From Fat to Brain: Adiponectin as a Mediator of Neuroplasticity in Depression
Abstract
1. Introduction
2. Methods
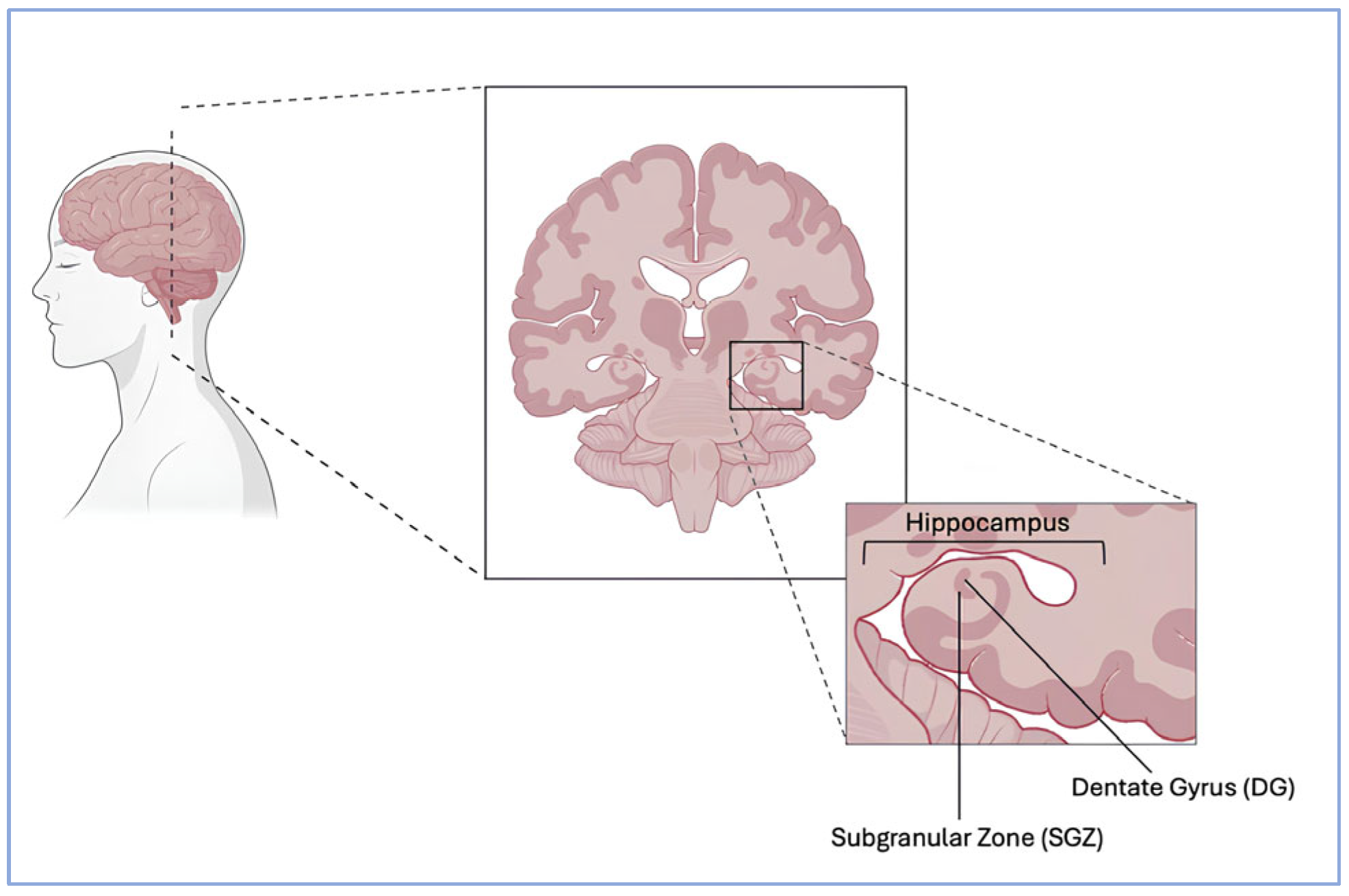
3. Neuroplasticity Dysregulation in Depression
4. Adiponectin: From Peripheral Hormone to Brain Modulator
5. Linking Exercise, Adiponectin, and Neuroplasticity
5.1. How Exercise Enhances Adiponectin Levels: Cellular and Molecular Mechanisms
5.2. Molecular Mechanisms Underlying Adiponectin’s Effects on Neurogenesis and Neuroplasticity
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BDNF | Brain-Derived Neurotrophic Factor |
| CNS | Central Nervous System |
| LTP | Long-Term Potentiation |
| AHN | Adult Hippocampal Neurogenesis |
| NSC | Neural Stem Cells |
| SGZ | Subgranular Zone |
| DG | Dentate Gyrus |
| LTD | Long-Term Depression |
| L-LTP | Late Phase Long-Term Potentiation |
| TRKB | Tropomyosin Receptor Kinase B |
| PLC | Phospholipase C |
| PI3K | Phosphatidylinositol-3 Kinase |
| mTOR | Mammalian Target Of Rapamycin |
| AMPK | AMP-Activated Protein Kinase |
| SSRI | Selective Serotonin Reuptake Inhibitor |
| WAT | White Adipose Tissue |
| HMW | High Molecular Weight |
| ER | Endoplasmatic Reticulum |
| PPAR | Proliferator-Activated Receptor |
| TZD | Thiazolidinedione |
| TNF-α | Tumour Necrosis Factor Alpha |
| IL-6 | Interleukin-6 |
| NF-κB | Nuclear Factor kB |
| BBB | Blood–brain barrier |
| CSF | Cerebrospinal Fluid |
| IFN-α | Interferon Alpha |
| HCV | Hepatitis C Virus |
| TPH2 | Tryptophan Hydroxylase 2 |
| SERT | Serotonin Transporter |
| BMI | Body Mass Index |
| BAT | Brown Adipose Tissue |
| GLUT4 | Glucose Transporter Type 4 |
| LPS | Lipopolysaccharide |
| ROS | Reactive Oxygen Species |
| SOD | Superoxide Dismutase |
References
- Tartt, A.N.; Mariani, M.B.; Hen, R.; Mann, J.J.; Boldrini, M. Dysregulation of Adult Hippocampal Neuroplasticity in Major Depression: Pathogenesis and Therapeutic Implications. Mol. Psychiatry 2022, 27, 2689–2699. [Google Scholar] [CrossRef]
- Tian, L.; Nie, H.; Zhang, Y.; Chen, Y.; Peng, Z.; Cai, M.; Wei, H.; Qin, P.; Dong, H.; Xiong, L. Recombinant Human Thioredoxin-1 Promotes Neurogenesis and Facilitates Cognitive Recovery Following Cerebral Ischemia in Mice. Neuropharmacology 2014, 77, 453–464. [Google Scholar] [CrossRef]
- Borsini, A.; Giacobbe, J.; Mandal, G.; Boldrini, M. Acute and Long-Term Effects of Adolescence Stress Exposure on Rodent Adult Hippocampal Neurogenesis, Cognition, and Behaviour. Mol. Psychiatry 2023, 28, 4124–4137. [Google Scholar] [CrossRef]
- Du Preez, A.; Onorato, D.; Eiben, I.; Musaelyan, K.; Egeland, M.; Zunszain, P.A.; Fernandes, C.; Thuret, S.; Pariante, C.M. Chronic Stress Followed by Social Isolation Promotes Depressive-like Behaviour, Alters Microglial and Astrocyte Biology and Reduces Hippocampal Neurogenesis in Male Mice. Brain. Behav. Immun. 2021, 91, 24–47. [Google Scholar] [CrossRef]
- Lucassen, P.J.; Meerlo, P.; Naylor, A.S.; van Dam, A.M.; Dayer, A.G.; Fuchs, E.; Oomen, C.A.; Czéh, B. Regulation of Adult Neurogenesis by Stress, Sleep Disruption, Exercise and Inflammation: Implications for Depression and Antidepressant Action. Eur. Neuropsychopharmacol. 2010, 20, 1–17. [Google Scholar] [CrossRef]
- Boldrini, M.; Underwood, M.D.; Hen, R.; Rosoklija, G.B.; Dwork, A.J.; John Mann, J.; Arango, V. Antidepressants Increase Neural Progenitor Cells in the Human Hippocampus. Neuropsychopharmacology 2009, 34, 2376–2389. [Google Scholar] [CrossRef]
- Boldrini, M.; Hen, R.; Underwood, M.D.; Rosoklija, G.B.; Dwork, A.J.; Mann, J.J.; Arango, V. Hippocampal Angiogenesis and Progenitor Cell Proliferation Are Increased with Antidepressant Use in Major Depression. Biol. Psychiatry 2012, 72, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Agostino, D.; Wu, Y.-T.; Daskalopoulou, C.; Hasan, M.T.; Huisman, M.; Prina, M. Global Trends in the Prevalence and Incidence of Depression:A Systematic Review and Meta-Analysis. J. Affect. Disord. 2021, 281, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Church, T.S.; Thomas, D.M.; Tudor-Locke, C.; Katzmarzyk, P.T.; Earnest, C.P.; Rodarte, R.Q.; Martin, C.K.; Blair, S.N.; Bouchard, C. Trends over 5 Decades in U.S. Occupation-Related Physical Activity and Their Associations with Obesity. PLoS ONE 2011, 6, e19657. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M.T.; Hamilton, D.G.; Zderic, T.W. Sedentary Behavior as a Mediator of Type 2 Diabetes. Med. Sport Sci. 2014, 60, 11–26. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Hood, S.D.; Drummond, P.D. A Review of Lifestyle Factors That Contribute to Important Pathways Associated with Major Depression: Diet, Sleep and Exercise. J. Affect. Disord. 2013, 148, 12–27. [Google Scholar] [CrossRef]
- Yau, S.-Y.; Lee, T.H.-Y.; Li, A.; Xu, A.; So, K.-F. Adiponectin Mediates Running-Restored Hippocampal Neurogenesis in Streptozotocin-Induced Type 1 Diabetes in Mice. Front. Neurosci. 2018, 12, 679. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The Anti-Inflammatory Effects of Exercise: Mechanisms and Implications for the Prevention and Treatment of Disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Cao, L.; Zhou, D. The Impact of Physical Activities on Adolescents’ Rule Consciousness: The Chain Mediation Effect of Friendship Quality and Emotional Intelligence. Front. Public Health 2025, 13, 1581016. [Google Scholar] [CrossRef] [PubMed]
- Pearce, M.; Garcia, L.; Abbas, A.; Strain, T.; Schuch, F.B.; Golubic, R.; Kelly, P.; Khan, S.; Utukuri, M.; Laird, Y.; et al. Association Between Physical Activity and Risk of Depression: A Systematic Review and Meta-Analysis. JAMA Psychiatry 2022, 79, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Dishman, R.K.; McDowell, C.P.; Herring, M.P. Customary Physical Activity and Odds of Depression: A Systematic Review and Meta-Analysis of 111 Prospective Cohort Studies. Br. J. Sports Med. 2021, 55, 926–934. [Google Scholar] [CrossRef]
- Roshanaei-Moghaddam, B.; Katon, W.J.; Russo, J. The Longitudinal Effects of Depression on Physical Activity. Gen. Hosp. Psychiatry 2009, 31, 306–315. [Google Scholar] [CrossRef]
- Vallance, J.K.; Winkler, E.A.H.; Gardiner, P.A.; Healy, G.N.; Lynch, B.M.; Owen, N. Associations of Objectively-Assessed Physical Activity and Sedentary Time with Depression: NHANES (2005–2006). Prev. Med. 2011, 53, 284–288. [Google Scholar] [CrossRef]
- Berlin, A.A.; Kop, W.J.; Deuster, P.A. Depressive Mood Symptoms and Fatigue After Exercise Withdrawal: The Potential Role of Decreased Fitness. Psychosom. Med. 2006, 68, 224–230. [Google Scholar] [CrossRef]
- Soini, E.; Rosenström, T.; Määttänen, I.; Jokela, M. Physical Activity and Specific Symptoms of Depression: A Pooled Analysis of Six Cohort Studies. J. Affect. Disord. 2024, 348, 44–53. [Google Scholar] [CrossRef]
- Blume, G.R.; Royes, L.F.F. Peripheral to Brain and Hippocampus Crosstalk Induced by Exercise Mediates Cognitive and Structural Hippocampal Adaptations. Life Sci. 2024, 352, 122799. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, X.; Lu, X.-Y. Adiponectin Exerts Neurotrophic Effects on Dendritic Arborization, Spinogenesis, and Neurogenesis of the Dentate Gyrus of Male Mice. Endocrinology 2016, 157, 2853–2869. [Google Scholar] [CrossRef]
- Nicolas, S.; Cazareth, J.; Zarif, H.; Guyon, A.; Heurteaux, C.; Chabry, J.; Petit-Paitel, A. Globular Adiponectin Limits Microglia Pro-Inflammatory Phenotype through an AdipoR1/NF-κB Signaling Pathway. Front. Cell. Neurosci. 2017, 11, 352. [Google Scholar] [CrossRef]
- Wang, P.; Liang, Y.; Chen, K.; Yau, S.-Y.; Sun, X.; Cheng, K.K.-Y.; Xu, A.; So, K.-F.; Li, A. Potential Involvement of Adiponectin Signaling in Regulating Physical Exercise-Elicited Hippocampal Neurogenesis and Dendritic Morphology in Stressed Mice. Front. Cell. Neurosci. 2020, 14, 189. [Google Scholar] [CrossRef] [PubMed]
- Kempermann, G.; Gage, F.H.; Aigner, L.; Song, H.; Curtis, M.A.; Thuret, S.; Kuhn, H.G.; Jessberger, S.; Frankland, P.W.; Cameron, H.A.; et al. Human Adult Neurogenesis: Evidence and Remaining Questions. Cell Stem Cell 2018, 23, 25–30. [Google Scholar] [CrossRef]
- Zheng, M.; Zhang, B.; Yau, S.S.Y.; So, K.-F.; Zhang, L.; Ou, H. Exercise Preconditioning Alleviates Ischemia-Induced Memory Deficits by Increasing Circulating Adiponectin. Neural Regen. Res. 2025, 20, 1445–1454. [Google Scholar] [CrossRef]
- Mukherjee, S.; Manahan-Vaughan, D. Role of Metabotropic Glutamate Receptors in Persistent Forms of Hippocampal Plasticity and Learning. Neuropharmacology 2013, 66, 65–81. [Google Scholar] [CrossRef]
- Minal, N.; Nilesh, W.; Akanksha, K. Epigenetic Regulation in Neuroplasticity: Key to Understanding and Treating Neurological Diseases. Chin. J. Appl. Physiol. 2025, 41, e20250011. [Google Scholar] [CrossRef]
- Cramer, S.C.; Sur, M.; Dobkin, B.H.; O’Brien, C.; Sanger, T.D.; Trojanowski, J.Q.; Rumsey, J.M.; Hicks, R.; Cameron, J.; Chen, D.; et al. Harnessing Neuroplasticity for Clinical Applications. Brain J. Neurol. 2011, 134, 1591–1609. [Google Scholar] [CrossRef] [PubMed]
- Mudgal, S.K.; Nath, S.; Chaturvedi, J.; Sharma, S.K.; Joshi, J. Neuroplasticity in Depression: A Narrative Review with Evidence-Based Insights. Psychiatr. Danub. 2022, 34, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Ferrarelli, F. Is Neuroplasticity Key to Treatment Response in Depression? Maybe So. Am. J. Psychiatry 2022, 179, 451–453. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Yang, B.; Rothschild, G.; Mann, J.J.; Sanford, L.D.; Tang, X.; Huang, C.; Wang, C.; Zhang, W. Epigenetic Regulation in Major Depression and Other Stress-Related Disorders: Molecular Mechanisms, Clinical Relevance and Therapeutic Potential. Signal Transduct. Target. Ther. 2023, 8, 309. [Google Scholar] [CrossRef]
- Chen, F.; Bertelsen, A.B.; Holm, I.E.; Nyengaard, J.R.; Rosenberg, R.; Dorph-Petersen, K.-A. Hippocampal Volume and Cell Number in Depression, Schizophrenia, and Suicide Subjects. Brain Res. 2020, 1727, 146546. [Google Scholar] [CrossRef]
- Cotter, D.; Mackay, D.; Chana, G.; Beasley, C.; Landau, S.; Everall, I.P. Reduced Neuronal Size and Glial Cell Density in Area 9 of the Dorsolateral Prefrontal Cortex in Subjects with Major Depressive Disorder. Cereb. Cortex 2002, 12, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Rajkowska, G.; O’Dwyer, G.; Teleki, Z.; Stockmeier, C.A.; Miguel-Hidalgo, J.J. GABAergic Neurons Immunoreactive for Calcium Binding Proteins Are Reduced in the Prefrontal Cortex in Major Depression. Neuropsychopharmacology 2007, 32, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Tunc-Ozcan, E.; Peng, C.-Y.; Zhu, Y.; Dunlop, S.R.; Contractor, A.; Kessler, J.A. Activating Newborn Neurons Suppresses Depression and Anxiety-like Behaviors. Nat. Commun. 2019, 10, 3768. [Google Scholar] [CrossRef]
- Surget, A.; Belzung, C. Adult Hippocampal Neurogenesis Shapes Adaptation and Improves Stress Response: A Mechanistic and Integrative Perspective. Mol. Psychiatry 2022, 27, 403–421. [Google Scholar] [CrossRef]
- Nicolas, S.; Veyssière, J.; Gandin, C.; Zsürger, N.; Pietri, M.; Heurteaux, C.; Glaichenhaus, N.; Petit-Paitel, A.; Chabry, J. Neurogenesis-Independent Antidepressant-like Effects of Enriched Environment Is Dependent on Adiponectin. Psychoneuroendocrinology 2015, 57, 72–83. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, F.; Zhai, M.; He, M.; Hu, Y.; Feng, L.; Li, Y.; Yang, J.; Wu, C. Hyperactive Neuronal Autophagy Depletes BDNF and Impairs Adult Hippocampal Neurogenesis in a Corticosterone-Induced Mouse Model of Depression. Theranostics 2023, 13, 1059–1075. [Google Scholar] [CrossRef]
- Abe, Y.; Erchinger, V.J.; Ousdal, O.T.; Oltedal, L.; Tanaka, K.F.; Takamiya, A. Neurobiological Mechanisms of Electroconvulsive Therapy for Depression: Insights into Hippocampal Volumetric Increases from Clinical and Preclinical Studies. J. Neurochem. 2024, 168, 1738–1750. [Google Scholar] [CrossRef]
- Luo, J.; Feng, Y.; Hong, Z.; Yin, M.; Zheng, H.; Zhang, L.; Hu, X. High-Frequency Repetitive Transcranial Magnetic Stimulation Promotes Neural Stem Cell Proliferation after Ischemic Stroke. Neural Regen. Res. 2024, 19, 1772–1780. [Google Scholar] [CrossRef]
- Zhao, R.; Tian, X.; Xu, H.; Wang, Y.; Lin, J.; Wang, B. Aerobic Exercise Restores Hippocampal Neurogenesis and Cognitive Function by Decreasing Microglia Inflammasome Formation Through Irisin/NLRP3 Pathway. Aging Cell 2025, 24, e70061. [Google Scholar] [CrossRef]
- Bliss, T.V.P.; Cooke, S.F. Long-Term Potentiation and Long-Term Depression: A Clinical Perspective. Clin. Sao Paulo Braz. 2011, 66 (Suppl. S1), 3–17. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Aghajanian, G.K. Synaptic Dysfunction in Depression: Potential Therapeutic Targets. Science 2012, 338, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Marsden, W.N. Synaptic Plasticity in Depression: Molecular, Cellular and Functional Correlates. Prog. Neuropsychopharmacology Biol. Psychiatry 2013, 43, 168–184. [Google Scholar] [CrossRef] [PubMed]
- Bin Ibrahim, M.Z.; Benoy, A.; Sajikumar, S. Long-term Plasticity in the Hippocampus: Maintaining within and ‘Tagging’ between Synapses. FEBS J. 2022, 289, 2176–2201. [Google Scholar] [CrossRef]
- Li, K.; Liu, L.; Zhang, G.; Wang, X.; Gu, T.; Luo, Q.; Sha, S.; Du, Y.; Wu, C.; Chen, L. Activation of Transient Receptor Potential Vanilloid 4 Impairs Long-Term Depression in Nucleus Accumbens and Induces Depressive-like Behavior. Neuropharmacology 2025, 273, 110429. [Google Scholar] [CrossRef]
- Abraham, W.C.; Jones, O.D.; Glanzman, D.L. Is Plasticity of Synapses the Mechanism of Long-Term Memory Storage? Npj Sci. Learn. 2019, 4, 9. [Google Scholar] [CrossRef]
- Liu, W.; Ge, T.; Leng, Y.; Pan, Z.; Fan, J.; Yang, W.; Cui, R. The Role of Neural Plasticity in Depression: From Hippocampus to Prefrontal Cortex. Neural Plast. 2017, 2017, 6871089. [Google Scholar] [CrossRef]
- Csabai, D.; Wiborg, O.; Czéh, B. Reduced Synapse and Axon Numbers in the Prefrontal Cortex of Rats Subjected to a Chronic Stress Model for Depression. Front. Cell. Neurosci. 2018, 12, 24. [Google Scholar] [CrossRef]
- Mahati, K.; Bhagya, V.; Christofer, T.; Sneha, A.; Rao, B.S. Enriched Environment Ameliorates Depression-Induced Cognitive Deficits and Restores Abnormal Hippocampal Synaptic Plasticity. Neurobiol. Learn. Mem. 2016, 134, 379–391. [Google Scholar] [CrossRef]
- Ely, B.A.; Nguyen, T.N.B.; Tobe, R.H.; Walker, A.M.; Gabbay, V. Multimodal Investigations of Reward Circuitry and Anhedonia in Adolescent Depression. Front. Psychiatry 2021, 12, 678709. [Google Scholar] [CrossRef] [PubMed]
- Rygvold, T.W.; Hatlestad-Hall, C.; Elvsåshagen, T.; Moberget, T.; Andersson, S. Long Term Potentiation-like Neural Plasticity and Performance-Based Memory Function. Neurobiol. Learn. Mem. 2022, 196, 107696. [Google Scholar] [CrossRef]
- Baltaci, S.B.; Mogulkoc, R.; Baltaci, A.K. Molecular Mechanisms of Early and Late LTP. Neurochem. Res. 2019, 44, 281–296. [Google Scholar] [CrossRef]
- Kandel, E.R.; Dudai, Y.; Mayford, M.R. The Molecular and Systems Biology of Memory. Cell 2014, 157, 163–186. [Google Scholar] [CrossRef] [PubMed]
- Kowiański, P.; Lietzau, G.; Czuba, E.; Waśkow, M.; Steliga, A.; Moryś, J. BDNF: A Key Factor with Multipotent Impact on Brain Signaling and Synaptic Plasticity. Cell. Mol. Neurobiol. 2018, 38, 579–593. [Google Scholar] [CrossRef]
- Panja, D.; Bramham, C.R. BDNF Mechanisms in Late LTP Formation: A Synthesis and Breakdown. Neuropharmacology 2014, 76, 664–676. [Google Scholar] [CrossRef]
- Cattaneo, A.; Cattane, N.; Begni, V.; Pariante, C.M.; Riva, M.A. The Human BDNF Gene: Peripheral Gene Expression and Protein Levels as Biomarkers for Psychiatric Disorders. Transl. Psychiatry 2016, 6, e958. [Google Scholar] [CrossRef]
- Cavaleri, D.; Moretti, F.; Bartoccetti, A.; Mauro, S.; Crocamo, C.; Carrà, G.; Bartoli, F. The Role of BDNF in Major Depressive Disorder, Related Clinical Features, and Antidepressant Treatment: Insight from Meta-Analyses. Neurosci. Biobehav. Rev. 2023, 149, 105159. [Google Scholar] [CrossRef]
- Correia, A.S.; Cardoso, A.; Vale, N. BDNF Unveiled: Exploring Its Role in Major Depression Disorder Serotonergic Imbalance and Associated Stress Conditions. Pharmaceutics 2023, 15, 2081. [Google Scholar] [CrossRef] [PubMed]
- Kishi, T.; Yoshimura, R.; Ikuta, T.; Iwata, N. Brain-Derived Neurotrophic Factor and Major Depressive Disorder: Evidence from Meta-Analyses. Front. Psychiatry 2018, 8, 308. [Google Scholar] [CrossRef]
- Molendijk, M.L.; Spinhoven, P.; Polak, M.; Bus, B.A.A.; Penninx, B.W.J.H.; Elzinga, B.M. Serum BDNF Concentrations as Peripheral Manifestations of Depression: Evidence from a Systematic Review and Meta-Analyses on 179 Associations (N=9484). Mol. Psychiatry 2014, 19, 791–800. [Google Scholar] [CrossRef]
- Bocchio-Chiavetto, L.; Bagnardi, V.; Zanardini, R.; Molteni, R.; Gabriela Nielsen, M.; Placentino, A.; Giovannini, C.; Rillosi, L.; Ventriglia, M.; Riva, M.A.; et al. Serum and Plasma BDNF Levels in Major Depression: A Replication Study and Meta-Analyses. World J. Biol. Psychiatry 2010, 11, 763–773. [Google Scholar] [CrossRef]
- Wolkowitz, O.M.; Wolf, J.; Shelly, W.; Rosser, R.; Burke, H.M.; Lerner, G.K.; Reus, V.I.; Nelson, J.C.; Epel, E.S.; Mellon, S.H. Serum BDNF Levels before Treatment Predict SSRI Response in Depression. Prog. Neuropsychopharmacology Biol. Psychiatry 2011, 35, 1623–1630. [Google Scholar] [CrossRef]
- Odaira, T.; Nakagawasai, O.; Takahashi, K.; Nemoto, W.; Sakuma, W.; Lin, J.-R.; Tan-No, K. Mechanisms Underpinning AMP-Activated Protein Kinase-Related Effects on Behavior and Hippocampal Neurogenesis in an Animal Model of Depression. Neuropharmacology 2019, 150, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Cao, J.; Liu, X.; Meng, F.; Li, M.; Chen, B.; Zhang, J. AMPK Plays a Dual Role in Regulation of CREB/BDNF Pathway in Mouse Primary Hippocampal Cells. J. Mol. Neurosci. 2015, 56, 782–788. [Google Scholar] [CrossRef]
- Calabrese, F.; Rossetti, A.C.; Racagni, G.; Gass, P.; Riva, M.A.; Molteni, R. Brain-Derived Neurotrophic Factor: A Bridge between Inflammation and Neuroplasticity. Front. Cell. Neurosci. 2014, 8, 430. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, A.; Macchi, F.; Plazzotta, G.; Veronica, B.; Bocchio-Chiavetto, L.; Riva, M.A.; Pariante, C.M. Inflammation and Neuronal Plasticity: A Link between Childhood Trauma and Depression Pathogenesis. Front. Cell. Neurosci. 2015, 9, 40. [Google Scholar] [CrossRef]
- Pedraz-Petrozzi, B.; Insan, S.; Spangemacher, M.; Reinwald, J.; Lamadé, E.K.; Gilles, M.; Deuschle, M.; Sartorius, A. Association between rTMS-Induced Changes in Inflammatory Markers and Improvement in Psychiatric Diseases: A Systematic Review. Ann. Gen. Psychiatry 2024, 23, 31. [Google Scholar] [CrossRef]
- Qian, X.; Zhong, Z.; Zhang, Y.; Qiu, L.; Tan, H. Fluoxetine Mitigates Depressive-like Behaviors in Mice via Anti-Inflammation and Enhancing Neuroplasticity. Brain Res. 2024, 1825, 148723. [Google Scholar] [CrossRef]
- Xiao, K.; Luo, Y.; Liang, X.; Tang, J.; Wang, J.; Xiao, Q.; Qi, Y.; Li, Y.; Zhu, P.; Yang, H.; et al. Beneficial Effects of Running Exercise on Hippocampal Microglia and Neuroinflammation in Chronic Unpredictable Stress-Induced Depression Model Rats. Transl. Psychiatry 2021, 11, 461. [Google Scholar] [CrossRef] [PubMed]
- Holmes, S.E.; Scheinost, D.; Finnema, S.J.; Naganawa, M.; Davis, M.T.; DellaGioia, N.; Nabulsi, N.; Matuskey, D.; Angarita, G.A.; Pietrzak, R.H.; et al. Lower Synaptic Density Is Associated with Depression Severity and Network Alterations. Nat. Commun. 2019, 10, 1529. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Redondo-Flórez, L.; Beltrán-Velasco, A.I.; Martín-Rodríguez, A.; Martínez-Guardado, I.; Navarro-Jiménez, E.; Laborde-Cárdenas, C.C.; Tornero-Aguilera, J.F. The Role of Adipokines in Health and Disease. Biomedicines 2023, 11, 1290. [Google Scholar] [CrossRef]
- Guenther, M.; James, R.; Marks, J.; Zhao, S.; Szabo, A.; Kidambi, S. Adiposity Distribution Influences Circulating Adiponectin Levels. Transl. Res. 2014, 164, 270–277. [Google Scholar] [CrossRef]
- Jonas, M.I.; Kurylowicz, A.; Bartoszewicz, Z.; Lisik, W.; Jonas, M.; Domienik-Karlowicz, J.; Puzianowska-Kuznicka, M. Adiponectin/Resistin Interplay in Serum and in Adipose Tissue of Obese and Normal-Weight Individuals. Diabetol. Metab. Syndr. 2017, 9, 95. [Google Scholar] [CrossRef]
- Yu, N.; Ruan, Y.; Gao, X.; Sun, J. Systematic Review and Meta-Analysis of Randomized, Controlled Trials on the Effect of Exercise on Serum Leptin and Adiponectin in Overweight and Obese Individuals. Horm. Metab. Res. 2017, 49, 164–173. [Google Scholar] [CrossRef]
- Hu, Y.; Dong, X.; Chen, J. Adiponectin and Depression: A Meta-Analysis. Biomed. Rep. 2015, 3, 38–42. [Google Scholar] [CrossRef]
- Arias, C.; Álvarez-Indo, J.; Cifuentes, M.; Morselli, E.; Kerr, B.; Burgos, P.V. Enhancing Adipose Tissue Functionality in Obesity: Senotherapeutics, Autophagy and Cellular Senescence as a Target. Biol. Res. 2024, 57, 51. [Google Scholar] [CrossRef] [PubMed]
- Waki, H.; Yamauchi, T.; Kamon, J.; Ito, Y.; Uchida, S.; Kita, S.; Hara, K.; Hada, Y.; Vasseur, F.; Froguel, P.; et al. Impaired Multimerization of Human Adiponectin Mutants Associated with Diabetes. J. Biol. Chem. 2003, 278, 40352–40363. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, F. Regulation of Adiponectin Multimerization, Signaling and Function. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef]
- Tripathi, Y.B.; Pandey, V. Obesity and Endoplasmic Reticulum (ER) Stresses. Front. Immunol. 2012, 3, 240. [Google Scholar] [CrossRef]
- Khoramipour, K.; Chamari, K.; Hekmatikar, A.A.; Ziyaiyan, A.; Taherkhani, S.; Elguindy, N.M.; Bragazzi, N.L. Adiponectin: Structure, Physiological Functions, Role in Diseases, and Effects of Nutrition. Nutrients 2021, 13, 1180. [Google Scholar] [CrossRef]
- Li, J.; Zou, B.; Yeo, Y.H.; Feng, Y.; Xie, X.; Lee, D.H.; Fujii, H.; Wu, Y.; Kam, L.Y.; Ji, F.; et al. Prevalence, Incidence, and Outcome of Non-Alcoholic Fatty Liver Disease in Asia, 1999–2019: A Systematic Review and Meta-Analysis. Lancet Gastroenterol. Hepatol. 2019, 4, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.W.; Feng, S.; Covinsky, K.E.; Hayssen, H.; Zhou, L.-Q.; Yeh, B.M.; Lai, J.C. A Comparison of Muscle Function, Mass, and Quality in Liver Transplant Candidates: Results From the Functional Assessment in Liver Transplantation Study. Transplantation 2016, 100, 1692–1698. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Y.; Shao, H.; Liu, S.; Niu, Y.; Fu, L. Globular Adiponectin Ameliorates Insulin Resistance in Skeletal Muscle by Enhancing the LKB1-Mediated AMPK Activation via SESN2. Sports Med. Health Sci. 2023, 5, 34–41. [Google Scholar] [CrossRef]
- Blandin, A.; Amosse, J.; Froger, J.; Hilairet, G.; Durcin, M.; Fizanne, L.; Ghesquière, V.; Prieur, X.; Chaigneau, J.; Vergori, L.; et al. Extracellular Vesicles Are Carriers of Adiponectin with Insulin-Sensitizing and Anti-Inflammatory Properties. Cell Rep. 2023, 42, 112866. [Google Scholar] [CrossRef]
- Litvinova, L.; Atochin, D.; Vasilenko, M.; Fattakhov, N.; Zatolokin, P.; Vaysbeyn, I.; Kirienkova, E. Role of Adiponectin and Proinflammatory Gene Expression in Adipose Tissue Chronic Inflammation in Women with Metabolic Syndrome. Diabetol. Metab. Syndr. 2014, 6, 137. [Google Scholar] [CrossRef]
- Ouchi, N.; Walsh, K. Adiponectin as an Anti-Inflammatory Factor. Clin. Chim. Acta 2007, 380, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Ramírez, P.; Malmhäll, C.; Tliba, O.; Rådinger, M.; Bossios, A. Adiponectin/AdipoR1 Axis Promotes IL-10 Release by Human Regulatory T Cells. Front. Immunol. 2021, 12, 677550. [Google Scholar] [CrossRef]
- Basak, S.; Murmu, A.; Matore, B.W.; Roy, P.P.; Singh, J. Thiazolidinedione an Auspicious Scaffold as PPAR-γ Agonist: Its Possible Mechanism to Manoeuvre against Insulin Resistant Diabetes Mellitus. Eur. J. Med. Chem. Rep. 2024, 11, 100160. [Google Scholar] [CrossRef]
- Hui, X.; Lam, K.S.; Vanhoutte, P.M.; Xu, A. Adiponectin and Cardiovascular Health: An Update. Br. J. Pharmacol. 2012, 165, 574–590. [Google Scholar] [CrossRef]
- Honda, M.; Tsuboi, A.; Minato, S.; Kitaoka, K.; Takeuchi, M.; Yano, M.; Kurata, M.; Wu, B.; Kazumi, T.; Fukuo, K. Association of Age and Anemia With Adiponectin Serum Levels in Normal-Weight Japanese Women. J. Clin. Med. Res. 2019, 11, 367–374. [Google Scholar] [CrossRef]
- Bloemer, J.; Pinky, P.D.; Govindarajulu, M.; Hong, H.; Judd, R.; Amin, R.H.; Moore, T.; Dhanasekaran, M.; Reed, M.N.; Suppiramaniam, V. Role of Adiponectin in Central Nervous System Disorders. Neural Plast. 2018, 2018, 4593530. [Google Scholar] [CrossRef]
- Yau, S.Y.; Li, A.; Hoo, R.L.C.; Ching, Y.P.; Christie, B.R.; Lee, T.M.C.; Xu, A.; So, K.-F. Physical Exercise-Induced Hippocampal Neurogenesis and Antidepressant Effects Are Mediated by the Adipocyte Hormone Adiponectin. Proc. Natl. Acad. Sci. USA 2014, 111, 15810–15815. [Google Scholar] [CrossRef]
- Neumeier, M.; Weigert, J.; Buettner, R.; Wanninger, J.; Schäffler, A.; Müller, A.M.; Killian, S.; Sauerbruch, S.; Schlachetzki, F.; Steinbrecher, A.; et al. Detection of Adiponectin in Cerebrospinal Fluid in Humans. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E965–E969. [Google Scholar] [CrossRef] [PubMed]
- Kusminski, C.M.; McTernan, P.G.; Schraw, T.; Kos, K.; O’Hare, J.P.; Ahima, R.; Kumar, S.; Scherer, P.E. Adiponectin Complexes in Human Cerebrospinal Fluid: Distinct Complex Distribution from Serum. Diabetologia 2007, 50, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Clain, J.; Couret, D.; Planesse, C.; Krejbich-Trotot, P.; Meilhac, O.; Lefebvre d’Hellencourt, C.; Viranaicken, W.; Diotel, N. Distribution of Adiponectin Receptors in the Brain of Adult Mouse: Effect of a Single Dose of the Adiponectin Receptor Agonist, AdipoRON, on Ischemic Stroke. Brain Sci. 2022, 12, 680. [Google Scholar] [CrossRef] [PubMed]
- Bloemer, J.; Pinky, P.D.; Smith, W.D.; Bhattacharya, D.; Chauhan, A.; Govindarajulu, M.; Hong, H.; Dhanasekaran, M.; Judd, R.; Amin, R.H.; et al. Adiponectin Knockout Mice Display Cognitive and Synaptic Deficits. Front. Endocrinol. 2019, 10, 819. [Google Scholar] [CrossRef]
- Gorska-Ciebiada, M.; Ciebiada, M. Adiponectin and Inflammatory Marker Levels in the Elderly Patients with Diabetes, Mild Cognitive Impairment and Depressive Symptoms. Int. J. Mol. Sci. 2024, 25, 10804. [Google Scholar] [CrossRef]
- Lehto, S.M.; Huotari, A.; Niskanen, L.; Tolmunen, T.; Koivumaa-Honkanen, H.; Honkalampi, K.; Ruotsalainen, H.; Herzig, K.-H.; Viinamäki, H.; Hintikka, J. Serum Adiponectin and Resistin Levels in Major Depressive Disorder. Acta Psychiatr. Scand. 2010, 121, 209–215. [Google Scholar] [CrossRef]
- Leo, R.; Di Lorenzo, G.; Tesauro, M.; Cola, C.; Fortuna, E.; Zanasi, M.; Troisi, A.; Siracusano, A.; Lauro, R.; Romeo, F. Decreased Plasma Adiponectin Concentration in Major Depression. Neurosci. Lett. 2006, 407, 211–213. [Google Scholar] [CrossRef]
- Zeugmann, S.; Quante, A.; Heuser, I.; Schwarzer, R.; Anghelescu, I. Inflammatory Biomarkers in 70 Depressed Inpatients with and without the Metabolic Syndrome. J. Clin. Psychiatry 2010, 71, 1007–1016. [Google Scholar] [CrossRef]
- Fábregas, B.C.; Vieira, É.L.M.; Moura, A.S.; Carmo, R.A.; Ávila, R.E.; Abreu, M.N.S.; Prossin, A.R.; Teixeira, A.L. A Follow-Up Study of 50 Chronic Hepatitis C Patients: Adiponectin as a Resilience Biomarker for Major Depression. Neuroimmunomodulation 2016, 23, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Lei, Y.; You, J.; Li, C.; Sun, L.; Garza, J.; Zhang, D.; Guo, M.; Scherer, P.E.; Lodge, D.; et al. Adiponectin Modulates Ventral Tegmental Area Dopamine Neuron Activity and Anxiety-Related Behavior through AdipoR1. Mol. Psychiatry 2019, 24, 126–144. [Google Scholar] [CrossRef]
- D’Aprile, I.; Petrillo, G.; Zonca, V.; Mazzelli, M.; De Cillis, F.; Di Benedetto, M.G.; Riva, M.A.; Cattaneo, A. Sex-Specific Metabolic and Inflammatory Alterations in Adult Animals Vulnerable to Prenatal Stress Exposure. Prog. Neuropsychopharmacol. Biol. Psychiatry 2025, 138, 111344. [Google Scholar] [CrossRef]
- Liu, L.; Tang, J.; Liang, X.; Li, Y.; Zhu, P.; Zhou, M.; Qin, L.; Deng, Y.; Li, J.; Wang, Y.; et al. Running Exercise Alleviates Hippocampal Neuroinflammation and Shifts the Balance of Microglial M1/M2 Polarization through Adiponectin/AdipoR1 Pathway Activation in Mice Exposed to Chronic Unpredictable Stress. Mol. Psychiatry 2024, 29, 2031–2042. [Google Scholar] [CrossRef] [PubMed]
- Pousti, F.; Ahmadi, R.; Mirahmadi, F.; Hosseinmardi, N.; Rohampour, K. Adiponectin Modulates Synaptic Plasticity in Hippocampal Dentate Gyrus. Neurosci. Lett. 2018, 662, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.R.; Fasano, R.; Paolisso, G. Adiponectin and Cognitive Decline. Int. J. Mol. Sci. 2020, 21, 2010. [Google Scholar] [CrossRef]
- Li, C.; Meng, F.; Garza, J.C.; Liu, J.; Lei, Y.; Kirov, S.A.; Guo, M.; Lu, X.-Y. Modulation of Depression-Related Behaviors by Adiponectin AdipoR1 Receptors in 5-HT Neurons. Mol. Psychiatry 2021, 26, 4205–4220. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, M.; Xu, L.; Li, Q.; Zhao, Z.; Liu, X.; Meng, F.; Liu, J.; Wang, W.; Li, C.; et al. PPARγ/Adiponectin Axis Attenuates Methamphetamine-Induced Conditional Place Preference via the Hippocampal AdipoR1 Signaling Pathway. Prog. Neuropsychopharmacol. Biol. Psychiatry 2023, 125, 110758. [Google Scholar] [CrossRef]
- van Himbergen, T.M.; Beiser, A.S.; Ai, M.; Seshadri, S.; Otokozawa, S.; Au, R.; Thongtang, N.; Wolf, P.A.; Schaefer, E.J. Biomarkers for Insulin Resistance and Inflammation and the Risk for All-Cause Dementia and Alzheimer Disease: Results from the Framingham Heart Study. Arch. Neurol. 2012, 69, 594–600. [Google Scholar] [CrossRef]
- Wennberg, A.M.V.; Gustafson, D.; Hagen, C.E.; Roberts, R.O.; Knopman, D.; Jack, C.; Petersen, R.C.; Mielke, M.M. Serum Adiponectin Levels, Neuroimaging, and Cognition in the Mayo Clinic Study of Aging. J. Alzheimer’s Dis. 2016, 53, 573–581. [Google Scholar] [CrossRef]
- Kim, K.Y.; Ha, J.; Kim, M.; Cho, S.Y.; Kim, H.; Kim, E.; for the Alzheimer’s Disease Neuroimaging Initiative. Plasma Adiponectin Levels Predict Cognitive Decline and Cortical Thinning in Mild Cognitive Impairment with Beta-Amyloid Pathology. Alzheimer’s Res. Ther. 2022, 14, 165. [Google Scholar] [CrossRef]
- Liu, F.; Xu, H.; Yin, Y.; Chen, Y.; Xie, L.; Li, H.; Wang, D.; Shi, B. Decreased Adiponectin Levels Are a Risk Factor for Cognitive Decline in Spinal Cord Injury. Dis. Markers 2022, 2022, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.L.; Diniz, B.S.; Campos, A.C.; Miranda, A.S.; Rocha, N.P.; Talib, L.L.; Gattaz, W.F.; Forlenza, O.V. Decreased Levels of Circulating Adiponectin in Mild Cognitive Impairment and Alzheimer’s Disease. NeuroMolecular Med. 2013, 15, 115–121. [Google Scholar] [CrossRef]
- Furman, J.L.; Soyombo, A.; Czysz, A.H.; Jha, M.K.; Carmody, T.J.; Mason, B.L.; Scherer, P.E.; Trivedi, M.H. Adiponectin Moderates Antidepressant Treatment Outcome in the Combining Medications to Enhance Depression Outcomes Randomized Clinical Trial. Pers. Med. Psychiatry 2018, 9–10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Machado-Vieira, R.; Gold, P.W.; Luckenbaugh, D.A.; Ballard, E.D.; Richards, E.M.; Henter, I.D.; De Sousa, R.T.; Niciu, M.J.; Yuan, P.; Zarate, C.A. The Role of Adipokines in the Rapid Antidepressant Effects of Ketamine. Mol. Psychiatry 2017, 22, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Permoda-Pachuta, A.; Malewska-Kasprzak, M.; Skibińska, M.; Rzepski, K.; Dmitrzak-Węglarz, M. Changes in Adipokine, Resitin, and BDNF Concentrations in Treatment-Resistant Depression after Electroconvulsive Therapy. Brain Sci. 2023, 13, 1358. [Google Scholar] [CrossRef]
- Chow, L.S.; Gerszten, R.E.; Taylor, J.M.; Pedersen, B.K.; Van Praag, H.; Trappe, S.; Febbraio, M.A.; Galis, Z.S.; Gao, Y.; Haus, J.M.; et al. Exerkines in Health, Resilience and Disease. Nat. Rev. Endocrinol. 2022, 18, 273–289. [Google Scholar] [CrossRef]
- Sirico, F.; Bianco, A.; D’Alicandro, G.; Castaldo, C.; Montagnani, S.; Spera, R.; Di Meglio, F.; Nurzynska, D. Effects of Physical Exercise on Adiponectin, Leptin, and Inflammatory Markers in Childhood Obesity: Systematic Review and Meta-Analysis. Child. Obes. Print 2018, 14, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Bruun, J.M.; Helge, J.W.; Richelsen, B.; Stallknecht, B. Diet and Exercise Reduce Low-Grade Inflammation and Macrophage Infiltration in Adipose Tissue but Not in Skeletal Muscle in Severely Obese Subjects. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E961–E967. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, T.R.; Mendes, T.T.; Ramos, G.P.; Cabido, C.E.T.; Morandi, R.F.; Ferraz, F.O.; Miranda, A.S.; Mendonça, V.A.; Teixeira, A.L.; Silami-Garcia, E.; et al. Aerobic Training Modulates the Increase in Plasma Concentrations of Cytokines in Response to a Session of Exercise. J. Environ. Public Health 2021, 2021, 1304139. [Google Scholar] [CrossRef]
- Jürimäe, J.; Purge, P.; Jürimäe, T. Adiponectin and Stress Hormone Responses to Maximal Sculling after Volume-Extended Training Season in Elite Rowers. Metabolism 2006, 55, 13–19. [Google Scholar] [CrossRef]
- Kraemer, R.R.; Aboudehen, K.S.; Carruth, A.K.; Durand, R.T.J.; Acevedo, E.O.; Hebert, E.P.; Johnson, L.G.; Castracane, V.D. Adiponectin Responses to Continuous and Progressively Intense Intermittent Exercise. Med. Sci. Sports Exerc. 2003, 35, 1320–1325. [Google Scholar] [CrossRef]
- Mallardo, M.; Tommasini, E.; Missaglia, S.; Pecci, C.; Rampinini, E.; Bosio, A.; Morelli, A.; Daniele, A.; Nigro, E.; Tavian, D. Effects of Exhaustive Exercise on Adiponectin and High-Molecular-Weight Oligomer Levels in Male Amateur Athletes. Biomedicines 2024, 12, 1743. [Google Scholar] [CrossRef]
- Bouassida, A.; Chamari, K.; Zaouali, M.; Feki, Y.; Zbidi, A.; Tabka, Z. Review on Leptin and Adiponectin Responses and Adaptations to Acute and Chronic Exercise. Br. J. Sports Med. 2010, 44, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Higaki, Y.; Taguchi, N.; Hara, M.; Nakamura, K.; Nanri, H.; Imaizumi, T.; Sakamoto, T.; Shimanoe, C.; Horita, M.; et al. Intensity-Specific and Modified Effects of Physical Activity on Serum Adiponectin in a Middle-Aged Population. J. Endocr. Soc. 2019, 3, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, M.A.; White, L.J.; McCoy, S.; Kim, H.-W.; Petty, T.; Wilsey, J. Plasma Adiponectin Response to Acute Exercise in Healthy Subjects. Eur. J. Appl. Physiol. 2004, 91, 324–329. [Google Scholar] [CrossRef]
- Jamurtas, A.Z.; Theocharis, V.; Koukoulis, G.; Stakias, N.; Fatouros, I.G.; Kouretas, D.; Koutedakis, Y. The Effects of Acute Exercise on Serum Adiponectin and Resistin Levels and Their Relation to Insulin Sensitivity in Overweight Males. Eur. J. Appl. Physiol. 2006, 97, 122–126. [Google Scholar] [CrossRef]
- Becic, T.; Studenik, C.; Hoffmann, G. Exercise Increases Adiponectin and Reduces Leptin Levels in Prediabetic and Diabetic Individuals: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Med. Sci. 2018, 6, 97. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; You, T.; Murphy, K.; Lyles, M.F.; Nicklas, B.J. Addition of Exercise Increases Plasma Adiponectin and Release from Adipose Tissue. Med. Sci. Sports Exerc. 2015, 47, 2450–2455. [Google Scholar] [CrossRef]
- Blüher, M.; Bullen, J.W.; Lee, J.H.; Kralisch, S.; Fasshauer, M.; Klöting, N.; Niebauer, J.; Schön, M.R.; Williams, C.J.; Mantzoros, C.S. Circulating Adiponectin and Expression of Adiponectin Receptors in Human Skeletal Muscle: Associations with Metabolic Parameters and Insulin Resistance and Regulation by Physical Training. J. Clin. Endocrinol. Metab. 2006, 91, 2310–2316. [Google Scholar] [CrossRef] [PubMed]
- Andarianto, A.; Rejeki, P.S.; Pranoto, A.; Izzatunnisa, N.; Rahmanto, I.; Muhammad, M.; Halim, S. Effects of Moderate-Intensity Combination Exercise on Increase Adiponectin Levels, Muscle Mass, and Decrease Fat Mass in Obese Women. Retos 2024, 55, 296–301. [Google Scholar] [CrossRef]
- Putra, D.P.; Wibawa, J.C.; Rossa, M.; Riyono, A. Effect of Physical Exercise on Adiponectin Levels in Humans: A Systematic Review. Fizjoterapia Pol. 2025, 25, 436–441. [Google Scholar] [CrossRef]
- Simpson, K.A.; Singh, M.A.F. Effects of Exercise on Adiponectin: A Systematic Review. Obesity 2008, 16, 241–256. [Google Scholar] [CrossRef]
- Mallardo, M.; D’Alleva, M.; Lazzer, S.; Giovanelli, N.; Graniero, F.; Billat, V.; Fiori, F.; Marinoni, M.; Parpinel, M.; Daniele, A.; et al. Improvement of Adiponectin in Relation to Physical Performance and Body Composition in Young Obese Males Subjected to Twenty-Four Weeks of Training Programs. Heliyon 2023, 9, e15790. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Kang, H.-S.; Shin, Y.-A. Exercise Improves Adiponectin Concentrations Irrespective of the Adiponectin Gene Polymorphisms SNP45 and the SNP276 in Obese Korean Women. Gene 2013, 516, 271–276. [Google Scholar] [CrossRef]
- Leońska-Duniec, A.; Grzywacz, A.; Jastrzębski, Z.; Jażdżewska, A.; Lulińska-Kuklik, E.; Moska, W.; Leźnicka, K.; Ficek, K.; Rzeszutko, A.; Dornowski, M.; et al. ADIPOQ Polymorphisms Are Associated with Changes in Obesityrelated traits in Response to Aerobic Training Programme in Women. Biol. Sport 2018, 35, 165–173. [Google Scholar] [CrossRef]
- Cardozo Gasparin, C.; Leite, N.; Lehtonen Rodrigues De Souza, R.; Viater Tureck, L.; E. Milano-Gai, G.; Pizzi, J.; R. Silva, L.; De Fátima Aguiar Lopes, M.; Lopes, W.A.; Furtado-Alle, L. A Relationship between Single Nucleotide Polymorphism (SNP) in HSD11β1 and ADIPOQ Genes and Obesity Related Features in Children and Adolescents Submitted on Physical Exercises. Braz. J. Implant. Health Sci. 2024, 6, 1791–1810. [Google Scholar] [CrossRef]
- Siitonen, N.; Pulkkinen, L.; Lindström, J.; Kolehmainen, M.; Eriksson, J.G.; Venojärvi, M.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Tuomilehto, J.; Uusitupa, M. Association of ADIPOQ Gene Variants with Body Weight, Type 2 Diabetes and Serum Adiponectin Concentrations: The Finnish Diabetes Prevention Study. BMC Med. Genet. 2011, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Shari, M.; Yusof, S.M.; Raja Hussain, R.N.J.; Kek, T.L.; Mohd Idris N, N.; Aiman, S.; Radzi, N.A.A.M.; Abu Kasim, N.A.; Mohamed, M.N.; Mazaulan, M.; et al. Aqua Exercises and Adipoq Gene Polymorphism: Impacts on The Metabolic and Obesity-Related Traits Among Obese Women. Int. J. Acad. Res. Bus. Soc. Sci. 2024, 14, 390–401. [Google Scholar] [CrossRef]
- Corbi, G.; Polito, R.; Monaco, M.L.; Cacciatore, F.; Scioli, M.; Ferrara, N.; Daniele, A.; Nigro, E. Adiponectin Expression and Genotypes in Italian People with Severe Obesity Undergone a Hypocaloric Diet and Physical Exercise Program. Nutrients 2019, 11, 2195. [Google Scholar] [CrossRef]
- De Luis Roman, D.; Izaola Jauregui, O.; Primo Martin, D. The Polymorphism Rs17300539 in the Adiponectin Promoter Gene Is Related to Metabolic Syndrome, Insulin Resistance, and Adiponectin Levels in Caucasian Patients with Obesity. Nutrients 2023, 15, 5028. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Liu, L.; Wei, Y.; Fang, C.; Zhou, F.; Chen, J.; Han, Q.; Huang, M.; Tan, X.; Liu, Q.; et al. Exercise Ameliorates the FGF21-Adiponectin Axis Impairment in Diet-Induced Obese Mice. Endocr. Connect. 2019, 8, 596–604. [Google Scholar] [CrossRef]
- Sadier, N.S.; El Hajjar, F.; Al Sabouri, A.A.K.; Abou-Abbas, L.; Siomava, N.; Almutary, A.G.; Tambuwala, M.M. Irisin: An Unveiled Bridge between Physical Exercise and a Healthy Brain. Life Sci. 2024, 339, 122393. [Google Scholar] [CrossRef]
- Pilozzi, A.; Carro, C.; Huang, X. Roles of β-Endorphin in Stress, Behavior, Neuroinflammation, and Brain Energy Metabolism. Int. J. Mol. Sci. 2020, 22, 338. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, V.; Vandoni, M.; Rossi, V.; Berardo, C.; Grazi, R.; Cordaro, E.; Tranfaglia, V.; Carnevale Pellino, V.; Cereda, C.; Zuccotti, G. Use of Physical Activity and Exercise to Reduce Inflammation in Children and Adolescents with Obesity. Int. J. Environ. Res. Public Health 2022, 19, 6908. [Google Scholar] [CrossRef]
- Passos, M.; Gonçalves, M. Regulation of Insulin Sensitivity by Adiponectin and Its Receptors in Response to Physical Exercise. Horm. Metab. Res. 2014, 46, 603–608. [Google Scholar] [CrossRef]
- Nowacka-Chmielewska, M.; Grabowska, K.; Grabowski, M.; Meybohm, P.; Burek, M.; Małecki, A. Running from Stress: Neurobiological Mechanisms of Exercise-Induced Stress Resilience. Int. J. Mol. Sci. 2022, 23, 13348. [Google Scholar] [CrossRef]
- Cano-Montoya, J.; Bentes, A.; Pavez, Y.; Rubilar, P.; Lavoz, C.; Ehrenfeld, P.; Sandoval, V.; Martínez-Huenchullán, S. Metabolic Response After a Single Maximal Exercise Session in Physically Inactive Young Adults (EASY Study): Relevancy of Adiponectin Isoforms. Biomolecules 2025, 15, 314. [Google Scholar] [CrossRef]
- Narciso, P.H.; von Ah Morano, A.E.; Agostinete, R.R.; Werneck, A.O.; Giannopoulos, A.J.; Bell, M.; Antunes, B.M.; Lira, F.S.; Fernandes, R.A.; Klentrou, P. Cytokine and Adipokine Response Following High-Intensity Interval Running and Cycling in Female Adolescents. Eur. J. Appl. Physiol. 2025. Online ahead of print. [Google Scholar] [CrossRef]
- Vidal, P.; Stanford, K.I. Exercise-Induced Adaptations to Adipose Tissue Thermogenesis. Front. Endocrinol. 2020, 11, 270. [Google Scholar] [CrossRef]
- Baldelli, S.; Aiello, G.; Mansilla Di Martino, E.; Campaci, D.; Muthanna, F.M.S.; Lombardo, M. The Role of Adipose Tissue and Nutrition in the Regulation of Adiponectin. Nutrients 2024, 16, 2436. [Google Scholar] [CrossRef]
- Félix-Soriano, E.; Sáinz, N.; Gil-Iturbe, E.; Castilla-Madrigal, R.; Celay, J.; Fernández-Galilea, M.; Pejenaute, Á.; Lostao, M.P.; Martínez-Climent, J.A.; Moreno-Aliaga, M.J. Differential Remodeling of Subcutaneous White and Interscapular Brown Adipose Tissue by Long-Term Exercise Training in Aged Obese Female Mice. J. Physiol. Biochem. 2023, 79, 451–465. [Google Scholar] [CrossRef]
- Ryan, B.J.; Schleh, M.W.; Ahn, C.; Ludzki, A.C.; Gillen, J.B.; Varshney, P.; Van Pelt, D.W.; Pitchford, L.M.; Chenevert, T.L.; Gioscia-Ryan, R.A.; et al. Moderate-Intensity Exercise and High-Intensity Interval Training Affect Insulin Sensitivity Similarly in Obese Adults. J. Clin. Endocrinol. Metab. 2020, 105, e2941–e2959. [Google Scholar] [CrossRef] [PubMed]
- Blümer, R.M.E.; van Roomen, C.P.; Meijer, A.J.; Houben-Weerts, J.H.P.M.; Sauerwein, H.P.; Dubbelhuis, P.F. Regulation of Adiponectin Secretion by Insulin and Amino Acids in 3T3-L1 Adipocytes. Metabolism 2008, 57, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Hajri, T.; Tao, H.; Wattacheril, J.; Marks-Shulman, P.; Abumrad, N.N. Regulation of Adiponectin Production by Insulin: Interactions with Tumor Necrosis Factor-α and Interleukin-6. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E350–E360. [Google Scholar] [CrossRef]
- Westerbacka, J.; Cornér, A.; Kannisto, K.; Kolak, M.; Makkonen, J.; Korsheninnikova, E.; Nyman, T.; Hamsten, A.; Fisher, R.M.; Yki-Järvinen, H. Acute in Vivo Effects of Insulin on Gene Expression in Adipose Tissue in Insulin-Resistant and Insulin-Sensitive Subjects. Diabetologia 2006, 49, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Motta, V.F.; Aguila, M.B.; Mandarim-DE-Lacerda, C.A. High-Intensity Interval Training (Swimming) Significantly Improves the Adverse Metabolism and Comorbidities in Diet-Induced Obese Mice. J. Sports Med. Phys. Fitness 2016, 56, 655–663. [Google Scholar]
- Pala, R.; Genc, E.; Tuzcu, M.; Orhan, C.; Sahin, N.; Er, B.; Cinar, V.; Sahin, K. L-Carnitine Supplementation Increases Expression of PPAR-Î3 and Glucose Transporters in Skeletal Muscle of Chronically and Acutely Exercised Rats. Cell. Mol. Biol. 2018, 64, 1–6. [Google Scholar] [CrossRef]
- Shirvani, H.; Mirnejad, R.; Soleimani, M.; Arabzadeh, E. Swimming Exercise Improves Gene Expression of PPAR-γ and Downregulates the Overexpression of TLR4, MyD88, IL-6, and TNF-α after High-Fat Diet in Rat Skeletal Muscle Cells. Gene 2021, 775, 145441. [Google Scholar] [CrossRef] [PubMed]
- Korbecki, J.; Bobiński, R.; Dutka, M. Self-Regulation of the Inflammatory Response by Peroxisome Proliferator-Activated Receptors. Inflamm. Res. 2019, 68, 443–458. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Gao, X.; Ge, Q.; Tai, W.; Hao, X.; Shao, Q.; Fang, Z.; Chen, M.; Song, Y.; Gao, W.; et al. Tumor Necrosis Factor-α Reduces Adiponectin Production by Decreasing Transcriptional Activity of Peroxisome Proliferator-Activated Receptor-γ in Calf Adipocytes. J. Dairy Sci. 2023, 106, 5182–5195. [Google Scholar] [CrossRef]
- Monsalve, F.A.; Pyarasani, R.D.; Delgado-Lopez, F.; Moore-Carrasco, R. Peroxisome Proliferator-Activated Receptor Targets for the Treatment of Metabolic Diseases. Mediat. Inflamm. 2013, 2013, 549627. [Google Scholar] [CrossRef]
- Pajvani, U.B.; Hawkins, M.; Combs, T.P.; Rajala, M.W.; Doebber, T.; Berger, J.P.; Wagner, J.A.; Wu, M.; Knopps, A.; Xiang, A.H.; et al. Complex Distribution, Not Absolute Amount of Adiponectin, Correlates with Thiazolidinedione-Mediated Improvement in Insulin Sensitivity. J. Biol. Chem. 2004, 279, 12152–12162. [Google Scholar] [CrossRef]
- Jin, D.; Sun, J.; Huang, J.; Yu, X.; Yu, A.; He, Y.; Li, Q.; Yang, Z. Peroxisome Proliferator-Activated Receptor γ Enhances Adiponectin Secretion via up-Regulating DsbA-L Expression. Mol. Cell. Endocrinol. 2015, 411, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lam, K.S.L.; Yau, M.; Xu, A. Post-Translational Modifications of Adiponectin: Mechanisms and Functional Implications. Biochem. J. 2008, 409, 623–633. [Google Scholar] [CrossRef]
- Mohammadi, A.; Gholamhoseinian, A.; Fallah, H. Zataria Multiflora Increases Insulin Sensitivity and PPARγ Gene Expression in High Fructose Fed Insulin Resistant Rats. Iran. J. Basic Med. Sci. 2014, 17, 263–270. [Google Scholar]
- Kjøbsted, R.; Hingst, J.R.; Fentz, J.; Foretz, M.; Sanz, M.N.; Pehmøller, C.; Shum, M.; Marette, A.; Mounier, R.; Treebak, J.T.; et al. AMPK in skeletal muscle function and metabolism. FASEB J. 2018, 32, 1741–1777. [Google Scholar] [CrossRef]
- Garcia, D.; Shaw, R.J. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol. Cell 2017, 66, 789–800. [Google Scholar] [CrossRef]
- Fang, H.; Judd, R.L. Adiponectin Regulation and Function. Compr. Physiol. 2018, 8, 1031–1063. [Google Scholar] [CrossRef] [PubMed]
- Tishinsky, J.M.; Dyck, D.J.; Robinson, L.E. Lifestyle Factors Increasing Adiponectin Synthesis and Secretion. Vitam. Horm. 2012, 90, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Christie, B.R.; van Praag, H.; Lin, K.; Siu, P.M.-F.; Xu, A.; So, K.-F.; Yau, S.-Y. AdipoRon Treatment Induces a Dose-Dependent Response in Adult Hippocampal Neurogenesis. Int. J. Mol. Sci. 2021, 22, 2068. [Google Scholar] [CrossRef]
- You, J.; Sun, L.; Wang, J.; Sun, F.; Wang, W.; Wang, D.; Fan, X.; Liu, D.; Xu, Z.; Qiu, C.; et al. Role of Adiponectin-Notch Pathway in Cognitive Dysfunction Associated with Depression and in the Therapeutic Effect of Physical Exercise. Aging Cell 2021, 20, e13387. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, J.; Wang, J.-G.; Liu, C.-L.; Yan, H.-J. AdipoRon Improves Cognitive Dysfunction of Alzheimer’s Disease and Rescues Impaired Neural Stem Cell Proliferation through AdipoR1/AMPK Pathway. Exp. Neurol. 2020, 327, 113249. [Google Scholar] [CrossRef]
- Whitaker, R.H.; Cook, J.G. Stress Relief Techniques: P38 MAPK Determines the Balance of Cell Cycle and Apoptosis Pathways. Biomolecules 2021, 11, 1444. [Google Scholar] [CrossRef]
- Zhang, D.; Guo, M.; Zhang, W.; Lu, X.-Y. Adiponectin Stimulates Proliferation of Adult Hippocampal Neural Stem/Progenitor Cells through Activation of P38 Mitogen-Activated Protein Kinase (p38MAPK)/Glycogen Synthase Kinase 3β (GSK-3β)/β-Catenin Signaling Cascade. J. Biol. Chem. 2011, 286, 44913–44920. [Google Scholar] [CrossRef]
- Song, J.; Kang, S.M.; Kim, E.; Kim, C.-H.; Song, H.-T.; Lee, J.E. Adiponectin Receptor-Mediated Signaling Ameliorates Cerebral Cell Damage and Regulates the Neurogenesis of Neural Stem Cells at High Glucose Concentrations: An in Vivo and in Vitro Study. Cell Death Dis. 2015, 6, e1844. [Google Scholar] [CrossRef]
- Lampada, A.; Taylor, V. Notch Signaling as a Master Regulator of Adult Neurogenesis. Front. Neurosci. 2023, 17, 1179011. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.; Yoon, G.H.; Chung, S.S.; Abid, M.N.; Kim, T.H.; Lee, H.Y.; Kim, M.O. Novel Osmotin Inhibits SREBP2 via the AdipoR1/AMPK/SIRT1 Pathway to Improve Alzheimer’s Disease Neuropathological Deficits. Mol. Psychiatry 2017, 22, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhu, S.; Chen, S.; Zou, J.; Zeng, P.; Tan, S. Neurological Mechanism-Based Analysis of the Role and Characteristics of Physical Activity in the Improvement of Depressive Symptoms. Rev. Neurosci. 2025, 36, 455–478. [Google Scholar] [CrossRef]
- Yu, L.; Wang, J.; Xia, Y.; Hou, W.; Yao, X.; Guo, Y.; Wang, J.; Wei, H.; Wang, S. Adiponectin Promotes Neurogenesis After Transient Cerebral Ischemia Through STAT3 Mediated BDNF Upregulation in Astrocytes. Neurochem. Res. 2023, 48, 641–657. [Google Scholar] [CrossRef]
- Nicolas, S.; Debayle, D.; Béchade, C.; Maroteaux, L.; Gay, A.-S.; Bayer, P.; Heurteaux, C.; Guyon, A.; Chabry, J. Adiporon, an Adiponectin Receptor Agonist Acts as an Antidepressant and Metabolic Regulator in a Mouse Model of Depression. Transl. Psychiatry 2018, 8, 159. [Google Scholar] [CrossRef]
- Chabry, J.; Nicolas, S.; Cazareth, J.; Murris, E.; Guyon, A.; Glaichenhaus, N.; Heurteaux, C.; Petit-Paitel, A. Enriched Environment Decreases Microglia and Brain Macrophages Inflammatory Phenotypes through Adiponectin-Dependent Mechanisms: Relevance to Depressive-like Behavior. Brain. Behav. Immun. 2015, 50, 275–287. [Google Scholar] [CrossRef]
- Kadowaki, T. Adiponectin and Adiponectin Receptors in Insulin Resistance, Diabetes, and the Metabolic Syndrome. J. Clin. Investig. 2006, 116, 1784–1792. [Google Scholar] [CrossRef]
- Gradinaru, D.; Margina, D.; Borsa, C.; Ionescu, C.; Ilie, M.; Costache, M.; Dinischiotu, A.; Prada, G.-I. Adiponectin: Possible Link between Metabolic Stress and Oxidative Stress in the Elderly. Aging Clin. Exp. Res. 2017, 29, 621–629. [Google Scholar] [CrossRef]
- Iwabu, M.; Yamauchi, T.; Okada-Iwabu, M.; Sato, K.; Nakagawa, T.; Funata, M.; Yamaguchi, M.; Namiki, S.; Nakayama, R.; Tabata, M.; et al. Adiponectin and AdipoR1 Regulate PGC-1alpha and Mitochondria by Ca(2+) and AMPK/SIRT1. Nature 2010, 464, 1313–1319. [Google Scholar] [CrossRef]
- Yu, J.; Zheng, J.; Lu, J.; Sun, Z.; Wang, Z.; Zhang, J. AdipoRon Protects Against Secondary Brain Injury After Intracerebral Hemorrhage via Alleviating Mitochondrial Dysfunction: Possible Involvement of AdipoR1–AMPK–PGC1α Pathway. Neurochem. Res. 2019, 44, 1678–1689. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Guo, H.; Li, X.; Yue, L.; Liu, H.; Zhao, L.; Bai, H.; Liu, X.; Wu, X.; Qu, Y. Adiponectin Attenuates Oxygen–Glucose Deprivation-Induced Mitochondrial Oxidative Injury and Apoptosis in Hippocampal HT22 Cells via the JAK2/STAT3 Pathway. Cell Transplant. 2018, 27, 1731–1743. [Google Scholar] [CrossRef]
- Wu, X.; Luo, J.; Liu, H.; Cui, W.; Guo, W.; Zhao, L.; Guo, H.; Bai, H.; Guo, K.; Feng, D.; et al. Recombinant adiponectin peptide promotes neuronal survival after intracerebral haemorrhage by suppressing mitochondrial and ATF4-CHOP apoptosis pathways in diabetic mice via Smad3 signalling inhibition. Cell Prolif. 2020, 53, e12759. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.-H.; Lam, K.S.-L.; Cheng, O.-Y.; Kwan, J.S.-C.; Ho, P.W.-L.; Cheng, K.K.-Y.; Chung, S.K.; Ho, J.W.-M.; Guo, V.Y.; Xu, A. Adiponectin Is Protective against Oxidative Stress Induced Cytotoxicity in Amyloid-Beta Neurotoxicity. PLoS ONE 2012, 7, e52354. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.-H.; Ou, H.-N.; Yu, J.-S.; Yau, S.-Y.; Tsang, H.W.-H. Pharmacological Blocking of Adiponectin Receptors Induces Alzheimer’s Disease-like Neuropathology and Impairs Hippocampal Function. Biomedicines 2025, 13, 1056. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genini, P.; D’Aprile, I.; Petrillo, G.; Di Benedetto, M.G.; Begni, V.; Cattane, N.; Cattaneo, A. From Fat to Brain: Adiponectin as a Mediator of Neuroplasticity in Depression. Biomolecules 2025, 15, 1642. https://doi.org/10.3390/biom15121642
Genini P, D’Aprile I, Petrillo G, Di Benedetto MG, Begni V, Cattane N, Cattaneo A. From Fat to Brain: Adiponectin as a Mediator of Neuroplasticity in Depression. Biomolecules. 2025; 15(12):1642. https://doi.org/10.3390/biom15121642
Chicago/Turabian StyleGenini, Patrizia, Ilari D’Aprile, Giulia Petrillo, Maria Grazia Di Benedetto, Veronica Begni, Nadia Cattane, and Annamaria Cattaneo. 2025. "From Fat to Brain: Adiponectin as a Mediator of Neuroplasticity in Depression" Biomolecules 15, no. 12: 1642. https://doi.org/10.3390/biom15121642
APA StyleGenini, P., D’Aprile, I., Petrillo, G., Di Benedetto, M. G., Begni, V., Cattane, N., & Cattaneo, A. (2025). From Fat to Brain: Adiponectin as a Mediator of Neuroplasticity in Depression. Biomolecules, 15(12), 1642. https://doi.org/10.3390/biom15121642






