Protein Engineering and Drug Discovery: Importance, Methodologies, Challenges, and Prospects
Abstract
1. Introduction
2. Technologies for Structural Determination of Proteins
3. Advanced Computational Tools in Protein Engineering
4. Biologics: The New Frontier
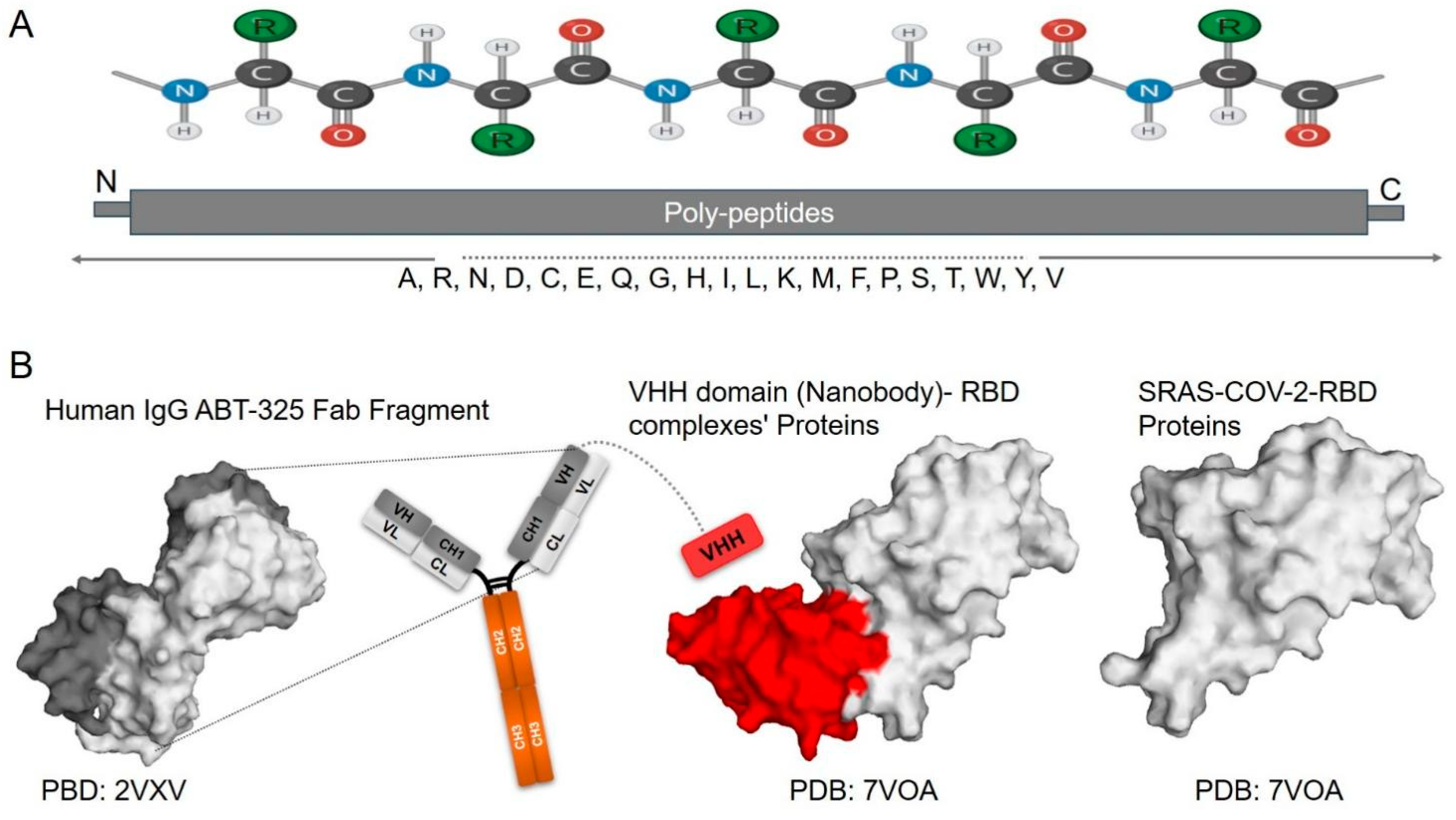
4.1. Antibodies
4.2. Nanobodies
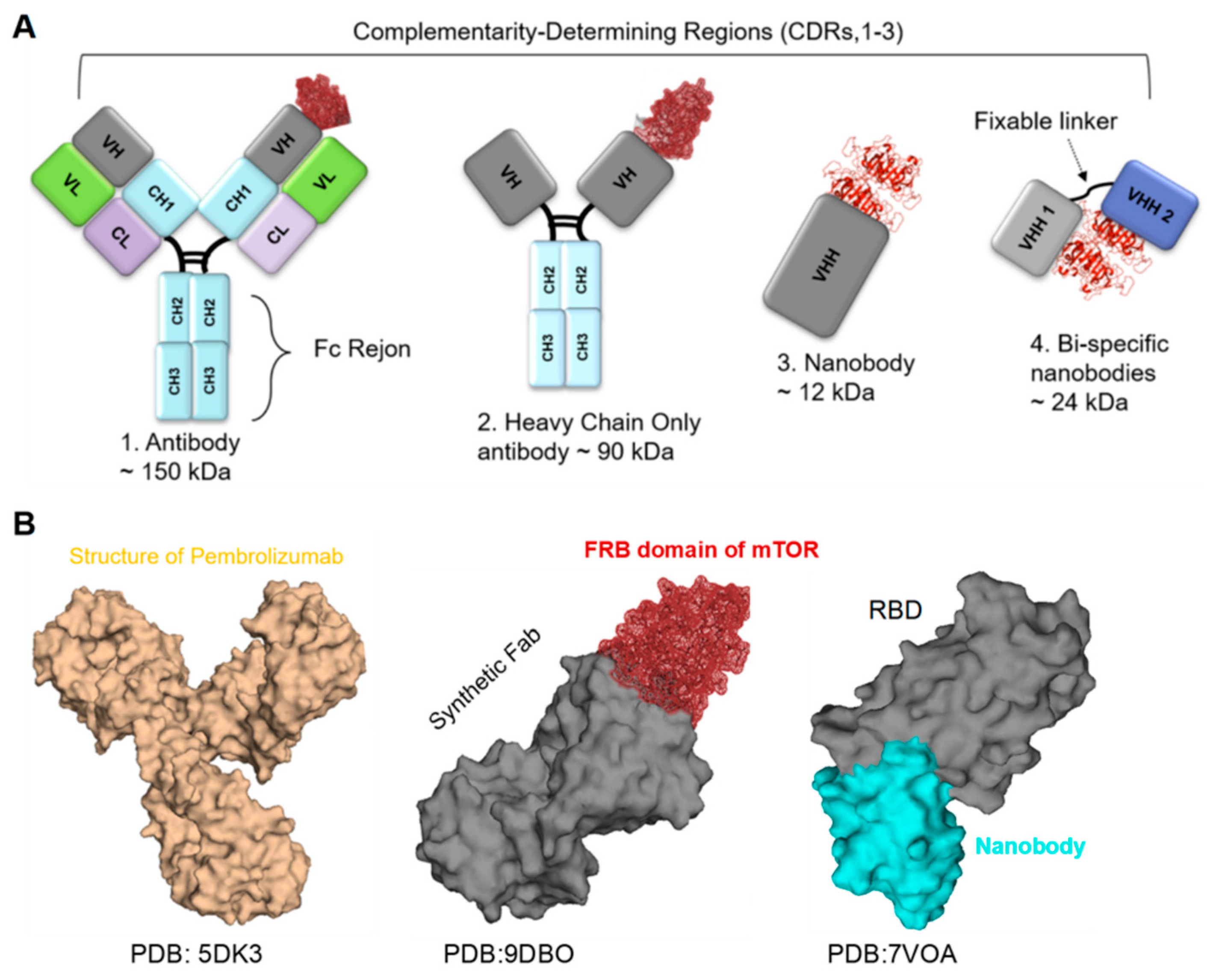
4.3. Cytokine-Based Therapy
5. Innovations of Protein Engineering-Based Therapies
6. Methodology
6.1. Molecular Cloning
6.2. Protein Expression Systems
6.2.1. Types of Protein Expression Systems
6.2.2. Vectors in Protein Expression
6.2.3. Types of Vectors
6.2.4. Cloning Vectors
6.2.5. Delivery Vectors
6.2.6. Key Features of Vectors
| Linker Type | Linker Name | Sequence | Features | Ref. |
|---|---|---|---|---|
| Flexible | (Gly4Ser)3 | GGGSGGGSGGGSGGGS | Highly flexible; promotes solubility and reduces steric hindrance; commonly used in fusions. | [91,106,107] |
| Flexible | (Gly)3 | GGG | Short flexible linker; enhances solubility; often used in simple fusions. | [107] |
| Rigid | (EAAAK)3 | EAAKEAAKEAAK | Rigid structure; minimizes interaction between fused proteins; functional for complex fusions. | [108,109] |
| Flexible | Conventional Gly linker | GGGGSGGGG | Provides flexibility with moderate length; suitable for various protein fusions. | [110,111] |
| Mixed | (Gly/Ser) Linker | GGGSGS | A combination of glycine and serine offers both flexibility and some rigidity. | [112] |
| Flexible | (Gly)10 | GGGGGGGGGG | Very flexible; enhances solubility and minimizes steric clashes, making it suitable for large proteins. | [91] |
| Rigid | (EAAAK)2 | EAAAKEAAK | Rigid; reduces potential steric hindrance; helpful in maintaining structural integrity. | [109] |
6.3. Therapeutic Protein Purification
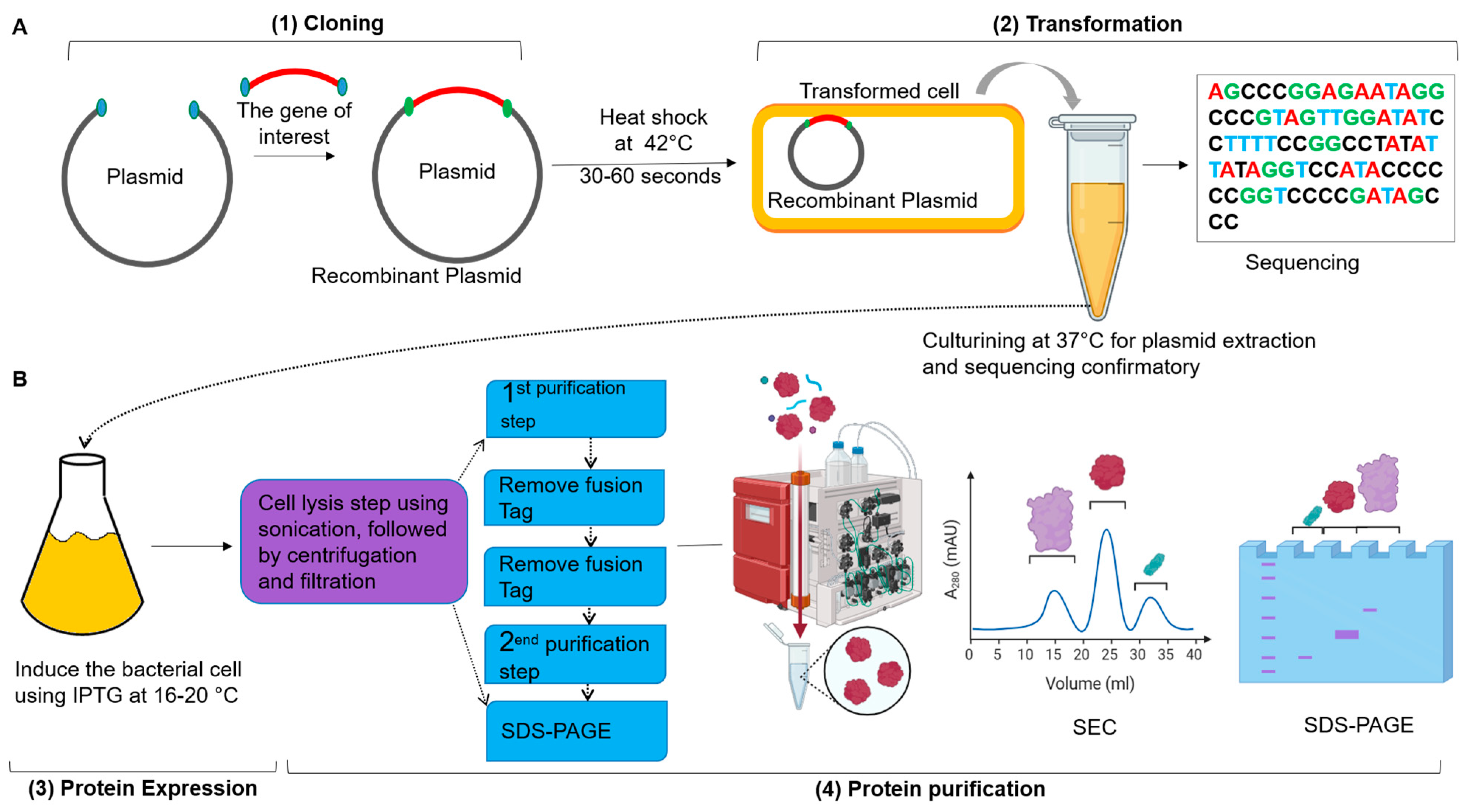
6.4. Characterization of Recombinant Proteins
| Technique | Purpose | Methodology | Applications | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|---|
| Separates proteins based on molecular weight | Proteins are denatured and coated with SDS, then subjected to electrophoresis in a polyacrylamide gel | Assessing purity, estimating molecular weight. | Simple, quick, and cost-effective | Does not provide information on protein activity | [61,117,118] |
| Detects specific proteins | Proteins from SDS-PAGE are transferred to a membrane and probed with specific antibodies | Confirming identity and expression levels | Highly specific detection | Requires high-quality antibodies; can be time-consuming | [117,119] |
| Analyzes protein mass and structure | Proteins are ionized and fragmented; the mass-to-charge ratio of ions is analyzed. | Identifying proteins, studying modifications | High sensitivity and specificity | Sample preparation can be complex | [120] |
| Measures the functional activity of enzymes | Quantifies the rate of reaction catalyzed by the enzyme using substrates and measuring products | Evaluating enzymatic activity and kinetics. | Directly assesses enzyme functionality. | Requires specific substrates; conditions must be optimized. | [114] |
| Analyzes the secondary structure of a protein | Measures the differential absorption of circularly polarized light to assess folding | Assessing protein folding and stability | Quick and requires small sample amounts | Limited to secondary structure; cannot provide 3D structures | [61,121] |
| Provides structural information in solution | Detects magnetic properties of atomic nuclei to determine 3D structures and dynamics | Understanding protein conformation and dynamics | Can analyze proteins in solution, retaining the native state | Limited to smaller proteins; requires high concentrations | [122,123] |
| Determines high-resolution structures | Crystals of proteins are bombarded with X-rays, producing a diffraction pattern that is analyzed for structure | Revealing atomic-level architecture | Provides high-resolution structures | Requires crystallization, which can be difficult | [90,124] |
| Assesses protein stability | Measures changes in fluorescence or absorbance as temperature increases | Identifying optimal storage conditions. | Simple and rapid; requires minimal equipment | May not provide detailed stability profiles. | [125,126] |
| Measures real-time interactions | Detects changes in refractive index as proteins bind to ligands on a sensor surface | Studying protein–protein and ligand binding interactions | Real-time measurement of interactions | Requires specific equipment and may need optimization | [61,127,128] |
| Measures binding interactions and thermodynamics | Monitors heat changes during binding events to determine affinity and stoichiometry | Analyzing binding interactions of protein–protein and ligands | Provides thermodynamic data in a single experiment | Requires large amounts of protein; can be expensive | [61,129] |
| Provides structural information in native states | Samples are rapidly frozen and imaged using electron microscopy to obtain 3D reconstructions | Studying large complexes and membrane proteins | Does not require crystallization; it can study large complexes | Requires specialized equipment and sample preparation | [130,131] |
| Analyzes protein conformation and dynamics | Proteins labeled with fluorescent tags are excited, and emission spectra are measured | Monitoring folding and binding events | Sensitive and versatile; can provide dynamic information | Requires labeling; may alter protein behavior | [61,132,133] |
7. Clinical Cases: Successful Examples
7.1. Recombinant Protein Fighting Infectious Diseases
7.2. Recombinant Protein as Cancer Therapy
7.3. Cytokine Therapies
7.4. CAR T-Cell Therapy
7.5. Nanobodies in Disease Treatment
| RPT*1 Name | Target Diseases | Technique | Mechanism of Action | Ref. |
|---|---|---|---|---|
| Recombinant Insulin | Diabetes Mellitus | rDNA*2 | Regulates blood sugar levels | [159] |
| Erythropoietin (EPO) | Anemia | rDNA | Stimulates red blood cell production | [160,161] |
| Adalimumab (Humira®) | Rheumatoid arthritis | rDNA | Inhibits TNF-alpha | [162] |
| Nivolumab (Opdivo®) | Melanoma, lung cancer | rDNA | Blocks PD-1 receptor to enhance immune response | [49,163] |
| Bevacizumab (Avastin®) | Various cancers (e.g., colorectal cancer) | rDNA | Inhibits VEGF to prevent tumor blood supply | [164] |
| Tocilizumab (Actemra®) | Rheumatoid arthritis, COVID-19 | rDNA | Inhibits IL-6 receptor | [165] |
| Atezolizumab (Tecentriq®) | Various cancers (e.g., hepato cellular cancer) | rDNA | Blocks PD-L1 to enhance the immune response | [166,167] |
| Rituximab (Rituxan®) | Non-Hodgkin lymphoma | Hybridoma technology | Targets CD20 on B-cells | [168,169] |
| Blinatumomab (Blincyto®) | Acute lymphoblastic leukemia | Bispecific T-cell engager (BiTEs)*3 | Engages T-cells to target leukemia cells | [170,171] |
| Tisagenlecleucel (Kymriah®) | Acute lymphoblastic leukemia | CAR T-cell therapy | Redirects T-cells to target leukemia cells | [172,173] |
| Axicabtagene Ciloleucel (Yescarta®) | Large B-cell lymphoma | CAR T-cell therapy | Redirects T-cells to target lymphoma cells | [174] |
| Zanubrutinib (Brukinsa®) | Chronic lymphocytic leukemia | Small molecule inhibitor | Inhibits BTK to reduce B-cell activation | [175,176] |
| Ciltacabtagene | Multiple Myeloma | Phage display technology | Targets the B-cell maturation antigen (BCMA) on multiple myeloma cells. | [177] |
| Ablynx ALX-0171 (nanobody) | Respiratory syncytial virus (RSV) | Phage display technology | Binds to RSV F protein to neutralize the virus, effectively inhibited replication below the detection limit for 87% of the tested viruses | [178] |
| Caplacizumab (Cablivi®) | Thrombotic thrombocytopenic purpura (TTP) | Phage display technology | Inhibits von Willebrand factor (vWF) | [179,180] |
| aSA3-Fc (nanobody) | SARS-COV-2 | Phage display Technology | Binds and potently neutralizes SARS-CoV-1, 2, and Omicron; in pre-clinical trials | [65] |
8. Challenges and Prospects
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jena, R.; Samal, H.B.; Sharma, J.; Suresh, P.; Mishra, A.P.; Nigam, M. Biotechnology in Drug Discovery and Development for Cancer. In Biotechnology and Cancer Therapeutics; Springer: Berlin/Heidelberg, Germany, 2025; pp. 447–478. [Google Scholar]
- Talwar, A. Principles of Pharmaceutical Biotechnology; Educohack Press: Delhi, India, 2025. [Google Scholar]
- Akhtar, Z.B.; Gupta, A.D. Advancements within molecular engineering for regenerative medicine and biomedical applications an investigation analysis towards a computing retrospective. J. Electron. Electromed. Eng. Med. Inform. 2024, 6, 54–72. [Google Scholar] [CrossRef]
- Schmid, A.S.; Neri, D. Advances in antibody engineering for rheumatic diseases. Nat. Rev. Rheumatol. 2019, 15, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Udeabor, S.E. Monoclonal antibodies in modern medicine: Their therapeutic potential and future directions. Trends Pharm. Biotechnol. 2024, 2, 12–20. [Google Scholar] [CrossRef]
- Deckers, J.; Anbergen, T.; Hokke, A.M.; de Dreu, A.; Schrijver, D.P.; de Bruin, K.; Toner, Y.C.; Beldman, T.J.; Spangler, J.B.; de Greef, T.F. Engineering cytokine therapeutics. Nat. Rev. Bioeng. 2023, 1, 286–303. [Google Scholar] [CrossRef]
- Wu, K.; Kwon, S.H.; Zhou, X.; Fuller, C.; Wang, X.; Vadgama, J.; Wu, Y. Overcoming challenges in small-molecule drug bioavailability: A review of key factors and approaches. Int. J. Mol. Sci. 2024, 25, 13121. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.; Muustafa, M.I. Nanobodies: A New Frontier in Antiviral Therapies. SLAS Discov. 2025, 35, 100251. [Google Scholar] [CrossRef]
- Listov, D.; Goverde, C.A.; Correia, B.E.; Fleishman, S.J. Opportunities and challenges in design and optimization of protein function. Nat. Rev. Mol. Cell Biol. 2024, 25, 639–653. [Google Scholar] [CrossRef]
- Shakhnovich, E. Protein folding thermodynamics and dynamics: Where physics, chemistry, and biology meet. Chem. Rev. 2006, 106, 1559–1588. [Google Scholar] [CrossRef]
- Marinko, J.T.; Huang, H.; Penn, W.D.; Capra, J.A.; Schlebach, J.P.; Sanders, C.R. Folding and misfolding of human membrane proteins in health and disease: From single molecules to cellular proteostasis. Chem. Rev. 2019, 119, 5537–5606. [Google Scholar] [CrossRef]
- Sweeney, P.; Park, H.; Baumann, M.; Dunlop, J.; Frydman, J.; Kopito, R.; McCampbell, A.; Leblanc, G.; Venkateswaran, A.; Nurmi, A. Protein misfolding in neurodegenerative diseases: Implications and strategies. Transl. Neurodegener. 2017, 6, 6. [Google Scholar] [CrossRef]
- Ho, R.J. Biotechnology and Biopharmaceuticals: Transforming Proteins and Genes into Drugs; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Kumar, V.; Barwal, A.; Sharma, N.; Mir, D.S.; Kumar, P.; Kumar, V. Therapeutic proteins: Developments, progress, challenges, and future perspectives. 3 Biotech 2024, 14, 112. [Google Scholar] [CrossRef]
- Burnett, M.J.; Burnett, A.C. Therapeutic recombinant protein production in plants: Challenges and opportunities. Plants People Planet 2020, 2, 121–132. [Google Scholar] [CrossRef]
- Appell, M.; Hurst, W.J.; Finley, J.W.; deMan, J.M. Amino acids and proteins. In Principles of Food Chemistry; Springer: Berlin/Heidelberg, Germany, 2018; pp. 117–164. [Google Scholar]
- Schulz, G.E.; Schirmer, R.H. Principles of Protein Structure; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Selvaraj, C.; Rudhra, O.; Alothaim, A.S.; Alkhanani, M.; Singh, S.K. Structure and chemistry of enzymatic active sites that play a role in the switch and conformation mechanism. Adv. Protein Chem. Struct. Biol. 2022, 130, 59–83. [Google Scholar]
- Gandhi, J.; Antonelli, A.C.; Afridi, A.; Vatsia, S.; Joshi, G.; Romanov, V.; Murray, I.V.; Khan, S.A. Protein misfolding and aggregation in neurodegenerative diseases: A review of pathogeneses, novel detection strategies, and potential therapeutics. Rev. Neurosci. 2019, 30, 339–358. [Google Scholar] [CrossRef]
- Wang, W. The Latest Progress of Cryo Electron Microscopy Technology in Protein Structure Analysis. Genom. Appl. Biol. 2024, 15, e0005. [Google Scholar] [CrossRef]
- Argiriadi, M.A.; Xiang, T.; Wu, C.; Ghayur, T.; Borhani, D.W. Unusual water-mediated antigenic recognition of the proinflammatory cytokine interleukin-18. J. Biol. Chem. 2009, 284, 24478–24489. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Zhang, X.; Zheng, P.; Dube, P.H.; Zeng, W.; Chen, S.; Cheng, Q.; Yang, Y.; Wu, Y.; Zhou, J. Hetero-bivalent nanobodies provide broad-spectrum protection against SARS-CoV-2 variants of concern including Omicron. Cell Res. 2022, 32, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Lupala, C.S.; Li, X.; Lei, J.; Chen, H.; Qi, J.; Liu, H.; Su, X.D. Computational simulations reveal the binding dynamics between human ACE2 and the receptor binding domain of SARS-CoV-2 spike protein. Quant. Biol. 2021, 9, 61–72. [Google Scholar] [CrossRef]
- Antony, P.; Vijayan, R. Molecular dynamics simulation study of the interaction between human angiotensin converting enzyme 2 and spike protein receptor binding domain of the SARS-CoV-2 B. 1.617 variant. Biomolecules 2021, 11, 1244. [Google Scholar] [CrossRef]
- Movahedpour, A.; Ahmadi, K.; Taheri-Anganeh, M.; Amiri, A.; Ahmadi, N.; Khatami, S.H.; Zafaranchi zm, S.; Soltani Fard, E.; Moazamfard, M.; Ghasemi, H. Designing a humanized immunotoxin based on HER2 specific scFv and DFF40 toxin against breast cancer: An in-silico study. Int. J. Pept. Res. Ther. 2022, 28, 130. [Google Scholar] [CrossRef]
- Åqvist, J.; Luzhkov, V.B.; Brandsdal, B.O. Ligand binding affinities from MD simulations. Acc. Chem. Res. 2002, 35, 358–365. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Tondar, A.; Sánchez-Herrero, S.; Bepari, A.K.; Bahmani, A.; Calvet Liñán, L.; Hervás-Marín, D. Virtual screening of small molecules targeting BCL2 with machine learning, molecular docking, and MD simulation. Biomolecules 2024, 14, 544. [Google Scholar] [CrossRef]
- Son, A.; Park, J.; Kim, W.; Yoon, Y.; Lee, S.; Park, Y.; Kim, H. Revolutionizing molecular design for innovative therapeutic applications through artificial intelligence. Molecules 2024, 29, 4626. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, S.B.; Samanta, D. Engineering protein-based therapeutics through structural and chemical design. Nat. Commun. 2023, 14, 2411. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Dahiya, V.; Vibhuti, A.; Pandey, R.P.; Tripathi, M.K.; Yadav, M.K. Therapeutic protein-based vaccines. In Protein-Based Therapeutics; Springer: Berlin/Heidelberg, Germany, 2023; pp. 355–384. [Google Scholar]
- Yadav, M.K.; Sahu, A.; Anu; Kasturria, N.; Priyadarshini, A.; Gupta, A.; Gupta, K.; Tomar, A.K. Clinical applications of protein-based therapeutics. In Protein-Based Therapeutics; Springer: Berlin/Heidelberg, Germany, 2023; pp. 23–47. [Google Scholar]
- Carter, P.J.; Quarmby, V. Immunogenicity risk assessment and mitigation for engineered antibody and protein therapeutics. Nat. Rev. Drug Discov. 2024, 23, 898–913. [Google Scholar] [CrossRef] [PubMed]
- Rondon, A.; Mahri, S.; Morales-Yanez, F.; Dumoulin, M.; Vanbever, R. Protein engineering strategies for improved pharmacokinetics. Adv. Funct. Mater. 2021, 31, 2101633. [Google Scholar] [CrossRef]
- Tan, P.K.; Kuppusamy, U.R.; Chua, K.H.; Arumugam, B. Emerging strategies to improve the stability and bioavailability of insulin: An update on formulations and delivery approaches. Curr. Drug Deliv. 2023, 20, 1141–1162. [Google Scholar] [CrossRef]
- Rahban, M.; Ahmad, F.; Piatyszek, M.A.; Haertlé, T.; Saso, L.; Saboury, A.A. Stabilization challenges and aggregation in protein-based therapeutics in the pharmaceutical industry. RSC Adv. 2023, 13, 35947–35963. [Google Scholar] [CrossRef]
- Monnier, P.P.; Vigouroux, R.J.; Tassew, N.G. In vivo applications of single chain Fv (variable domain) (scFv) fragments. Antibodies 2013, 2, 193–208. [Google Scholar] [CrossRef]
- Unverdorben, F.; Richter, F.; Hutt, M.; Seifert, O.; Malinge, P.; Fischer, N.; Kontermann, R.E. Pharmacokinetic properties of IgG and various Fc fusion proteins in mice. mAbs 2016, 8, 120–128. [Google Scholar] [CrossRef]
- Fitzpatrick, E.A.; Wang, J.; Strome, S. Engineering of Fc multimers as a protein therapy for autoimmune disease. Front. Immunol. 2020, 11, 496. [Google Scholar] [CrossRef]
- Jafari, R.; M Zolbanin, N.; Rafatpanah, H.; Majidi, J.; Kazemi, T. Fc-fusion proteins in therapy: An updated view. Curr. Med. Chem. 2017, 24, 1228–1237. [Google Scholar] [CrossRef] [PubMed]
- Kandari, D.; Bhatnagar, R. Antibody engineering and its therapeutic applications. Int. Rev. Immunol. 2023, 42, 156–183. [Google Scholar] [CrossRef] [PubMed]
- Victoir, B.; Croix, C.; Gouilleux, F.; Prié, G. Targeted therapeutic strategies for the treatment of cancer. Cancers 2024, 16, 461. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ukidve, A.; Kim, J.; Mitragotri, S. Targeting strategies for tissue-specific drug delivery. Cell 2020, 181, 151–167. [Google Scholar] [CrossRef]
- Kreutzfeldt, J.; Rozeboom, B.; Dey, N.; De, P. The trastuzumab era: Current and upcoming targeted HER2+ breast cancer therapies. Am. J. Cancer Res. 2020, 10, 1045. [Google Scholar]
- Chaudhari, R.; Patel, V.; Kumar, A. Cutting-edge approaches for targeted drug delivery in breast cancer: Beyond conventional therapies. Nanoscale Adv. 2024, 6, 2270–2286. [Google Scholar] [CrossRef]
- Pavlasova, G.; Mraz, M. The regulation and function of CD20: An “enigma” of B-cell biology and targeted therapy. Haematologica 2020, 105, 1494. [Google Scholar] [CrossRef]
- Parvez, A.; Choudhary, F.; Mudgal, P.; Khan, R.; Qureshi, K.A.; Farooqi, H.; Aspatwar, A. PD-1 and PD-L1: Architects of immune symphony and immunotherapy breakthroughs in cancer treatment. Front. Immunol. 2023, 14, 1296341. [Google Scholar] [CrossRef]
- Butt, L. Measuring Markers of Immune Response in Patients Treated with Nivolumab (Opdivo®) and Pembrolizumab (Keytruda®). Diploma Thesis, University of Otago, Dunedin, New Zealand, 2017. [Google Scholar]
- Shiravand, Y.; Khodadadi, F.; Kashani, S.M.A.; Hosseini-Fard, S.R.; Hosseini, S.; Sadeghirad, H.; Ladwa, R.; O’Byrne, K.; Kulasinghe, A. Immune checkpoint inhibitors in cancer therapy. Curr. Oncol. 2022, 29, 3044–3060. [Google Scholar] [CrossRef]
- Makuku, R.; Khalili, N.; Razi, S.; Keshavarz-Fathi, M.; Rezaei, N. Current and future perspectives of PD-1/PDL-1 blockade in cancer immunotherapy. J. Immunol. Res. 2021, 2021, 6661406. [Google Scholar] [CrossRef]
- Yan, T.; Zhu, L.; Chen, J. Current advances and challenges in CAR T-Cell therapy for solid tumors: Tumor-associated antigens and the tumor microenvironment. Exp. Hematol. Oncol. 2023, 12, 14. [Google Scholar] [CrossRef]
- Zhang, T.; Lin, Y.; Gao, Q. Bispecific antibodies targeting immunomodulatory checkpoints for cancer therapy. Cancer Biol. Med. 2023, 20, 181–195. [Google Scholar] [CrossRef]
- van de Donk, N.W.; Zweegman, S. T-cell-engaging bispecific antibodies in cancer. Lancet 2023, 402, 142–158. [Google Scholar] [CrossRef]
- Marei, H.E.; Cenciarelli, C.; Hasan, A. Potential of antibody–drug conjugates (ADCs) for cancer therapy. Cancer Cell Int. 2022, 22, 255. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Bordeau, B.M.; Balthasar, J.P. Mechanisms of ADC toxicity and strategies to increase ADC tolerability. Cancers 2023, 15, 713. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H. The biology of neutralizing antibody therapy with REGEN-COV. In Features, Transmission, Detection, and Case Studies in COVID-19; Elsevier: Amsterdam, The Netherlands, 2024; pp. 415–426. [Google Scholar]
- Singh, D.D.; Sharma, A.; Lee, H.-J.; Yadav, D.K. SARS-CoV-2: Recent variants and clinical efficacy of antibody-based therapy. Front. Cell. Infect. Microbiol. 2022, 12, 839170. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.; Peacock, T.P.; Harvey, W.T.; Hughes, J.; Wright, D.W.; Consortium, C.-G.U.; Willett, B.J.; Thomson, E.; Gupta, R.K.; Peacock, S.J. SARS-CoV-2 variant evasion of monoclonal antibodies based on in vitro studies. Nat. Rev. Microbiol. 2023, 21, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Tripathi, P.; Kumar, P.; Shekhar, R.; Pathak, R. From detection to protection: Antibodies and their crucial role in diagnosing and combatting SARS-CoV-2. Vaccines 2024, 12, 459. [Google Scholar] [CrossRef]
- Mohammed, A.; Zeng, W.; Mengist, H.M.; Kombe, A.J.K.; Ou, H.; Yang, Y.; Dan, Z.; Xu, Z.; Ma, H.; Jin, T. Generation, biochemical characterizations and validation of potent nanobodies derived from alpaca specific for human receptor of advanced glycation end product. Biochem. Biophys. Res. Commun. 2021, 581, 38–45. [Google Scholar] [CrossRef]
- Ma, H.; Zeng, W.; Meng, X.; Huang, X.; Yang, Y.; Zhao, D.; Zhou, P.; Wang, X.; Zhao, C.; Sun, Y. Potent neutralization of SARS-CoV-2 by hetero-bivalent alpaca nanobodies targeting the spike receptor-binding domain. J. Virol. 2021, 95, e02185-18. [Google Scholar] [CrossRef]
- Huo, J.; Mikolajek, H.; Le Bas, A.; Clark, J.J.; Sharma, P.; Kipar, A.; Dormon, J.; Norman, C.; Weckener, M.; Clare, D.K. A potent SARS-CoV-2 neutralising nanobody shows therapeutic efficacy in the Syrian golden hamster model of COVID-19. Nat. Commun. 2021, 12, 5469. [Google Scholar] [CrossRef]
- Ye, G.; Gallant, J.P.; Massey, C.; Shi, K.; Tai, W.; Zheng, J.; Odle, A.E.; Vickers, M.A.; Shang, J.; Wan, Y. The development of a novel nanobody therapeutic for SARS-CoV-2. BioRxiv 2020. preprint. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, X.; Zeng, W.; Zhou, J.; Chi, X.; Chen, S.; Zheng, P.; Wang, M.; Wu, Y.; Zhao, D. A bispecific nanobody dimer broadly neutralizes SARS-CoV-1 & 2 variants of concern and offers substantial protection against Omicron via low-dose intranasal administration. Cell Discov. 2022, 8, 132. [Google Scholar] [PubMed]
- Olga, P.; Yu, L.D. Nanobodies are potential therapeutic agents for the Ebola virus infection. Acta Naturae (aнглoязычная версия) 2021, 13, 53–63. [Google Scholar]
- Jin, H.; Tang, X.; Li, L.; Chen, Y.; Zhu, Y.; Chong, H.; He, Y. Generation of HIV-resistant cells with a single-domain antibody: Implications for HIV-1 gene therapy. Cell. Mol. Immunol. 2021, 18, 660–674. [Google Scholar] [CrossRef]
- Duan, Q.; Ai, T.; Ma, Y.; Li, R.; Jin, H.; Chen, X.; Zhang, R.; Bao, K.; Chen, Q. Research Progress on the Application of Neutralizing Nanobodies in the Prevention and Treatment of Viral Infections. Microorganisms 2025, 13, 1352. [Google Scholar] [CrossRef]
- Mahomed, S. Broadly neutralizing antibodies for HIV prevention: A comprehensive review and future perspectives. Clin. Microbiol. Rev. 2024, 37, e00152-22. [Google Scholar] [CrossRef]
- Cunningham, S.; Piedra, P.A.; Martinon-Torres, F.; Szymanski, H.; Brackeva, B.; Dombrecht, E.; Detalle, L.; Fleurinck, C.; Piedra, P.A.; Verhulst, S. Nebulised ALX-0171 for respiratory syncytial virus lower respiratory tract infection in hospitalised children: A double-blind, randomised, placebo-controlled, phase 2b trial. Lancet Respir. Med. 2021, 9, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Sinha, N.; Raja, A. Nanoscale warriors against viral invaders: A comprehensive review of Nanobodies as potential antiviral therapeutics. mAbs 2025, 17, 2486390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, N.; Xiao, H.; Wang, C.; He, L. Small antibodies with big applications: Nanobody-based cancer diagnostics and therapeutics. Cancers 2023, 15, 5639. [Google Scholar] [CrossRef]
- Abdolvahab, M.H.; Karimi, P.; Mohajeri, N.; Abedini, M.; Zare, H. Targeted drug delivery using nanobodies to deliver effective molecules to breast cancer cells: The most attractive application of nanobodies. Cancer Cell Int. 2024, 24, 67. [Google Scholar] [CrossRef]
- Zeven, K.; Lauwers, Y.; De Mey, L.; Debacker, J.M.; De Pauw, T.; De Groof, T.W.; Devoogdt, N. Advancements in nuclear imaging using radiolabeled nanobody tracers to support cancer immunotherapy. Immunother. Adv. 2024, 4, ltae006. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, C.; Wang, Y.; Sun, Y.; Huang, X.; Huang, M.; Xu, H.; Fan, H.; Chen, D.; Zhao, F. Dose escalation biodistribution, positron emission tomography/computed tomography imaging and dosimetry of a highly specific radionuclide-labeled non-blocking nanobody. EJNMMI Res. 2021, 11, 113. [Google Scholar] [CrossRef]
- Maksymova, L.; Pilger, Y.A.; Nuhn, L.; Van Ginderachter, J.A. Nanobodies targeting the tumor microenvironment and their formulation as nanomedicines. Mol. Cancer 2025, 24, 65. [Google Scholar] [CrossRef]
- Xia, B.; Lin, K.; Wang, X.; Chen, F.; Zhou, M.; Li, Y.; Lin, Y.; Qiao, Y.; Li, R.; Zhang, W. Nanobody-derived bispecific CAR-T cell therapy enhances the anti-tumor efficacy of T cell lymphoma treatment. Mol. Ther.-Oncolytics 2023, 30, 86–102. [Google Scholar] [CrossRef]
- Scapin, G.; Yang, X.; Prosise, W.W.; McCoy, M.; Reichert, P.; Johnston, J.M.; Kashi, R.S.; Strickland, C. Structure of full-length human anti-PD1 therapeutic IgG4 antibody pembrolizumab. Nat. Struct. Mol. Biol. 2015, 22, 953–958. [Google Scholar] [CrossRef]
- O’Leary, K.M.; Slezak, T.; Kossiakoff, A.A. Conformation-specific synthetic intrabodies modulate mTOR signaling with subcellular spatial resolution. Proc. Natl. Acad. Sci. USA 2025, 122, e2424679122. [Google Scholar] [CrossRef]
- Kaur, H.; Ghorai, S.M. Role of cytokines as immunomodulators. In Immunomodulators and Human Health; Springer, 2022; pp. 371–414. [Google Scholar]
- Aloi, N.; Drago, G.; Ruggieri, S.; Cibella, F.; Colombo, P.; Longo, V. Extracellular vesicles and immunity: At the crossroads of cell communication. Int. J. Mol. Sci. 2024, 25, 1205. [Google Scholar] [CrossRef]
- Abraha, R. Review on the role and biology of cytokines in adaptive and innate immune system. Arch Vet Anim Sci 2020, 2, 2. [Google Scholar]
- Laha, D.; Grant, R.; Mishra, P.; Nilubol, N. The role of tumor necrosis factor in manipulating the immunological response of tumor microenvironment. Front. Immunol. 2021, 12, 656908. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-h.; Wang, Y.-n.; Chang, Q.-y.; Ma, P.; Hu, Y.; Cao, X. Type III interferons in viral infection and antiviral immunity. Cell. Physiol. Biochem. 2018, 51, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Sokol, C.L.; Luster, A.D. The chemokine system in innate immunity. Cold Spring Harb. Perspect. Biol. 2015, 7, a016303. [Google Scholar] [CrossRef] [PubMed]
- Muta, K.; Krantz, S.B.; Bondurant, M.C.; Wickrema, A. Distinct roles of erythropoietin, insulin-like growth factor I, and stem cell factor in the development of erythroid progenitor cells. J. Clin. Investig. 1994, 94, 34–43. [Google Scholar] [CrossRef]
- Yadav, P.; Chandra, V.; Raghuvanshi, V.; Yadav, A.; Yadav, A.; Ali, S.; Tripathi, V.M. Interferons as a Potential Therapeutic Drug for COVID-19: A Literature Review of Mechanisms, Current Clinical Trials, and Challenges. J. Community Med. Health Solut. 2023, 4, 48–56. [Google Scholar]
- Song, K. Current development status of cytokines for cancer immunotherapy. Biomol. Ther. 2024, 32, 13. [Google Scholar] [CrossRef]
- Miller, C.H.; Maher, S.G.; Young, H.A. Clinical use of interferon-γ. Ann. N. Y. Acad. Sci. 2009, 1182, 69–79. [Google Scholar] [CrossRef]
- Jin, T.; Yin, Q. Structural Immunology; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Reddy Chichili, V.P.; Kumar, V.; Sivaraman, J. Linkers in the structural biology of protein–protein interactions. Protein Sci. 2013, 22, 153–167. [Google Scholar] [CrossRef]
- Manghwar, H.; Li, B.; Ding, X.; Hussain, A.; Lindsey, K.; Zhang, X.; Jin, S. CRISPR/Cas systems in genome editing: Methodologies and tools for sgRNA design, off-target evaluation, and strategies to mitigate off-target effects. Adv. Sci. 2020, 7, 1902312. [Google Scholar] [CrossRef]
- Mustafa, M.I.; Alzebair, A.A.; Mohammed, A. Development of recombinant antibody by yeast surface display technology. Curr. Res. Pharmacol. Drug Discov. 2024, 6, 100174. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, M.I.; Mohammed, A. Developing recombinant antibodies by phage display technology to neutralize viral infectious diseases. SLAS Discov. 2024, 29, 100140. [Google Scholar] [CrossRef] [PubMed]
- Kathirvel, I.; Gayathri Ganesan, N. Computational Strategies to Enhance Cell-Free Protein Synthesis Efficiency. BioMedInformatics 2024, 4, 2022–2042. [Google Scholar] [CrossRef]
- Goshisht, M.K. Machine learning and deep learning in synthetic biology: Key architectures, applications, and challenges. ACS Omega 2024, 9, 9921–9945. [Google Scholar] [CrossRef]
- Pardon, E.; Laeremans, T.; Triest, S.; Rasmussen, S.G.; Wohlkönig, A.; Ruf, A.; Muyldermans, S.; Hol, W.G.; Kobilka, B.K.; Steyaert, J. A general protocol for the generation of Nanobodies for structural biology. Nat. Protoc. 2014, 9, 674–693. [Google Scholar] [CrossRef]
- Chandrashekar, M.; Maralappanavar, M.; Patil, P.; Patil, N.; Sivaram, A. Selection, Screening, and Analysis of Recombinant Clones. In A Complete Guide to Gene Cloning: From Basic to Advanced; Springer: Berlin/Heidelberg, Germany, 2022; pp. 97–117. [Google Scholar]
- Datta, N. Unlocking the Power of Molecular Cloning: Revolutionizing Medical Microbiology Procedures. Univ. Mich. Undergrad. Res. J. 2024, 17, e5509. [Google Scholar] [CrossRef]
- Liu, M.; Xiao, R.; Li, X.; Zhao, Y.; Huang, J. A comprehensive review of recombinant technology in the food industry: Exploring expression systems, application, and future challenges. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70078. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Li, T.; Xue, W.; Zhang, S.; Cui, L.; Wang, H.; Zhang, Y.; Zhou, L.; Gu, Y.; Xia, N. Genetic engineering of baculovirus-insect cell system to improve protein production. Front. Bioeng. Biotechnol. 2022, 10, 994743. [Google Scholar] [CrossRef]
- Nora, L.C.; Westmann, C.A.; Martins-Santana, L.; Alves, L.d.F.; Monteiro, L.M.O.; Guazzaroni, M.E.; Silva-Rocha, R. The art of vector engineering: Towards the construction of next-generation genetic tools. Microb. Biotechnol. 2019, 12, 125–147. [Google Scholar] [CrossRef]
- Jin, T.; Chuenchor, W.; Jiang, J.; Cheng, J.; Li, Y.; Fang, K.; Huang, M.; Smith, P.; Xiao, T.S. Design of an expression system to enhance MBP-mediated crystallization. Sci. Rep. 2017, 7, 40991. [Google Scholar] [CrossRef]
- Feng, Y.; Xu, Q.; Yang, T.; Sun, E.; Li, J.; Shi, D.; Wu, D. A novel self-cleavage system for production of soluble recombinant protein in Escherichia coli. Protein Expr. Purif. 2014, 99, 64–69. [Google Scholar] [CrossRef]
- Butt, M.H.; Zaman, M.; Ahmad, A.; Khan, R.; Mallhi, T.H.; Hasan, M.M.; Khan, Y.H.; Hafeez, S.; Massoud, E.E.S.; Rahman, M.H. Appraisal for the potential of viral and nonviral vectors in gene therapy: A review. Genes 2022, 13, 1370. [Google Scholar] [CrossRef]
- Fang, Y.; Chen, X.; Sun, Z.; Yan, X.; Shi, L.; Jin, C. Discovery and investigation of the truncation of the (GGGGS) n linker and its effect on the productivity of bispecific antibodies expressed in mammalian cells. Bioprocess Biosyst. Eng. 2025, 48, 159–170. [Google Scholar] [CrossRef]
- Chatrdooz, H.; Sargolzaei, J. An Overview of Property, Design, and Functionality of Linkers for Fusion Protein Construction. Proteins Struct. Funct. Bioinform. 2025, 93, 1411–1425. [Google Scholar] [CrossRef]
- Chen, X.; Zaro, J.L.; Shen, W.-C. Fusion protein linkers: Property, design and functionality. Adv. Drug Deliv. Rev. 2013, 65, 1357–1369. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Huang, Z.; Zhang, C.; Dong, B.-J.; Guo, R.-H.; Yue, H.-W.; Yan, L.-T.; Xing, X.-H. Construction of a linker library with widely controllable flexibility for fusion protein design. Appl. Microbiol. Biotechnol. 2016, 100, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Quek, J.-P.; Ser, Z.; Chew, B.L.A.; Li, X.; Wang, L.; Sobota, R.M.; Luo, D.; Phoo, W.W. Dynamic interactions of post cleaved NS2B cofactor and NS3 protease identified by integrative structural approaches. Viruses 2022, 14, 1440. [Google Scholar] [CrossRef] [PubMed]
- Peyret, H.; Gehin, A.; Thuenemann, E.C.; Blond, D.; El Turabi, A.; Beales, L.; Clarke, D.; Gilbert, R.J.; Fry, E.E.; Stuart, D.I. Tandem fusion of hepatitis B core antigen allows assembly of virus-like particles in bacteria and plants with enhanced capacity to accommodate foreign proteins. PLoS ONE 2015, 10, e0120751. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Feng, Y.; Yuan, Q. Improving trehalose synthase activity by adding the C-terminal domain of trehalose synthase from Thermus thermophilus. Bioresour. Technol. 2017, 245, 1749–1756. [Google Scholar] [CrossRef]
- Hekmat, D. Large-scale crystallization of proteins for purification and formulation. Bioprocess Biosyst. Eng. 2015, 38, 1209–1231. [Google Scholar] [CrossRef]
- Bisswanger, H. Enzyme assays. Perspect. Sci. 2014, 1, 41–55. [Google Scholar] [CrossRef]
- Iacobucci, I.; Monaco, V.; Cozzolino, F.; Monti, M. From classical to new generation approaches: An excursus of-omics methods for investigation of protein-protein interaction networks. J. Proteom. 2021, 230, 103990. [Google Scholar] [CrossRef] [PubMed]
- Banari, A.; Samanta, A.K.; Munke, A.; Laugks, T.; Bajt, S.; Grünewald, K.; Marlovits, T.C.; Küpper, J.; Maia, F.R.; Chapman, H.N. Advancing time-resolved structural biology: Latest strategies in cryo-EM and X-ray crystallography. Nat. Methods 2025, 22, 1420–1435. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, M.; Rai, V.; Kumar, A.; Kiran; Yadav, A.K.; Rajak, K.K.; Gupta, V.; Chander, V.; Avasthe, R. SDS-PAGE and western blotting: Basic principles and protocol. In Protocols for the Diagnosis of Pig Viral Diseases; Springer: Berlin/Heidelberg, Germany, 2022; pp. 313–328. [Google Scholar]
- Smith, J.P.; Ralbovsky, N.M.; Lauro, M.L.; Hoyt, E.; Guetschow, E.D.; Wang, F.; McIntosh, J.A.; Liu, Z.; Mangion, I.; Variankaval, N. Quantitation and speciation of residual protein within active pharmaceutical ingredients using image analysis with SDS-PAGE. J. Pharm. Biomed. Anal. 2022, 207, 114393. [Google Scholar] [CrossRef]
- Kang, C.-C.; Yamauchi, K.A.; Vlassakis, J.; Sinkala, E.; Duncombe, T.A.; Herr, A.E. Single cell–resolution western blotting. Nat. Protoc. 2016, 11, 1508–1530. [Google Scholar] [CrossRef]
- Bruce, C.; Stone, K.; Gulcicek, E.; Williams, K. Proteomics and the analysis of proteomic data: 2013 overview of current protein-profiling technologies. Curr. Protoc. Bioinform. 2013, 41, 13.21.11–13.21.17. [Google Scholar] [CrossRef]
- Li, C.H.; Nguyen, X.; Narhi, L.; Chemmalil, L.; Towers, E.; Muzammil, S.; Gabrielson, J.; Jiang, Y. Applications of circular dichroism (CD) for structural analysis of proteins: Qualification of near-and far-UV CD for protein higher order structural analysis. J. Pharm. Sci. 2011, 100, 4642–4654. [Google Scholar] [CrossRef]
- Cavanagh, J. Protein NMR Spectroscopy: Principles and Practice; Academic Press: Cambridge, MA, USA, 1996. [Google Scholar]
- Wüthrich, K. NMR studies of structure and function of biological macromolecules (Nobel Lecture). Angew. Chem. Int. Ed. 2003, 42, 3340–3363. [Google Scholar] [CrossRef]
- Glusker, J.P.; Trueblood, K.N. Crystal Structure Analysis: A Primer; Oxford University Press: Oxford, UK, 2010; Volume 14. [Google Scholar]
- Senisterra, G.; Chau, I.; Vedadi, M. Thermal denaturation assays in chemical biology. Assay Drug Dev. Technol. 2012, 10, 128–136. [Google Scholar] [CrossRef]
- Kazlauskas, E.; Petrauskas, V.; Paketurytė, V.; Matulis, D. Standard operating procedure for fluorescent thermal shift assay (FTSA) for determination of protein–ligand binding and protein stability. Eur. Biophys. J. 2021, 50, 373–379. [Google Scholar] [CrossRef]
- Douzi, B. Protein–protein interactions: Surface plasmon resonance. In Bacterial Protein Secretion Systems: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2017; pp. 257–275. [Google Scholar]
- Xu, W.; Meng, D.; Li, M.; Wang, X.; Xu, C.; Zhang, Y.; Lu, D.; Ren, R. Recent Advances in the Quantitative Determination of Protein Receptor–Ligand Interaction Kinetics. Crit. Rev. Anal. Chem. 2024, 55, 1723–1734. [Google Scholar] [CrossRef] [PubMed]
- Perozzo, R.; Folkers, G.; Scapozza, L. Thermodynamics of protein–ligand interactions: History, presence, and future aspects. J. Recept. Signal Transduct. 2004, 24, 1–52. [Google Scholar] [CrossRef]
- Piper, S.J.; Johnson, R.M.; Wootten, D.; Sexton, P.M. Membranes under the magnetic lens: A dive into the diverse world of membrane protein structures using Cryo-EM. Chem. Rev. 2022, 122, 13989–14017. [Google Scholar] [CrossRef]
- Chua, E.Y.; Mendez, J.H.; Rapp, M.; Ilca, S.L.; Tan, Y.Z.; Maruthi, K.; Kuang, H.; Zimanyi, C.M.; Cheng, A.; Eng, E.T. Better, faster, cheaper: Recent advances in cryo–electron microscopy. Annu. Rev. Biochem. 2022, 91, 1–32. [Google Scholar] [CrossRef]
- dos Santos Rodrigues, F.H.; Delgado, G.G.; da Costa, T.S.; Tasic, L. Applications of fluorescence spectroscopy in protein conformational changes and intermolecular contacts. BBA Adv. 2023, 3, 100091. [Google Scholar] [CrossRef]
- Mocz, G.; Ross, J.A. Fluorescence techniques in analysis of protein–ligand interactions. In Protein-ligand interactions: Methods and Applications; Springer: Berlin/Heidelberg, Germany, 2013; pp. 169–210. [Google Scholar]
- Orders, M. An EUA for Bamlanivimab-A monoclonal anti-body for COVID-19. Med Lett Drugs Ther 2020, 62, 185–186. [Google Scholar]
- Ganesh, R.; Philpot, L.M.; Bierle, D.M.; Anderson, R.J.; Arndt, L.L.; Arndt, R.F.; Culbertson, T.L.; Destro Borgen, M.J.; Hanson, S.N.; Kennedy, B.D. Real-world clinical outcomes of bamlanivimab and casirivimab-imdevimab among high-risk patients with mild to moderate coronavirus disease 2019. J. Infect. Dis. 2021, 224, 1278–1286. [Google Scholar] [CrossRef]
- Deeks, E.D. Casirivimab/imdevimab: First approval. Drugs 2021, 81, 2047–2055. [Google Scholar] [CrossRef] [PubMed]
- Emens, L.A. Trastuzumab: Targeted therapy for the management of HER-2/neu-overexpressing metastatic breast cancer. Am. J. Ther. 2005, 12, 243–253. [Google Scholar] [PubMed]
- Wang, J.; Xu, B. Targeted therapeutic options and future perspectives for HER2-positive breast cancer. Signal Transduct. Target. Ther. 2019, 4, 34. [Google Scholar] [CrossRef] [PubMed]
- Kwok, G.; Yau, T.C.; Chiu, J.W.; Tse, E.; Kwong, Y.-L. Pembrolizumab (keytruda). Hum. Vaccines Immunother. 2016, 12, 2777–2789. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S. Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non–small-cell lung cancer with PD-L1 tumor proportion score≥ 50%. J. Clin. Oncol. 2021, 39, 2339–2349. [Google Scholar] [CrossRef]
- Alqahtani, S.A.; Sulkowski, M.S. The role of interferon for the treatment of chronic hepatitis C virus infection. In HCV: The Journey from Discovery to a Cure: Volume I; Springer: Cham, Switzerland, 2019; pp. 97–113. [Google Scholar]
- Crisà, E.; Cerrano, M.; Beggiato, E.; Benevolo, G.; Lanzarone, G.; Manzini, P.M.; Borchiellini, A.; Riera, L.; Boccadoro, M.; Ferrero, D. Can pegylated interferon improve the outcome of polycythemia vera patients? J. Hematol. Oncol. 2017, 10, 15. [Google Scholar] [CrossRef]
- Hasanov, E.; Diab, A.; Tannir, N. Back to interleukin 2 after four decades: Review of the history, biology, novel approaches and clinical trials. Kidney Cancer J. 2020, 18, 19–24. [Google Scholar]
- Rotte, A.; Bhandaru, M. Interleukin-2. In Immunotherapy of Melanoma; Springer: Berlin/Heidelberg, Germany, 2016; pp. 257–273. [Google Scholar]
- Miliotou, A.N.; Papadopoulou, L.C. CAR T-cell therapy: A new era in cancer immunotherapy. Curr. Pharm. Biotechnol. 2018, 19, 5–18. [Google Scholar] [CrossRef]
- Ali, S.; Kjeken, R.; Niederlaender, C.; Markey, G.; Saunders, T.S.; Opsata, M.; Moltu, K.; Bremnes, B.; Grønevik, E.; Muusse, M. The European medicines agency review of Kymriah (Tisagenlecleucel) for the treatment of acute lymphoblastic leukemia and diffuse large B-cell lymphoma. Oncologist 2020, 25, e321–e327. [Google Scholar] [CrossRef]
- Awasthi, R.; Maier, H.J.; Zhang, J.; Lim, S. Kymriah®(tisagenlecleucel)–an overview of the clinical development journey of the first approved CAR-T therapy. Hum. Vaccines Immunother. 2023, 19, 2210046. [Google Scholar] [CrossRef] [PubMed]
- Fala, L. Yescarta (Axicabtagene Ciloleucel) second CAR T-cell therapy approved for patients with certain types of large B-cell lymphoma. J. Hematol. Oncol. Pharm. 2018, 59–61. Available online: https://jhoponline.com/issues/special-issues/yescarta-axicabtagene-ciloleucel-second-car-t-cell-therapy-approved-for-patients-with-certain-types-of-large-b-cell-lymphoma (accessed on 1 August 2025).
- Hegde, M. CAR-T-Cell Therapy: Success and Advancements in the Treatment of Hematological Malignancies. In Cell-Based Immunotherapies for Cancer; Springer: Cham, Switzerland, 2025; pp. 127–159. [Google Scholar]
- Laramy, C.R.B. The CAR-T Cell Therapy Innovation Drivers: A Yescarta Case Study. Berkeley Tech. LJ 2024, 39, 553. [Google Scholar]
- Dushenkov, A.; Jungsuwadee, P. Chimeric antigen receptor T-cell therapy: Foundational science and clinical knowledge for pharmacy practice. J. Oncol. Pharm. Pract. 2019, 25, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Ong, M.Z.; Kimberly, S.A.; Lee, W.-H.; Ling, M.; Lee, M.; Tan, K.-W.; Foo, J.-B.; Yow, H.-Y.; Sellappans, R.; Hamzah, S. FDA-approved CAR T-cell Therapy: A Decade of Progress and Challenges. Curr. Pharm. Biotechnol. 2024, 25, 1377–1393. [Google Scholar] [CrossRef]
- Tanaka, Y. Ozoralizumab: First Nanobody® therapeutic for rheumatoid arthritis. Expert Opin. Biol. Ther. 2023, 23, 579–587. [Google Scholar] [CrossRef]
- Keam, S.J. Ozoralizumab: First approval. Drugs 2023, 83, 87–92. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kawanishi, M.; Nakanishi, M.; Yamasaki, H.; Takeuchi, T. Efficacy and safety of the anti-TNF multivalent NANOBODY® compound ozoralizumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: A 52-week result of a phase II/III study (OHZORA trial). Mod. Rheumatol. 2023, 33, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Peyvandi, F.; Scully, M.; Hovinga, J.K.; Knöbl, P.; Cataland, S.; De Beuf, K.; Callewaert, F.; De Winter, H.; Zeldin, R. Caplacizumab reduces the frequency of major thromboembolic events, exacerbations and death in patients with acquired thrombotic thrombocytopenic purpura. J. Thromb. Haemost. 2017, 15, 1448–1452. [Google Scholar] [CrossRef]
- Zheng, X.L. Novel mechanisms of action of emerging therapies of hereditary thrombotic thrombocytopenic purpura. Expert Rev. Hematol. 2024, 17, 341–351. [Google Scholar] [CrossRef]
- Mei, H.; Li, C.; Jiang, H.; Zhao, X.; Huang, Z.; Jin, D.; Guo, T.; Kou, H.; Liu, L.; Tang, L. A bispecific CAR-T cell therapy targeting BCMA and CD38 in relapsed or refractory multiple myeloma. J. Hematol. Oncol. 2021, 14, 161. [Google Scholar] [CrossRef]
- Alyas, J.; Rafiq, A.; Amir, H.; Khan, S.U.; Sultana, T.; Ali, A.; Hameed, A.; Ahmad, I.; Kazmi, A.; Sajid, T. Human insulin: History, recent advances, and expression systems for mass production. Biomed. Res. Ther. 2021, 8, 4540–4561. [Google Scholar] [CrossRef]
- Singh, A.K.; Carroll, K.; Perkovic, V.; Solomon, S.; Jha, V.; Johansen, K.L.; Lopes, R.D.; Macdougall, I.C.; Obrador, G.T.; Waikar, S.S. Daprodustat for the treatment of anemia in patients undergoing dialysis. N. Engl. J. Med. 2021, 385, 2325–2335. [Google Scholar] [CrossRef] [PubMed]
- Moradi, Z.; Maali, A.; Shad, J.S.; Farasat, A.; Kouchaki, R.; Moghadami, M.; Ahmadi, M.H.; Azad, M. Updates on novel erythropoiesis-stimulating agents: Clinical and molecular approach. Indian J. Hematol. Blood Transfus. 2020, 36, 26–36. [Google Scholar] [CrossRef]
- Fleischmann, R.M.; Alvarez, D.F.; Bock, A.E.; Cronenberger, C.; Vranic, I.; Zhang, W.; Alten, R. Long-term efficacy, safety, and immunogenicity of the adalimumab biosimilar, PF-06410293, in patients with rheumatoid arthritis after switching from reference adalimumab (Humira®) or continuing biosimilar therapy: Week 52–92 data from a randomized, double-blind, phase 3 trial. Arthritis Res. Ther. 2021, 23, 248. [Google Scholar]
- Torrente-López, A.; Hermosilla, J.; Salmerón-García, A.; Cabeza, J.; Navas, N. Comprehensive analysis of nivolumab, a therapeutic anti-Pd-1 monoclonal antibody: Impact of handling and stress. Pharmaceutics 2022, 14, 692. [Google Scholar] [CrossRef]
- Chitoran, E.; Rotaru, V.; Stefan, D.-C.; Gullo, G.; Simion, L. Blocking Tumoral Angiogenesis VEGF/VEGFR Pathway: Bevacizumab—20 Years of Therapeutic Success and Controversy. Cancers 2025, 17, 1126. [Google Scholar] [CrossRef] [PubMed]
- Parisi, S.; Ditto, M.C.; Ghellere, F.; Panaro, S.; Piccione, F.; Borrelli, R.; Fusaro, E. Update on tocilizumab in rheumatoid arthritis: A narrative review. Front. Immunol. 2025, 16, 1470488. [Google Scholar] [CrossRef] [PubMed]
- Inman, B.A.; Longo, T.A.; Ramalingam, S.; Harrison, M.R. Atezolizumab: A PD-L1–blocking antibody for bladder cancer. Clin. Cancer Res. 2017, 23, 1886–1890. [Google Scholar] [CrossRef]
- Phetphoung, T. Production of PD-L1 Immune Checkpoint Inhibitor in Nicotiana Benthamiana. Master’s Thesis, Chulalongkorn University, Bangkok, Thailand, 2022. [Google Scholar]
- Adams, G.P.; Weiner, L.M. Monoclonal antibody therapy of cancer. Nat. Biotechnol. 2005, 23, 1147–1157. [Google Scholar] [CrossRef]
- Shin, H.G.; Yang, H.R.; Yoon, A.; Lee, S. Bispecific antibody-based immune-cell engagers and their emerging therapeutic targets in cancer immunotherapy. Int. J. Mol. Sci. 2022, 23, 5686. [Google Scholar] [CrossRef]
- Gaballa, M.R.; Banerjee, P.; Milton, D.R.; Jiang, X.; Ganesh, C.; Khazal, S.; Nandivada, V.; Islam, S.; Kaplan, M.; Daher, M. Blinatumomab maintenance after allogeneic hematopoietic cell transplantation for B-lineage acute lymphoblastic leukemia. Blood J. Am. Soc. Hematol. 2022, 139, 1908–1919. [Google Scholar] [CrossRef] [PubMed]
- Mirfakhraie, R.; Dehaghi, B.K.; Ghorbi, M.D.; Ghaffari-Nazari, H.; Mohammadian, M.; Salimi, M.; Ardakani, M.T.; Parkhideh, S. All about blinatumomab: The bispecific T cell engager immunotherapy for B cell acute lymphoblastic leukemia. Hematol. Transfus. Cell Ther. 2024, 46, 192–200. [Google Scholar] [CrossRef]
- Halford, Z.; Anderson, M.K.; Bennett, L.L.; Moody, J. Tisagenlecleucel in acute lymphoblastic leukemia: A review of the literature and practical considerations. Ann. Pharmacother. 2021, 55, 466–479. [Google Scholar] [CrossRef]
- Sheykhhasan, M.; Manoochehri, H.; Dama, P. Use of CAR T-cell for acute lymphoblastic leukemia (ALL) treatment: A review study. Cancer Gene Ther. 2022, 29, 1080–1096. [Google Scholar] [CrossRef]
- Halford, Z.; Anderson, M.K.; Bennett, L.L. Axicabtagene ciloleucel: Clinical data for the use of CAR T-cell therapy in relapsed and refractory large B-cell lymphoma. Ann. Pharmacother. 2021, 55, 390–405. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-H.; Zheng, X.; Rao, G.-W.; Zheng, Q. Targeted small molecule therapy and inhibitors for lymphoma. Future Med. Chem. 2024, 16, 1465–1484. [Google Scholar] [CrossRef] [PubMed]
- Robak, T.; Witkowska, M.; Smolewski, P. The role of Bruton’s kinase inhibitors in chronic lymphocytic leukemia: Current status and future directions. Cancers 2022, 14, 771. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, R.; Liang, T.; Ren, H.; Park, C.; Tai, C.-H.; Ni, W.; Zhou, J.; Mackay, S.; Edmondson, E. Camel nanobody-based B7-H3 CAR-T cells show high efficacy against large solid tumours. Nat. Commun. 2023, 14, 5920. [Google Scholar] [CrossRef]
- Detalle, L.; Stohr, T.; Palomo, C.; Piedra, P.A.; Gilbert, B.E.; Mas, V.; Millar, A.; Power, U.F.; Stortelers, C.; Allosery, K. Generation and characterization of ALX-0171, a potent novel therapeutic nanobody for the treatment of respiratory syncytial virus infection. Antimicrob. Agents Chemother. 2016, 60, 6–13. [Google Scholar] [CrossRef]
- Coppo, P.; Bubenheim, M.; Benhamou, Y.; Völker, L.; Brinkkötter, P.; Kühne, L.; Knöbl, P.; Mingot-Castellano, M.E.; Pascual-Izquierdo, C.; de la Rubia, J. Caplacizumab use in immune-mediated thrombotic thrombocytopenic purpura: An international multicentre retrospective Cohort study (The Capla 1000+ project). eClinicalMedicine 2025, 82, 103168. [Google Scholar] [CrossRef]
- Arbabi-Ghahroudi, M. Camelid single-domain antibodies: Promises and challenges as lifesaving treatments. Int. J. Mol. Sci. 2022, 23, 5009. [Google Scholar] [CrossRef]
- Kazlauskas, R. Engineering more stable proteins. Chem. Soc. Rev. 2018, 47, 9026–9045. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, V.B.; Menta, A.K.; Blum, J.; Wahida, A.; Subbiah, V. AlphaFold’s Predictive Revolution in Precision Oncology. AI Precis. Oncol. 2024, 1, 160–167. [Google Scholar] [CrossRef]
- Pak, M.A.; Markhieva, K.A.; Novikova, M.S.; Petrov, D.S.; Vorobyev, I.S.; Maksimova, E.S.; Kondrashov, F.A.; Ivankov, D.N. Using AlphaFold to predict the impact of single mutations on protein stability and function. PLoS ONE 2023, 18, e0282689. [Google Scholar] [CrossRef]
- Car, E.; Barbier, L.; Huys, I.; Simoens, S.; Vulto, A.G. Evolving global regulatory landscape for approval of biosimilars: Current challenges and opportunities for convergence. Expert Opin. Biol. Ther. 2025, 25, 649–668. [Google Scholar] [CrossRef]
- Gupta, D.K.; Tiwari, A.; Yadav, Y.; Soni, P.; Joshi, M. Ensuring safety and efficacy in combination products: Regulatory challenges and best practices. Front. Med. Technol. 2024, 6, 1377443. [Google Scholar] [CrossRef]
- Scully, M.; Cataland, S.R.; Peyvandi, F.; Coppo, P.; Knöbl, P.; Kremer Hovinga, J.A.; Metjian, A.; de la Rubia, J.; Pavenski, K.; Callewaert, F. Caplacizumab treatment for acquired thrombotic thrombocytopenic purpura. N. Engl. J. Med. 2019, 380, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Cernaro, V.; Lacquaniti, A.; Buemi, A.; Lupica, R.; Buemi, M. Does erythropoietin always win? Curr. Med. Chem. 2014, 21, 849–854. [Google Scholar] [CrossRef]
- Creangă, E.-C.; Stan, R.; Nicolae, A.-C.; Drăgoi, C.M.; Dumitrescu, I.-B. Personalized Therapeutic Advances in Erythropoietin Signaling: From Anemia Management to Extensive Clinical Applications. Pharmaceutics 2025, 17, 1190. [Google Scholar] [CrossRef]
- Masloh, S.; Culot, M.; Gosselet, F.; Chevrel, A.; Scapozza, L.; Zeisser Labouebe, M. Challenges and opportunities in the oral delivery of recombinant biologics. Pharmaceutics 2023, 15, 1415. [Google Scholar] [CrossRef]
- Slastnikova, T.A.; Ulasov, A.; Rosenkranz, A.; Sobolev, A. Targeted intracellular delivery of antibodies: The state of the art. Front. Pharmacol. 2018, 9, 1208. [Google Scholar] [CrossRef]
- Zelikin, A.N.; Ehrhardt, C.; Healy, A.M. Materials and methods for delivery of biological drugs. Nat. Chem. 2016, 8, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Gupta, U.; Pranav, A.; Kohli, A.; Ghosh, S.; Singh, D. The contribution of artificial intelligence to drug discovery: Current progress and prospects for the future. In Microbial Data Intelligence and Computational Techniques for Sustainable Computing; Springer: Berlin/Heidelberg, Germany, 2024; pp. 1–23. [Google Scholar]
- Navaei, O. Personalized Medicine: Tailoring Treatment Plans Based on Genetic Profile. Eurasian J. Chem. Med. Pet. Res. 2025, 4, 129–151. [Google Scholar]
- Rishishwar, S.; Asokan, N. Innovations in Drug Delivery Systems for Biologics: Enhancing Stability and Targeted Delivery for Next-Generation Therapeutics. Chin. J. Appl. Physiol. 2025, 41, e20250001. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Malhotra, J.; Kulkarni, P.; Horne, D.; Salgia, R.; Singhal, S.S. Emerging therapeutic strategies to overcome drug resistance in cancer cells. Cancers 2024, 16, 2478. [Google Scholar] [CrossRef] [PubMed]
- Zhra, M.; Akhund, S.A.; Mohammad, K.S. Advancements in Osteosarcoma Therapy: Overcoming Chemotherapy Resistance and Exploring Novel Pharmacological Strategies. Pharmaceuticals 2025, 18, 520. [Google Scholar] [CrossRef] [PubMed]
- Eon-Duval, A.; Broly, H.; Gleixner, R. Quality attributes of recombinant therapeutic proteins: An assessment of impact on safety and efficacy as part of a quality by design development approach. Biotechnol. Prog. 2012, 28, 608–622. [Google Scholar] [CrossRef]
- Beck, A.; Wagner-Rousset, E.; Ayoub, D.; Van Dorsselaer, A.; Sanglier-Cianferani, S. Characterization of therapeutic antibodies and related products. Anal. Chem. 2013, 85, 715–736. [Google Scholar] [CrossRef]
- Jahn, E.-M.; Schneider, C.K. How to systematically evaluate immunogenicity of therapeutic proteins–regulatory considerations. New Biotechnol. 2009, 25, 280–286. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, A.; Ibrahim, N.A.; Basher, N.S. Protein Engineering and Drug Discovery: Importance, Methodologies, Challenges, and Prospects. Biomolecules 2025, 15, 1628. https://doi.org/10.3390/biom15111628
Mohammed A, Ibrahim NA, Basher NS. Protein Engineering and Drug Discovery: Importance, Methodologies, Challenges, and Prospects. Biomolecules. 2025; 15(11):1628. https://doi.org/10.3390/biom15111628
Chicago/Turabian StyleMohammed, Ahmed, Nasir A. Ibrahim, and Nosiba S. Basher. 2025. "Protein Engineering and Drug Discovery: Importance, Methodologies, Challenges, and Prospects" Biomolecules 15, no. 11: 1628. https://doi.org/10.3390/biom15111628
APA StyleMohammed, A., Ibrahim, N. A., & Basher, N. S. (2025). Protein Engineering and Drug Discovery: Importance, Methodologies, Challenges, and Prospects. Biomolecules, 15(11), 1628. https://doi.org/10.3390/biom15111628






