Translational Molecular and Fluid Biomarkers for Age-Related Macular Degeneration: Practical Insights from Animal Models and Humans
Abstract
1. Introduction
2. Methods
2.1. Literature Search Strategy
2.2. Study Selection
2.3. Evidence Grading
2.4. Data Synthesis
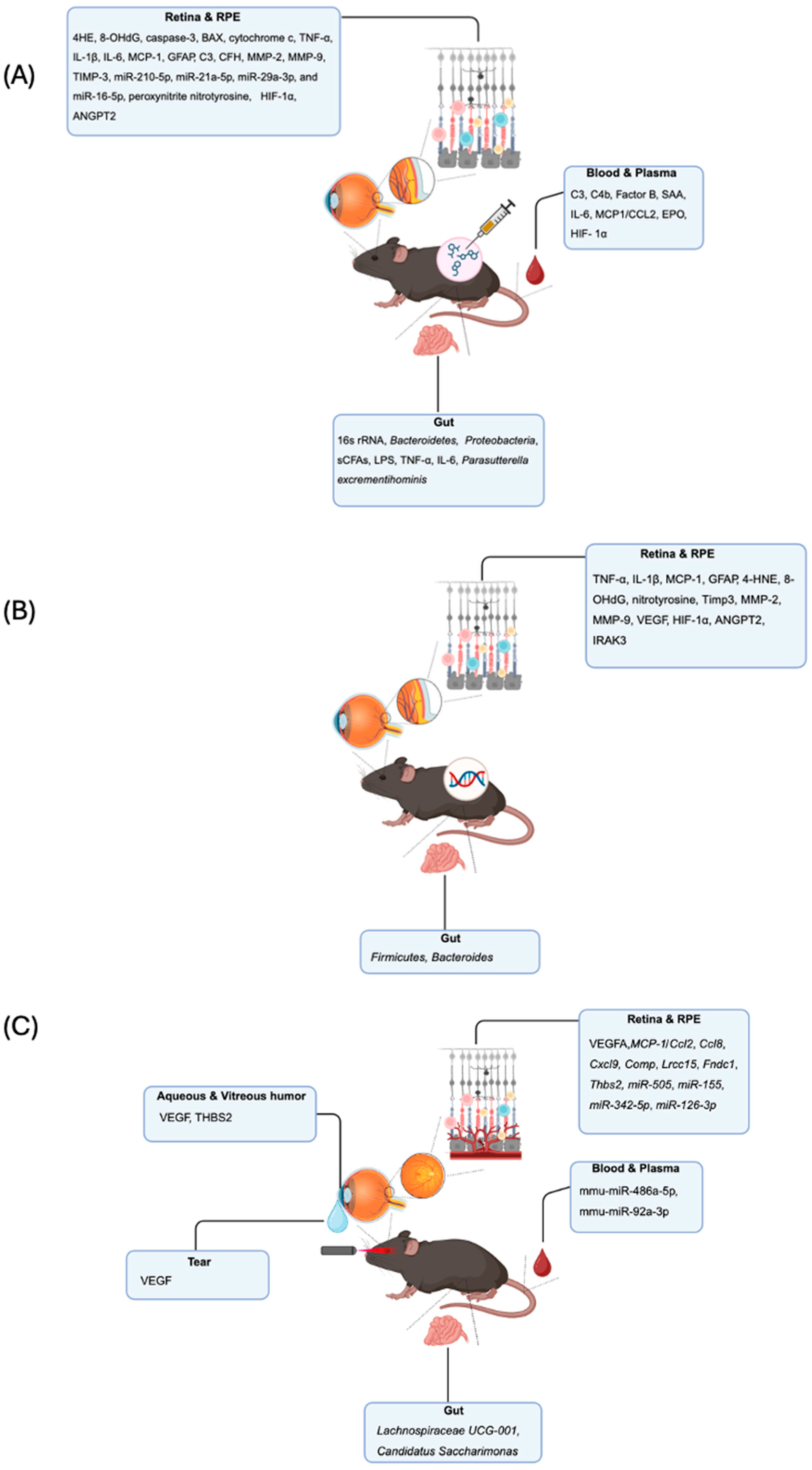
3. Preclinical Studies
3.1. Chemical Dry AMD Models for Mechanistic Discovery
3.1.1. Retina and RPE—Choroid Tissue Markers in Chemical Models
3.1.2. Measurable Systemic Biomarkers in Chemical Models
3.1.3. Directly Measurable Microbiome Changes in Chemical Models
3.2. Genetic Mouse Models for Dry AMD: Pathogenic Mechanisms and Biomarker Potential
3.2.1. Retina and RPE-Choroid Alteration in Genetic Models
3.2.2. Measurable Systemic and Microbiome Biomarkers from Genetic Models
3.3. Laser-Induced Neovascularization Wet AMD Model: Translational Biomarker Discovery
3.3.1. Retina and RPE Biomarkers in Laser-Induced Models
3.3.2. Directly Measurable Serum and Plasma Biomarkers
3.3.3. Biomarkers Directly Measurable in Tear Fluid
3.3.4. Directly Measurable Aqueous and Vitreous Humor Biomarkers
3.3.5. Directly Measurable Microbiome Biomarkers
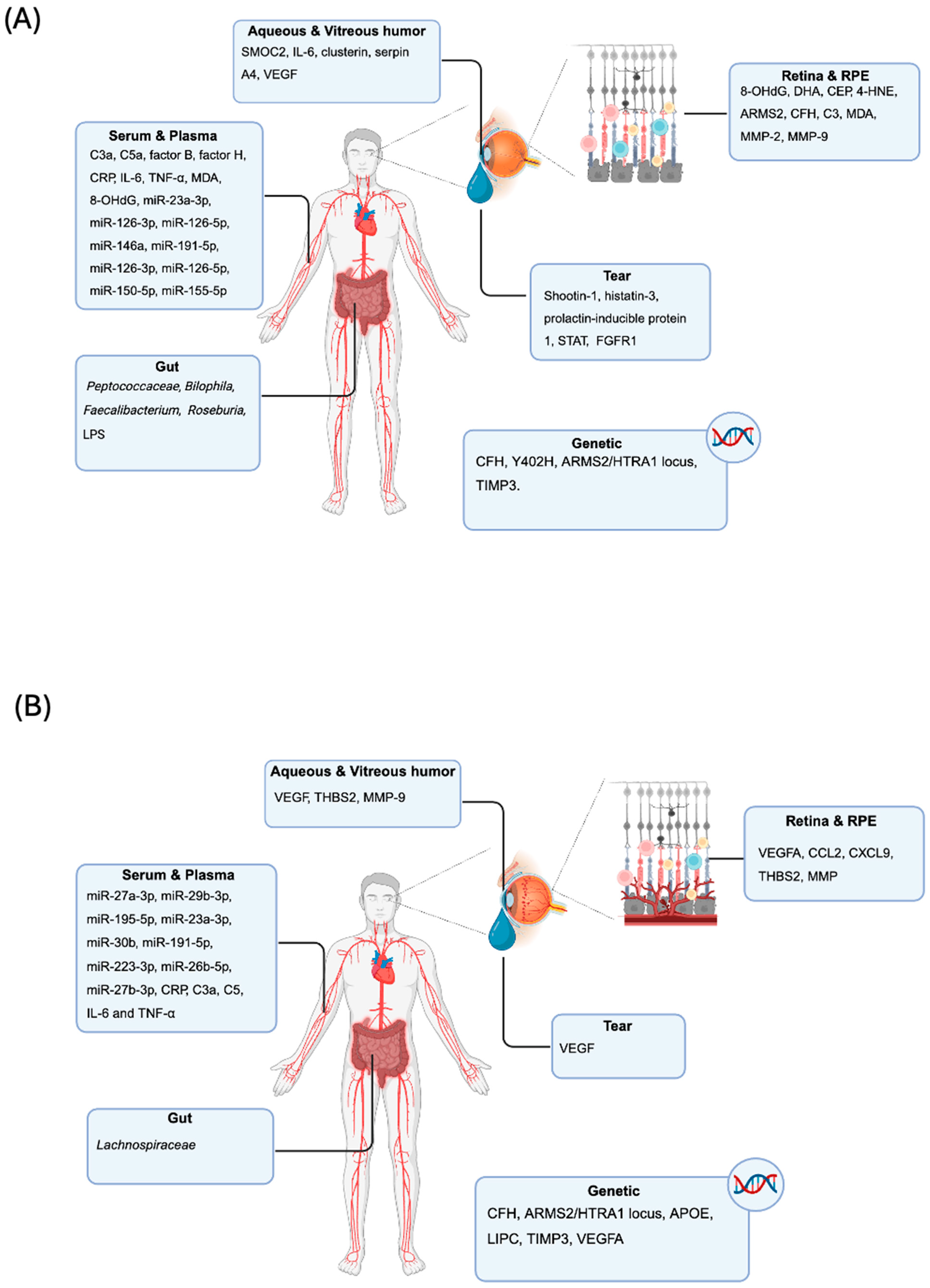
4. Human Studies
4.1. Biomarkers in “Dry” AMD in Humans: From Tissue to Biofluids
4.1.1. Non-Directly Measurable Tissue Mechanistic: Markers from Pathology
4.1.2. Directly Measurable Serum and Plasma Biomarkers
4.1.3. Directly Measurable Tear Fluid Biomarkers
4.1.4. Directly Measurable Aqueous and Vitreous Humor Biomarkers
4.1.5. Directly Measurable Microbiome Biomarkers
4.2. Biomarkers in “Wet” AMD in Humans: Clinical and Biofluids Applications
4.2.1. Retinal Imaging Markers and Tissue Pathology
4.2.2. Serum and Plasma Biomarkers
4.2.3. Directly Measurable Tear Fluid Biomarkers
4.2.4. Directly Measurable Aqueous and Vitreous Humor Biomarkers
4.2.5. Directly Measurable Microbiome Biomarkers
- Understanding Confounding: Foundations and Assumptions of Mendelian Randomization
4.2.6. Directly Measurable Genetic Biomarkers
5. Discussion: Translational Integration and Limits
6. Conclusions: Toward Practical Biomarker Strategies for AMD
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMD | Age-related Macular Degeneration |
| GA | Geographic Atrophy |
| RPE | Retinal Pigment Epithelium |
| VEGF | Vascular Endothelial Growth Factor |
| FDA | Food and Drug Administration |
| CFH | Complement Factor H |
| ARMS2 | Age-Related Maculopathy Susceptibility 2 |
| HTRA1 | HtrA serine peptidase 1 |
| C3 | Complement component 3 |
| miRNAs | microRNAs |
| MNU | N-methyl-N-nitrosourea |
| ROS | Reactive oxygen species |
| BAX | Bcl-2-associated X protein |
| MCP | Monocyte Chemoattractant Protein |
| GFAP | Glial Fibrillary Acidic Protein |
| MMP | Matrix metalloproteinase |
| TIMP-3 | Tissue Inhibitor of Metalloproteinases-3 |
| ECM | Extracellular matrix |
| ANGP | Angiopoietin |
| SAA | Serum Amyloid A |
| CCL2 | Chemokine ligand 2 |
| EPO | Erythropoietin |
| SCFAs | Short-Chain Fatty Acids |
| LPS | Lipopolysaccharide |
| AAE | Aronia Anthocyanidin Extract |
| C-X3-C | Motif Chemokine Receptor 1 |
| VLDRL | Very low-density lipoprotein receptor |
| 8-OHDG | 8-hydroxy-2′-deoxyguanosine |
| IRAK-M | interleukin-1 receptor–associated kinase M |
| AAV | Adeno-Associated Virus |
| BrM | Bruch’s membrane |
| SNP | Single Nucleotide Polymorphism |
| MNV | Macular Neovascularization |
| THBS2 | Thrombospondin 2 |
| DHA | Docosahexaenoic Acid |
| CEP | Carboxyethyl Pyrrole |
| MDA | Malondialdehyde |
| CRP | C-reactive protein |
| SMOC2 | Secreted Modular Calcium-binding protein 2 |
| SD-OCT | Spectral-Domain Optical Coherence Tomography |
| IRF | Intraretinal Fluid |
| SRF | Subretinal Fluid |
| PEDs | Pigment Epithelial Detachments |
References
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.-Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Curcio, C.A.; Kar, D.; Owsley, C.; Sloan, K.R.; Ach, T. Age-Related Macular Degeneration, a Mathematically Tractable Disease. Investig. Ophthalmol. Vis. Sci 2024, 65, 4. [Google Scholar] [CrossRef]
- Choudhary, M.; Malek, G. A Review of Pathogenic Drivers of Age-Related Macular Degeneration, Beyond Complement, and Potential Endpoints to Test Therapeutic Interventions in Preclinical Studies. Adv. Exp. Med. Biol. 2019, 1185, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.H.C.; Ma, J.Y.W.; Jobling, A.I.; Brandli, A.; Greferath, U.; Fletcher, E.L.; Vessey, K.A. Exploring the pathogenesis of age-related macular degeneration: A review of the interplay between retinal pigment epithelium dysfunction and the innate immune system. Front. Neurosci. 2022, 16, 1009599. [Google Scholar] [CrossRef] [PubMed]
- Bowes Rickman, C.; Farsiu, S.; Toth, C.A.; Klingeborn, M. Dry Age-Related Macular Degeneration: Mechanisms, Therapeutic Targets, and Imaging. Investig. Ophthalmol. Vis. Sci. 2013, 54, ORSF68–ORSF80. [Google Scholar] [CrossRef]
- Fritsche, L.G.; Igl, W.; Cooke Bailey, J.N.; Grassmann, F.; Sengupta, S.; Bragg-Gresham, J.L.; Burdon, K.P.; Hebbring, S.J.; Wen, C.; Gorski, M.; et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat. Genet. 2016, 48, 134–143. [Google Scholar] [CrossRef]
- Hageman, G.S.; Gehrs, K.; Lejnine, S.; Bansal, A.T.; DeAngelis, M.M.; Guymer, R.H.; Baird, P.N.; Allikmets, R.; Deciu, C.; Oeth, P.; et al. Clinical validation of a genetic model to estimate the risk of developing choroidal neovascular age-related macular degeneration. Hum. Genom. 2011, 5, 420–440. [Google Scholar] [CrossRef]
- Lad, E.M.; Finger, R.P.; Guymer, R. Biomarkers for the Progression of Intermediate Age-Related Macular Degeneration. Ophthalmol. Ther. 2023, 12, 2917–2941. [Google Scholar] [CrossRef]
- Li, S.; Qiu, Y.; Li, Y.; Wu, J.; Yin, N.; Ren, J.; Shao, M.; Yu, J.; Song, Y.; Sun, X.; et al. Serum metabolite biomarkers for the early diagnosis and monitoring of age-related macular degeneration. J. Adv. Res. 2024, 74, 443–454. [Google Scholar] [CrossRef]
- Borrelli, E.; Serafino, S.; Ricardi, F.; Coletto, A.; Neri, G.; Olivieri, C.; Ulla, L.; Foti, C.; Marolo, P.; Toro, M.D.; et al. Deep Learning in Neovascular Age-Related Macular Degeneration. Medicina 2024, 60, 990. [Google Scholar] [CrossRef]
- Exploring the Role of Exosomal miRNA-146a-5p in Sodium Iodate-Induced Retinal Pigment Epithelial Dysfunction | IOVS | ARVO Journals. Available online: https://iovs.arvojournals.org/article.aspx?articleid=2795919 (accessed on 29 July 2025).
- Chen, Y.; Liu, S.; Hu, D.; Xing, Y.; Shen, Y. N -methyl- N -nitrosourea-induced retinal degeneration in mice. Exp. Eye Res. 2014, 121, 102–113. [Google Scholar] [CrossRef]
- Zhao, J.; Liao, Y.; Chen, J.; Dong, X.; Gao, Z.; Zhang, H.; Wu, X.; Liu, Z.; Wu, Y. Aberrant Buildup of All-Trans-Retinal Dimer, a Nonpyridinium Bisretinoid Lipofuscin Fluorophore, Contributes to the Degeneration of the Retinal Pigment Epithelium. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1063–1075. [Google Scholar] [CrossRef]
- Zhang, J.; Bai, Y.; Huang, L.; Qi, Y.; Zhang, Q.; Li, S.; Wu, Y.; Li, X. Protective effect of autophagy on human retinal pigment epithelial cells against lipofuscin fluorophore A2E: Implications for age-related macular degeneration. Cell Death Dis. 2015, 6, e1972. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Tzekov, R.; Su, M.; Zhu, Y.; Han, A.; Li, W. Hydrogen peroxide-induced oxidative damage and protective role of peroxiredoxin 6 protein via EGFR/ERK signaling pathway in RPE cells. Front. Aging Neurosci. 2023, 15, 1169211. [Google Scholar] [CrossRef] [PubMed]
- Kaczara, P.; Sarna, T.; Burke, J.M. Dynamics of H2O2 Availability to ARPE-19 Cultures in Models of Oxidative Stress. Free Radic. Biol. Med. 2010, 48, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Zhao, W.; Li, X.; Yang, C.; Guo, P. Tyrosol protects RPE cells from H2O2-induced oxidative damage in vitro and in vivo through activation of the Nrf2/HO-1 pathway. Eur. J. Pharmacol. 2025, 991, 177316. [Google Scholar] [CrossRef]
- Hara, A.; Niwa, M.; Aoki, H.; Kumada, M.; Kunisada, T.; Oyama, T.; Yamamoto, T.; Kozawa, O.; Mori, H. A new model of retinal photoreceptor cell degeneration induced by a chemical hypoxia-mimicking agent, cobalt chloride. Brain Res. 2006, 1109, 192–200. [Google Scholar] [CrossRef]
- Muñoz-Sánchez, J.; Chánez-Cárdenas, M.E. The use of cobalt chloride as a chemical hypoxia model. J. Appl. Toxicol. 2019, 39, 556–570. [Google Scholar] [CrossRef]
- Ramkumar, H.L.; Zhang, J.; Chan, C.-C. Retinal Ultrastructure of Murine Models of Dry Age-related Macular Degeneration (AMD). Prog. Retin. Eye Res. 2010, 29, 169–190. [Google Scholar] [CrossRef]
- Montezuma, S.R.; Sobrin, L.; Seddon, J.M. Review of Genetics in Age Related Macular Degeneration. Semin. Ophthalmol. 2007, 22, 229–240. [Google Scholar] [CrossRef]
- Hanus, J.; Anderson, C.; Wang, S. RPE Necroptosis in Response to Oxidative Stress and in AMD. Ageing Res. Rev. 2015, 24, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Enzbrenner, A.; Zulliger, R.; Biber, J.; Pousa, A.M.Q.; Schäfer, N.; Stucki, C.; Giroud, N.; Berrera, M.; Kortvely, E.; Schmucki, R.; et al. Sodium Iodate-Induced Degeneration Results in Local Complement Changes and Inflammatory Processes in Murine Retina. Int. J. Mol. Sci. 2021, 22, 9218. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.T.; Gao, J.; Cao, S.; Sandhu, N.; Cui, J.Z.; Chou, C.L.; Fang, E.; Matsubara, J.A. Inflammatory Mediators Induced by Amyloid-Beta in the Retina and RPE In Vivo: Implications for Inflammasome Activation in Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2225–2237. [Google Scholar] [CrossRef] [PubMed]
- Cervellati, F.; Cervellati, C.; Romani, A.; Cremonini, E.; Sticozzi, C.; Belmonte, G.; Pessina, F.; Valacchi, G. Hypoxia induces cell damage via oxidative stress in retinal epithelial cells. Free Radic. Res. 2014, 48, 303–312. [Google Scholar] [CrossRef]
- Lazzara, F.; Trotta, M.C.; Platania, C.B.M.; D’Amico, M.; Petrillo, F.; Galdiero, M.; Gesualdo, C.; Rossi, S.; Drago, F.; Bucolo, C. Stabilization of HIF-1α in Human Retinal Endothelial Cells Modulates Expression of miRNAs and Proangiogenic Growth Factors. Front. Pharmacol. 2020, 11, 1063. [Google Scholar] [CrossRef]
- Zhang, C.-J.; Xiang, L.; Chen, X.-J.; Wang, X.-Y.; Wu, K.-C.; Zhang, B.-W.; Chen, D.-F.; Jin, G.-H.; Zhang, H.; Chen, Y.-C.; et al. Ablation of Mature miR-183 Leads to Retinal Dysfunction in Mice. Investig. Ophthalmol. Vis. Sci. 2020, 61, 12. [Google Scholar] [CrossRef]
- Fasanaro, P.; D’Alessandra, Y.; Di Stefano, V.; Melchionna, R.; Romani, S.; Pompilio, G.; Capogrossi, M.C.; Martelli, F. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J. Biol. Chem. 2008, 283, 15878–15883. [Google Scholar] [CrossRef]
- Shu, Y.; Li, Z.; Zong, T.; Mu, T.; Zhou, H.; Yang, Q.; Wu, M.; Liu, Y.; Xie, T.; Tan, C.; et al. MiR-21-5p promotes RPE cell necroptosis by targeting Peli1 in a rat model of AMD. In. Vitro Cell Dev. Biol. Anim. 2025, 61, 801–815. [Google Scholar] [CrossRef]
- Tong, Y.; Wu, Y.; Ma, J.; Ikeda, M.; Ide, T.; Griffin, C.T.; Ding, X.-Q.; Wang, S. Comparative mechanistic study of RPE cell death induced by different oxidative stresses. Redox Biol. 2023, 65, 102840. [Google Scholar] [CrossRef]
- Yang, H.-J.; Hu, R.; Sun, H.; Chen, B.; Li, X.; Chen, J.-B. 4-HNE induces proinflammatory cytokines of human retinal pigment epithelial cells by promoting extracellular efflux of HSP70. Exp. Eye Res. 2019, 188, 107792. [Google Scholar] [CrossRef]
- Penn, J.S.; Madan, A.; Caldwell, R.B.; Bartoli, M.; Caldwell, R.W.; Hartnett, M.E. Vascular Endothelial Growth Factor in Eye Disease. Prog. Retin. Eye Res. 2008, 27, 331–371. [Google Scholar] [CrossRef]
- Spilsbury, K.; Garrett, K.L.; Shen, W.Y.; Constable, I.J.; Rakoczy, P.E. Overexpression of vascular endothelial growth factor (VEGF) in the retinal pigment epithelium leads to the development of choroidal neovascularization. Am. J. Pathol. 2000, 157, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Uehara, H.; Zhang, X.; Das, S.K.; Olsen, T.; Holt, D.; Simonis, J.M.; Jackman, K.; Singh, N.; Miya, T.R.; et al. Photoreceptor avascular privilege is shielded by soluble VEGF receptor-1. eLife 2013, 2, e00324. [Google Scholar] [CrossRef] [PubMed]
- Hanus, J.; Anderson, C.; Sarraf, D.; Ma, J.; Wang, S. Retinal pigment epithelial cell necroptosis in response to sodium iodate. Cell Death Discov. 2016, 2, 16054. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Liu, W.; Liu, G.; Li, X.; Lu, P. Assessing the protective effects of cryptotanshinone on CoCl2-induced hypoxia in RPE cells. Mol. Med. Rep. 2021, 24, 739. [Google Scholar] [CrossRef]
- Xing, Y.; Liang, S.; Zhang, L.; Ni, H.; Zhang, X.; Wang, J.; Yang, L.; Song, S.; Li, H.-H.; Jia, C.; et al. Combination of Lactobacillus fermentum NS9 and aronia anthocyanidin extract alleviates sodium iodate-induced retina degeneration. Sci. Rep. 2023, 13, 8380. [Google Scholar] [CrossRef]
- Nguyen, Y.; Rudd Zhong Manis, J.; Ronczkowski, N.M.; Bui, T.; Oxenrider, A.; Jadeja, R.N.; Thounaojam, M.C. Unveiling the gut-eye axis: How microbial metabolites influence ocular health and disease. Front. Med. 2024, 11, 1377186. [Google Scholar] [CrossRef]
- Raoul, W.; Auvynet, C.; Camelo, S.; Guillonneau, X.; Feumi, C.; Combadière, C.; Sennlaub, F. CCL2/CCR2 and CX3CL1/CX3CR1 chemokine axes and their possible involvement in age-related macular degeneration. J. Neuroinflammation 2010, 7, 87. [Google Scholar] [CrossRef]
- Vessey, K.A.; Greferath, U.; Jobling, A.I.; Phipps, J.A.; Ho, T.; Waugh, M.; Fletcher, E.L. Ccl2/Cx3cr1 knockout mice have inner retinal dysfunction but are not an accelerated model of AMD. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7833–7846. [Google Scholar] [CrossRef]
- Ross, R.J.; Zhou, M.; Shen, D.; Fariss, R.N.; Ding, X.; Bojanowski, C.M.; Tuo, J.; Chan, C.-C. Immunological protein expression profile in Ccl2/Cx3cr1 deficient mice with lesions similar to age-related macular degeneration. Exp. Eye Res. 2008, 86, 675–683. [Google Scholar] [CrossRef]
- Imamura, Y.; Noda, S.; Hashizume, K.; Shinoda, K.; Yamaguchi, M.; Uchiyama, S.; Shimizu, T.; Mizushima, Y.; Shirasawa, T.; Tsubota, K. Drusen, choroidal neovascularization, and retinal pigment epithelium dysfunction in SOD1-deficient mice: A model of age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2006, 103, 11282–11287. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, K.; Hirasawa, M.; Imamura, Y.; Noda, S.; Shimizu, T.; Shinoda, K.; Kurihara, T.; Noda, K.; Ozawa, Y.; Ishida, S.; et al. Retinal Dysfunction and Progressive Retinal Cell Death in SOD1-Deficient Mice. Am. J. Pathol. 2008, 172, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Aredo, B.; Chen, B.; Zhao, C.X.; He, Y.-G.; Ufret-Vincenty, R.L. Mice with a Combined Deficiency of Superoxide Dismutase 1 (Sod1), DJ-1 (Park7), and Parkin (Prkn) Develop Spontaneous Retinal Degeneration with Aging. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3740–3751. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.K.; Yang, J.; Hsu, C.W.; Gore, A.; Bassuk, A.G.; Brown, L.M.; Colligan, R.; Sengillo, J.D.; Mahajan, V.B.; Tsang, S.H. HTRA1, an age-related macular degeneration protease, processes extracellular matrix proteins EFEMP1 and TSP1. Aging Cell 2018, 17, e12710. [Google Scholar] [CrossRef]
- Vierkotten, S.; Muether, P.S.; Fauser, S. Overexpression of HTRA1 leads to ultrastructural changes in the elastic layer of Bruch’s membrane via cleavage of extracellular matrix components. PLoS ONE 2011, 6, e22959. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Melo, E.; Jakob, P.; Friedlein, A.; Elsässer, B.; Goettig, P.; Kueppers, V.; Delobel, F.; Stucki, C.; Dunkley, T.; et al. N-Terminomics identifies HtrA1 cleavage of thrombospondin-1 with generation of a proangiogenic fragment in the polarized retinal pigment epithelial cell model of age-related macular degeneration. Matrix Biol. 2018, 70, 84–101. [Google Scholar] [CrossRef]
- Zinkernagel, M.S.; Zysset-Burri, D.C.; Keller, I.; Berger, L.E.; Leichtle, A.B.; Largiadèr, C.R.; Fiedler, G.M.; Wolf, S. Association of the Intestinal Microbiome with the Development of Neovascular Age-Related Macular Degeneration. Sci. Rep. 2017, 7, 40826. [Google Scholar] [CrossRef]
- Bringer, M.-A.; Gabrielle, P.-H.; Bron, A.M.; Creuzot-Garcher, C.; Acar, N. The gut microbiota in retinal diseases. Exp. Eye Res. 2022, 214, 108867. [Google Scholar] [CrossRef]
- Pennesi, M.E.; Neuringer, M.; Courtney, R.J. Animal models of age related macular degeneration. Mol. Aspects Med. 2012, 33, 487–509. [Google Scholar] [CrossRef]
- Badia, A.; Salas, A.; Duarri, A.; Ferreira-de-Souza, B.; Zapata, M.Á.; Fontrodona, L.; García-Arumí, J. Transcriptomics analysis of Ccl2/Cx3cr1/Crb1rd8 deficient mice provides new insights into the pathophysiology of progressive retinal degeneration. Exp. Eye Res. 2021, 203, 108424. [Google Scholar] [CrossRef]
- Liu, J.; Copland, D.A.; Clare, A.J.; Gorski, M.; Richards, B.T.; Scott, L.; Theodoropoulou, S.; Greferath, U.; Cox, K.; Shi, G.; et al. Replenishing IRAK-M expression in retinal pigment epithelium attenuates outer retinal degeneration. Sci. Transl. Med. 2024, 16, eadi4125. [Google Scholar] [CrossRef]
- Liu, J.; Copland, D.A.; Clare, A.J.; Gorski, M.; Richards, B.T.; Scott, L.; Theodoropoulou, S.; Greferath, U.; Cox, K.; Bell, O.H.; et al. Replenishing Age-Related Decline of IRAK-M Expression in Retinal Pigment Epithelium Attenuates Outer Retinal Degeneration. bioRxiv 2023, 2023.09.27.559733. [Google Scholar] [CrossRef]
- Jones, A.; Kumar, S.; Zhang, N.; Tong, Z.; Yang, J.-H.; Watt, C.; Anderson, J.; Amrita; Fillerup, H.; McCloskey, M.; et al. Increased expression of multifunctional serine protease, HTRA1, in retinal pigment epithelium induces polypoidal choroidal vasculopathy in mice. Proc. Natl. Acad. Sci. USA 2011, 108, 14578–14583. [Google Scholar] [CrossRef]
- Zysset-Burri, D.C.; Keller, I.; Berger, L.E.; Largiadèr, C.R.; Wittwer, M.; Wolf, S.; Zinkernagel, M.S. Associations of the intestinal microbiome with the complement system in neovascular age-related macular degeneration. NPJ Genom. Med. 2020, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Rowan, S.; Taylor, A. Gut microbiota modify risk for dietary glycemia-induced age-related macular degeneration. Gut Microbes 2018, 9, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.S.; Soetikno, B.T.; Lajko, M.; Fawzi, A.A. A Mouse Model for Laser-induced Choroidal Neovascularization. J. Vis. Exp. 2015, 106, 53502. [Google Scholar] [CrossRef]
- Jimenez, A.I.; Bernabeu-Zornoza, A.; Esteve, J.J.; Gombau, A.; Matei, A.; Ramos, F.; Martínez-Navarrete, G.; Fernandez, E.; Sylentis, T. Optimization and characterization of an improved laser-induced choroidal neovascularization animal model for the study of retinal diseases. Investig. Ophthalmol. Vis. Sci. 2023, 64, 2112. [Google Scholar]
- Gong, Y.; Li, J.; Sun, Y.; Fu, Z.; Liu, C.-H.; Evans, L.; Tian, K.; Saba, N.; Fredrick, T.; Morss, P.; et al. Optimization of an Image-Guided Laser-Induced Choroidal Neovascularization Model in Mice. PLoS ONE 2015, 10, e0132643. [Google Scholar] [CrossRef]
- Salas, A.; Badia, A.; Fontrodona, L.; Zapata, M.; García-Arumí, J.; Duarri, A. Neovascular Progression and Retinal Dysfunction in the Laser-Induced Choroidal Neovascularization Mouse Model. Biomedicines 2023, 11, 2445. [Google Scholar] [CrossRef]
- Liu, Y.-S.; Pan, J.-Q.; Pan, X.-B.; Kong, F.-S.; Zhang, J.-Q.; Wei, Z.-Y.; Xu, Z.-H.; Rao, J.-H.; Wang, J.-H.; Chen, J.-H. Comparative Analysis of Molecular Landscape in Mouse Models and Patients Reveals Conserved Inflammation Pathways in Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2024, 65, 13. [Google Scholar] [CrossRef]
- Wolf, J.; Schlecht, A.; Rosmus, D.-D.; Boneva, S.; Agostini, H.; Schlunck, G.; Wieghofer, P.; Lange, C. Comparative transcriptome analysis of human and murine choroidal neovascularization identifies fibroblast growth factor inducible-14 as phylogenetically conserved mediator of neovascular age-related macular degeneration. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166340. [Google Scholar] [CrossRef]
- Toma, H.S.; Barnett, J.M.; Penn, J.S.; Kim, S.J. Improved assessment of laser-induced choroidal neovascularization. Microvasc. Res. 2010, 80, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Iwanishi, H.; Yamanaka, O.; Sumioka, T.; Yasuda, S.; Miyajima, M.; Saika, S. Delayed regression of laser-induced choroidal neovascularization in TNFα-null mice. J. Cell. Mol. Med. 2022, 26, 5315–5325. [Google Scholar] [CrossRef] [PubMed]
- Lambert, V.; Lecomte, J.; Hansen, S.; Blacher, S.; Gonzalez, M.-L.A.; Struman, I.; Sounni, N.E.; Rozet, E.; de Tullio, P.; Foidart, J.M.; et al. Laser-induced choroidal neovascularization model to study age-related macular degeneration in mice. Nat. Protoc. 2013, 8, 2197–2211. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Qi, S.; Zhuang, H.; Zhuo, Q.; Liang, Y.; Kong, H.; Zhao, C.; Zhang, S. Proteotranscriptomic analyses reveal distinct interferon-beta signaling pathways and therapeutic targets in choroidal neovascularization. Front. Immunol. 2023, 14, 1163739. [Google Scholar] [CrossRef]
- Apte, R.S.; Richter, J.; Herndon, J.; Ferguson, T.A. Macrophages Inhibit Neovascularization in a Murine Model of Age-Related Macular Degeneration. PLoS Med. 2006, 3, e310. [Google Scholar] [CrossRef]
- Brandli, A.; Khong, F.L.; Kong, R.C.K.; Kelly, D.J.; Fletcher, E.L. Transcriptomic analysis of choroidal neovascularization reveals dysregulation of immune and fibrosis pathways that are attenuated by a novel anti-fibrotic treatment. Sci. Rep. 2022, 12, 859. [Google Scholar] [CrossRef]
- Kiel, C.; Berber, P.; Karlstetter, M.; Aslanidis, A.; Strunz, T.; Langmann, T.; Grassmann, F.; Weber, B.H.F. A Circulating MicroRNA Profile in a Laser-Induced Mouse Model of Choroidal Neovascularization. Int. J. Mol. Sci. 2020, 21, 2689. [Google Scholar] [CrossRef]
- Moshtaghion, S.M.M.; Locri, F.; Reyes, A.P.; Plastino, F.; Kvanta, A.; Morillo-Sanchez, M.J.; Rodríguez-de-la-Rúa, E.; Gutierrez-Sanchez, E.; Montero-Sánchez, A.; Lucena-Padros, H.; et al. VEGF in Tears as a Biomarker for Exudative Age-Related Macular Degeneration: Molecular Dynamics in a Mouse Model and Human Samples. Int. J. Mol. Sci. 2025, 26, 3855. [Google Scholar] [CrossRef]
- Dos Santos, F.M.; Ciordia, S.; Mesquita, J.; de Sousa, J.P.C.; Paradela, A.; Tomaz, C.T.; Passarinha, L.A.P. Vitreous humor proteome: Unraveling the molecular mechanisms underlying proliferative and neovascular vitreoretinal diseases. Cell Mol. Life Sci. 2022, 80, 22. [Google Scholar] [CrossRef]
- Oca, A.I.; Pérez-Sala, Á.; Pariente, A.; Ochoa, R.; Velilla, S.; Peláez, R.; Larráyoz, I.M. Predictive Biomarkers of Age-Related Macular Degeneration Response to Anti-VEGF Treatment. J. Pers. Med. 2021, 11, 1329. [Google Scholar] [CrossRef]
- Ratnapriya, R.; Chew, E.Y. Age-related macular degeneration-clinical review and genetics update. Clin. Genet. 2013, 84, 160–166. [Google Scholar] [CrossRef]
- Andriessen, E.M.; Wilson, A.M.; Mawambo, G.; Dejda, A.; Miloudi, K.; Sennlaub, F.; Sapieha, P. Gut microbiota influences pathological angiogenesis in obesity-driven choroidal neovascularization. EMBO Mol. Med. 2016, 8, 1366–1379. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Mo, Y. The gut-retina axis: A new perspective in the prevention and treatment of diabetic retinopathy. Front. Endocrinol. 2023, 14, 1205846. [Google Scholar] [CrossRef] [PubMed]
- Bakri, S.J.; Bektas, M.; Sharp, D.; Luo, R.; Sarda, S.P.; Khan, S. Geographic atrophy: Mechanism of disease, pathophysiology, and role of the complement system. J. Manag. Care Spec. Pharm. 2023, 29, S2–S11. [Google Scholar] [CrossRef] [PubMed]
- Lau, L.-I.; Liu, C.J.; Wei, Y.-H. Increase of 8-hydroxy-2’-deoxyguanosine in aqueous humor of patients with exudative age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5486–5490. [Google Scholar] [CrossRef]
- Shen, J.K.; Dong, A.; Hackett, S.F.; Bell, W.R.; Green, W.R.; Campochiaro, P.A. Oxidative damage in age-related macular degeneration. Histol. Histopathol. 2007, 22, 1301–1308. [Google Scholar] [CrossRef]
- Crabb, J.W.; Miyagi, M.; Gu, X.; Shadrach, K.; West, K.A.; Sakaguchi, H.; Kamei, M.; Hasan, A.; Yan, L.; Rayborn, M.E.; et al. Drusen proteome analysis: An approach to the etiology of age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2002, 99, 14682–14687. [Google Scholar] [CrossRef]
- Lu, L.; Gu, X.; Hong, L.; Laird, J.; Jaffe, K.; Choi, J.; Crabb, J.; Salomon, R.G. Synthesis and structural characterization of carboxyethylpyrrole-modified proteins: Mediators of age-related macular degeneration. Bioorg. Med. Chem. 2009, 17, 7548–7561. [Google Scholar] [CrossRef]
- Gu, X.; Meer, S.G.; Miyagi, M.; Rayborn, M.E.; Hollyfield, J.G.; Crabb, J.W.; Salomon, R.G. Carboxyethylpyrrole protein adducts and autoantibodies, biomarkers for age-related macular degeneration. J. Biol. Chem. 2003, 278, 42027–42035. [Google Scholar] [CrossRef]
- Renganathan, K.; Gu, J.; Rayborn, M.E.; Crabb, J.S.; Salomon, R.G.; Collier, R.J.; Kapin, M.A.; Romano, C.; Hollyfield, J.G.; Crabb, J.W. CEP Biomarkers as Potential Tools for Monitoring Therapeutics. PLoS ONE 2013, 8, e76325. [Google Scholar] [CrossRef]
- Ethen, C.M.; Reilly, C.; Feng, X.; Olsen, T.W.; Ferrington, D.A. Age-related macular degeneration and retinal protein modification by 4-hydroxy-2-nonenal. Investig. Ophthalmol. Vis. Sci. 2007, 48, 3469–3479. [Google Scholar] [CrossRef]
- Gu, J.; Pauer, G.J.T.; Yue, X.; Narendra, U.; Sturgill, G.M.; Bena, J.; Gu, X.; Peachey, N.S.; Salomon, R.G.; Hagstrom, S.A.; et al. Proteomic and genomic biomarkers for age-related macular degeneration. Adv. Exp. Med. Biol. 2010, 664, 411–417. [Google Scholar] [CrossRef]
- Totan, Y.; Yağci, R.; Bardak, Y.; Ozyurt, H.; Kendir, F.; Yilmaz, G.; Sahin, S.; Sahin Tiğ, U. Oxidative macromolecular damage in age-related macular degeneration. Curr. Eye Res. 2009, 34, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Nita, M.; Strzałka-Mrozik, B.; Grzybowski, A.; Mazurek, U.; Romaniuk, W. Age-related macular degeneration and changes in the extracellular matrix. Med. Sci. Monit. 2014, 20, 1003–1016. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.A.; Lee, Y.; Zhang, J.-J.; Marshall, J. Disturbed Matrix Metalloproteinase Activity of Bruch’s Membrane in Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4459–4466. [Google Scholar] [CrossRef] [PubMed]
- Abokyi, S.; To, C.-H.; Lam, T.T.; Tse, D.Y. Central Role of Oxidative Stress in Age-Related Macular Degeneration: Evidence from a Review of the Molecular Mechanisms and Animal Models. Oxid. Med. Cell Longev. 2020, 2020, 7901270. [Google Scholar] [CrossRef]
- Chen, W.; Li, Z.; Zhou, X.; Li, C.; Lin, Y. Identification of Biomarkers for Oxidative Stress in Age-Related Macular Degeneration: Combining Transcriptomics and Mendelian Randomization Analysis. Transl. Vis. Sci. Technol. 2025, 14, 42. [Google Scholar] [CrossRef]
- Hageman, G.S.; Anderson, D.H.; Johnson, L.V.; Hancox, L.S.; Taiber, A.J.; Hardisty, L.I.; Hageman, J.L.; Stockman, H.A.; Borchardt, J.D.; Gehrs, K.M.; et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2005, 102, 7227–7232. [Google Scholar] [CrossRef]
- Reynolds, R.; Hartnett, M.E.; Atkinson, J.P.; Giclas, P.C.; Rosner, B.; Seddon, J.M. Plasma complement components and activation fragments: Associations with age-related macular degeneration genotypes and phenotypes. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5818–5827. [Google Scholar] [CrossRef]
- Ildefonso, C.J.; Biswal, M.R.; Ahmed, C.M.; Lewin, A.S. The NLRP3 Inflammasome and its Role in Age-Related Macular Degeneration. Adv. Exp. Med. Biol. 2016, 854, 59–65. [Google Scholar] [CrossRef]
- Szaflik, J.P.; Janik-Papis, K.; Synowiec, E.; Ksiazek, D.; Zaras, M.; Wozniak, K.; Szaflik, J.; Blasiak, J. DNA damage and repair in age-related macular degeneration. Mutat. Res. 2009, 669, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Martinez, B.; Peplow, P.V. MicroRNAs as diagnostic and prognostic biomarkers of age-related macular degeneration: Advances and limitations. Neural Regen. Res. 2020, 16, 440–447. [Google Scholar] [CrossRef]
- Berber, P.; Grassmann, F.; Kiel, C.; Weber, B.H.F. An Eye on Age-Related Macular Degeneration: The Role of MicroRNAs in Disease Pathology. Mol. Diagn. Ther. 2017, 21, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Aguilar, M.; Groman-Lupa, S.; Jiménez-Martínez, M.C. MicroRNAs as potential biomarkers and therapeutic targets in age-related macular degeneration. Front. Ophthalmol. 2023, 3, 1023782. [Google Scholar] [CrossRef]
- Zafrilla, P.; Losada, M.; Perez, A.; Caravaca, G.; Mulero, J. Biomarkers of oxidative stress in patients with wet age related macular degeneration. J. Nutr. Health Aging 2013, 17, 219–222. [Google Scholar] [CrossRef]
- Yuan, L.-Y.; Su, W.-M.; Li, L.-P.; Tian, X.-F.; Zheng, X.-L.; Yuan, X.-Y. Causal role of oxidative stress in age-related macular degeneration: A bidirectional Mendelian randomization study. Int. J. Ophthalmol. 2025, 18, 1307. [Google Scholar] [CrossRef]
- Winiarczyk, M.; Kaarniranta, K.; Winiarczyk, S.; Adaszek, Ł.; Winiarczyk, D.; Mackiewicz, J. Tear film proteome in age-related macular degeneration. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 1127–1139. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Tokarz, P.; Koskela, A.; Paterno, J.; Blasiak, J. Autophagy regulates death of retinal pigment epithelium cells in age-related macular degeneration. Cell Biol. Toxicol. 2017, 33, 113–128. [Google Scholar] [CrossRef]
- Zhou, L.; Beuerman, R.W. The power of tears: How tear proteomics research could revolutionize the clinic. Expert Rev. Proteom. 2017, 14, 189–191. [Google Scholar] [CrossRef]
- Zhou, L.; Beuerman, R.W.; Chan, C.M.; Zhao, S.Z.; Li, X.R.; Yang, H.; Tong, L.; Liu, S.; Stern, M.E.; Tan, D. Identification of tear fluid biomarkers in dry eye syndrome using iTRAQ quantitative proteomics. J. Proteome Res. 2009, 8, 4889–4905. [Google Scholar] [CrossRef]
- Baek, J.-H.; Lim, D.; Park, K.H.; Chae, J.-B.; Jang, H.; Lee, J.; Chung, H. Quantitative proteomic analysis of aqueous humor from patients with drusen and reticular pseudodrusen in age-related macular degeneration. BMC Ophthalmol. 2018, 18, 289. [Google Scholar] [CrossRef] [PubMed]
- Rinsky, B.; Beykin, G.; Grunin, M.; Amer, R.; Khateb, S.; Tiosano, L.; Almeida, D.; Hagbi-Levi, S.; Elbaz-Hayoun, S.; Chowers, I. Analysis of the Aqueous Humor Proteome in Patients with Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2021, 62, 18. [Google Scholar] [CrossRef]
- Yoon, E.G.; Nam, K.T.; Choi, M.; Choi, K.-E.; Yun, C. Aqueous Humor Levels of Vascular Endothelial Growth Factor in Patients With Dry Age-Related Macular Degeneration and Subretinal Drusenoid Deposits. Investig. Ophthalmol. Vis. Sci. 2025, 66, 10. [Google Scholar] [CrossRef] [PubMed]
- García-Quintanilla, L.; Rodríguez-Martínez, L.; Bandín-Vilar, E.; Gil-Martínez, M.; González-Barcia, M.; Mondelo-García, C.; Fernández-Ferreiro, A.; Mateos, J. Recent Advances in Proteomics-Based Approaches to Studying Age-Related Macular Degeneration: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 14759. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-Y.; Wang, J.; Qin, B.; Tan, Y. Investigating the causal link between gut microbiota and dry age-related macular degeneration: A bidirectional Mendelian randomization study. Int. J. Ophthalmol. 2024, 17, 1723–1730. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, J.Y.; Luo, W.; He, P.C.; Skondra, D. The Emerging Role of Gut Microbiota in Age-Related Macular Degeneration. Am. J. Pathol. 2023, 193, 1627–1637. [Google Scholar] [CrossRef]
- Ambati, J.; Fowler, B.J. Mechanisms of age-related macular degeneration. Neuron 2012, 75, 26–39. [Google Scholar] [CrossRef]
- Wong, D.T.; Berger, A.R.; Bourgault, S.; Chen, J.; Colleaux, K.; Cruess, A.F.; Dookeran, R.I.; Gauthier, D.; Hurley, B.; Kapusta, M.A.; et al. Imaging Biomarkers and Their Impact on Therapeutic Decision-Making in the Management of Neovascular Age-Related Macular Degeneration. Ophthalmologica 2021, 244, 265–280. [Google Scholar] [CrossRef]
- Kaiser, P.K.; Wykoff, C.C.; Singh, R.P.; Khanani, A.M.; Do, D.V.; Patel, H.; Patel, N. Retinal fluid and thickness as measures of disease activity in neovascular age-related macular degeneration. Retina 2021, 41, 1579–1586. [Google Scholar] [CrossRef]
- Rispoli, M.; Cennamo, G.; Antonio, L.D.; Lupidi, M.; Parravano, M.; Pellegrini, M.; Veritti, D.; Vujosevic, S.; Savastano, M.C. Practical guidance for imaging biomarkers in exudative age-related macular degeneration. Surv. Ophthalmol. 2023, 68, 615–627. [Google Scholar] [CrossRef]
- Litts, K.M.; Ach, T.; Hammack, K.M.; Sloan, K.R.; Zhang, Y.; Freund, K.B.; Curcio, C.A. Quantitative Analysis of Outer Retinal Tubulation in Age-Related Macular Degeneration from Spectral-Domain Optical Coherence Tomography and Histology. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2647–2656. [Google Scholar] [CrossRef] [PubMed]
- Hammer, M.; Oertel, J.; Alderzy, H.; Tarhan, M.; Meller, D.; Curcio, C.A. Fundus autofluorescence intensity, lifetime, and spectral imaging in age-related macular degeneration. Exp. Eye Res. 2025, 258, 110500. [Google Scholar] [CrossRef] [PubMed]
- Schlecht, A.; Boneva, S.; Gruber, M.; Zhang, P.; Horres, R.; Bucher, F.; Auw-Haedrich, C.; Hansen, L.; Stahl, A.; Hilgendorf, I.; et al. Transcriptomic Characterization of Human Choroidal Neovascular Membranes Identifies Calprotectin as a Novel Biomarker for Patients with Age-Related Macular Degeneration. Am. J. Pathol. 2020, 190, 1632–1642. [Google Scholar] [CrossRef] [PubMed]
- ElShelmani, H.; Brennan, I.; Kelly, D.J.; Keegan, D. Differential Circulating MicroRNA Expression in Age-Related Macular Degeneration. Int. J. Mol. Sci. 2021, 22, 12321. [Google Scholar] [CrossRef]
- Kauppinen, A.; Paterno, J.J.; Blasiak, J.; Salminen, A.; Kaarniranta, K. Inflammation and its role in age-related macular degeneration. Cell. Mol. Life Sci. 2016, 73, 1765–1786. [Google Scholar] [CrossRef]
- Beatty, S.; Koh, H.; Phil, M.; Henson, D.; Boulton, M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv. Ophthalmol. 2000, 45, 115–134. [Google Scholar] [CrossRef]
- Caban, M.; Owczarek, K.; Lewandowska, U. The Role of Metalloproteinases and Their Tissue Inhibitors on Ocular Diseases: Focusing on Potential Mechanisms. Int. J. Mol. Sci. 2022, 23, 4256. [Google Scholar] [CrossRef]
- Wilson, S.; Siebourg-Polster, J.; Titz, B.; Jiang, Z.; Bartolo, F.; Lavergne, V.; Gayán, J.; Garweg, J.G.; Fauser, S.; Dieckmann, A. Correlation of Aqueous, Vitreous, and Serum Protein Levels in Patients with Retinal Diseases. Transl. Vis. Sci. Technol. 2023, 12, 9. [Google Scholar] [CrossRef]
- Nobl, M.; Reich, M.; Dacheva, I.; Siwy, J.; Mullen, W.; Schanstra, J.P.; Choi, C.Y.; Kopitz, J.; Kretz, F.T.A.; Auffarth, G.U.; et al. Proteomics of vitreous in neovascular age-related macular degeneration. Exp. Eye Res. 2016, 146, 107–117. [Google Scholar] [CrossRef]
- Schiavone, N.; Isoldi, G.; Calcagno, S.; Rovida, E.; Antiga, E.; De Almeida, C.V.; Lulli, M. Exploring the Gut Microbiota–Retina Axis: Implications for Health and Disease. Microorganisms 2025, 13, 1101. [Google Scholar] [CrossRef]
- Li, C.; Lu, P. Association of Gut Microbiota with Age-Related Macular Degeneration and Glaucoma: A Bidirectional Mendelian Randomization Study. Nutrients 2023, 15, 4646. [Google Scholar] [CrossRef]
- Liu, K.; Zou, J.; Yuan, R.; Fan, H.; Hu, H.; Cheng, Y.; Liu, J.; Zou, H.; You, Z. Exploring the Effect of the Gut Microbiome on the Risk of Age-Related Macular Degeneration from the Perspective of Causality. Investig. Ophthalmol. Vis. Sci. 2023, 64, 22. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Gao, S.; Han, G. Evidence for Genetic Causal Association Between the Gut Microbiome, Derived Metabolites, and Age-Related Macular Degeneration: A Mediation Mendelian Randomization Analysis. Biomedicines 2025, 13, 639. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, L.G.; Chen, W.; Schu, M.; Yaspan, B.L.; Yu, Y.; Thorleifsson, G.; Zack, D.J.; Arakawa, S.; Cipriani, V.; Ripke, S.; et al. Seven new loci associated with age-related macular degeneration. Nat. Genet. 2013, 45, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Kaur, I.; Rathi, S.; Chakrabarti, S. Variations in TIMP3 are associated with age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2010, 107, E112–E113. [Google Scholar] [CrossRef]
- Churchill, A.J.; Carter, J.G.; Lovell, H.C.; Ramsden, C.; Turner, S.J.; Yeung, A.; Escardo, J.; Atan, D. VEGF polymorphisms are associated with neovascular age-related macular degeneration. Hum. Mol. Genet. 2006, 15, 2955–2961. [Google Scholar] [CrossRef]
- Yu, C.; Robman, L.; He, W.; Woods, R.L.; Phuong Thao, L.T.; Wolfe, R.; Phung, J.; Makeyeva, G.A.; Hodgson, L.A.B.; McNeil, J.J.; et al. Predictive Performance of an Updated Polygenic Risk Score for Age-Related Macular Degeneration. Ophthalmology 2024, 131, 880–891. [Google Scholar] [CrossRef]
- Cai, X.; Seal, S.; McGinnis, J.F. Sustained inhibition of neovascularization in vldlr-/- mice following intravitreal injection of cerium oxide nanoparticles and the role of the ASK1-P38/JNK-NF-κB pathway. Biomaterials 2014, 35, 249–258. [Google Scholar] [CrossRef]
- Sennlaub, F.; Auvynet, C.; Calippe, B.; Lavalette, S.; Poupel, L.; Hu, S.J.; Dominguez, E.; Camelo, S.; Levy, O.; Guyon, E.; et al. CCR2(+) monocytes infiltrate atrophic lesions in age-related macular disease and mediate photoreceptor degeneration in experimental subretinal inflammation in Cx3cr1 deficient mice. EMBO Mol. Med. 2013, 5, 1775–1793. [Google Scholar] [CrossRef]
- Choi, Y.J.; Lim, D.; Byeon, S.H.; Shin, E.-C.; Chung, H. Chemokine Receptor Profiles of T Cells in Patients with Age-Related Macular Degeneration. Yonsei Med. J. 2022, 63, 357–364. [Google Scholar] [CrossRef]
- Merkle, C.W.; Augustin, M.; Harper, D.J.; Glösmann, M.; Baumann, B. Degeneration of Melanin-Containing Structures Observed Longitudinally in the Eyes of SOD1-/- Mice Using Intensity, Polarization, and Spectroscopic OCT. Transl. Vis. Sci. Technol. 2022, 11, 28. [Google Scholar] [CrossRef]
- Enserro, D.M.; Demler, O.V.; Pencina, M.J.; D’Agostino, R.B. Measures for evaluation of prognostic improvement under multivariate normality for nested and nonnested models. Stat. Med. 2019, 38, 3817–3831. [Google Scholar] [CrossRef]
- Sideri, O.; Correa, V.; Ziakas, N.; Tsinopoulos, I.; Miller, J.W.; Vavvas, D.G. Systematic Review of Proteomics in Age-Related Macular Degeneration and Pathway Analysis of Significant Protein Changes. Ophthalmol. Sci. 2025, 5, 100793. [Google Scholar] [CrossRef]
- Masli, S.; Sheibani, N.; Cursiefen, C.; Zieske, J. Matricellular protein thrombospondin: Influence on ocular angiogenesis, wound healing and immuneregulation. Curr. Eye Res. 2014, 39, 759–774. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Intonti, S.; Olivieri, C.; Reibaldi, M.; Borrelli, E.; Curcio, C.; Conedera, F.M. Translational Molecular and Fluid Biomarkers for Age-Related Macular Degeneration: Practical Insights from Animal Models and Humans. Biomolecules 2025, 15, 1571. https://doi.org/10.3390/biom15111571
Intonti S, Olivieri C, Reibaldi M, Borrelli E, Curcio C, Conedera FM. Translational Molecular and Fluid Biomarkers for Age-Related Macular Degeneration: Practical Insights from Animal Models and Humans. Biomolecules. 2025; 15(11):1571. https://doi.org/10.3390/biom15111571
Chicago/Turabian StyleIntonti, Simona, Chiara Olivieri, Michele Reibaldi, Enrico Borrelli, Claudia Curcio, and Federica Maria Conedera. 2025. "Translational Molecular and Fluid Biomarkers for Age-Related Macular Degeneration: Practical Insights from Animal Models and Humans" Biomolecules 15, no. 11: 1571. https://doi.org/10.3390/biom15111571
APA StyleIntonti, S., Olivieri, C., Reibaldi, M., Borrelli, E., Curcio, C., & Conedera, F. M. (2025). Translational Molecular and Fluid Biomarkers for Age-Related Macular Degeneration: Practical Insights from Animal Models and Humans. Biomolecules, 15(11), 1571. https://doi.org/10.3390/biom15111571




