Mitochondrial Dysfunction in Cardiomyopathy and Heart Failure: From Energetic Collapse to Therapeutic Opportunity
Abstract
1. Introduction
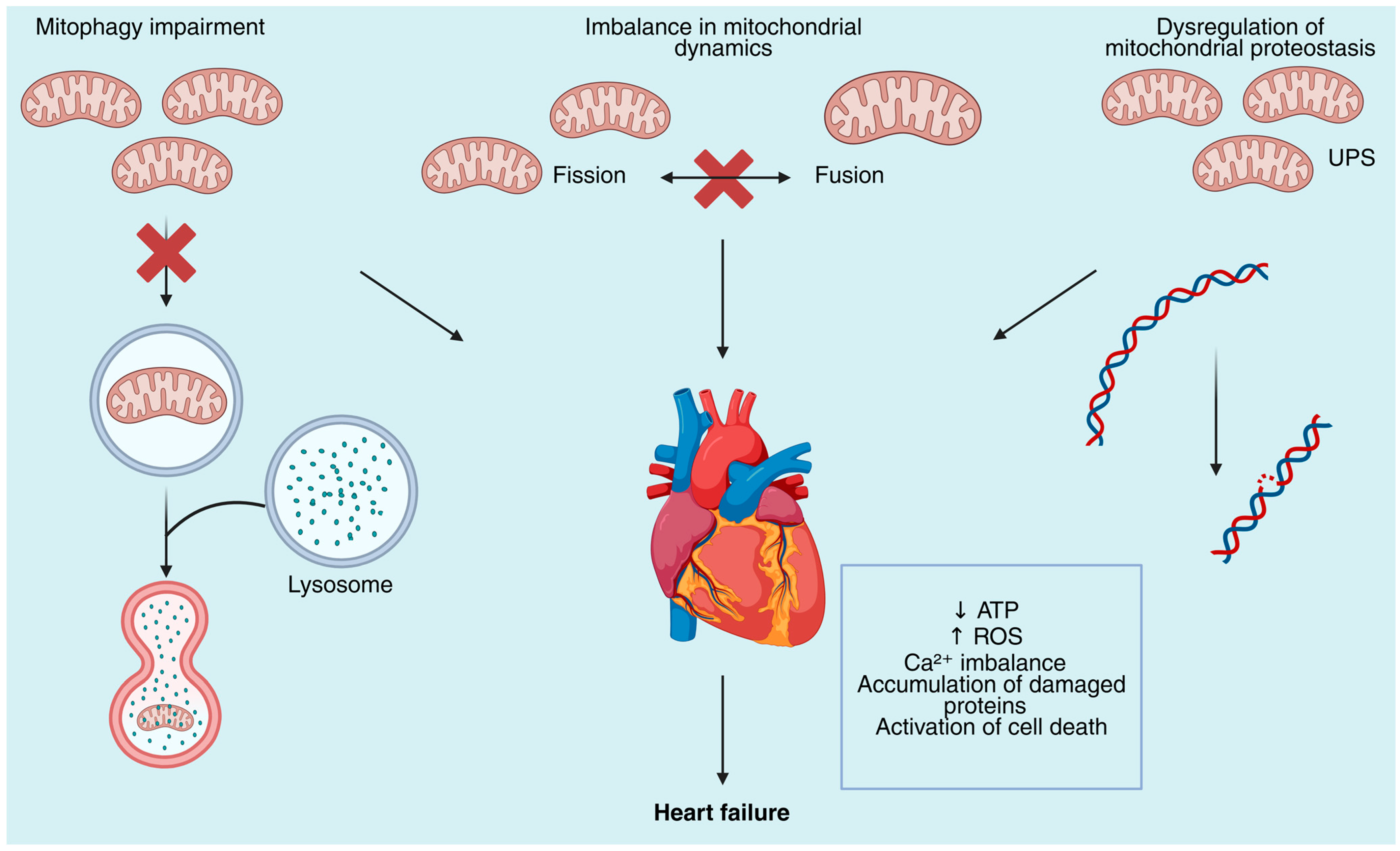
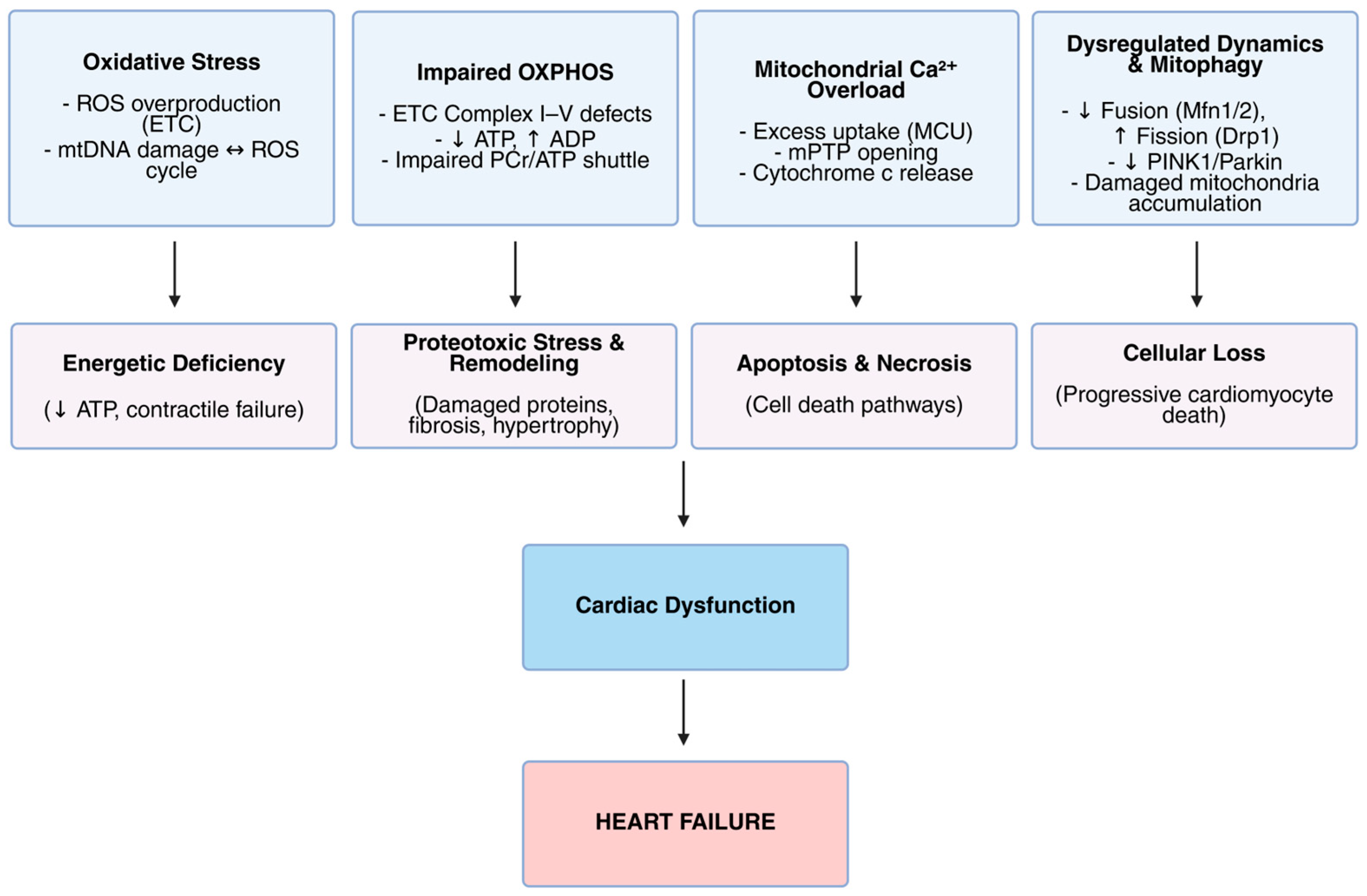
2. Mechanisms of Mitochondrial Dysfunction in Heart Failure
3. Mitochondrial Dysfunction Across Different Types of Cardiomyopathy
4. Mitochondrial-Driven Cell Death Pathways in Heart Failure Progression
5. Therapeutic Strategies Targeting Mitochondrial Dysfunction
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nguyen, B.Y.; Ruiz-Velasco, A.; Bui, T.; Collins, L.; Wang, X.; Liu, W. Mitochondrial Function in the Heart: The Insight into Mechanisms and Therapeutic Potentials. Br. J. Pharmacol. 2019, 176, 4302–4318. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Karwi, Q.G.; Tian, R.; Wende, A.R.; Abel, E.D. Cardiac Energy Metabolism in Heart Failure. Circ. Res. 2021, 128, 1487–1513. [Google Scholar] [CrossRef] [PubMed]
- Doenst, T.; Nguyen, T.D.; Abel, E.D. Cardiac Metabolism in Heart Failure: Implications beyond Atp Production. Circ. Res. 2013, 113, 709–724. [Google Scholar] [CrossRef]
- Sun, Q.; Karwi, Q.G.; Wong, N.; Lopaschuk, G.D. Advances in Myocardial Energy Metabolism: Metabolic Remodelling in Heart Failure and Beyond. Cardiovasc. Res. 2024, 120, 1996–2016. [Google Scholar] [CrossRef] [PubMed]
- Vega, R.B.; Kelly, D.P. Cardiac Nuclear Receptors: Architects of Mitochondrial Structure and Function. J. Clin. Investig. 2017, 127, 1155–1164. [Google Scholar] [CrossRef]
- Pavlović, N.; Križanac, M.; Kumrić, M.; Vukojević, K.; Božić, J. Mitochondrial Dysfunction: The Silent Catalyst of Kidney Disease Progression. Cells 2025, 14, 794. [Google Scholar] [CrossRef]
- Bayeva, M.; Gheorghiade, M.; Ardehali, H. Mitochondria as a Therapeutic Target in Heart Failure. J. Am. Coll. Cardiol. 2013, 61, 599–610. [Google Scholar] [CrossRef]
- Schwemmlein, J.; Maack, C.; Bertero, E. Mitochondria as Therapeutic Targets in Heart Failure. Curr. Heart Fail. Rep. 2022, 19, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.K.; Lackey, A.; Snyder, J.; Karhadkar, S.; Rao, A.D.; DiCarlo, A.; Sato, P.Y. Mitochondrial Membrane Intracellular Communication in Healthy and Diseased Myocardium. Front. Cell Dev. Biol. 2020, 8, 609241. [Google Scholar] [CrossRef]
- Tokuyama, T.; Yanagi, S. Role of Mitochondrial Dynamics in Heart Diseases. Genes 2023, 14, 1876. [Google Scholar] [CrossRef]
- Federico, M.; De la Fuente, S.; Palomeque, J.; Sheu, S.S. The Role of Mitochondria in Metabolic Disease: A Special Emphasis on Heart Dysfunction. J. Physiol. 2021, 599, 3477–3493. [Google Scholar] [CrossRef]
- Nan, J.; Zhu, W.; Rahman, M.S.; Liu, M.; Li, D.; Su, S.; Zhang, N.; Hu, X.; Yu, H.; Gupta, M.P.; et al. Molecular Regulation of Mitochondrial Dynamics in Cardiac Disease. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1260–1273. [Google Scholar] [CrossRef]
- Morciano, G.; Vitto, V.A.M.; Bouhamida, E.; Giorgi, C.; Pinton, P. Mitochondrial Bioenergetics and Dynamism in the Failing Heart. Life 2021, 11, 436. [Google Scholar] [CrossRef] [PubMed]
- Stanley, W.C.; Recchia, F.A.; Lopaschuk, G.D. Myocardial Substrate Metabolism in the Normal and Failing Heart. Physiol. Rev. 2005, 85, 1093–1129. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Ussher, J.R.; Folmes, C.D.L.; Jaswal, J.S.; Stanley, W.C. Myocardial Fatty Acid Metabolism in Health and Disease. Physiol. Rev. 2010, 90, 207–258. [Google Scholar] [CrossRef]
- Tuomainen, T.; Tavi, P. The Role of Cardiac Energy Metabolism in Cardiac Hypertrophy and Failure. Exp. Cell Res. 2017, 360, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.A.; O’Rourke, B. Cardiac Mitochondria and Arrhythmias. Cardiovasc. Res. 2010, 88, 241–249. [Google Scholar] [CrossRef]
- Rich, P.R. The Molecular Machinery of Keilin’s Respiratory Chain. Proc. Biochem. Soc. Trans. 2003, 31, 1095–1105. [Google Scholar] [CrossRef]
- Murphy, E.; Ardehali, H.; Balaban, R.S.; DiLisa, F.; Dorn, G.W.; Kitsis, R.N.; Otsu, K.; Ping, P.; Rizzuto, R.; Sack, M.N.; et al. Mitochondrial Function, Biology, and Role in Disease. Circ. Res. 2016, 118, 1960–1991. [Google Scholar] [CrossRef]
- Campos, J.C.; Bozi, L.H.M.; Bechara, L.R.G.; Lima, V.M.; Ferreira, J.C.B. Mitochondrial Quality Control in Cardiac Diseases. Front. Physiol. 2016, 7, 479. [Google Scholar] [CrossRef]
- Youle, R.J.; Van Der Bliek, A.M. Mitochondrial Fission, Fusion, and Stress. Science 2012, 337, 1062–1065. [Google Scholar] [CrossRef]
- Puigserver, P.; Spiegelman, B.M. Peroxisome Proliferator-Activated Receptor-γ Coactivator 1α (PGC-1α): Transcriptional Coactivator and Metabolic Regulator. Endocr. Rev. 2003, 24, 78–90. [Google Scholar] [CrossRef]
- Ashrafi, G.; Schwarz, T.L. The Pathways of Mitophagy for Quality Control and Clearance of Mitochondria. Cell Death Differ. 2013, 20, 31–42. [Google Scholar] [CrossRef]
- Kubli, D.A.; Gustafsson, Å.B. Mitochondria and Mitophagy: The Yin and Yang of Cell Death Control. Circ. Res. 2012, 111, 1208–1221. [Google Scholar] [CrossRef]
- Dorn, G.W. Mitochondrial Dynamics in Heart Disease. Biochim. Biophys. Acta Mol. Cell Res. 2013, 1833, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Elliott, P.; Andersson, B.; Arbustini, E.; Bilinska, Z.; Cecchi, F.; Charron, P.; Dubourg, O.; Kühl, U.; Maisch, B.; McKenna, W.J.; et al. Classification of the Cardiomyopathies: A Position Statement from the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2008, 29, 270–276. [Google Scholar] [CrossRef]
- Maron, B.J.; Towbin, J.A.; Thiene, G.; Antzelevitch, C.; Corrado, D.; Arnett, D.; Moss, A.J.; Seidman, C.E.; Young, J.B. Contemporary Definitions and Classification of the Cardiomyopathies. Circulation 2006, 113, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, G.; Steinberg, C.; Dubois, M.; Sénéchal, M. What the Cardiologist Should Know About Mitochondrial Cardiomyopathy? Can. J. Cardiol. 2019, 35, 221–224. [Google Scholar] [CrossRef] [PubMed]
- El-Hattab, A.W.; Scaglia, F. Mitochondrial Cardiomyopathies. Front. Cardiovasc. Med. 2016, 3, 25. [Google Scholar] [CrossRef]
- Neubauer, S. The Failing Heart—An Engine Out of Fuel. N. Engl. J. Med. 2007, 356, 1140–1151. [Google Scholar] [CrossRef]
- Paillard, M.; Abdellatif, M.; Andreadou, I.; Bär, C.; Bertrand, L.; Brundel, B.J.J.M.; Chiva-Blanch, G.; Davidson, S.M.; Dawson, D.; Di Lisa, F.; et al. Mitochondrial Targets in Ischaemic Heart Disease and Heart Failure, and Their Potential for a More Efficient Clinical Translation. A Scientific Statement of the ESC Working Group on Cellular Biology of the Heart and the ESC Working Group on Myocardial Function. Eur. J. Heart Fail. 2025, 27, 1720–1736. [Google Scholar] [CrossRef]
- Luan, Y.; Luan, Y.; Feng, Q.; Chen, X.; Ren, K.D.; Yang, Y. Emerging Role of Mitophagy in the Heart: Therapeutic Potentials to Modulate Mitophagy in Cardiac Diseases. Oxid. Med. Cell. Longev. 2021, 2021, 3259963. [Google Scholar] [CrossRef]
- Carinci, M.; Vezzani, B.; Patergnani, S.; Ludewig, P.; Lessmann, K.; Magnus, T.; Casetta, I.; Pugliatti, M.; Pinton, P.; Giorgi, C. Different Roles of Mitochondria in Cell Death and Inflammation: Focusing on Mitochondrial Quality Control in Ischemic Stroke and Reperfusion. Biomedicines 2021, 9, 169. [Google Scholar] [CrossRef]
- Maack, C.; Dudek, J.; Bertero, E.; Tampakakis, E.; Vernon, H.J. Mitochondrial Cardiomyopathies: Pathogenesis, Diagnosis, and Treatment. Eur. Heart J. 2025, 46, 4060–4075. [Google Scholar] [CrossRef]
- Yang, J.; Guo, Q.; Feng, X.; Liu, Y.; Zhou, Y. Mitochondrial Dysfunction in Cardiovascular Diseases: Potential Targets for Treatment. Front. Cell Dev. Biol. 2022, 10, 841523. [Google Scholar] [CrossRef]
- Tsutsui, H.; Kinugawa, S.; Matsushima, S. Oxidative Stress and Mitochondrial DNA Damage in Heart Failure. Circ. J. 2008, 72, A31–A37. [Google Scholar] [CrossRef]
- Dubois-Deruy, E.; Peugnet, V.; Turkieh, A.; Pinet, F. Oxidative Stress in Cardiovascular Diseases. Antioxidants 2020, 9, 864. [Google Scholar] [CrossRef]
- Werbner, B.; Tavakoli-Rouzbehani, O.M.; Fatahian, A.N.; Boudina, S. The Dynamic Interplay between Cardiac Mitochondrial Health and Myocardial Structural Remodeling in Metabolic Heart Disease, Aging, and Heart Failure. J. Cardiovasc. Aging 2023, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Marquez, J.; Lee, S.R.; Kim, N.; Han, J. Rescue of Heart Failure by Mitochondrial Recovery. Int. Neurourol. J. 2016, 20, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Peoples, J.N.; Saraf, A.; Ghazal, N.; Pham, T.T.; Kwong, J.Q. Mitochondrial Dysfunction and Oxidative Stress in Heart Disease. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Tian, R. Mitochondrial Dysfunction in Pathophysiology of Heart Failure. J. Clin. Investig. 2018, 128, 3716–3726. [Google Scholar] [CrossRef]
- Bhullar, S.K.; Dhalla, N.S. Status of Mitochondrial Oxidative Phosphorylation during the Development of Heart Failure. Antioxidants 2023, 12, 1941. [Google Scholar] [CrossRef]
- ten Hove, M.; Neubauer, S. MR Spectroscopy in Heart Failure—Clinical and Experimental Findings. Heart Fail. Rev. 2007, 12, 48–57. [Google Scholar] [CrossRef]
- Hao, Y.D.; Zhao, Y.X.; Yang, S.W.; Zhou, Y.J. High-Energy Phosphates and Ischemic Heart Disease: From Bench to Bedside. Front. Cardiovasc. Med. 2021, 8, 675608. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, D.J. The Failing Heart: Energy Supply, Processing, and Transfer. Methodist DeBakey Cardiovasc. J. 2017, 13, 3. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dridi, H.; Santulli, G.; Bahlouli, L.; Miotto, M.C.; Weninger, G.; Marks, A.R. Mitochondrial Calcium Overload Plays a Causal Role in Oxidative Stress in the Failing Heart. Biomolecules 2023, 13, 1409. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.; Albakri, J.S.; Allemailem, K.S.; Sultan, A.; Alwanian, W.M.; Alrumaihi, F.; Almansour, N.M.; Aldakheel, F.M.; Khalil, F.M.A.; Abduallah, A.M.; et al. Mitochondrial Dysfunction and Calcium Homeostasis in Heart Failure: Exploring the Interplay between Oxidative Stress and Cardiac Remodeling for Future Therapeutic Innovations. Curr. Probl. Cardiol. 2025, 50, 102968. [Google Scholar] [CrossRef]
- Manolis, A.S.; Manolis, A.A.; Manolis, T.A.; Apostolaki, N.E.; Apostolopoulos, E.J.; Melita, H.; Katsiki, N. Mitochondrial Dysfunction in Cardiovascular Disease: Current Status of Translational Research/Clinical and Therapeutic Implications. Med. Res. Rev. 2021, 41, 275–313. [Google Scholar] [CrossRef]
- Santulli, G.; Xie, W.; Reiken, S.R.; Marks, A.R. Mitochondrial Calcium Overload Is a Key Determinant in Heart Failure. Proc. Natl. Acad. Sci. USA 2015, 112, 11389–11394. [Google Scholar] [CrossRef]
- Popoiu, T.-A.; Maack, C.; Bertero, E. Mitochondrial Calcium Signaling and Redox Homeostasis in Cardiac Health and Disease. Front. Mol. Med. 2023, 3, 1235188. [Google Scholar] [CrossRef]
- Xu, H.X.; Cui, S.M.; Zhang, Y.M.; Ren, J. Mitochondrial Ca2+ Regulation in the Etiology of Heart Failure: Physiological and Pathophysiological Implications. Acta Pharmacol. Sin. 2020, 41, 1301–1309. [Google Scholar] [CrossRef]
- Quiles, J.M.; Gustafsson, Å.B. The Role of Mitochondrial Fission in Cardiovascular Health and Disease. Nat. Rev. Cardiol. 2022, 19, 723–736. [Google Scholar] [CrossRef]
- Svaguša, T.; Martinić, M.; Martinić, M.; Kovačević, L.; Šepac, A.; Miličić, D.; Bulum, J.; Starčević, B.; Sirotković-Skerlev, M.; Seiwerth, F.; et al. Mitochondrial Unfolded Protein Response, Mitophagy and Other Mitochondrial Quality Control Mechanisms in Heart Disease and Aged Heart. Croat. Med. J. 2020, 61, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ren, T.; Li, C.; Wu, Q.; Liu, J.; Guan, X.; Chang, X.; Liu, Z.; Liu, R. Mechanisms Involved in the Regulation of Mitochondrial Quality Control by PGAM5 in Heart Failure. Cell Stress. Chaperones 2024, 29, 510–518. [Google Scholar] [CrossRef]
- Javadifar, A.; Tahani, M.; Khayat, S.; Nasab, S.R.; Karav, S.; Kesharwani, P.; Sahebkar, A. Targeting Mitophagy in the Heart: Exploring the Therapeutic Potential of MicroRNAs. Mech. Ageing Dev. 2025, 226, 112082. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Bi, Y.; Zhao, Z.; Wang, S.; Lin, S.; Yang, Z.; Wang, X.; Mao, J. Emerging Role of Mitophagy in Heart Failure: From Molecular Mechanism to Targeted Therapy. Cell Cycle 2023, 22, 906–918. [Google Scholar] [CrossRef] [PubMed]
- Shires, S.E.; Gustafsson, Å.B. Mitophagy and Heart Failure. J. Mol. Med. 2015, 93, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Duan, J.; Wang, Q.; Xu, S.; Zhou, S.; Yao, K. Mitochondrial Dynamics and Mitophagy in Cardiometabolic Disease. Front. Cardiovasc. Med. 2022, 9, 917135. [Google Scholar] [CrossRef]
- Vásquez-Trincado, C.; García-Carvajal, I.; Pennanen, C.; Parra, V.; Hill, J.A.; Rothermel, B.A.; Lavandero, S. Mitochondrial Dynamics, Mitophagy and Cardiovascular Disease. J. Physiol. 2016, 594, 509–525. [Google Scholar] [CrossRef]
- Qiu, Z.; Wei, Y.; Song, Q.; Du, B.; Wang, H.; Chu, Y.; Hu, Y. The Role of Myocardial Mitochondrial Quality Control in Heart Failure. Front. Pharmacol. 2019, 10, 1404. [Google Scholar] [CrossRef]
- Ng, S.M.; Neubauer, S.; Rider, O.J. Myocardial Metabolism in Heart Failure. Curr. Heart Fail. Rep. 2023, 20, 63–75. [Google Scholar] [CrossRef]
- Soares, R.O.S.; Losada, D.M.; Jordani, M.C.; Évora, P.; Castro-e-Silva, O. Ischemia/Reperfusion Injury Revisited: An Overview of the Latest Pharmacological Strategies. Int. J. Mol. Sci. 2019, 20, 5034. [Google Scholar] [CrossRef]
- Dyck, J.R.B.; Cheng, J.F.; Stanley, W.C.; Barr, R.; Chandler, M.P.; Brown, S.; Wallace, D.; Arrhenius, T.; Harmon, C.; Yang, G.; et al. Malonyl Coenzyme a Decarboxylase Inhibition Protects the Ischemic Heart by Inhibiting Fatty Acid Oxidation and Stimulating Glucose Oxidation. Circ. Res. 2004, 94, e78–e84. [Google Scholar] [CrossRef]
- Li, X.; Wu, F.; Günther, S.; Looso, M.; Kuenne, C.; Zhang, T.; Wiesnet, M.; Klatt, S.; Zukunft, S.; Fleming, I.; et al. Inhibition of Fatty Acid Oxidation Enables Heart Regeneration in Adult Mice. Nature 2023, 622, 619–626. [Google Scholar] [CrossRef]
- Ying, C. Viral Myocarditis. Yale J. Biol. Med. 2024, 97, 515–520. [Google Scholar] [CrossRef]
- Qi, Y.; Yin, J.; Xia, W.; Yang, S. Exploring the Role of Mitochondrial Antiviral Signaling Protein in Cardiac Diseases. Front. Immunol. 2025, 16, 1540774. [Google Scholar] [CrossRef]
- Chen, J.; Shao, J.; Wang, Y.; Wu, K.; Huang, M. OPA1, a Molecular Regulator of Dilated Cardiomyopathy. J. Cell. Mol. Med. 2023, 27, 3017–3025. [Google Scholar] [CrossRef]
- Hinton, A.; Claypool, S.M.; Neikirk, K.; Senoo, N.; Wanjalla, C.N.; Kirabo, A.; Williams, C.R. Mitochondrial Structure and Function in Human Heart Failure. Circ. Res. 2024, 135, 372–396. [Google Scholar] [CrossRef] [PubMed]
- Da Dalt, L.; Cabodevilla, A.G.; Goldberg, I.J.; Norata, G.D. Cardiac Lipid Metabolism, Mitochondrial Function, and Heart Failure. Cardiovasc. Res. 2023, 119, 1905–1914. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, V.; Waddingham, M.T.; Tsuchimochi, H.; Maack, C.; Pearson, J.T. Mechano-Energetic Uncoupling in Hypertrophic Cardiomyopathy: Pathophysiological Mechanisms and Therapeutic Opportunities. J. Mol. Cell. Cardiol. Plus 2023, 4, 100036. [Google Scholar] [CrossRef] [PubMed]
- Witjas-Paalberends, E.R.; Güclü, A.; Germans, T.; Knaapen, P.; Harms, H.J.; Vermeer, A.M.C.; Christiaans, I.; Wilde, A.A.M.; DosRemedios, C.; Lammertsma, A.A.; et al. Gene-Specific Increase in the Energetic Cost of Contraction in Hypertrophic Cardiomyopathy Caused by Thick Filament Mutations. Cardiovasc. Res. 2014, 103, 248–257. [Google Scholar] [CrossRef]
- Tsampasian, V.; Cameron, D.; Sobhan, R.; Bazoukis, G.; Vassiliou, V.S. Phosphorus Magnetic Resonance Spectroscopy (31P MRS) and Cardiovascular Disease: The Importance of Energy. Medicina 2023, 59, 174. [Google Scholar] [CrossRef]
- Crilley, J.G.; Boehm, E.A.; Blair, E.; Rajagopalan, B.; Blamire, A.M.; Styles, P.; McKenna, W.J.; Östman-Smith, I.; Clarke, K.; Watkins, H. Hypertrophic Cardiomyopathy Due to Sarcomeric Gene Mutations Is Characterized by Impaired Energy Metabolism Irrespective of the Degree of Hypertrophy. J. Am. Coll. Cardiol. 2003, 41, 1776–1782. [Google Scholar] [CrossRef]
- Nollet, E.E.; Duursma, I.; Rozenbaum, A.; Eggelbusch, M.; Wüst, R.C.I.; Schoonvelde, S.A.C.; Michels, M.; Jansen, M.; Van Der Wel, N.N.; Bedi, K.C.; et al. Mitochondrial Dysfunction in Human Hypertrophic Cardiomyopathy Is Linked to Cardiomyocyte Architecture Disruption and Corrected by Improving NADH-Driven Mitochondrial Respiration. Eur. Heart J. 2023, 44, 1170–1185. [Google Scholar] [CrossRef]
- Christiansen, L.B.; Dela, F.; Koch, J.; Hansen, C.N.; Leifsson, P.S.; Yokota, T. Impaired Cardiac Mitochondrial Oxidative Phosphorylation and Enhanced Mitochondrial Oxidative Stress in Feline Hypertrophic Cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H1237–H1247. [Google Scholar] [CrossRef]
- Porcari, A.; Fontana, M.; Gillmore, J.D. Transthyretin Cardiac Amyloidosis. Cardiovasc. Res. 2022, 118, 3517–3535. [Google Scholar] [CrossRef] [PubMed]
- Dittloff, K.T.; Spanghero, E.; Solís, C.; Banach, K.; Russell, B. Transthyretin Deposition Alters Cardiomyocyte Sarcomeric Architecture, Calcium Transients, and Contractile Force. Physiol. Rep. 2022, 10, e15207. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Guan, J.; Jiang, B.; Brenner, D.A.; Del Monte, F.; Ward, J.E.; Connors, L.H.; Sawyer, D.B.; Semigran, M.J.; Macgillivray, T.E.; et al. Amyloidogenic Light Chains Induce Cardiomyocyte Contractile Dysfunction and Apoptosis via a Non-Canonical P38α MAPK Pathway. Proc. Natl. Acad. Sci. USA 2010, 107, 4188–4193. [Google Scholar] [CrossRef]
- Brenner, D.A.; Jain, M.; Pimentel, D.R.; Wang, B.; Connors, L.H.; Skinner, M.; Apstein, C.S.; Liao, R. Human Amyloidogenic Light Chains Directly Impair Cardiomyocyte Function Through an Increase in Cellular Oxidant Stress. Circ. Res. 2004, 94, 1008–1010. [Google Scholar] [CrossRef]
- McWilliams-Koeppen, H.P.; Foster, J.S.; Hackenbrack, N.; Ramirez-Alvarado, M.; Donohoe, D.; Williams, A.; Macy, S.; Wooliver, C.; Wortham, D.; Morrell-Falvey, J.; et al. Light Chain Amyloid Fibrils Cause Metabolic Dysfunction in Human Cardiomyocytes. PLoS ONE 2015, 10, e0137716. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Chen, W. Molecular Mechanisms and Emerging Therapies in Wild-Type Transthyretin Amyloid Cardiomyopathy. Heart Fail. Rev. 2024, 29, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Pieroni, M.; Moon, J.C.; Arbustini, E.; Barriales-Villa, R.; Camporeale, A.; Vujkovac, A.C.; Elliott, P.M.; Hagege, A.; Kuusisto, J.; Linhart, A.; et al. Cardiac Involvement in Fabry Disease: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2021, 77, 922–936. [Google Scholar] [CrossRef]
- Weissman, D.; Dudek, J.; Sequeira, V.; Maack, C. Fabry Disease: Cardiac Implications and Molecular Mechanisms. Curr. Heart Fail. Rep. 2024, 21, 81–100. [Google Scholar] [CrossRef]
- Majamaa, K.; Moilanen, J.S.; Uimonen, S.; Remes, A.M.; Salmela, P.I.; Kärppä, M.; Majamaa-Voltti, K.A.M.; Rusanen, H.; Sorri, M.; Peuhkurinen, K.J.; et al. Epidemiology of A3243G, the Mutation for Mitochondrial Encephalomyopathy, Lactic Acidosis, and Strokelike Episodes: Prevalence of the Mutation in an Adult Population. Am. J. Hum. Genet. 1998, 63, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Ryytty, S.; Modi, S.R.; Naumenko, N.; Shakirzyanova, A.; Rahman, M.O.; Vaara, M.; Suomalainen, A.; Tavi, P.; Hämäläinen, R.H. Varied Responses to a High m.3243A>G Mutation Load and Respiratory Chain Dysfunction in Patient-Derived Cardiomyocytes. Cells 2022, 11, 2593. [Google Scholar] [CrossRef]
- Lopes, L.R.; Macken, W.L.; Preez, S.D.; Kotwal, H.; Savvatis, K.; Sekhri, N.; Mohiddin, S.A.; Kabiljo, R.; Pitceathly, R.D.S. An Analysis of Mitochondrial Variation in Cardiomyopathy Patients from the 100,000 Genomes Cohort: M.4300A>G as a Cause of Genetically Elusive Hypertrophic Cardiomyopathy. Hum. Genom. 2024, 18, 136. [Google Scholar] [CrossRef] [PubMed]
- Berardo, A.; Domínguez-González, C.; Engelstad, K.; Hirano, M. Advances in Thymidine Kinase 2 Deficiency: Clinical Aspects, Translational Progress, and Emerging Therapies. J. Neuromuscul. Dis. 2022, 9, 225–235. [Google Scholar] [CrossRef]
- Dudek, J.; Maack, C. Barth Syndrome Cardiomyopathy. Cardiovasc. Res. 2017, 113, 399–410. [Google Scholar] [CrossRef]
- Gallo, G.; Rubattu, S.; Volpe, M. Mitochondrial Dysfunction in Heart Failure: From Pathophysiological Mechanisms to Therapeutic Opportunities. Int. J. Mol. Sci. 2024, 25, 2667. [Google Scholar] [CrossRef]
- Qi, X.; Zhu, Z.; Wang, Y.; Wen, Z.; Jiang, Z.; Zhang, L.; Pang, Y.; Lu, J. Research Progress on the Relationship between Mitochondrial Function and Heart Failure: A Bibliometric Study from 2002 to 2021. Front. Mol. Biosci. 2022, 9, 1036364. [Google Scholar] [CrossRef]
- Watson, W.D.; Arvidsson, P.M.; Miller, J.J.J.; Lewis, A.J.; Rider, O.J. A Mitochondrial Basis for Heart Failure Progression. Cardiovasc. Drugs Ther. 2024, 38, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Xiao, W.; Shi, Y.; Liu, M.; Xiao, X. Heat Shock Pretreatment Inhibited the Release of Smac/DIABLO from Mitochondria and Apoptosis Induced by Hydrogen Peroxide in Cardiomyocytes and C2C12 Myogenic Cells. Cell Stress. Chaperones 2005, 10, 252–262. [Google Scholar] [CrossRef]
- Kim, N.H.; Kang, P.M. Apoptosis in Cardiovascular Diseases: Mechanism and Clinical Implications. Korean Circ. J. 2010, 40, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidis, K.; Whelan, R.S.; Kitsis, R.N. Mechanisms of Cell Death in Heart Disease. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1552–1562. [Google Scholar] [CrossRef]
- Guerrero, A.D.; Schmitz, I. Promotion of Caspase Activation by Caspase-9-Mediated Feedback Amplification of Mitochondrial Damage. J. Clin. Cell Immunol. 2012, 3, 1000126. [Google Scholar] [CrossRef]
- Chen, L.; Knowlton, A.A. Mitochondria and Heart Failure: New Insights into an Energetic Problem. Minerva Cardioangiol. 2010, 58, 213–229. [Google Scholar]
- He, X.; Du, T.; Long, T.; Liao, X.; Dong, Y.; Huang, Z.P. Signaling Cascades in the Failing Heart and Emerging Therapeutic Strategies. Signal Transduct. Target. Ther. 2022, 7, 134. [Google Scholar] [CrossRef]
- Javadov, S.; Jang, S.; Parodi-Rullán, R.; Khuchua, Z.; Kuznetsov, A.V. Mitochondrial Permeability Transition in Cardiac Ischemia–Reperfusion: Whether Cyclophilin D Is a Viable Target for Cardioprotection? Cell. Mol. Life Sci. 2017, 74, 2795–2813. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Y.; Cui, M.; Jin, L.; Wang, Y.; Lv, F.; Liu, Y.; Zheng, W.; Shang, H.; Zhang, J.; et al. CaMKII Is a RIP3 Substrate Mediating Ischemia- and Oxidative Stress-Induced Myocardial Necroptosis. Nat. Med. 2016, 22, 175–182. [Google Scholar] [CrossRef]
- Ying, L.; Benjanuwattra, J.; Chattipakorn, S.C.; Chattipakorn, N. The Role of RIPK3-Regulated Cell Death Pathways and Necroptosis in the Pathogenesis of Cardiac Ischaemia-Reperfusion Injury. Acta Physiol. 2021, 231, e13541. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Wan, K.; Yin, M.; Hu, P.; Que, Y.; Zhou, X.; Zhang, L.; Li, T.; Du, Y.; Xu, G.; et al. RIPK3 Induces Cardiomyocyte Necroptosis via Inhibition of AMPK-Parkin-Mitophagy in Cardiac Remodelling after Myocardial Infarction. Oxid. Med. Cell Longev. 2021, 2021, 6635955. [Google Scholar] [CrossRef]
- Lemasters, J.J. V. Necrapoptosis and the Mitochondrial Permeability Transition: Shared Pathways to Necrosis and Apoptosis. Am. J. Physiol. 1999, 276, G1–G6. [Google Scholar] [CrossRef]
- Nakayama, H.; Otsu, K. Mitochondrial DNA as an Inflammatory Mediator in Cardiovascular Diseases. Biochem. J. 2018, 475, 839–852. [Google Scholar] [CrossRef] [PubMed]
- Silvis, M.J.M.; Kaffka genaamd Dengler, S.E.; Odille, C.A.; Mishra, M.; van der Kaaij, N.P.; Doevendans, P.A.; Sluijter, J.P.G.; de Kleijn, D.P.V.; de Jager, S.C.A.; Bosch, L.; et al. Damage-Associated Molecular Patterns in Myocardial Infarction and Heart Transplantation: The Road to Translational Success. Front. Immunol. 2020, 11, 599511. [Google Scholar] [CrossRef]
- Mortensen, S.A.; Rosenfeldt, F.; Kumar, A.; Dolliner, P.; Filipiak, K.J.; Pella, D.; Alehagen, U.; Steurer, G.; Littarru, G.P. The Effect of Coenzyme Q10 on Morbidity and Mortality in Chronic Heart Failure: Results from Q-SYMBIO: A Randomized Double-Blind Trial. JACC Heart Fail. 2014, 2, 641–649. [Google Scholar] [CrossRef]
- Leshnower, B.G.; Kanemoto, S.; Matsubara, M.; Sakamoto, H.; Hinmon, R.; Gorman, J.H.; Gorman, R.C. Cyclosporine Preserves Mitochondrial Morphology After Myocardial Ischemia/Reperfusion Independent of Calcineurin Inhibition. Ann. Thorac. Surg. 2008, 86, 1286–1292. [Google Scholar] [CrossRef]
- Cung, T.-T.; Morel, O.; Cayla, G.; Rioufol, G.; Garcia-Dorado, D.; Angoulvant, D.; Bonnefoy-Cudraz, E.; Guérin, P.; Elbaz, M.; Delarche, N.; et al. Cyclosporine before PCI in Patients with Acute Myocardial Infarction. N. Engl. J. Med. 2015, 373, 1021–1031. [Google Scholar] [CrossRef]
- Ottani, F.; Latini, R.; Staszewsky, L.; La Vecchia, L.; Locuratolo, N.; Sicuro, M.; Masson, S.; Barlera, S.; Milani, V.; Lombardi, M.; et al. Cyclosporine A in Reperfused Myocardial Infarction the Multicenter, Controlled, Open-Label CYCLE Trial. J. Am. Coll. Cardiol. 2016, 67, 365–374. [Google Scholar] [CrossRef]
- Arany, Z.; He, H.; Lin, J.; Hoyer, K.; Handschin, C.; Toka, O.; Ahmad, F.; Matsui, T.; Chin, S.; Wu, P.H.; et al. Transcriptional Coactivator PGC-1α Controls the Energy State and Contractile Function of Cardiac Muscle. Cell Metab. 2005, 1, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Zhu, Y.; Deng, C.; Liang, Z.; Chen, J.; Chen, Y.; Wang, X.; Liu, Y.; Tian, Y.; Yang, Y. Peroxisome Proliferator-Activated Receptor Gamma Coactivator-1 (PGC-1) Family in Physiological and Pathophysiological Process and Diseases. Signal Transduct. Target. Ther. 2024, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, K.; Takegawa, R.; Shoaib, M.; Aoki, T.; Choudhary, R.C.; Kuschner, C.E.; Nishikimi, M.; Miyara, S.J.; Rolston, D.M.; Guevara, S.; et al. Mitochondrial Transplantation Therapy for Ischemia Reperfusion Injury: A Systematic Review of Animal and Human Studies. J. Transl. Med. 2021, 19, 214. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour, P.; Sadeghsoltani, F.; Saghebasl, S.; Boroumand, S.; Khanicheragh, P.; Tafti, S.H.A.; Rahbarghazi, R.; Rahmati, M. Mitochondrial Transplantation for Cardioprotection and Induction of Angiogenesis in Ischemic Heart Disease. Stem Cell Res. Ther. 2025, 16, 54. [Google Scholar] [CrossRef]
- Wisløff, U.; Støylen, A.; Loennechen, J.P.; Bruvold, M.; Rognmo, Ø.; Haram, P.M.; Tjønna, A.E.; Helgerud, J.; Slørdahl, S.A.; Lee, S.J.; et al. Superior Cardiovascular Effect of Aerobic Interval Training versus Moderate Continuous Training in Heart Failure Patients: A Randomized Study. Circulation 2007, 115, 3086–3094. [Google Scholar] [CrossRef]
- Eisenberg, T.; Abdellatif, M.; Schroeder, S.; Primessnig, U.; Stekovic, S.; Pendl, T.; Harger, A.; Schipke, J.; Zimmermann, A.; Schmidt, A.; et al. Cardioprotection and Lifespan Extension by the Natural Polyamine Spermidine. Nat. Med. 2016, 22, 1428–1438. [Google Scholar] [CrossRef]
- Fragasso, G.; Salerno, A.; Lattuada, G.; Cuko, A.; Calori, G.; Scollo, A.; Ragogna, F.; Arioli, F.; Bassanelli, G.; Spoladore, R.; et al. Effect of Partial Inhibition of Fatty Acid Oxidation by Trimetazidine on Whole Body Energy Metabolism in Patients with Chronic Heart Failure. Heart 2011, 97, 1495–1500. [Google Scholar] [CrossRef] [PubMed]
- Ferry, A. Dapagliflozin for Patients with Heart Failure and Reduced Ejection Fraction. J. Am. Acad. Physician Assist. 2022, 35, 51–53. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner–La Rocca, H.-P.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef]
- Saucedo-Orozco, H.; Voorrips, S.N.; de Boer, R.A.; Westenbrink, B.D.; Yurista, S.R. SGLT2 Inhibitors and Ketone Metabolism in Heart Failure. J. Lipid Atheroscler. 2022, 11, 1. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Verma, S. Mechanisms of Cardiovascular Benefits of Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors: A State-of-the-Art Review. JACC Basic Transl. Sci. 2020, 5, 632–644. [Google Scholar] [CrossRef]
- Bonora, M.; Wieckowski, M.R.; Sinclair, D.A.; Kroemer, G.; Pinton, P.; Galluzzi, L. Targeting Mitochondria for Cardiovascular Disorders: Therapeutic Potential and Obstacles. Nat. Rev. Cardiol. 2019, 16, 33–55. [Google Scholar] [CrossRef]
- Kiyuna, L.A.; e Albuquerque, R.P.; Chen, C.H.; Mochly-Rosen, D.; Ferreira, J.C.B. Targeting Mitochondrial Dysfunction and Oxidative Stress in Heart Failure: Challenges and Opportunities. Free Radic. Biol. Med. 2018, 129, 155–168. [Google Scholar] [CrossRef]
- Trnka, J.; Elkalaf, M.; Andě, M. Lipophilic Triphenylphosphonium Cations Inhibit Mitochondrial Electron Transport Chain and Induce Mitochondrial Proton Leak. PLoS ONE 2015, 10, e0121837. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.A.; Perry, J.B.; Allen, M.E.; Sabbah, H.N.; Stauffer, B.L.; Shaikh, S.R.; Cleland, J.G.F.; Colucci, W.S.; Butler, J.; Voors, A.A.; et al. Expert Consensus Document: Mitochondrial Function as a Therapeutic Target in Heart Failure. Nat. Rev. Cardiol. 2017, 14, 238–250. [Google Scholar] [CrossRef]
- Paraskevaidis, I.; Kourek, C.; Farmakis, D.; Tsougos, E. Mitochondrial Dysfunction in Cardiac Disease: The Fort Fell. Biomolecules 2024, 14, 1534. [Google Scholar] [CrossRef] [PubMed]
- Shayota, B.J. Biomarkers of Mitochondrial Disorders. Neurotherapeutics 2024, 21, e00325. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Raoof, M.; Chen, Y.; Sumi, Y.; Sursal, T.; Junger, W.; Brohi, K.; Itagaki, K.; Hauser, C.J. Circulating Mitochondrial DAMPs Cause Inflammatory Responses to Injury. Nature 2010, 464, 104–107. [Google Scholar] [CrossRef]
- Iyer, S.S.; He, Q.; Janczy, J.R.; Elliott, E.I.; Zhong, Z.; Olivier, A.K.; Sadler, J.J.; Knepper-Adrian, V.; Han, R.; Qiao, L.; et al. Mitochondrial Cardiolipin Is Required for Nlrp3 Inflammasome Activation. Immunity 2013, 39, 311–323. [Google Scholar] [CrossRef]
- Pustylnikov, S.; Costabile, F.; Beghi, S.; Facciabene, A. Targeting Mitochondria in Cancer: Current Concepts and Immunotherapy Approaches. Transl. Res. 2018, 202, 35–51. [Google Scholar] [CrossRef]
- Schroeder, M.A.; Clarke, K.; Neubauer, S.; Tyler, D.J. Hyperpolarized Magnetic Resonance: A Novel Technique for the in Vivo Assessment of Cardiovascular Disease. Circulation 2011, 124, 1580–1594. [Google Scholar] [CrossRef]
- Murphy, M.P.; Hartley, R.C. Mitochondria as a Therapeutic Target for Common Pathologies. Nat. Rev. Drug Discov. 2018, 17, 865–886. [Google Scholar] [CrossRef]
- Daubert, M.A.; Yow, E.; Dunn, G.; Marchev, S.; Barnhart, H.; Douglas, P.S.; O’Connor, C.; Goldstein, S.; Udelson, J.E.; Sabbah, H.N. Novel Mitochondria-Targeting Peptide in Heart Failure Treatment: A Randomized, Placebo-Controlled Trial of Elamipretide. Circ. Heart Fail. 2017, 10, e004389. [Google Scholar] [CrossRef]
- Kloner, R.A.; Hale, S.L.; Dai, W.; Gorman, R.C.; Shuto, T.; Koomalsingh, K.J.; Gorman, J.H.; Sloan, R.C.; Frasier, C.R.; Watson, C.A.; et al. Reduction of Ischemia/Reperfusion Injury with Bendavia, a Mitochondria-Targeting Cytoprotective Peptide. J. Am. Heart Assoc. 2012, 1, e001644. [Google Scholar] [CrossRef]
- Diguet, N.; Trammell, S.A.J.; Tannous, C.; Deloux, R.; Piquereau, J.; Mougenot, N.; Gouge, A.; Gressette, M.; Manoury, B.; Blanc, J.; et al. Nicotinamide Riboside Preserves Cardiac Function in a Mouse Model of Dilated Cardiomyopathy. Circulation 2018, 137, 2256–2273. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Longo, V.D.; Harvie, M. Impact of Intermittent Fasting on Health and Disease Processes. Ageing Res. Rev. 2017, 39, 46–58. [Google Scholar] [CrossRef] [PubMed]
- López-Lluch, G.; Navas, P. Calorie Restriction as an Intervention in Ageing. J. Physiol. 2016, 594, 2043–2060. [Google Scholar] [CrossRef] [PubMed]
| Therapeutic Strategy | Mechanism/Rationale | Evidence/Key Findings | References |
|---|---|---|---|
| Antioxidant supplementation (Coenzyme Q10) | Improves electron transport, reduces oxidative stress | Q-SYMBIO trial (420 patients): reduced major CV events and mortality | [105] |
| mPTP inhibition (Cyclosporine A) | Prevents mitochondrial permeability transition pore opening during reperfusion | Preclinical benefit; CIRCUS and CYCLE trials: no clinical efficacy in STEMI patients | [106,107,108] |
| PGC-1 family activation (PGC-1α, PGC-1β, PRC) | Enhances mitochondrial biogenesis, OXPHOS, fatty acid oxidation, antioxidant defense | Knockout studies: PGC-1α deficiency reduces mitochondrial activity and cardiac energy supply | [109,110] |
| Mitochondrial transplantation | Delivery of healthy mitochondria to restore ATP production and reduce ROS | Preclinical: improved cardiomyocyte survival; Human feasibility: improved myocardial bioenergetics in pediatric ischemic cardiomyopathy | [111,112] |
| Exercise training | Enhances mitochondrial capacity, reverse remodeling, endothelial function | RCT: Aerobic interval training > moderate continuous training in post-infarction HF | [113] |
| Caloric restriction mimetic (Spermidine) | Promotes mitophagy, mitochondrial respiration, and cardioprotection | Animal studies: reduced hypertrophy, preserved diastolic function; Human data: higher intake → lower BP, fewer CV events | [114] |
| Metabolic modulation (Trimetazidine) | Shifts substrate utilization toward glucose oxidation | RCTs: improved EF, NYHA class, QoL; reduced resting energy expenditure | [115] |
| SGLT2 inhibitors (Dapagliflozin, Empagliflozin) | Enhance ketone utilization, mitochondrial respiration, and reduce oxidative stress | DAPA-HF, EMPEROR-Reduced, EMPEROR-Preserved, DELIVER trials: reduced CV death and HF hospitalization across HFrEF, HFpEF, HFmrEF | [116,117,118,119,120,121] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlović, N.; Todorović, P.; Maglica, M.; Kumrić, M.; Vukojević, K.; Pogorelić, Z.; Božić, J. Mitochondrial Dysfunction in Cardiomyopathy and Heart Failure: From Energetic Collapse to Therapeutic Opportunity. Biomolecules 2025, 15, 1572. https://doi.org/10.3390/biom15111572
Pavlović N, Todorović P, Maglica M, Kumrić M, Vukojević K, Pogorelić Z, Božić J. Mitochondrial Dysfunction in Cardiomyopathy and Heart Failure: From Energetic Collapse to Therapeutic Opportunity. Biomolecules. 2025; 15(11):1572. https://doi.org/10.3390/biom15111572
Chicago/Turabian StylePavlović, Nikola, Petar Todorović, Mirko Maglica, Marko Kumrić, Katarina Vukojević, Zenon Pogorelić, and Joško Božić. 2025. "Mitochondrial Dysfunction in Cardiomyopathy and Heart Failure: From Energetic Collapse to Therapeutic Opportunity" Biomolecules 15, no. 11: 1572. https://doi.org/10.3390/biom15111572
APA StylePavlović, N., Todorović, P., Maglica, M., Kumrić, M., Vukojević, K., Pogorelić, Z., & Božić, J. (2025). Mitochondrial Dysfunction in Cardiomyopathy and Heart Failure: From Energetic Collapse to Therapeutic Opportunity. Biomolecules, 15(11), 1572. https://doi.org/10.3390/biom15111572












