The miR-125a-5p/IRF4 Axis Mediates Sodium Arsenite-Induced M2 Macrophage Polarization
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experimental Model
2.2. Reagents and Antibodies
2.3. Paraffin Embedding and Sectioning
2.4. Cell Culture
2.5. The Establishment and Identification of the Macrophage Model
2.6. Macrophage Viability
2.7. Flow Cytometry
2.8. Cell Transfection
2.9. Immunofluorescence Staining
2.10. RNA Extraction and Real-Time Quantitative PCR
2.11. Western Blot
2.12. Statistical Analysis
3. Results
3.1. Macrophages Polarize to M2 Type in Liver and Bladder Tissues of Arsenic-Exposed Rats
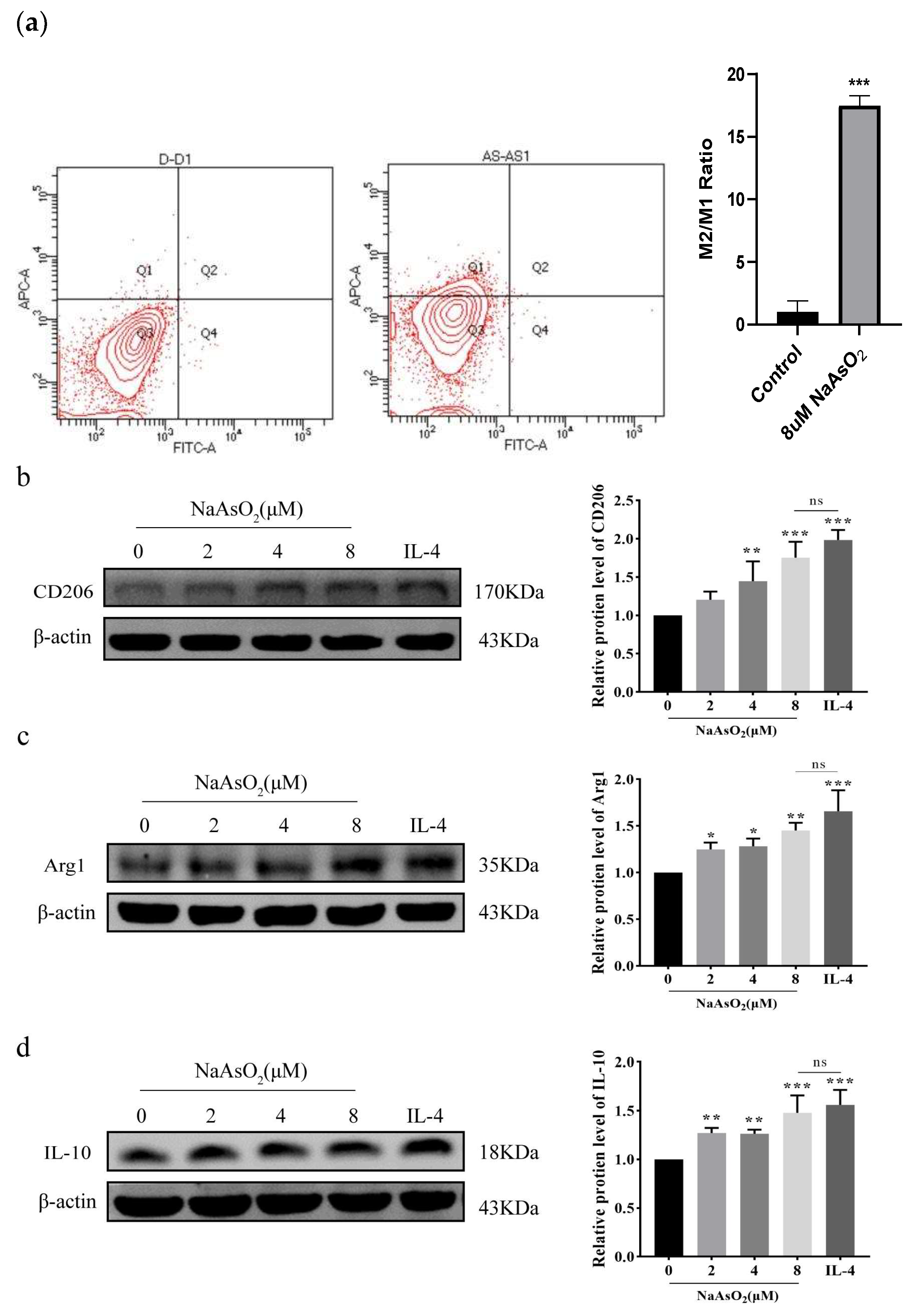
3.2. NaAsO2 Induced the Expression of THP-1-Derived Macrophage-Related Genes In Vitro
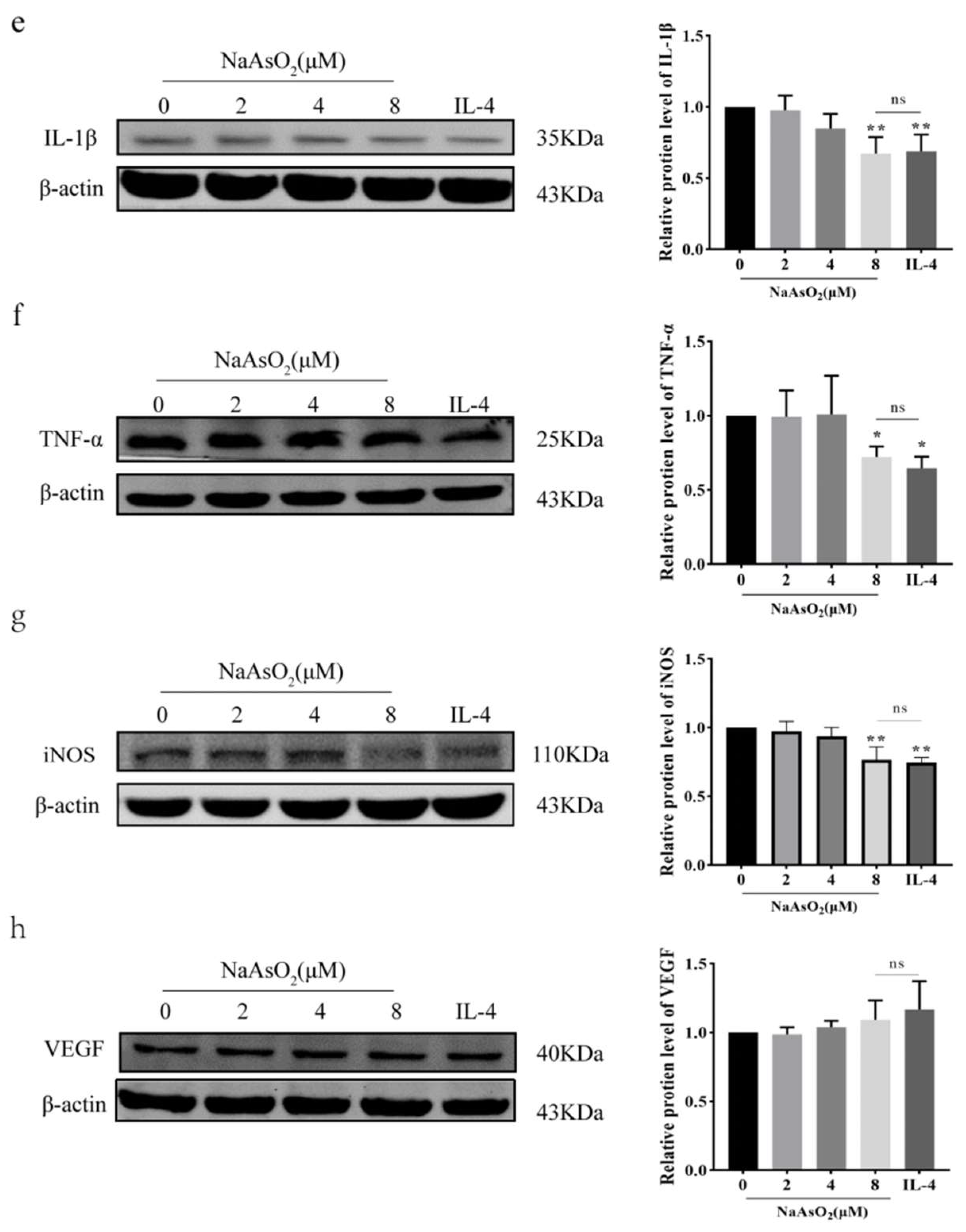
3.3. NaAsO2 Induced the Expression of THP-1-Derived Macrophage-Related Proteins
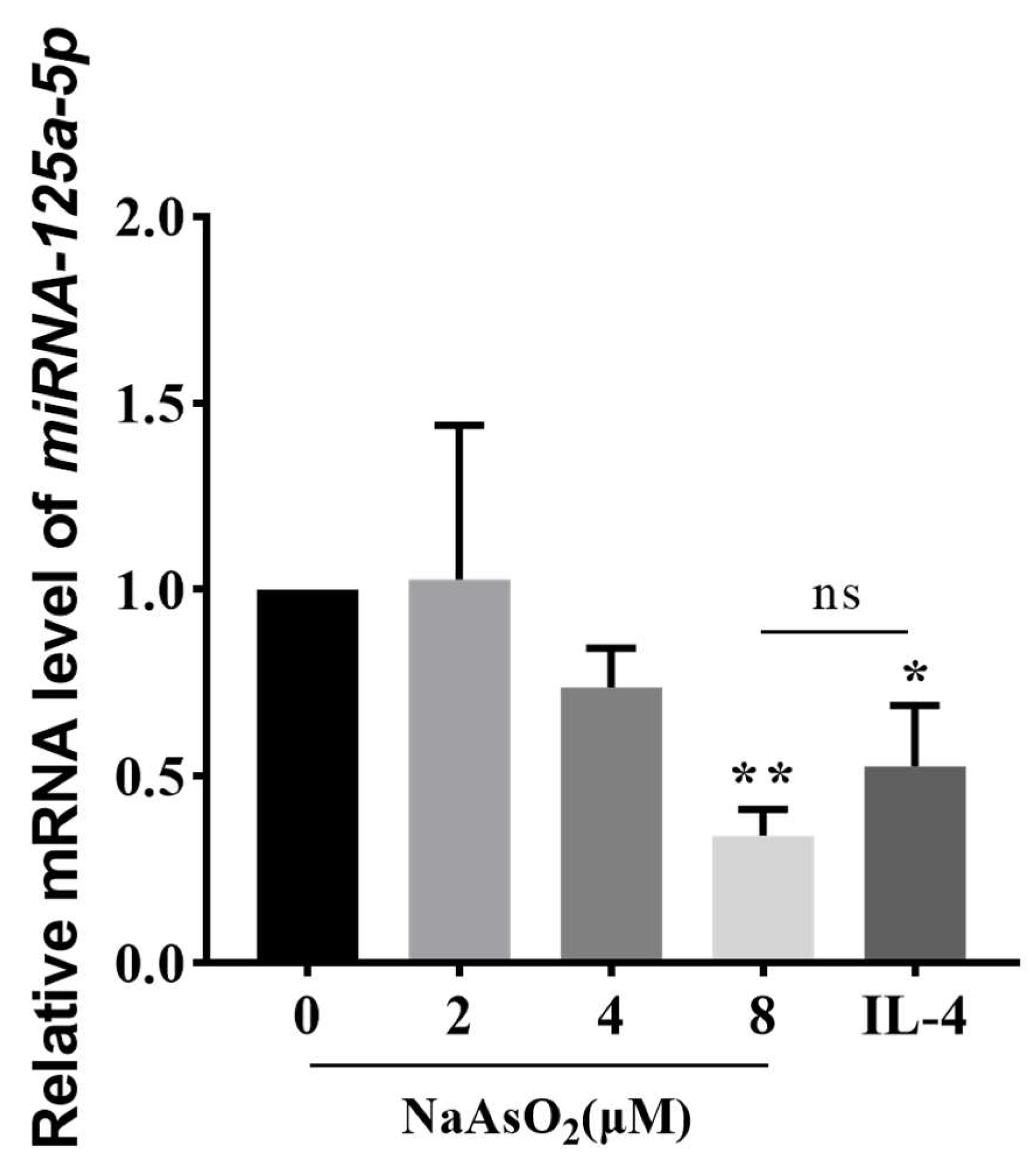
3.4. NaAsO2 Suppresses miR-125a-5p Expression in Polarized Macrophages
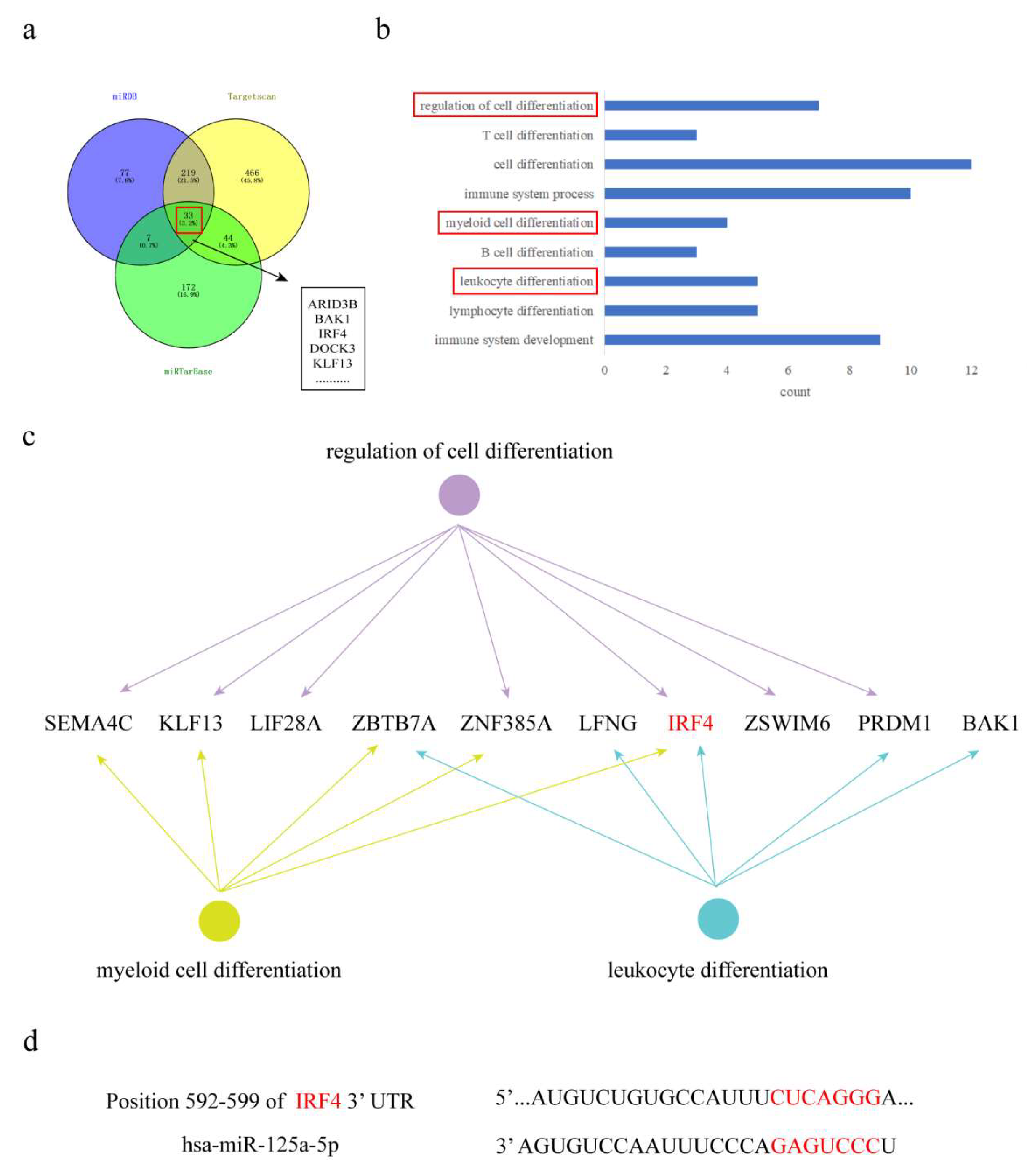
3.5. Bioinformatics Prediction of MiR-125a-5p Target Genes
3.6. MiR-125a-5p/IRF4 Axis Mediates Arsenic-Driven Macrophage Polarization via Epigenetic Regulation
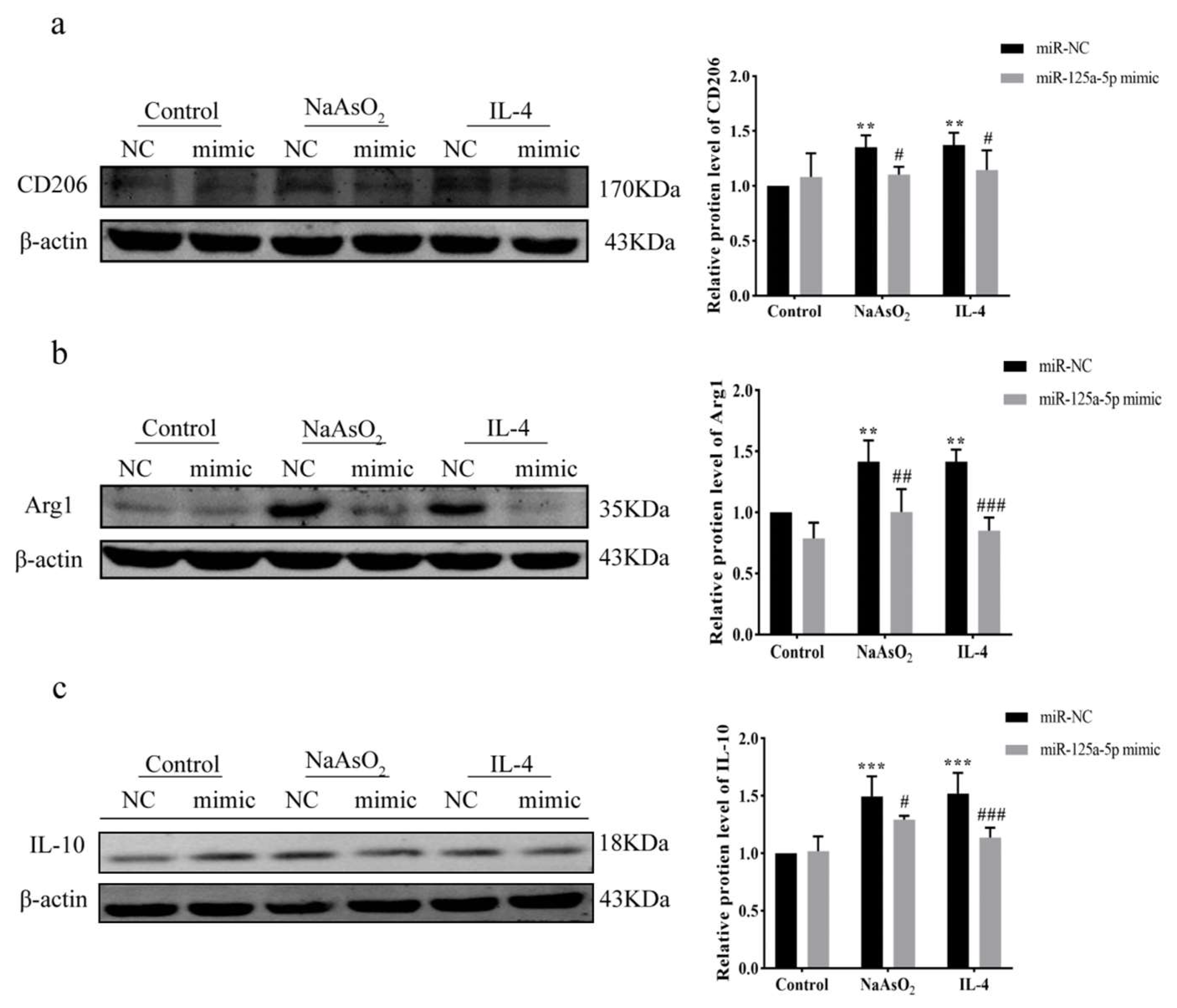
3.7. MiR-125a-5p Overexpression Inhibited NaAsO2-Induced M2 Polarization via IRF4 Suppression
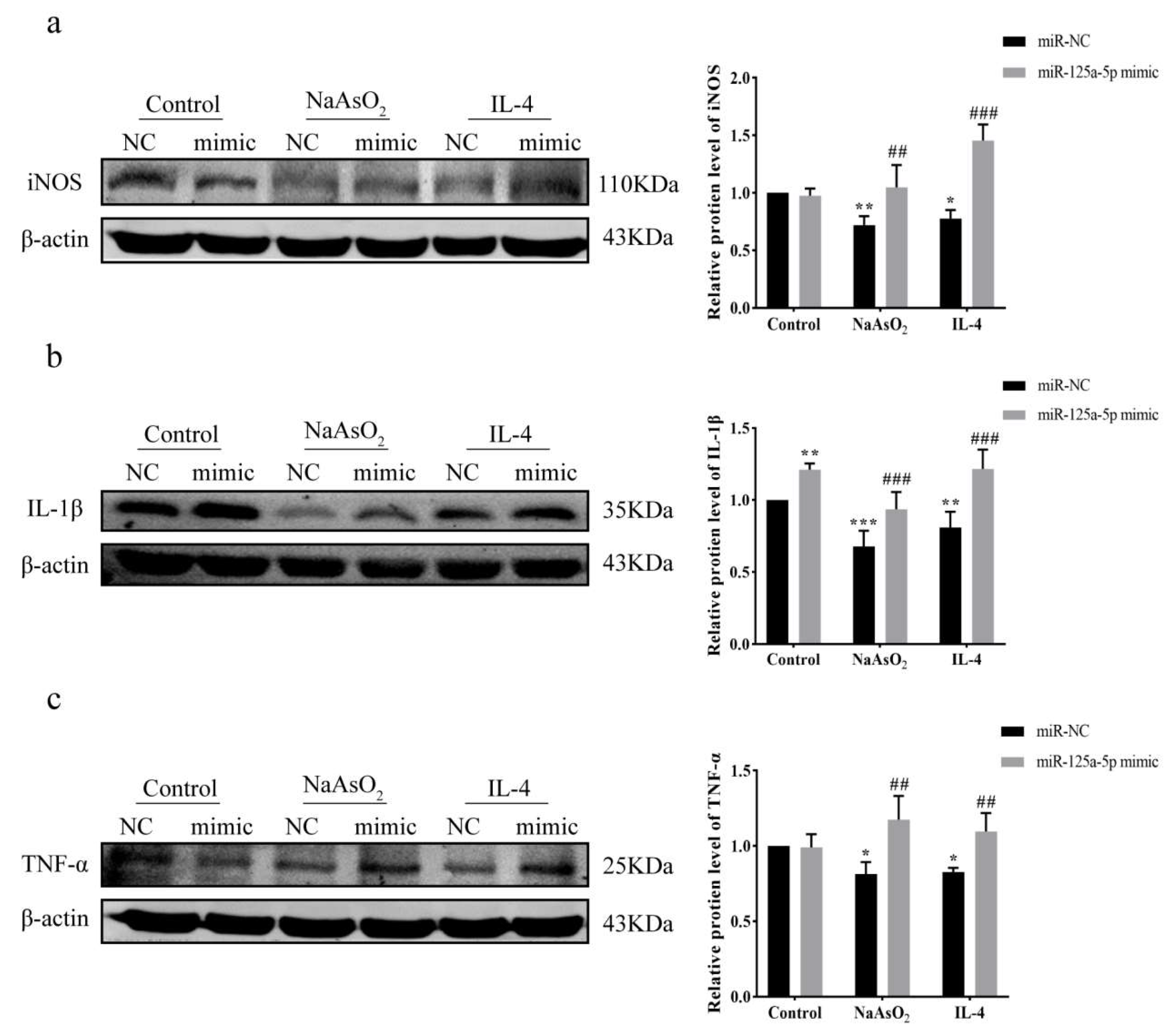
3.8. MiR-125a-5p Overexpression Restores Inflammation via Attenuating NaAsO2-Mediated Suppression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hughes, M.F. Biomarkers of exposure: A case study with inorganic arsenic. Environ. Health Perspect. 2006, 114, 1790–1796. [Google Scholar] [CrossRef]
- Rahaman, M.S.; Yamasaki, S.; Hossain, K.F.B.; Hosokawa, T.; Saito, T.; Kurasaki, M. Effects of curcumin, D-pinitol alone or in combination in cytotoxicity induced by arsenic in PC12 cells. Food Chem. Toxicol. 2020, 144, 111577. [Google Scholar] [CrossRef]
- Baris, D.R.; Waddell, R.; Freeman, L.B.; Schwenn, M.; Silverman, D. Elevated Bladder Cancer in Northern New England: The Role of Drinking Water and Arsenic. J. Natl. Cancer Inst. 2016, 108, djw099. [Google Scholar] [CrossRef]
- Kim, T.H.; Seo, J.W.; Hong, Y.S.; Song, K.H. Case-control study of chronic low-level exposure of inorganic arsenic species and non-melanoma skin cancer. J. Dermatol. 2017, 44, 1374–1379. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef]
- Wang, N.; Liang, H.; Zen, K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front. Immunol. 2014, 5, 614. [Google Scholar] [CrossRef]
- Essandoh, K.; Li, Y.; Huo, J.; Fan, G.C. MiRNA-Mediated Macrophage Polarization and its Potential Role in the Regulation of Inflammatory Response. Shock 2016, 46, 122–131. [Google Scholar] [CrossRef]
- Graff, J.W.; Dickson, A.M.; Clay, G.; McCaffrey, A.P.; Wilson, M.E. Identifying functional microRNAs in macrophages with polarized phenotypes. J. Biol. Chem. 2012, 287, 21816–21825. [Google Scholar] [CrossRef]
- Zhao, J.; Huang, F.; He, F.; Gao, C.; Liang, S.; Ma, P.; Dong, G.; Han, H.; Qin, H. Forced Activation of Notch in Macrophages Represses Tumor Growth by Upregulating miR-125a and Disabling Tumor-Associated Macrophages. Cancer Res. 2016, 76, 1403–1415. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Yu, H.R.; Huang, L.T.; Huang, H.C.; Chen, R.F.; Lin, I.C.; Ou, C.Y.; Hsu, T.; Yang, K. MiRNA-125b regulates TNF-α production in CD14+ neonatal monocytes via post-transcriptional regulation. J. Leukoc. Biol. 2012, 92, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.A.; So, Y.L.; Sinha, N.; Gibson, W.; Baltimore, D. MicroRNA-125b potentiates macrophage activation. J. Immunol. 2011, 187, 5062–5068. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.K.; Wang, Z.; Li, G. MicroRNA-125 in immunity and cancer. Cancer Lett. 2019, 454, 134–145. [Google Scholar] [CrossRef]
- Chang, J.; Tang, C. The role of macrophage polarization in rheumatoid arthritis and osteoarthritis: Pathogenesis and therapeutic strategies. Int. Immunopharmacol. 2024, 142, 113056. [Google Scholar] [CrossRef] [PubMed]
- Babaev, V.R.; Hebron, K.E.; Wiese, C.B.; Toth, C.L.; Ding, L.; Zhang, Y.; May, J.M.; Fazio, S.; Vickers, K.C.; Linton, M.F. Macrophage deficiency of Akt2 reduces atherosclerosis in Ldlr null mice. J. Lipid Res. 2014, 55, 2296–2308. [Google Scholar] [CrossRef]
- Barrett, T.J. Macrophages in Atherosclerosis Regression. Arter. Thromb. Vasc. Biol. 2020, 40, 20–33. [Google Scholar] [CrossRef]
- Wang, S.; Yang, S.; Chen, Y.; Li, R.; Han, S.; Kamili, A.; Wu, Y.; Zhang, W. Ginsenoside Rb2 Alleviated Atherosclerosis by Inhibiting M1 Macrophages Polarization Induced by MicroRNA-216a. Front. Pharmacol. 2022, 12, 764130. [Google Scholar] [CrossRef]
- Herbein, G.; Varin, A. The macrophage in HIV-1 infection: From activation to deactivation? Retrovirology 2010, 7, 33. [Google Scholar] [CrossRef]
- Sica, A.; Erreni, M.; Allavena, P.; Porta, C. Macrophage polarization in pathology. Cell Mol. Life Sci. 2015, 72, 4111–4126. [Google Scholar] [CrossRef]
- Rees, P.A.; Greaves, N.S.; Baguneid, M.; Bayat, A. Chemokines in Wound Healing and as Potential Therapeutic Targets for Reducing Cutaneous Scarring. Adv. Wound Care 2015, 4, 687–703. [Google Scholar] [CrossRef]
- Abdelaziz, M.H.; Abdelwahab, S.F.; Wan, J.; Cai, W.; Huixuan, W.; Jianjun, C.; Kumar, K.D.; Vasudevan, A.; Sadek, A.; Su, Z.; et al. Alternatively activated macrophages; a double-edged sword in allergic asthma. J. Transl. Med. 2020, 18, 58. [Google Scholar] [CrossRef] [PubMed]
- Hesketh, M.; Sahin, K.B.; West, Z.E.; Murray, R.Z. Macrophage Phenotypes Regulate Scar Formation and Chronic Wound Healing. Int. J. Mol. Sci. 2017, 18, 1545. [Google Scholar] [CrossRef]
- White, M.J.; Gomer, R.H. Trypsin, Tryptase, and Thrombin Polarize Macrophages towards a Pro-Fibrotic M2a Phenotype. PLoS ONE 2015, 10, e0138748. [Google Scholar] [CrossRef]
- Xue, J.D.; Gao, J.; Tang, A.F.; Feng, C. Shaping the immune landscape: Multidimensional environmental stimuli refine macrophage polarization and foster revolutionary approaches in tissue regeneration. Heliyon 2024, 10, e37192. [Google Scholar] [CrossRef]
- Anders, C.B.; Lawton, T.M.W.; Ammons, M.C. Metabolic immunomodulation of macrophage functional plasticity in nonhealing wounds. Curr. Opin. Infect. Dis. 2019, 32, 204–209. [Google Scholar] [CrossRef]
- Hung, C.H.; Hsu, H.Y.; Chiou, H.Y.; Tsai, M.Y.; You, H.Y.; Lin, Y.C.; Liao, W.T.; Lin, Y.C. Arsenic Induces M2 Macrophage Polarization and Shifts M1/M2 Cytokine Production via Mitophagy. Int. J. Mol. Sci. 2022, 23, 13879. [Google Scholar] [CrossRef]
- Sun, Y.M.; Lin, K.Y.; Chen, Y.Q. Diverse functions of miR-125 family in different cell contexts. J. Hematol. Oncol. 2013, 6, 6. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Myasoedova, V.A.; Revin, V.V.; Orekhov, A.N.; Bobryshev, Y.V. The impact of interferon-regulatory factors to macrophage differentiation and polarization into M1 and M2. Immunobiology 2018, 223, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Khayati, S.; Dehnavi, S.; Sadeghia, M.; Afshari, J.T.; Esmaeili, S.A.; Mohammadi, M. The potential role of miRNA in regulating macrophage polarization. Heliyon 2023, 9, e21615. [Google Scholar] [CrossRef] [PubMed]
- Günthner, R.; Anders, H.J. Interferon-regulatory factors determine macrophage phenotype polarization. Mediat. Inflamm. 2013, 2013, 731023. [Google Scholar] [CrossRef]
- Mamuna, A.A.; Chauhana, A.; Qia, S.; Ngwaa, C.; Xua, Y.; Sharmeena, P.; Hazenb, A.L.; Lia, J.; Aronowskia, J.A.; McCullougha, L.D.; et al. Microglial IRF5-IRF4 regulatory axis regulates neuroinflammation after cerebral ischemia and impacts stroke outcomes. Proc. Natl. Acad. Sci. USA 2020, 117, 1742–1752. [Google Scholar] [CrossRef]
- Fang, H.; Yang, M.; Pan, Q.; Jin, H.; Li, H.; Wang, R.; Wang, Q.; Zhang, J. MicroRNA-22-3p alleviates spinal cord ischemia/reperfusion injury by modulating M2 macrophage polarization via IRF5. J. Neurochem. 2021, 156, 106–120. [Google Scholar] [CrossRef]
- Liang, C.; Tang, Y.; Gao, X.; Lei, N.; Luo, Y.; Chen, P.; Duan, S.; Cao, Y.; Yang, Y.; Zhang, Y. Depression Exacerbates Dextran Sulfate Sodium-Induced Colitis via IRF5-Mediated Macrophage Polarization. Dig. Dis. Sci. 2023, 68, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Terker, A.S.; Pan, Y.; Li, Z.; Harris, R.C. Deletion of Myeloid Interferon Regulatory Factor 4 (Irf4) in Mouse Model Protects against Kidney Fibrosis after Ischemic Injury by Decreased Macrophage Recruitment and Activation. J. Am. Soc. Nephrol. 2021, 32, 1037–1052. [Google Scholar] [CrossRef] [PubMed]
- Boutilier, A.J.; Elsawa, S.F. Macrophage Polarization States in the Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 6995. [Google Scholar] [CrossRef]
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumor-Associated Macrophages in Tumor Immunity. Front. Immunol. 2020, 11, 583084. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhang, X.; Li, Z.; Zhu, B. Metabolic regulatory crosstalk between tumor microenvironment and tumor-associated macrophages. Theranostics 2021, 11, 1016–1030. [Google Scholar] [CrossRef]




| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| CD206 | GCAAAGTGGATTACGTGTCTTG | CTGTTATGTCGCTGGCAAATG |
| Arg1 | GTCTGTGGGAAAAGCAAGCG | CACCAGGCTGATTCTTCCGT |
| IL-10 | AAGACCCAGACATCAAGGCG | AGGCATTCTTCACCTGCTCC |
| IL-1β | CTGCTCTGGGATTCTCTTCAG | ATCTGTTTAGGGCCATCAGC |
| TNF-α | ACTTTGGAGTGATCGGCC | GCTTGAGGGTTTGCTACAAC |
| VEGF | AGGGCAGAATCATCACGAAG | GGATGGCTTGAAGATGTACTCG |
| GAPDH | TGTTGCCATCAATGACCCCTT | CTCCACGACGTACTCAGCG |
| miR-125a-5p | GCGTCCCTGAGACCCTTTAAC | AGTGCAGGGTCCGAGGTATT |
| U6 | AGAGAAGATTAGCATGGCCCCTG | AGTGCAGGGTCCGAGGTATT |
| miR-125a-5p Stem-Loop primer | GTCGTATCCAGTGCAGGGTCCGAGGTAT TCGCACTGGATACGACTCACAG | |
| U6 Stem-Loop primer | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAAAATATG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Yao, F.; Tong, S.; Li, M.; Liao, Q.; Wang, F.; Xi, S. The miR-125a-5p/IRF4 Axis Mediates Sodium Arsenite-Induced M2 Macrophage Polarization. Biomolecules 2025, 15, 1630. https://doi.org/10.3390/biom15111630
Yu Y, Yao F, Tong S, Li M, Liao Q, Wang F, Xi S. The miR-125a-5p/IRF4 Axis Mediates Sodium Arsenite-Induced M2 Macrophage Polarization. Biomolecules. 2025; 15(11):1630. https://doi.org/10.3390/biom15111630
Chicago/Turabian StyleYu, Yan, Fan Yao, Suyuan Tong, Mingzheng Li, Qilong Liao, Fei Wang, and Shuhua Xi. 2025. "The miR-125a-5p/IRF4 Axis Mediates Sodium Arsenite-Induced M2 Macrophage Polarization" Biomolecules 15, no. 11: 1630. https://doi.org/10.3390/biom15111630
APA StyleYu, Y., Yao, F., Tong, S., Li, M., Liao, Q., Wang, F., & Xi, S. (2025). The miR-125a-5p/IRF4 Axis Mediates Sodium Arsenite-Induced M2 Macrophage Polarization. Biomolecules, 15(11), 1630. https://doi.org/10.3390/biom15111630








