Definition and Discovery of Tandem SH3-Binding Motifs Interacting with Members of the p47phox-Related Protein Family
Abstract
1. Introduction
2. Methods
2.1. Identification of Tandem SH3 Domains
2.2. Multiple Sequence Alignments
2.3. Search for Structures and Structural Analysis
2.4. Structure Prediction
2.5. Residue-Wise Contributions to Gibbs Free Energies in the Tandem SH3 Motif
2.6. Conformational Ensemble Prediction
2.7. Motif Prediction
3. Results
3.1. Multiple Lines of Evidence Support the Cooperation of Tandem SH3 Domains in Motif Binding for Members of the p47phox-Related Organizer Superfamily
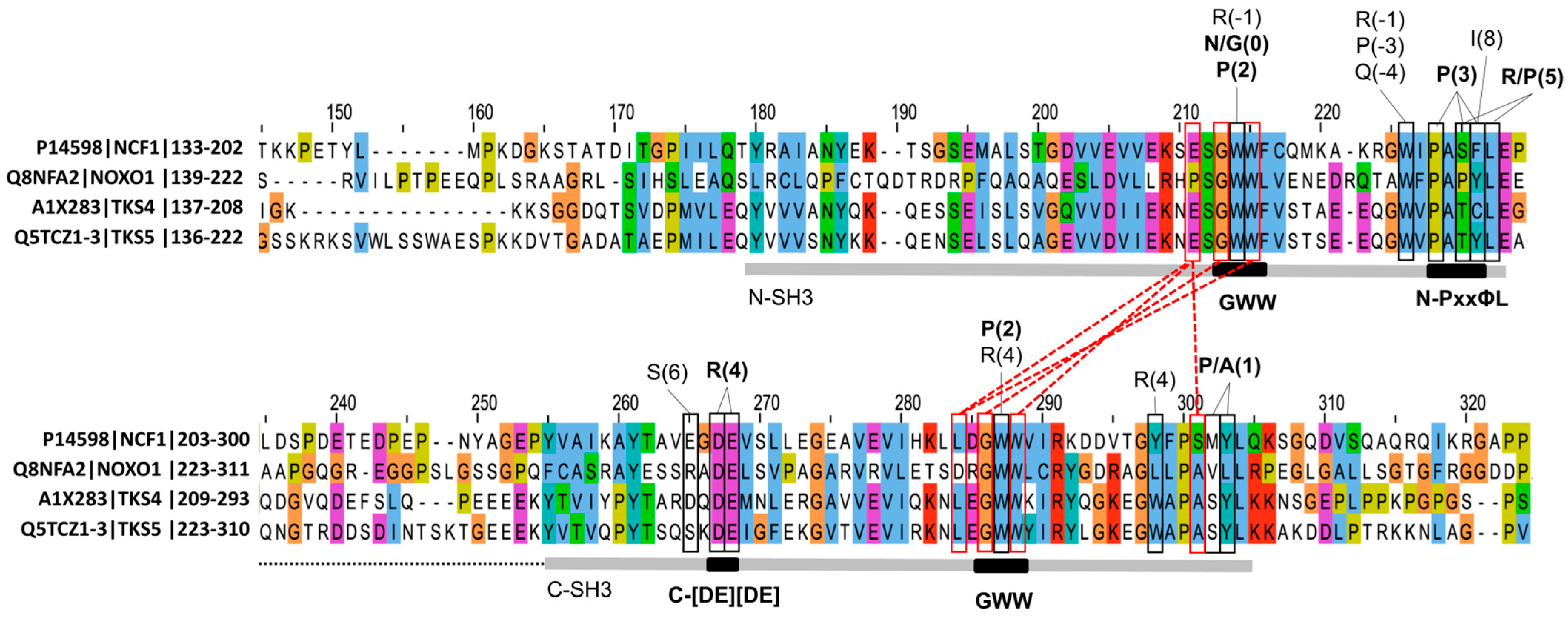
3.2. The Binding Preference of the Tandem SH3 Domains of the NCF1 Family: Definition of the tSH3-Binding Motif
3.3. Detection of Tandem SH3-Binding Motif Candidates Within the NCF1 Family and Their Binding Partners
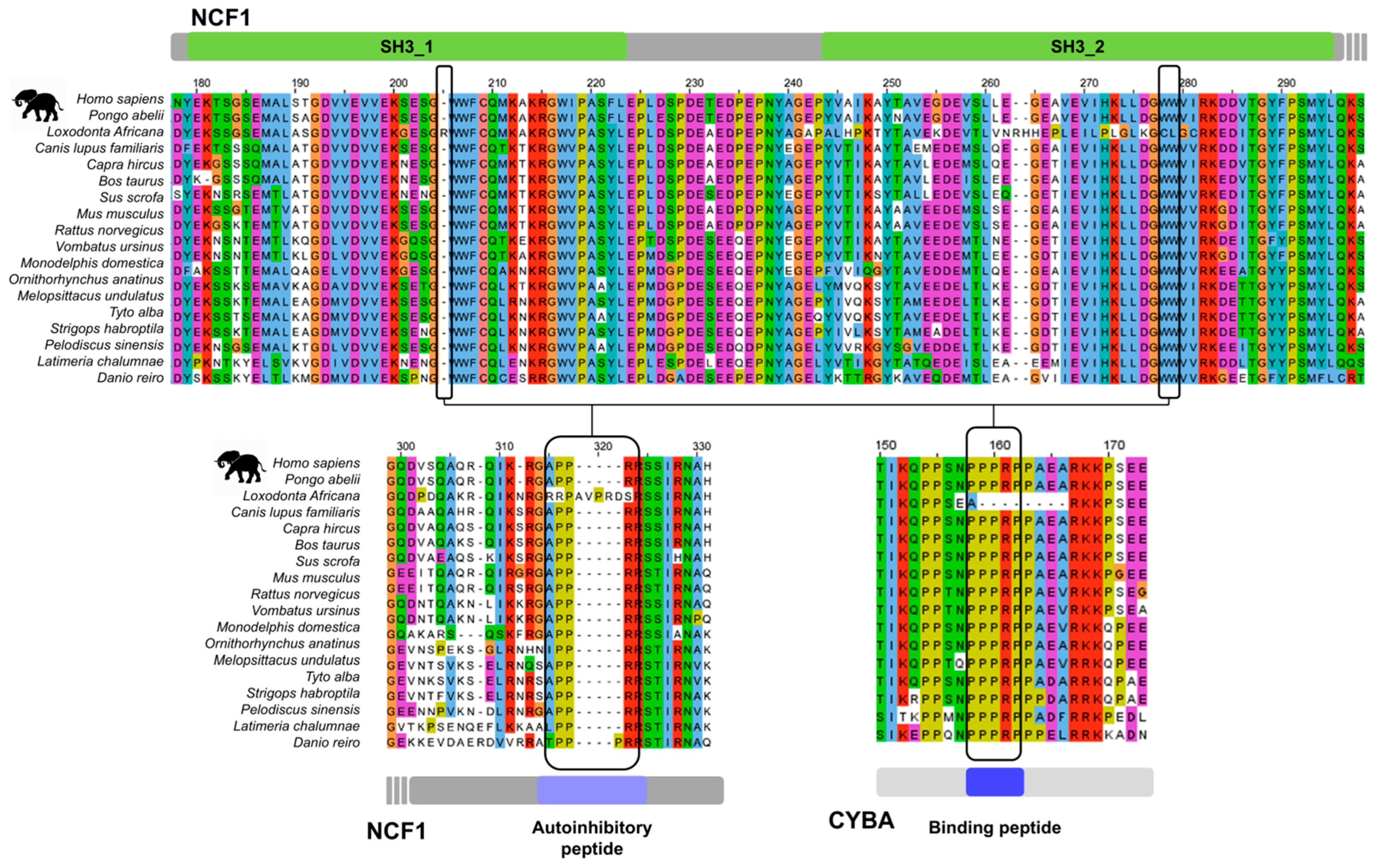
3.4. Sequence Signatures of SH3 Tandemization and Motif Binding Within the NCF1 Family
3.5. Does SH3 Tandemization and Joint Binding of a Single Motif Occur Outside the NCF1 Family?
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AF | AlphaFold |
| AIR | Autoinhibitory region |
| CGD | Chronic granulomatous disease |
| ELM | Eukaryotic linear motif resource |
| IDR | Intrinsically disordered region |
| ITC | Isothermal titration calorimetry |
| PDB | Protein data bank |
| PPI | Protein–protein interaction |
| PPII | Polyproline type II |
| SLiM | Short linear motif |
| tSH3s | tandem SH3 domains |
References
- Mehrabipour, M.; Jasemi, N.S.K.; Dvorsky, R.; Ahmadian, M.R. A Systematic Compilation of Human SH3 Domains: A Versatile Superfamily in Cellular Signaling. Cells 2023, 12, 2054. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, T.; Li, L.; Li, S.S.-C. The SH3 Domain—A Family of Versatile Peptide- and Protein-Recognition Module. Front. Biosci. 2008, 13, 4938–4952. [Google Scholar] [CrossRef]
- Kapeller, R.; Prasad, K.V.; Janssen, O.; Hou, W.; Schaffhausen, B.S.; Rudd, C.E.; Cantley, L.C. Identification of Two SH3-Binding Motifs in the Regulatory Subunit of Phosphatidylinositol 3-Kinase. J. Biol. Chem. 1994, 269, 1927–1933. [Google Scholar] [CrossRef]
- Merő, B.; Radnai, L.; Gógl, G.; Tőke, O.; Leveles, I.; Koprivanacz, K.; Szeder, B.; Dülk, M.; Kudlik, G.; Vas, V.; et al. Structural Insights into the Tyrosine Phosphorylation-Mediated Inhibition of SH3 Domain-Ligand Interactions. J. Biol. Chem. 2019, 294, 4608–4620. [Google Scholar] [CrossRef]
- Rao, Y.; Ma, Q.; Vahedi-Faridi, A.; Sundborger, A.; Pechstein, A.; Puchkov, D.; Luo, L.; Shupliakov, O.; Saenger, W.; Haucke, V. Molecular Basis for SH3 Domain Regulation of F-BAR-Mediated Membrane Deformation. Proc. Natl. Acad. Sci. USA 2010, 107, 8213–8218. [Google Scholar] [CrossRef]
- Ghosh, A.; Mazarakos, K.; Zhou, H.-X. Three Archetypical Classes of Macromolecular Regulators of Protein Liquid-Liquid Phase Separation. Proc. Natl. Acad. Sci. USA 2019, 116, 19474–19483. [Google Scholar] [CrossRef] [PubMed]
- Tateno, K.; Ando, T.; Tabata, M.; Sugasawa, H.; Hayashi, T.; Yu, S.; Pm, S.; Inomata, K.; Mikawa, T.; Ito, Y.; et al. Different Molecular Recognition by Three Domains of the Full-Length GRB2 to SOS1 Proline-Rich Motifs and EGFR Phosphorylated Sites. Chem. Sci. 2024, 15, 15858–15872. [Google Scholar] [CrossRef]
- Amaya, J.; Ryan, V.H.; Fawzi, N.L. The SH3 Domain of Fyn Kinase Interacts with and Induces Liquid-Liquid Phase Separation of the Low-Complexity Domain of hnRNPA2. J. Biol. Chem. 2018, 293, 19522–19531. [Google Scholar] [PubMed]
- Kazemein Jasemi, N.S.; Mehrabipour, M.; Magdalena Estirado, E.; Brunsveld, L.; Dvorsky, R.; Ahmadian, M.R. Functional Classification and Interaction Selectivity Landscape of the Human SH3 Domain Superfamily. Cells 2024, 13, 195. [Google Scholar] [CrossRef]
- Teyra, J.; Huang, H.; Jain, S.; Guan, X.; Dong, A.; Liu, Y.; Tempel, W.; Min, J.; Tong, Y.; Kim, P.M.; et al. Comprehensive Analysis of the Human SH3 Domain Family Reveals a Wide Variety of Non-Canonical Specificities. Structure 2017, 25, 1598–1610.e3. [Google Scholar] [CrossRef]
- Feng, S.; Chen, J.K.; Yu, H.; Simon, J.A.; Schreiber, S.L. Two Binding Orientations for Peptides to the Src SH3 Domain: Development of a General Model for SH3-Ligand Interactions. Science 1994, 266, 1241–1247. [Google Scholar] [CrossRef]
- Lim, W.A.; Richards, F.M.; Fox, R.O. Structural Determinants of Peptide-Binding Orientation and of Sequence Specificity in SH3 Domains. Nature 1994, 372, 375–379. [Google Scholar] [CrossRef]
- Yu, H.; Chen, J.K.; Feng, S.; Dalgarno, D.C.; Brauer, A.W.; Schreiber, S.L. Structural Basis for the Binding of Proline-Rich Peptides to SH3 Domains. Cell 1994, 76, 933–945. [Google Scholar] [CrossRef]
- Aitio, O.; Hellman, M.; Kesti, T.; Kleino, I.; Samuilova, O.; Pääkkönen, K.; Tossavainen, H.; Saksela, K.; Permi, P. Structural Basis of PxxDY Motif Recognition in SH3 Binding. J. Mol. Biol. 2008, 382, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Berry, D.; Nash, P.; Pawson, T.; McGlade, C.J.; Li, S.S.-C. Structural Basis for Specific Binding of the Gads SH3 Domain to an RxxK Motif-Containing SLP-76 Peptide: A Novel Mode of Peptide Recognition. Mol. Cell 2003, 11, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Kurakin, A.V.; Wu, S.; Bredesen, D.E. Atypical Recognition Consensus of CIN85/SETA/Ruk SH3 Domains Revealed by Target-Assisted Iterative Screening. J. Biol. Chem. 2003, 278, 34102–34109. [Google Scholar] [CrossRef]
- Dionne, U.; Chartier, F.J.M.; López de Los Santos, Y.; Lavoie, N.; Bernard, D.N.; Banerjee, S.L.; Otis, F.; Jacquet, K.; Tremblay, M.G.; Jain, M.; et al. Direct Phosphorylation of SRC Homology 3 Domains by Tyrosine Kinase Receptors Disassembles Ligand-Induced Signaling Networks. Mol. Cell 2018, 70, 995–1007.e11. [Google Scholar] [CrossRef] [PubMed]
- Sumimoto, H.; Kage, Y.; Nunoi, H.; Sasaki, H.; Nose, T.; Fukumaki, Y.; Ohno, M.; Minakami, S.; Takeshige, K. Role of Src Homology 3 Domains in Assembly and Activation of the Phagocyte NADPH Oxidase. Proc. Natl. Acad. Sci. USA 1994, 91, 5345–5349. [Google Scholar] [CrossRef]
- Groemping, Y.; Lapouge, K.; Smerdon, S.J.; Rittinger, K. Molecular Basis of Phosphorylation-Induced Activation of the NADPH Oxidase. Cell 2003, 113, 343–355. [Google Scholar] [CrossRef]
- Yuzawa, S.; Suzuki, N.N.; Fujioka, Y.; Ogura, K.; Sumimoto, H.; Inagaki, F. A Molecular Mechanism for Autoinhibition of the Tandem SH3 Domains of p47phox, the Regulatory Subunit of the Phagocyte NADPH Oxidase. Genes Cells 2004, 9, 443–456. [Google Scholar] [CrossRef]
- Rufer, A.C.; Rumpf, J.; von Holleben, M.; Beer, S.; Rittinger, K.; Groemping, Y. Isoform-Selective Interaction of the Adaptor Protein Tks5/FISH with Sos1 and Dynamins. J. Mol. Biol. 2009, 390, 939–950. [Google Scholar] [CrossRef]
- Ago, T.; Nunoi, H.; Ito, T.; Sumimoto, H. Mechanism for Phosphorylation-Induced Activation of the Phagocyte NADPH Oxidase Protein p47(phox). Triple Replacement of Serines 303, 304, and 328 with Aspartates Disrupts the SH3 Domain-Mediated Intramolecular Interaction in p47(phox), Thereby Activating the Oxidase. J. Biol. Chem. 1999, 274, 33644–33653. [Google Scholar] [PubMed]
- Huang, J.; Kleinberg, M.E. Activation of the Phagocyte NADPH Oxidase Protein p47(phox). Phosphorylation Controls SH3 Domain-Dependent Binding to p22(phox). J. Biol. Chem. 1999, 274, 19731–19737. [Google Scholar] [CrossRef] [PubMed]
- Nobuhisa, I.; Takeya, R.; Ogura, K.; Ueno, N.; Kohda, D.; Inagaki, F.; Sumimoto, H. Activation of the Superoxide-Producing Phagocyte NADPH Oxidase Requires Co-Operation between the Tandem SH3 Domains of p47phox in Recognition of a Polyproline Type II Helix and an Adjacent Alpha-Helix of p22phox. Biochem. J. 2006, 396, 183–192. [Google Scholar] [CrossRef]
- Takeya, R.; Ueno, N.; Kami, K.; Taura, M.; Kohjima, M.; Izaki, T.; Nunoi, H.; Sumimoto, H. Novel Human Homologues of p47phox and p67phox Participate in Activation of Superoxide-Producing NADPH Oxidases. J. Biol. Chem. 2003, 278, 25234–25246. [Google Scholar] [CrossRef]
- Merő, B.; Koprivanacz, K.; Cserkaszky, A.; Radnai, L.; Vas, V.; Kudlik, G.; Gógl, G.; Sok, P.; Póti, Á.L.; Szeder, B.; et al. Characterization of the Intramolecular Interactions and Regulatory Mechanisms of the Scaffold Protein Tks4. Int. J. Mol. Sci. 2021, 22, 8103. [Google Scholar] [CrossRef]
- Diaz, B.; Shani, G.; Pass, I.; Anderson, D.; Quintavalle, M.; Courtneidge, S.A. Tks5-Dependent, Nox-Mediated Generation of Reactive Oxygen Species Is Necessary for Invadopodia Formation. Sci. Signal. 2009, 2, ra53. [Google Scholar] [CrossRef]
- Kumar, M.; Michael, S.; Alvarado-Valverde, J.; Zeke, A.; Lazar, T.; Glavina, J.; Nagy-Kanta, E.; Donagh, J.M.; Kalman, Z.E.; Pascarelli, S.; et al. ELM-the Eukaryotic Linear Motif Resource-2024 Update. Nucleic Acids Res. 2024, 52, D442–D455. [Google Scholar] [CrossRef] [PubMed]
- Gouw, M.; Alvarado-Valverde, J.; Čalyševa, J.; Diella, F.; Kumar, M.; Michael, S.; Van Roey, K.; Dinkel, H.; Gibson, T.J. How to Annotate and Submit a Short Linear Motif to the Eukaryotic Linear Motif Resource. Methods Mol. Biol. 2020, 2141, 73–102. [Google Scholar]
- Blum, M.; Andreeva, A.; Florentino, L.C.; Chuguransky, S.R.; Grego, T.; Hobbs, E.; Pinto, B.L.; Orr, A.; Paysan-Lafosse, T.; Ponamareva, I.; et al. InterPro: The Protein Sequence Classification Resource in 2025. Nucleic Acids Res. 2025, 53, D444–D456. [Google Scholar] [CrossRef]
- Altenhoff, A.M.; Warwick Vesztrocy, A.; Bernard, C.; Train, C.-M.; Nicheperovich, A.; Prieto Baños, S.; Julca, I.; Moi, D.; Nevers, Y.; Majidian, S.; et al. OMA Orthology in 2024: Improved Prokaryote Coverage, Ancestral and Extant GO Enrichment, a Revamped Synteny Viewer and More in the OMA Ecosystem. Nucleic Acids Res. 2024, 52, D513–D521. [Google Scholar] [CrossRef]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, Scalable Generation of High-Quality Protein Multiple Sequence Alignments Using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef]
- Procter, J.B.; Carstairs, G.M.; Soares, B.; Mourão, K.; Ofoegbu, T.C.; Barton, D.; Lui, L.; Menard, A.; Sherstnev, N.; Roldan-Martinez, D.; et al. Alignment of Biological Sequences with Jalview. Methods Mol. Biol. 2021, 2231, 203–224. [Google Scholar]
- Mosca, R.; Céol, A.; Stein, A.; Olivella, R.; Aloy, P. 3did: A Catalog of Domain-Based Interactions of Known Three-Dimensional Structure. Nucleic Acids Res. 2014, 42, D374–D379. [Google Scholar] [CrossRef]
- van Kempen, M.; Kim, S.S.; Tumescheit, C.; Mirdita, M.; Lee, J.; Gilchrist, C.L.M.; Söding, J.; Steinegger, M. Fast and Accurate Protein Structure Search with Foldseek. Nat. Biotechnol. 2024, 42, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Burley, S.K.; Bhikadiya, C.; Bi, C.; Bittrich, S.; Chen, L.; Crichlow, G.V.; Duarte, J.M.; Dutta, S.; Fayazi, M.; Feng, Z.; et al. RCSB Protein Data Bank: Celebrating 50 Years of the PDB with New Tools for Understanding and Visualizing Biological Macromolecules in 3D. Protein Sci. 2022, 31, 187–208. [Google Scholar] [CrossRef]
- Del Conte, A.; Camagni, G.F.; Clementel, D.; Minervini, G.; Monzon, A.M.; Ferrari, C.; Piovesan, D.; Tosatto, S.C.E. RING 4.0: Faster Residue Interaction Networks with Novel Interaction Types across over 35,000 Different Chemical Structures. Nucleic Acids Res. 2024, 52, W306–W312. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Lee, S.; Levy Karin, E.; Kim, H.; Moriwaki, Y.; Ovchinnikov, S.; Steinegger, M.; Mirdita, M. Easy and Accurate Protein Structure Prediction Using ColabFold. Nat. Protoc. 2025, 20, 620–642. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Bret, H.; Gao, J.; Zea, D.J.; Andreani, J.; Guerois, R. From Interaction Networks to Interfaces, Scanning Intrinsically Disordered Regions Using AlphaFold2. Nat. Commun. 2024, 15, 597. [Google Scholar] [CrossRef]
- Lee, C.Y.; Hubrich, D.; Varga, J.K.; Schäfer, C.; Welzel, M.; Schumbera, E.; Djokic, M.; Strom, J.M.; Schönfeld, J.; Geist, J.L.; et al. Systematic Discovery of Protein Interaction Interfaces Using AlphaFold and Experimental Validation. Mol. Syst. Biol. 2024, 20, 75–97. [Google Scholar] [CrossRef]
- Varga, J.K.; Ovchinnikov, S.; Schueler-Furman, O. actifpTM: A Refined Confidence Metric of AlphaFold2 Predictions Involving Flexible Regions. Bioinformatics 2025, 41, btaf107. [Google Scholar] [CrossRef]
- Zeke, A.; Gibson, T.J.; Dobson, L. Linear Motifs Regulating Protein Secretion, Sorting and Autophagy in Leishmania Parasites Are Diverged with Respect to Their Host Equivalents. PLoS Comput. Biol. 2024, 20, e1011902. [Google Scholar] [CrossRef] [PubMed]
- Meng, E.C.; Goddard, T.D.; Pettersen, E.F.; Couch, G.S.; Pearson, Z.J.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Tools for Structure Building and Analysis. Protein Sci. 2023, 32, e4792. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate Structure Prediction of Biomolecular Interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
- Dutta, S.; Rittinger, K. Regulation of NOXO1 Activity through Reversible Interactions with p22 and NOXA1. PLoS ONE 2010, 5, e10478. [Google Scholar] [CrossRef]
- Gohlke, H.; Case, D.A. Converging Free Energy Estimates: MM-PB(GB)SA Studies on the Protein-Protein Complex Ras-Raf. J. Comput. Chem. 2004, 25, 238–250. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, L.; Weng, G.; Shen, C.; Zhang, O.; Liu, M.; Zhang, C.; Gu, S.; Wang, J.; Wang, X.; et al. HawkDock Version 2: An Updated Web Server to Predict and Analyze the Structures of Protein-Protein Complexes. Nucleic Acids Res. 2025, 53, W306–W315. [Google Scholar] [CrossRef]
- Sun, H.; Li, Y.; Tian, S.; Xu, L.; Hou, T. Assessing the Performance of MM/PBSA and MM/GBSA Methods. 4. Accuracies of MM/PBSA and MM/GBSA Methodologies Evaluated by Various Simulation Protocols Using PDBbind Data Set. Phys. Chem. Chem. Phys. 2014, 16, 16719–16729. [Google Scholar] [PubMed]
- Mirdita, M.; Schütze, K.; Moriwaki, Y.; Heo, L.; Ovchinnikov, S.; Steinegger, M. ColabFold: Making Protein Folding Accessible to All. Nat. Methods 2022, 19, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.; Hempel, T.; Jiménez-Luna, J.; Gastegger, M.; Xie, Y.; Foong, A.Y.K.; Satorras, V.G.; Abdin, O.; Veeling, B.S.; Zaporozhets, I.; et al. Scalable Emulation of Protein Equilibrium Ensembles with Generative Deep Learning. Science 2025, 389, eadv9817. [Google Scholar] [CrossRef] [PubMed]
- UniProt Consortium UniProt: The Universal Protein Knowledgebase in 2025. Nucleic Acids Res. 2025, 53, D609–D617. [CrossRef] [PubMed]
- Erdős, G.; Dosztányi, Z. AIUPred: Combining Energy Estimation with Deep Learning for the Enhanced Prediction of Protein Disorder. Nucleic Acids Res. 2024, 52, W176–W181. [Google Scholar] [CrossRef]
- Varga, J.; Dobson, L.; Tusnády, G.E. TOPDOM: Database of Conservatively Located Domains and Motifs in Proteins. Bioinformatics 2016, 32, 2725–2726. [Google Scholar] [CrossRef]
- Oughtred, R.; Stark, C.; Breitkreutz, B.-J.; Rust, J.; Boucher, L.; Chang, C.; Kolas, N.; O’Donnell, L.; Leung, G.; McAdam, R.; et al. The BioGRID Interaction Database: 2019 Update. Nucleic Acids Res. 2019, 47, D529–D541. [Google Scholar] [CrossRef]
- Del Toro, N.; Shrivastava, A.; Ragueneau, E.; Meldal, B.; Combe, C.; Barrera, E.; Perfetto, L.; How, K.; Ratan, P.; Shirodkar, G.; et al. The IntAct Database: Efficient Access to Fine-Grained Molecular Interaction Data. Nucleic Acids Res. 2022, 50, D648–D653. [Google Scholar] [CrossRef]
- László, L.; Kurilla, A.; Tilajka, Á.; Pancsa, R.; Takács, T.; Novák, J.; Buday, L.; Vas, V. Unveiling Epithelial Plasticity Regulation in Lung Cancer: Exploring the Cross-Talk among Tks4 Scaffold Protein Partners. Mol. Biol. Cell 2024, 35, ar111. [Google Scholar] [CrossRef]
- Yamamoto, A.; Kami, K.; Takeya, R.; Sumimoto, H. Interaction between the SH3 Domains and C-Terminal Proline-Rich Region in NADPH Oxidase Organizer 1 (Noxo1). Biochem. Biophys. Res. Commun. 2007, 352, 560–565. [Google Scholar] [CrossRef]
- Iizuka, S.; Abdullah, C.; Buschman, M.D.; Diaz, B.; Courtneidge, S.A. The Role of Tks Adaptor Proteins in Invadopodia Formation, Growth and Metastasis of Melanoma. Oncotarget 2016, 7, 78473–78486. [Google Scholar] [CrossRef]
- Courtneidge, S.A. Cell Migration and Invasion in Human Disease: The Tks Adaptor Proteins. Biochem. Soc. Trans. 2012, 40, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Buschman, M.D.; Bromann, P.A.; Cejudo-Martin, P.; Wen, F.; Pass, I.; Courtneidge, S.A. The Novel Adaptor Protein Tks4 (SH3PXD2B) Is Required for Functional Podosome Formation. Mol. Biol. Cell 2009, 20, 1302–1311. [Google Scholar] [CrossRef] [PubMed]
- Stapley, B.J.; Creamer, T.P. A Survey of Left-Handed Polyproline II Helices. Protein Sci. 1999, 8, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Hekkelman, M.L.; Salmoral, D.Á.; Perrakis, A.; Joosten, R.P. DSSP 4: FAIR Annotation of Protein Secondary Structure. Protein Sci. 2025, 34, e70208. [Google Scholar] [CrossRef]
- Davey, N.E.; Van Roey, K.; Weatheritt, R.J.; Toedt, G.; Uyar, B.; Altenberg, B.; Budd, A.; Diella, F.; Dinkel, H.; Gibson, T.J. Attributes of Short Linear Motifs. Mol. Biosyst. 2012, 8, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Van Roey, K.; Uyar, B.; Weatheritt, R.J.; Dinkel, H.; Seiler, M.; Budd, A.; Gibson, T.J.; Davey, N.E. Short Linear Motifs: Ubiquitous and Functionally Diverse Protein Interaction Modules Directing Cell Regulation. Chem. Rev. 2014, 114, 6733–6778. [Google Scholar] [CrossRef]
- Fuxreiter, M.; Tompa, P.; Simon, I. Local Structural Disorder Imparts Plasticity on Linear Motifs. Bioinformatics 2007, 23, 950–956. [Google Scholar] [CrossRef]
- Hornbeck, P.V.; Kornhauser, J.M.; Latham, V.; Murray, B.; Nandhikonda, V.; Nord, A.; Skrzypek, E.; Wheeler, T.; Zhang, B.; Gnad, F. 15 Years of PhosphoSitePlus®: Integrating Post-Translationally Modified Sites, Disease Variants and Isoforms. Nucleic Acids Res. 2019, 47, D433–D441. [Google Scholar] [CrossRef]
- Varadi, M.; Bertoni, D.; Magana, P.; Paramval, U.; Pidruchna, I.; Radhakrishnan, M.; Tsenkov, M.; Nair, S.; Mirdita, M.; Yeo, J.; et al. AlphaFold Protein Structure Database in 2024: Providing Structure Coverage for over 214 Million Protein Sequences. Nucleic Acids Res. 2024, 52, D368–D375. [Google Scholar] [CrossRef]
- Oughtred, R.; Rust, J.; Chang, C.; Breitkreutz, B.-J.; Stark, C.; Willems, A.; Boucher, L.; Leung, G.; Kolas, N.; Zhang, F.; et al. The BioGRID Database: A Comprehensive Biomedical Resource of Curated Protein, Genetic, and Chemical Interactions. Protein Sci. 2021, 30, 187–200. [Google Scholar] [CrossRef]
- Finan, P.; Shimizu, Y.; Gout, I.; Hsuan, J.; Truong, O.; Butcher, C.; Bennett, P.; Waterfield, M.D.; Kellie, S. An SH3 Domain and Proline-Rich Sequence Mediate an Interaction between Two Components of the Phagocyte NADPH Oxidase Complex. J. Biol. Chem. 1994, 269, 13752–13755. [Google Scholar] [CrossRef]
- Gu, Y.; Xu, Y.C.; Wu, R.F.; Nwariaku, F.E.; Souza, R.F.; Flores, S.C.; Terada, L.S. p47phox Participates in Activation of RelA in Endothelial Cells. J. Biol. Chem. 2003, 278, 17210–17217. [Google Scholar] [CrossRef]
- Kleino, I.; Järviluoma, A.; Hepojoki, J.; Huovila, A.P.; Saksela, K. Preferred SH3 Domain Partners of ADAM Metalloproteases Include Shared and ADAM-Specific SH3 Interactions. PLoS ONE 2015, 10, e0121301. [Google Scholar] [CrossRef] [PubMed]
- Abram, C.L.; Seals, D.F.; Pass, I.; Salinsky, D.; Maurer, L.; Roth, T.M.; Courtneidge, S.A. The Adaptor Protein Fish Associates with Members of the ADAMs Family and Localizes to Podosomes of Src-Transformed Cells. J. Biol. Chem. 2003, 278, 16844–16851. [Google Scholar] [CrossRef]
- Onofri, F.; Giovedi, S.; Kao, H.T.; Valtorta, F.; Bongiorno Borbone, L.; De Camilli, P.; Greengard, P.; Benfenati, F. Specificity of the Binding of Synapsin I to Src Homology 3 Domains. J. Biol. Chem. 2000, 275, 29857–29867. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, F.; Ishii, K.J.; Kobiyama, K.; Kojima, Y.; Coban, C.; Sasaki, S.; Ishii, N.; Klinman, D.M.; Okuda, K.; Akira, S.; et al. TRAF4 Acts as a Silencer in TLR-Mediated Signaling through the Association with TRAF6 and TRIF. Eur. J. Immunol. 2005, 35, 2477–2485. [Google Scholar] [CrossRef]
- Cho, N.H.; Cheveralls, K.C.; Brunner, A.-D.; Kim, K.; Michaelis, A.C.; Raghavan, P.; Kobayashi, H.; Savy, L.; Li, J.Y.; Canaj, H.; et al. OpenCell: Endogenous Tagging for the Cartography of Human Cellular Organization. Science 2022, 375, eabi6983. [Google Scholar] [CrossRef]
- Parrini, M.C.; Sadou-Dubourgnoux, A.; Aoki, K.; Kunida, K.; Biondini, M.; Hatzoglou, A.; Poullet, P.; Formstecher, E.; Yeaman, C.; Matsuda, M.; et al. SH3BP1, an Exocyst-Associated RhoGAP, Inactivates Rac1 at the Front to Drive Cell Motility. Mol. Cell 2011, 42, 650–661. [Google Scholar] [CrossRef]
- Kurilla, A.; László, L.; Takács, T.; Tilajka, Á.; Lukács, L.; Novák, J.; Pancsa, R.; Buday, L.; Vas, V. Studying the Association of TKS4 and CD2AP Scaffold Proteins and Their Implications in the Partial Epithelial-Mesenchymal Transition (EMT) Process. Int. J. Mol. Sci. 2023, 24, 15136. [Google Scholar] [CrossRef] [PubMed]
- Elbediwy, A.; Zihni, C.; Terry, S.J.; Clark, P.; Matter, K.; Balda, M.S. Epithelial Junction Formation Requires Confinement of Cdc42 Activity by a Novel SH3BP1 Complex. J. Cell Biol. 2012, 198, 677–693. [Google Scholar] [CrossRef]
- Boldt, K.; van Reeuwijk, J.; Lu, Q.; Koutroumpas, K.; Nguyen, T.-M.T.; Texier, Y.; van Beersum, S.E.C.; Horn, N.; Willer, J.R.; Mans, D.A.; et al. An Organelle-Specific Protein Landscape Identifies Novel Diseases and Molecular Mechanisms. Nat. Commun. 2016, 7, 11491. [Google Scholar] [CrossRef]
- Noack, D.; Rae, J.; Cross, A.R.; Ellis, B.A.; Newburger, P.E.; Curnutte, J.T.; Heyworth, P.G. Autosomal Recessive Chronic Granulomatous Disease Caused by Defects in NCF-1, the Gene Encoding the Phagocyte p47-Phox: Mutations Not Arising in the NCF-1 Pseudogenes. Blood 2001, 97, 305–311. [Google Scholar] [CrossRef]
- Ababou, A.; Pfuhl, M.; Ladbury, J.E. Novel Insights into the Mechanisms of CIN85 SH3 Domains Binding to Cbl Proteins: Solution-Based Investigations and in Vivo Implications. J. Mol. Biol. 2009, 387, 1120–1136. [Google Scholar] [CrossRef] [PubMed]
- Havrylov, S.; Rzhepetskyy, Y.; Malinowska, A.; Drobot, L.; Redowicz, M.J. Proteins Recruited by SH3 Domains of Ruk/CIN85 Adaptor Identified by LC-MS/MS. Proteome Sci. 2009, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Jozic, D.; Cárdenes, N.; Deribe, Y.L.; Moncalián, G.; Hoeller, D.; Groemping, Y.; Dikic, I.; Rittinger, K.; Bravo, J. Cbl Promotes Clustering of Endocytic Adaptor Proteins. Nat. Struct. Mol. Biol. 2005, 12, 972–979. [Google Scholar] [CrossRef]
- Ceregido, M.A.; Garcia-Pino, A.; Ortega-Roldan, J.L.; Casares, S.; López Mayorga, O.; Bravo, J.; van Nuland, N.A.J.; Azuaga, A.I. Multimeric and Differential Binding of CIN85/CD2AP with Two Atypical Proline-Rich Sequences from CD2 and Cbl-B*. FEBS J. 2013, 280, 3399–3415. [Google Scholar] [CrossRef] [PubMed]
- Desrochers, G.; Cappadocia, L.; Lussier-Price, M.; Ton, A.-T.; Ayoubi, R.; Serohijos, A.; Omichinski, J.G.; Angers, A. Molecular Basis of Interactions between SH3 Domain-Containing Proteins and the Proline-Rich Region of the Ubiquitin Ligase Itch. J. Biol. Chem. 2017, 292, 6325–6338. [Google Scholar] [CrossRef]
- Hashimoto, S.; Hirose, M.; Hashimoto, A.; Morishige, M.; Yamada, A.; Hosaka, H.; Akagi, K.-I.; Ogawa, E.; Oneyama, C.; Agatsuma, T.; et al. Targeting AMAP1 and Cortactin Binding Bearing an Atypical Src Homology 3/proline Interface for Prevention of Breast Cancer Invasion and Metastasis. Proc. Natl. Acad. Sci. USA 2006, 103, 7036–7041. [Google Scholar] [CrossRef]
- Kristensen, O.; Guenat, S.; Dar, I.; Allaman-Pillet, N.; Abderrahmani, A.; Ferdaoussi, M.; Roduit, R.; Maurer, F.; Beckmann, J.S.; Kastrup, J.S.; et al. A Unique Set of SH3-SH3 Interactions Controls IB1 Homodimerization. EMBO J. 2006, 25, 785–797. [Google Scholar] [CrossRef]
- Ogura, K.; Nobuhisa, I.; Yuzawa, S.; Takeya, R.; Torikai, S.; Saikawa, K.; Sumimoto, H.; Inagaki, F. NMR Solution Structure of the Tandem Src Homology 3 Domains of p47phox Complexed with a p22phox-Derived Proline-Rich Peptide. J. Biol. Chem. 2006, 281, 3660–3668. [Google Scholar] [CrossRef]
- Leto, T.L.; Adams, A.G.; de Mendez, I. Assembly of the Phagocyte NADPH Oxidase: Binding of Src Homology 3 Domains to Proline-Rich Targets. Proc. Natl. Acad. Sci. USA 1994, 91, 10650–10654. [Google Scholar] [CrossRef]
- Evans, R.; O’Neill, M.; Pritzel, A.; Antropova, N.; Senior, A.; Green, T.; Žídek, A.; Bates, R.; Blackwell, S.; Yim, J.; et al. Protein Complex Prediction with AlphaFold-Multimer. bioRxiv 2021. [Google Scholar] [CrossRef]
- Omidi, A.; Møller, M.H.; Malhis, N.; Bui, J.M.; Gsponer, J. AlphaFold-Multimer Accurately Captures Interactions and Dynamics of Intrinsically Disordered Protein Regions. Proc. Natl. Acad. Sci. USA 2024, 121, e2406407121. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, T.; Khandare, V.; Mirkin, F.G.; Tumtas, Y.; Bubeck, D.; Bozkurt, T.O. AlphaFold2-Multimer Guided High-Accuracy Prediction of Typical and Atypical ATG8-Binding Motifs. PLoS Biol. 2023, 21, e3001962. [Google Scholar] [CrossRef]
- Tsuboyama, K.; Dauparas, J.; Chen, J.; Laine, E.; Mohseni Behbahani, Y.; Weinstein, J.J.; Mangan, N.M.; Ovchinnikov, S.; Rocklin, G.J. Mega-Scale Experimental Analysis of Protein Folding Stability in Biology and Design. Nature 2023, 620, 434–444. [Google Scholar] [CrossRef]
- Kudlik, G.; Takács, T.; Radnai, L.; Kurilla, A.; Szeder, B.; Koprivanacz, K.; Merő, B.L.; Buday, L.; Vas, V. Advances in Understanding TKS4 and TKS5: Molecular Scaffolds Regulating Cellular Processes from Podosome and Invadopodium Formation to Differentiation and Tissue Homeostasis. Int. J. Mol. Sci. 2020, 21, 8117. [Google Scholar] [CrossRef]
- Tilajka, Á.; Kurilla, A.; László, L.; Lovrics, A.; Novák, J.; Takács, T.; Buday, L.; Vas, V. Predictive Value Analysis of the Interaction Network of Tks4 Scaffold Protein in Colon Cancer. Front. Mol. Biosci. 2024, 11, 1414805. [Google Scholar] [CrossRef]
- Jacksi, M.; Schad, E.; Buday, L.; Tantos, A. Absence of Scaffold Protein Tks4 Disrupts Several Signaling Pathways in Colon Cancer Cells. Int. J. Mol. Sci. 2023, 24, 1310. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Bruck, S.; Cemerski, S.; Zhang, L.; Butler, B.; Dani, A.; Cooper, J.A.; Shaw, A.S. CD2AP Links Cortactin and Capping Protein at the Cell Periphery to Facilitate Formation of Lamellipodia. Mol. Cell. Biol. 2013, 33, 38–47. [Google Scholar] [CrossRef] [PubMed]
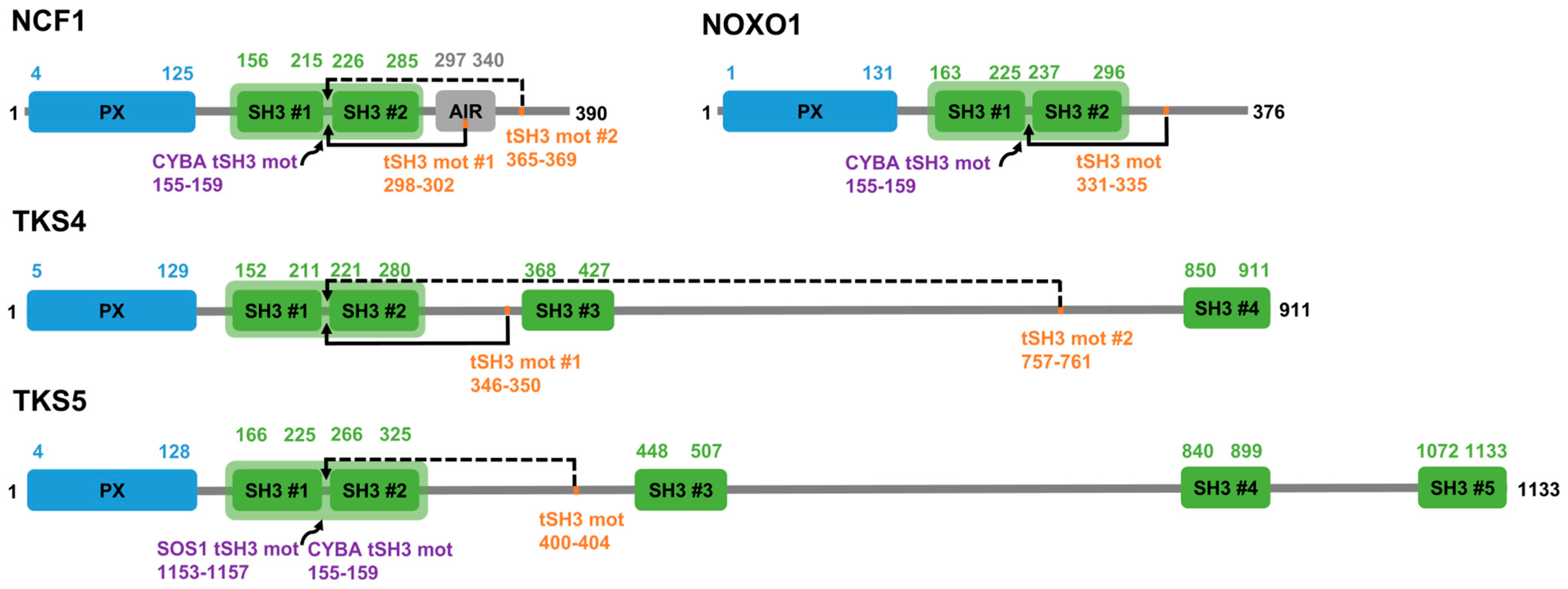



| Partner with SH3s (by Gene Name) | UniProt AC; Tandem SH3 Positions | Partner with Motif (by Gene Name) | UniProt AC; Peptide Positions | Peptide Sequence | Autoregulatory/Partner (A/P) | Kd/Not Measured (NM) | PDB Structures | Reference | Status: Validated/Proposed |
|---|---|---|---|---|---|---|---|---|---|
| NCF1 | P14598 158-283 | NCF1 | P14598 296-304 | RGAPPRRSS | A | 29 μM | 1NG2, 1UEC | [19] | Validated |
| NCF1 | P14598 158-283 | CYBA | P13498 153-161 | SNPPPRPPA | P | 0.19 μM | 1OV3, 1WLP, 7YXW | [18,19] | Validated |
| NOXO1 | Q8NFA2 165-294 | NOXO1 | Q8NFA2 329-337 | PTVPTRPSP | A | 50 µM | N/A | [46] | Validated |
| NOXO1 | Q8NFA2 165-294 | CYBA | P13498 153-161 | SNPPPRPPA | P | 0.15 µM | N/A | [46] | Validated |
| TKS4 | A1X283 155-278 | TKS4 | A1X283 344-352 | QRPPPRRDM | A | 12 ± 4 µM | N/A | [26] | Validated |
| TKS5 | Q5TCZ1-3 168-297 | CYBA | P13498 153-161 | SNPPPRPPA | P | NM | N/A | [27] | Validated |
| TKS5 | Q5TCZ1-3 168-297 | SOS1 | Q07889 1151-1159 | PPVPPRRRP | P | NM | N/A | [21] | Validated |
| NCF1 | P14598 158-283 | NCF1 | P14598 363-371 | PAVPPRPSA | A | N/A | N/A | N/A | Proposed |
| TKS4 | A1X283 155-278 | TKS4 | A1X283 755-763 | PVVPPRRPP | A | N/A | N/A | N/A | Proposed |
| TKS5 | Q5TCZ1-3 168-297 | TKS5 | Q5TCZ1-3 398-406 | TRPPPRRES | A | N/A | N/A | N/A | Proposed |
| NCF1 family protein | Identified partners with the motif positions indicated |
| NCF1 | NCF2 (227-233), SRSF2 (105-111), ADAM19 (789-795), RELA (326-332), SYN1 (676-682) |
| NOXO1 | ARHGAP8 (213-219) |
| TKS4 | SH3BP1 (538-544) * |
| TKS5 | GAB2 (351-357), PLEKHA7 (919-925), ADAM19 (789-795), RIN3 (385-391), DENND1A (950-956), INPP5E (189-195) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalman, Z.E.; Lazar, T.; Dobson, L.; Pancsa, R. Definition and Discovery of Tandem SH3-Binding Motifs Interacting with Members of the p47phox-Related Protein Family. Biomolecules 2025, 15, 1641. https://doi.org/10.3390/biom15121641
Kalman ZE, Lazar T, Dobson L, Pancsa R. Definition and Discovery of Tandem SH3-Binding Motifs Interacting with Members of the p47phox-Related Protein Family. Biomolecules. 2025; 15(12):1641. https://doi.org/10.3390/biom15121641
Chicago/Turabian StyleKalman, Zsofia E., Tamas Lazar, Laszlo Dobson, and Rita Pancsa. 2025. "Definition and Discovery of Tandem SH3-Binding Motifs Interacting with Members of the p47phox-Related Protein Family" Biomolecules 15, no. 12: 1641. https://doi.org/10.3390/biom15121641
APA StyleKalman, Z. E., Lazar, T., Dobson, L., & Pancsa, R. (2025). Definition and Discovery of Tandem SH3-Binding Motifs Interacting with Members of the p47phox-Related Protein Family. Biomolecules, 15(12), 1641. https://doi.org/10.3390/biom15121641







