Plasma Extracellular Vesicles Contain Protein Biomarkers for Capturing Stages of Metabolic Dysfunction-Associated Steatotic Liver Disease: A Preliminary Exploratory Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Blood Sampling
2.3. Liver Biopsy
2.4. Liver MRI Acquisition and Post-Processing
2.5. EV Isolation
2.6. Nanoparticle Tracking Analysis
2.7. Western Blot Analysis
2.8. Mass Spectrometry Analysis
2.9. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Plasma EV Proteins as Markers for Steatosis
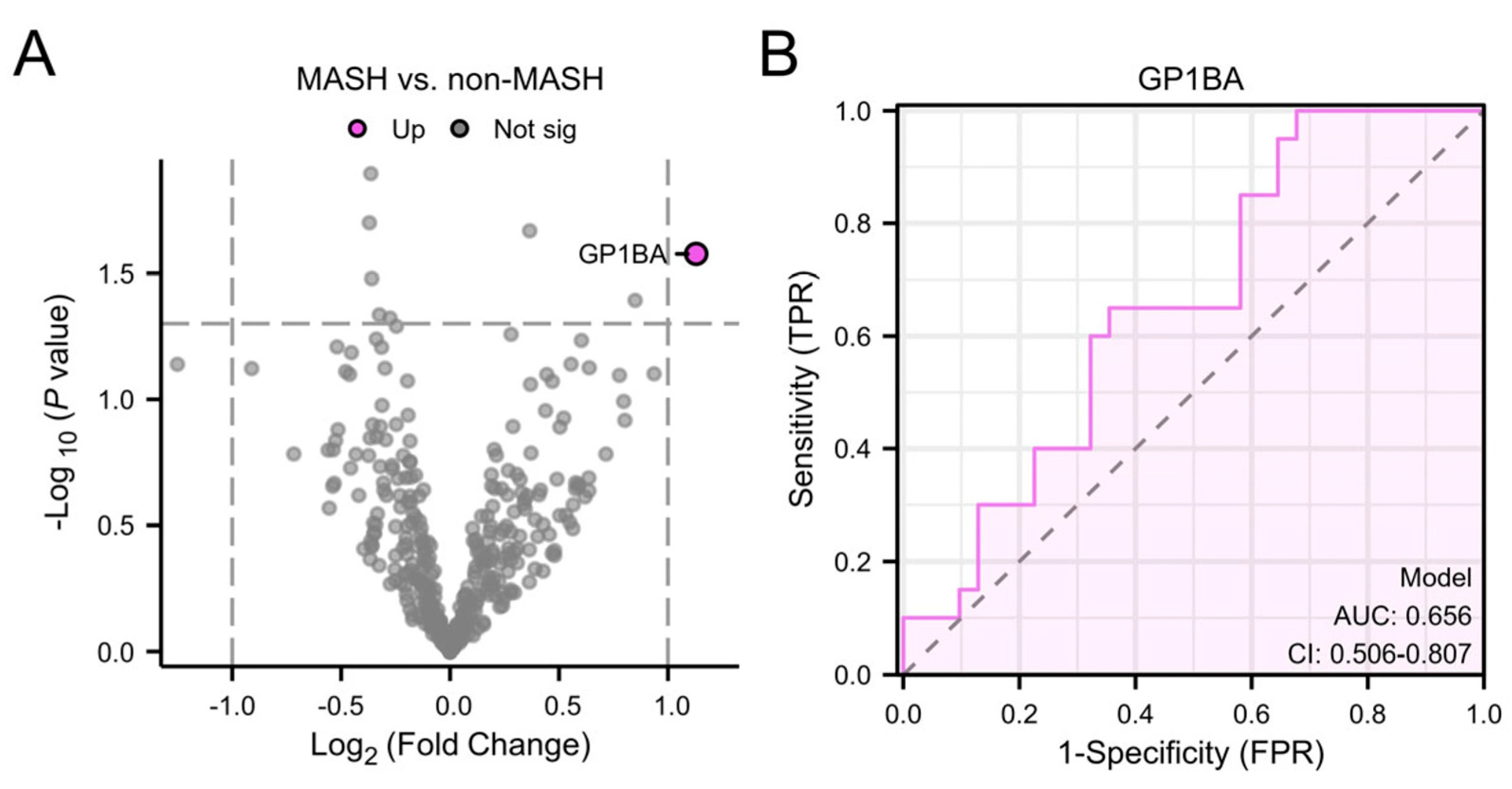
3.3. Plasma EV Proteins as Markers for MASH
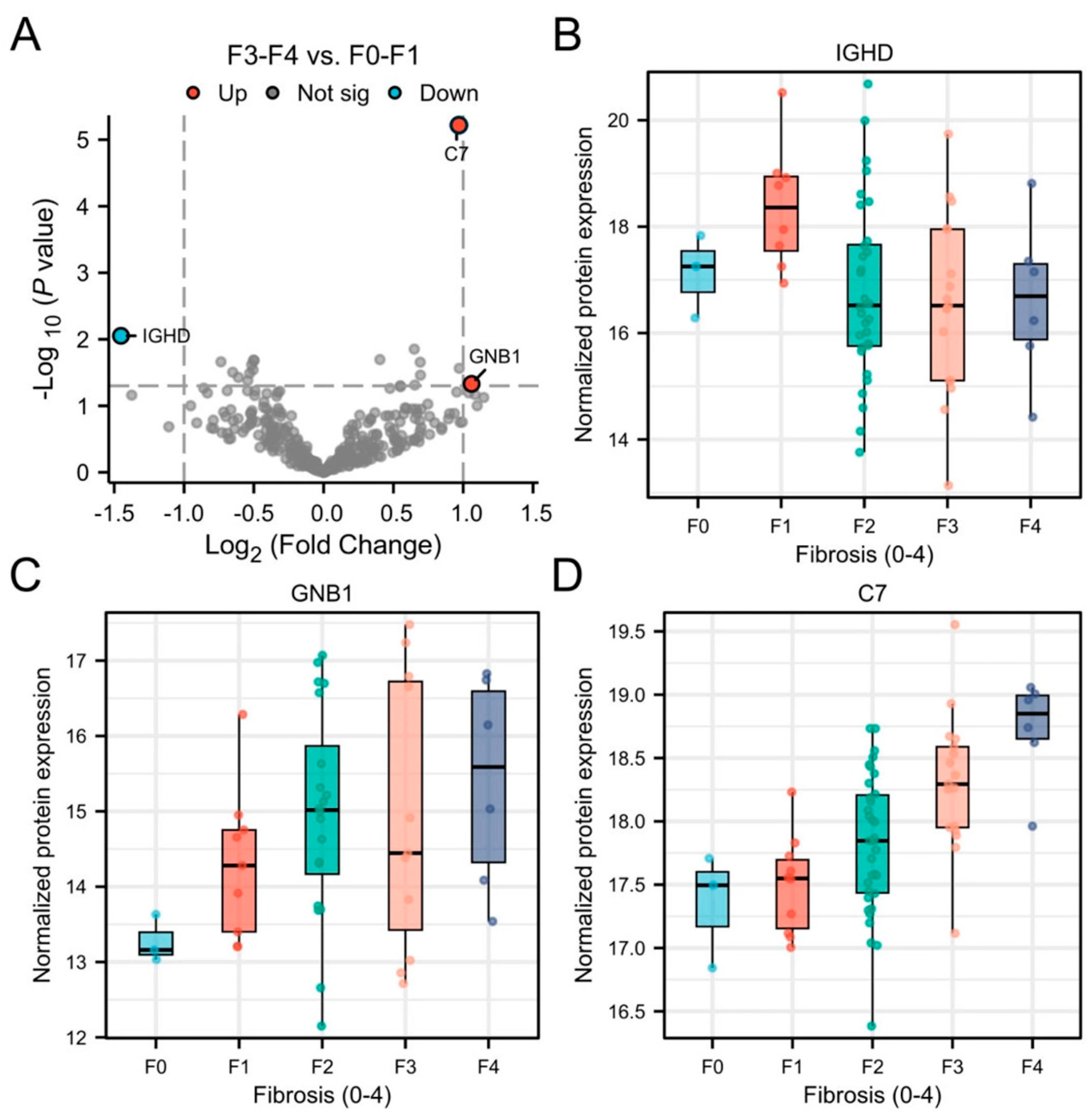
3.4. Plasma EV Proteins as Markers for Fibrosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANCHOR | Amsterdam MASLD-MASH cohort |
| ANPEP | Alanyl aminopeptidase |
| AUC | Area under the curve |
| C7 | Complement component 7 |
| CAP | Controlled attenuation parameter |
| CCDC25 | Coiled-coil domain-containing protein 25 |
| CI CRN | Confidence interval Clinical research network |
| cT1 DIA DTT | Iron-corrected T1 mapping Data-independent acquisition Dithiothreitol |
| ELF | Enhanced liver fibrosis-test |
| EV FAIMS | Extracellular vesicle Field asymmetric waveform ion mobility spectrometry |
| F0 | No fibrosis |
| F1 | Mild fibrosis |
| F2 | Significant fibrosis |
| F3 | Advanced fibrosis |
| F4 | Cirrhosis |
| FIB4 | Fibrosis-4 score |
| GNB1 | Guanine nucleotide-binding protein subunit beta-1 |
| GP1BA | Glycoprotein Ib alpha chain |
| H4C1 | Histone H4 |
| HCC | Hepatocellular carcinoma |
| IGHD | Immunoglobulin heavy constant delta |
| IQR | Interquartile range |
| KLHL41 LC-MS | Kelch-like protein 41 Liquid chromatography- mass spectrometer |
| LSM | Liver stiffness measurement |
| MASH | Metabolic dysfunction-associated steatohepatitis |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| MR | Magnetic resonance |
| MRE | Magnetic resonance elastography |
| NIT | Non-invasive test |
| OIT3 | Oncoprotein-induced transcript 3 |
| PDFF | Proton density fat fraction |
| ROC | Receiver operating characteristic |
| ROI | Region of interest |
| S1 | Mild steatosis |
| S2 | Moderate steatosis |
| S3 SAF | Advanced steatosis Steatosis, activity and fibrosis |
| SD | Standard deviation |
| T2DM | Type 2 diabetes mellitus |
| VCTE | Vibration-controlled transient elastography |
References
- Younossi, Z.M.; Kalligeros, M.; Henry, L. Epidemiology of Metabolic Dysfunction-Associated Steatotic Liver Disease. Clin. Mol. Hepatol. 2024, 31, S32. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Hardy, T.; Oakley, F.; Anstee, Q.M.; Day, C.P. Nonalcoholic Fatty Liver Disease: Pathogenesis and Disease Spectrum. Annu. Rev. Pathol. 2016, 11, 451–496. [Google Scholar] [CrossRef]
- Diehl, A.M.; Day, C. Cause, Pathogenesis, and Treatment of Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2017, 377, 2063–2072. [Google Scholar] [CrossRef]
- Sumida, Y.; Nakajima, A.; Itoh, Y. Limitations of liver biopsy and non-invasive diagnostic tests for the diagnosis of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J. Gastroenterol. 2014, 20, 475–485. [Google Scholar] [CrossRef]
- Ratziu, V.; Charlotte, F.; Heurtier, A.; Gombert, S.; Giral, P.; Bruckert, E.; Grimaldi, A.; Capron, F.; Poynard, T.; LIDO Study Group. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 2005, 128, 1898–1906. [Google Scholar] [CrossRef]
- Davison, B.A.; Harrison, S.A.; Cotter, G.; Alkhouri, N.; Sanyal, A.; Edwards, C.; Colca, J.R.; Iwashita, J.; Koch, G.G.; Dittrich, H.C. Suboptimal reliability of liver biopsy evaluation has implications for randomized clinical trials. J. Hepatol. 2020, 73, 1322–1332. [Google Scholar] [CrossRef]
- Castera, L.; Friedrich-Rust, M.; Loomba, R. Noninvasive Assessment of Liver Disease in Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1264–1281.e4. [Google Scholar] [CrossRef]
- Bril, F.; Ortiz-Lopez, C.; Lomonaco, R.; Orsak, B.; Freckleton, M.; Chintapalli, K.; Hardies, J.; Lai, S.; Solano, F.; Tio, F.; et al. Clinical value of liver ultrasound for the diagnosis of nonalcoholic fatty liver disease in overweight and obese patients. Liver Int. 2015, 35, 2139–2146. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.D.M.; Cotrim, H.P.; Barbosa, D.B.V.; De Athayde, L.G.M.; Santos, A.S.; Bitencourt, A.G.V.; De Freitas, L.A.R.; Rios, A.; Alves, E. Fatty liver disease in severe obese patients: Diagnostic value of abdominal ultrasound. World J. Gastroenterol. 2008, 14, 1415–1418. [Google Scholar] [CrossRef]
- Eddowes, P.J.; Sasso, M.; Allison, M.; Tsochatzis, E.; Anstee, Q.M.; Sheridan, D.; Guha, I.N.; Cobbold, J.F.; Deeks, J.J.; Paradis, V.; et al. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1717–1730. [Google Scholar] [CrossRef]
- Vuppalanchi, R.; Siddiqui, M.S.; Van Natta, M.L.; Hallinan, E.; Brandman, D.; Kowdley, K.; A Neuschwander-Tetri, B.; Loomba, R.; Dasarathy, S.; Abdelmalek, M.; et al. Performance characteristics of vibration-controlled transient elastography for evaluation of nonalcoholic fatty liver disease. Hepatology 2018, 67, 134–144. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef]
- Imajo, K.; Kessoku, T.; Honda, Y.; Tomeno, W.; Ogawa, Y.; Mawatari, H.; Fujita, K.; Yoneda, M.; Taguri, M.; Hyogo, H.; et al. Magnetic Resonance Imaging More Accurately Classifies Steatosis and Fibrosis in Patients with Nonalcoholic Fatty Liver Disease Than Transient Elastography. Gastroenterology 2016, 150, 626–637.e7. [Google Scholar] [CrossRef] [PubMed]
- Bohte, A.E.; de Niet, A.; Jansen, L.; Bipat, S.; Nederveen, A.J.; Verheij, J.; Terpstra, V.; Sinkus, R.; van Nieuwkerk, K.M.J.; de Knegt, R.J.; et al. Non-invasive evaluation of liver fibrosis: A comparison of ultrasound-based transient elastography and MR elastography in patients with viral hepatitis B and C. Eur. Radiol. 2014, 24, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Caussy, C.; Reeder, S.B.; Sirlin, C.B.; Loomba, R. Noninvasive, Quantitative Assessment of Liver Fat by MRI-PDFF as an Endpoint in NASH Trials. Hepatology 2018, 68, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Alkhouri, N.; Beyer, C.; Shumbayawonda, E.; Andersson, A.; Yale, K.; Rolph, T.; Chung, R.T.; Vuppalanchi, R.; Cusi, K.; Loomba, R.; et al. Decreases in cT1 and liver fat content reflect treatment-induced histological improvements in MASH. J. Hepatol. 2025, 82, 438–445. [Google Scholar] [CrossRef]
- Hirsova, P.; Ibrahim, S.H.; Verma, V.K.; Morton, L.A.; Shah, V.H.; LaRusso, N.F.; Gores, G.J.; Malhi, H. Extracellular vesicles in liver pathobiology: Small particles with big impact. Hepatology 2016, 64, 2219–2233. [Google Scholar] [CrossRef]
- Srinivas, A.N.; Suresh, D.; Kaur, S.; Kumar, D.P. The promise of small particles: Extracellular vesicles as biomarkers in liver pathology. J. Physiol. 2023, 601, 4953–4971. [Google Scholar] [CrossRef]
- Newman, L.A.; Sorich, M.J.; Rowland, A. Role of Extracellular Vesicles in the Pathophysiology, Diagnosis and Tracking of Non-Alcoholic Fatty Liver Disease. J. Clin. Med. 2020, 9, 2032. [Google Scholar] [CrossRef]
- Troelstra, M.A.; Witjes, J.J.; van Dijk, A.; Mak, A.L.; Gurney-Champion, O.; Runge, J.H.; Zwirs, D.; Stols-Gonçalves, D.; Zwinderman, A.H.; Wolde, M.T.; et al. Assessment of Imaging Modalities Against Liver Biopsy in Nonalcoholic Fatty Liver Disease: The Amsterdam NAFLD-NASH Cohort. J. Magn. Reson. Imaging 2021, 54, 1937–1949. [Google Scholar] [CrossRef]
- Bedossa, P.; Poitou, C.; Veyrie, N.; Bouillot, J.-L.; Basdevant, A.; Paradis, V.; Tordjman, J.; Clement, K. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology 2012, 56, 1751–1759. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.-C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Yokoo, T.; Shiehmorteza, M.; Hamilton, G.; Wolfson, T.; Schroeder, M.E.; Middleton, M.S.; Bydder, M.; Gamst, A.C.; Kono, Y.; Kuo, A.; et al. Estimation of hepatic proton-density fat fraction by using MR imaging at 3.0 T. Radiology 2011, 258, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Guenthner, C.; Sethi, S.; Troelstra, M.; Dokumaci, A.S.; Sinkus, R.; Kozerke, S. Ristretto MRE: A generalized multi-shot GRE-MRE sequence. NMR Biomed. 2019, 32, e4049. [Google Scholar] [CrossRef]
- Runge, J.H.; Hoelzl, S.H.; Sudakova, J.; Dokumaci, A.S.; Nelissen, J.L.; Guenthner, C.; Lee, J.; Troelstra, M.; Fovargue, D.; Stoker, J.; et al. A novel magnetic resonance elastography transducer concept based on a rotational eccentric mass: Preliminary experiences with the gravitational transducer. Phys. Med. Biol. 2019, 64, 045007. [Google Scholar] [CrossRef] [PubMed]
- Sinkus, R.; Lambert, S.; Abd-Elmoniem, K.Z.; Morse, C.; Heller, T.; Guenthner, C.; Ghanem, A.M.; Holm, S.; Gharib, A.M. Rheological determinants for simultaneous staging of hepatic fibrosis and inflammation in patients with chronic liver disease. NMR Biomed. 2018, 31, e3956. [Google Scholar] [CrossRef]
- Mojtahed, A.; Kelly, C.J.; Herlihy, A.H.; Kin, S.; Wilman, H.R.; McKay, A.; Kelly, M.; Milanesi, M.; Neubauer, S.; Thomas, E.L.; et al. Reference range of liver corrected T1 values in a population at low risk for fatty liver disease-a UK Biobank sub-study, with an appendix of interesting cases. Abdom. Radiol. 2019, 44, 72–84. [Google Scholar] [CrossRef]
- Martínez-Aguilar, M.; Trillos-Almanza, M.C.; Wolters, J.C.; Buist-Homan, M.; van Vilsteren, F.; Blokzijl, H.; Moshage, H. Optimization of protocols for blood-derived extracellular vesicles for studies in liver diseases. Explor. Dig. Dis. 2024, 3, 143–162. [Google Scholar] [CrossRef]
- Frankenfield, A.M.; Ni, J.; Ahmed, M.; Hao, L. Protein Contaminants Matter: Building Universal Protein Contaminant Libraries for DDA and DIA Proteomics. J. Proteome Res. 2022, 21, 2104–2113. [Google Scholar] [CrossRef]
- Shen, J.; Hovhannisyan, H.; Lian, J.B.; Montecino, M.A.; Stein, G.S.; Stein, J.L.; van Wijnen, A.J. Transcriptional induction of the osteocalcin gene during osteoblast differentiation involves acetylation of histones h3 and h4. Mol. Endocrinol. 2003, 17, 743–756. [Google Scholar] [CrossRef]
- Agricola, E.; Verdone, L.; Di Mauro, E.; Caserta, M. H4 acetylation does not replace H3 acetylation in chromatin remodelling and transcription activation of Adr1-dependent genes. Mol. Microbiol. 2006, 62, 1433–1446. [Google Scholar] [CrossRef]
- Gralla, M.; Camporeale, G.; Zempleni, J. Holocarboxylase synthetase regulates expression of biotin transporters by chromatin remodeling events at the SMVT locus. J. Nutr. Biochem. 2008, 19, 400–408. [Google Scholar] [CrossRef][Green Version]
- Wen, J.; Yang, S.; Yan, G.; Lei, J.; Liu, X.; Zhang, N.; Zhang, J.; Deng, H.; Wu, L.; Li, Y. Increased OIT3 in macrophages promotes PD-L1 expression and hepatocellular carcinogenesis via NF-κB signaling. Exp. Cell Res. 2023, 428, 113651. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, J.; Xu, Y.; Wang, J.; Zhao, H.; Lei, J.; Zhou, Y.; Chen, Y.; Wu, L.; Zhou, M.; et al. OIT3 mediates macrophage polarization and facilitates hepatocellular carcinoma progression. Cancer Immunol. Immunother. 2022, 71, 2677–2689. [Google Scholar] [CrossRef]
- Niu, L.; E Geyer, P.; Albrechtsen, N.J.W.; Gluud, L.L.; Santos, A.; Doll, S.; Treit, P.V.; Holst, J.J.; Knop, F.K.; Vilsbøll, T.; et al. Plasma proteome profiling discovers novel proteins associated with non-alcoholic fatty liver disease. Mol. Syst. Biol. 2019, 15, e8793. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liang, X.; Li, H.; Zou, J.; Xu, L.; Zhang, Y.; Lin, J.; Zeng, J.; Zhong, X.; Liu, X.; et al. The NET-DNA-CCDC25 inhibitor di-Pal-MTO suppresses tumor progression and promotes the innate immune response. Cell Mol. Immunol. 2025, 22, 628–644. [Google Scholar] [CrossRef]
- Deng, H.; Zhang, J.; Zheng, Y.; Li, J.; Xiao, Q.; Wei, F.; Han, W.; Xu, X.; Zhang, Y. CCDC25 may be a potential diagnostic and prognostic marker of hepatocellular carcinoma: Results from microarray analysis. Front. Surg. 2022, 9, 878648. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Martinez, A.; Cenik, B.K.; Bezprozvannaya, S.; Chen, B.; Bassel-Duby, R.; Liu, N.; Olson, E.N. KLHL41 stabilizes skeletal muscle sarcomeres by nonproteolytic ubiquitination. Elife 2017, 6, e26439. [Google Scholar] [CrossRef] [PubMed]
- Malehmir, M.; Pfister, D.; Gallage, S.; Szydlowska, M.; Inverso, D.; Kotsiliti, E.; Leone, V.; Peiseler, M.; Surewaard, B.G.J.; Rath, D.; et al. Platelet GPIbα is a mediator and potential interventional target for NASH and subsequent liver cancer. Nat. Med. 2019, 25, 641–655. [Google Scholar] [CrossRef]
- Heijnen, H.; van der Sluijs, P. Platelet secretory behaviour: As diverse as the granules... or not? J. Thromb. Haemost. 2015, 13, 2141–2151. [Google Scholar] [CrossRef] [PubMed]
- Boccatonda, A.; Del Cane, L.; Marola, L.; D’Ardes, D.; Lessiani, G.; di Gregorio, N.; Ferri, C.; Cipollone, F.; Serra, C.; Santilli, F.; et al. Platelet, Antiplatelet Therapy and Metabolic Dysfunction-Associated Steatotic Liver Disease: A Narrative Review. Life 2024, 14, 473. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, Y.; Lu, P.; Wu, X.; Han, J.; Shi, Y.; Liu, Y.; Cheng, Y.; Gao, L.; Zhao, J.; et al. Association of complement components with the risk and severity of NAFLD: A systematic review and meta-analysis. Front. Immunol. 2022, 13, 1054159. [Google Scholar] [CrossRef]
- Prado, L.G.; Nagy, L.E. Role of Complement in Liver Diseases. Semin. Liver Dis. 2024, 44, 510–522. [Google Scholar] [CrossRef]
- Hou, W.; Janech, M.G.; Sobolesky, P.M.; Bland, A.M.; Samsuddin, S.; Alazawi, W.; Syn, W.K. Proteomic screening of plasma identifies potential noninvasive biomarkers associated with significant/advanced fibrosis in patients with nonalcoholic fatty liver disease. Biosci. Rep. 2020, 40, BSR20190395. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.I.; Parra, E.R.; Jiao, J.; Solis Soto, L.M.; Ledesma, D.A.; Saldarriaga, O.A.; Stevenson, H.L.; Beretta, L. Cellular and Molecular Mechanisms of Liver Fibrosis in Patients with NAFLD. Cancers 2023, 15, 2871. [Google Scholar] [CrossRef] [PubMed]
- McPherson, S.; Henderson, E.; Burt, A.D.; Day, C.P.; Anstee, Q.M. Serum immunoglobulin levels predict fibrosis in patients with non-alcoholic fatty liver disease. J. Hepatol. 2014, 60, 1055–1062. [Google Scholar] [CrossRef]
- Kimura, T.; Singh, S.; Tanaka, N.; Umemura, T. Role of G Protein-Coupled Receptors in Hepatic Stellate Cells and Approaches to Anti-Fibrotic Treatment of Non-Alcoholic Fatty Liver Disease. Front. Endocrinol. 2021, 12, 773432. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, C.Y. G protein-coupled receptors as potential targets for nonalcoholic fatty liver disease treatment. World J. Gastroenterol. 2021, 27, 677–691. [Google Scholar] [CrossRef]


| All Participants (n = 70) | MASLD Without Steatohepatitis (n = 23) | MASH (n = 47) | p-Value | ||
|---|---|---|---|---|---|
| Age, years | 49.0 (37.2–60.2) | 49.0 (38.5–55.5) | 50.0 (37.5–61.0) | 0.754 * | |
| Sex | Men, n (%) | 39 (55.7) | 13 (56.5) | 26 (55.3) | 0.924 ** |
| Women, n (%) | 31 (44.3) | 10 (43.5) | 21 (44.7) | ||
| BMI, kg/m2 | 32.8 (29.5–36.2) | 32.0 (29.8–35.4) | 33.0 (29.4–36.3) | 0.408 * | |
| Waist circumference, cm | 112.6 (±13.2) | 109.6 (±10.8) | 114.1 (±14.1) | 0.179 *** | |
| Hip circumference, cm | 112.7 (±11.8) | 111.2 (±9.8) | 113.4 (±12.7) | 0.477 *** | |
| T2DM | Yes, n (%) | 29 (41.4) | 6 (26.1) | 23 (48.9) | 0.068 ** |
| No, n (%) | 41 (58.6) | 17 (73.9) | 24 (51.1) | ||
| Hypertension | Yes, n (%) | 27 (38.6) | 6 (26.1) | 21 (44.7) | 0.133 ** |
| No, n (%) | 43 (61.4) | 17 (73.9) | 26 (55.3) | ||
| Total bilirubin, µmol/L | 9.0 (6.8–12.0) | 8.0 (6.5–11.0) | 9.0 (7.0–12.0) | 0.495 * | |
| ALP, U/L | 87.5 (70.0–104.2) | 86.0 (68.0–104.0) | 88.0 (71.0–105.0) | 0.683 * | |
| γGT, U/L | 66.0 (36.5–94.0) | 66.0 (39.0–95.0) | 64.0 (36.5–91.8) | 0.817 * | |
| AST, U/L | 42.0 (35.0–57.0) | 37.0 (29.0–41.2) | 50.0 (38.0–74.8) | <0.001 * | |
| ALT, U/L | 62.0 (48.0–82.0) | 60.0 (48.0–74.5) | 62.0 (47.5–107.0) | 0.269 * | |
| Steatosis grade | 1, n (%) | 22 (31.4) | 14 (60.9) | 8 (17.0) | <0.001 ** |
| 2, n (%) | 28 (40.0) | 6 (26.1) | 22 (46.8) | ||
| 3, n (%) | 20 (28.6) | 3 (13.0) | 17 (36.2) | ||
| Fibrosis stage | 0, n (%) | 3 (4.3) | 3 (13.0) | 0 (0.0) | <0.001 ** |
| 1, n (%) | 10 (14.3) | 9 (39.1) | 1 (2.1) | ||
| 2, n (%) | 34 (48.6) | 10 (43.5) | 24 (51.1) | ||
| 3, n (%) | 17 (24.3) | 1 (4.3) | 16 (34.0) | ||
| 4, n (%) | 6 (8.6) | 0 (0.0) | 6 (12.8) | ||
| PDFF, % | 17.7 (14.6–24.8) | 17.2 (8.4–22.6) | 20.2 (15.4–25.0) | 0.158 * | |
| cT1, ms | 901.0 (846.0–974.0) | 850.0 (786.5–890.5) | 926.0 (889.0–990.0) | <0.001 * | |
| MRE, kPa | 1.8 (1.7–2.0) | 1.7 (1.5–1.9) | 1.9 (1.7–2.2) | 0.034 * | |
| Protein | AUC (95% CI) | Correlation with PDFF | ||
|---|---|---|---|---|
| <S2 vs. ≥S2 | <S3 vs. ≥S3 | Spearman’s R | p-Value | |
| H4C1 | 0.589 (0.433–0.746) | 0.679 (0.511–0.847) | −0.01 | 0.953 |
| OIT3 | 0.602 (0.439–0.765) | 0.689 (0.527–0.851) | 0.08 | 0.586 |
| ANPEP | 0.631 (0.481–0.781) | 0.688 (0.551–0.824) | 0.16 | 0.232 |
| CCDC25 | 0.723 (0.594–0.851) | 0.590 (0.427–0.751) | −0.04 | 0.765 |
| KLHL41 | 0.606 (0.452–0.761) | 0.658 (0.479–0.836) | −0.34 | 0.016 |
| Protein | AUC (95% CI) | Correlation with MRE-Derived Elasticity | ||
|---|---|---|---|---|
| <F2 vs. ≥F2 | <F3 vs. ≥F3 | Spearman’s R | p-Value | |
| C7 | 0.802 (0.689–0.914) | 0.827 (0.723–0.931) | 0.38 | 0.004 |
| IGHD | 0.757 (0.629–0.885) | 0.582 (0.436–0.729) | −0.06 | 0.782 |
| GNB1 | 0.712 (0.560–0.864) | 0.577 (0.393–0.761) | −0.04 | 0.746 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Son, K.C.v.; Serna-Salas, S.; Wolters, J.C.; Wassenaar, N.P.M.; Driessen, S.; Mak, A.L.; Dijk, A.-M.v.; Houttu, V.A.T.; Witjes, J.J.; et al. Plasma Extracellular Vesicles Contain Protein Biomarkers for Capturing Stages of Metabolic Dysfunction-Associated Steatotic Liver Disease: A Preliminary Exploratory Study. Biomolecules 2025, 15, 1596. https://doi.org/10.3390/biom15111596
Li Y, Son KCv, Serna-Salas S, Wolters JC, Wassenaar NPM, Driessen S, Mak AL, Dijk A-Mv, Houttu VAT, Witjes JJ, et al. Plasma Extracellular Vesicles Contain Protein Biomarkers for Capturing Stages of Metabolic Dysfunction-Associated Steatotic Liver Disease: A Preliminary Exploratory Study. Biomolecules. 2025; 15(11):1596. https://doi.org/10.3390/biom15111596
Chicago/Turabian StyleLi, Yakun, Koen C. van Son, Sandra Serna-Salas, Justina C. Wolters, Nienke P. M. Wassenaar, Stan Driessen, Anne Linde Mak, Anne-Marieke van Dijk, Veera A. T. Houttu, Julia J. Witjes, and et al. 2025. "Plasma Extracellular Vesicles Contain Protein Biomarkers for Capturing Stages of Metabolic Dysfunction-Associated Steatotic Liver Disease: A Preliminary Exploratory Study" Biomolecules 15, no. 11: 1596. https://doi.org/10.3390/biom15111596
APA StyleLi, Y., Son, K. C. v., Serna-Salas, S., Wolters, J. C., Wassenaar, N. P. M., Driessen, S., Mak, A. L., Dijk, A.-M. v., Houttu, V. A. T., Witjes, J. J., Zwirs, D., Doukas, M., Verheij, J., Dullaart, R. P. F., Blokzijl, H., Holleboom, A. G., & Moshage, H. (2025). Plasma Extracellular Vesicles Contain Protein Biomarkers for Capturing Stages of Metabolic Dysfunction-Associated Steatotic Liver Disease: A Preliminary Exploratory Study. Biomolecules, 15(11), 1596. https://doi.org/10.3390/biom15111596








