou, E.I.; Bogdanos, D.P. Human peripheral blood mononuclear cell culture for flow cytometric analysis of phosphorylated mitogen-activated protein kinases. Methods Mol. Biol. 2012, 806, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Zakrajsek, B.A. Effect of experimental treatment on housekeeping gene expression: Validation by real-time, quantitative RT-PCR. J. Biochem. Biophys. Methods 2000, 46, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Untergasser, A.; Nijveen, H.; Rao, X.; Bisseling, T.; Geurts, R.; Leunissen, J.A. Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res. 2007, 35, W71–W74. [Google Scholar] [CrossRef] [PubMed]
- Bookout, A.L.; Mangelsdorf, D.J. Quantitative real-time PCR protocol for analysis of nuclear receptor signaling pathways. Nucl. Recept. Signal. 2003, 1, e012. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Diggle, P.J.; Liang, K.Y.; Zeger, S.L. Analysis of Longitudinal Data; Clarendon Press: Oxford, UK, 1994. [Google Scholar]
- Sawilowsky, S.S. Fermat, Schubert, Einstein, and Behrens-Fisher: The Probable Difference Between Two Means When σ_1^2≠σ_2^2. J. Mod. Appl. Stat. Methods 2002, 1, 55. [Google Scholar] [CrossRef]
- Erdeljan, P.; MacDonald, J.F.; Matthews, S.G. Glucocorticoids and serotonin alter glucocorticoid receptor (GR) but not mineralocorticoid receptor (MR) mRNA levels in fetal mouse hippocampal neurons, in vitro. Brain Res. 2001, 896, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Gunnar, M.R.; DePasquale, C.E.; Reid, B.M.; Donzella, B.; Miller, B.S. Pubertal stress recalibration reverses the effects of early life stress in postinstitutionalized children. Proc. Natl. Acad. Sci. USA 2019, 116, 23984–23988. [Google Scholar] [CrossRef]
- Heim, C.; Newport, D.J.; Mletzko, T.; Miller, A.H.; Nemeroff, C.B. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology 2008, 33, 693–710. [Google Scholar] [CrossRef] [PubMed]
- Panagiotakopoulos, L.; Kelly, S.; Neigh, G.N. HIV-1 proteins accelerate HPA axis habituation in female rats. Physiol. Behav. 2015, 150, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Scharf, S.H.; Liebl, C.; Binder, E.B.; Schmidt, M.V.; Muller, M.B. Expression and regulation of the Fkbp5 gene in the adult mouse brain. PLoS ONE 2011, 6, e16883. [Google Scholar] [CrossRef]
- Lee, R.S.; Tamashiro, K.L.; Yang, X.; Purcell, R.H.; Harvey, A.; Willour, V.L.; Huo, Y.; Rongione, M.; Wand, G.S.; Potash, J.B. Chronic corticosterone exposure increases expression and decreases deoxyribonucleic acid methylation of Fkbp5 in mice. Endocrinology 2010, 151, 4332–4343. [Google Scholar] [CrossRef]
- Binder, E.B. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology 2009, 34 (Suppl. S1), S186–S195. [Google Scholar] [CrossRef]
- Taves, M.D.; Ashwell, J.D. Glucocorticoids in T cell development, differentiation and function. Nat. Rev. Immunol. 2021, 21, 233–243. [Google Scholar] [CrossRef]
- Shirtcliff, E.A.; Coe, C.L.; Pollak, S.D. Early childhood stress is associated with elevated antibody levels to herpes simplex virus type 1. Proc. Natl. Acad. Sci. USA 2009, 106, 2963–2967. [Google Scholar] [CrossRef]
- Sommershof, A.; Aichinger, H.; Engler, H.; Adenauer, H.; Catani, C.; Boneberg, E.M.; Elbert, T.; Groettrup, M.; Kolassa, I.T. Substantial reduction of naive and regulatory T cells following traumatic stress. Brain Behav. Immun. 2009, 23, 1117–1124. [Google Scholar] [CrossRef]
- Tyrka, A.R.; Parade, S.H.; Eslinger, N.M.; Marsit, C.J.; Lesseur, C.; Armstrong, D.A.; Philip, N.S.; Josefson, B.; Seifer, R. Methylation of exons 1D, 1F, and 1H of the glucocorticoid receptor gene promoter and exposure to adversity in preschool-aged children. Dev. Psychopathol. 2015, 27, 577–585. [Google Scholar] [CrossRef] [PubMed]
- van der Knaap, L.J.; Oldehinkel, A.J.; Verhulst, F.C.; van Oort, F.V.; Riese, H. Glucocorticoid receptor gene methylation and HPA-axis regulation in adolescents. The TRAILS study. Psychoneuroendocrinology 2015, 58, 46–50. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez, M.M.; Panagiotakopoulos, L.; Hayes, T.; Howell, B.R.; Ethun, K.; Easley, K.A.; Silvestri, G.; Carnathan, D.G.; McCandless, J.; Meyer, J.; et al. Long-Term Alterations of Glucocorticoid Receptor Expression and CD4+ T Cells in Adolescent Rhesus Macaques Following Early-Life Adversity. Biomolecules 2025, 15, 1701. https://doi.org/10.3390/biom15121701
Sanchez MM, Panagiotakopoulos L, Hayes T, Howell BR, Ethun K, Easley KA, Silvestri G, Carnathan DG, McCandless J, Meyer J, et al. Long-Term Alterations of Glucocorticoid Receptor Expression and CD4+ T Cells in Adolescent Rhesus Macaques Following Early-Life Adversity. Biomolecules. 2025; 15(12):1701. https://doi.org/10.3390/biom15121701
Chicago/Turabian StyleSanchez, Mar M., Leonidas Panagiotakopoulos, Timothy Hayes, Brittany R. Howell, Kelly Ethun, Kirk A. Easley, Guido Silvestri, Diane G. Carnathan, Jackson McCandless, Jerrold Meyer, and et al. 2025. "Long-Term Alterations of Glucocorticoid Receptor Expression and CD4+ T Cells in Adolescent Rhesus Macaques Following Early-Life Adversity" Biomolecules 15, no. 12: 1701. https://doi.org/10.3390/biom15121701
APA StyleSanchez, M. M., Panagiotakopoulos, L., Hayes, T., Howell, B. R., Ethun, K., Easley, K. A., Silvestri, G., Carnathan, D. G., McCandless, J., Meyer, J., & Neigh, G. N. (2025). Long-Term Alterations of Glucocorticoid Receptor Expression and CD4+ T Cells in Adolescent Rhesus Macaques Following Early-Life Adversity. Biomolecules, 15(12), 1701. https://doi.org/10.3390/biom15121701







