Mechanism of Action of Zinc Oxide Nanoparticles as an Antibacterial Agent Against Streptococcus mutans
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization of ZnO NPs by DLS, SEM and STEM
2.3. Bacteria and Cultivation
2.4. MTT Metabolic Assay of Biofilms and CV Biofilm Biomass Staining
2.5. Colony Forming Units (CFU)
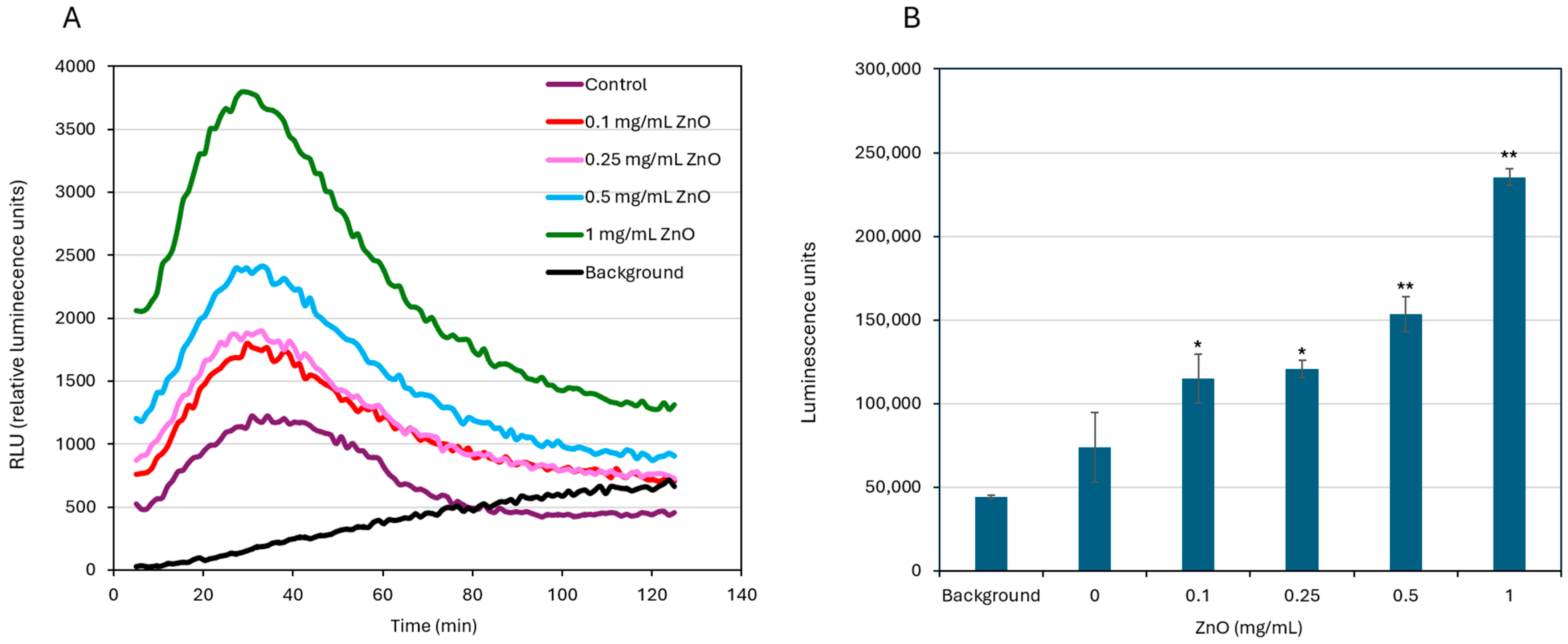
2.6. ATP Content
2.7. pH Measurements
2.8. SYTO 9/PI Live/Dead Staining
2.9. Morphological Imaging by High-Resolution Scanning Electron Microscopy (HR-SEM)
2.10. Energy Dispersive X-Ray Spectroscopy (EDS)
2.11. Determination of Extracellular Polysaccharide (EPS) Production by Congo Red
2.12. ROS Production
2.13. Membrane Potential Determination by Flow Cytometry
2.14. RNA Extraction
2.15. Reverse Transcription (RT) and Quantitative Real-Time PCR
2.16. Cytotoxicity Assay
2.17. Statistical Analysis
3. Results
3.1. Antibacterial and Anti-Biofilm Effects of ZnO NPs on S. mutans
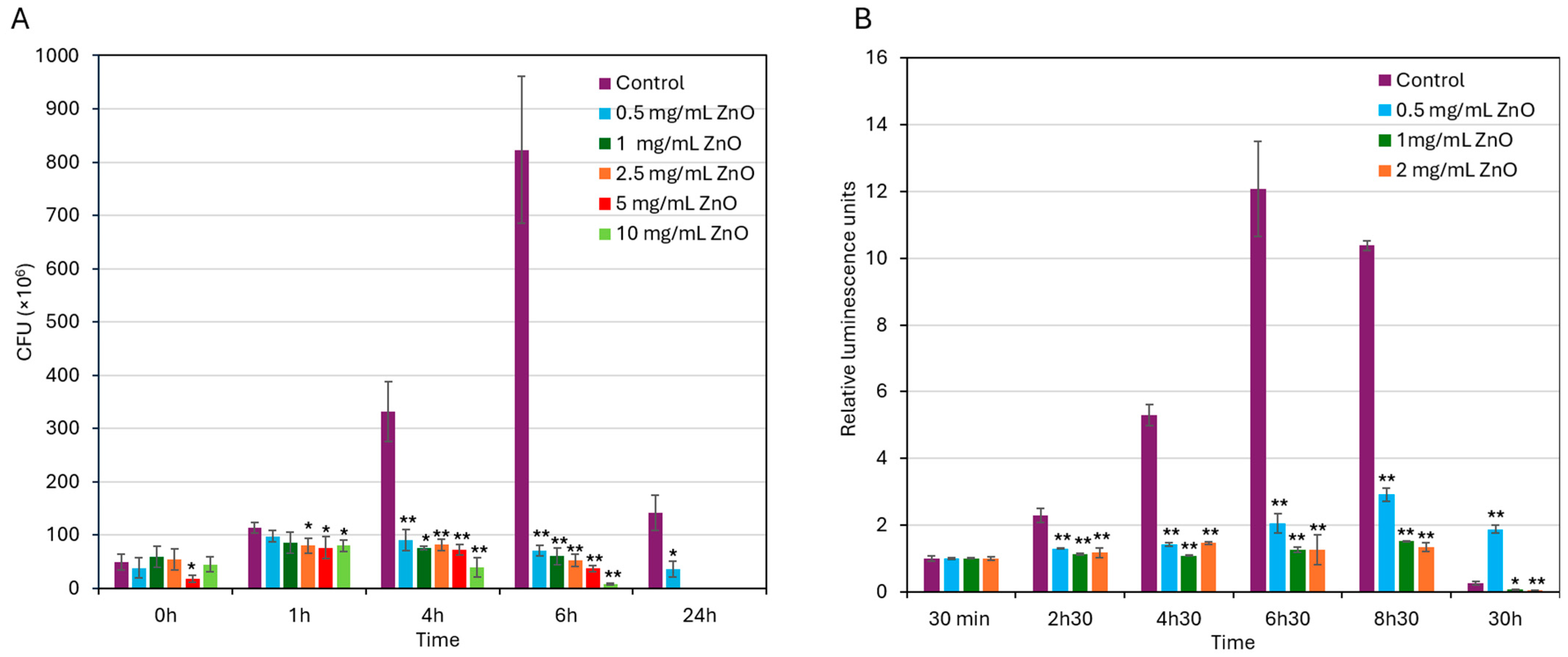
3.2. ZnO NPs Exert a Bacteriostatic Effect That Progresses into a Bactericidal Effect upon Prolonged Exposure
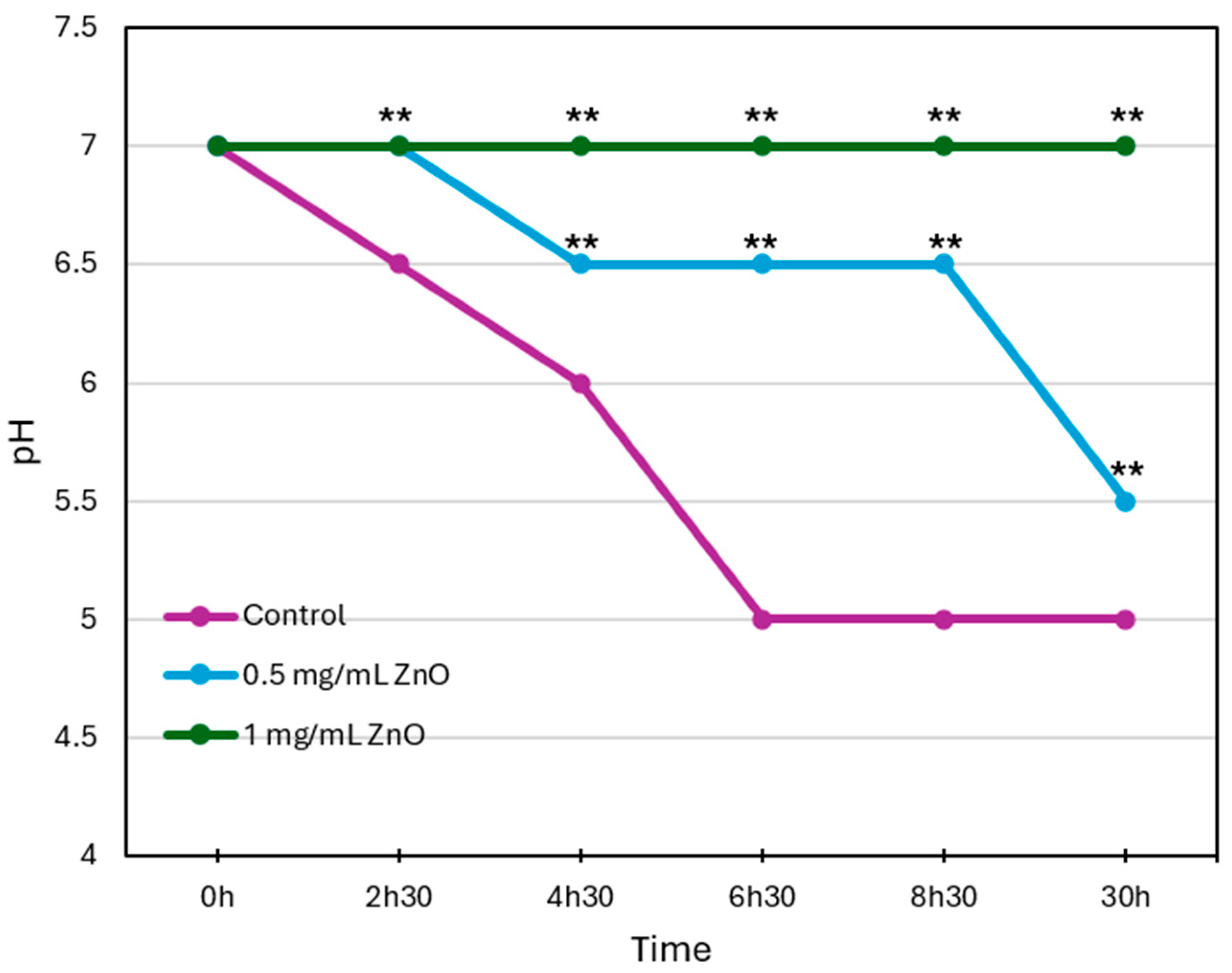
3.3. ZnO NPs Inhibit Acid Production by S. mutans in a Concentration-Dependent Manner
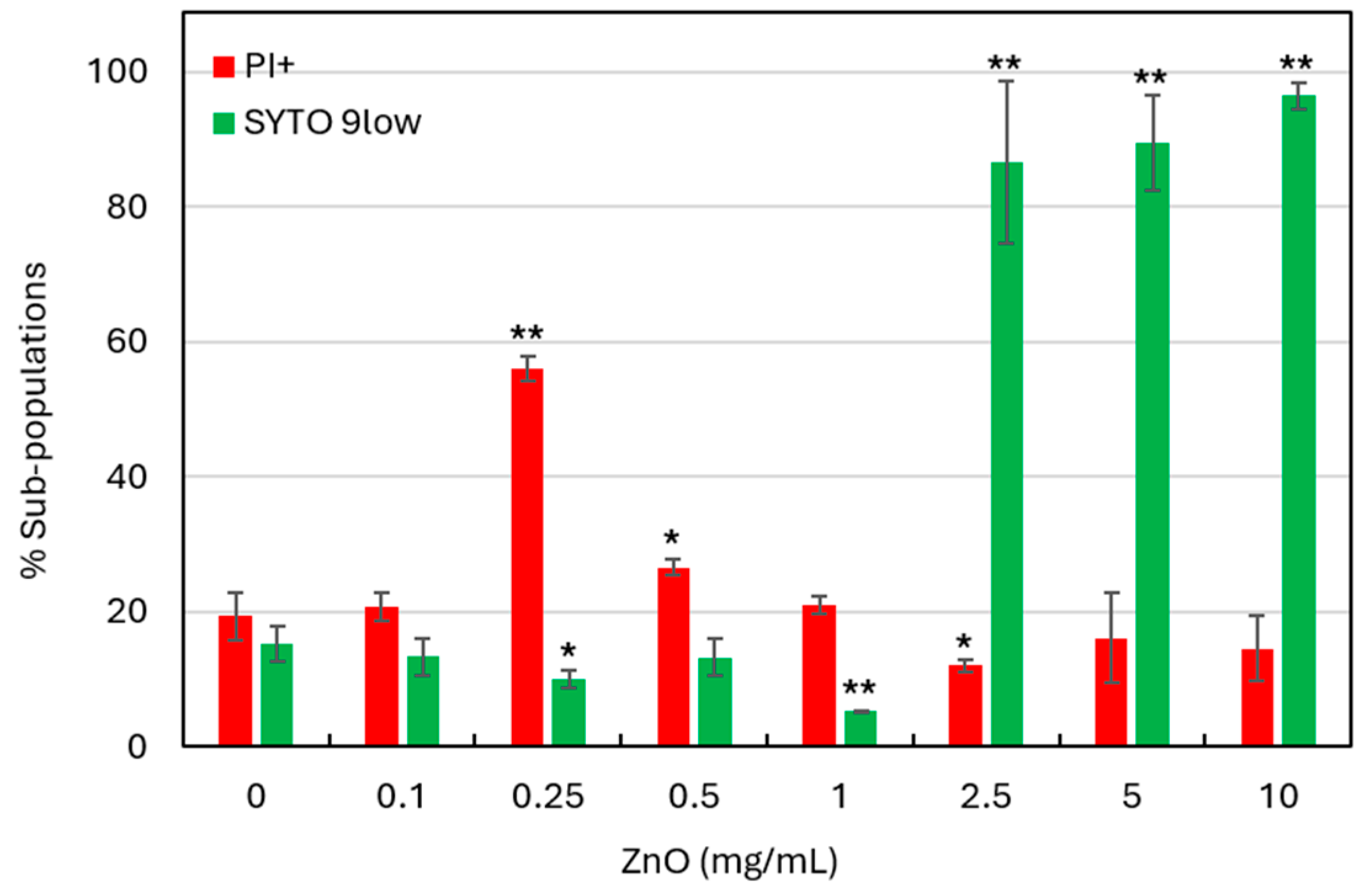
3.4. SYTO 9/PI Live/Dead Staining Suggests That ZnO NPs Induce Cytoplasmic Leakage
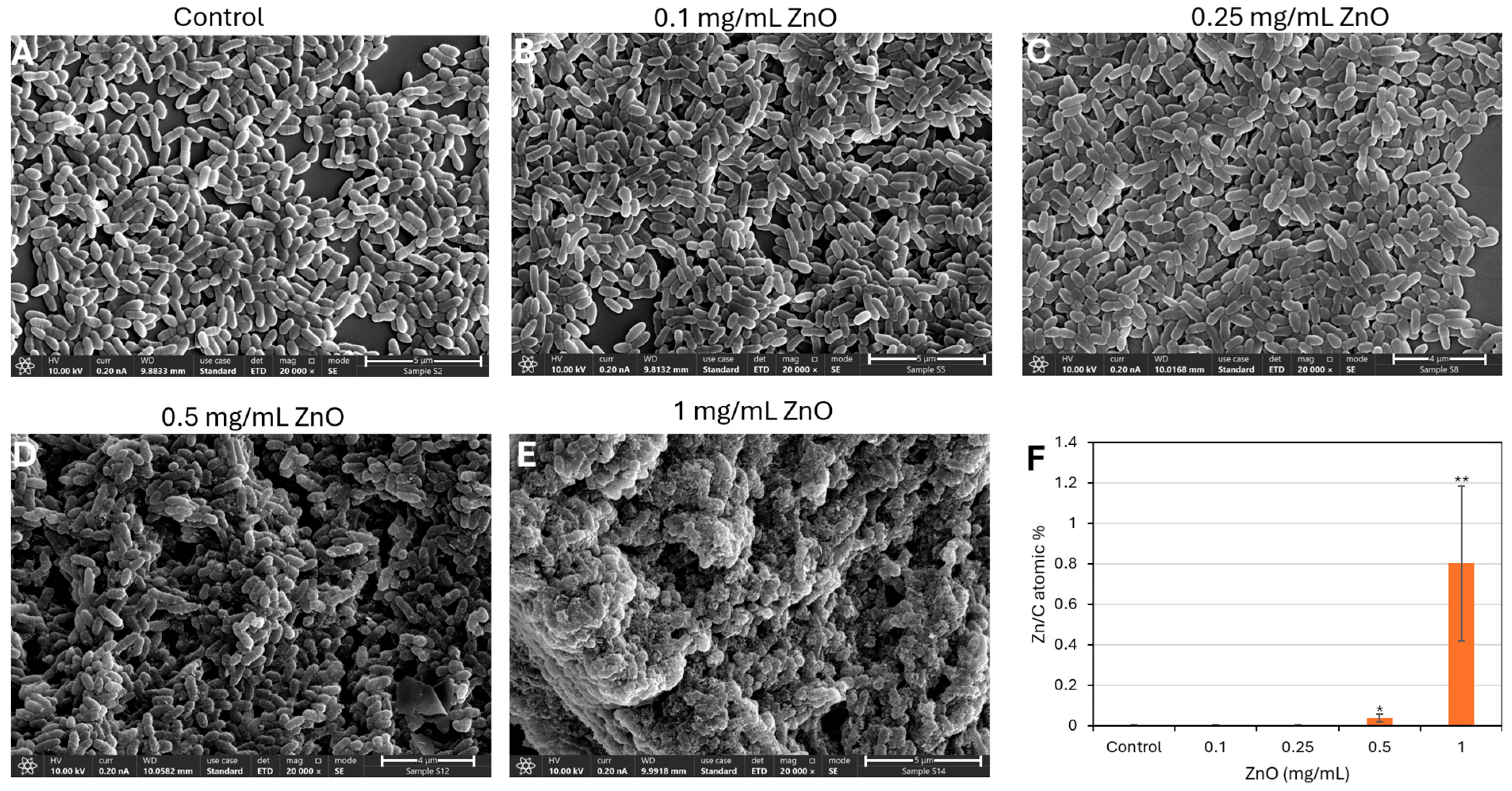
3.5. ZnO NPs-Induced Morphological Changes in S. mutans as Demonstrated by HR-SEM Imaging
3.6. ZnO NPs Induce ROS Production
3.7. ZnO NPs Treatment Causes a Slight Increase in Membrane Potential
3.8. Treatment with ZnO NPs Leads to Reduced EPS Production
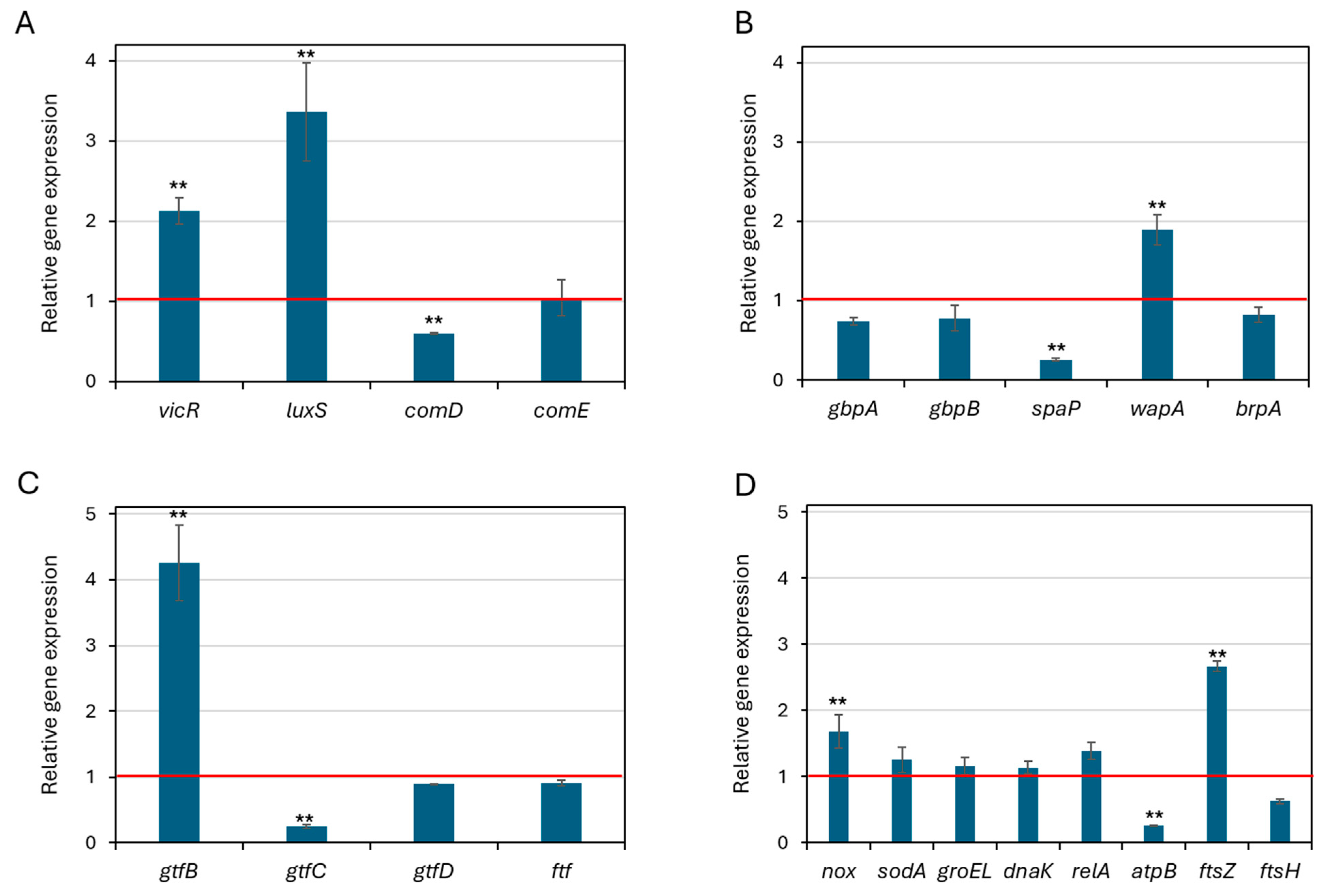
3.9. Effect of ZnO NPs on Gene Expression
3.10. Cytotoxic Effect of ZnO NPs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CFU | Colony forming units |
| CV | Crystal violet |
| EDS | Energy Dispersive X-ray Spectroscopy |
| EPS | Extracellular polysaccharides substance |
| HR-SEM | High-resolution scanning electron microscopy |
| MBIC | Minimum biofilm inhibitory concentration |
| MIC | Minimum inhibitory concentration |
| ROS | Reactive oxygen species |
| S. mutans | Streptococcus mutans |
| ZnO NPs | Zinc oxide nanoparticles |
References
- Valm, A.M. The Structure of Dental Plaque Microbial Communities in the Transition from Health to Dental Caries and Periodontal Disease. J. Mol. Biol. 2019, 431, 2957–2969. [Google Scholar] [CrossRef]
- Wang, C.; Hou, J.; van der Mei, H.C.; Busscher, H.J.; Ren, Y. Emergent Properties in Streptococcus mutans Biofilms Are Controlled through Adhesion Force Sensing by Initial Colonizers. mBio 2019, 10, 10–1128. [Google Scholar] [CrossRef]
- Lemos, J.A.; Palmer, S.R.; Zeng, L.; Wen, Z.T.; Kajfasz, J.K.; Freires, I.A.; Abranches, J.; Brady, L.J. The Biology of Streptococcus mutans. Microbiol. Spectr. 2019, 7, 10-1128. [Google Scholar] [CrossRef]
- Koo, H.; Xiao, J.; Klein, M.I.; Jeon, J.G. Exopolysaccharides produced by Streptococcus mutans glucosyltransferases modulate the establishment of microcolonies within multispecies biofilms. J. Bacteriol. 2010, 192, 3024–3032. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, E.; Imai, S.; Hanada, N.; Hayakawa, T. Biofilm Bormation on Titanium and Hydroxyapatite Surface using Artificial Mouth System. J. Hard Tissue Biol. 2013, 22, 419–424. [Google Scholar] [CrossRef]
- Laosuwan, K.; Epasinghe, D.J.; Wu, Z.; Leung, W.K.; Green, D.W.; Jung, H.S. Comparison of biofilm formation and migration of Streptococcus mutans on tooth roots and titanium miniscrews. Clin. Exp. Dent. Res. 2018, 4, 40–47. [Google Scholar] [CrossRef]
- Dani, S.; Prabhu, A.; Chaitra, K.R.; Desai, N.C.; Patil, S.R.; Rajeev, R. Assessment of Streptococcus mutans in healthy versus gingivitis and chronic periodontitis: A clinico-microbiological study. Contemp. Clin. Dent. 2016, 7, 529–534. [Google Scholar] [CrossRef]
- Fitri, D.K.; Tuygunov, N.; Wan Harun, W.H.A.; Purwasena, I.A.; Cahyanto, A.; Zakaria, M.N. Key virulence genes associated with Streptococcus mutans biofilm formation: A systematic review. Front. Oral Health 2025, 6, 1654428. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Jing, M.; Gong, T.; Yan, J.; Wang, X.; Xu, M.; Zhou, X.; Zeng, J.; Li, Y. Regulatory mechanisms of exopolysaccharide synthesis and biofilm formation in Streptococcus mutans. J. Oral Microbiol. 2023, 15, 2225257. [Google Scholar] [CrossRef]
- Nilsson, M.; Jakobsen, T.H.; Givskov, M.; Twetman, S.; Tolker-Nielsen, T. Oxidative stress response plays a role in antibiotic tolerance of Streptococcus mutans biofilms. Microbiology 2019, 165, 334–342. [Google Scholar] [CrossRef]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef]
- Bowen, W.H.; Koo, H. Biology of Streptococcus mutans-derived glucosyltransferases: Role in extracellular matrix formation of cariogenic biofilms. Caries Res. 2011, 45, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Krzyściak, W.; Jurczak, A.; Kościelniak, D.; Bystrowska, B.; Skalniak, A. The virulence of Streptococcus mutans and the ability to form biofilms. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 499–515. [Google Scholar] [CrossRef]
- Sims, K.R., Jr.; Maceren, J.P.; Liu, Y.; Rocha, G.R.; Koo, H.; Benoit, D.S.W. Dual antibacterial drug-loaded nanoparticles synergistically improve treatment of Streptococcus mutans biofilms. Acta Biomater. 2020, 115, 418–431. [Google Scholar] [CrossRef] [PubMed]
- DeCesaris, D.S.; Hayashi, M.A.L.; Vickerman, M.M.; Rickard, A.H.; Tenuta, L.M.A. Dextranase enhances nanoparticle penetration of S. mutans biofilms. J. Oral Microbiol. 2025, 17, 2528561. [Google Scholar] [CrossRef]
- Sahli, C.; Moya, S.E.; Lomas, J.S.; Gravier-Pelletier, C.; Briandet, R.; Hémadi, M. Recent advances in nanotechnology for eradicating bacterial biofilm. Theranostics 2022, 12, 2383–2405. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. A mini review of antibacterial properties of ZnO nanoparticles. Front. Phys. 2021, 9, 641481. [Google Scholar] [CrossRef]
- Lallo da Silva, B.; Caetano, B.L.; Chiari-Andréo, B.G.; Pietro, R.; Chiavacci, L.A. Increased antibacterial activity of ZnO nanoparticles: Influence of size and surface modification. Colloids Surf. B Biointerfaces 2019, 177, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Vitasovic, T.; Caniglia, G.; Eghtesadi, N.; Ceccato, M.; Bo Jesen, E.D.; Gosewinkel, U.; Neusser, G.; Rupp, U.; Walther, P.; Kranz, C.; et al. Antibacterial Action of Zn2+ Ions Driven by the in vivo Formed ZnO Nanoparticles. ACS Appl. Mater. Interfaces 2024, 16, 30847–30859. [Google Scholar] [CrossRef]
- Lahiri, D.; Ray, R.R.; Sarkar, T.; Upadhye, V.J.; Ghosh, S.; Pandit, S.; Pati, S.; Edinur, H.A.; Abdul Kari, Z.; Nag, M.; et al. Anti-biofilm efficacy of green-synthesized ZnO nanoparticles on oral biofilm: In vitro and in silico study. Front. Microbiol. 2022, 13, 939390. [Google Scholar] [CrossRef]
- Riahi, S.; Moussa, N.B.; Lajnef, M.; Jebari, N.; Dabek, A.; Chtourou, R.; Guisbiers, G.; Vimont, S.; Herth, E. Bactericidal activity of ZnO nanoparticles against multidrug-resistant bacteria. J. Mol. Liq. 2023, 387, 122596. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; Ding, Y.; Povey, M.; York, D. Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids). J. Nanopart. Res. 2007, 9, 479–489. [Google Scholar] [CrossRef]
- Tiwari, V.; Mishra, N.; Gadani, K.; Solanki, P.S.; Shah, N.A.; Tiwari, M. Mechanism of Anti-bacterial Activity of Zinc Oxide Nanoparticle Against Carbapenem-Resistant Acinetobacter baumannii. Front. Microbiol. 2018, 9, 1218. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Dwivedi, S.; Wahab, R.; Khan, F.; Mishra, Y.K.; Musarrat, J.; Al-Khedhairy, A.A. Reactive oxygen species mediated bacterial biofilm inhibition via zinc oxide nanoparticles and their statistical determination. PLoS ONE 2014, 9, e111289. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.G.; Cho, M.H.; Lee, J. ZnO nanoparticles inhibit Pseudomonas aeruginosa biofilm formation and virulence factor production. Microbiol. Res. 2014, 169, 888–896. [Google Scholar] [CrossRef]
- McQuillan, J.S.; Shaw, A.M. Whole-cell Escherichia coli-based bio-sensor assay for dual zinc oxide nanoparticle toxicity mechanisms. Biosens. Bioelectron. 2014, 51, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Pelgrift, R.Y.; Friedman, A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815. [Google Scholar] [CrossRef]
- Zhang, R.; Carlsson, F.; Edman, M.; Hummelgård, M.; Jonsson, B.G.; Bylund, D.; Olin, H. Escherichia coli Bacteria Develop Adaptive Resistance to Antibacterial ZnO Nanoparticles. Adv. Biosyst. 2018, 2, e1800019. [Google Scholar] [CrossRef] [PubMed]
- Andrade, V.; Martínez, A.; Rojas, N.; Bello-Toledo, H.; Flores, P.; Sánchez-Sanhueza, G.; Catalán, A. Antibacterial activity against Streptococcus mutans and diametrical tensile strength of an interim cement modified with zinc oxide nanoparticles and terpenes: An in vitro study. J. Prosthet. Dent. 2018, 119, 862.e861–862.e867. [Google Scholar] [CrossRef]
- Barma, M.D.; Muthupandiyan, I.; Samuel, S.R.; Amaechi, B.T. Inhibition of Streptococcus mutans, antioxidant property and cytotoxicity of novel nano-zinc oxide varnish. Arch. Oral Biol. 2021, 126, 105132. [Google Scholar] [CrossRef]
- Hamad, A.M.; Atiyea, Q.M. In vitro study of the effect of zinc oxide nanoparticles on Streptococcus mutans isolated from human dental caries. J. Phys. Conf. Ser. 2021, 1879, 022041. [Google Scholar] [CrossRef]
- Mirhosseini, F.; Amiri, M.; Daneshkazemi, A.; Zandi, H.; Javadi, Z.S. Antimicrobial Effect of Different Sizes of Nano Zinc Oxide on Oral Microorganisms. Front. Dent. 2019, 16, 105–112. [Google Scholar] [CrossRef]
- Soni, J.; Revathi, D.; Dhanraj, G.; Ramasubburayan, R. Bioinspired green synthesis of ZnO nanoparticles by marine-derived Streptomyces plicatus and its multifaceted biomedicinal properties. Microb. Pathog. 2024, 193, 106758. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Jha, S.; Singh, A.K.; Mishra, S.K.; Pathak, A.K.; Ojha, R.P.; Yadav, R.S.; Dikshit, A. Innovative Investigation of Zinc Oxide Nanoparticles Used in Dentistry. Crystals 2022, 12, 1063. [Google Scholar] [CrossRef]
- Shaik, M.R.; Kandaswamy, K.; Guru, A.; Khan, H.; Giri, J.; Mallik, S.; Shah, M.A.; Arockiaraj, J. Piperine-coated zinc oxide nanoparticles target biofilms and induce oral cancer apoptosis via BCl-2/BAX/P53 pathway. BMC Oral Health 2024, 24, 715. [Google Scholar] [CrossRef]
- Jiang, N.; Zhao, S.; Wang, S.; Lu, Z. Proteomics of Streptococcus mutans to Reveal the Antibiofilm Formation Mechanism of Ag/ZnO Nanocomposites with Light-Emitting Diode Radiation. Int. J. Nanomed. 2021, 16, 7741–7757. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Pi, J.; Cai, J. The Advancing of Zinc Oxide Nanoparticles for Biomedical Applications. Bioinorg. Chem. Appl. 2018, 2018, 1062562. [Google Scholar] [CrossRef]
- Xiao, D.; Huang, Y.; Fang, Z.; Liu, D.; Wang, Q.; Xu, Y.; Li, P.; Li, J. Zinc oxide nanoparticles for skin wound healing: A systematic review from the perspective of disease types. Mater. Today Bio 2025, 34, 102221. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, D.K.; Mohanarangam, M.; Kadirvelu, L.; Sivaramalingam, S.S.; Jothivel, D.; Ravichandran, A.; Periasamy, S.; Kandaswamy, K. Biofilms and oral health: Nanotechnology for biofilm control. Discov. Nano 2025, 20, 114. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hua, H.; Li, W.; Wang, R.; Jiang, X.; Zhu, M. Strong antibacterial dental resin composites containing cellulose nanocrystal/zinc oxide nanohybrids. J. Dent. 2019, 80, 23–29. [Google Scholar] [CrossRef]
- Alves, M.J.; Grenho, L.; Lopes, C.; Borges, J.; Vaz, F.; Vaz, I.P.; Fernandes, M.H. Antibacterial effect and biocompatibility of a novel nanostructured ZnO-coated gutta-percha cone for improved endodontic treatment. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 92, 840–848. [Google Scholar] [CrossRef]
- Eshed, M.; Lellouche, J.; Matalon, S.; Gedanken, A.; Banin, E. Sonochemical coatings of ZnO and CuO nanoparticles inhibit Streptococcus mutans biofilm formation on teeth model. Langmuir 2012, 28, 12288–12295. [Google Scholar] [CrossRef]
- Mohd Bakhori, S.K.; Mahmud, S.; Ling, C.A.; Sirelkhatim, A.H.; Hasan, H.; Mohamad, D.; Masudi, S.M.; Seeni, A.; Abd Rahman, R. In-vitro efficacy of different morphology zinc oxide nanopowders on Streptococcus sobrinus and Streptococcus mutans. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 868–877. [Google Scholar] [CrossRef]
- Zada, M.H.; Kubek, M.; Khan, W.; Kumar, A.; Domb, A. Dispersible hydrolytically sensitive nanoparticles for nasal delivery of thyrotropin releasing hormone (TRH). J. Control. Release 2019, 295, 278–289. [Google Scholar] [CrossRef]
- Chamlagain, M.; Hu, J.; Sionov, R.V.; Steinberg, D. Anti-bacterial and anti-biofilm activities of arachidonic acid against the cariogenic bacterium Streptococcus mutans. Front. Microbiol. 2024, 15, 1333274. [Google Scholar] [CrossRef]
- Sionov, R.V.; Siag, A.; Mersini, E.T.; Kogan, N.M.; Alkhazov, T.; Koman, I.; Rowlo, P.; Gutkin, V.; Gross, M.; Steinberg, D. The Incorporation of CBD into Biodegradable DL-Lactide/Glycolide Copolymers Creates a Persistent Antibacterial Environment: An In Vitro Study on Streptococcus mutans and Staphylococcus aureus. Pharmaceutics 2025, 17, 463. [Google Scholar] [CrossRef]
- Melkam, A.; Sionov, R.V.; Shalish, M.; Steinberg, D. Enhanced Anti-Bacterial Activity of Arachidonic Acid against the Cariogenic Bacterium Streptococcus mutans in Combination with Triclosan and Fluoride. Antibiotics 2024, 13, 540. [Google Scholar] [CrossRef] [PubMed]
- Sionov, R.V.; Tsavdaridou, D.; Aqawi, M.; Zaks, B.; Steinberg, D.; Shalish, M. Tooth mousse containing casein phosphopeptide-amorphous calcium phosphate prevents biofilm formation of Streptococcus mutans. BMC Oral Health 2021, 21, 136. [Google Scholar] [CrossRef] [PubMed]
- Wolfson, G.; Sionov, R.V.; Smoum, R.; Korem, M.; Polacheck, I.; Steinberg, D. Anti-Bacterial and Anti-Biofilm Activities of Anandamide against the Cariogenic Streptococcus mutans. Int. J. Mol. Sci. 2023, 24, 6177. [Google Scholar] [CrossRef] [PubMed]
- Sionov, R.V.; Korem, M.; Polacheck, I.; Steinberg, D. Cannabidiol (CBD) Acts as an Antioxidant on Gardnerella vaginalis, Resulting in Reduced Metabolic Activity, Loss of Survivability, and Elimination of Biofilms. Antibiotics 2025, 14, 136. [Google Scholar] [CrossRef] [PubMed]
- Aqawi, M.; Sionov, R.V.; Gallily, R.; Friedman, M.; Steinberg, D. Anti-Biofilm Activity of Cannabigerol against Streptococcus mutans. Microorganisms 2021, 9, 2031. [Google Scholar] [CrossRef]
- Sionov, R.V.; Assi, S.; Gershkovitz, M.; Sagiv, J.Y.; Polyansky, L.; Mishalian, I.; Fridlender, Z.G.; Granot, Z. Isolation and Characterization of Neutrophils with Anti-Tumor Properties. J. Vis. Exp. 2015, 100, e52933. [Google Scholar] [CrossRef]
- Bernabè, G.; Pauletto, A.; Zamuner, A.; Cassari, L.; Castagliuolo, I.; Brun, P.; Dettin, M. Exploiting Conserved Quorum Sensing Signals in Streptococcus mutans and Streptococcus pneumoniae. Microorganisms 2022, 10, 2386. [Google Scholar] [CrossRef]
- Aqawi, M.; Sionov, R.V.; Friedman, M.; Steinberg, D. The Antibacterial Effect of Cannabigerol toward Streptococcus mutans Is Influenced by the Autoinducers 21-CSP and AI-2. Biomedicines 2023, 11, 668. [Google Scholar] [CrossRef]
- Zamakhaeva, S.; Chaton, C.T.; Rush, J.S.; Ajay Castro, S.; Kenner, C.W.; Yarawsky, A.E.; Herr, A.B.; van Sorge, N.M.; Dorfmueller, H.C.; Frolenkov, G.I.; et al. Modification of cell wall polysaccharide guides cell division in Streptococcus mutans. Nat. Chem. Biol. 2021, 17, 878–887. [Google Scholar] [CrossRef]
- Wright, P.P.; Ramachandra, S.S. Quorum Sensing and Quorum Quenching with a Focus on Cariogenic and Periodontopathic Oral Biofilms. Microorganisms 2022, 10, 1783. [Google Scholar] [CrossRef]
- Zhu, L.; Kreth, J.; Cross, S.E.; Gimzewski, J.K.; Shi, W.; Qi, F. Functional characterization of cell-wall-associated protein WapA in Streptococcus mutans. Microbiology 2006, 152, 2395–2404. [Google Scholar] [CrossRef]
- Yang, J.; Deng, D.; Brandt, B.W.; Nazmi, K.; Wu, Y.; Crielaard, W.; Ligtenberg, A.J.M. Diversity of SpaP in genetic and salivary agglutinin mediated adherence among Streptococcus mutans strains. Sci. Rep. 2019, 9, 19943. [Google Scholar] [CrossRef] [PubMed]
- Lasserre, J.F.; Brecx, M.C.; Toma, S. Oral Microbes, Biofilms and Their Role in Periodontal and Peri-Implant Diseases. Materials 2018, 11, 1802. [Google Scholar] [CrossRef] [PubMed]
- Colombo, A.P.V.; Tanner, A.C.R. The Role of Bacterial Biofilms in Dental Caries and Periodontal and Peri-implant Diseases: A Historical Perspective. J. Dent. Res. 2019, 98, 373–385. [Google Scholar] [CrossRef]
- Busscher, H.J.; Rinastiti, M.; Siswomihardjo, W.; van der Mei, H.C. Biofilm formation on dental restorative and implant materials. J. Dent. Res. 2010, 89, 657–665. [Google Scholar] [CrossRef]
- Arweiler, N.B.; Netuschil, L. The Oral Microbiota. Adv. Exp. Med. Biol. 2016, 902, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Berger, D.; Rakhamimova, A.; Pollack, A.; Loewy, Z. Oral Biofilms: Development, Control, and Analysis. High Throughput 2018, 7, 24. [Google Scholar] [CrossRef]
- Peterson, S.N.; Snesrud, E.; Liu, J.; Ong, A.C.; Kilian, M.; Schork, N.J.; Bretz, W. The dental plaque microbiome in health and disease. PLoS ONE 2013, 8, e58487. [Google Scholar] [CrossRef]
- Colon, G.; Ward, B.C.; Webster, T.J. Increased osteoblast and decreased Staphylococcus epidermidis functions on nanophase ZnO and TiO2. J. Biomed. Mater. Res. A 2006, 78, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhu, L.; Lin, D. Toxicity of ZnO nanoparticles to Escherichia coli: Mechanism and the influence of medium components. Environ. Sci. Technol. 2011, 45, 1977–1983. [Google Scholar] [CrossRef]
- Hernández-Sierra, J.F.; Ruiz, F.; Pena, D.C.; Martínez-Gutiérrez, F.; Martínez, A.E.; Guillén Ade, J.; Tapia-Pérez, H.; Castañón, G.M. The antimicrobial sensitivity of Streptococcus mutans to nanoparticles of silver, zinc oxide, and gold. Nanomedicine 2008, 4, 237–240. [Google Scholar] [CrossRef]
- Barak, T.; Sharon, E.; Steinberg, D.; Feldman, M.; Sionov, R.V.; Shalish, M. Anti-Bacterial Effect of Cannabidiol against the Cariogenic Streptococcus mutans Bacterium: An In Vitro Study. Int. J. Mol. Sci. 2022, 23, 15878. [Google Scholar] [CrossRef]
- Suzuki, T.; Tagami, J.; Hanada, N. Role of F1F0-ATPase in the growth of Streptococcus mutans GS5. J. Appl. Microbiol. 2000, 88, 555–562. [Google Scholar] [CrossRef]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm Matrixome: Extracellular Components in Structured Microbial Communities. Trends Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef]
- Cheng, X.; Xu, X.; Zhou, X.; Ning, J. Oxidative stress response: A critical factor affecting the ecological competitiveness of Streptococcus mutans. J. Oral Microbiol. 2024, 16, 2292539. [Google Scholar] [CrossRef]
- Baker, J.L.; Derr, A.M.; Karuppaiah, K.; MacGilvray, M.E.; Kajfasz, J.K.; Faustoferri, R.C.; Rivera-Ramos, I.; Bitoun, J.P.; Lemos, J.A.; Wen, Z.T.; et al. Streptococcus mutans NADH oxidase lies at the intersection of overlapping regulons controlled by oxygen and NAD+ levels. J. Bacteriol. 2014, 196, 2166–2177. [Google Scholar] [CrossRef]
- Ahn, S.J.; Deep, K.; Turner, M.E.; Ishkov, I.; Waters, A.; Hagen, S.J.; Rice, K.C. Characterization of LrgAB as a stationary phase-specific pyruvate uptake system in Streptococcus mutans. BMC Microbiol. 2019, 19, 223. [Google Scholar] [CrossRef]
- Matsui, R.; Cvitkovitch, D. Acid tolerance mechanisms utilized by Streptococcus mutans. Future Microbiol. 2010, 5, 403–417. [Google Scholar] [CrossRef]
- Wen, Z.T.; Burne, R.A. LuxS-mediated signaling in Streptococcus mutans is involved in regulation of acid and oxidative stress tolerance and biofilm formation. J. Bacteriol. 2004, 186, 2682–2691. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, A.; Ansai, T.; Takehara, T.; Kuramitsu, H.K. LuxS-based signaling affects Streptococcus mutans biofilm formation. Appl. Environ. Microbiol. 2005, 71, 2372–2380. [Google Scholar] [CrossRef]
- Sztajer, H.; Lemme, A.; Vilchez, R.; Schulz, S.; Geffers, R.; Yip, C.Y.; Levesque, C.M.; Cvitkovitch, D.G.; Wagner-Döbler, I. Autoinducer-2-regulated genes in Streptococcus mutans UA159 and global metabolic effect of the luxS mutation. J. Bacteriol. 2008, 190, 401–415. [Google Scholar] [CrossRef]
- Sekiya, M.; Izumisawa, S.; Iwamoto-Kihara, A.; Fan, Y.; Shimoyama, Y.; Sasaki, M.; Nakanishi-Matsui, M. Proton-pumping F-ATPase plays an important role in Streptococcus mutans under acidic conditions. Arch. Biochem. Biophys. 2019, 666, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, J.M.; Asally, M. The Microbiologist’s Guide to Membrane Potential Dynamics. Trends Microbiol. 2020, 28, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Bottomley, A.L.; Liew, A.T.F.; Kusuma, K.D.; Peterson, E.; Seidel, L.; Foster, S.J.; Harry, E.J. Coordination of Chromosome Segregation and Cell Division in Staphylococcus aureus. Front. Microbiol. 2017, 8, 1575. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Lutkenhaus, J. At the Heart of Bacterial Cytokinesis: The Z Ring. Trends Microbiol. 2019, 27, 781–791. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Yuan, C.; Liu, S.; Xin, F.; Deng, X.; Wang, X. Streptococcus mutans cell division protein FtsZ has higher GTPase and polymerization activities in acidic environment. Mol. Oral Microbiol. 2022, 37, 97–108. [Google Scholar] [CrossRef]
- Mozaffari, A.; Mirzapour, S.M.; Rad, M.S.; Ranjbaran, M. Cytotoxicity of PLGA-zinc oxide nanocomposite on human gingival fibroblasts. J. Adv. Periodontol. Implant. Dent. 2023, 15, 28–34. [Google Scholar] [CrossRef]
- Seker, S.; Elçin, A.E.; Yumak, T.; Sınağ, A.; Elçin, Y.M. In vitro cytotoxicity of hydrothermally synthesized ZnO nanoparticles on human periodontal ligament fibroblast and mouse dermal fibroblast cells. Toxicol. In Vitr. 2014, 28, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Selim, Y.A.; Azb, M.A.; Ragab, I.; HM Abd El-Azim, M. Green Synthesis of Zinc Oxide Nanoparticles Using Aqueous Extract of Deverra tortuosa and their Cytotoxic Activities. Sci. Rep. 2020, 10, 3445. [Google Scholar] [CrossRef] [PubMed]
- Pushpalatha, C.; Suresh, J.; Gayathri, V.S.; Sowmya, S.V.; Augustine, D.; Alamoudi, A.; Zidane, B.; Mohammad Albar, N.H.; Patil, S. Zinc Oxide Nanoparticles: A Review on Its Applications in Dentistry. Front. Bioeng. Biotechnol. 2022, 10, 917990. [Google Scholar] [CrossRef]



| Genes | Forward Primers | Reverse Primers | Biological Function |
|---|---|---|---|
| gyrA | TACAGGTGATGTCATGGGTAAATA C | CCG GGT AGT ACT TCC ATT AGG TCA C | DNA gyrase subunit; housekeeping gene. |
| gtfB | AGCAATGCAGCCAATCTACAAAT | ACGAACTTTGCCGTTATTGTCA | Glycosyltransferase-I; makes water-insoluble glucans (EPS). |
| gtfC | GGTTTAACGTCAAAATTAGCTGTATT | CTCAACCAACCGCCACTGTT | Glycosyltransferase-SI; makes both water soluble and water-insoluble glucans (EPS). |
| gtfD | CAGGCAGCCAACGCATTAA | AGCCCTCGCTCATCATAAGC | Glucosyltransferase-D, makes water-soluble glucans. |
| ftf | AAATATGAAGGCGGCTACAACG | CTTCACCAGTCTTAGCATCCTGAA | Fructosyltransferase; makes fructans (EPS). |
| gbpA | GGTGGTTCTGTGCCTGATGA | TTGCCAGCCTGATACACGTT | Glucan-binding protein, promotes adhesion and biofilm stability. |
| gbpB | AGGGCAATGTACTTGGGGTG | TTTGGCCACCTTGAACACCT | Glucan-binding protein B; binds glucans, regulates biofilm formation. |
| spaP | GACTTTGGTAATGGTTATGCATCAA | TTTGTATCAGCCGGATCAAGTG | Surface antigen I/II; adhesion (sucrose-independent biofilm). |
| wapA | GCACGCTTGCAGTACATTGC | CATAAGGTCGCGAGCAGCT | Wall protein; adhesion and biofilm |
| brpA | GGAGGAGCTGCATCAGGATTC | AACTCCAGCACATCCAGCAAG | Biofilm-regulating protein A; controls biofilm formation, acid and oxidative stress, and cell division. |
| nox | GGGTTGTGGAATGGCACTTTGG | CAATGGCTGTCACTGGCGATTC | NADH oxidase; ROS scavenger. |
| sodA | GCAGTGCTAAGACTCCCGAATC | TTGCGGAAGTGTGAGATTGGC | Superoxide dismutase; oxidative stress defense. |
| groEL | CCAGGAGCTTTGACTGCGAC | TTGCGGATGATGATGTAGATGGT | Chaperone (Hsp60); stress response. |
| dnaK | GCAGGTCAAGAGGGAGCTCA | CCGCCCTTGTCTGAGAATC | Chaperone (Hsp70); stress response. |
| relA | ACAAAAAGGGTATCGTCCGTACAT | AATCACGCTTGGTATTGCTAATTG | GTP diphosphokinase; stringent response, biofilm regulation. |
| atpB | AGCCAACCTTGGCAACTGAAA | TGTCAGACGGCGTTCAAGGTT | β-subunit of F1F0-ATPase, ATP synthesis and acid tolerance. |
| ftsZ | CAACCAAGAGCACAACAGCAAG | ACGACGAAGATTCCAATCGCC | Cell division protein; Z-ring formation. |
| ftsH | TGTTCCGTTCTTCTCTATTTCTGG | GCACGCTCTGCTTTCTTAGC | ATP-dependent protease, stress response and cell division. |
| vicR | CGCAGTGGCTGAGGAAAATG | ACCTGTGTGTGTCGCTAAGTGATG | Response regulator of cell wall and biofilm genes. |
| luxS | ACTGTTCCCCTTTTGGCTGTC | AACTTGCTTTGATGACTGTGGC | Autoinducer-2 synthase; quorum sensing, biofilm. |
| comD | TGAAAATAGCATAGGTGAGTCAAAG | ATTTAGGTTAGCTGATTAACACTATACAC | Histidine kinase, part of ComDE quorum sensing system. |
| comE | CACAACAACTTATTGACGCTATCCC | TGATTGGCTACTTCCAGTCCTTTC | Response regulator of ComDE, controls competence/biofilm genes. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emram, R.; Sionov, R.V.; Gutkin, V.; Wilensky, A.; Steinberg, D.; Assad, R. Mechanism of Action of Zinc Oxide Nanoparticles as an Antibacterial Agent Against Streptococcus mutans. Biomolecules 2025, 15, 1660. https://doi.org/10.3390/biom15121660
Emram R, Sionov RV, Gutkin V, Wilensky A, Steinberg D, Assad R. Mechanism of Action of Zinc Oxide Nanoparticles as an Antibacterial Agent Against Streptococcus mutans. Biomolecules. 2025; 15(12):1660. https://doi.org/10.3390/biom15121660
Chicago/Turabian StyleEmram, Raphaelle, Ronit Vogt Sionov, Vitaly Gutkin, Asaf Wilensky, Doron Steinberg, and Rawi Assad. 2025. "Mechanism of Action of Zinc Oxide Nanoparticles as an Antibacterial Agent Against Streptococcus mutans" Biomolecules 15, no. 12: 1660. https://doi.org/10.3390/biom15121660
APA StyleEmram, R., Sionov, R. V., Gutkin, V., Wilensky, A., Steinberg, D., & Assad, R. (2025). Mechanism of Action of Zinc Oxide Nanoparticles as an Antibacterial Agent Against Streptococcus mutans. Biomolecules, 15(12), 1660. https://doi.org/10.3390/biom15121660





