Human Mutant Dynactin Subunit 1 Causes Profound Motor Neuron Disease Consistent with Possible Mechanisms Involving Axonopathy, Mitochondriopathy, Protein Nitration, and T-Cell-Mediated Cytolysis
Abstract
1. Introduction
2. Material and Methods
2.1. Ethics Statement
2.2. Tg Mice and Treatments
2.3. Characterization of Clinical Disease
2.4. Histology and Neuropathology
2.5. Immunohistochemistry
2.6. SDS-PAGE and Immunoblotting
2.7. Data Presentation and Statistical Analyses
3. Results
3.1. Verification of Tg Mice Expressing Human DCTN1
3.2. G59S-DCTN1 Tg Mice Develop a Tremorous, Disequilibrium Clumsy and Spastic Phenotype That Evolves into Fatal Paralysis
3.3. Motor Neurons Degenerate in Mutant Dynactin Tg Mice
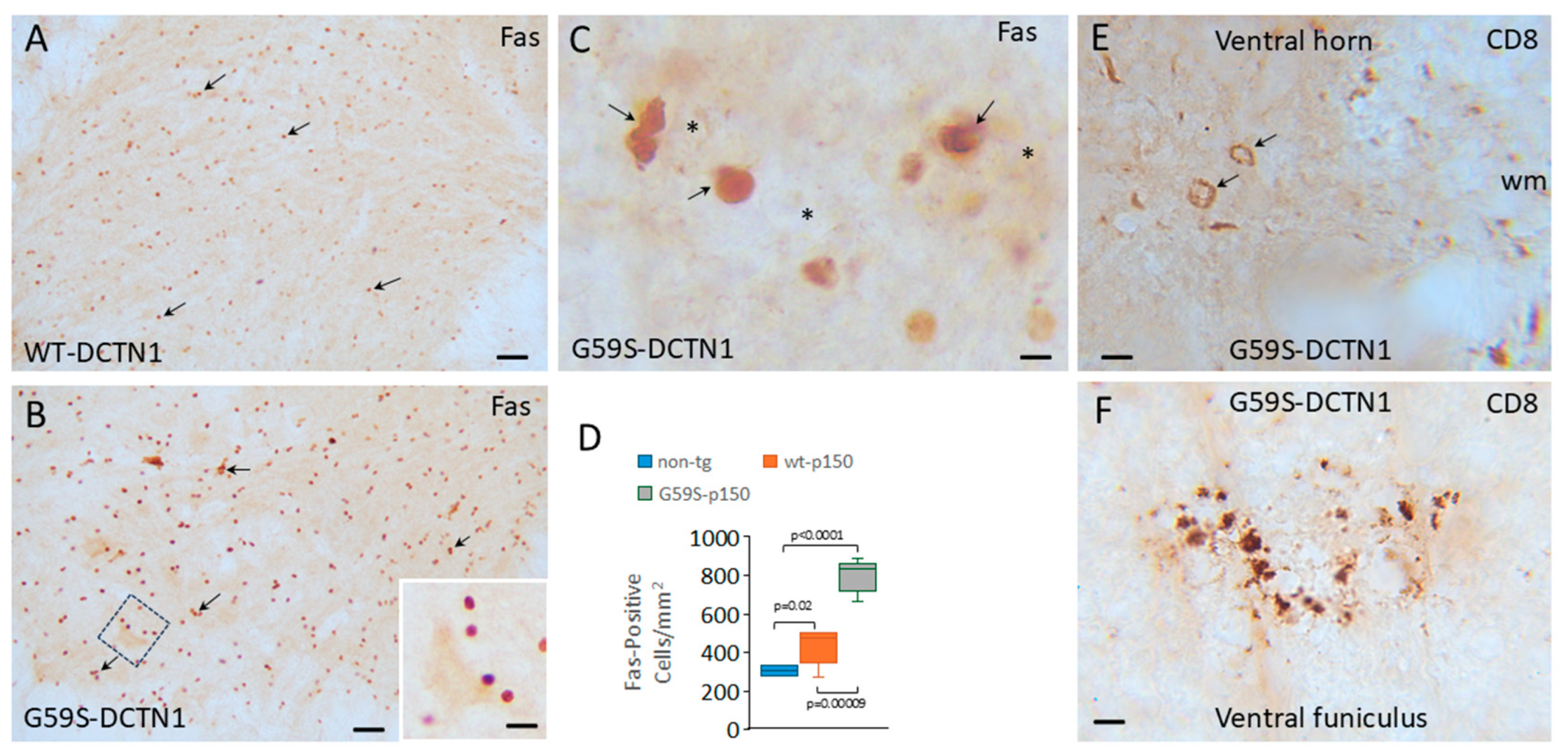
3.4. Cytotoxic T Lymphocytes and Inflammatory Cytokines Accumulate in Dynactin Transgenic Mouse Spinal Cord
3.5. Mutant Dynactin Mice Develop Axon Pathology
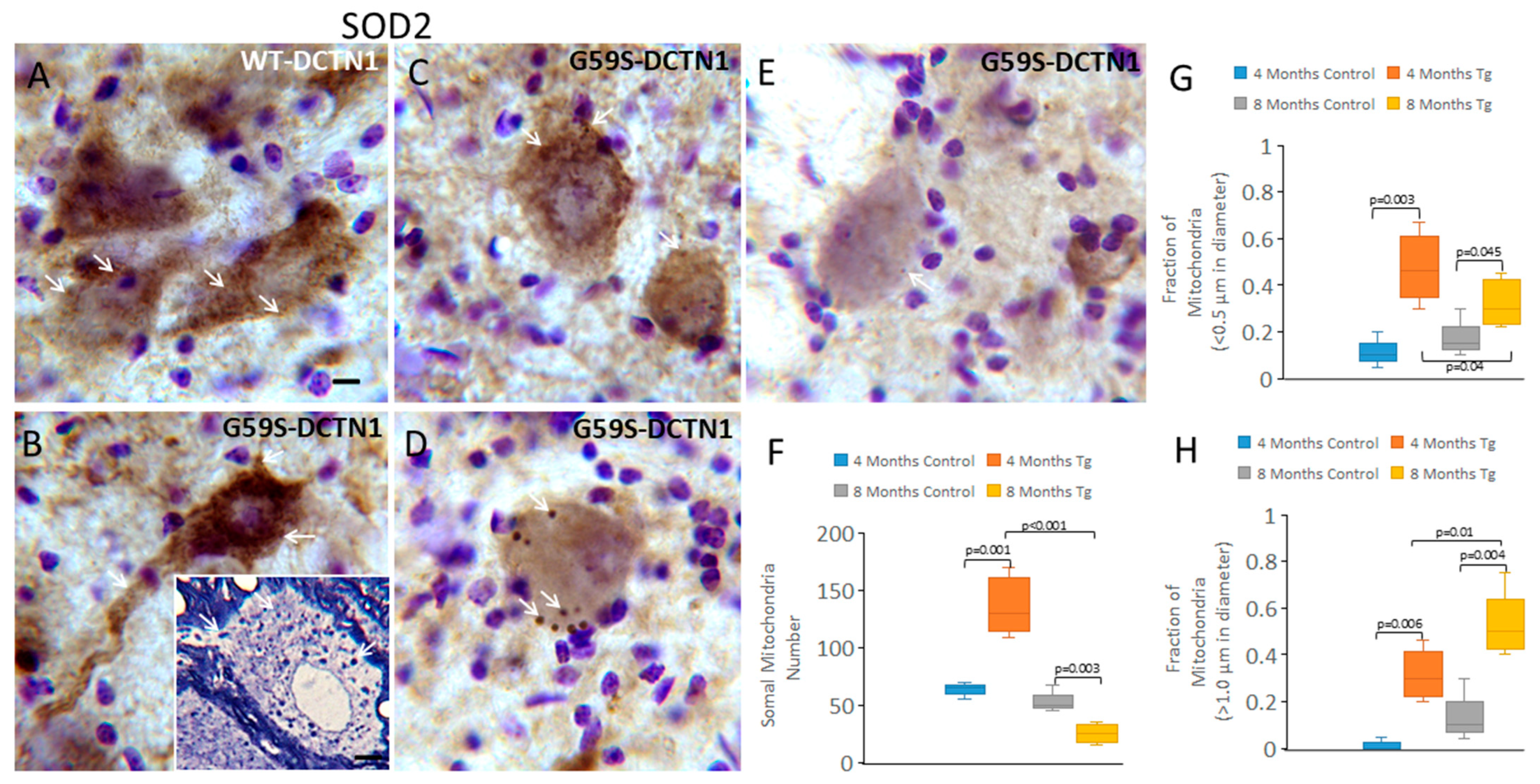
3.6. Mutant Dynactin Mice Have Extensive Mitochondrial Pathology
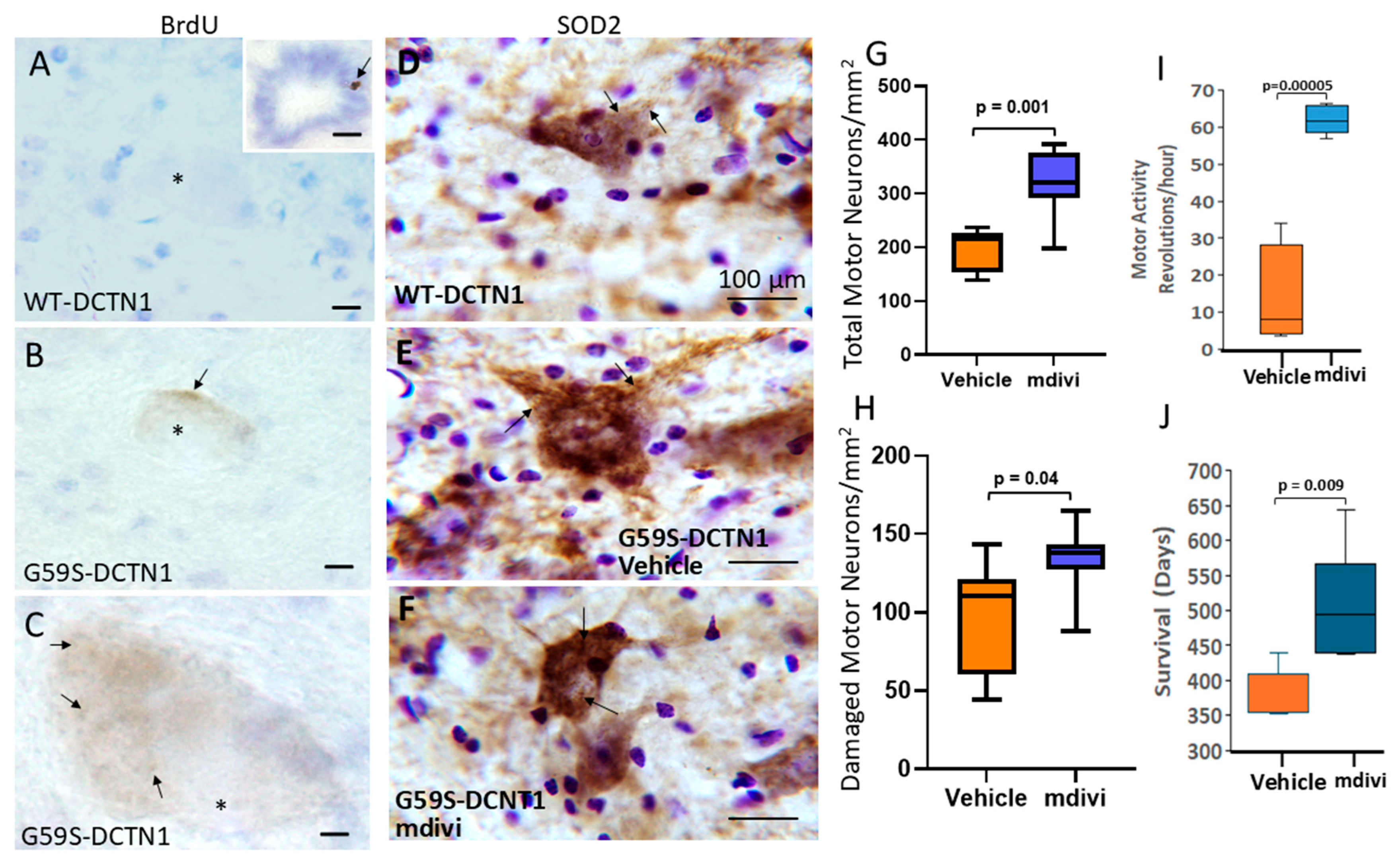
3.7. Targeting Mitochondria with Small-Molecule Drugs Mdivi-1 and GNX-4728 Protects Motor Neurons in Mutant Dynactin Mice
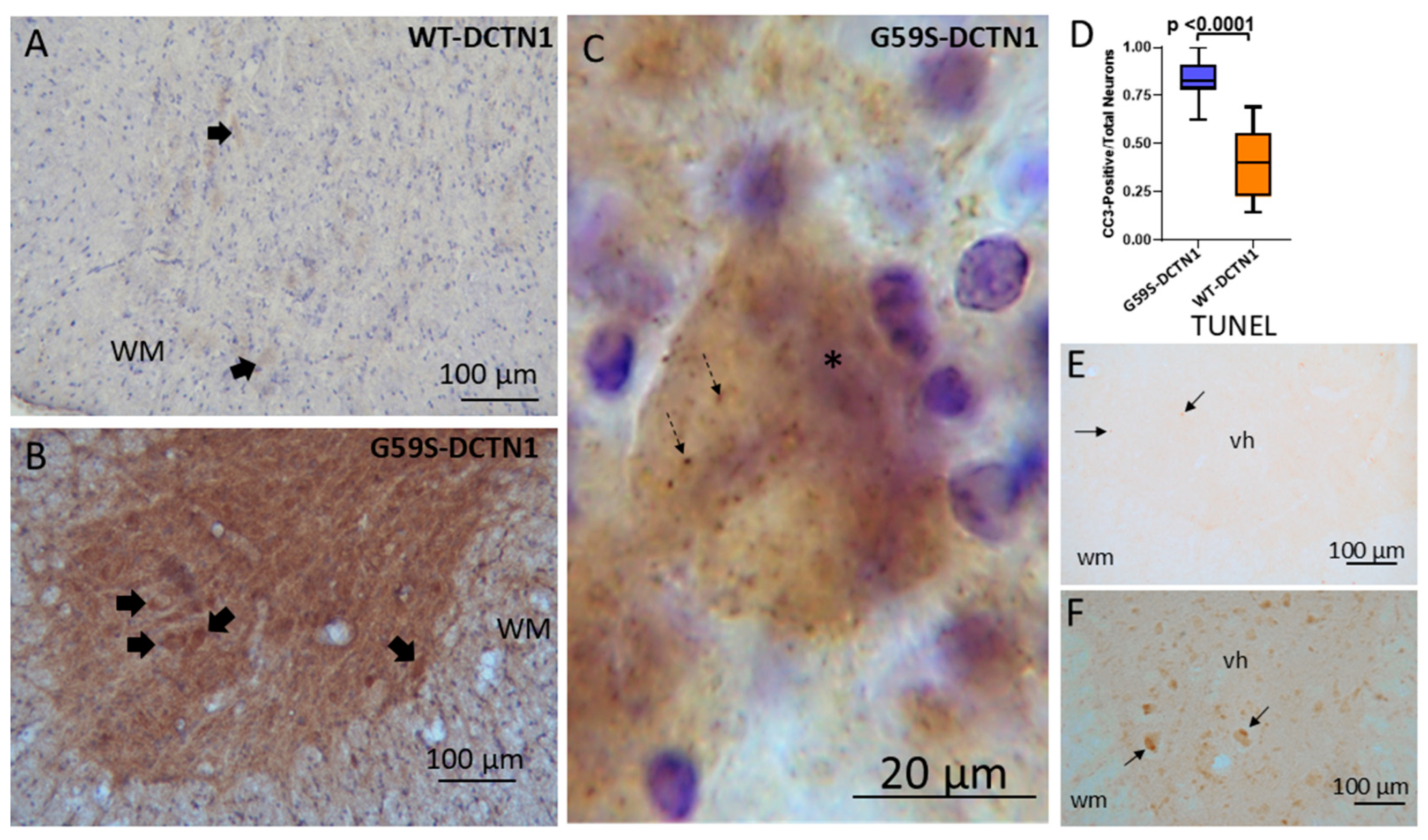
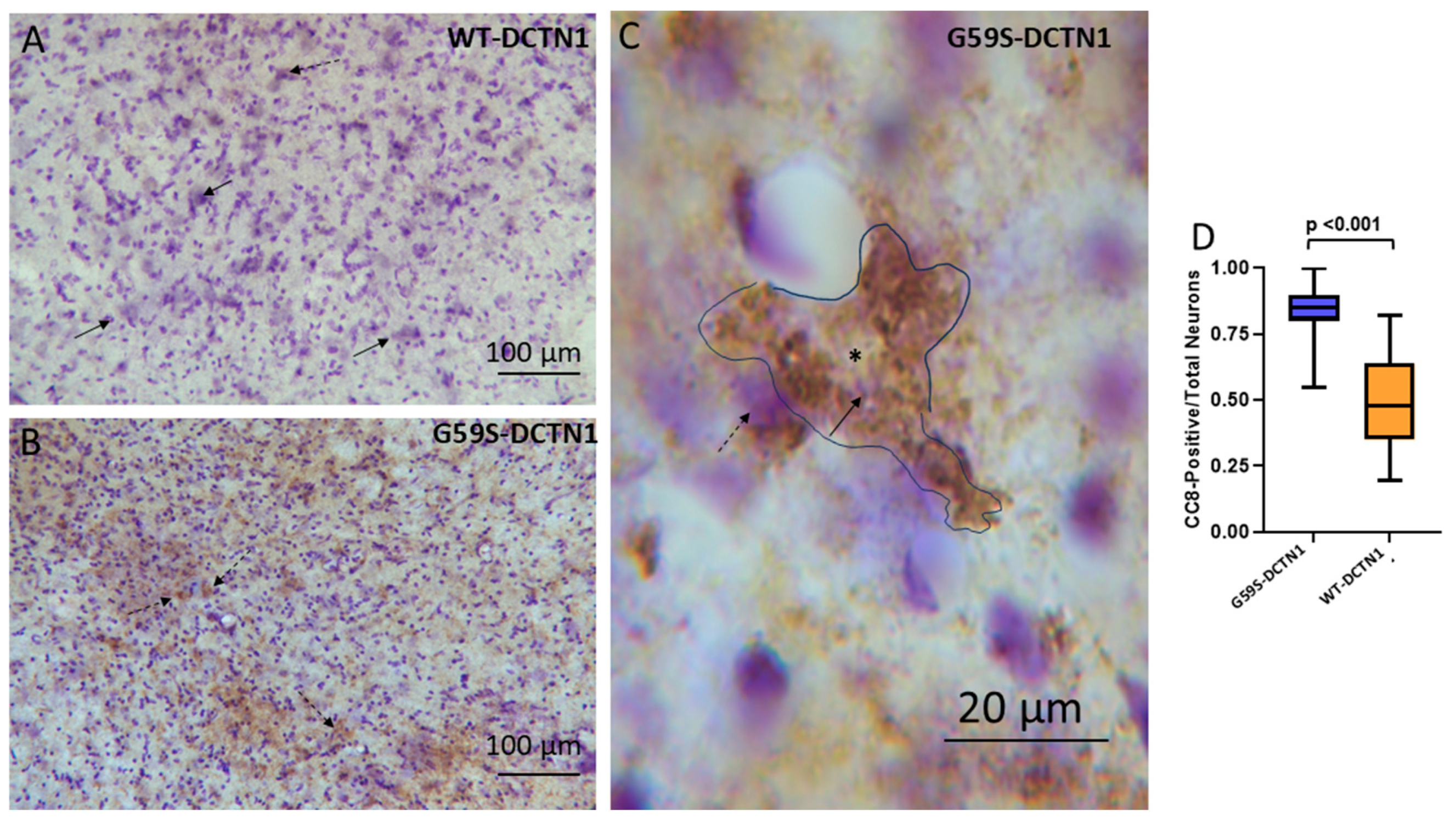
3.8. Motor Neurons in Mutant Dynactin Have Activated Caspases
3.9. Motor Neurons in Mutant Dynactin Tg Mice Develop Hsp90 Nitration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Puls, I.; Jonnakuty, C.; LaMonte, B.H.; Holzbaur, E.L.; Tokito, M.; Mann, E.; Floeter, M.K.; Bidus, K.; Drayna, D.; Oh, S.J.; et al. Mutant dynactin in motor neuron disease. Nat. Genet. 2003, 33, 455–456. [Google Scholar] [CrossRef]
- Puls, I.; Oh, S.J.; Sumner, C.J.; Wallace, K.E.; Floeter, M.K.; Mann, E.A.; Kennedy, W.R.; Wendelschafer-Crabb, G.; Vortmeyer, A.; Powers, R.; et al. Distal spinal and bulbar muscular atrophy caused by dynactin mutation. Ann. Neurol. 2005, 57, 687–694. [Google Scholar] [CrossRef]
- Münch, C.; Sedlmeier, R.; Meyer, T.; Homberg, V.; Sperfeld, A.D.; Kurt, A.; Prudlo, J.; Peraus, G.; Hanemann, C.O.; Stumm, G.; et al. Point mutations of the p150 subunit of dynactin (DCTN1) gene in ALS. Neurology 2004, 63, 724–726. [Google Scholar] [CrossRef]
- Tsuboi, Y.; Mishima, T.; Fujioka, S. Perry Disease: Concept of a New Disease and Clinical Diagnostic Criteria. J. Mov. Disord. 2021, 14, 1–9. [Google Scholar] [CrossRef]
- Gill, S.R.; Schroer, T.A.; Szilak, I.; Steuer, E.R.; Sheetz, M.P.; Cleveland, D.W. Dynactin, a conserved, ubiquitously expressed component of an activator of vesicle motility mediated by cytoplasmic dynein. J. Cell Biol. 1991, 115, 1639–1650. [Google Scholar] [CrossRef]
- Schroer, T.A.; Sheetz, M.P. Functions of microtubule-based motors. Annu. Rev. Physiol. 1991, 53, 629–652. [Google Scholar] [CrossRef] [PubMed]
- Schroer, T.A. Dynactin. Annu. Rev. Cell Dev. Biol. 2004, 20, 759–779. [Google Scholar] [CrossRef]
- Hirokawa, N.; Takemura, R. Molecular Motors and mechanisms of directional transport in neurons. Nat. Rev. Neurosci. 2005, 6, 201–214. [Google Scholar] [CrossRef] [PubMed]
- LaMonte, B.H.; Wallace, K.E.; Holloway, B.A.; Shelly, S.S.; Ascaño, J.; Tokito, M.; Van Winkle, T.; Howland, D.S.; Holzbaur, E.L.F. Disruption of dynein/dynactin inhibits axonal transport in motor neurons causing late-onset progressive degeneration. Neuron 2002, 34, 715–727. [Google Scholar] [CrossRef]
- Ross, J.L.; Wallace, K.; Shuman, H.; Goldman, Y.E.; Holzbaur, E.L.F. Processive bidirectional motion of dynein–dynactin complexes in vitro. Nat. Cell Biol. 2006, 8, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, B.; Acevedo-Arozena, A.; Imarisio, S.; Berger, Z.; Vacher, C.; O’Kane, C.J.; Brown, S.D.; Rubinsztein, D.C. Dynein mutations impair autophagic clearance of aggregate-prone proteins. Nat. Genet. 2005, 37, 771–776. [Google Scholar] [CrossRef]
- Moore, J.K.; Sept, D.; Cooper, J.A. Neurodegeneration mutations in dynactin impair dynein-dependent nuclear migration. Proc. Natl. Acad. Sci. USA 2009, 106, 5147–5152. [Google Scholar] [CrossRef] [PubMed]
- Pathak, A.; Stanley, E.M.; Hickman, F.E.; Wallace, N.; Brewer, B.; Li, D.; Gluska, S.; Perlson, E.; Fuhrmann, S.; Akassoglou, K.; et al. Retrograde degenerative signaling mediated by the P75 neurotrophin receptor requires p150glued deacetylation by axonal HDAC1. Dev. Cell 2018, 46, 376–387.e7. [Google Scholar] [CrossRef] [PubMed]
- Stykel, M.G.; Humphries, K.; Kirby, M.P.; Czaniecki, C.; Wang, T.; Ryan, T.; Bamm, V.; Ryan, S.D. Nitration of microtubules blocks axonal mitochondrial transport in a human pluripotent stem cell model of Parkinson’s disease. FASEB J. 2018, 32, 5350–5364. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.R.; Sumner, C.J.; Caviston, J.P.; Tokito, M.K.; Ranganathan, S.; Ligon, L.A.; Wallace, K.E.; LaMonte, B.H.; Harmison, G.G.; Puls, I.; et al. A motor neuron disease-associated mutation in p150Glued perturbs dynactin function and induces protein aggregation. J. Cell Biol. 2006, 172, 733–745. [Google Scholar] [CrossRef]
- Moughamian, A.J.; Holzbaur, E.L. Dynactin is required for transport initiation from the distal axon. Neuron 2012, 74, 331–343. [Google Scholar] [CrossRef]
- Lloyd, T.E.; Machamer, J.; O’Hara, K.; Kim, J.H.; Collins, S.E.; Wong, M.Y.; Sahin, B.; Imlach, W.; Yang, Y.; Levitan, E.S.; et al. The p150Glued CAP-Gly domain regulates initiation of retrograde transport at synaptic termini. Neuron 2012, 74, 344–360. [Google Scholar] [CrossRef]
- Pilling, A.D.; Horiuchi, D.; Lively, C.M.; Saxton, W.M. Kinesin-1 and Dynein are the primary motors for fast transport of mitochondria in Drosophila motor axons. Mol. Biol. Cell 2006, 17, 2057–2068. [Google Scholar] [CrossRef]
- Lieberman, A.R. The axon reaction: A review of the principal features of perikaryal responses to axon injury. Int. Rev. Neurobiol. 1971, 14, 49–124. [Google Scholar] [CrossRef]
- Price, D.L.; Porter, K.R. The response of ventral horn neurons to axonal transection. J. Cell Biol. 1972, 53, 24–37. [Google Scholar] [CrossRef]
- Martin, L.J.; Al-Abdulla, N.A.; Brambrink, A.M.; Kirsch, J.R.; Sieber, F.E.; Portera-Cailliau, C. Neurodegeneration in excitotoxicity, lobal cerebral ischemia, and target deprivation: A perspective on the contributions of apoptosis and necrosis. Brain Res. Bull. 1998, 46, 281–309. [Google Scholar] [CrossRef]
- Caroni, P. Overexpression of growth-associated proteins in the neurons of adult transgenic mice. J. Neurosc. Meth. 1997, 71, 3–9. [Google Scholar] [CrossRef]
- Laird, F.M.; Farah, M.H.; Ackerley, S.; Hoke, A.; Maragakis, N.; Rothstein, J.D.; Griffin, J.; Price, D.L.; Martin, L.J.; Wong, P.C. Motor neuron disease occurring in a mutant dynactin mouse model is characterized by defects in vesicular trafficking. J. Neurosci. 2008, 28, 1997–2005. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, Y.; Price, A.; Martin, L.J. Generation and characterization of transgenic mice expressing mitochondrial targeted red fluorescent protein selectively in neurons: Modeling mitochondriopathy in excitotoxicity and amyotrophic lateral sclerosis. Mol. Neurodegener. 2011, 6, 75. [Google Scholar] [CrossRef]
- Feng, G.; Mellor, R.H.; Bernstein, M.; Keller-Peck, C.; Nguyen, Q.T.; Wallace, M.; Nerbonne, J.M.; Lichtman, J.W.; Sanes, J.R. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 2000, 28, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.F.; Clayton, D.A. In situ localization of mitochondrial DNA replication in intact mammalian cells. J. Cell Biol. 1996, 135, 883–893. [Google Scholar] [CrossRef]
- Liu, Z.; Martin, L.J. The adult neural stem and progenitor cell niche is altered in amyotrophic lateral sclerosis mouse brain. J. Comp. Neurol. 2006, 497, 468–488. [Google Scholar] [CrossRef]
- Martin, L.J.; Adams, N.A.; Pan, Y.; Price, A.; Wong, M. The mitochondrial permeability transition pore regulates nitric oxide-mediated apoptosis of neurons induced by target deprivation. J. Neurosci. 2011, 31, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.J.; Fancelli, D.; Wong, M.; Niedzwiecki, M.; Ballarini, M.; Plyte, S.; Chang, Q. GNX-4728, a novel small molecule drug inhibitor of mitochondrial permeability transition, is therapeutic in a mouse model of amyotrophic lateral sclerosis. Front. Cell. Neurosci. 2014, 8, 433. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.J.; Liu, Z. Adult olfactory bulb neural precursor cell grafts provide temporary protection from motor neuron degeneration, improve motor function, and extend survival in amyotrophic lateral sclerosis mice. J. Neuropathol. Exp. Neurol. 2007, 66, 1002–1018. [Google Scholar] [CrossRef][Green Version]
- Wong, M.; Martin, L.J. Skeletal muscle-restricted expression of human SOD1 causes motor neuron degeneration in transgenic mice. Hum. Mol. Genet. 2010, 19, 2284–2302. [Google Scholar] [CrossRef]
- Martin, L.J.; Pardo, C.A.; Cork, L.C.; Price, D.L. Synaptic pathology and glial responses to neuronal injury precede the formation of senile plaques and amyloid deposits in the aging cerebral cortex. Am. J. Pathol. 1994, 145, 1358–1381. [Google Scholar]
- Portera-Cailliau, C.; Price, D.L.; Martin, L.J. Non-NMDA and NMDA receptor-mediated excitotoxic neuronal deaths in adult brain are morphologically distinct: Further evidence for an apoptosis-necrosis continuum. J. Comp. Neurol. 1997, 378, 88–104. [Google Scholar] [CrossRef]
- Martin, L.J.; Liu, Z.; Chen, K.; Price, A.C.; Pan, Y.; Swaby, J.A.; Golden, W.C. Motor neuron degeneration in amyotrophic lateral sclerosis mutant superoxide dismutase-1 transgenic mice: Mechanisms of mitochondriopathy and cell death. J. Comp. Neurol. 2007, 500, 20–46. [Google Scholar] [CrossRef]
- Sternberger, L.A.; Petrali, J.P. The unlabeled antibody enzyme method. Attempted use of peroxidase-conjugated antigen as the third layer in the technique. J. Histochem. Cytochem. 1977, 25, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Bratthauer, G.L. The peroxidase-antiperoxidase (PAP) method. Methods Mol. Biol. 1994, 34, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Franco, M.C.; Ye, Y.; Refakis, C.A.; Feldman, J.L.; Stokes, A.L.; Basso, M.; Melero Fernández De Mera, R.M.; Sparrow, N.A.; Calingasan, N.Y.; Kiaei, M.; et al. Nitration of Hsp90 induces cell death. Proc. Natl. Acad. Sci. USA 2013, 110, E1102–E1111. [Google Scholar] [CrossRef]
- Holzbaur, E.L.F.; Hammarback, J.A.; Paschal, B.M.; Kravit, N.G.; Pfister, K.K.; Vallee, R.B. Homology of a 150K cytoplasmic dynein-associated polypeptide with the drosophila gene glued. Nature 1991, 351, 579–583. [Google Scholar] [CrossRef]
- Martin, L.J.; Chen, K.; Liu, Z. Adult motor neuron apoptosis is mediated by nitric oxide and Fas death receptor linked by DNA damage and p53 activation. J. Neurosci. 2005, 25, 6449–6459. [Google Scholar] [CrossRef]
- Torvik, A. Central chromatolysis and the axon reaction: A reappraisal. Neuropathol. Appl. Neurobiol. 1976, 2, 423–432. [Google Scholar] [CrossRef]
- Martin, L.J.; Kaiser, A.; Price, A.C. Motor neuron degeneration after sciatic nerve avulsion in adult rat evolves with oxidative stress and is apoptosis. J. Neurobiol. 1999, 40, 185–201. [Google Scholar] [CrossRef]
- Weisiger, R.A.; Fridovich, I. Mitochondrial superoxide dismutase site of synthesis and intramitochondrial localization. J. Biol. Chem. 1973, 248, 4793–4796. [Google Scholar] [CrossRef]
- Narendra, D.; Tanaka, A.; Suen, D.F.; Youle, R.J. Parkin-induced mitophagy in the pathogenesis of Parkinson disease. Autophagy 2009, 5, 706–708. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.J. The mitochondrial permeability transition pore: A molecular target for amyotrophic lateral sclerosis therapy. Biochim. Biophys. Acta. 2010, 1802, 186–197. [Google Scholar] [CrossRef]
- Martin, L.J. Mitochondriopathy in Parkinson disease and amyotrophic lateral sclerosis. J. Neuropathol. Exp. Neurol. 2006, 65, 1103–1110. [Google Scholar] [CrossRef]
- Martin, L.J.; Liu, Z. Injury-induced spinal motor neuron apoptosis is preceded by DNA single-strand breaks and is p53- and Bax-dependent. J. Neurobiol. 2002, 50, 181–197. [Google Scholar] [CrossRef]
- Martin, L.J. Mitochondrial and cell death mechanisms in neurodegenerative diseases. Pharmaceuticals 2010, 3, 839–915. [Google Scholar] [CrossRef]
- Parrish, A.B.; Freel, C.D.; Kornbluth, S. Cellular mechanisms controlling caspase activation and function. Cold Spring Harb. Perspect. Biol. 2013, 5, a008672. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.S.; Carson, M.; Smith, C.D.; Koppenol, W.H. ALS, SOD and peroxynitrite. Nature 1993, 364, 584. [Google Scholar] [CrossRef] [PubMed]
- Estévez, A.G.; Crow, J.P.; Sampson, J.B.; Reiter, C.; Zhuang, Y.; Richardson, G.J.; Tarpey, M.M.; Barbeito, L.; Beckman, J.S. Induction of nitric oxide—Dependent apoptosis in motor neurons by zinc-deficient superoxide dismutase. Science 1999, 286, 2498–2500. [Google Scholar] [CrossRef]
- Chen, K.; Northington, F.J.; Martin, L.J. Inducible nitric oxide synthase is present in motor neuron mitochondria and schwann cells and contributes to disease mechanisms in ALS mice. Brain Struct. Funct. 2010, 214, 219–234. [Google Scholar] [CrossRef]
- Liu, Z.-J.; Li, H.-F.; Tan, G.-H.; Tao, Q.-Q.; Ni, W.; Cheng, X.-W.; Xiong, Z.-Q.; Wu, Z.-Y. Identify mutation in amyotrophic lateral sclerosis cases using HaloPlex target enrichment system. Neurobiol. Aging 2014, 35, e11–e2881. [Google Scholar] [CrossRef] [PubMed]
- Dulski, J.; Konno, T.; Wszolek, Z. DCTN1-related neurodegeneration. In GeneReviews®; University of Washington: Seattle, WA, USA, 2010. [Google Scholar]
- Farrer, M.J.; Hulihan, M.M.; Kachergus, J.M.; Dächsel, J.C.; Stoessl, A.J.; Grantier, L.L.; Calne, S.; Calne, D.B.; Lechevalier, B.; Chapon, F.; et al. DCTN1 mutations in Perry syndrome. Nat. Genet. 2009, 41, 163–165. [Google Scholar] [CrossRef]
- Konno, T.; Ross, O.A.; Teive, H.A.G.; Sławek, J.; Dickson, D.W.; Wszolek, Z.K. DCTN1-related neurodegeneration: Perry syndrome and beyond. Park. Relat. Disord. 2017, 41, 14–24. [Google Scholar] [CrossRef]
- Vilariño-Güell, C.; Wider, C.; Soto-Ortolaza, A.I.; Cobb, S.A.; Kachergus, J.M.; Keeling, B.H.; Dachsel, J.C.; Hulihan, M.M.; Dickson, D.W.; Wszolek, Z.K.; et al. Characterization of DCTN1 genetic variability in neurodegeneration. Neurology 2009, 72, 2024–2028. [Google Scholar] [CrossRef]
- Drerup, C.M.; Herbert, A.L.; Monk, K.R.; Nechiporuk, A.V. Regulation of mitochondria-dynactin interaction and mitochondrial retrograde transport in axons. eLife 2017, 6, e22234. [Google Scholar] [CrossRef]
- Kononenko, N.L.; Claßen, G.A.; Kuijpers, M.; Puchkov, D.; Maritzen, T.; Tempes, A.; Malik, A.R.; Skalecka, A.; Bera, S.; Jaworski, J.; et al. Retrograde transport of TrkB-containing autophagosomes via the adaptor AP-2 mediates neuronal complexity and prevents neurodegeneration. Nat. Commun. 2017, 8, 14819. [Google Scholar] [CrossRef]
- Ayloo, S.; Lazarus, J.E.; Dodda, A.; Tokito, M.; Ostap, E.M.; Holzbaur, E.L. Dynactin functions as both a dynamic tether and brake during dynein-driven motility. Nat. Comm. 2014, 5, 4807. [Google Scholar] [CrossRef]
- Lai, C.; Lin, X.; Chandran, J.; Shim, H.; Yang, W.J.; Cai, H. The G59S mutation in p150glued causes dysfunction of dynactin in mice. J. Neurosci. 2007, 27, 13982–13990. [Google Scholar] [CrossRef] [PubMed]
- Chevalier-Larsen, E.S.; Wallace, K.E.; Pennise, C.R.; Holzbaur, E.L. Lysosomal proliferation and distal degeneration in motor neurons expressing the G59S mutation in the p150Glued subunit of dynactin. Hum. Mol. Genet. 2008, 17, 1946–1955. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Xu, Z. Massive mitochondrial degeneration in motor neurons triggers the onset of amyotrophic lateral sclerosis in mice expressing a mutant SOD1. J. Neurosci. 1998, 18, 3241–3250. [Google Scholar] [CrossRef] [PubMed]
- Jaarsma, D.; Haasdijk, E.D.; Grashorn, J.A.C.; Hawkins, R.; Van Duijn, W.; Verspaget, H.W.; London, J.; Holstege, J.C. Human Cu/Zn superoxide dismutase (SOD1) overexpression in mice causes mitochondrial vacuolization, axonal degeneration, and premature motoneuron death and accelerates motoneuron disease in mice expressing a familial amyotrophic lateral sclerosis mutant SOD1. Neurobiol. Dis. 2000, 7, 623–643. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.J.; Gertz, B.; Pan, Y.; Price, A.C.; Molkentin, J.D.; Chang, Q. The mitochondrial permeability transition pore in motor neurons: Involvement in the pathobiology of ALS mice. Exp. Neurol. 2009, 218, 333–346. [Google Scholar] [CrossRef]
- Perlson, E.; Maday, S.; Fu, M.M.; Moughamian, A.J.; Holzbaur, E.L. Retrograde axonal transport: Pathways to cell death? Trends Neurosci. 2010, 33, 335–344. [Google Scholar] [CrossRef]
- Fenton, A.R.; Jongens, T.A.; Holzbaur, E.L.F. Mitochondrial adaptor TRAK2 activates and functionally links opposing kinesin and dynein motors. Nat. Comm. 2021, 12, 4578. [Google Scholar] [CrossRef]
- Tradewell, M.L.; Cooper, L.A.; Minotti, S.; Durham, H.D. Calcium dysregulation, mitochondrial pathology and protein aggregation in a culture model of amyotrophic lateral sclerosis: Mechanistic relationship and differential sensitivity to intervention. Neurobiol. Dis. 2011, 42, 265–275. [Google Scholar] [CrossRef]
- Manfredi, A.A.; Rovere-Querini, P. The mitochondrion—A trojan horse that kicks off inflammation? N. Engl. J. Med. 2010, 362, 2132–2134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wei, H.; Zhao, A.; Yan, X.; Zhang, X.; Gan, J.; Guo, M.; Wang, J.; Zhang, F.; Jiang, Y.; et al. Mitochondrial DNA leakage: Underlying mechanisms and therapeutic implications in neurological disorders. J. Neuroinflammation 2025, 22, 34. [Google Scholar] [CrossRef]
- He, D.; Xu, Y.; Liu, M.; Cui, L. The inflammatory puzzle: Piecing together the links between neuroinflammation and amyotrophic lateral sclerosis. Aging Dis. 2024, 15, 96. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, B.; Sinha, S.C.; Amin, S.; Gan, L. Mechanism and therapeutic potential of targeting cGAS-STING signaling in neurological disorders. Mol. Neurodegener. 2023, 18, 79. [Google Scholar] [CrossRef]
- Cunha-Oliveira, T.; Montezinho, L.; Simões, R.F.; Carvalho, M.; Ferreiro, E.; Silva, F.S.G. Mitochondria: A Promising Convergent Target for the Treatment of Amyotrophic Lateral Sclerosis. Cells 2024, 13, 248. [Google Scholar] [CrossRef] [PubMed]
- Zeytun, A.; Hassuneh, M.; Nagarkatti, M.; Nagarkatti, P.S. Fas-Fas ligand-based interactions between tumor cells and tumor-specific cytotoxic T lymphocytes: A lethal two-way street. Blood 1997, 90, 1952–1959. [Google Scholar] [CrossRef]
- Kotov, D.I.; Kotov, J.A.; Goldberg, M.F.; Jenkins, M.K. Many Th Cell Subsets Have Fas Ligand-Dependent Cytotoxic Potential. J. Immunol. 2018, 200, 2004–2012. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Duan, Z.; Cui, Y. CD8+ T cells in brain injury and neurodegeneration. Front. Cell. Neurosci. 2023, 17, 1281763. [Google Scholar] [CrossRef] [PubMed]
- Gowing, G.; Dequen, F.; Soucy, G.; Julien, J.P. Absence of tumor necrosis factor-alpha does not affect motor neuron disease caused by superoxide dismutase 1 mutations. J. Neurosci. 2006, 26, 11397–11402. [Google Scholar] [CrossRef]
- Rojas-Zuleta, W.G.; Sanchez, E. IL-9: Function, Sources, and Detection. In Methods in Molecular Biology; Clifton, N.J., Ed.; Humana Press: New York, NY, USA, 2017; Volume 1585, pp. 21–35. [Google Scholar] [CrossRef]
- Purwar, R.; Schlapbach, C.; Xiao, S.; Kang, H.S.; Elyaman, W.; Jiang, X.; Jetten, A.M.; Khoury, S.J.; Fuhlbrigge, R.C.; Kuchroo, V.K.; et al. Robust tumor immunity to melanoma mediated by interleukin-9-producing T cells. Nat. Med. 2012, 18, 1248–1253. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Cao, F.; Cui, L.; Ciric, B.; Zhang, G.X.; Rostami, A. IL-9 signaling affects central nervous system resident cells during inflammatory stimuli. Exp. Mol. Pathol. 2015, 99, 570–574. [Google Scholar] [CrossRef]
- Kawamata, T.; Akiyama, H.; Yamada, T.; McGeer, P.L. Immunologic reactions in amyotrophic lateral sclerosis brain and spinal cord tissue. Am. J. Pathol. 1992, 140, 691–707. [Google Scholar]
- Liu, L.; Chahroudi, A.; Silvestri, G.; Wernett, M.E.; Kaiser, W.J.; Safrit, J.T.; Komoriya, A.; Altman, J.D.; Packard, B.Z.; Feinberg, M.B. Visualization and quantification of T cell-mediated cytotoxicity using cell-permeable fluorogenic caspase substrates. Nat. Med. 2002, 8, 185–189. [Google Scholar] [CrossRef]
- Martin, L.J. DNA damage and repair: Relevance to mechanisms of neurodegeneration. J. Neuropathol. Exp. Neurol. 2008, 67, 377–387. [Google Scholar] [CrossRef]
- Acehan, D.; Jiang, X.; Morgan, D.G.; Heuser, J.E.; Wang, X.; Akey, C.W. Three-dimensional structure of the apoptosome: Implications for assembly, procaspase-9 binding, and activation. Mol. Cell 2002, 9, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.C.; Hong, C.; Akey, I.V.; Yuan, S.; Akey, C.W. A near atomic structure of the active human apoptosome. eLife 2016, 5, e17755. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.-I.; Saiki, S.; Furuya, N.; Yamada, D.; Imamichi, Y.; Li, Y.; Kawajiri, S.; Sasaki, H.; Koike, M.; Tsuboi, Y.; et al. P150glued-associated disorders are caused by activation of intrinsic apoptotic pathway. PLoS ONE 2014, 9, e94645. [Google Scholar] [CrossRef]
- Liu, X.; Song, L.; Yu, J.; Huang, F.; Li, Y.; Ma, C. Mdivi-1: A promising drug and its underlying mechanisms in the treatment of neurodegenerative diseases. Histol. Histopathol. 2022, 37, 505–512. [Google Scholar] [CrossRef]
- Smirnova, E.; Griparic, L.; Shurland, D.-L.; Van Der Bliek, A.M. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. MBoC 2001, 12, 2245–2256. [Google Scholar] [CrossRef]
- Joshi, A.U.; Saw, N.L.; Vogel, H.; Cunnigham, A.D.; Shamloo, M.; Mochly-Rosen, D. Inhibition of Drp1/Fis1 interaction slows progression of amyotrophic lateral sclerosis. EMBO Mol. Med. 2018, 10, e8166. [Google Scholar] [CrossRef]
- Ciuro, M.; Sangiorgio, M.; Cacciato, V.; Cantone, G.; Fichera, C.; Salvatorelli, L.; Magro, G.; Leanza, G.; Vecchio, M.; Valle, M.S.; et al. Mitigating the functional deficit after neurotoxic motoneuronal loss by an inhibitor of mitochondrial fission. IJMS 2024, 25, 7059. [Google Scholar] [CrossRef]
- Bido, S.; Soria, F.N.; Fan, R.Z.; Bezard, E.; Tieu, K. Mitochondrial division inhibitor-1 is neuroprotective in the A53T-α-synuclein rat model of Parkinson’s disease. Sci. Rep. 2017, 7, 7495. [Google Scholar] [CrossRef]
- Peluffo, H.; Shacka, J.J.; Ricart, K.; Bisig, C.G.; Martìnez-Palma, L.; Pritsch, O.; Kamaid, A.; Eiserich, J.P.; Crow, J.P.; Barbeito, L.; et al. Induction of motor neuron apoptosis by free 3-nitro-L-tyrosine. J. Neurochem. 2004, 89, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Martin, L.J. Motor neurons rapidly accumulate DNA single-strand breaks after in vitro exposure to nitric oxide and peroxynitrite and in vivo axotomy. J. Comp. Neurol. 2001, 432, 35–60. [Google Scholar] [CrossRef]
- Abe, K.; Pan, L.-H.; Watanabe, M.; Kato, T.; Itoyama, Y. Induction of nitrotyrosine-like immunoreactivity in the lower motor neuron of amyotrophic lateral sclerosis. Neurosci. Lett. 1995, 199, 152–154. [Google Scholar] [CrossRef]
- Chou, S.M.; Wang, H.S.; Komai, K. Colocalization of NOS and SOD1 in neurofilament accumulation within motor neurons of amyotrophic lateral sclerosis: An immunohistochemical Study. J. Chem. Neuroanat. 1996, 10, 249–258. [Google Scholar] [CrossRef]
- Beal, M.F.; Ferrante, R.J.; Browne, S.E.; Matthews, R.T.; Kowall, N.W.; Brown, R.H. Incrased 3-nitrotyrosine in both sporadic and familial amyotrophic lateral sclerosis. Ann. Neurol. 1997, 42, 644–654. [Google Scholar] [CrossRef]
- Cha, C.I.; Chung, Y.H.; Shin, C.M.; Shin, D.H.; Kim, Y.S.; Gurney, M.E.; Lee, K.W. Immunocytochemical study on the distribution of nitrotyrosine in the brain of the transgenic mice expressing a human Cu/Zn SOD mutation. Brain Res. 2000, 853, 156–161. [Google Scholar] [CrossRef]
- Gersh, I.; Bodian, D. Some chemical mechanisms in chromatolysis. J. Cell. Comp. Physiol. 1943, 21, 253–279. [Google Scholar] [CrossRef]
- Cragg, B.G. What is the signal for chromatolysis? Brain Res. 1970, 23, 1–21. [Google Scholar] [CrossRef]
- Kusaka, H.; Imai, T.; Hashimoto, S.; Yamamoto, T.; Maya, K.; Yamasaki, M. Ultrastructural study of chromatolytic neurons in an adult-onset sporadic case of amyotrophic lateral sclerosis. Acta Neuropathol. 1988, 75, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.J. Neuronal death in amyotrophic lateral sclerosis is apoptosis: Possible contribution of a programmed cell death mechanism. J. Neuropathol. Exp. Neurol. 1999, 58, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Logan, I.E.; Nguyen, K.T.; Chatterjee, T.; Manivannan, B.; Paul, N.P.; Kim, S.R.; Sixta, E.M.; Bastian, L.P.; Marean-Reardon, C.; Karajannis, M.A.; et al. Selective nitration of Hsp90 acts as a metabolic switch promoting tumor cell proliferation. Redox Biol. 2024, 75, 103249. [Google Scholar] [CrossRef] [PubMed]
- Harguindey, S.; Stanciu, D.; Devesa, J.; Alfarouk, K.; Cardone, R.A.; Polo Orozco, J.D.; Devesa, P.; Rauch, C.; Orive, G.; Anitua, E.; et al. Cellular acidification as a new approach to cancer treatment and to the understanding and therapeutics of neurodegenerative diseases. Semin. Cancer Biol. 2017, 43, 157–179. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, V.; Franco, M.C.; Martin, L.J. Human Mutant Dynactin Subunit 1 Causes Profound Motor Neuron Disease Consistent with Possible Mechanisms Involving Axonopathy, Mitochondriopathy, Protein Nitration, and T-Cell-Mediated Cytolysis. Biomolecules 2025, 15, 1637. https://doi.org/10.3390/biom15121637
Xie V, Franco MC, Martin LJ. Human Mutant Dynactin Subunit 1 Causes Profound Motor Neuron Disease Consistent with Possible Mechanisms Involving Axonopathy, Mitochondriopathy, Protein Nitration, and T-Cell-Mediated Cytolysis. Biomolecules. 2025; 15(12):1637. https://doi.org/10.3390/biom15121637
Chicago/Turabian StyleXie, Victor, Maria Clara Franco, and Lee J. Martin. 2025. "Human Mutant Dynactin Subunit 1 Causes Profound Motor Neuron Disease Consistent with Possible Mechanisms Involving Axonopathy, Mitochondriopathy, Protein Nitration, and T-Cell-Mediated Cytolysis" Biomolecules 15, no. 12: 1637. https://doi.org/10.3390/biom15121637
APA StyleXie, V., Franco, M. C., & Martin, L. J. (2025). Human Mutant Dynactin Subunit 1 Causes Profound Motor Neuron Disease Consistent with Possible Mechanisms Involving Axonopathy, Mitochondriopathy, Protein Nitration, and T-Cell-Mediated Cytolysis. Biomolecules, 15(12), 1637. https://doi.org/10.3390/biom15121637







