Prenatal Maternal Immune Activation with Lipopolysaccharide Accelerates the Developmental Acquisition of Neonatal Reflexes in Rat Offspring Without Affecting Maternal Care Behaviors
Abstract
1. Introduction
1.1. Developmental Reflex Acquisition
1.2. Maternal Care Behavior
1.3. Investigating the Effect of MIA on Neonatal Reflex Development in Offspring and Maternal Care Behaviors
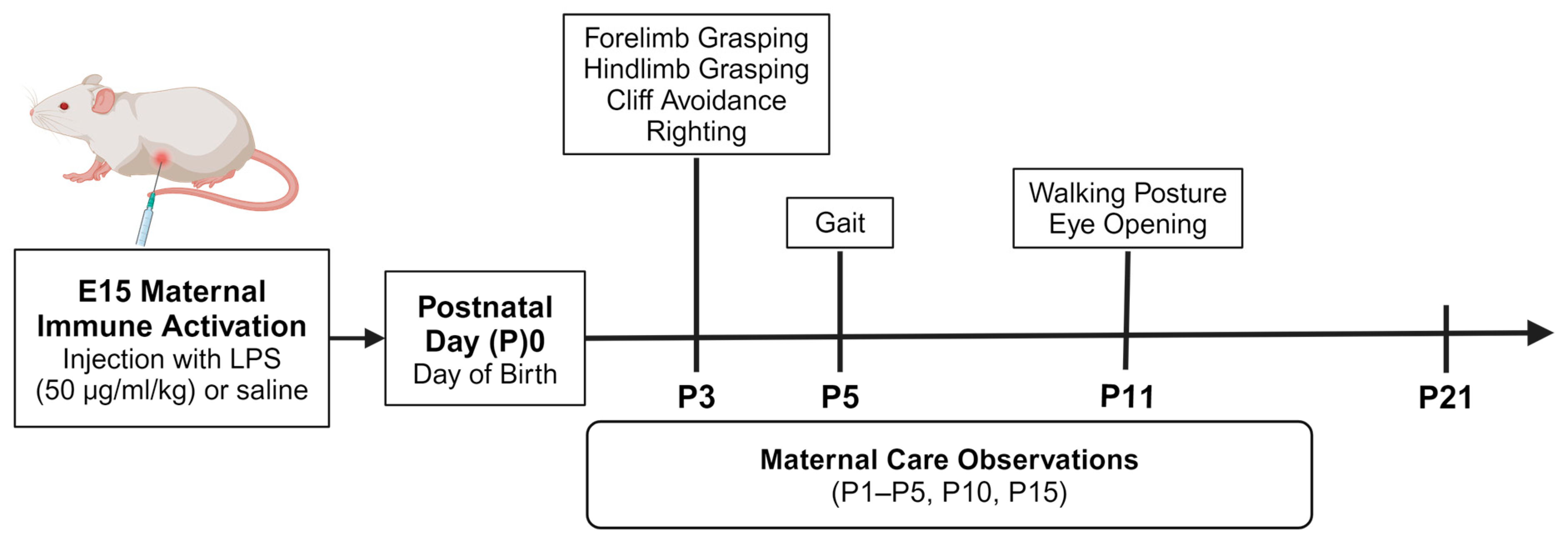
2. Materials and Methods
2.1. Experimental Subjects
2.2. Maternal Immune Activation
2.2.1. Lipopolysaccharide Preparation
2.2.2. MIA Injection on E15
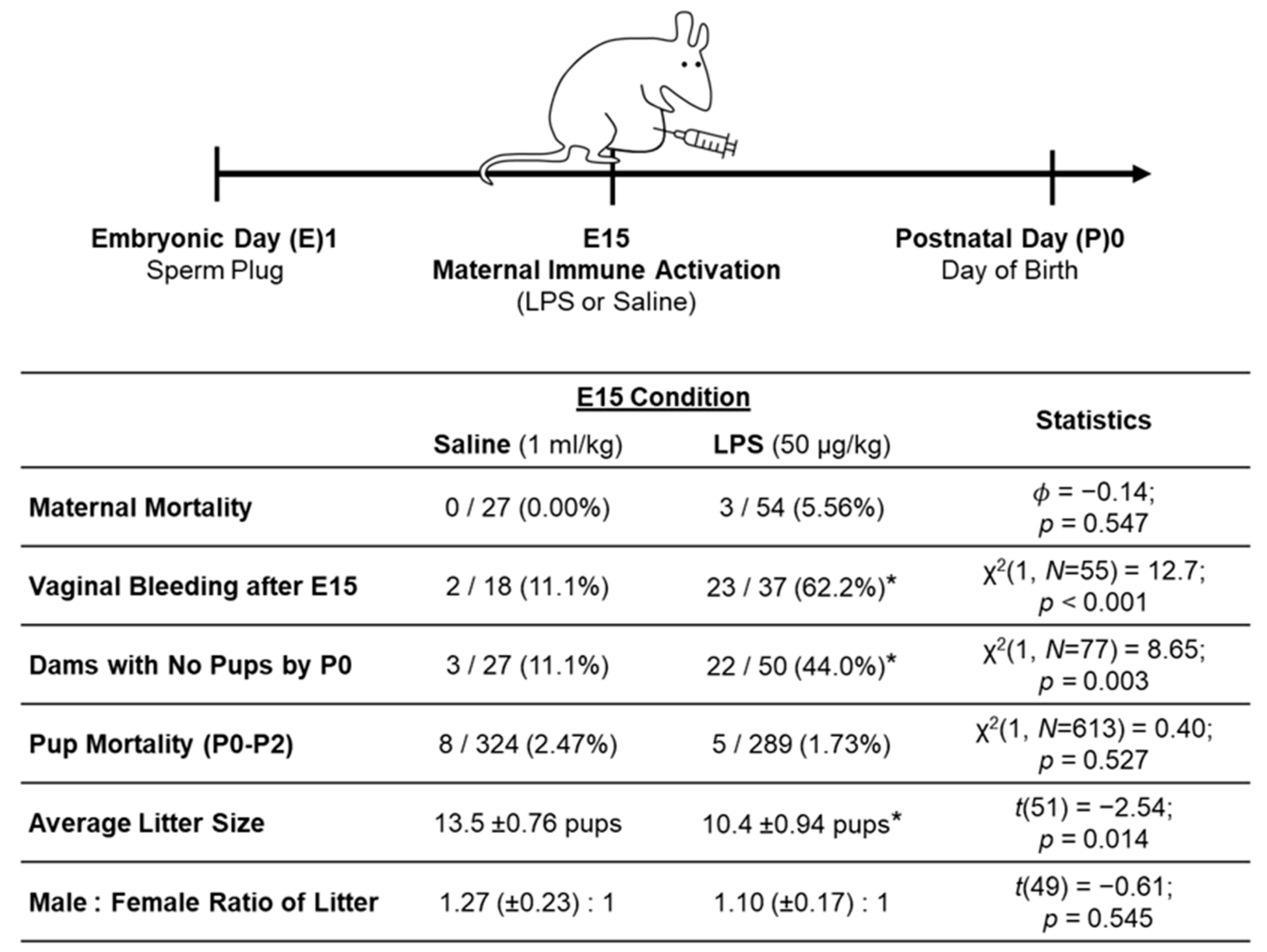
2.2.3. MIA Model Outcomes
2.3. Neonatal Reflex Behaviors
2.4. Maternal Care Observations
2.5. Statistical Analyses
3. Results
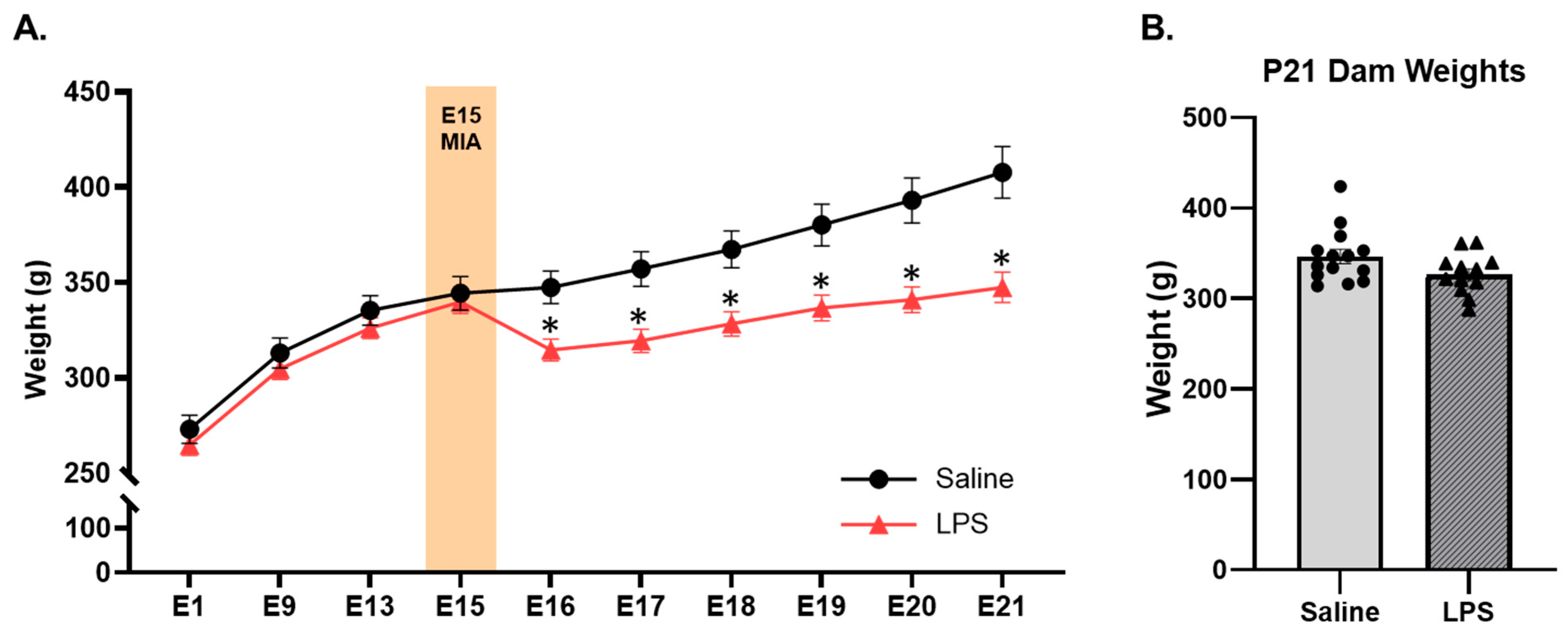
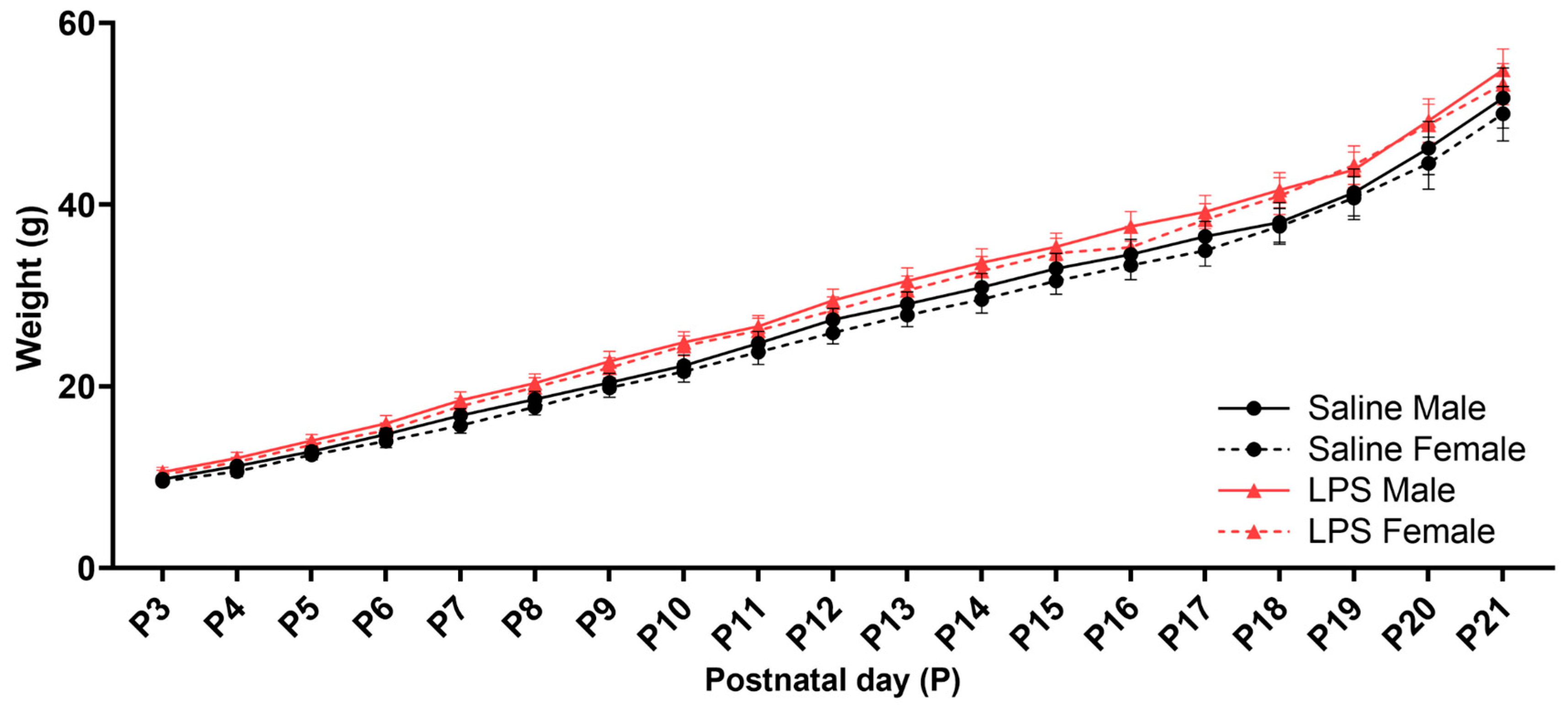
3.1. Offspring Weights
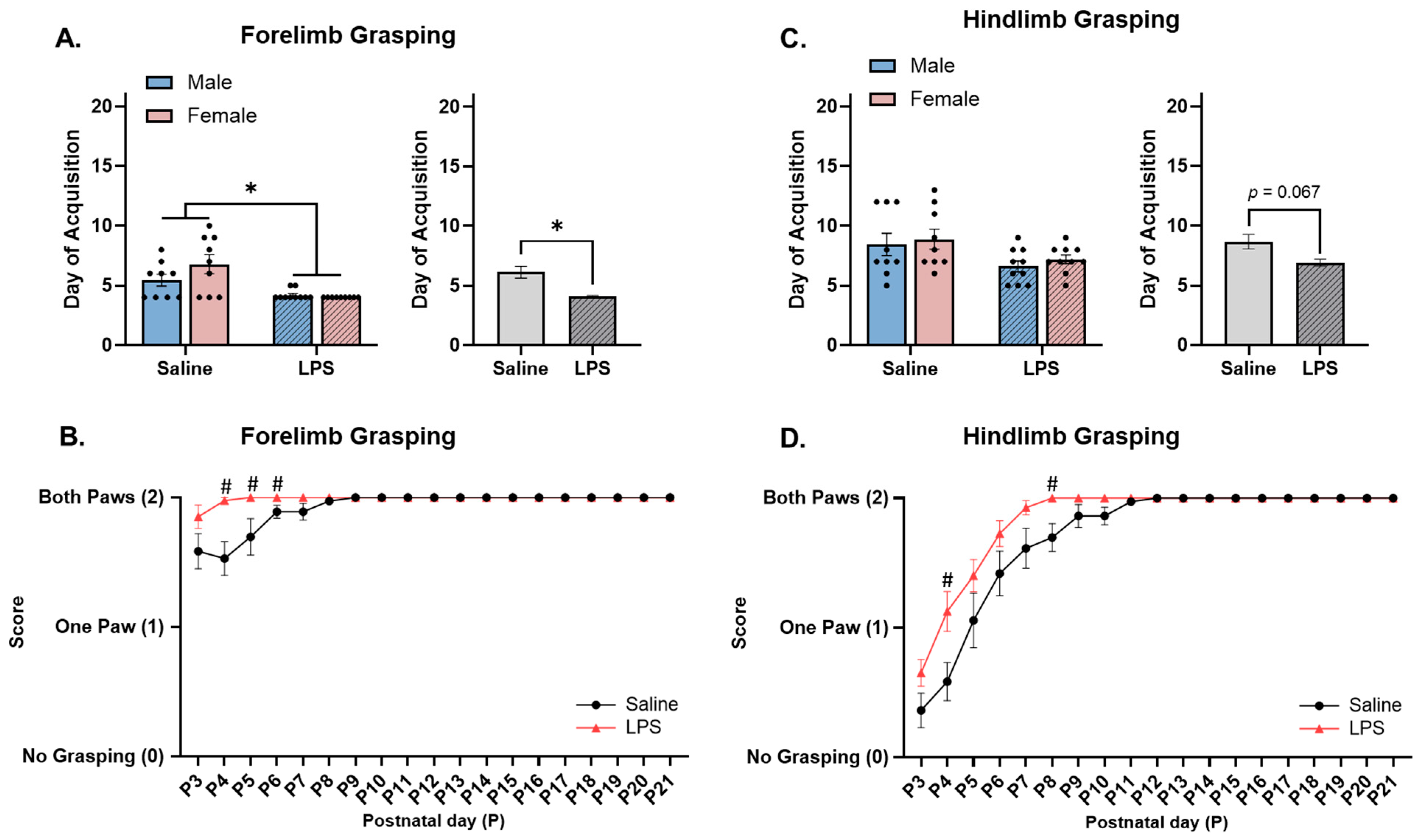
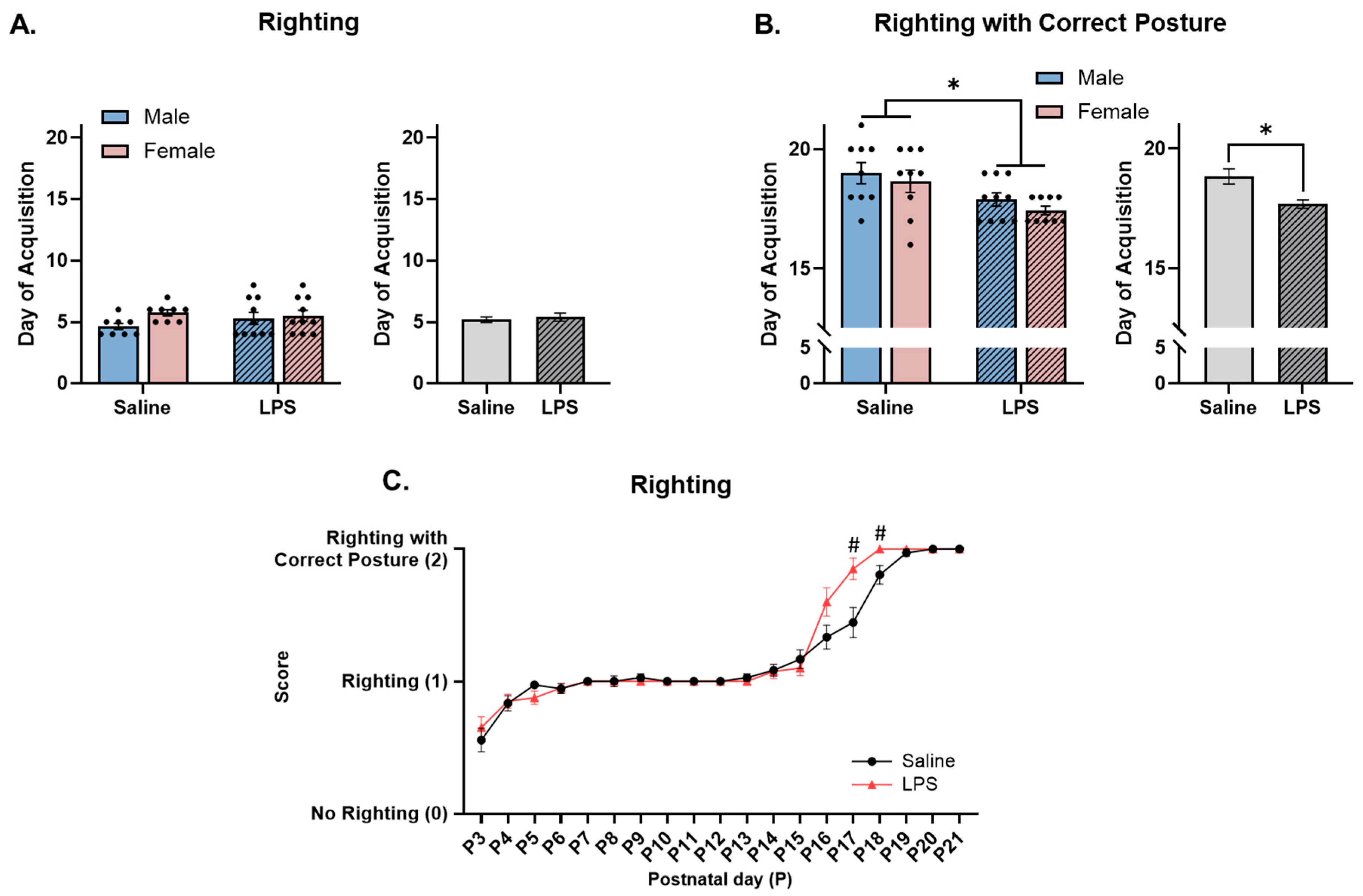
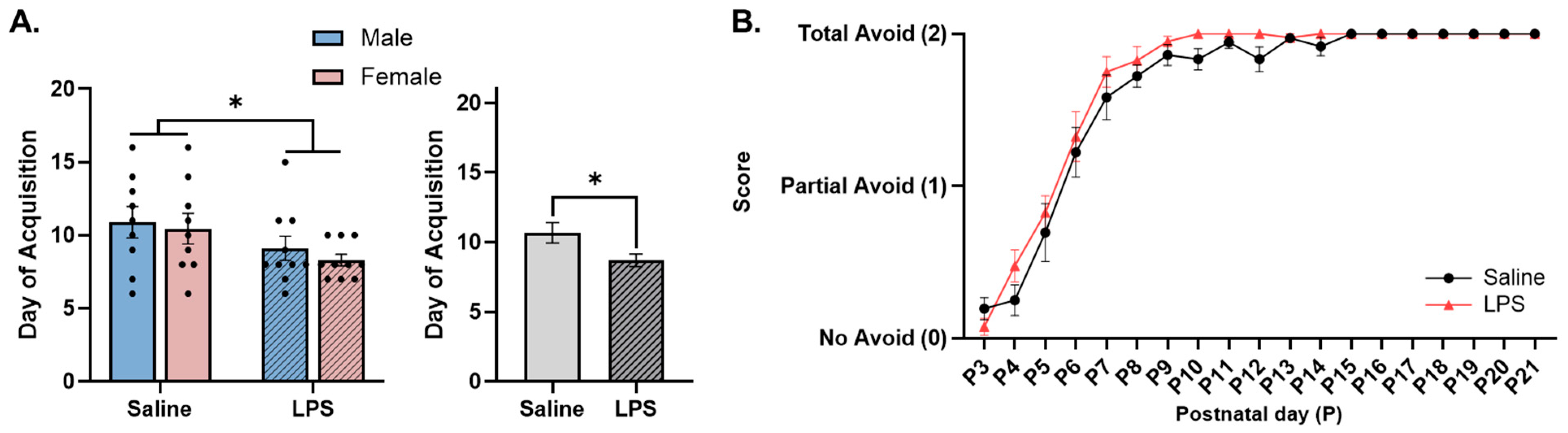
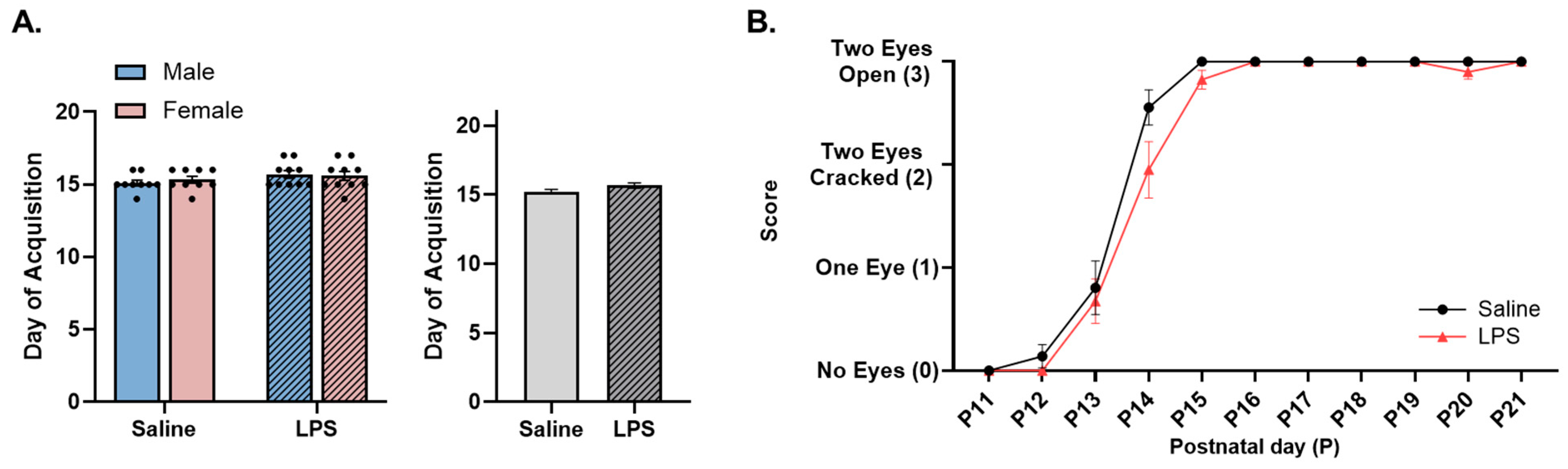
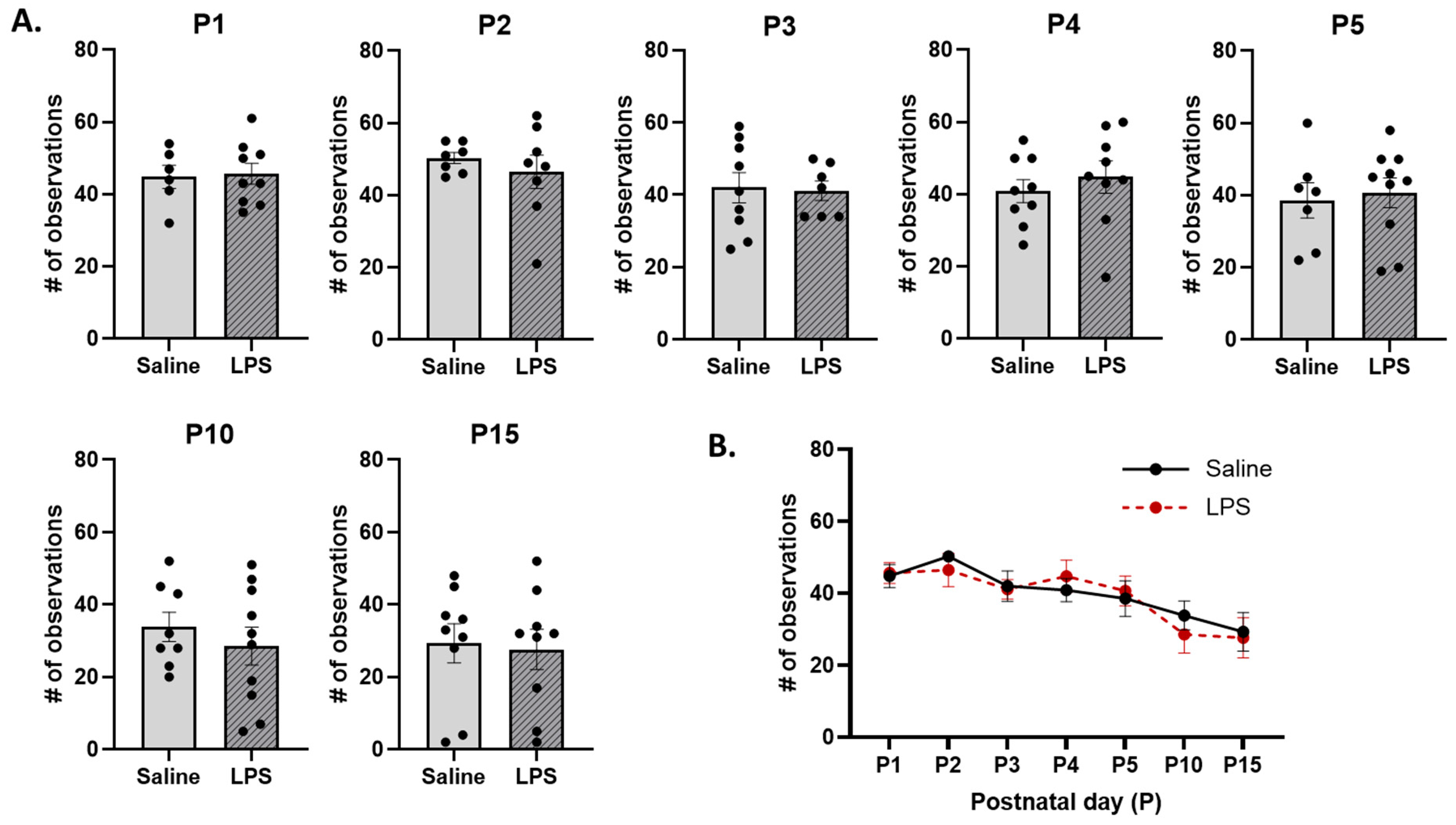
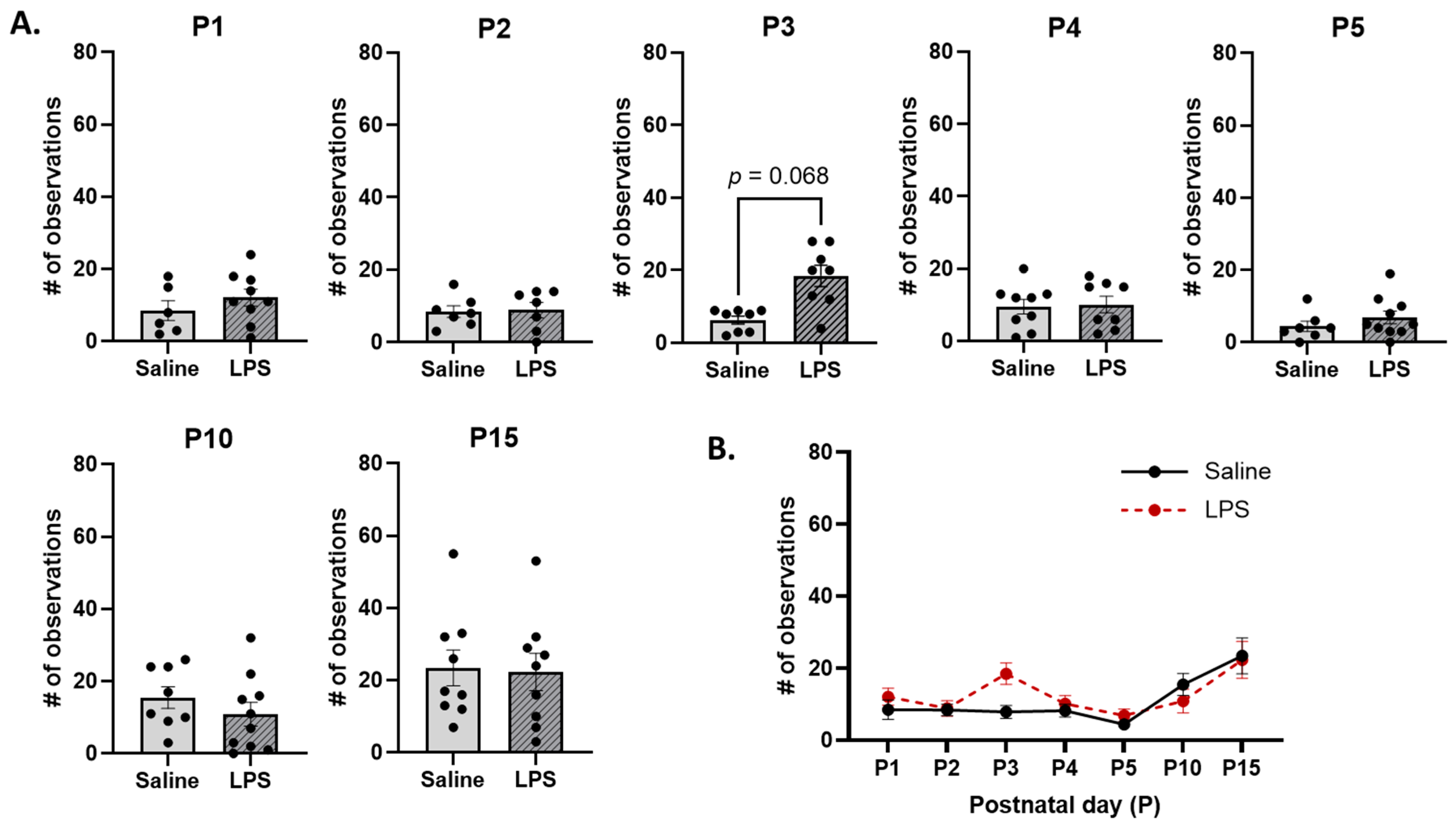
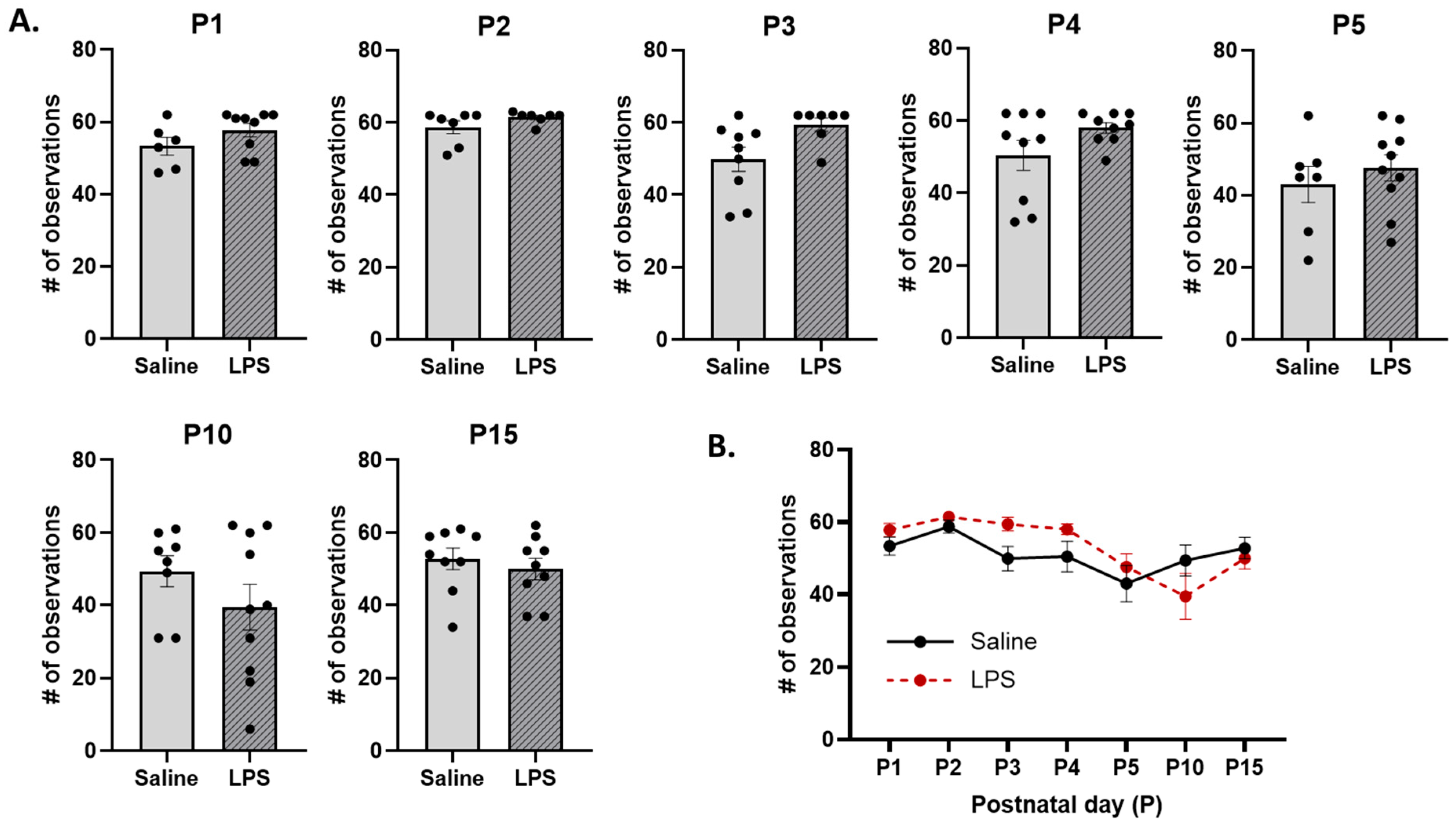
3.2. Neonatal Reflex Acquisition
3.3. Maternal Care
4. Discussion
4.1. MIA Accelerates Acquisition and Improves Performance of Developmental Reflexes in Neonatal Offspring
4.2. Maternal Care Toward Pups Was Not Affected by MIA
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MIA | Maternal immune activation |
| NDDs | Neurodevelopmental disorders |
| LPS | Lipopolysaccharide |
| Poly I:C | Polyinosinic:polycytidylic acid |
| ABN | Arched-back nursing |
| LG | Licking/grooming |
| Non-ABN | Non-arched-back nursing |
References
- Boksa, P. Maternal infection during pregnancy and schizophrenia. J. Psychiatry Neurosci. 2008, 33, 183–185. [Google Scholar] [PubMed]
- Brown, A.S. Epidemiologic studies of exposure to prenatal infection and risk of schizophrenia and autism. Dev. Neurobiol. 2012, 72, 1272–1276. [Google Scholar] [CrossRef] [PubMed]
- Cheslack-Postava, K.; Brown, A.S. Prenatal infection and schizophrenia: A decade of further progress. Schizophr. Res. 2022, 247, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Massarali, A.; Adhya, D.; Srivastava, D.P.; Baron-Cohen, S.; Kotter, M.R. Virus-induced maternal immune activation as an environmental factor in the etiology of autism and schizophrenia. Front. Neuroscience 2022, 16, 834058. [Google Scholar] [CrossRef]
- Hall, M.B.; Willis, D.E.; Rodriguez, E.L.; Schwarz, J.M. Maternal immune activation as an epidemiological risk factor for neurodevelopmental disorders: Considerations of timing, severity, individual differences, and sex in human and rodent studies. Front. Neurosci. 2023, 17, 507. [Google Scholar] [CrossRef]
- Ashdown, H.; Dumont, Y.; Ng, M.; Poole, S.; Boksa, P.; Luheshi, G.N. The role of cytokines in mediating effects of prenatal infection on the fetus: Implications for schizophrenia. Mol. Psychiatry 2005, 11, 47–55. [Google Scholar] [CrossRef]
- Solek, C.M.; Farooqi, N.; Verly, M.; Lim, T.K.; Ruthazer, E.S. Maternal immune activation in neurodevelopmental disorders. Dev. Dyn. 2018, 247, 588–619. [Google Scholar] [CrossRef]
- Boksa, P. Effects of prenatal infection on brain development and behavior: A review of findings from animal models. Brain Behav. Immun. 2010, 24, 881–897. [Google Scholar] [CrossRef]
- Morris-Rosendahl, D.J.; Crocq, M.A. Neurodevelopmental disorders—The history and future of a diagnostic concept. Dialogues Clin. Neurosci. 2020, 22, 65–72. [Google Scholar] [CrossRef]
- CDC. Developmental Disability Basics. U.S. Centers for Disease Control and Prevention, 2024. Available online: https://www.cdc.gov/childdevelopment/about/developmental-disabilitybasics.html?CDC_AAref_Val=https%3A%2F%2Fwww.cdc.gov%2Fncbd%0Add%2Fdevelopmentaldisabilities%2Findex.html (accessed on 17 May 2024).
- Abel, K.M.; Drake, R.; Goldstein, J.M. Sex differences in schizophrenia. Int. Rev. Psychiatry 2010, 22, 417–428. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, S.; Zhang, M.; Xiang, M.; Zhao, J.; Chen, S.; Wang, H.; Han, L.; Ran, J. Prevalence of neurodevelopmental disorders among US children and adolescents in 2019 and 2020. Front. Psychol. 2022, 13, 997648. [Google Scholar] [CrossRef] [PubMed]
- Brašić, J.R.; Holland, J.A. A qualitative and quantitative review of obstetric complications and autistic disorder. J. Dev. Phys. Disabil. 2007, 19, 337–364. [Google Scholar] [CrossRef]
- Fatemi, S.H.; Folsom, T.D. The neurodevelopmental hypothesis of schizophrenia, revisited. Schizophr. Bull. 2009, 35, 528–548. [Google Scholar] [CrossRef] [PubMed]
- Heyer, D.B.; Meredith, R.M. Environmental toxicology: Sensitive periods of development and neurodevelopmental disorders. NeuroToxicology 2017, 58, 23–41. [Google Scholar] [CrossRef]
- Rapoport, J.L.; Giedd, J.N.; Gogtay, N. Neurodevelopmental model of schizophrenia: Update 2012. Mol. Psychiatry 2012, 17, 1228–1238. [Google Scholar] [CrossRef]
- Jain, S.; Baer, R.J.; McCulloch, C.E.; Rogers, E.; Rand, L.; Jelliffe-Pawlowski, L.; Piao, X. Association of maternal immune activation during pregnancy and neurologic outcomes in offspring. J. Pediatr. 2021, 238, 87–93. [Google Scholar] [CrossRef]
- Meredith, R.M. Sensitive and critical periods during neurotypical and aberrant neurodevelopment: A framework for neurodevelopmental disorders. Neurosci. Biobehav. Rev. 2015, 50, 180–188. [Google Scholar] [CrossRef]
- Bonti, E.; Zerva, I.K.; Koundourou, C.; Sofologi, M. The high rates of comorbidity among neurodevelopmental disorders: Reconsidering the clinical utility of distinct diagnostic categories. J. Pers. Med. 2024, 14, 300. [Google Scholar] [CrossRef]
- Harris, S.R. Early motor delays as diagnostic clues in autism spectrum disorder. Eur. J. Pediatr. 2017, 176, 1259–1262. [Google Scholar] [CrossRef]
- Lim, Y.H.; Licari, M.; Spittle, A.J.; Watkins, R.E.; Zwicker, J.G.; Downs, J.; Finlay-Jones, A. Early motor function of children with autism spectrum disorder: A systematic review. Pediatrics 2021, 147, 2020011270. [Google Scholar] [CrossRef]
- Ming, X.; Brimacombe, M.; Wagner, G.C. Prevalence of motor impairment in autism spectrum disorders. Brain Dev. 2007, 29, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Provost, B.; Lopez, B.R.; Heimerl, S. A comparison of motor delays in young children: Autism spectrum disorder, developmental delay, and developmental concerns. J. Autism Dev. Disord. 2007, 37, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, P.; Teitelbaum, O.; Nye, J.; Fryman, J.; Maurer, R.G. Movement analysis in infancy may be useful for early diagnosis of autism. Proc. Natl. Acad. Sci. USA 1998, 95, 13982–13987. [Google Scholar] [CrossRef] [PubMed]
- Filatova, S.; Koivumaa-Honkanen, H.; Hirvonen, N.; Freeman, A.; Ivandic, I.; Hurtig, T.; Khandaker, G.; Jones, P.; Moilanen, K.; Miettunen, J. Early motor developmental milestones and schizophrenia: A systematic review and meta-analysis. Schizophr. Res. 2017, 188, 13–20. [Google Scholar] [CrossRef]
- Keskinen, E.; Marttila, A.; Marttila, R.; Jones, P.B.; Murray, G.K.; Moilanen, K.; Koivumaa-Honkanen, H.; Mäki, P.; Isohanni, M.; Jääskeläinen, E.; et al. Interaction between parental psychosis and early motor development and the risk of schizophrenia in a general population birth cohort. Eur. Psychiatry 2015, 30, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, H.J.; Mortensen, E.L.; Schiffman, J.; Reinisch, J.M.; Maeda, J.; Mednick, S.A. Early developmental milestones and risk of schizophrenia: A 45-year follow-up of the Copenhagen Perinatal Cohort. Schizophr. Res. 2010, 118, 41–47. [Google Scholar] [CrossRef]
- Gabis, L.V.; Shaham, M.; Leon Attia, O.; Shefer, S.; Rosenan, R.; Gabis, T.; Daloya, M. The weak link: Hypotonia in infancy and autism early identification. Front. Neurol. 2021, 12, 612674. [Google Scholar] [CrossRef]
- Straathof, E.J.M.; Heineman, K.R.; Hamer, E.G.; Hadders-Algra, M. Patterns of atypical muscle tone in the general infant population—Prevalence and associations with perinatal risk and neurodevelopmental status. Early Hum. Dev. 2021, 152, 105276. [Google Scholar] [CrossRef]
- Haida, O.; Al Sagheer, T.; Balbous, A.; Francheteau, M.; Matas, E.; Soria, F.; Fernagut, P.O.; Jaber, M. Sex-dependent behavioral deficits and neuropathology in a maternal immune activation model of autism. Transl. Psychiatry 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Arsenault, D.; St-Amour, I.; Cisbani, G.; Rousseau, L.S.; Cicchetti, F. The different effects of LPS and poly I: C prenatal immune challenges on the behavior, development and inflammatory responses in pregnant mice and their offspring. Brain Behav. Immun. 2014, 38, 77–90. [Google Scholar] [CrossRef]
- Malkova, N.V.; Yu, C.Z.; Hsiao, E.Y.; Moore, M.J.; Patterson, P.H. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav. Immun. 2012, 26, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Harvey, L.; Boksa, P. Do prenatal immune activation and maternal iron deficiency interact to affect neurodevelopment and early behavior in rat offspring? Brain Behav. Immun. 2014, 35, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Straley, M.E.; Van Oeffelen, W.; Theze, S.; Sullivan, A.M.; O’Mahony, S.M.; Cryan, J.F.; O’Keeffe, G.W. Distinct alterations in motor & reward seeking behavior are dependent on the gestational age of exposure to LPS-induced maternal immune activation. Brain Behav. Immun. 2017, 63, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Meaney, M.J. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu. Rev. Neurosci. 2001, 24, 1161–1192. [Google Scholar] [CrossRef]
- Smith, J.W.; Seckl, J.R.; Evans, A.T.; Costall, B.; Smythe, J.W. Gestational stress induces post-partum depression-like behaviour and alters maternal care in rats. Psychoneuroendocrinology 2004, 29, 227–244. [Google Scholar] [CrossRef]
- Champagne, F.A.; Francis, D.D.; Mar, A.; Meaney, M.J. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol. Behav. 2003, 79, 359–371. [Google Scholar] [CrossRef]
- Kirsten, T.B.; Chaves, G.P.; Taricano, M.; Martins, D.O.; Flório, J.C.; Britto, L.R.G.; Torrão, A.d.S.; Palermo-Neto, J.; Bernardi, M.M. Prenatal LPS exposure reduces olfactory perception in neonatal and adult rats. Physiol. Behav. 2011, 104, 417–422. [Google Scholar] [CrossRef]
- Penteado, S.; Gomes, C.d.O.M.-S.; Kirsten, T.; Reis-Silva, T.; de Melo, R.C.; Acenjo, M.; Queiroz-Hazarbassanov, N.; Bernardi, M.M. Prenatal lipopolysaccharide increases maternal behavior, decreases maternal odor preference, and induces lipopolysaccharide hyporesponsiveness. Psychol. Neurosci. 2013, 6, 31–38. [Google Scholar] [CrossRef]
- Bernardi, M.M.; Kirsten, T.B.; Matsuoka, S.M.; Teodorov, E.; Habr, S.F.; Penteado, S.H.W.N.; Palermo-Neto, J. Prenatal lipopolysaccharide exposure affects maternal behavior and male offspring sexual behavior in adulthood. Neuroimmunomodulation 2010, 17, 47–55. [Google Scholar] [CrossRef]
- Ronovsky, M.; Berger, S.; Zambon, A.; Reisinger, S.N.; Horvath, O.; Pollak, A.; Lindtner, C.; Berger, A.; Pollak, D.D. Maternal immune activation transgenerationally modulates maternal care and offspring depression-like behavior. Brain Behav. Immun. 2017, 63, 127–136. [Google Scholar] [CrossRef]
- Guma, E.; Plitman, E.; Chakravarty, M.M. The role of maternal immune activation in altering the neurodevelopmental trajectories of offspring: A translational review of neuroimaging studies with implications for autism spectrum disorder and schizophrenia. Neurosci. Biobehav. Rev. 2019, 104, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Pinares-Garcia, P.; Stratikopoulos, M.; Zagato, A.; Loke, H.; Lee, J. Sex: A significant risk factor for neurodevelopmental and neurodegenerative disorders. Brain Sci. 2018, 8, 154. [Google Scholar] [CrossRef] [PubMed]
- Kentner, A.C.; Bilbo, S.D.; Brown, A.S.; Hsiao, E.Y.; McAllister, A.K.; Meyer, U.; Pearce, B.D.; Pletnikov, M.V.; Snyder, S.H.; Yolken, R.H.; et al. Maternal immune activation: Reporting guidelines to improve the rigor, reproducibility, and transparency of the model. Neuropsychopharmacology 2019, 44, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.; Hofsink, N.; Plösch, T. LPS vs. Poly I:C model: Comparison of long-term effects of bacterial and viral maternal immune activation (MIA) on the offspring. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2022, 322, R99–R111. [Google Scholar] [CrossRef]
- Fortier, M.E.; Luheshi, G.N.; Boksa, P. Effects of prenatal infection on prepulse inhibition in the rat depend on the nature of the infectious agent and the stage of pregnancy. Behav. Brain Res. 2007, 181, 270–277. [Google Scholar] [CrossRef]
- French, S.S.; Chester, E.M.; Demas, G.E. Maternal immune activation affects litter success, size and neuroendocrine responses related to behavior in adult offspring. Physiol. Behav. 2013, 119, 175–184. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Armstrong, E.A.; Yager, J.Y. Neurodevelopmental reflex testing in neonatal rat pups. J. Vis. Exp. 2017, 2017, 55261. [Google Scholar] [CrossRef]
- Orso, R.; Creutzberg, K.C.; Wearick-Silva, L.E.; Wendt Viola, T.; Tractenberg, S.G.; Benetti, F.; Grassi-Oliveira, R. How early life stress impact maternal care: A systematic review of rodent studies. Front. Behav. Neurosci. 2019, 13, 197. [Google Scholar] [CrossRef]
- Chen, F.; Du, S.; Bian, J.; You, Z.B.; Wu, Y. Chronic hypoxia exposure during pregnancy is associated with a decreased active nursing activity in mother and an abnormal birth weight and postnatal growth in offspring of rats. Horm. Behav. 2012, 61, 504–511. [Google Scholar] [CrossRef]
- Foley, K.A.; Ossenkopp, K.P.; Kavaliers, M.; MacFabe, D.F. Pre- and neonatal exposure to lipopolysaccharide or the enteric metabolite, propionic acid, alters development and behavior in adolescent rats in a sexually dimorphic manner. PLoS ONE 2014, 9, e87072. [Google Scholar] [CrossRef]
- Hameete, B.C.; Fernández-Calleja, J.M.S.; de Groot, M.W.G.D.M.; Oppewal, T.R.; Tiemessen, M.M.; Hogenkamp, A.; de Vries, R.B.; Groenink, L. The poly(I:C)-induced maternal immune activation model; a systematic review and meta-analysis of cytokine levels in the offspring. Brain Behav. Immun.-Health 2021, 11, 100192. [Google Scholar] [CrossRef] [PubMed]
- Meyer, U. Prenatal Poly(I:C) exposure and other developmental immune activation models in rodent systems. Biol. Psychiatry 2014, 75, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Meyer, U.; Yee, B.K.; Feldon, J. The neurodevelopmental impact of prenatal infections at different times of pregnancy: The earlier the worse? Neuroscientist 2007, 13, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.W.; Pang, Y.; Lin, S.; Tien, L.T.; Ma, T.; Rhodes, P.G.; Cai, Z. Minocycline reduces lipopolysaccharide-induced neurological dysfunction and brain injury in the neonatal rat. J. Neurosci. Res. 2005, 82, 71–82. [Google Scholar] [CrossRef]
- Fan, L.W.; Pang, Y.; Lin, S.; Rhodes, P.G.; Cai, Z. Minocycline attenuates lipopolysaccharide-induced white matter injury in the neonatal rat brain. Neuroscience 2005, 133, 159–168. [Google Scholar] [CrossRef]
- Faas, M.M.; Kunnen, A.; Dekker, D.C.; Harmsen, H.J.M.; Aarnoudse, J.G.; Abbas, F.; De Vos, P.; Van Pampus, M.G. Porphyromonas gingivalis and E-coli induce different cytokine production patterns in pregnant women. PLoS ONE 2014, 9, e86355. [Google Scholar] [CrossRef]
- Migale, R.; Herbert, B.R.; Lee, Y.S.; Sykes, L.; Waddington, S.N.; Peebles, D.; Hagberg, H.; Johnson, M.R.; Bennett, P.R.; MacIntyre, D.A. Specific lipopolysaccharide serotypes induce differential maternal and neonatal inflammatory responses in a murine model of preterm labor. Am. J. Pathol. 2015, 185, 2390–2401. [Google Scholar] [CrossRef]
- Schwarz, J.M.; Bilbo, S.D. The immune system and the developing brain. In Colloquium Series on the Developing Brain; Morgan & Claypool Life Sciences: San Rafael, CA, USA, 2011; pp. 1–128. [Google Scholar] [CrossRef]
- Cunningham, C.L.; Martínez-Cerdeño, V.; Noctor, S.C. Diversity of neural precursor cell types in the prenatal macaque cerebral cortex exists largely within the astroglial cell lineage. PLoS ONE 2013, 8, e63848. [Google Scholar] [CrossRef]
- Cunningham, C.L.; Martínez-Cerdeño, V.; Noctor, S.C. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J. Neurosci. 2013, 33, 4216–4233. [Google Scholar] [CrossRef]
- Filipello, F.; Morini, R.; Corradini, I.; Zerbi, V.; Canzi, A.; Michalski, B.; Erreni, M.; Markicevic, M.; Starvaggi-Cucuzza, C.; Otero, K.; et al. The microglial innate immune receptor TREM2 is required for synapse elimination and normal brain connectivity. Immunity 2018, 48, 979–991.e8. [Google Scholar] [CrossRef]
- Li, Q.; Cheng, Z.; Zhou, L.; Darmanis, S.; Neff, N.F.; Okamoto, J.; Gulati, G.; Bennett, M.L.; Sun, L.O.; Clarke, L.E.; et al. Developmental heterogeneity of microglia and brain myeloid cells revealed by deep single-cell RNA sequencing. Neuron 2019, 101, 207–223.e10. [Google Scholar] [CrossRef] [PubMed]
- Lukens, J.R.; Eyo, U.B. Microglia and neurodevelopmental disorders. Annu. Rev. Neurosci. 2022, 45, 425–445. [Google Scholar] [CrossRef] [PubMed]
- Schafer, D.P.; Lehrman, E.K.; Kautzman, A.G.; Koyama, R.; Mardinly, A.R.; Yamasaki, R.; Ransohoff, R.M.; Greenberg, M.E.; Barres, B.A.; Stevens, B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 2012, 74, 691–705. [Google Scholar] [CrossRef]
- Bauman, M.D.; Van de Water, J. Translational opportunities in the prenatal immune environment: Promises and limitations of the maternal immune activation model. Neurobiol. Dis. 2020, 141, 104864. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Bolasco, G.; Pagani, F.; Maggi, L.; Scianni, M.; Panzanelli, P.; Giustetto, M.; Ferreira, T.A.; Guiducci, E.; Dumas, L.; et al. Synaptic pruning by microglia is necessary for normal brain development. Science 2011, 333, 1456–1458. [Google Scholar] [CrossRef]
- Tay, T.L.; Savage, J.C.; Hui, C.W.; Bisht, K.; Tremblay, M.È. Microglia across the lifespan: From origin to function in brain development, plasticity and cognition. J. Physiol. 2017, 595, 1929–1945. [Google Scholar] [CrossRef]
- Zhan, Y.; Paolicelli, R.C.; Sforazzini, F.; Weinhard, L.; Bolasco, G.; Pagani, F.; Vyssotski, A.L.; Bifone, A.; Gozzi, A.; Ragozzino, D.A.; et al. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat. Neurosci. 2014, 17, 400–406. [Google Scholar] [CrossRef]
- Fernández de Cossío, L.; Guzmán, A.; van der Veldt, S.; Luheshi, G.N. Prenatal infection leads to ASD-like behavior and altered synaptic pruning in the mouse offspring. Brain Behav. Immun. 2017, 63, 88–98. [Google Scholar] [CrossRef]
- Matcovitch-Natan, O.; Winter, D.R.; Giladi, A.; Aguilar, S.V.; Spinrad, A.; Sarrazin, S.; Ben-Yehuda, H.; David, E.; González, F.Z.; Perrin, P.; et al. Microglia development follows a stepwise program to regulate brain homeostasis. Science 2016, 353, aad8670. [Google Scholar] [CrossRef]
- Callaghan, B.L.; Tottenham, N. The stress acceleration hypothesis: Effects of early-life adversity on emotion circuits and behavior. Curr. Opin. Behav. Sci. 2016, 7, 76–81. [Google Scholar] [CrossRef]
- Ellis, B.J.; Sheridan, M.A.; Belsky, J.; McLaughlin, K.A. Why and how does early adversity influence development? Toward an integrated model of dimensions of environmental experience. Dev. Psychopathol. 2022, 34, 447–471. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Erickson, M.; Mohammed, R.; Kentner, A.C. Maternal immune activation accelerates puberty initiation and alters mechanical allodynia in male and female C57BL6/J mice. Dev. Psychobiol. 2022, 64, e22278. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B.H.; Oerbeck, B.; Skirbekk, B.; Petrovski, B.É.; Kristensen, H. Neurodevelopmental disorders: Prevalence and comorbidity in children referred to mental health services. Nord. J. Psychiatry 2018, 72, 285–291. [Google Scholar] [CrossRef] [PubMed]
- King-Dowling, S.; Proudfoot, N.A.; Obeid, J. Comorbidity among chronic physical health conditions and neurodevelopmental disorders in childhood. Curr. Dev. Disord. Rep. 2019, 6, 248–258. [Google Scholar] [CrossRef]
- Sparks, A.; Evans, S.G.; Javadi, M.; Lasalandra, B.; Martens, E.; Venkatesh, R.; Vaccarino, I.T.; Vaccarino, A.L. Assessment of anxiety in children with neurodevelopment disorders: Rasch analysis of the Spence Children’s Anxiety Scale. Front. Psychiatry 2024, 15, 1240357. [Google Scholar] [CrossRef]
- Kong, L.; Chen, X.; Liang, Y.; Forsell, Y.; Gissler, M.; Lavebratt, C. Association of preeclampsia and perinatal complications with offspring neurodevelopmental and psychiatric disorders. JAMA Netw. Open 2022, 5, E2145719. [Google Scholar] [CrossRef]
- Nieves, C.; Victoria da Costa Ghignatti, P.; Aji, N.; Bertagnolli, M. Immune cells and infectious diseases in preeclampsia susceptibility. Can. J. Cardiol. 2024, 40, 2340–2355. [Google Scholar] [CrossRef]
- Freitag, C.M.; Rohde, L.A.; Lempp, T.; Romanos, M. Phenotypic and measurement influences on heritability estimates in childhood ADHD. Eur. Child Adolesc. Psychiatry 2010, 19, 311–323. [Google Scholar] [CrossRef]
- Sandin, S.; Lichtenstein, P.; Kuja-Halkola, R.; Hultman, C.; Larsson, H.; Reichenberg, A. The heritability of autism spectrum disorder. JAMA-J. Am. Med. Assoc. 2017, 318, 1182–1184. [Google Scholar] [CrossRef]
- Rommelse, N.N.J.; Franke, B.; Geurts, H.M.; Hartman, C.A.; Buitelaar, J.K. Shared heritability of attention-deficit/hyperactivity disorder and autism spectrum disorder. Eur. Child Adolesc. Psychiatry 2010, 19, 281–295. [Google Scholar] [CrossRef]
- Parenti, I.; Rabaneda, L.G.; Schoen, H.; Novarino, G. Neurodevelopmental disorders: From genetics to functional pathways. Trends Neurosci. 2020, 43, 608–621. [Google Scholar] [CrossRef] [PubMed]
- Bowie, C.R.; Harvey, P.D. Communication abnormalities predict functional outcomes in chronic schizophrenia: Differential associations with social and adaptive functions. Schizophr. Res. 2008, 103, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Mody, M.; Shui, A.M.; Nowinski, L.A.; Golas, S.B.; Ferrone, C.; O’Rourke, J.A.; McDougle, C.J. Communication deficits and the motor system: Exploring patterns of associations in Autism Spectrum Disorder (ASD). J. Autism Dev. Disord. 2017, 47, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Cascio, C.J. Somatosensory processing in neurodevelopmental disorders. J. Neurodev. Disord. 2010, 2, 62–69. [Google Scholar] [CrossRef]
- Javitt, D.C. Sensory processing in schizophrenia: Neither simple nor intact. Schizophr. Bull. 2009, 35, 1059–1064. [Google Scholar] [CrossRef]
- Suarez, M.A. Sensory processing in children with autism spectrum disorders and impact on functioning. Pediatr. Clin. N. Am. 2012, 59, 203–214. [Google Scholar] [CrossRef]
- Guma, E.; Snook, E.; Spring, S.; Lerch, J.P.; Nieman, B.J.; Devenyi, G.A.; Chakravarty, M.M. Subtle alterations in neonatal neurodevelopment following early or late exposure to prenatal maternal immune activation in mice. NeuroImage Clin. 2021, 32, 102868. [Google Scholar] [CrossRef]
- Moriceau, S.; Sullivan, R.M. Unique neural circuitry for neonatal olfactory learning. J. Neurosci. 2004, 24, 1182–1189. [Google Scholar] [CrossRef]
- Tosta, A.; Fonseca, A.S.; Messender, D.; Siqueira, P.; Ferreira, S.T.; Lourenco, M.V.; Pandolfo, P. Gestational physical exercise prevents early-life behavioral impairments and normalizes dopamine D2 receptor expression in the frontal cortex in the offspring of a rat model of attention deficit hyperactivity disorder. bioRxiv 2023. [Google Scholar] [CrossRef]
- DeRosa, H.; Caradonna, S.G.; Tran, H.; Marrocco, J.; Kentner, A.C. Got milk? Maternal immune activation during the mid-lactational period affects nutritional milk quality and adolescent offspring sensory processing in male and female rats. Mol. Psychiatry 2022, 27, 4829–4842. [Google Scholar] [CrossRef]
- Pendyala, G.; Chou, S.; Jung, Y.; Coiro, P.; Spartz, E.; Padmashri, R.; Li, M.; Dunaevsky, A. Maternal immune activation causes behavioral impairments and altered cerebellar cytokine and synaptic protein expression. Neuropsychopharmacology 2017, 42, 1435–1446. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.L.; Portugal, C.C.; Summavielle, T.; Barbosa, F.; Magalhães, A. Maternal separation effects on mother rodents’ behaviour: A systematic review. Neurosci. Biobehav. Rev. 2020, 117, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.R.; de Azevedo, M.S.; de Souza, M.A.; Lutz, M.L.; Alves, M.B.; Izquierdo, I.; Cammarota, M.; Silveira, P.; Lucion, A. Neonatal handling alters the structure of maternal behavior and affects mother-pup bonding. Behav. Brain Res. 2014, 265, 216–228. [Google Scholar] [CrossRef]
- Barha, C.K.; Pawluski, J.L.; Galea, L.A.M. Maternal care affects male and female offspring working memory and stress reactivity. Physiol. Behav. 2007, 92, 939–950. [Google Scholar] [CrossRef]
- Pan, P.; Fleming, A.S.; Lawson, D.; Jenkins, J.M.; McGowan, P.O. Within- and between-litter maternal care alter behavior and gene regulation in female offspring. Behav. Neurosci. 2014, 128, 736–748. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hall, M.B.; Lemanski, E.A.; Schwarz, J.M. Prenatal Maternal Immune Activation with Lipopolysaccharide Accelerates the Developmental Acquisition of Neonatal Reflexes in Rat Offspring Without Affecting Maternal Care Behaviors. Biomolecules 2025, 15, 347. https://doi.org/10.3390/biom15030347
Hall MB, Lemanski EA, Schwarz JM. Prenatal Maternal Immune Activation with Lipopolysaccharide Accelerates the Developmental Acquisition of Neonatal Reflexes in Rat Offspring Without Affecting Maternal Care Behaviors. Biomolecules. 2025; 15(3):347. https://doi.org/10.3390/biom15030347
Chicago/Turabian StyleHall, Mary Beth, Elise A. Lemanski, and Jaclyn M. Schwarz. 2025. "Prenatal Maternal Immune Activation with Lipopolysaccharide Accelerates the Developmental Acquisition of Neonatal Reflexes in Rat Offspring Without Affecting Maternal Care Behaviors" Biomolecules 15, no. 3: 347. https://doi.org/10.3390/biom15030347
APA StyleHall, M. B., Lemanski, E. A., & Schwarz, J. M. (2025). Prenatal Maternal Immune Activation with Lipopolysaccharide Accelerates the Developmental Acquisition of Neonatal Reflexes in Rat Offspring Without Affecting Maternal Care Behaviors. Biomolecules, 15(3), 347. https://doi.org/10.3390/biom15030347





