Comparative Ultrasonic Bath and Probe Extraction of Piperine from Piper nigrum L. Using Natural Deep Eutectic Solvents: RSM Optimization, Characterization, and In Vitro Bioactivity
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
2.3. Preparation of NADES
2.4. Preliminary Extraction of Piperine by UBE and UPE
2.5. RSM Optimization of Piperine Extraction Using UBE and UPE
2.5.1. One-Factor Preliminary Experiments (OFAT)
2.5.2. RSM Experiments of UBE- and UPE-Piperine
2.6. Isolation and Purification of Piperine
2.7. Characterization of Purified Piperine
2.7.1. Confirm Chemical Identity and Purity
2.7.2. Melting Point Determination
2.7.3. Verify Characteristic Absorbance and Purity
2.7.4. Identify Functional Groups
2.7.5. Quantify Piperine Concentration
2.7.6. Compare Chemical Profiles and Semi-Quantitative Content
2.7.7. Determine Crystalline Structure
2.7.8. Confirm Molecular Vibrations and Structure
2.7.9. Observation of Surface Morphology and Particle Size
2.7.10. Elemental Composition Analysis
2.7.11. Analysis of Proton Chemical Structure
2.7.12. Analysis of Carbon Chemical Structure
2.8. In Vitro Studies
2.8.1. Cytotoxicity of UBE- and UPE-Derived Piperine in C2C12 Cells
2.8.2. Anticancer Activity Assessment
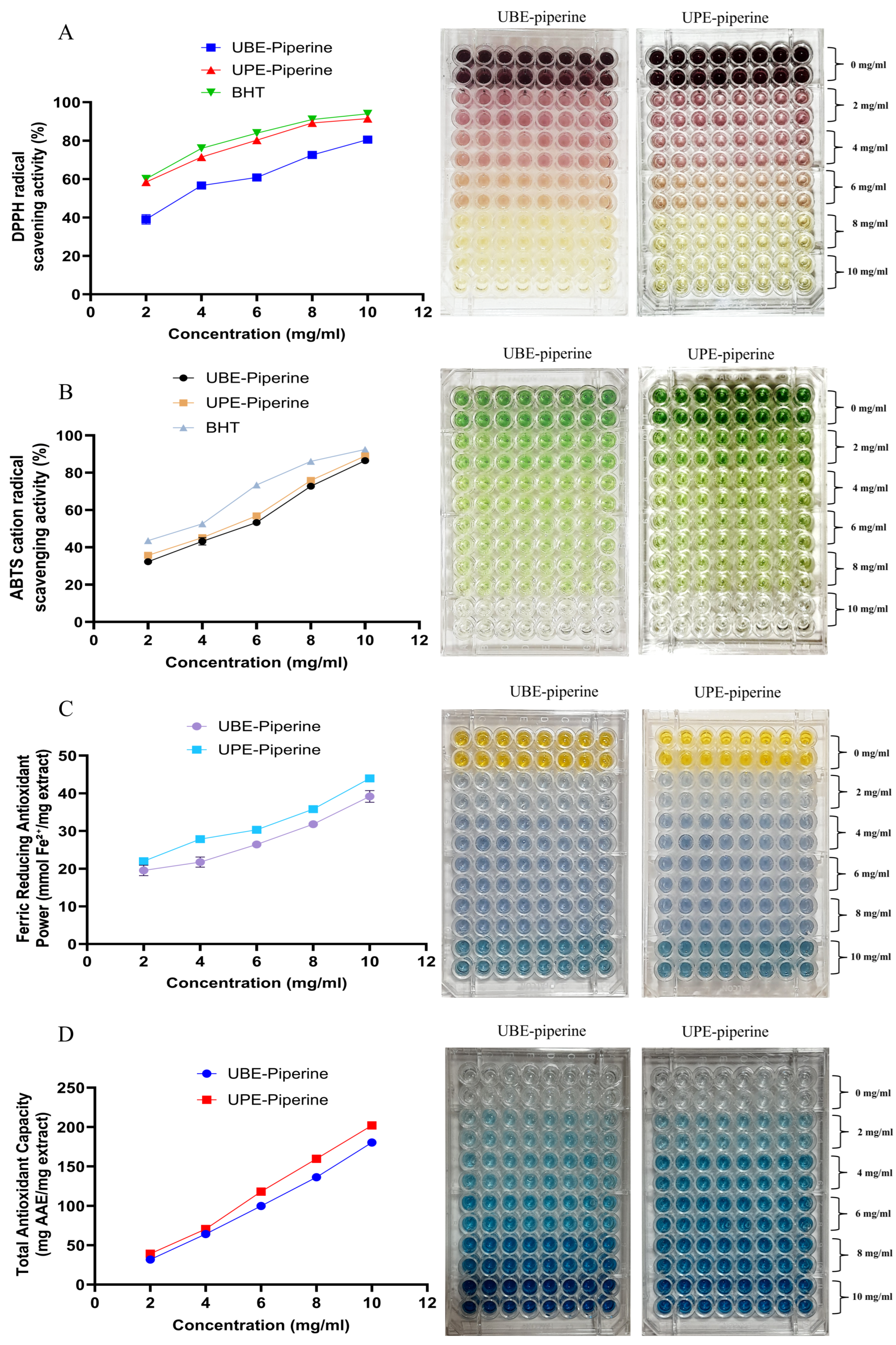
2.8.3. Antioxidant Activity
In Vitro Antioxidant Assays
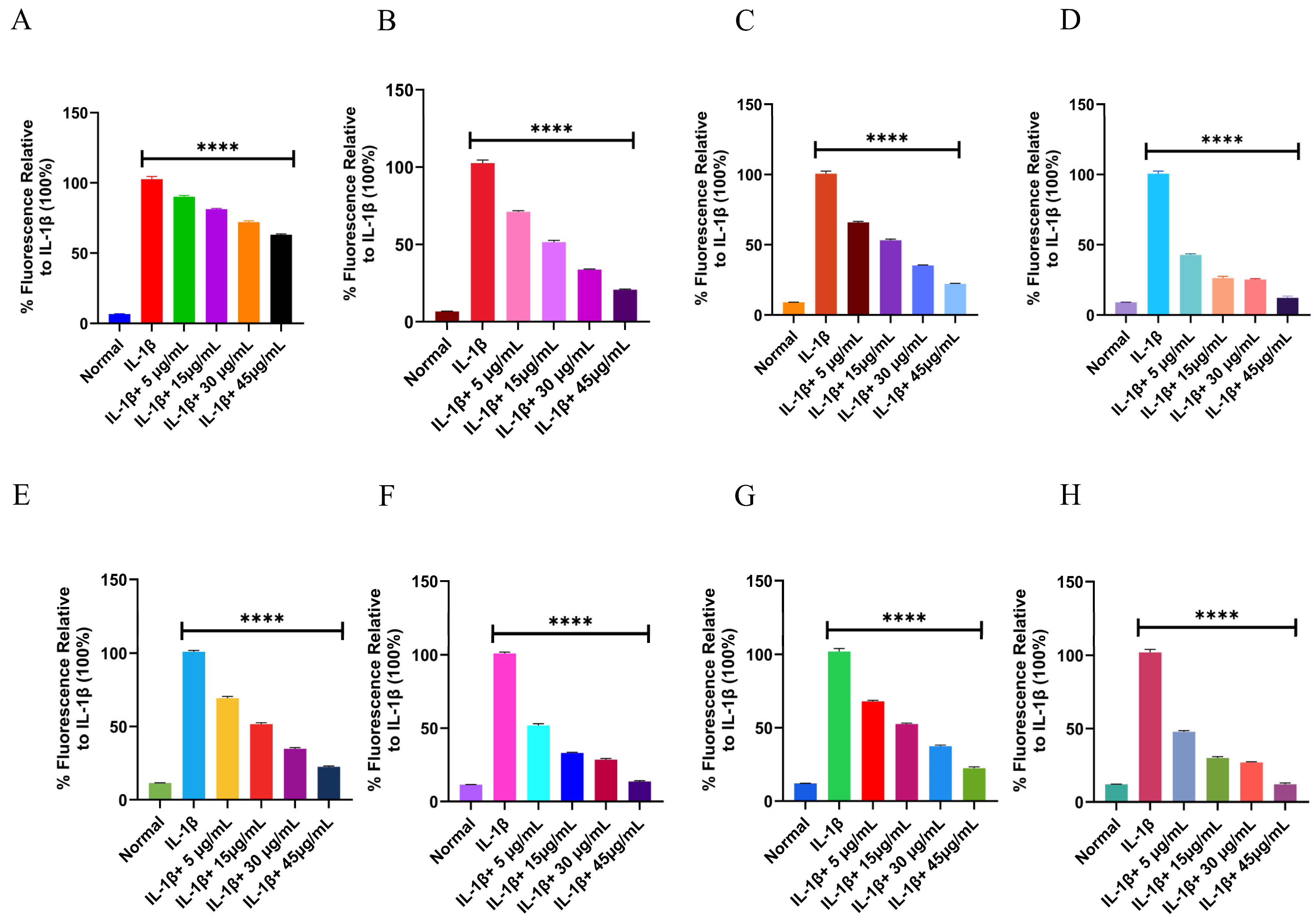
Intracellular ROS and RNS Evaluation in THP-1 and RAW 264.7 Cells
2.9. Statistical Analysis
3. Results and Discussion
3.1. Efficacy of Piperine Extraction Using NADES
3.1.1. Piperine Yield from UBE
3.1.2. Piperine Yield from UPE
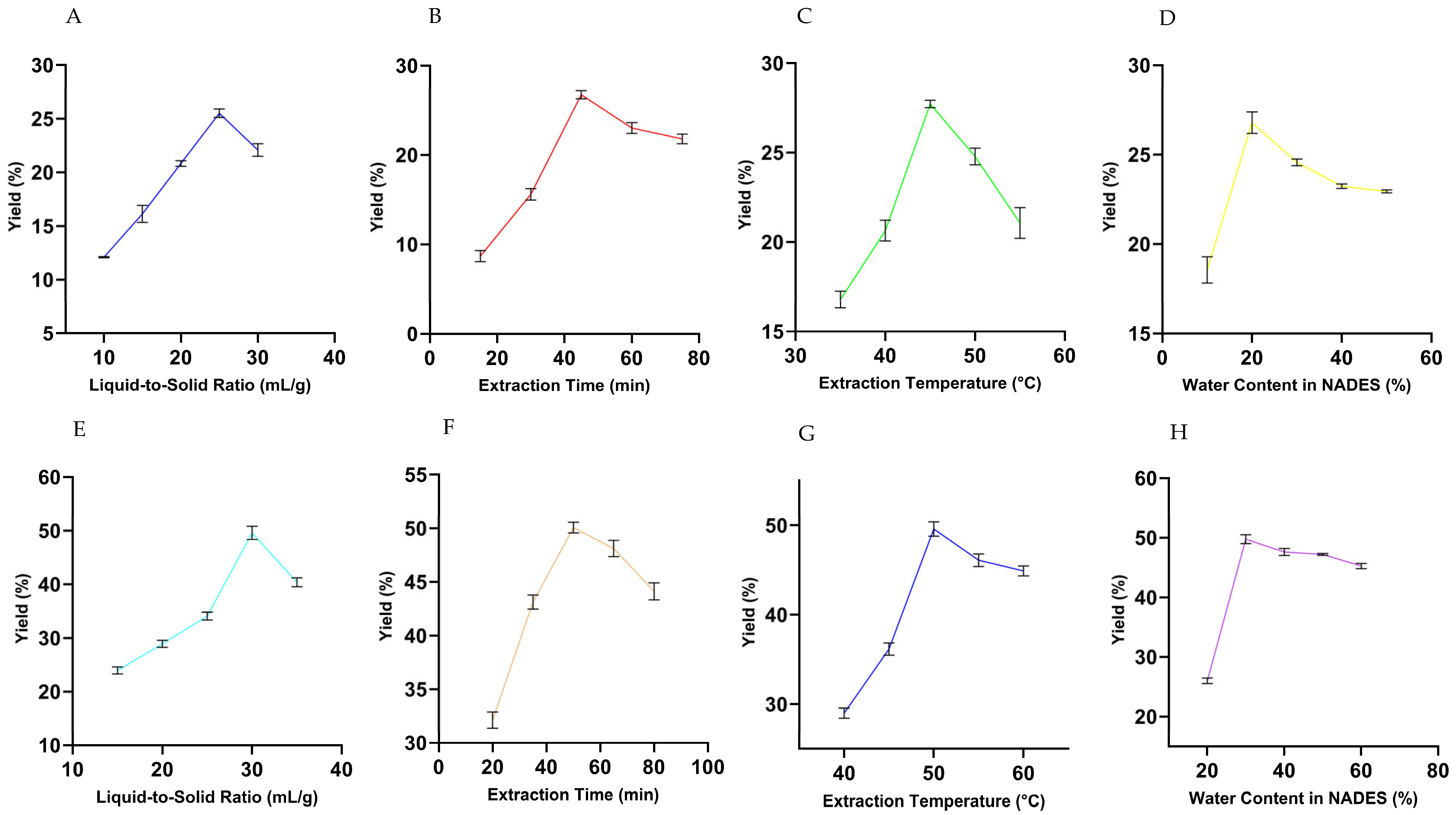
3.2. One-Factor Preliminary Experiments of UBE and UPE
3.2.1. Effect of Liquid-to-Solid Ratio
3.2.2. Effect of Extraction Time
3.2.3. Effect of Extraction Temperature
3.2.4. Effect of Water Content in NADES
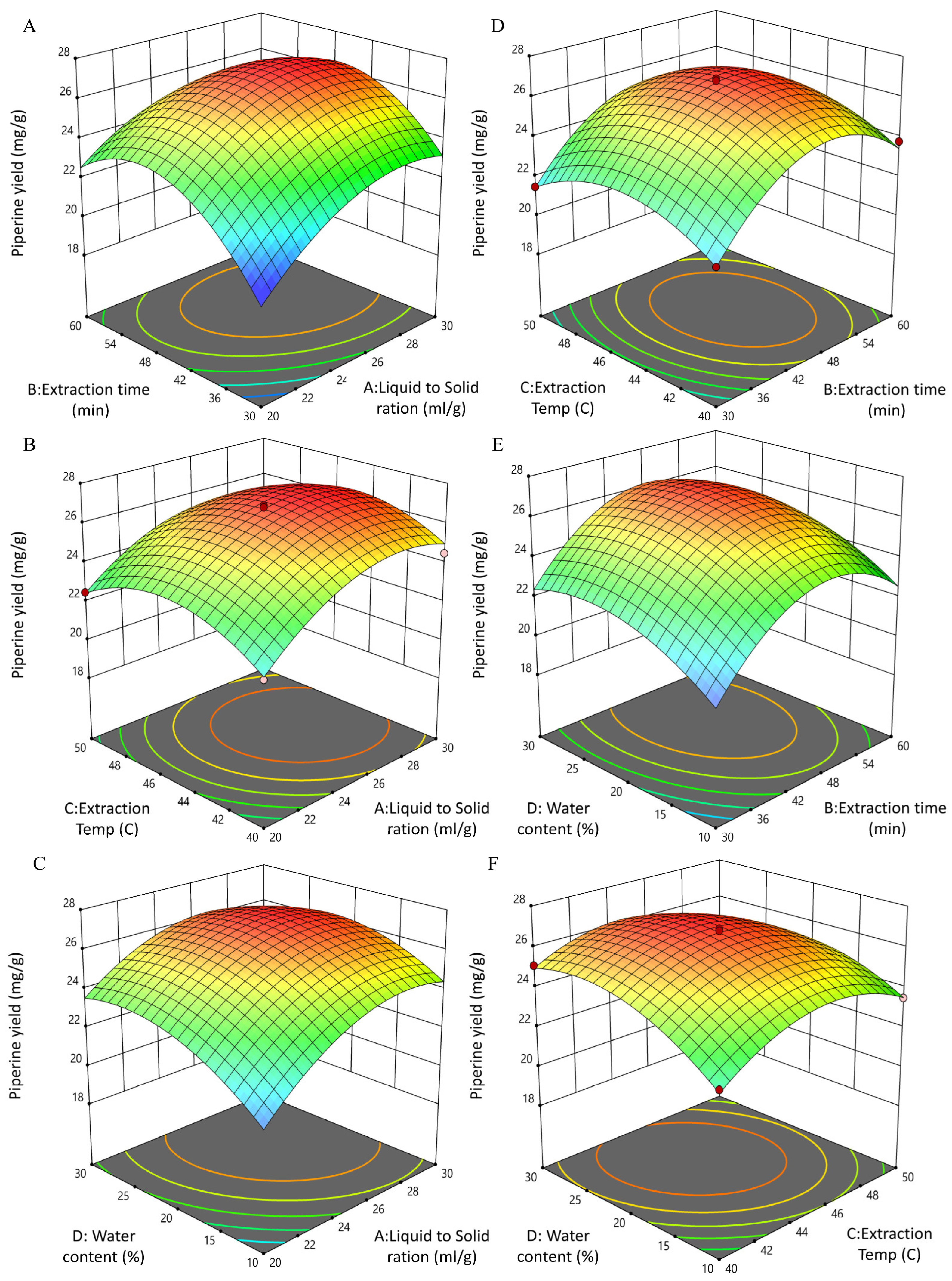
3.3. BBD and RSM Optimization of UBE-Piperine
3.3.1. Fitting of the UBE-Piperine Regression Equation and Analysis of Variance
−0.063067A2 − 0.011841B2 − 0.064567C2 − 0.013267D2
−0.006333AB − 0.008000AC − 0.009000AD
−0.000333BC − 0.000333BD − 0.009500CD
3.3.2. Interactive Effects of UBE Process Variables on Piperine Yield
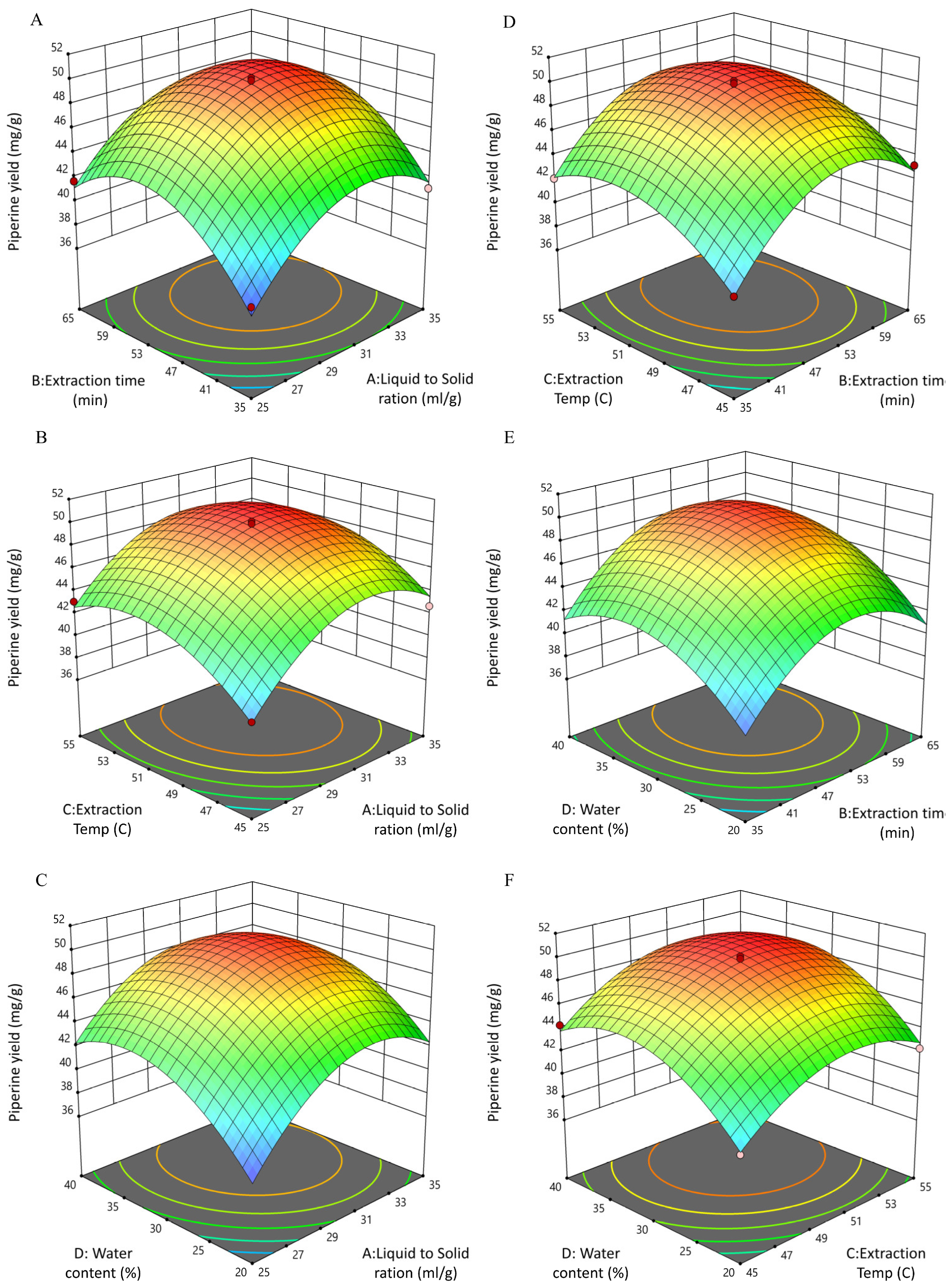
3.4. BBD and RSM Optimization of UPE-Piperine
3.4.1. Fitting of the UPE-Piperine Regression Equation and Analysis of Variance
−0.072133A2 − 0.012267B2 − 0.072067C2 − 0.014533D2
−0.007667AB − 0.008667AC − 0.010333AD
−0.000667BC − 0.000333BD − 0.010500CD
3.4.2. Interactive Effects of UPE Process Variables on Piperine Yield
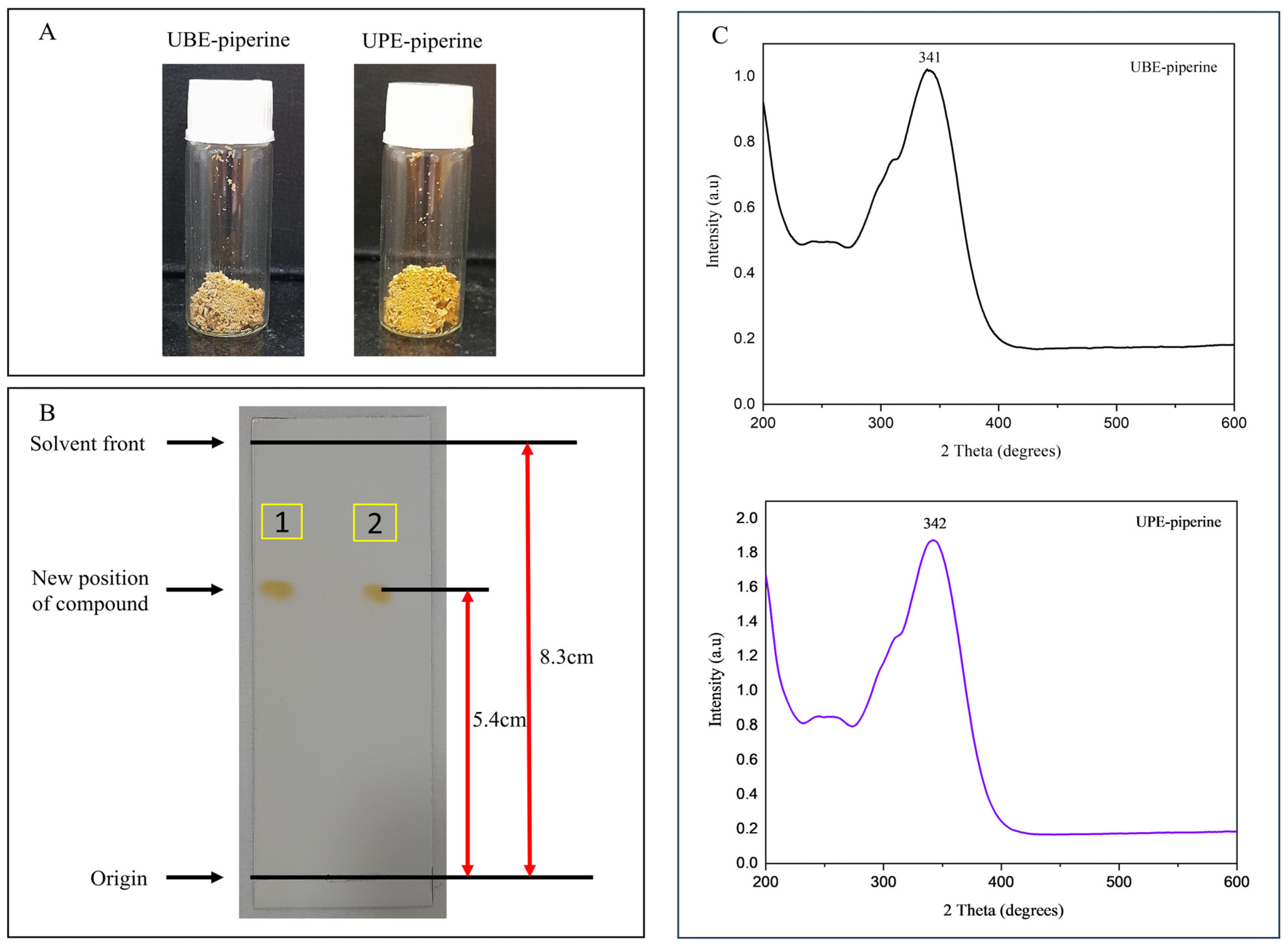
3.5. Isolation and Purification of Piperine Obtained from UBE and UPE
3.6. Characterization of Purified UBE- and UPE-Derived Piperine
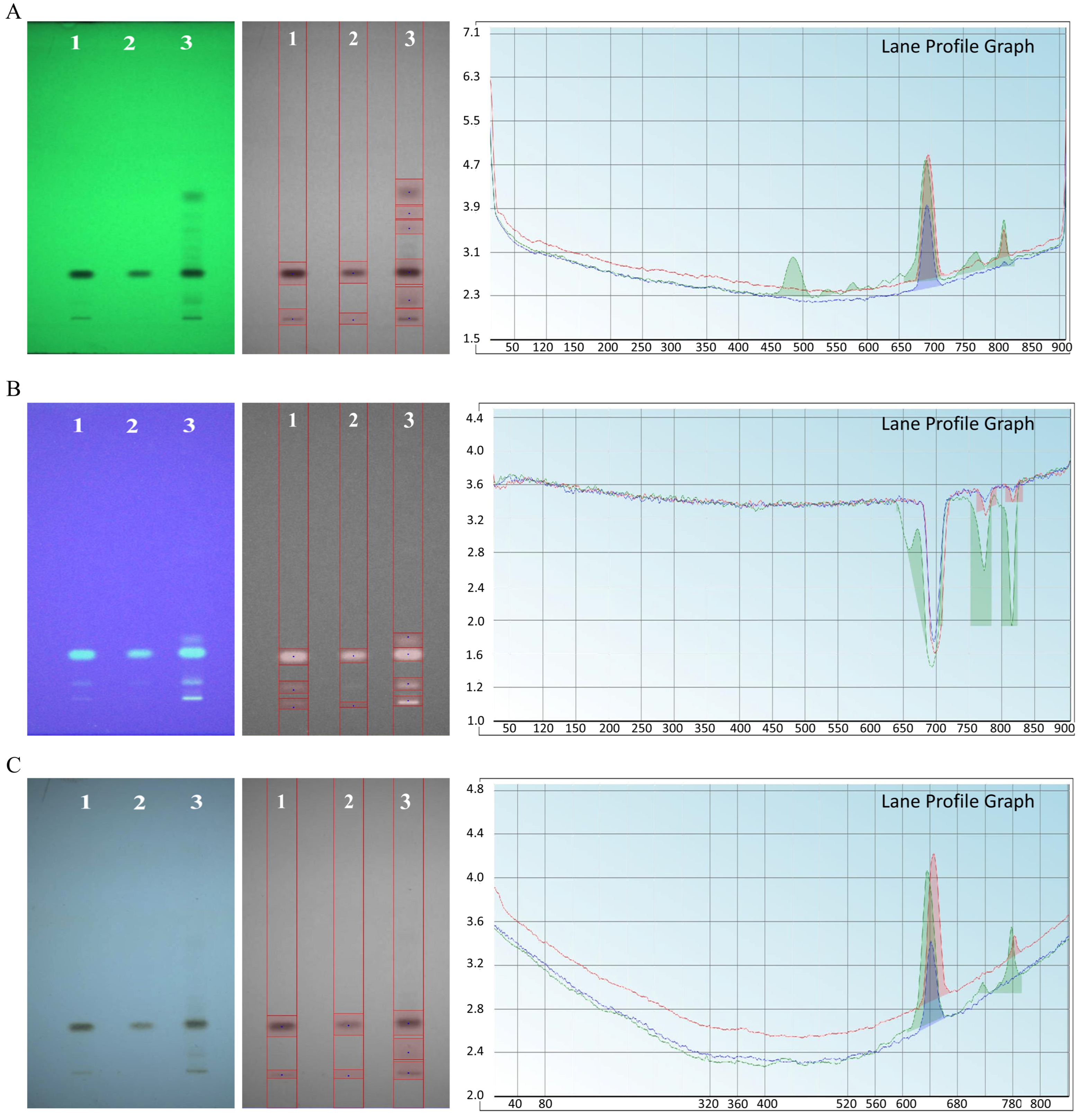
3.6.1. TLC Analysis
3.6.2. Melting Point Determination
3.6.3. UV-Vis Spectral Analysis
3.6.4. FTIR Spectral Analysis
3.6.5. HPLC Chromatographic Analysis
3.6.6. HPTLC Fingerprinting Analysis
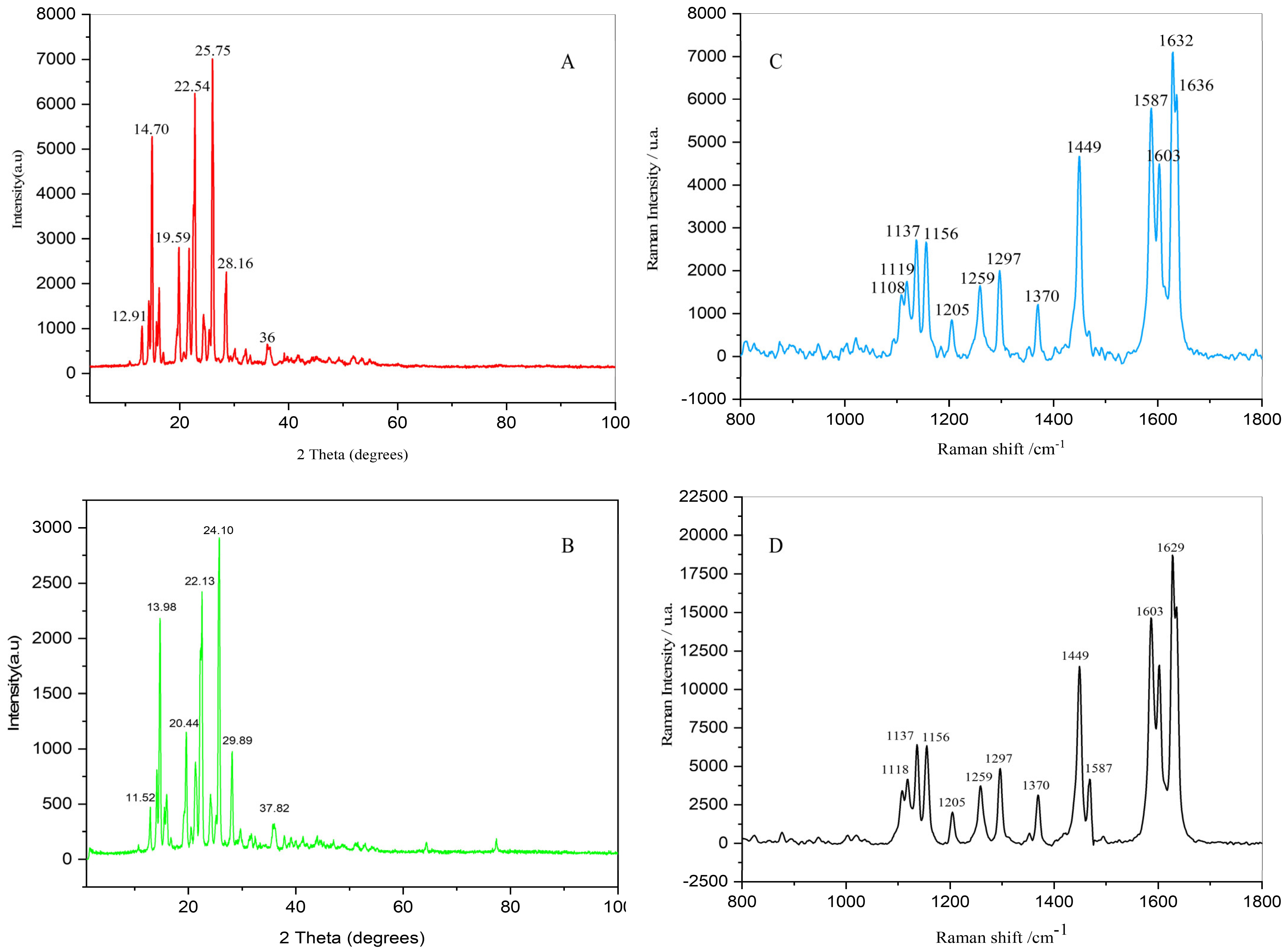
3.6.7. XRD Analysis
3.6.8. Raman Spectroscopy Analysis
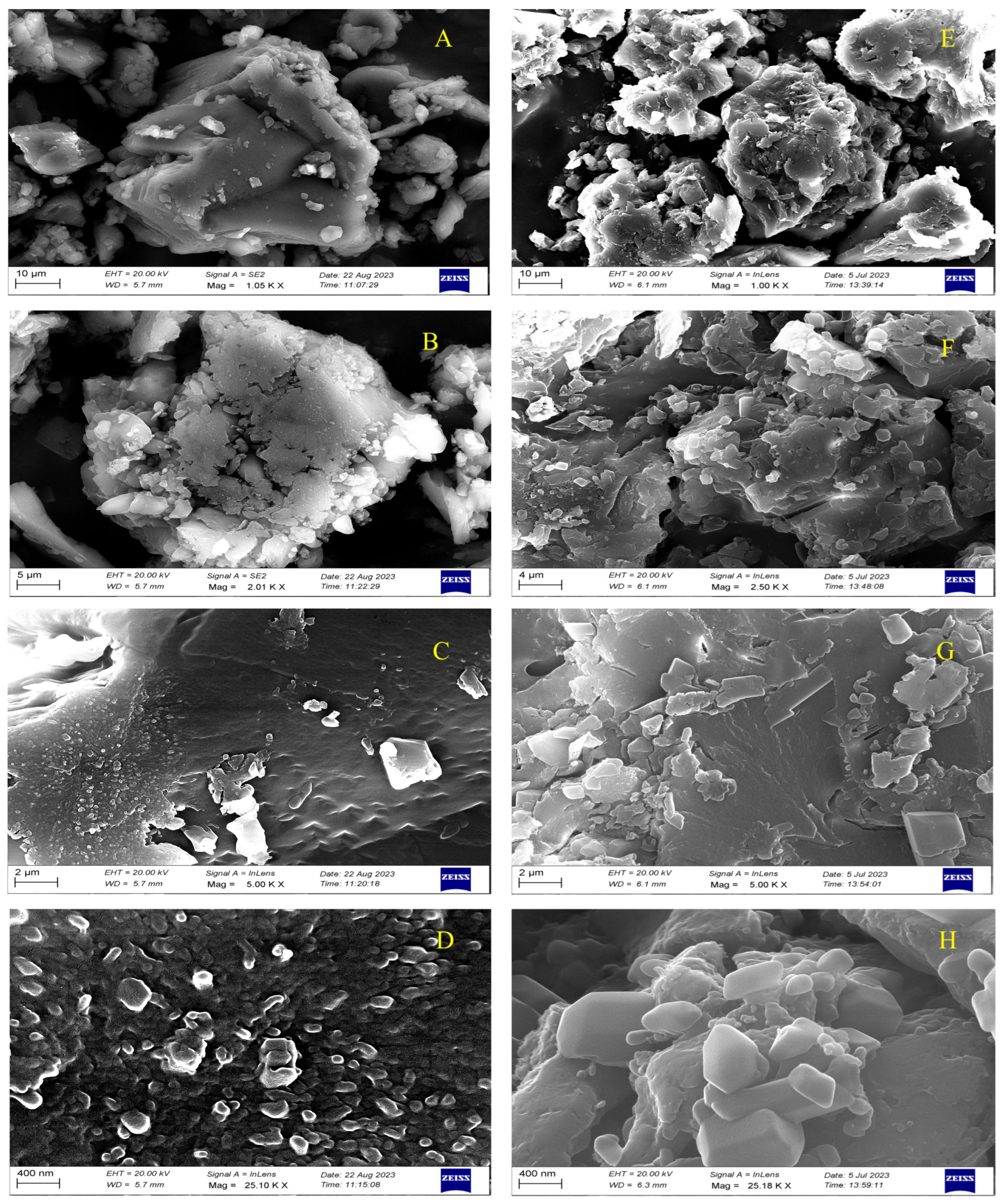
3.6.9. SEM Morphological Analysis
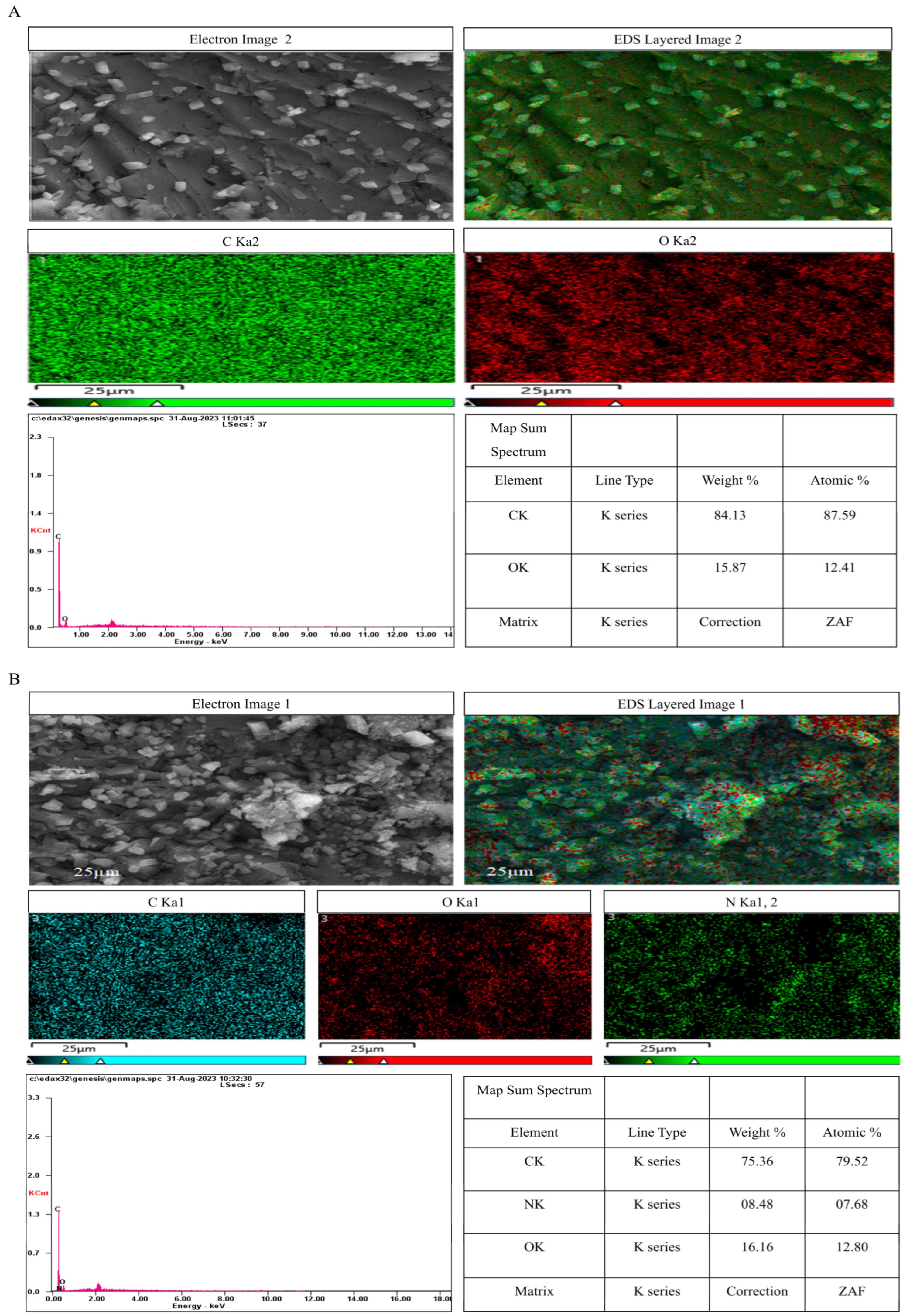
3.6.10. EDS Elemental Analysis
3.6.11. NMR Spectroscopy
3.7. In Vitro Evaluation
3.7.1. Cytotoxicity of UBE- and UPE-Derived Piperine
3.7.2. Anticancer Activity Assessment
3.7.3. Antioxidant Assays
3.7.4. Intracellular ROS and RNS Evaluation in THP-1 and RAW 264.7 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hashimoto, K.; Yaoi, T.; Koshiba, H.; Yoshida, T.; Maoka, T.; Fujiwara, Y.; Yamamoto, Y.; Mori, K. Photochemical Isomerization of Piperine, a Pungent Constituent in Pepper. Food Sci. Technol. Int. Tokyo 1996, 2, 24–29. [Google Scholar] [CrossRef]
- Rahman Khan, Z.; Moni, F.; Sharmin, S.; Al-Mansur, M.A.; Gafur, A.; Rahman, O.; Afroz, F. Isolation of Bulk Amount of Piperine as Active Pharmaceutical Ingredient (API) from Black Pepper and White Pepper (Piper nigrum L.). Pharmacol. Pharm. 2017, 8, 253–262. [Google Scholar] [CrossRef]
- Shingate, P.N.; Dongre, P.P.; Kannur, D.M. New method development for extraction and isolation of piperine from black pepper. Int. J. Pharm. Sci. Res. 2013, 4, 3165. [Google Scholar]
- Tiwari, A.; Mahadik, K.R.; Gabhe, S.Y. Piperine: A comprehensive review of methods of isolation, purification, and biological properties. Med. Drug Discov. 2020, 7, 100027. [Google Scholar] [CrossRef]
- Raman, A.; Lin, Z. Use of Piperine for Treating Skin Pigmentation Disorders. EP1094813A2, 2 May 2001. [Google Scholar]
- Zhang, C.; Tian, Q.; Li, Y. Design, synthesis, and insecticidal activity evaluation of piperine derivatives. Front. Chem. 2022, 10, 973630. [Google Scholar] [CrossRef] [PubMed]
- Vasavirama, K.; Upender, M. Piperine: A valuable alkaloid from piper species. Int. J. Pharm. Pharm. Sci. 2014, 6, 34–38. [Google Scholar]
- Alshehri, S.; Imam, S.S.; Hussain, A.; Altamimi, M.A. Formulation of piperine ternary inclusion complex using β CD and HPMC: Physicochemical characterization, molecular docking, and antimicrobial testing. Processes 2020, 8, 1450. [Google Scholar] [CrossRef]
- Elnaggar, Y.S.R.; Etman, S.M.; Abdelmonsif, D.A.; Abdallah, O.Y. Intranasal Piperine-Loaded Chitosan Nanoparticles as Brain-Targeted Therapy in Alzheimer’s Disease: Optimization, Biological Efficacy, and Potential Toxicity. J. Pharm. Sci. 2015, 104, 3544–3556. [Google Scholar] [CrossRef]
- Arora, S.; Singh, B.; Kumar, S.; Kumar, A.; Singh, A.; Singh, C. Piperine loaded drug delivery systems for improved biomedical applications: Current status and future directions. Health Sci. Rev. 2023, 9, 100138. [Google Scholar] [CrossRef]
- Yu, J.W.; Yuan, H.W.; Bao, L.D.; Si, L.G. Interaction between piperine and genes associated with sciatica and its mechanism based on molecular docking technology and network pharmacology. Mol. Divers. 2021, 25, 233–248. [Google Scholar] [CrossRef]
- Mitra, S.; Anand, U.; Jha, N.K.; Shekhawat, M.S.; Saha, S.C.; Nongdam, P.; Rengasamy, K.R.R.; Proćków, J.; Dey, A. Anticancer Applications and Pharmacological Properties of Piperidine and Piperine: A Comprehensive Review on Molecular Mechanisms and Therapeutic Perspectives. Front. Pharmacol. 2022, 12, 772418. [Google Scholar] [CrossRef]
- Itharat, A.; Kanokkangsadal, P.; Khemawoot, P.; Wanichsetakul, P.; Davies, N. Pharmacokinetics of piperine after oral administration of Sahastara remedy capsules in healthy volunteers. Res. Pharm. Sci. 2020, 15, 410–417. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Ray, A.K.; Mishra, S.K. Molecular and pharmacological aspects of piperine as a potential molecule for disease prevention and management: Evidence from clinical trials. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Poojar, B.; Ommurugan, B.; Adiga, S.; Thomas, H.; Sori, R.K.; Poojar, B.; Hodlur, N.; Tilak, A.; Korde, R.; Gandigawad, P.; et al. Methodology Used in the Study. Asian J. Pharm. Clin. Res. 2017, 7, 1–5. [Google Scholar]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Pandit Patil, S.; Dasharath Lavate, K.; Bendgude, R.R.; MBhosale, M.; Ganpati, S. Review: Conventional and Modern Extraction Methods of Herbal Drugs. Int. J. Pharm. Res. Appl. 2023, 8, 2445. [Google Scholar]
- Sun, S.; Yu, Y.; Jo, Y.; Han, J.H.; Xue, Y.; Cho, M.; Bae, S.-J.; Ryu, D.; Park, W.; Ha, K.-T.; et al. Impact of extraction techniques on phytochemical composition and bioactivity of natural product mixtures. Front. Pharmacol. 2025, 16, 1615338. [Google Scholar] [CrossRef]
- Bitwell, C.; Indra, S.S.; Luke, C.; Kakoma, M.K. A review of modern and conventional extraction techniques and their applications for extracting phytochemicals from plants. Sci. Afr. 2023, 19, e01585. [Google Scholar] [CrossRef]
- Cao, S.; Liang, J.; Chen, M.; Xu, C.; Wang, X.; Qiu, L.; Zhao, X.; Hu, W. Comparative analysis of extraction technologies for plant extracts and absolutes. Front. Chem. 2025, 13, 1536590. [Google Scholar] [CrossRef]
- Garcia-Larez, F.L.; Esquer, J.; Guzmán, H.; Zepeda-Quintana, D.S.; Moreno-Vásquez, M.J.; Rodríguez-Félix, F.; Del-Toro-Sánchez, C.L.; López-Corona, B.E.; Tapia-Hernández, J.A. Effect of Ultrasound-Assisted Extraction (UAE) parameters on the recovery of polyphenols from pecan nutshell waste biomass and its antioxidant activity. Biomass Convers. Biorefinery 2025, 15, 10977–10995. [Google Scholar] [CrossRef]
- Shen, L.; Pang, S.; Zhong, M.; Sun, Y.; Qayum, A.; Liu, Y.; Rashid, A.; Xu, B.; Liang, Q.; Ma, H.; et al. A comprehensive review of ultrasonic assisted extraction (UAE) for bioactive components: Principles, advantages, equipment, and combined technologies. Ultrason. Sonochem. 2023, 101, 106646. [Google Scholar] [CrossRef]
- Song, Z.; Huang, G.; Huang, H. The ultrasonic-assisted enzymatic extraction, characteristics and antioxidant activities of lychee nuclear polysaccharide. Ultrason. Sonochem. 2024, 110, 107038. [Google Scholar] [CrossRef]
- Zorrilla, J.G.; Rial, C.; Martínez-González, M.I.; Molinillo, J.M.G.; Macías, F.A.; Varela, R.M. Ginger Phytotoxicity: Potential Efficacy of Extracts, Metabolites and Derivatives for Weed Control. Agronomy 2024, 14, 2353. [Google Scholar] [CrossRef]
- Shaterabadi, D.; Aboonajmi, M.; Ghorbani Javid, M.; Arabhosseini, A. Effect of power ultrasound on the extraction of black caraway (Carum carvi L.) and evaluation of their qualitative properties using response surface methodology. Food Sci. Nutr. 2020, 8, 4361–4369. [Google Scholar] [CrossRef]
- Sganzerla, M.; Coutinho, J.P.; de Melo, A.M.T.; Godoy, H.T. Fast method for capsaicinoids analysis from Capsicum chinense fruits. Food Res. Int. 2014, 64, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Freitas, D.S.; Ribeiro, A.; Cavaco-paulo, A. The Versatility of NADES Across Applications. Molecules 2025, 30, 3862. [Google Scholar] [CrossRef] [PubMed]
- Kocanci, F.G.; Dolanbay, S.N.; Aslim, B. Comparison of three different protocols of alkaloid extraction from Glaucium corniculatum plant. Int. J. Second. Metab. 2022, 9, 43–51. [Google Scholar] [CrossRef]
- Masala, V.; Jokić, S.; Aladić, K.; Molnar, M.; Tuberoso, C.I.G. Exploring Phenolic Compounds Extraction from Saffron Water Extraction. Molecules 2024, 29, 2600. [Google Scholar] [CrossRef]
- Lwamba, C.; Aboushanab, S.A.; Ambati, R.R.; Kovaleva, E.G. Innovative Green Approach for Extraction of Piperine from Black Pepper Based on Response Surface Methodology. Sustain. Chem. 2023, 4, 40–53. [Google Scholar] [CrossRef]
- Stasiłowicz-Krzemień, A.; Wójcik, J.; Gościniak, A.; Szymański, M.; Szulc, P.; Górecki, K.; Cielecka-Piontek, J. Natural Deep Eutectic Solvents Combined with Supercritical Carbon Dioxide for the Extraction of Curcuminoids from Turmeric. Pharmaceuticals 2024, 17, 1596. [Google Scholar] [CrossRef]
- Chevé-Kools, E.; Choi, Y.H.; Roullier, C.; Ruprich-Robert, G.; Grougnet, R.; Chapeland-Leclerc, F.; Hollmann, F. Natural deep eutectic solvents (NaDES): Green solvents for pharmaceutical applications and beyond. Green Chem. 2025, 27, 8360–8385. [Google Scholar] [CrossRef]
- Jiménez-Ortega, L.A.; Bastidas-Bastidas, P.d.J.; Angulo-Escalante, M.A.; Mota-Morales, J.D.; Heredia, J.B. Optimized Extraction of Glycosylated Flavonoids from Pepper (Capsicum annuum L.) Agricultural Biomass Residues through Ultrasonic Pulse-Assisted Natural Deep Eutectic Solvents. ACS Sustain. Resour. Manag. 2024, 1, 165–177. [Google Scholar] [CrossRef]
- Wu, K.; Ren, J.; Wang, Q.; Nuerjiang, M.; Xia, X.; Bian, C. Research Progress on the Preparation and Action Mechanism of Natural Deep Eutectic Solvents and Their Application in Food. Foods 2022, 11, 3528. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.; Sarraguça, M. A Comprehensive Review on Deep Eutectic Solvents and Its Use to Extract Bioactive Compounds of Pharmaceutical Interest. Pharmaceuticals 2024, 17, 124. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Hernández, G.; Zepeda-Vallejo, L.G.; García-Magaña, M.D.L.; Vivar-Vera, M.D.L.Á.; Pérez-Larios, A.; Girón-Pérez, M.I.; Coria-Tellez, A.V.; Rodríguez-Aguayo, C.; Montalvo-González, E. Extraction of alkaloids using ultrasound from pulp and by-products of soursop fruit (Annona muricata L.). Appl. Sci. 2020, 10, 4869. [Google Scholar] [CrossRef]
- Kazmi, I.; Al-Abbasi, F.A.; Imam, S.S.; Afzal, M.; Nadeem, M.S.; Altayb, H.N.; Alshehri, S. Formulation of Piperine Nanoparticles: In Vitro Breast Cancer Cell Line and In Vivo Evaluation. Polymers 2022, 14, 1349. [Google Scholar] [CrossRef]
- Latiff, N.A.; Ong, P.Y.; Abd Rashid, S.N.A.; Abdullah, L.C.; Mohd Amin, N.A.; Fauzi, N.A.M. Enhancing recovery of bioactive compounds from Cosmos caudatus leaves via ultrasonic extraction. Sci. Rep. 2021, 11, 17297. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, Y.; Huang, G.; Huang, H. Ultrasound-assisted extraction, analysis and antioxidant activity of polysaccharide from the rinds of Garcinia mangostana L. Ultrason. Sonochem. 2023, 97, 106474. [Google Scholar] [CrossRef]
- Grozdanova, T.; Trusheva, B.; Alipieva, K.; Popova, M.; Dimitrova, L.; Najdenski, H.; Zaharieva, M.M.; Ilieva, Y.; Vasileva, B.; Miloshev, G.; et al. Extracts of medicinal plants with natural deep eutectic solvents: Enhanced antimicrobial activity and low genotoxicity. BMC Chem. 2020, 14, 73. [Google Scholar] [CrossRef]
- Jeliński, T.; Przybyłek, M.; Cysewski, P. Natural Deep Eutectic Solvents as Agents for Improving Solubility, Stability and Delivery of Curcumin. Pharm. Res. 2019, 36, 116. [Google Scholar] [CrossRef]
- Deng, L.; Huang, G. Ultrasound-assisted extraction, optimization, characteristics and antioxidant activity of Piper nigrum L. polysaccharides. Ultrason. Sonochem. 2025, 116, 107309. [Google Scholar] [CrossRef] [PubMed]
- Paarakh, P.M.; Sreeram, D.C.; Shruthi, S.D.; Ganapathy, S.P. In vitro cytotoxic and in silico activity of piperine isolated from Piper nigrum fruits Linn. Silico Pharmacol. 2015, 3, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.; Chapman, V. Genetic control of the cytologically diploid behaviour of hexaploid wheat. Nature 1958, 182, 713–715. [Google Scholar] [CrossRef]
- Stämpfli, R.; Brühwiler, P.; Mourad, S.; Verdejo, R.; Shaffer, M. Development and characterisation of carbon nanotube-reinforced polyurethane foams. EMPA Act. 2007, 26, 51. [Google Scholar]
- Salari, S.; Bahabadi, S.E.; Samzadeh-Kermani, A.; Yosefzaei, F. In-vitro evaluation of antioxidant and antibacterial potential of green synthesized silver nanoparticles using prosopis farcta fruit extract. Iran. J. Pharm. Res. 2019, 18, 430–445. [Google Scholar]
- Das, P.E.; Abu-Yousef, I.A.; Majdalawieh, A.F.; Narasimhan, S.; Poltronieri, P. Green Synthesis of Encapsulated Copper Nanoparticles Using a Hydroalcoholic Extract of Moringa oleifera Leaves and Assessment of Their Antioxidant and Antimicrobial Activities. Molecules 2020, 25, 555. [Google Scholar] [CrossRef]
- Zhong, G.; Yang, X.; Jiang, X.; Kumar, A.; Long, H.; Xie, J.; Zheng, L.; Zhao, J. Dopamine-melanin nanoparticles scavenge reactive oxygen and nitrogen species and activate autophagy for osteoarthritis therapy. Nanoscale 2019, 11, 11605–11616. [Google Scholar] [CrossRef]
- Bencresciuto, G.F.; Carnevale, M.; Paris, E.; Gallucci, F.; Santangelo, E.; Migliori, C.A. A Sustainable Alternative for Cosmetic Applications: NADES Extraction of Bioactive Compounds from Hazelnut By-Products. Sustainability 2025, 17, 1516. [Google Scholar] [CrossRef]
- García-Roldán, A.; Piriou, L.; Jauregi, P. Natural deep eutectic solvents as a green extraction of polyphenols from spent coffee ground with enhanced bioactivities. Front. Plant Sci. 2023, 13, 1072592. [Google Scholar] [CrossRef]
- Spaggiari, C.; Carbonell-Rozas, L.; Zuilhof, H.; Costantino, G.; Righetti, L. Structural elucidation and long-term stability of synthesized NADES: A detailed physicochemical analysis. J. Mol. Liq. 2025, 424, 127105. [Google Scholar] [CrossRef]
- Maimulyanti, A.; Prihadi, A.R.; Mellisani, B.; Nurhidayati, I.; Putri, F.A.R.; Puspita, F.; Widarsih, R.W. Green Extraction Technique To Separate Tannin From Coffee Husk Waste Using Natural Deep Eutectic Solvent (Nades). Rasayan J. Chem. 2023, 16, 2002–2008. [Google Scholar] [CrossRef]
- Putu, N.; Hikmawanti, E.; Ramadon, D.; Jantan, I. Natural Deep Eutectic Solvents (NADES): Phytochemical Extraction Performance Enhancer for Pharmaceutical and Nutraceutical Product Development. Plants 2021, 10, 2091. [Google Scholar] [CrossRef]
- Ramos, I.N.d.F.; da Silva, M.F.; Lopes, J.M.S.; Cruz, J.N.; Alves, F.S.; do Rego, J.d.A.R.; Costa, M.L.d.; Assumpção, P.P.d.; Barros Brasil, D.d.S.; Khayat, A.S. Extraction, Characterization, and Evaluation of the Cytotoxic Activity of Piperine in Its Isolated form and in Combination with Chemotherapeutics against Gastric Cancer. Molecules 2023, 28, 5587. [Google Scholar] [CrossRef]
- Chumanee, S.; Thunta, J.; Khoomsab, R.; Winyakul, C. Optimizing ultrasound-assisted extraction for enhanced quantification of 7-hydroxymitragynine and mitragynine in kratom. Creat. Sci. 2024, 17, 258707. [Google Scholar] [CrossRef]
- Yohannes, A.; Zhang, B.; Dong, B.; Yao, S. Ultrasonic extraction of tropane alkaloids from radix physochlainae using as extractant an ionic liquid with similar structure. Molecules 2019, 24, 2897. [Google Scholar] [CrossRef]
- Jauregi, P.; Esnal-Yeregi, L.; Labidi, J. Natural deep eutectic solvents (NADES) for the extraction of bioactives: Emerging opportunities in biorefinery applications. PeerJ Anal. Chem. 2024, 6, e32. [Google Scholar] [CrossRef]
- Nguyen, K.V.; Le, N.T.; Dang, V.T.T.; Koshovyi, O.; Raal, A.; Nguyen, H.T. Alkaloid Extraction from Coptis chinensis Franch. Using Ultrasound-Assisted Aqueous Solutions of Surfactants, Organic Acids, Deep Eutectic Solvents, and Supramolecular Deep Eutectic Solvents. Molecules 2025, 30, 1418. [Google Scholar] [CrossRef]
- Le, X.T.; Nguyen, N.T.; Nguyen-Thi, B.T.; Dang, M.K.; Ngo-Thi, T.N.; Vu, D.P.; Duong-Nguyen, H.N.; Pham-Vu, M.C. Optimization of Piperine Extraction Process from Vietnamese White Pepper. IOP Conf. Ser. Earth Environ. Sci. 2024, 1340, 012020. [Google Scholar] [CrossRef]
- Singh Kumar, N.; Kumar, P.K.; Gupta Kumar, D.; Singh, S.; Kumar Singh, V. Scholars Research Library UV-spectrophotometric method development for estimation of piperine in Chitrakadi Vati. Sch. Res. Libr. 2011, 3, 178–182. [Google Scholar]
- Al-Mamun, M.R.; Maniruzzaman, M.; Rahman Badal, M.M.; Haque, M.A. Comparison of piperine content, antimicrobial and antioxidant activity of Piper chaba root and stem. Heliyon 2024, 10, e38709. [Google Scholar] [CrossRef]
- Han Jeong, Y.; Van Kien, N.; Jin Han Seog, D.; Ryoo, J.J. Comparison between the use of polyether ether ketone and stainless steel columns for ultrasonic-assisted extraction under various ultrasonic conditions. Ultrason. Sonochem. 2022, 90, 106125. [Google Scholar] [CrossRef] [PubMed]
- Rathod, S.S.; Rathod, V.K. Extraction of piperine from Piper longum using ultrasound. Ind. Crops Prod. 2014, 58, 259–264. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef] [PubMed]
- Alves, F.S.; Rodrigues Do Rego, J.d.A.; Da Costa, M.L.; Lobato Da Silva, L.F.; Da Costa, R.A.; Cruz, J.N.; Brasil, D.D.S.B. Spectroscopic methods and in silico analyses using density functional theory to characterize and identify piperine alkaloid crystals isolated from pepper (Piper nigrum L.). J. Biomol. Struct. Dyn. 2020, 38, 2792–2799. [Google Scholar] [CrossRef]
- Imam, S.S.; Alzahrani, T.A.; Hussain, A.; Altamimi, M.A. Formulation and Evaluation of Supramolecular Food-Grade Piperine HP β CD and TPGS Complex: Dissolution, Physicochemical Characterization, Molecular Docking, In Vitro Antioxidant Activity, and Antimicrobial Assessment. Molecules 2020, 25, 4716. [Google Scholar] [CrossRef]
- Zhao, S.; Kwok, K.C.; Liang, H. Investigation on ultrasound assisted extraction of saikosaponins from Radix Bupleuri. Sep. Purif. Technol. 2007, 55, 307–312. [Google Scholar] [CrossRef]
- Bucur, M.P.; Radulescu, M.C.; Radu, G.L.; Bucur, B. Cavitation-Effect-Based Treatments and Extractions for Superior Fruit and Milk Valorisation. Molecules 2023, 28, 4677. [Google Scholar] [CrossRef]
- Tan, S.X.; Andriyana, A.; Lim, S.; Ong, H.C.; Pang, Y.L.; Ngoh, G.C. Rapid Ultrasound-Assisted Starch Extraction from Sago Pith Waste (SPW) for the Fabrication of Sustainable Bioplastic Film. Polymers 2021, 13, 4398. [Google Scholar] [CrossRef]
- Mohanraj, V.; Aravindan, B.; Jayaprakash, C.; Thenmozhi, M. In Silico Drug Design and Extraction of Piperine an inhibitor for fernesyltransferase in Cryptococcus neoformans. World J. Pharm. Res. 2014, 3, 1107–1120. [Google Scholar]
- Haiss, M.A.; Maraie, N.K. Utilization of ultrasonication technique for the preparation of apigenin nanocrystals. Int. J. Drug Deliv. Technol. 2021, 11, 964–973. [Google Scholar] [CrossRef]
- Naik, A.S.; Suryawanshi, D.; Kumar, M.; Waghmare, R. Ultrasonic treatment: A cohort review on bioactive compounds, allergens and physico-chemical properties of food. Curr. Res. Food Sci. 2021, 4, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, C.; Tiwari, B.K. Ultrasound: A Clean, Green Extraction Technology for Bioactives and Contaminants; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; Volume 76. [Google Scholar]
- Mehta, N.; S, J.; Kumar, P.; Verma, A.K.; Umaraw, P.; Khatkar, S.K.; Khatkar, A.B.; Pathak, D.; Kaka, U.; Sazili, A.Q. Extracción asistida por ultrasonidos y encapsulación de componentes bioactivos para aplicaciones alimentarias. Foods 2022, 11, 2973. [Google Scholar] [CrossRef] [PubMed Central]
- Giri, S.; Dash, K.K.; Bhagya Raj, G.V.S.; Kovács, B.; Ayaz Mukarram, S. Ultrasound assisted phytochemical extraction of persimmon fruit peel: Integrating ANN modeling and genetic algorithm optimization. Ultrason. Sonochem. 2024, 102, 106759. [Google Scholar] [CrossRef]
- Cho, S.; Jung, Y.; Rho, S.J.; Kim, Y.R. Stability, bioavailability, and cellular antioxidant activity of piperine complexed with cyclic glucans. Food Sci. Biotechnol. 2025, 34, 2475–2488. [Google Scholar] [CrossRef]
- Chang, W.L.; Peng, J.Y.; Hong, C.L.; Li, P.C.; Chye, S.M.; Lu, F.J.; Lin, H.Y.; Chen, C.H. Piperine Induces Apoptosis and Cell Cycle Arrest via Multiple Oxidative Stress Mechanisms and Regulation of PI3K/Akt and MAPK Signaling in Colorectal Cancer Cells. Antioxidants 2025, 14, 892. [Google Scholar] [CrossRef]
- Uniyal, P.; Akhtar, A.; Rawat, R. Flavonoid-Based Combination Therapies and Nano-Formulations: An Emerging Frontier in Breast Cancer Treatment. Pharmaceuticals 2025, 18, 1486. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Zhang, H.; Dzah, C.S.; Zandile, M.; Duan, Y.; Ma, H.; Luo, X. Advances in ultrasound assisted extraction of bioactive compounds from cash crops—A review. Ultrason. Sonochem. 2018, 48, 538–549. [Google Scholar] [CrossRef]
- Bin Mokaizh, A.A.; Nour, A.H.; Kerboua, K. Ultrasonic-assisted extraction to enhance the recovery of bioactive phenolic compounds from Commiphora gileadensis leaves. Ultrason. Sonochem. 2024, 105, 106852. [Google Scholar] [CrossRef]

 , UPE-piperine (
, UPE-piperine ( ), and PNL extract (
), and PNL extract ( ).
).
 , UPE-piperine (
, UPE-piperine ( ), and PNL extract (
), and PNL extract ( ).
).

| NADES Code | HBA | HBD | Molar Ratio | Type |
|---|---|---|---|---|
| NADES-1 | L-Proline | Glycerin + Malic Acid | 1:2:2 | Ternary |
| NADES-2 | Choline Chloride | Urea | 1:1 | Binary |
| NADES-3 | Choline Chloride | 1,2-Propylene Glycol | 1:1 | Binary |
| NADES-4 | Choline Chloride | Malic Acid | 1:1 | Binary |
| NADES-5 | Choline Chloride | Glycerin + Urea | 1:1:1 | Ternary |
| NADES-6 | Choline Chloride | Citric Acid + 1,2-Propylene Glycol | 1:2:2 | Ternary |
| Independent Variable | Levels (UBE) | Levels (UPE) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Liquid-to-Solid Ratio (mL/g) | 10 | 15 | 20 | 25 | 30 | 15 | 20 | 25 | 30 | 35 |
| Extraction Time (min) | 15 | 30 | 45 | 60 | 75 | 20 | 35 | 50 | 65 | 80 |
| Extraction Temperature (°C) | 35 | 40 | 45 | 50 | 55 | 40 | 45 | 50 | 55 | 60 |
| Water Content in NADES (%) | 10 | 20 | 30 | 40 | 50 | 20 | 30 | 40 | 50 | 60 |
| Levels | Liquid-to-Solid Ratio (mL/g) | Extraction Time (min) | Extraction Temperature (°C) | Water Content in NADES (%) |
|---|---|---|---|---|
| −1 | 20 | 30 | 40 | 10 |
| 0 | 25 | 45 | 45 | 20 |
| 1 | 30 | 60 | 50 | 30 |
| Levels | Liquid-to-Solid Ratio (mL/g) | Extraction Time (min) | Extraction Temperature (°C) | Water Content in NADES (%) |
|---|---|---|---|---|
| −1 | 25 | 35 | 45 | 20 |
| 0 | 30 | 50 | 50 | 30 |
| 1 | 35 | 65 | 55 | 40 |
| Test | Chemicals and Experimental Setup | Reaction Time/Duration | Detection Wavelength (nm) | Control Standard | Data Analysis |
|---|---|---|---|---|---|
| DPPH | 0.1 mM DPPH | 35 min, dark | 517 nm | BHT | % Radical Scavenging (Equation (4)) [44]. |
| ABTS | 7 mM ABTS + 2.45 mM K2S2O8 | 20 min, dark | 734 nm | BHT | % Radical Scavenging (Equation (4)) [45]. |
| FRAP | FRAP solution | 15 min at 37 °C | 593 nm | FeSO4 | mmol Fe(II)/mg extract [46]. |
| TAC | Phosphomolybdate reagent | 95 min at 95 °C | 695 nm | Ascorbic acid | ppm ascorbic acid equivalents [47]. |
| Serial Number | A: Liquid-to-Solid Ratio (mL/g) | B: Extraction Time (min) | C: Extraction Temperature (°C) | D:Water Content in NADES (%) | Piperine Yield % (Predicted) |
|---|---|---|---|---|---|
| 1 | 30 | 30 | 45 | 20 | 23.8 |
| 2 | 25 | 45 | 40 | 30 | 25.1 |
| 3 | 20 | 60 | 45 | 20 | 22.2 |
| 4 | 30 | 45 | 40 | 20 | 24.5 |
| 5 | 30 | 45 | 45 | 10 | 25.2 |
| 6 | 25 | 30 | 40 | 20 | 21.3 |
| 7 | 20 | 45 | 45 | 30 | 23.7 |
| 8 | 25 | 45 | 45 | 20 | 26.8 |
| 9 | 25 | 45 | 45 | 20 | 26.7 |
| 10 | 25 | 60 | 50 | 20 | 23.9 |
| 11 | 25 | 30 | 45 | 10 | 20.2 |
| 12 | 25 | 45 | 40 | 10 | 22.6 |
| 13 | 30 | 45 | 50 | 20 | 24.4 |
| 14 | 25 | 45 | 45 | 20 | 26.6 |
| 15 | 25 | 60 | 45 | 10 | 22.8 |
| 16 | 20 | 45 | 40 | 20 | 21.8 |
| 17 | 30 | 60 | 45 | 20 | 24.2 |
| 18 | 25 | 60 | 45 | 30 | 24.7 |
| 19 | 25 | 30 | 50 | 20 | 21.5 |
| 20 | 20 | 30 | 45 | 20 | 19.9 |
| 21 | 30 | 45 | 45 | 30 | 25.5 |
| 22 | 20 | 45 | 45 | 10 | 21.6 |
| 23 | 25 | 30 | 45 | 30 | 22.3 |
| 24 | 25 | 45 | 50 | 30 | 24.1 |
| 25 | 25 | 45 | 50 | 10 | 23.5 |
| 26 | 25 | 60 | 40 | 20 | 23.8 |
| 27 | 25 | 45 | 45 | 20 | 26.9 |
| 28 | 25 | 45 | 45 | 20 | 26.6 |
| 29 | 20 | 45 | 50 | 20 | 22.5 |
| Serial Number | A: Liquid-to-Solid Ratio (mL/g) | B: Extraction Time (min) | C: Extraction Temperature (°C) | D: Water Content in NADES (%) | Piperine Yield % (Predicted) |
|---|---|---|---|---|---|
| 1 | 35 | 65 | 50 | 30 | 44.6 |
| 2 | 30 | 35 | 45 | 30 | 38.7 |
| 3 | 25 | 65 | 50 | 30 | 41.7 |
| 4 | 30 | 50 | 45 | 40 | 44.3 |
| 5 | 25 | 50 | 55 | 30 | 43.1 |
| 6 | 30 | 35 | 50 | 40 | 41.5 |
| 7 | 35 | 50 | 50 | 40 | 47.4 |
| 8 | 30 | 50 | 55 | 40 | 47.6 |
| 9 | 30 | 65 | 45 | 30 | 43.2 |
| 10 | 30 | 50 | 50 | 30 | 49.6 |
| 11 | 30 | 35 | 50 | 20 | 38.5 |
| 12 | 25 | 50 | 50 | 40 | 41.9 |
| 13 | 25 | 50 | 50 | 20 | 36.7 |
| 14 | 30 | 65 | 55 | 30 | 46.2 |
| 15 | 30 | 50 | 45 | 20 | 39.5 |
| 16 | 35 | 50 | 45 | 30 | 42.7 |
| 17 | 30 | 50 | 55 | 20 | 42.3 |
| 18 | 30 | 50 | 50 | 30 | 49.9 |
| 19 | 35 | 50 | 50 | 20 | 44.1 |
| 20 | 25 | 35 | 50 | 30 | 37.8 |
| 21 | 30 | 50 | 50 | 30 | 50.1 |
| 22 | 25 | 50 | 45 | 30 | 38.8 |
| 23 | 30 | 65 | 50 | 20 | 41.2 |
| 24 | 35 | 50 | 55 | 30 | 45.8 |
| 25 | 35 | 35 | 50 | 30 | 41.1 |
| 26 | 30 | 65 | 50 | 40 | 45.7 |
| 27 | 30 | 50 | 50 | 30 | 49.2 |
| 28 | 30 | 35 | 55 | 30 | 42.1 |
| 29 | 30 | 50 | 50 | 30 | 49.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayedh Al Adhreai, A.M.; Christyraj, J.R.S.S.; Yesudhason, B.V.; Arul Soundara Rajan, Y.A.P.; Bhaswant, M. Comparative Ultrasonic Bath and Probe Extraction of Piperine from Piper nigrum L. Using Natural Deep Eutectic Solvents: RSM Optimization, Characterization, and In Vitro Bioactivity. Biomolecules 2025, 15, 1631. https://doi.org/10.3390/biom15111631
Ayedh Al Adhreai AM, Christyraj JRSS, Yesudhason BV, Arul Soundara Rajan YAP, Bhaswant M. Comparative Ultrasonic Bath and Probe Extraction of Piperine from Piper nigrum L. Using Natural Deep Eutectic Solvents: RSM Optimization, Characterization, and In Vitro Bioactivity. Biomolecules. 2025; 15(11):1631. https://doi.org/10.3390/biom15111631
Chicago/Turabian StyleAyedh Al Adhreai, Abdullah Mohammed, Johnson Retnaraj Samuel Selvan Christyraj, Beryl Vedha Yesudhason, Yolin Angel Poomany Arul Soundara Rajan, and Maharshi Bhaswant. 2025. "Comparative Ultrasonic Bath and Probe Extraction of Piperine from Piper nigrum L. Using Natural Deep Eutectic Solvents: RSM Optimization, Characterization, and In Vitro Bioactivity" Biomolecules 15, no. 11: 1631. https://doi.org/10.3390/biom15111631
APA StyleAyedh Al Adhreai, A. M., Christyraj, J. R. S. S., Yesudhason, B. V., Arul Soundara Rajan, Y. A. P., & Bhaswant, M. (2025). Comparative Ultrasonic Bath and Probe Extraction of Piperine from Piper nigrum L. Using Natural Deep Eutectic Solvents: RSM Optimization, Characterization, and In Vitro Bioactivity. Biomolecules, 15(11), 1631. https://doi.org/10.3390/biom15111631






