Nanoparticle-Based Delivery of Resveratrol Suppresses Ehrlich Ascites Carcinoma and Protects Testicular Function via Antioxidant, Anti-Angiogenic, Anti-Inflammatory, and Pro-Apoptotic Mechanisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Molecular Docking
2.2. Preparation of Resveratrol-Loaded Phytosome Nanoparticles (RES-PNPs)
2.3. Preparation and Viability Determination of EAC Cells
2.4. Animal Care and Experimental Design
- •
- Group 1 (Control): Received only vehicle (distilled water).
- •
- Group 2 (RES): Administered resveratrol (10 mg/kg body weight/day, orally) for 20 days.
- •
- Group 3 (RES-PNPs): Received resveratrol-loaded phytosome nanoparticles (10 mg/kg/day, orally) for 20 days.
- •
- Group 4 (EAC): Inoculated intraperitoneally on day 1 with 0.2 mL of EAC cell suspension containing 2.5 × 106 cells and left untreated for 20 days.
- •
- Group 5 (EAC + RES): EAC-bearing mice treated with RES (10 mg/kg/day, orally) for 20 days post-inoculation.
- •
- Group 6 (EAC + RES-PNPs): EAC-bearing mice treated with RES-PNPs (10 mg/kg/day, orally) for 20 days post-inoculation.
2.5. Survival Monitoring and Analysis
2.6. Evaluation of Tumor Growth Parameters
2.7. VEGF Analysis in Ascitic Fluid
2.8. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
2.9. Sample Collection
2.10. Hormonal Analysis
2.11. Antioxidant and Inflammatory Status
2.12. Cyclooxygenase-2 (COX-2) Activity
2.13. Epididymal Sperm Analysis
2.14. Histopathological and Ultrastructural Examination
2.15. Statistical Analysis
3. Results
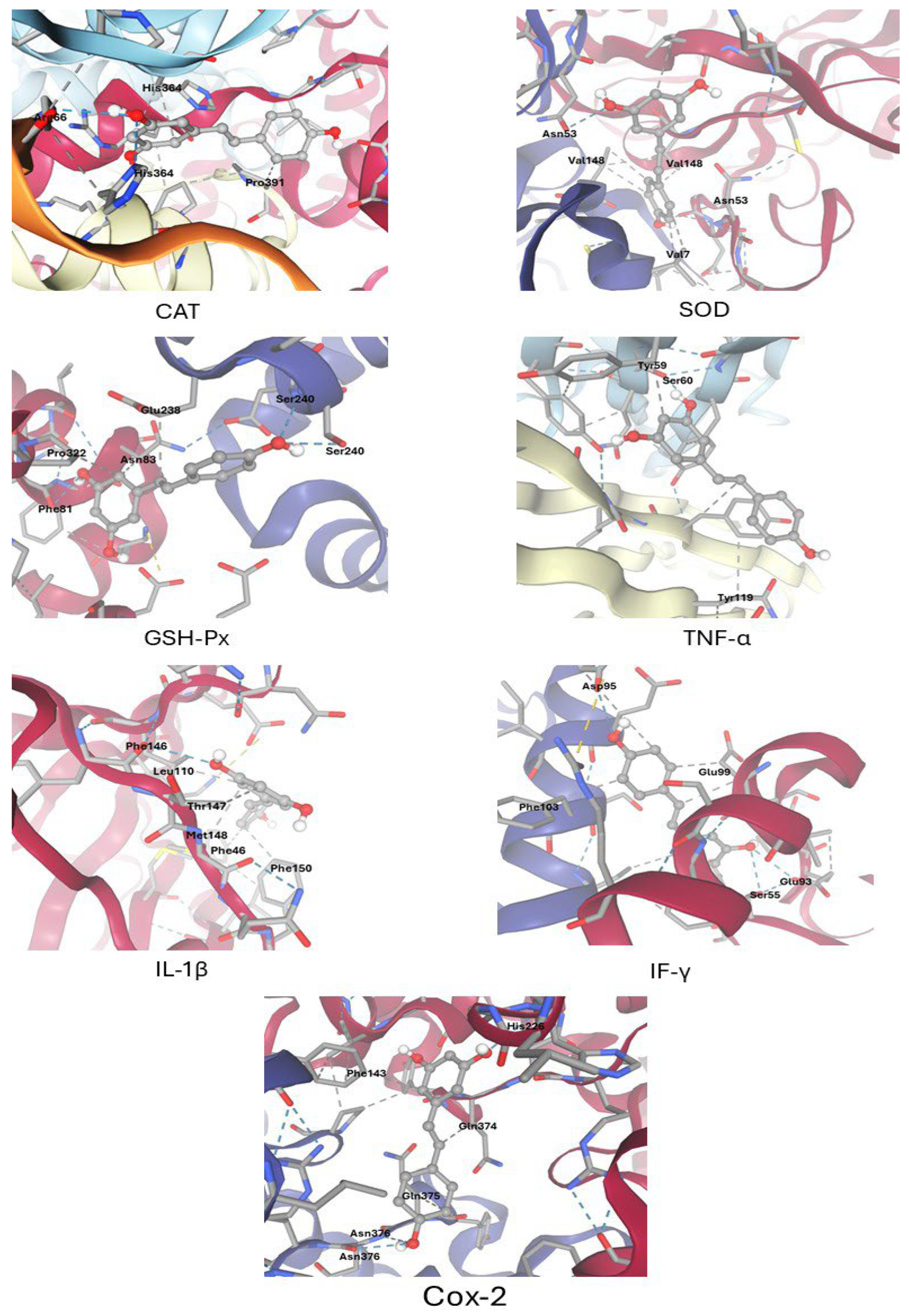
3.1. Molecular Docking Results
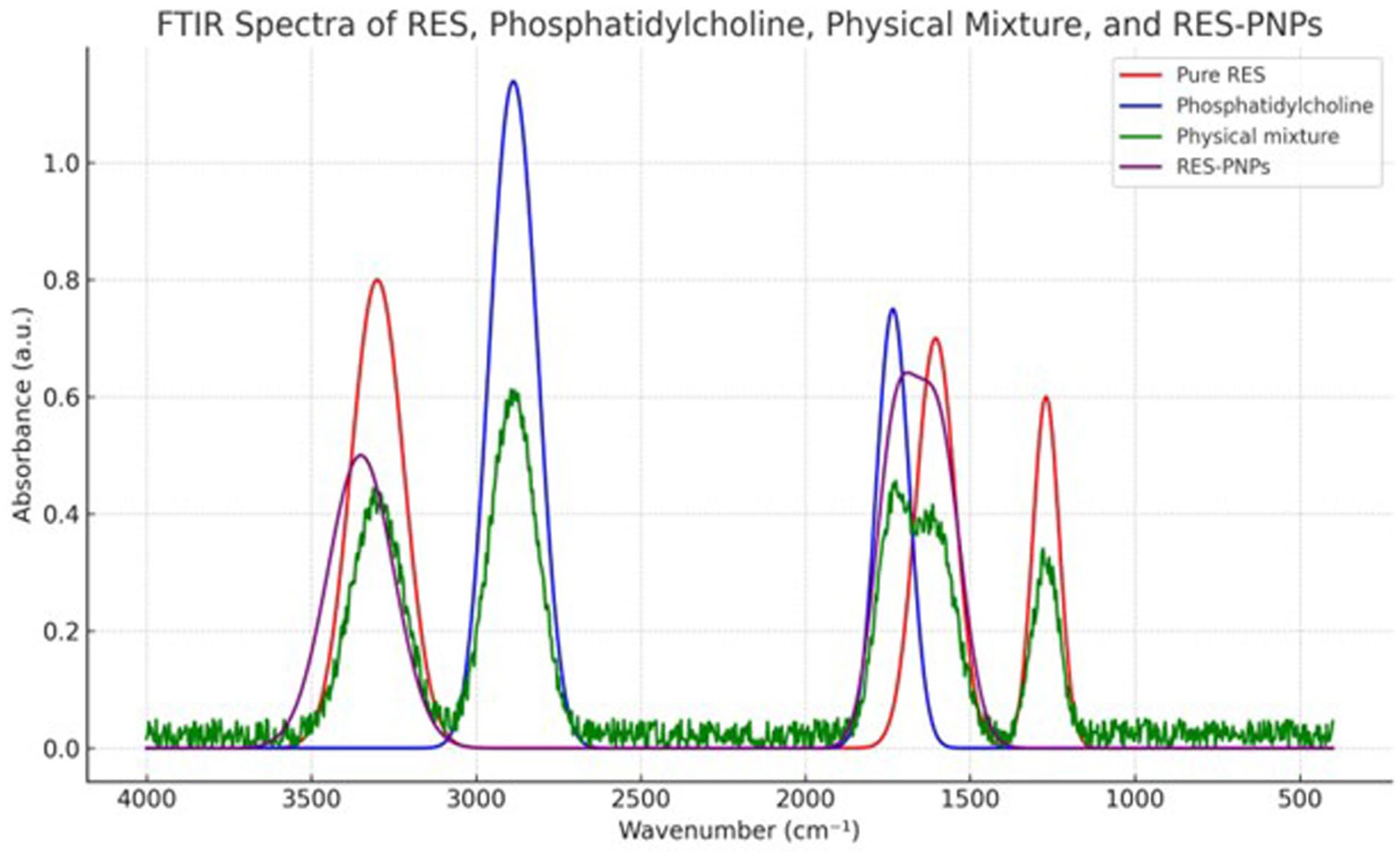
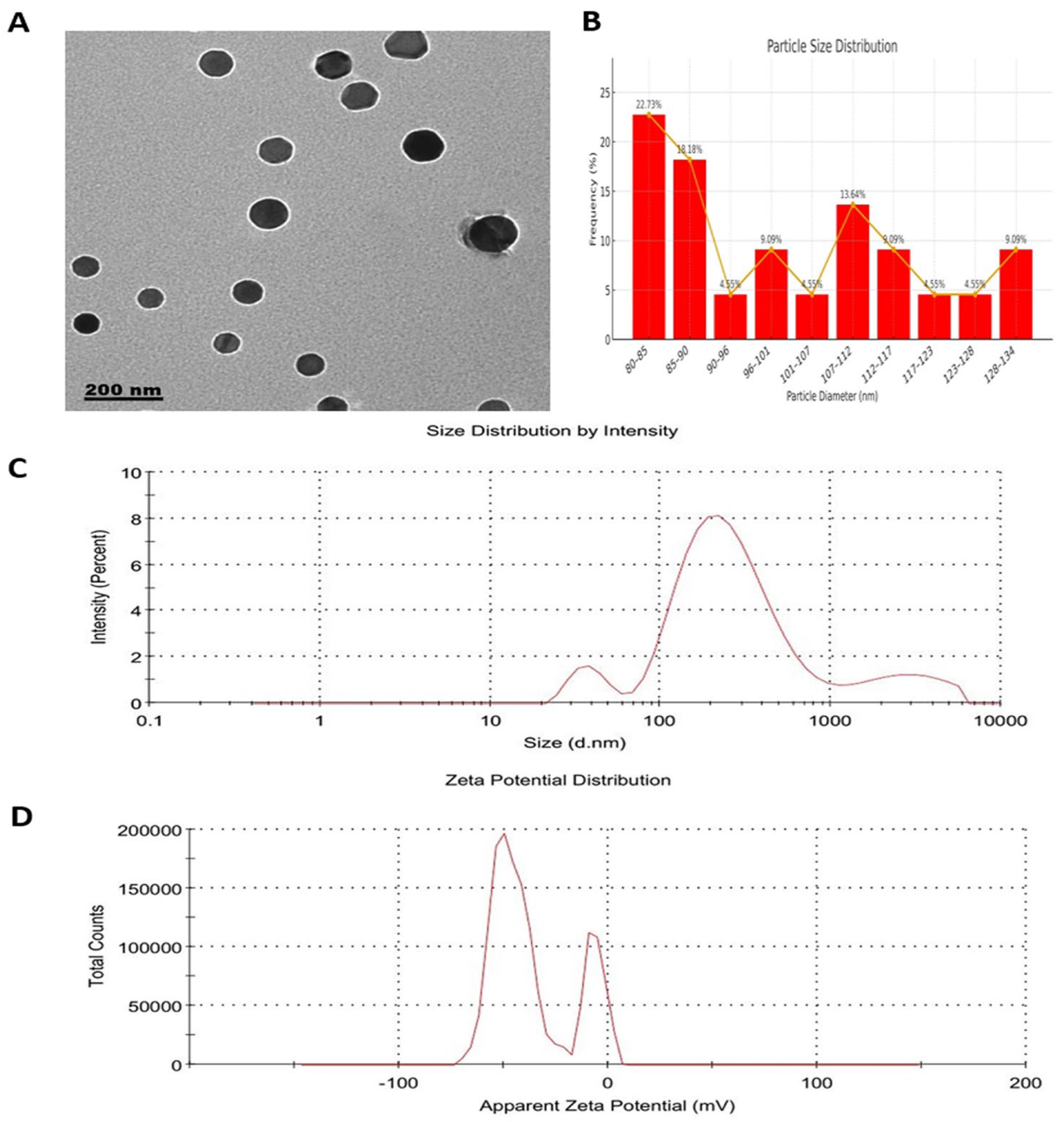
3.2. Characterization of Resveratrol-Loaded Phytosome Nanoparticles (RES-PNPs)
3.3. Changes in Tumor Growth and Survival Parameters
3.4. Expression Profiles of Apoptotic-Regulatory Genes
3.5. VEGF Levels in Ascitic Fluid
3.6. Semen Quality
3.7. Hormonal Profile
3.8. Redox Status in Testicular Tissue
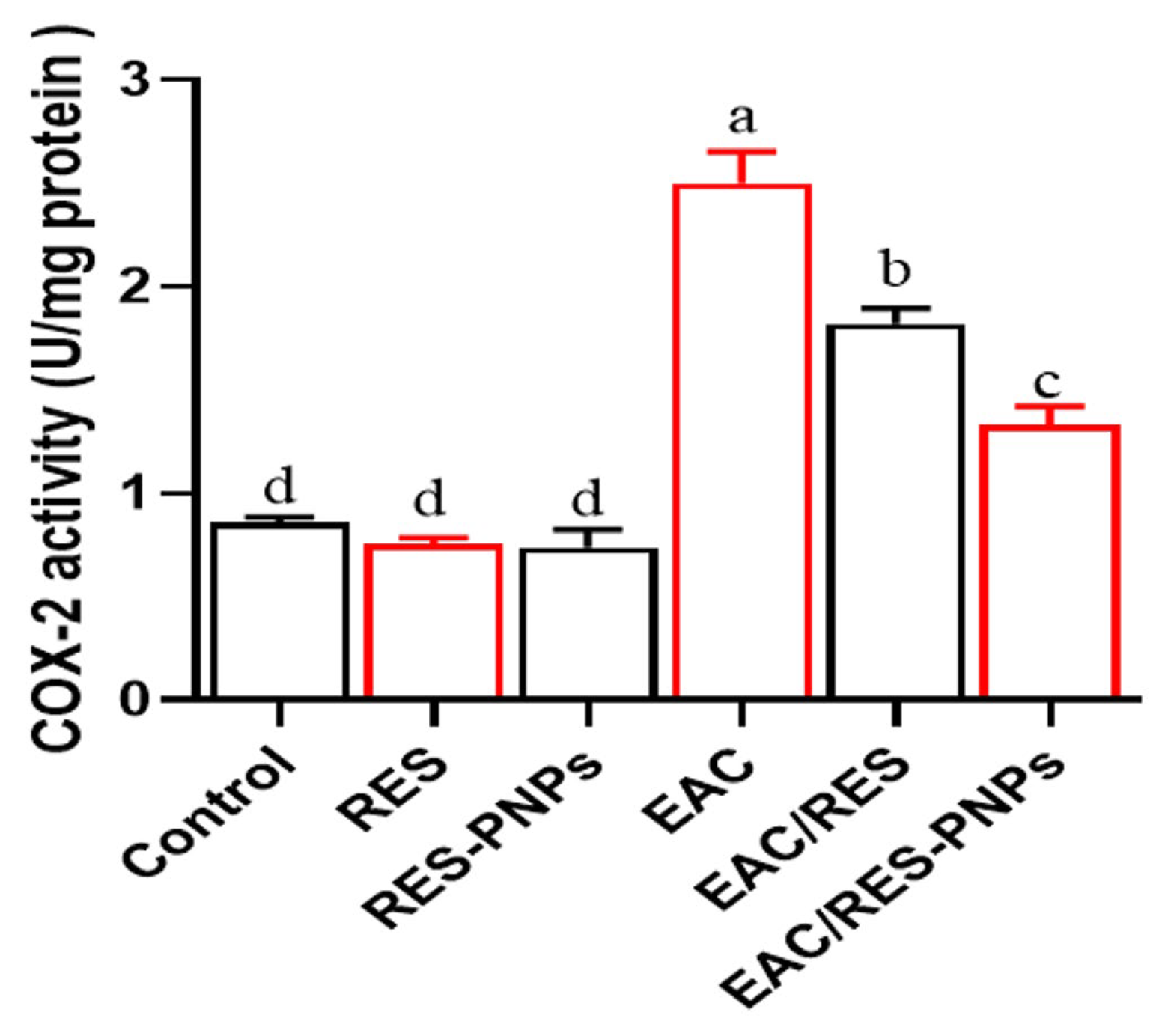
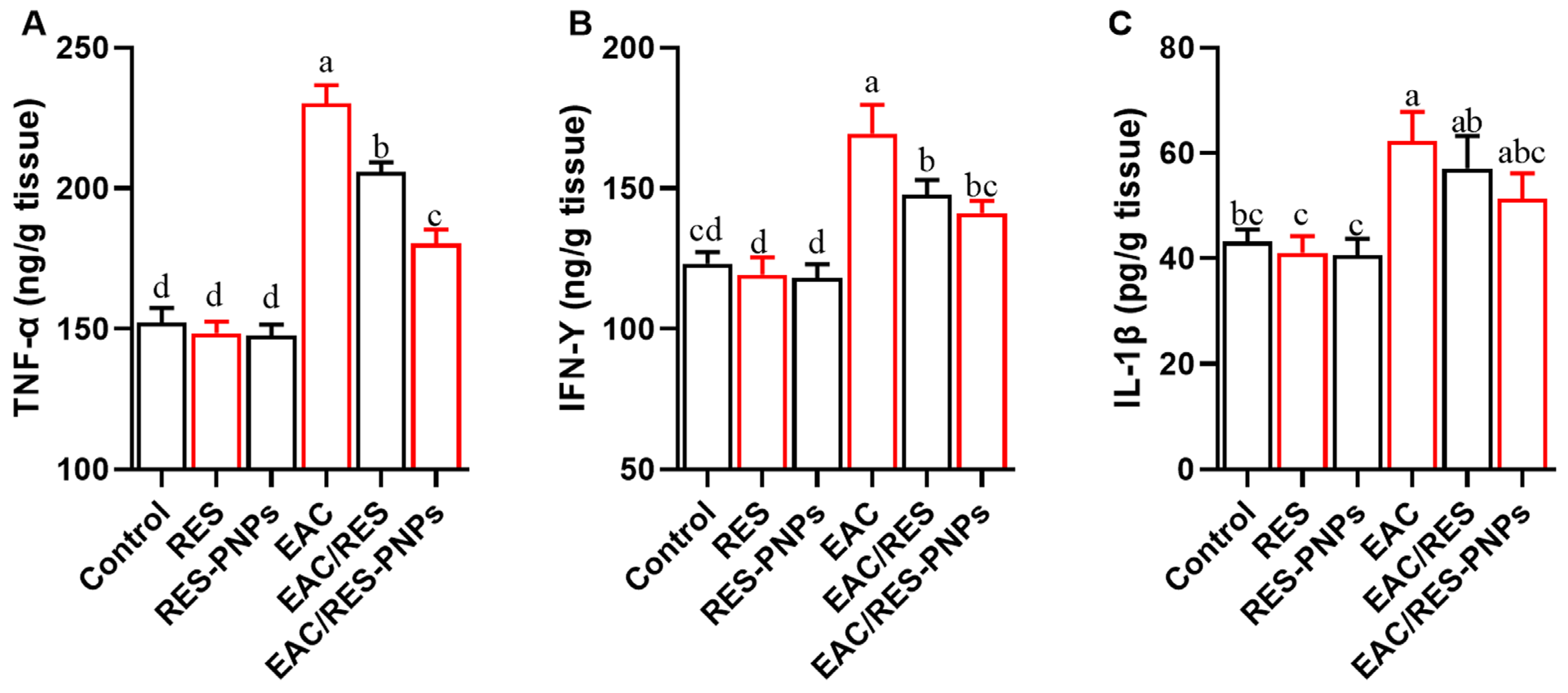
3.9. Testicular Inflammation and COX-2 Activity
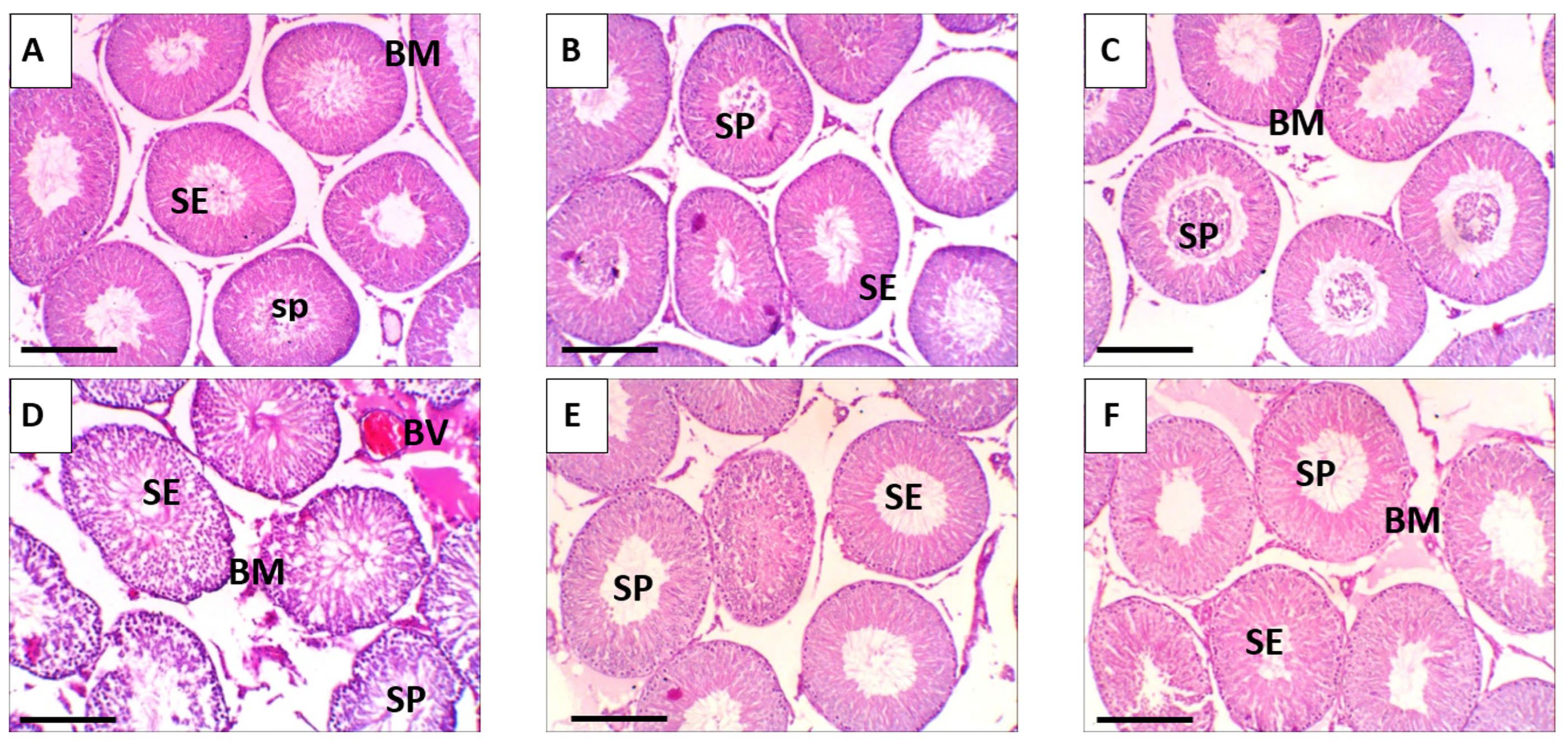
3.10. Histopathological Observations: Ultrastructural Alterations
3.11. Ultrastructural Alterations
4. Discussion
Study Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RES | Resveratrol |
| RES-PNPs | Resveratrol-loaded phytosome nanoparticles |
| EAC | Ehrlich Ascites Carcinoma |
| CAT | Catalase |
| SOD | Superoxide dismutase |
| GSH-Px | Glutathione peroxidase |
| TNF-α | Tumor necrosis factor-alpha |
| IL-1β | Interleukin-1 beta |
| IFN-γ | Interferon-gamma |
| COX-2 | Cyclooxygenase-2 |
| TEM | Transmission electron microscopy |
| DLS | Dynamic light scattering |
| PDI | Polydispersity index |
| EE or EE% | Encapsulation efficiency |
| DMSO | Dimethyl sulfoxide |
| PBS | Phosphate-buffered saline |
| MST | Mean survival time |
| ILS% | Percentage increase in lifespan |
| VEGF | Vascular endothelial growth factor |
| qRT-PCR | (Quantitative) real-time polymerase chain reaction |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| LH | Luteinizing hormone |
| FSH | Follicle-stimulating hormone |
| GSH | Glutathione |
| MDA | Malondialdehyde |
| PC | Protein carbonyl |
| FTIR | Fourier-transform infrared spectroscopy |
| KBr | Potassium bromide |
| SD | Standard deviation |
| SE | Standard error |
| ANOVA | Analysis of variance |
| H&E | Hematoxylin and eosin (stain) |
| MAPK | Mitogen-activated protein kinase |
| PI3K | Phosphoinositide 3-kinase |
| Akt | Protein kinase B (Akt) |
| mTOR | Mammalian target of rapamycin |
| AMPK | AMP-activated protein kinase |
| SIRT-1 | Sirtuin 1 |
| N/A | Not applicable |
| SEM | Standard error of the mean |
| HPLC | High-performance liquid chromatography |
References
- Bhat, G.R.; Sethi, I.; Sadida, H.Q.; Rah, B.; Mir, R.; Algehainy, N.; Albalawi, I.A.; Masoodi, T.; Subbaraj, G.K.; Jamal, F.; et al. Cancer cell plasticity: From cellular, molecular, and genetic mechanisms to tumor heterogeneity and drug resistance. Cancer Metastasis Rev. 2024, 43, 197–228. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Al-Ostoot, F.H.; Salah, S.; Khamees, H.A.; Khanum, S.A. Tumor angiogenesis: Current challenges and therapeutic opportunities. Cancer Treat. Res. Commun. 2021, 28, 100422. [Google Scholar] [CrossRef]
- Ghalehbandi, S.; Yuzugulen, J.; Pranjol, M.Z.I.; Pourgholami, M.H. The role of VEGF in cancer-induced angiogenesis and research progress of drugs targeting VEGF. Eur. J. Pharmacol. 2023, 949, 175586. [Google Scholar] [CrossRef]
- He, J.; Zhang, H.P. Research progress and treatment status of malignant ascites. Front. Oncol. 2024, 14, 1390426. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.M.; Preusser, M.; Berghoff, A.S.; Bergen, E.S. Malignant ascites: Current therapy options and treatment prospects. Cancer Treat. Rev. 2023, 121, 102646. [Google Scholar] [CrossRef] [PubMed]
- Radulski, D.R.; Stipp, M.C.; Galindo, C.M.; Acco, A. Features and applications of Ehrlich tumor model in cancer studies: A literature review. Transl. Breast Cancer Res. A J. Focus. Transl. Res. Breast Cancer 2023, 4, 22. [Google Scholar] [CrossRef]
- Qari, M.; Harakeh, S.; Akefe, I.O.; Saber, S.H.; Al-Raddadi, R.; Abd Elmageed, Z.Y.; Alamri, T.; El-Shitany, N.; Ali, S.S.; Almuhayawi, M.S.; et al. Pomegranate nanoparticle mitigates cisplatin-induced testicular toxicity and improves cisplatin anti-cancer efficacy in Ehrlich carcinoma model. J. King Saud. Univ. Sci. 2023, 35, 102631. [Google Scholar] [CrossRef]
- Yilmaz, S.; Göçmen, Y.; Tokpınar, A.; Ateş, Ş.; Nisari, M.; Caniklioğlu, A.; Öztekin, Ü. Effect of Curcumin on Testis in Mice with Ehrlich Ascites Tumor. New Trends Med. Sci. 2025, 1, 53–58. [Google Scholar]
- Abosharaf, H.A.; Gebreel, D.; Allam, S.; El-Atrash, A.; Tousson, E. Ehrlich ascites carcinoma provokes renal toxicity and DNA injury in mice: Therapeutic impact of chitosan and maitake nanoparticles. Basic. Clin. Pharmacol. Toxicol. 2024, 134, 472–484. [Google Scholar] [CrossRef]
- Zafar, A.; Khatoon, S.; Khan, M.J.; Abu, J.; Naeem, A. Advancements and limitations in traditional anti-cancer therapies: A comprehensive review of surgery, chemotherapy, radiation therapy, and hormonal therapy. Discov. Oncol. 2025, 16, 607. [Google Scholar] [CrossRef] [PubMed]
- Sflakidou, E.; Leonidis, G.; Foroglou, E.; Siokatas, C.; Sarli, V. Recent Advances in Natural Product-Based Hybrids as Anti-Cancer Agents. Molecules 2022, 27, 6632. [Google Scholar] [CrossRef] [PubMed]
- Alfawaz, M.; Elmorsy, E.M.; Samy, A.; Shams, A.S.; Salem, M.A.; Shaalan, A.A.M.; Fawzy, M.S.; Hosny, N. Therapeutic Potential of Food-Derived Rutin Phytosome Nanoparticles: Anti-Tumor, Antioxidant, and Anti-Inflammatory Activity in Ehrlich Ascites Carcinoma. Pharmaceuticals 2025, 18, 1410. [Google Scholar] [CrossRef] [PubMed]
- Bejenaru, L.E.; Biţă, A.; Belu, I.; Segneanu, A.-E.; Radu, A.; Dumitru, A.; Ciocîlteu, M.V.; Mogoşanu, G.D.; Bejenaru, C. Resveratrol: A Review on the Biological Activity and Applications. Appl. Sci. 2024, 14, 4534. [Google Scholar] [CrossRef]
- Vernousfaderani, E.K.; Akhtari, N.; Rezaei, S.; Rezaee, Y.; Shiranirad, S.; Mashhadi, M.; Hashemi, A.; Khankandi, H.P.; Behzad, S. Resveratrol and Colorectal Cancer: A Molecular Approach to Clinical Researches. Curr. Top. Med. Chem. 2021, 21, 2634–2646. [Google Scholar] [CrossRef]
- Doiphode, S.; Lokhande, K.; Ghosh, P.; Swamy, K.V.; Nagar, S. Dual inhibition of cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LOX) by resveratrol derivatives in cancer therapy: In silico approach. J. Biomol. Struct. Dyn. 2022, 41, 8571–8586. [Google Scholar] [CrossRef]
- Jang, J.Y.; Im, E.; Kim, N.D. Mechanism of Resveratrol-Induced Programmed Cell Death and New Drug Discovery against Cancer: A Review. Int. J. Mol. Sci. 2022, 23, 13689. [Google Scholar] [CrossRef]
- Shabani Nashtaei, M.; Amidi, F.; Sedighi Gilani, M.A.; Aleyasin, A.; Bakhshalizadeh, S.; Naji, M.; Nekoonam, S. Protective features of resveratrol on human spermatozoa cryopreservation may be mediated through 5’ AMP-activated protein kinase activation. Andrology 2017, 5, 313–326. [Google Scholar] [CrossRef]
- Illiano, E.; Trama, F.; Zucchi, A.; Iannitti, R.G.; Fioretti, B.; Costantini, E. Resveratrol-Based Multivitamin Supplement Increases Sperm Concentration and Motility in Idiopathic Male Infertility: A Pilot Clinical Study. J. Clin. Med. 2020, 9, 4017. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Alaee, S.; Namavar, M.R.; Khodabandeh, Z.; Ahmadi, N.; Rashidipour, N.; Karami-Mohajeri, S. The antioxidant properties of resveratrol on sperm parameters, testicular tissue, antioxidant capacity, and lipid peroxidation in isoflurane-induced toxicity in mice. Hum. Exp. Toxicol. 2023, 42, 9603271231215036. [Google Scholar] [CrossRef]
- Shi, A.; Wang, J.; Guo, R.; Feng, X.; Ge, Y.; Liu, H.; Agyei, D.; Wang, Q. Improving resveratrol bioavailability using water-in-oil-in-water (W/O/W) emulsion: Physicochemical stability, in vitro digestion resistivity and transport properties. J. Funct. Foods 2021, 87, 104717. [Google Scholar] [CrossRef]
- Talebi, M.; Shahbazi, K.; Dakkali, M.S.; Akbari, M.; Almasi Ghale, R.; Hashemi, S.; Sashourpour, M.; Mojab, F.; Aminzadeh, S. Phytosomes: A promising nanocarrier system for enhanced bioavailability and therapeutic efficacy of herbal products. Phytomedicine Plus 2025, 5, 100779. [Google Scholar] [CrossRef]
- Borges, S.C.; Ferreira, P.E.B.; da Silva, L.M.; de Paula Werner, M.F.; Irache, J.M.; Cavalcanti, O.A.; Buttow, N.C. Evaluation of the treatment with resveratrol-loaded nanoparticles in intestinal injury model caused by ischemia and reperfusion. Toxicology 2018, 396-397, 13–22. [Google Scholar] [CrossRef] [PubMed]
- El-Azab, M.; Hishe, H.; Moustafa, Y.; El-Awady, E.S. Anti-angiogenic effect of resveratrol or curcumin in Ehrlich ascites carcinoma-bearing mice. Eur. J. Pharmacol. 2011, 652, 7–14. [Google Scholar] [CrossRef]
- Zhang, X.; Hartmann, P. How to calculate sample size in animal and human studies. Front. Med. 2023, 10, 1215927. [Google Scholar] [CrossRef] [PubMed]
- Festing, M.F.W.; Altman, D.G. Guidelines for the Design and Statistical Analysis of Experiments Using Laboratory Animals. ILAR J. 2002, 43, 244–258. [Google Scholar] [CrossRef]
- Dolai, N.; Islam, A.; Haldar, P.K. Antiproliferative Activity and Apoptosis Inducing Mechanism of Anthocephalus cadamba on Dalton’s Lymphoma Ascites Cells. Iran. J. Pharm. Res. IJPR 2016, 15, 505–514. [Google Scholar]
- Bala, A.; Kar, B.; Haldar, P.K.; Mazumder, U.K.; Bera, S. Evaluation of anticancer activity of Cleome gynandra on Ehrlich’s Ascites Carcinoma treated mice. J. Ethnopharmacol. 2010, 129, 131–134. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Sakuma, Y. Gonadal steroid action and brain sex differentiation in the rat. J. Neuroendocrinol. 2009, 21, 410–414. [Google Scholar] [CrossRef]
- Jaffe, B. Methods of Hormone Radioimmunoassay; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Strauss, J.F.; Barbieri, R.L.; Dokras, A.; Williams, C.J.; Williams, S.Z. Yen & Jaffe’s Reproductive Endocrinology-E-Book: Physiology, Pathophysiology, and Clinical Management; Elsevier Health Sciences: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Cardoso, E.; Mathias, M.D.L.; Monarca, R.I.; Gabriel, S.I. Assessing Optimal Cell Counts in Sperm Shape Abnormality Assays in Rodents. Animals 2023, 13, 3324. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-L.; Chen, H.-H.; Zheng, L.-L.; Sun, L.-P.; Shi, L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct. Target. Ther. 2023, 8, 198. [Google Scholar] [CrossRef] [PubMed]
- Hedayati, N.; Safari, M.H.; Milasi, Y.E.; Kahkesh, S.; Farahani, N.; Khoshnazar, S.M.; Dorostgou, Z.; Alaei, E.; Alimohammadi, M.; Rahimzadeh, P.; et al. Modulation of the PI3K/Akt signaling pathway by resveratrol in cancer: Molecular mechanisms and therapeutic opportunity. Discov. Oncol. 2025, 16, 669. [Google Scholar] [CrossRef] [PubMed]
- Sarfraz, M.; Arafat, M.; Zaidi, S.H.H.; Eltaib, L.; Siddique, M.I.; Kamal, M.; Ali, A.; Asdaq, S.M.B.; Khan, A.; Aaghaz, S.; et al. Resveratrol-Laden Nano-Systems in the Cancer Environment: Views and Reviews. Cancers 2023, 15, 4499. [Google Scholar] [CrossRef]
- Trofin, A.-M.; Scripcariu, D.V.; Filipiuc, S.-I.; Neagu, A.-N.; Filipiuc, L.-E.; Tamba, B.-I.; Palaghia, M.M.; Uritu, C.M. From Nature to Nanomedicine: Enhancing the Antitumor Efficacy of Rhein, Curcumin, and Resveratrol. Medicina 2025, 61, 981. [Google Scholar] [CrossRef]
- Lee, D.; Lee, M.; Go, E.; Chung, N. Resveratrol-loaded gold nanoparticles enhance caspase-mediated apoptosis in PANC-1 pancreatic cells via mitochondrial intrinsic apoptotic pathway. Cancer Nanotechnol. 2022, 13, 34. [Google Scholar] [CrossRef]
- Zhao, Z.; Cao, Y.; Xu, R.; Fang, J.; Zhang, Y.; Xu, X.; Huang, L.; Li, R. Nanoparticles (NPs)-mediated targeted regulation of redox homeostasis for effective cancer therapy. Smart Mater. Med. 2024, 5, 291–320. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, M.; Xu, Y.; Peng, W.; Zhang, S.; Li, R.; Zhang, H.; Zhang, H.; Cheng, S.; Wang, Y.; et al. Resveratrol-Loaded TPGS-Resveratrol-Solid Lipid Nanoparticles for Multidrug-Resistant Therapy of Breast Cancer: In Vivo and In Vitro Study. Front. Bioeng. Biotechnol. 2021, 9, 762489. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P.; Slama, P.; Roychoudhury, S. Oxidative Stress, Testicular Inflammatory Pathways, and Male Reproduction. Int. J. Mol. Sci. 2021, 22, 10043. [Google Scholar] [CrossRef]
- Zhang, L.-X.; Li, C.-X.; Kakar, M.U.; Khan, M.S.; Wu, P.-F.; Amir, R.M.; Dai, D.-F.; Naveed, M.; Li, Q.-Y.; Saeed, M.; et al. Resveratrol (RV): A pharmacological review and call for further research. Biomed. Pharmacother. 2021, 143, 112164. [Google Scholar] [CrossRef]
- Rasha, F.; Mims, B.M.; Castro-Piedras, I.; Barnes, B.J.; Grisham, M.B.; Rahman, R.L.; Pruitt, K. The Versatility of Sirtuin-1 in Endocrinology and Immunology. Front. Cell Dev. Biol. 2020, 8, 589016. [Google Scholar] [CrossRef]
- Perrone, D.; Fuggetta, M.P.; Ardito, F.; Cottarelli, A.; De Filippis, A.; Ravagnan, G.; De Maria, S.; Lo Muzio, L. Resveratrol (3,5,4’-trihydroxystilbene) and its properties in oral diseases. Exp. Ther. Med. 2017, 14, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, F.I.; Yasmin, T.; Akter, R.; Islam, M.N.; Hossain, M.M.; Khan, F.; Aldhahrani, A.; Soliman, M.M.; Subhan, N.; Haque, M.A.; et al. Resveratrol treatment modulates several antioxidant and anti-inflammatory genes expression and ameliorated oxidative stress mediated fibrosis in the kidneys of high-fat diet-fed rats. Saudi Pharm. J. 2022, 30, 1454–1463. [Google Scholar] [CrossRef] [PubMed]
- Eo, S.H.; Kim, S.J. Resveratrol-mediated inhibition of cyclooxygenase-2 in melanocytes suppresses melanogenesis through extracellular signal-regulated kinase 1/2 and phosphoinositide 3-kinase/Akt signalling. Eur. J. Pharmacol. 2019, 860, 172586. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jiang, M.; Wang, L.; Yu, S. Combined chemotherapy with cyclooxygenase-2 (COX-2) inhibitors in treating human cancers: Recent advancement. Biomed. Pharmacother. 2020, 129, 110389. [Google Scholar] [CrossRef]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef]
- Varlamova, E.G.; Gudkov, S.V.; Blinova, E.V.; Blinov, D.S.; Turovsky, E.A. Anticancer signal transduction pathways of selenium nanoparticles in mouse colorectal cancer model. Biochem. Biophys. Res. Commun. 2025, 769, 151962. [Google Scholar] [CrossRef]



| Gene | Forward Primer 5′ → 3′ | Reverse Primer 5′ → 3′ |
|---|---|---|
| caspase-3 | GGGGAGCTTGGAACGCTAAG | GAGTCCACTGACTTGCTCCC |
| bax | CTGGATCCAAGACCAGGGTG | GTGAGGACTCCAGCCACAAA |
| bcl-2 | GAACTGGGGGAGGATTGTGG | GCATGCTGGGGCCATATAGT |
| GAPDH | AAATGAGAGAGGCCCAGCTAC | GAGGGCTGCAGTCCGTATTTA |
| Target Proteins | Binding Affinity (Kcal/M) | Hydrogen Bonds | Hydrophobic Contacts | Key Residues |
|---|---|---|---|---|
| CAT | −6.9 | 2 | 2 | Arg66, His364(2), Pro391 |
| SOD | −6.2 | 1 | 6 | Asn53(2),Val148(3), Val7(2) |
| GSH-Px | −6.4 | 3 | 3 | Phe81, Pro322, Asn83, Glu238, Ser240(2) |
| TNF-α | −6.6 | 1 | 2 | Tyr59, Ser60, Tyr119 |
| IL-1β | −5.4 | 1 | 5 | Phe46, Phe146, Phe150, Leu110, Ther147, Met148 |
| IF-γ | −6.4 | 3 | 4 | Asp95 (2), Glu99(2), Glu93, Ser55, Phe103 |
| Cox-2 | −7.6 | 3 | 3 | Asn376(2), Gln375, Gln374, Phe143, His228 |
| Time (Days) | Particle Size | PDI | Zeta Potential | Encapsulation Efficiency |
|---|---|---|---|---|
| Storage at 4 °C | ||||
| 0 | 190.01 ± 4.15 | 0.486 ± 0.02 | −36.0 ± 1.24 | 84.0 ± 2.01 |
| 15 | 192.63 ± 5.01 | 0.493 ± 0.01 | −35.4 ± 1.16 | 83.3 ± 2.14 |
| 30 | 197.42 ± 4.33 | 0.502 ± 0.03 | −35.1 ± 1.09 | 83.6 ± 1.76 |
| Storage at 25 °C | ||||
| 0 | 190.01 ± 4.15 | 0.486 ± 0.02 | −36.0 ± 1.24 | 84.0 ± 2.01 |
| 15 | 214.26 ± 5.12 | 0.522 ± 0.01 | −31.24 ± 1.36 | 78.26 ± 2.41 |
| 30 | 246.29 ± 6.01 | 0.549 ± 0.01 | −28.36 ± 1.23 | 72.21 ± 2.35 |
| Parameters | EAC | EAC/RES | EAC/RES-PNPs |
|---|---|---|---|
| Mean survival time (days) | 7.5 | 11 | 12 |
| Increased life span percentage (%) | -- | 46.67 | 60.00 |
| Change in body weight (%) | 42.15 ± 2.14 a | 35.52 ± 2.93 ab | 28.64 ± 3.29 b |
| Abdominal circumference (mm) | 10.11 ± 0.84 a | 8.81 ± 0.72 ab | 7.13 ± 0.53 b |
| Ascitic fluid volume (mL) | 8.76 ± 0.37 a | 5.19 ± 0.22 b | 3.37 ± 0.26 c |
| Viable EAC cells count (106/mL) | 33.27 ± 1.77 a | 21.17 ± 1.03 b | 12.43 ± 0.84 c |
| TRT | Sperm Count (×106) | Sperm Motility (%) | Sperm Abnormalities (%) |
|---|---|---|---|
| Control | 10.12 ± 0.86 a | 81.42 ± 2.13 ab | 3.57 ± 0.19 d |
| RES | 10.96 ± 0.94 a | 83.16 ± 3.11 ab | 3.16 ± 0.23 d |
| RES-PNPs | 11.14 ± 1.01 a | 84.45 ± 3.86 a | 2.88 ± 0.11 d |
| EAC | 6.24 ± 0.43 c | 52.32 ± 2.54 d | 16.29 ± 1.25 a |
| EAC/RES | 7.15 ± 0.59 bc | 63.34 ± 2.17 c | 12.37 ± 1.12 b |
| EAC/RES-PNPs | 8.93 ± 0.62 ab | 74.12 ± 3.29 b | 9.87 ± 0.87 c |
| TRT | Testosterone (ng/mL) | LH (ng/mL) | FSH (ng/mL) |
|---|---|---|---|
| Control | 3.22 ± 0.21 a | 6.07 ± 0.41 a | 5.14 ± 0.33 a |
| RES | 3.41 ± 0.29 a | 6.12 ± 0.38 a | 5.19 ± 0.35 a |
| RES-PNPs | 3.54 ± 0.18 a | 6.33 ± 0.53 a | 5.32 ± 0.42 a |
| EAC | 1.12 ± 0.08 c | 3.26 ± 0.18 b | 1.86 ± 0.05 c |
| EAC/RES | 1.83 ± 0.07 b | 4.12 ± 0.22 b | 2.17 ± 0.09 c |
| EAC/RES-PNPs | 2.24 ± 0.12 b | 5.37 ± 0.26 a | 3.11 ± 0.17 b |
| Parameters | SOD (U/mg protein) | CAT (U/mg protein) | GSH-Px (U/mg protein) | GSH (nmol/g protein) | PC (nmol/g protein) | MDA (nmol/g tissue) |
|---|---|---|---|---|---|---|
| Control | 8.41 ± 0.45 ab | 19.32 ± 1.12 ab | 14.12 ± 0.75 bc | 10.12 ± 0.63 a | 2.14 ± 0.12 d | 21.12 ± 1.33 cd |
| RES | 9.13 ± 0.71 a | 20.12 ± 1.39 a | 15.32 ± 0.83 ab | 10.69 ± 0.54 a | 1.95 ± 0.11 d | 20.12 ± 1.52 d |
| RES-PNPs | 9.83 ± 0.62 a | 21.95 ± 1.62 a | 16.47 ± 0.66 a | 10.95 ± 0.46 a | 1.84 ± 0.29 d | 19.41 ± 1.32 d |
| EAC | 3.16 ± 0.13 d | 7.16 ± 0.55 c | 6.69 ± 0.42 d | 4.82 ± 0.28 c | 4.85 ± 0.22 a | 35.96 ± 2.94 a |
| EAC/RES | 4.91 ± 0.21 c | 11.13 ± 0.93 c | 8.16 ± 0.51 d | 5.11 ± 0.33 c | 3.47 ± 0.23 b | 31.12 ± 3.11 ab |
| EAC/RES-PNPs | 7.49 ± 0.44 b | 15.93 ± 0.99 b | 12.74 ± 0.73 c | 8.14 ± 0.53 b | 2.75 ± 0.12 c | 27.93 ± 2.39 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfawaz, M.; Elmorsy, E.M.; Alshammari, A.N.; Emam, M.N.; Hegab, I.I.; Shaalan, A.A.M.; Fawzy, M.S.; Mohammed, L.A. Nanoparticle-Based Delivery of Resveratrol Suppresses Ehrlich Ascites Carcinoma and Protects Testicular Function via Antioxidant, Anti-Angiogenic, Anti-Inflammatory, and Pro-Apoptotic Mechanisms. Biomolecules 2025, 15, 1605. https://doi.org/10.3390/biom15111605
Alfawaz M, Elmorsy EM, Alshammari AN, Emam MN, Hegab II, Shaalan AAM, Fawzy MS, Mohammed LA. Nanoparticle-Based Delivery of Resveratrol Suppresses Ehrlich Ascites Carcinoma and Protects Testicular Function via Antioxidant, Anti-Angiogenic, Anti-Inflammatory, and Pro-Apoptotic Mechanisms. Biomolecules. 2025; 15(11):1605. https://doi.org/10.3390/biom15111605
Chicago/Turabian StyleAlfawaz, M., Ekramy M. Elmorsy, Ahmad Najem Alshammari, Marwa Nagy Emam, Islam Ibrahim Hegab, Aly A. M. Shaalan, Manal S. Fawzy, and Lina Abdelhady Mohammed. 2025. "Nanoparticle-Based Delivery of Resveratrol Suppresses Ehrlich Ascites Carcinoma and Protects Testicular Function via Antioxidant, Anti-Angiogenic, Anti-Inflammatory, and Pro-Apoptotic Mechanisms" Biomolecules 15, no. 11: 1605. https://doi.org/10.3390/biom15111605
APA StyleAlfawaz, M., Elmorsy, E. M., Alshammari, A. N., Emam, M. N., Hegab, I. I., Shaalan, A. A. M., Fawzy, M. S., & Mohammed, L. A. (2025). Nanoparticle-Based Delivery of Resveratrol Suppresses Ehrlich Ascites Carcinoma and Protects Testicular Function via Antioxidant, Anti-Angiogenic, Anti-Inflammatory, and Pro-Apoptotic Mechanisms. Biomolecules, 15(11), 1605. https://doi.org/10.3390/biom15111605







