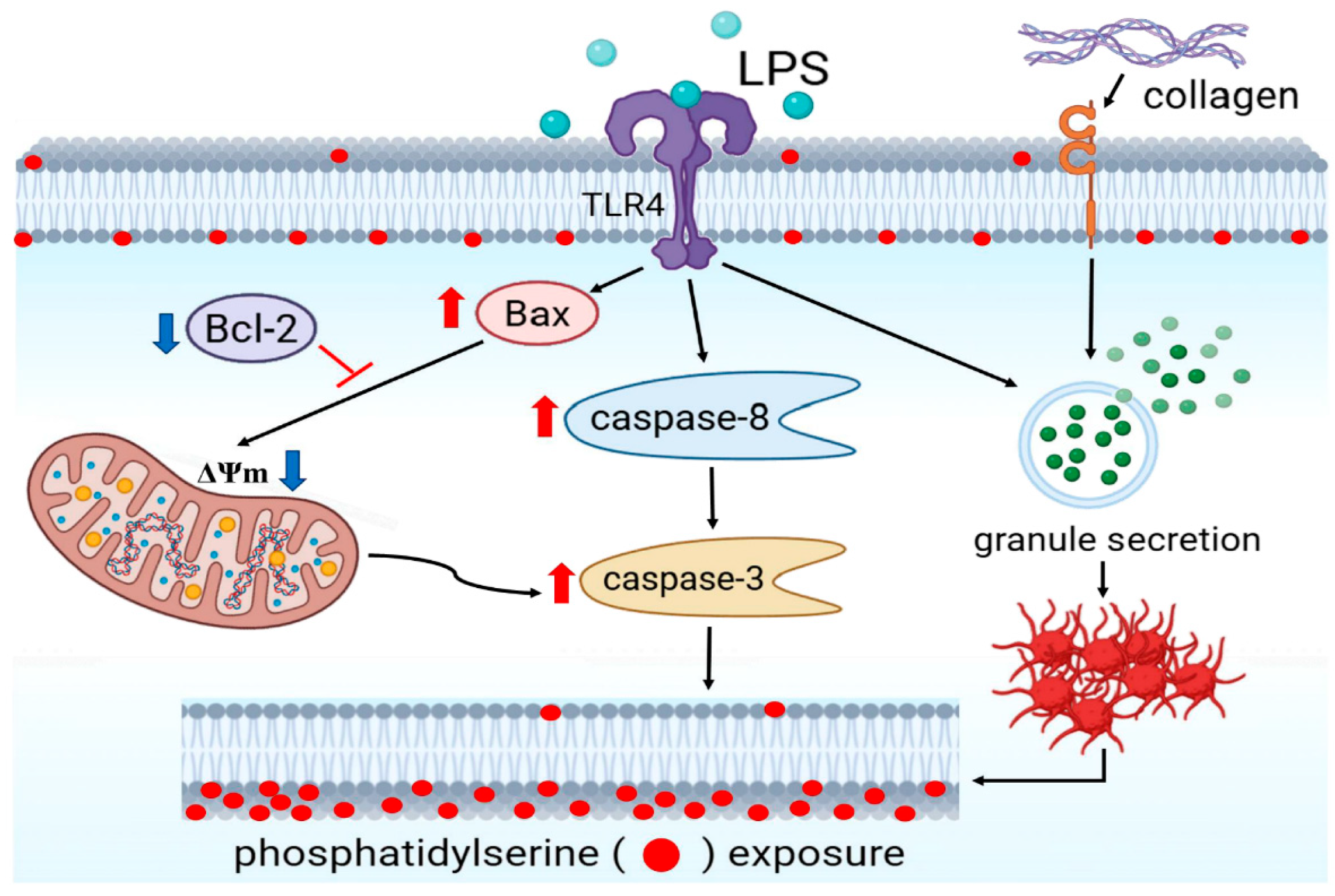
Lipopolysaccharide Potentiates Platelet Aggregation in Association with Apoptosis Through a Novel TLR4–Bax/Bcl-2-Mitochondrial Dysfunction Axis in Humans
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagent
2.2. Human Platelet Preparation and Aggregation
2.3. Detection of ATP Release and Cytosolic Ca2+ Mobilization
2.4. Scanning Electron Microscopic (SEM) Examination of Platelet Uultrastructure
2.5. Measurement of Mitochondrial Membrane Potential (ΔΨm) Dissipation
2.6. Measurement of PS Exposure and P-Selectin Expression in Washed Platelets
2.7. Confocal Microscopic Analysis of Fluorescently Labeled Platelets
2.8. Assessment of Protein Expression via Immunoblotting
2.9. Statistical Analysis
3. Results
3.1. LPS Potentiates Collagen-Induced Platelet Activation and Morphological Changes in Platelets
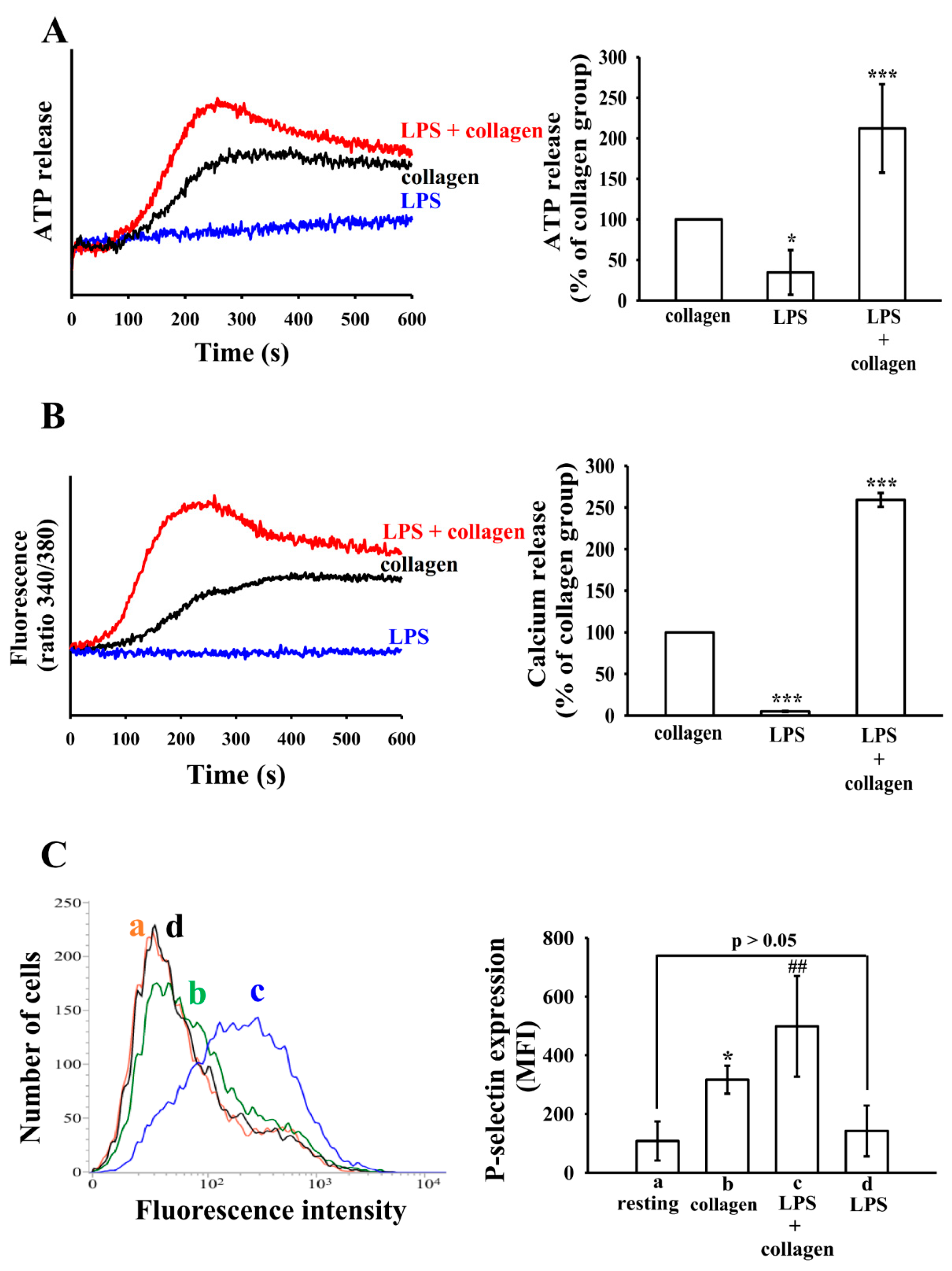
3.2. The Effect of LPS on ATP Release, Intracellular Calcium Levels ([Ca2+]i) and P-Selectin Expression
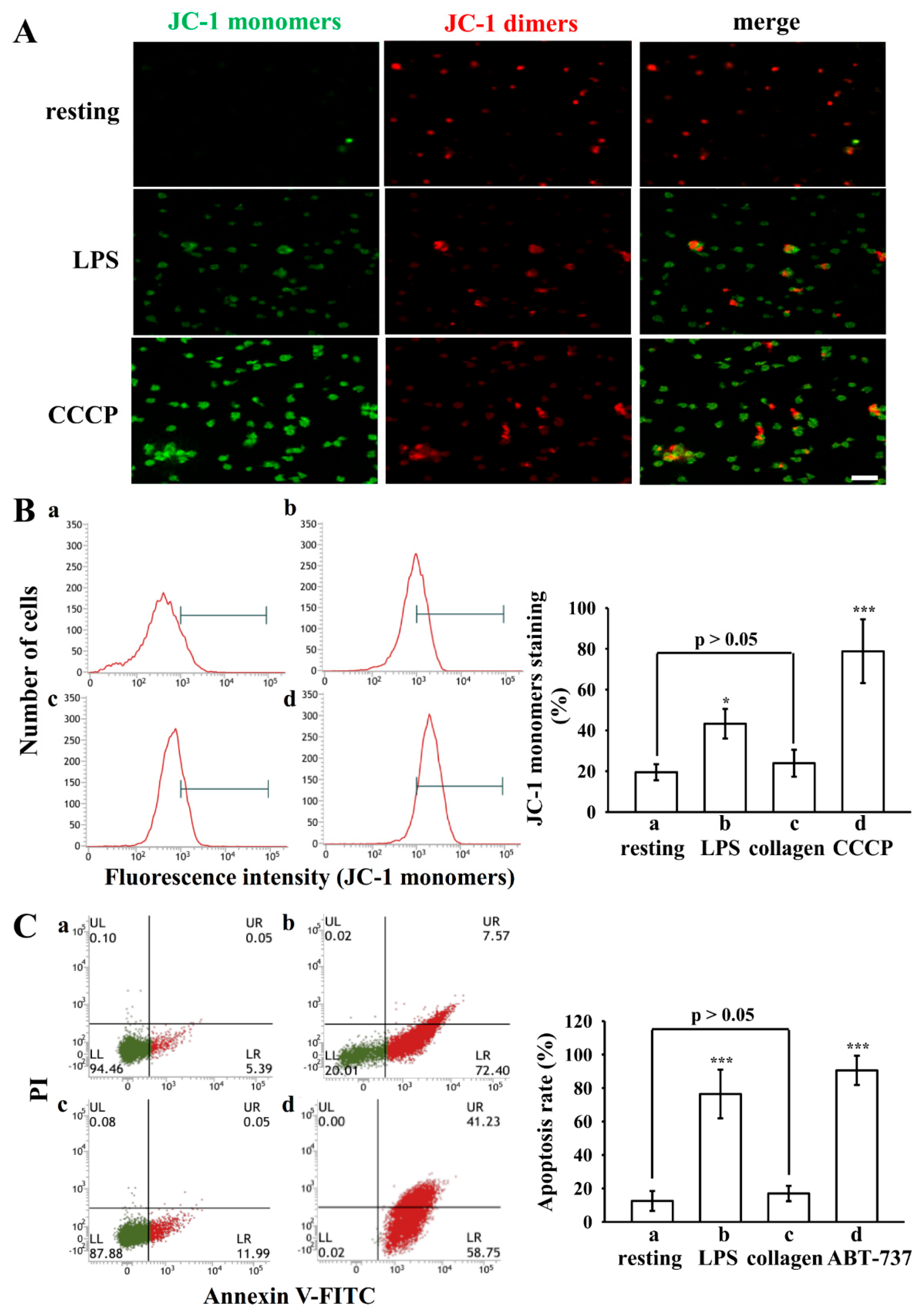
3.3. LPS Induced ΔΨm Change and PS Exposure in Platelets
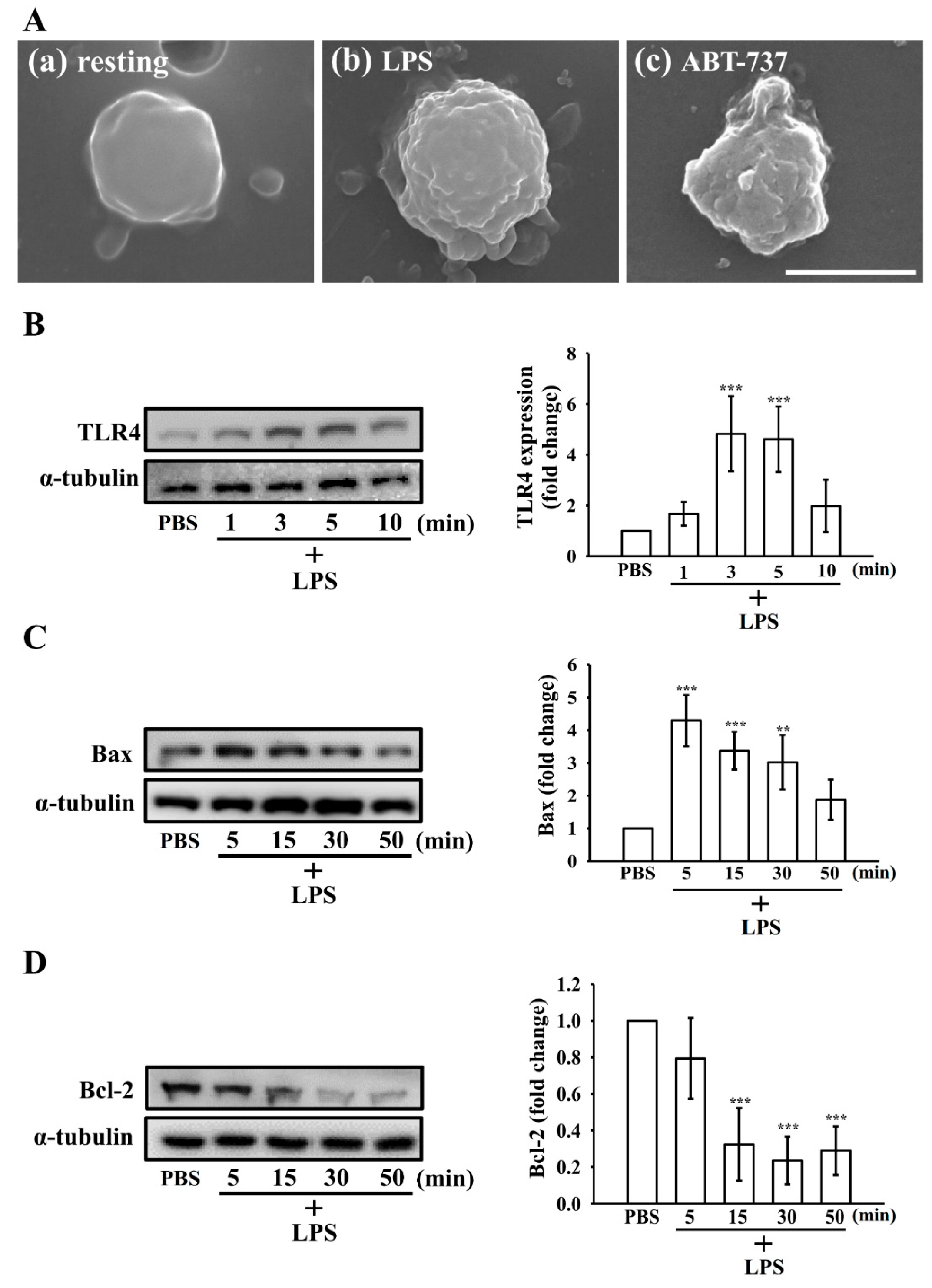
3.4. Morphologic Changes in Apoptotic Platelets by LPS
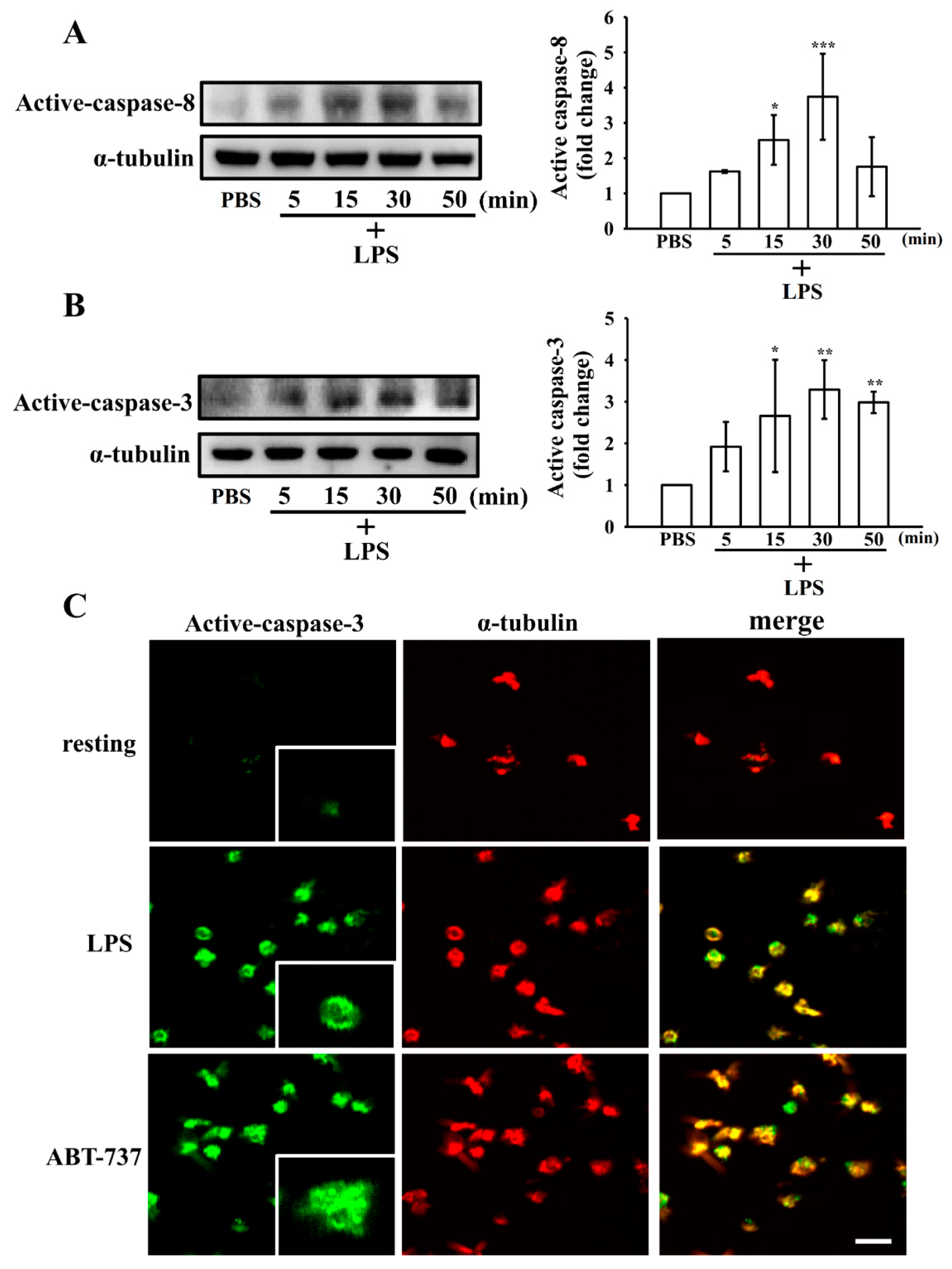
3.5. Effect of LPS on the Extrinsic and Intrinsic Apoptotic Pathway in Washed Human Platelets
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- La Via, L.; Sangiorgio, G.; Stefani, S.; Marino, A.; Nunnari, G.; Cocuzza, S.; La Mantia, I.; Cacopardo, B.; Stracquadanio, S.; Spampinato, S.; et al. The Global Burden of Sepsis and Septic Shock. Epidemiologia 2024, 5, 456–478. [Google Scholar] [CrossRef]
- Unar, A.; Bertolino, L.; Patauner, F.; Gallo, R.; Durante-Mangoni, E. Decoding Sepsis-Induced Disseminated Intravascular Coagulation: A Comprehensive Review of Existing and Emerging Therapies. J. Clin. Med. 2023, 12, 6128. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.; Zheng, X.; Zhao, S.; Liu, H.; Chen, E.; Xie, R.; Li, R.; Chen, Y. Disseminated Intravascular Coagulation: Cause, Molecular Mechanism, Diagnosis, and Therapy. MedComm 2025, 6, e70058. [Google Scholar] [CrossRef] [PubMed]
- Setarehaseman, A.; Mohammadi, A.; Maitta, R.W. Thrombocytopenia in Sepsis. Life 2025, 15, 274. [Google Scholar] [CrossRef] [PubMed]
- Hua, T.; Yao, F.; Wang, H.; Liu, W.; Zhu, X.; Yao, Y. Megakaryocyte in Sepsis: The Trinity of Coagulation, Inflammation and Immunity. Crit. Care 2024, 28, 442. [Google Scholar] [CrossRef]
- Koupenova, M.; Livada, A.C.; Morrell, C.N. Platelet and Megakaryocyte Roles in Innate and Adaptive Immunity. Circ. Res. 2022, 130, 288–308. [Google Scholar] [CrossRef]
- Zheng, S.S.; Perdomo, J.S. Desialylation and Apoptosis in Immune Thrombocytopenia: Implications for Pathogenesis and Treatment. Curr. Issues Mol. Biol. 2024, 46, 11942–11956. [Google Scholar] [CrossRef]
- Josefsson, E.C. Platelet Intrinsic Apoptosis. Thromb. Res. 2023, 231, 206–213. [Google Scholar] [CrossRef]
- Zhang, C.; Shang, X.; Yuan, Y.; Li, Y. Platelet-Related Parameters as Potential Biomarkers for the Prognosis of Sepsis. Exp. Ther. Med. 2023, 25, 133. [Google Scholar] [CrossRef]
- Das, U.N. Infection, Inflammation, and Immunity in Sepsis. Biomolecules 2023, 13, 1332. [Google Scholar] [CrossRef]
- Akele, O.; Rana, F.; Acharya, S.; LeDoux, D.; Chalhoub, M. Targeting Lipopolysaccharides in Gram-Negative Sepsis: Therapeutic Advances and Challenges. J. Drug Target. 2025, 1–13. [Google Scholar] [CrossRef]
- Wang, M.; Feng, J.; Zhou, D.; Wang, J. Bacterial Lipopolysaccharide-Induced Endothelial Activation and Dysfunction: A New Predictive and Therapeutic Paradigm for Sepsis. Eur. J. Med. Res. 2023, 28, 339. [Google Scholar] [CrossRef]
- Sheu, J.R.; Hung, W.C.; Wu, C.H.; Ma, M.C.; Kan, Y.C.; Lin, C.H.; Lin, M.S.; Luk, H.N.; Yen, M.H. Reduction in Lipopolysaccharide-Induced Thrombocytopenia by Triflavin in a Rat Model of Septicemia. Circulation 1999, 99, 3056–3062. [Google Scholar] [CrossRef] [PubMed]
- Galgano, L.; Guidetti, G.F.; Torti, M.; Canobbio, I. The Controversial Role of LPS in Platelet Activation In Vitro. Int. J. Mol. Sci. 2022, 23, 10900. [Google Scholar] [CrossRef]
- Violi, F.; Cammisotto, V.; Bartimoccia, S.; Pignatelli, P.; Carnevale, R.; Nocella, C. Gut-Derived Low-Grade Endotoxaemia, Atherothrombosis and Cardiovascular Disease. Nat. Rev. Cardiol. 2023, 20, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Han, J.; Welch, E.J.; Ye, R.D.; Voyno-Yasenetskaya, T.A.; Malik, A.B.; Du, X.; Li, Z. Lipopolysaccharide Stimulates Platelet Secretion and Potentiates Platelet Aggregation via TLR4/MyD88 and the cGMP-Dependent Protein Kinase Pathway. J. Immunol. 2009, 182, 7997–8004. [Google Scholar] [CrossRef] [PubMed]
- Claushuis, T.A.M.; Van Der Veen, A.I.P.; Horn, J.; Schultz, M.J.; Houtkooper, R.H.; Van ’t Veer, C.; Van Der Poll, T. Platelet Toll-like receptor expression and activation induced by lipopolysaccharide and sepsis. Platelets 2019, 30, 296–304. [Google Scholar] [CrossRef]
- Jayachandran, M.; Brunn, G.J.; Karnicki, K.; Miller, R.S.; Owen, W.G.; Miller, V.M. In vivo effects of lipopolysaccharide and TLR4 on platelet production and activity: Implications for thrombotic risk. J. Appl. Physiol. 2007, 102, 429–433. [Google Scholar] [CrossRef]
- Hsia, C.W.; Huang, W.C.; Jayakumar, T.; Hsia, C.H.; Hou, S.M.; Chang, C.C.; Yen, T.L.; Sheu, J.R. Garcinol Acts as a Novel Integrin αIIbβ3 Inhibitor in Human Platelets. Life Sci. 2023, 326, 121791. [Google Scholar] [CrossRef]
- Lin, K.H.; Hsiao, G.; Shih, C.M.; Chou, D.S.; Sheu, J.R. Mechanisms of Resveratrol-Induced Platelet Apoptosis. Cardiovasc. Res. 2009, 83, 575–585. [Google Scholar] [CrossRef]
- Shcherbina, A.; Remold-O’Donnell, E. Role of Caspase in a Subset of Human Platelet Activation Responses. Blood 1999, 93, 4222–4231. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.H.Y.; Kahr, W.H.A. Molecular Basis of Platelet Granule Defects. J. Thromb. Haemost. 2025, 23, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Escopy, S.; Chaikof, E.L. Targeting the P-Selectin/PSGL-1 Pathway: Discovery of Disease-Modifying Therapeutics for Disorders of Thromboinflammation. Blood Vessel Thromb. Hemost. 2024, 1, 100015. [Google Scholar] [CrossRef]
- Suzuki, T.; Kobayashi, M.; Isatsu, K.; Nishihara, T.; Aiuchi, T.; Nakaya, K.; Hasegawa, K. Mechanisms involved in apoptosis of human macrophages induced by lipopolysaccharide from Actinobacillus actinomycetemcomitans in the presence of cycloheximide. Infect. Immun. 2004, 72, 1856–1865. [Google Scholar] [CrossRef]
- Remenyi, G.; Szasz, R.; Friese, P.; Dale, G.L. Role of mitochondrial permeability transition pore in coated-platelet formation. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 467–471. [Google Scholar] [CrossRef]
- Assinger, A.; Schrottmaier, W.C.; Salzmann, M.; Rayes, J. Platelets in Sepsis: An Update on Experimental Models and Clinical Data. Front. Immunol. 2019, 10, 1687. [Google Scholar] [CrossRef]
- Rivadeneyra, L.; Carestia, A.; Etulain, J.; Pozner, R.G.; Fondevila, C.; Negrotto, S.; Schattner, M. Regulation of Platelet Responses Triggered by Toll-Like Receptor 2 and 4 Ligands Is Another Non-Genomic Role of Nuclear Factor-KappaB. Thromb. Res. 2014, 133, 235–243. [Google Scholar] [CrossRef]
- Lopes Pires, M.E.; Clarke, S.R.; Marcondes, S.; Gibbins, J.M. Lipopolysaccharide Potentiates Platelet Responses via Toll-Like Receptor 4-Stimulated Akt-Erk-PLA2 Signalling. PLoS ONE 2017, 12, e0186981. [Google Scholar] [CrossRef]
- Zhang, H.; Nimmer, P.M.; Tahir, S.K.; Chen, J.; Fryer, R.M.; Hahn, K.R.; Iciek, L.A.; Morgan, S.J.; Nasarre, M.C.; Nelson, R.; et al. Bcl-2 family proteins are essential for platelet survival. Cell Death Differ. 2007, 14, 943–951. [Google Scholar] [CrossRef]
- Mason, K.D.; Carpinelli, M.R.; Fletcher, J.I.; Collinge, J.E.; Hilton, A.A.; Ellis, S.; Kelly, P.N.; Ekert, P.G.; Metcalf, D.; Roberts, A.W.; et al. Programmed anuclear cell death delimits platelet life span. Cell 2007, 128, 1173–1186. [Google Scholar] [CrossRef] [PubMed]
- Leytin, V.; Gyulkhandanyan, A.V.; Freedman, J. Platelet Apoptosis Can Be Triggered Bypassing the Death Receptors. Clin. Appl. Thromb. Hemost. 2019, 25, 1076029619853641. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Yang, X.; Li, Y.; Wang, X.; Tan, S.; Chen, F. LPS Enhances Platelet Aggregation via TLR4, Which Is Related to Mitochondria Damage Caused by Intracellular ROS, but Not Extracellular ROS. Cell. Immunol. 2018, 328, 86–92. [Google Scholar] [CrossRef] [PubMed]
- McArthur, K.; Chappaz, S.; Kile, B.T. Apoptosis in Megakaryocytes and Platelets: The Life and Death of a Lineage. Blood 2018, 131, 605–610. [Google Scholar] [CrossRef]
- Schoenwaelder, S.M.; Jarman, K.E.; Gardiner, E.E.; Hua, M.; Qiao, J.; White, M.J.; Josefsson, E.C.; Alwis, I.; Ono, A.; Willcox, A.; et al. Bcl-xL-Inhibitory BH3 Mimetics Can Induce a Transient Thrombocytopathy That Undermines the Hemostatic Function of Platelets. Blood 2011, 118, 1663–1674. [Google Scholar] [CrossRef]
- Leytin, V. Apoptosis in the Anucleate Platelet. Blood Rev. 2012, 26, 51–63. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-C.; Hsia, C.-W.; Huang, W.-C.; Chang, C.-C.; Darmanto, A.G.; Sheu, J.-R. Lipopolysaccharide Potentiates Platelet Aggregation in Association with Apoptosis Through a Novel TLR4–Bax/Bcl-2-Mitochondrial Dysfunction Axis in Humans. Biomolecules 2025, 15, 1638. https://doi.org/10.3390/biom15121638
Chen C-C, Hsia C-W, Huang W-C, Chang C-C, Darmanto AG, Sheu J-R. Lipopolysaccharide Potentiates Platelet Aggregation in Association with Apoptosis Through a Novel TLR4–Bax/Bcl-2-Mitochondrial Dysfunction Axis in Humans. Biomolecules. 2025; 15(12):1638. https://doi.org/10.3390/biom15121638
Chicago/Turabian StyleChen, Chun-Chao, Chih-Wei Hsia, Wei-Chieh Huang, Chao-Chien Chang, Arief Gunawan Darmanto, and Joen-Rong Sheu. 2025. "Lipopolysaccharide Potentiates Platelet Aggregation in Association with Apoptosis Through a Novel TLR4–Bax/Bcl-2-Mitochondrial Dysfunction Axis in Humans" Biomolecules 15, no. 12: 1638. https://doi.org/10.3390/biom15121638
APA StyleChen, C.-C., Hsia, C.-W., Huang, W.-C., Chang, C.-C., Darmanto, A. G., & Sheu, J.-R. (2025). Lipopolysaccharide Potentiates Platelet Aggregation in Association with Apoptosis Through a Novel TLR4–Bax/Bcl-2-Mitochondrial Dysfunction Axis in Humans. Biomolecules, 15(12), 1638. https://doi.org/10.3390/biom15121638







