Molecular Pathogenesis of Arrhythmogenic Cardiomyopathy: Mechanisms and Therapeutic Perspectives
Abstract
1. Introduction
2. Genetic Basis of ACM
3. Molecular and Cellular Pathogenesis
3.1. Desmosomal Dysfunction
3.2. Signal Transduction Pathways
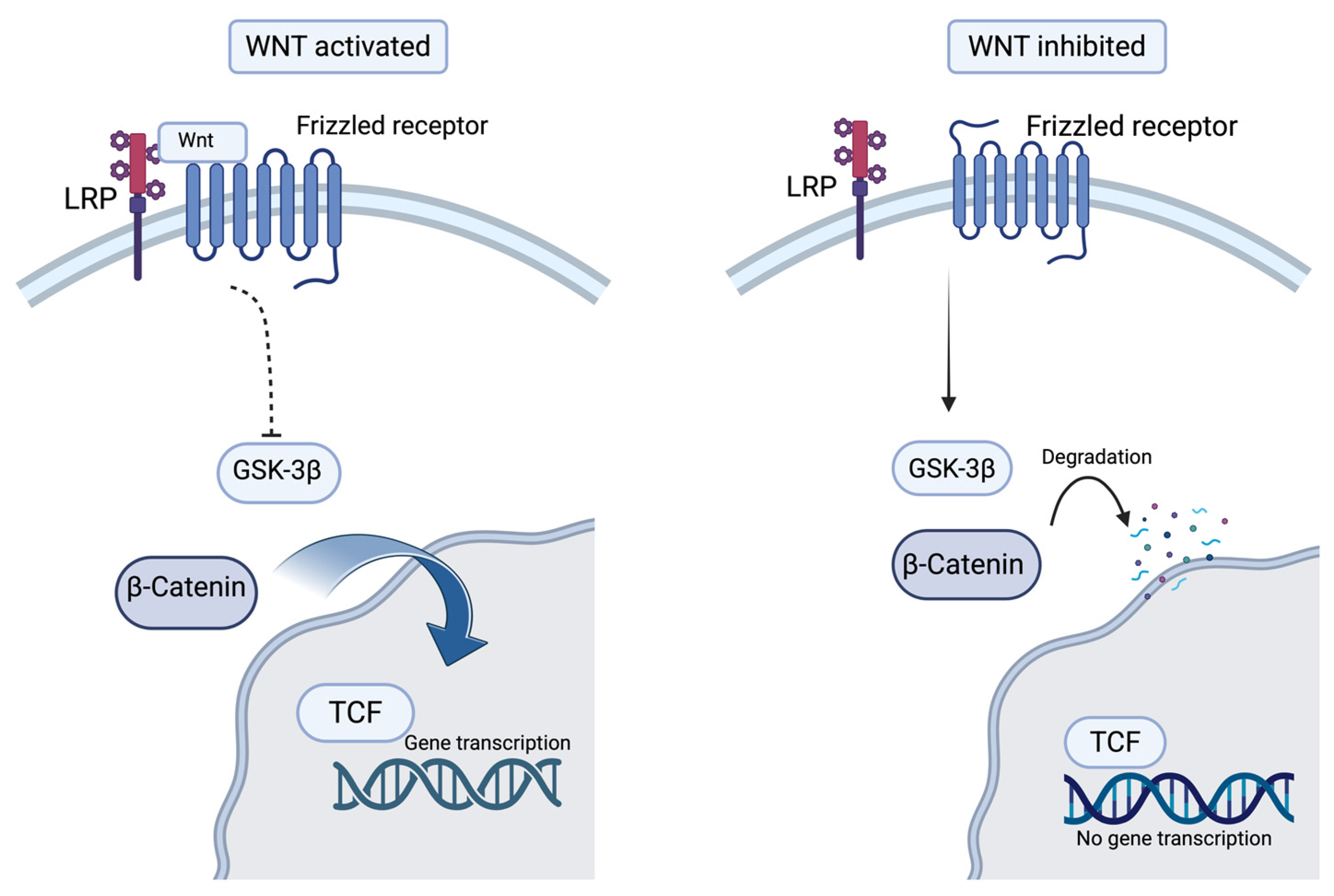
3.2.1. The Wnt/β-Catenin Signaling Pathway
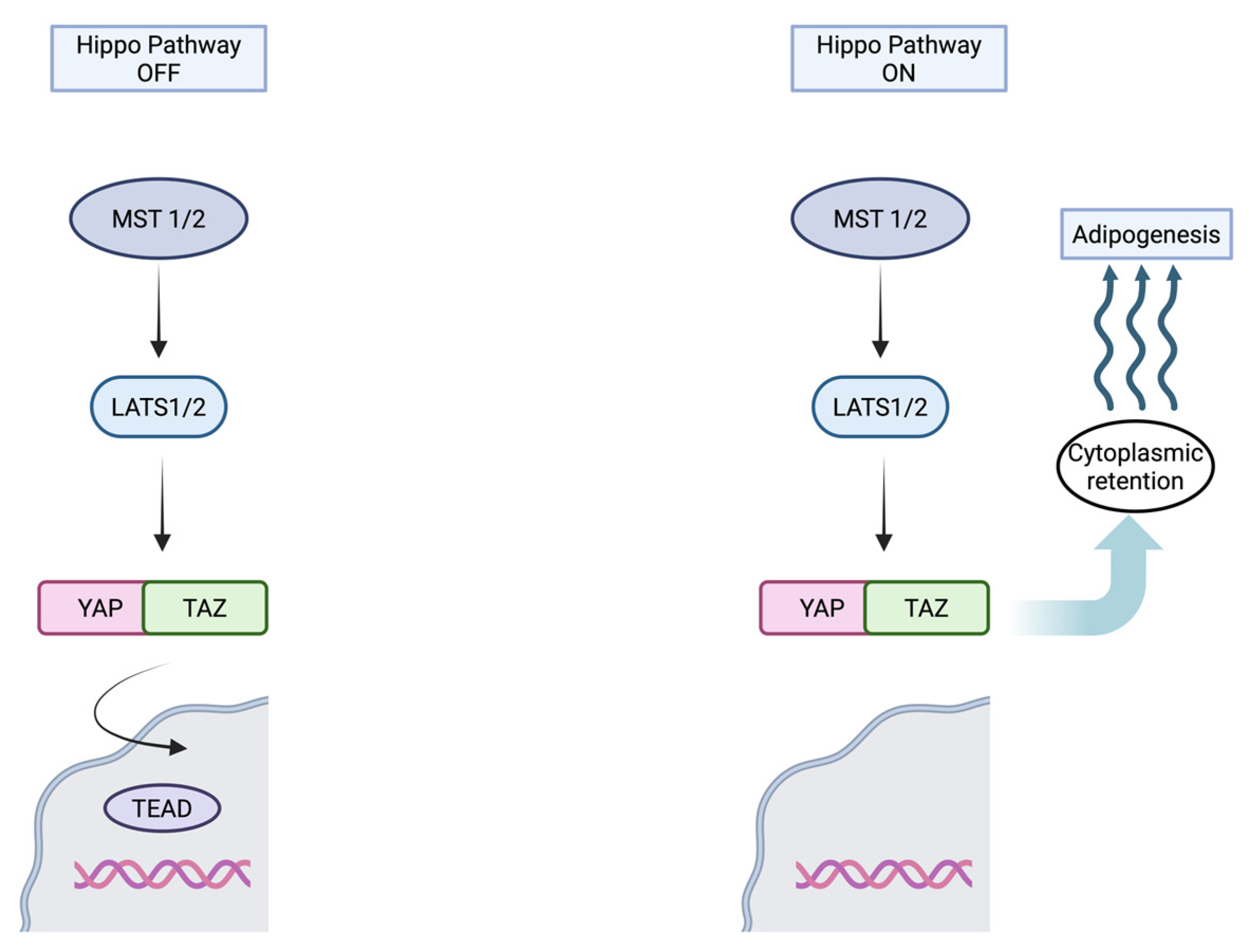
3.2.2. Hippo Pathway Activation
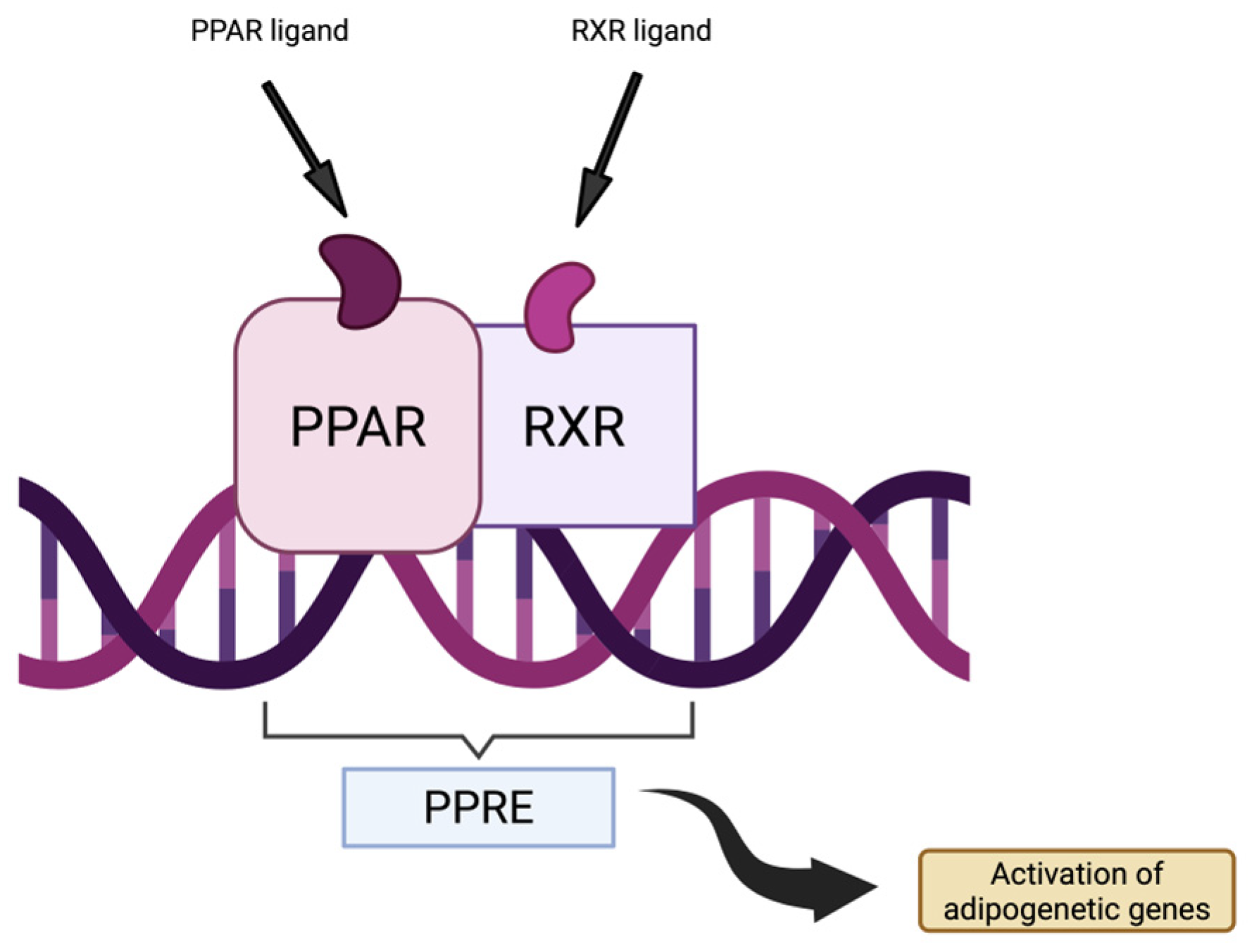
3.2.3. PPARγ Pathway
3.3. Cardiomyocyte Loss
3.4. Inflammation and Immune Activation
3.5. Adipogenesis
3.6. Arrhythmogenesis
4. Current Clinical Management
5. Emerging Therapeutic Approaches
5.1. Gene Therapy
5.2. Current Clinical Trials
5.3. Targeted Molecular Therapies
6. Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAV | Adeno-Associated Virus |
| AAV6 | Adeno-Associated Virus, Serotype 6 |
| AAV9 | Adeno-Associated Virus, Serotype 9 |
| ACM | Arrhythmogenic Cardiomyopathy |
| ALVC | Arrythmogenic Left Ventricular Cardiomyopathy |
| ARVC | Arrhythmogenic Right Ventricular Cardiomyopathy |
| ARVD | Arrhythmogenic Right Ventricular Dysplasia |
| β-catenin | Beta-Catenin (Transcription Factor) |
| C/EBP-α | CCAAT/Enhancer-Binding Protein Alpha |
| Cx43 | Connexin-43 |
| CDH2 | N-Cadherin |
| DCM | Dilated Cardiomyopathy |
| DES | Desmin |
| DSC2 | Desmocollin-2 |
| DSG2 | Desmoglein-2 |
| DSP | Desmoplakin |
| EKG | Electrocardiogram |
| ESC | European Society Of Cardiology |
| FDA | Food and Drug Administration |
| GSK-3β | Glycogen Synthase Kinase 3 Beta |
| ICD | Implantable Cardioverter-Defibrillator |
| IND | Investigational New Drug |
| IL-1β | Interleukin 1 Beta |
| IL-6 | Interleukin 6 |
| iPSC | Induced Pluripotent Stem Cells |
| iPSC-CM | Induced Pluripotent Stem Cell-Derived Cardiomyocyte |
| ISL1 | ISL LIM Homeobox 1 |
| JUP | Junctional plakoglobin |
| LATS1/2 | Large Tumor Suppressor Kinase 1/2 |
| LVEF | Left Ventricular Ejection Fraction |
| LV | Left Ventricle |
| MLC2v | Myosin Light Chain 2 (ventricular isoform) |
| MST1/2 | Mammalian STE20-like Protein Kinase |
| NSVT | Non-Sustained Ventricular Tachycardia |
| PES | Programmed Electrical Stimulation |
| PKC-α | Protein Kinase C Alpha |
| PKP2 | Plakophilin-2 |
| PLB | Phospholamban |
| PPARγ | Peroxisome Proliferator-Activated Receptor Gamma |
| rAAV | Recombinant Adeno-Associated Virus |
| RVEF | Right Ventricular Ejection Fraction |
| RV | Right Ventricle |
| SAP97 | Synapse-Associated Protein 97 |
| SCN5A | Sodium-Voltage-Gated Channel Alpha Subunit 5 |
| SB2 | Selective GSK-3β Inhibitor |
| SCD | Sudden Cardiac Death |
| SERCA | Sarco/Endoplasmic Reticulum Calcium ATPase |
| siRNA | Small Interfering RNA |
| SMVT | Sustained Monomorphic Ventricular Tachycardia |
| TAZ | Transcriptional Coactivator with PDZ-binding Motif |
| TCF | T-cell Factor |
| TEAD | TEA Domain Family Member |
| TGF-β | Transforming Growth Factor Beta |
| TGF3β | Transforming Growth Factor 3 Beta |
| TMEM43 | Transmembrane Protein 43 |
| TNF-α | Tumor Necrosis Factor Alpha |
| TTN | Titin |
| WT1 | Wilms Tumor 1 |
| Wnt | Wingless-related integration site (signaling pathway) |
| YAP | Yess-associated Protein |
References
- Corrado, D.; Basso, C.; Judge, D.P. Arrhythmogenic cardiomyopathy. Circ. Res. 2017, 121, 784–802. [Google Scholar] [PubMed]
- Corrado, D.; Perazzolo Marra, M.; Zorzi, A.; Beffagna, G.; Cipriani, A.; Lazzari, M.D.; Migliore, F.; Pilichou, K.; Rampazzo, A.; Rigato, I.; et al. Diagnosis of arrhythmogenic cardiomyopathy: The Padua criteria. Int. J. Cardiol. 2020, 319, 106–114. [Google Scholar] [CrossRef]
- Thiene, G.; Nava, A.; Corrado, D.; Rossi, L.; Pennelli, N. Right ventricular cardiomyopathy and sudden death in young people. N. Engl. J. Med. 1988, 318, 129–133. [Google Scholar] [PubMed]
- Vimalanathan, A.K.; Ehler, E.; Gehmlich, K. Genetics of and pathogenic mechanisms in arrhythmogenic right ventricular cardiomyopathy. Biophys. Rev. 2018, 10, 973–982. [Google Scholar] [CrossRef]
- Basso, C.; Corrado, D.; Marcus, F.I.; Nava, A.; Thiene, G. Arrhythmogenic right ventricular cardiomyopathy. Lancet 2009, 373, 1289–1300. [Google Scholar] [CrossRef]
- Engel, M.; Shiel, E.A.; Chelko, S.P. Basic and translational mechanisms in inflammatory arrhythmogenic cardiomyopathy. Int. J. Cardiol. 2024, 397, 131602. [Google Scholar]
- Kochi, A.N.; Vettor, G.; Dessanai, M.A.; Pizzamiglio, F.; Tondo, C. Sudden cardiac death in athletes: From the basics to the practical work-up. Medicina 2021, 57, 168. [Google Scholar] [CrossRef]
- Corrado, D.; Basso, C.; Thiene, G. Sudden cardiac death in young people with apparently normal heart. Cardiovasc. Res. 2001, 50, 399–408. [Google Scholar] [CrossRef] [PubMed]
- James, C.A.; Bhonsale, A.; Tichnell, C.; Murray, B.; Russell, S.D.; Tandri, H.; Tedford, R.J.; Judge, D.P.; Calkins, H. Exercise increases age-related penetrance and arrhythmic risk in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated desmosomal mutation carriers. J. Am. Coll. Cardiol. 2013, 62, 1290–1297. [Google Scholar]
- de la Guía-Galipienso, F.; Ugedo-Alzaga, K.; Grazioli, G.; Quesada-Ocete, F.J.; Feliu-Rey, E.; Perez, M.V.; Quesada-Dorador, A.; Sanchis-Gomar, F. Arrhythmogenic cardiomyopathy and athletes: A dangerous relationship. Curr. Probl. Cardiol. 2023, 48, 101799. [Google Scholar] [CrossRef]
- Austin, K.M.; Trembley, M.A.; Chandler, S.F.; Sanders, S.P.; Saffitz, J.E.; Abrams, D.J.; Pu, W.T. Molecular mechanisms of arrhythmogenic cardiomyopathy. Nat. Rev. Cardiol. 2019, 16, 519–537. [Google Scholar] [CrossRef]
- Morgan, J.A.; Corrigan, F.; Baune, B.T. Effects of physical exercise on central nervous system functions: A review of brain region specific adaptations. J. Mol. Psychiatry 2015, 3, 3. [Google Scholar] [CrossRef]
- Chelko, S.P.; Keceli, G.; Carpi, A.; Doti, N.; Agrimi, J.; Asimaki, A.; Beti, C.B.; Miyamoto, M.; Amat-Codina, N.; Bedja, D.; et al. Exercise triggers CAPN1-mediated AIF truncation, inducing myocyte cell death in arrhythmogenic cardiomyopathy. Sci. Transl. Med. 2021, 13, eabf0891. [Google Scholar] [CrossRef]
- Hammer, K.P.; Mustroph, J.; Stauber, T.; Birchmeier, W.; Wagner, S.; Maier, L.S. Beneficial effect of voluntary physical exercise in Plakophilin2 transgenic mice. PLoS ONE 2021, 16, e0252649. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, M.; Verrilli, V.; Pinto, A.; Popolo, A. Role of connexin 43 in cardiovascular diseases. Eur. J. Pharmacol. 2015, 768, 71–76. [Google Scholar] [CrossRef]
- Coscarella, I.L.; Landim-Vieira, M.; Pinto, J.R.; Chelko, S.P. Arrhythmogenic Cardiomyopathy: Exercise pitfalls, role of Connexin-43, and moving beyond antiarrhythmics. Int. J. Mol. Sci. 2022, 23, 8753. [Google Scholar] [CrossRef]
- Veeranki, S.; Givvimani, S.; Kundu, S.; Metreveli, N.; Pushpakumar, S.; Tyagi, S.C. Moderate intensity exercise prevents diabetic cardiomyopathy associated contractile dysfunction through restoration of mitochondrial function and connexin 43 levels in db/db mice. J. Mol. Cell Cardiol. 2016, 92, 163–173. [Google Scholar] [CrossRef]
- Sanchis-Gomar, F.; Malkani, K.; Perez-Quilis, C. Exercise training intensity and connexin 43 expression in hypertrophic cardiomyopathy. J. Mol. Cell Cardiol. 2017, 109, 60. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Yu, T.; Yang, H.; Peng, Z. Exhaustive exercise-induced cardiac conduction system injury and changes of cTnT and Cx43. Int. J. Sports Med. 2015, 36, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Corrado, D.; Fontaine, G.; Marcus, F.I.; McKenna, W.J.; Nava, A.; Thiene, G.; Wichter, T. Arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circulation 2000, 101, e101–e106. [Google Scholar] [CrossRef]
- Costa, S.; Cerrone, M.; Saguner, A.M.; Brunckhorst, C.; Delmar, M.; Duru, F. Arrhythmogenic cardiomyopathy: An in-depth look at molecular mechanisms and clinical correlates. Trends Cardiovasc. Med. 2021, 31, 395–402. [Google Scholar] [CrossRef]
- Gandjbakhch, E.; Redheuil, A.; Pousset, F.; Charron, P.; Frank, R. Clinical diagnosis, imaging, and genetics of arrhythmogenic right ventricular cardiomyopathy/dysplasia: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2018, 72, 784–804. [Google Scholar] [CrossRef]
- Delmar, M.; McKenna, W.J. The cardiac desmosome and arrhythmogenic cardiomyopathies: From gene to disease. Circ. Res. 2010, 107, 700–714. [Google Scholar] [CrossRef]
- Corrado, D.; Anastasakis, A.; Basso, C.; Bauce, B.; Blomström-Lundqvist, C.; Bucciarelli-Ducci, C.; Cipriani, A.; De Asmundis, C.; Gandjbakhch, E.; Jiménez-Jáimez, J.; et al. Proposed diagnostic criteria for arrhythmogenic cardiomyopathy: European Task Force consensus report. Int. J. Cardiol. 2024, 395, 131447. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Asatryan, B.; Siripanthong, B.; Munroe, P.B.; Tiku-Owens, A.; Lopes, L.R.; Khanji, M.Y.; Protonotarios, A.; Santangeli, P.; Muser, D.; et al. State of the art review on genetics and precision medicine in arrhythmogenic cardiomyopathy. Int. J. Mol. Sci. 2020, 21, 6615. [Google Scholar] [CrossRef] [PubMed]
- Akdis, D.; Brunckhorst, C.; Duru, F.; Saguner, A.M. Arrhythmogenic cardiomyopathy: Electrical and structural phenotypes. Arrhythm. Electrophysiol. Rev. 2016, 5, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Varrenti, M.; Preda, A.; Frontera, A.; Baroni, M.; Gigli, L.; Vargiu, S.; Colombo, G.; Carbonaro, M.; Paolucci, M.; Giordano, F.; et al. Arrhythmogenic cardiomyopathy: Definition, classification and arrhythmic risk stratification. J. Clin. Med. 2024, 13, 456. [Google Scholar] [CrossRef]
- Bariani, R.; Rigato, I.; Cason, M.; Marinas, M.B.; Celeghin, R.; Pilichou, K.; Bauce, B. Genetic background and clinical features in arrhythmogenic left ventricular cardiomyopathy: A systematic review. J. Clin. Med. 2022, 11, 4313. [Google Scholar] [CrossRef]
- Mattesi, G.; Cipriani, A.; Bauce, B.; Rigato, I.; Zorzi, A.; Corrado, D. Arrhythmogenic left ventricular cardiomyopathy: Genotype-phenotype correlations and new diagnostic criteria. J. Clin. Med. 2021, 10, 2212. [Google Scholar] [CrossRef]
- Pinamonti, B.; Brun, F.; Mestroni, L.; Sinagra, G. Arrhythmogenic right ventricular cardiomyopathy: From genetics to diagnostic and therapeutic challenges. World J. Cardiol. 2014, 6, 1234–1244. [Google Scholar] [CrossRef]
- Stevens, T.L.; Wallace, M.J.; Refaey, M.E.; Roberts, J.D.; Koenig, S.N.; Mohler, P.J. Arrhythmogenic cardiomyopathy: Molecular insights for improved therapeutic design. J. Cardiovasc. Dev. Dis. 2020, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Delva, E.; Tucker, D.K.; Kowalczyk, A.P. The desmosome. Cold Spring Harb. Perspect. Biol. 2009, 1, a002543. [Google Scholar] [CrossRef] [PubMed]
- Protonotarios, N.; Tsatsopoulou, A.; Patsourakos, P.; Alexopoulos, D.; Gezerlis, P.; Simitsis, S.; Scampardonis, G. Cardiac abnormalities in familial palmoplantar keratosis. Br. Heart J. 1986, 56, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Leopoulou, M.; Mattsson, G.; LeQuang, J.A.; Pergolizzi, J.V.; Varrassi, G.; Wallhagen, M.; Magnusson, P. Naxos disease—A narrative review. Expert. Rev. Cardiovasc. Ther. 2020, 18, 801–808. [Google Scholar] [CrossRef]
- van Tintelen, J.P.; Entius, M.M.; Bhuiyan, Z.A.; Jongbloed, R.; Wiesfeld, A.C.P.; Wilde, A.A.M.; van der Smagt, J.; Boven, L.G.; Mannens, M.M.A.M.; van Langen, I.M.; et al. Plakophilin-2 mutations are the major determinant of familial arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circulation 2006, 113, 1650–1658. [Google Scholar] [CrossRef]
- Groeneweg, J.A.; Bhonsale, A.; James, C.A.; te Riele, A.S.; Dooijes, D.; Tichnell, C.; Murray, B.; Wiesfeld, A.C.P.; Sawant, A.C.; Kassamali, B.; et al. Clinical presentation, long-term follow-up, and outcomes of 1001 arrhythmogenic right ventricular dysplasia/cardiomyopathy patients and family members. Circ. Cardiovasc. Genet. 2015, 8, 437–446. [Google Scholar] [CrossRef]
- Biernacka, E.K.; Borowiec, K.; Franaszczyk, M.; Szperl, M.; Rampazzo, A.; Woźniak, O.; Roszczynko, M.; Śmigielski, W.; Lutyńska, A.; Hoffman, P. Pathogenic variants in plakophilin-2 gene (PKP2) are associated with better survival in arrhythmogenic right ventricular cardiomyopathy. J. Appl. Genet. 2021, 62, 613–620. [Google Scholar] [CrossRef]
- James, C.A.; Jongbloed, J.D.H.; Hershberger, R.E.; Morales, A.; Judge, D.P.; Syrris, P.; Pilichou, K.; Domingo, A.M.; Murray, B.; Cadrin-Tourigny, J.; et al. International evidence based reappraisal of genes associated with arrhythmogenic right ventricular cardiomyopathy using the Clinical Genome Resource framework. Circ. Genom. Precis. Med. 2021, 14, e003273. [Google Scholar] [CrossRef]
- Alcalde, M.; Campuzano, O.; Berne, P.; García-Pavía, P.; Doltra, A.; Arbelo, E.; Sarquella-Brugada, G.; Iglesias, A.; Alonso-Pulpon, L.; Brugada, J.; et al. Stop-gain mutations in PKP2 are associated with a later age of onset of arrhythmogenic right ventricular cardiomyopathy. PLoS ONE 2014, 9, e100560. [Google Scholar] [CrossRef]
- Xu, Z.; Zhu, W.; Wang, C.; Huang, L.; Zhou, Q.; Hu, J.; Cheng, X.; Hong, K. Genotype-phenotype relationship in patients with arrhythmogenic right ventricular cardiomyopathy caused by desmosomal gene mutations: A systematic review and meta-analysis. Sci. Rep. 2017, 7, 41387. [Google Scholar] [CrossRef]
- Heuser, A.; Plovie, E.R.; Ellinor, P.T.; Grossmann, K.S.; Shin, J.T.; Wichter, T.; Basson, C.T.; Lerman, B.B.; Sasse-Klaassen, S.; Thierfelder, L.; et al. Mutant desmocollin-2 causes arrhythmogenic right ventricular cardiomyopathy. Am. J. Hum. Genet. 2006, 79, 1081–1088. [Google Scholar] [CrossRef]
- Li, D.; Liu, Y.; Maruyama, M.; Zhu, W.; Chen, H.; Zhang, W.; Reuter, S.; Lin, S.-F.; Haneline, L.S.; Field, L.J.; et al. Restrictive loss of plakoglobin in cardiomyocytes leads to arrhythmogenic cardiomyopathy. Hum. Mol. Genet. 2011, 20, 4582–4596. [Google Scholar] [CrossRef]
- Bauce, B.; Rampazzo, A.; Basso, C.; Mazzotti, E.; Rigato, I.; Steriotis, A.; Beffagna, G.; Lorenzon, A.; De Bortoli, M.; Pilichou, K.; et al. Clinical phenotype and diagnosis of arrhythmogenic right ventricular cardiomyopathy in pediatric patients carrying desmosomal gene mutations. Heart Rhythm. 2011, 8, 1686–1695. [Google Scholar] [CrossRef]
- Merner, N.D.; Hodgkinson, K.A.; Haywood, A.F.M.; Connors, S.; French, V.M.; Drenckhahn, J.-D.; Kupprion, C.; Ramadanova, K.; Thierfelder, L.; McKenna, W.; et al. Arrhythmogenic right ventricular cardiomyopathy type 5 is a fully penetrant, lethal arrhythmic disorder caused by a missense mutation in the TMEM43 gene. Am. J. Hum. Genet. 2008, 82, 809–821. [Google Scholar] [CrossRef]
- Bermúdez-Jiménez, F.J.; Carriel, V.; Brodehl, A.; Alaminos, M.; Campos, A.; Schirmer, I.; Milting, H.; Abril, B.Á.; Álvarez, M.; López-Fernández, S.; et al. Novel Desmin mutation p.Glu401Asp impairs filament formation, disrupts cell membrane integrity, and causes severe arrhythmogenic left ventricular cardiomyopathy/dysplasia. Circulation 2018, 137, 1595–1610. [Google Scholar] [CrossRef] [PubMed]
- van der Heijden, J.F.; Hassink, R.J. The phospholamban p.Arg14del founder mutation in Dutch patients with arrhythmogenic cardiomyopathy. Neth. Heart J. 2013, 21, 284–285. [Google Scholar] [CrossRef]
- Mayosi, B.M.; Fish, M.; Shaboodien, G.; Mastantuono, E.; Kraus, S.; Wieland, T.; Kotta, M.-C.; Chin, A.; Laing, N.; Ntusi, N.B.A.; et al. Identification of cadherin 2 (CDH2) mutations in arrhythmogenic right ventricular cardiomyopathy. Circ. Cardiovasc. Genet. 2017, 10, e001605. [Google Scholar] [CrossRef]
- Te Riele, A.S.J.M.; Agullo-Pascual, E.; James, C.A.; Leo-Macias, A.; Cerrone, M.; Zhang, M.; Lin, X.; Lin, B.; Sobreira, N.L.; Amat-Alarcon, N.; et al. Multilevel analyses of SCN5A mutations in arrhythmogenic right ventricular dysplasia/cardiomyopathy suggest non-canonical mechanisms for disease pathogenesis. Cardiovasc. Res. 2017, 113, 102–111. [Google Scholar] [CrossRef]
- Beffagna, G.; Occhi, G.; Nava, A.; Vitiello, L.; Ditadi, A.; Basso, C.; Bauce, B.; Carraro, G.; Thiene, G.; Towbin, J.A.; et al. Regulatory mutations in transforming growth factor-beta3 gene cause arrhythmogenic right ventricular cardiomyopathy type 1. Cardiovasc. Res. 2005, 65, 366–373. [Google Scholar] [CrossRef]
- Quarta, G.; Syrris, P.; Ashworth, M.; Jenkins, S.; Zuborne Alapi, K.; Morgan, J.; Muir, A.; Pantazis, A.; McKenna, W.J.; Elliott, P.M. Mutations in the Lamin A/C gene mimic arrhythmogenic right ventricular cardiomyopathy. Eur. Heart J. 2012, 33, 1128–1136. [Google Scholar] [CrossRef]
- Carruth, E.D.; Qureshi, M.; Alsaid, A.; Kelly, M.A.; Calkins, H.; Murray, B.; Tichnell, C.; Sturm, A.C.; Regeneron Genetics Center; Baras, A.; et al. Loss-of-function FLNC variants are associated with arrhythmogenic cardiomyopathy phenotypes when identified through exome sequencing of a general clinical population. Circ. Genom. Precis. Med. 2022, 15, e003645. [Google Scholar] [CrossRef]
- Al-Amoudi, A.; Frangakis, A.S. Structural studies on desmosomes. Biochem. Soc. Trans. 2008, 36, 181–187. [Google Scholar] [CrossRef]
- Dusek, R.L.; Godsel, L.M.; Green, K.J. Discriminating roles of desmosomal cadherins: Beyond desmosomal adhesion. J. Dermatol. Sci. 2007, 45, 7–21. [Google Scholar] [CrossRef]
- Green, K.J.; Simpson, C.L. Desmosomes: New perspectives on a classic. J. Investig. Dermatol. 2007, 127, 2499–2515. [Google Scholar] [CrossRef]
- Najor, N.A. Desmosomes in human disease. Annu. Rev. Pathol. 2018, 13, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Hoorntje, E.T.; Te Rijdt, W.P.; James, C.A.; Pilichou, K.; Basso, C.; Judge, D.P.; Bezzina, C.R.; van Tintelen, J.P. Arrhythmogenic cardiomyopathy: Pathology, genetics, and concepts in pathogenesis. Cardiovasc. Res. 2017, 113, 1521–1531. [Google Scholar] [CrossRef]
- Meraviglia, V.; Alcalde, M.; Campuzano, O.; Bellin, M. Inflammation in the pathogenesis of arrhythmogenic cardiomyopathy: Secondary event or active driver? Front. Cardiovasc. Med. 2021, 8, 784715. [Google Scholar] [CrossRef] [PubMed]
- McGregor, S.M.; Husain, A.N. A brief review and update of the clinicopathologic diagnosis of arrhythmogenic cardiomyopathy. Arch. Pathol. Lab. Med. 2015, 139, 1181–1186. [Google Scholar] [CrossRef]
- van der Voorn, S.M.; Te Riele, A.S.J.M.; Basso, C.; Calkins, H.; Remme, C.A.; van Veen, T.A.B. Arrhythmogenic cardiomyopathy: Pathogenesis, pro-arrhythmic remodelling, and novel approaches for risk stratification and therapy. Cardiovasc. Res. 2020, 116, 1571–1584. [Google Scholar] [CrossRef]
- Steinhart, Z.; Angers, S. Wnt signaling in development and tissue homeostasis. Development 2018, 145, dev146589. [Google Scholar] [CrossRef]
- Lorenzon, A.; Calore, M.; Poloni, G.; De Windt, L.J.; Braghetta, P.; Rampazzo, A. Wnt/β-catenin pathway in arrhythmogenic cardiomyopathy. Oncotarget 2017, 8, 60640–60655. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef]
- Gao, S.; Puthenvedu, D.; Lombardi, R.; Chen, S.N. Established and emerging mechanisms in the pathogenesis of arrhythmogenic cardiomyopathy: A multifaceted disease. Int. J. Mol. Sci. 2020, 21, 6320. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.E.; Hemati, N.; Longo, K.A.; Bennett, C.N.; Lucas, P.C.; Erickson, R.L.; MacDougald, O.A. Inhibition of adipogenesis by Wnt signaling. Science 2000, 289, 950–953. [Google Scholar] [CrossRef]
- Chen, S.; Guttridge, D.C.; You, Z.; Zhang, Z.; Fribley, A.; Mayo, M.W.; Kitajewski, J.; Wang, C.Y. Wnt-1 signaling inhibits apoptosis by activating beta-catenin/T cell factor-mediated transcription. J. Cell Biol. 2001, 152, 87–96. [Google Scholar] [CrossRef]
- Piquer-Gil, M.; Domenech-Dauder, S.; Sepúlveda-Gómez, M.; Machí-Camacho, C.; Braza-Boïls, A.; Zorio, E. Non coding RNAs as regulators of Wnt/β-catenin and Hippo pathways in arrhythmogenic cardiomyopathy. Biomedicines 2022, 10, 2619. [Google Scholar] [CrossRef]
- Yu, F.-X.; Zhao, B.; Guan, K.-L. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell 2015, 163, 811–828. [Google Scholar] [CrossRef]
- Han, Y. Analysis of the role of the Hippo pathway in cancer. J. Transl. Med. 2019, 17, 116. [Google Scholar] [CrossRef]
- Fu, M.; Hu, Y.; Lan, T.; Guan, K.-L.; Luo, T.; Luo, M. The Hippo signalling pathway and its implications in human health and diseases. Signal Transduct. Target. Ther. 2022, 7, 376. [Google Scholar] [CrossRef] [PubMed]
- Plouffe, S.W.; Meng, Z.; Lin, K.C.; Lin, B.; Hong, A.W.; Chun, J.V.; Guan, K.-L. Characterization of Hippo pathway components by gene inactivation. Mol. Cell 2016, 64, 993–1008. [Google Scholar] [CrossRef]
- Chen, S.N.; Gurha, P.; Lombardi, R.; Ruggiero, A.; Willerson, J.T.; Marian, A.J. The hippo pathway is activated and is a causal mechanism for adipogenesis in arrhythmogenic cardiomyopathy. Circ. Res. 2014, 114, 454–468. [Google Scholar] [CrossRef]
- Heallen, T.; Zhang, M.; Wang, J.; Bonilla-Claudio, M.; Klysik, E.; Johnson, R.L.; Martin, J.F. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science 2011, 332, 458–461. [Google Scholar] [CrossRef]
- Chandra, M.; Miriyala, S.; Panchatcharam, M. PPARγ and its role in cardiovascular diseases. PPAR Res. 2017, 2017, 6404638. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, M.; Suh, J.M.; Hah, N.; Liddle, C.; Atkins, A.R.; Downes, M.; Evans, R.M. PPARγ signaling and metabolism: The good, the bad and the future. Nat. Med. 2013, 19, 557–566. [Google Scholar] [CrossRef]
- Reisqs, J.-B.; Moreau, A.; Charrabi, A.; Sleiman, Y.; Meli, A.C.; Millat, G.; Briand, V.; Beauverger, P.; Richard, S.; Chevalier, P. The PPARγ pathway determines electrophysiological remodelling and arrhythmia risks in DSC2 arrhythmogenic cardiomyopathy. Clin. Transl. Med. 2022, 12, e748. [Google Scholar] [CrossRef] [PubMed]
- Sommariva, E.; Stadiotti, I.; Casella, M.; Catto, V.; Dello Russo, A.; Carbucicchio, C.; Arnaboldi, L.; De Metrio, S.; Milano, G.; Scopece, A.; et al. Oxidized LDL-dependent pathway as new pathogenic trigger in arrhythmogenic cardiomyopathy. EMBO Mol. Med. 2021, 13, e14365. [Google Scholar] [CrossRef]
- Wei, C.; Saguner, A.; Duru, F.; Marine, J.E.; James, C.A.; Calkins, H.; Judge, D.P.; Shou, W.; Chen, H.-S.V. Po-02-246 pparγ activation mediates cardiac pathologies in two mouse models of arrhythmogenic cardiomyopathy (acm) and its therapeutic implications. Heart Rhythm. 2023, 20, S286. [Google Scholar] [CrossRef]
- Lu, W.; Rao, Y.; Li, Y.; Dai, Y.; Chen, K. The landscape of cell death processes with associated immunogenic and fibrogenic effects in arrhythmogenic cardiomyopathy. J. Cardiovasc. Dev. Dis. 2022, 9, 301. [Google Scholar] [CrossRef]
- Mallat, Z.; Tedgui, A.; Fontaliran, F.; Frank, R.; Durigon, M.; Fontaine, G. Evidence of apoptosis in arrhythmogenic right ventricular dysplasia. N. Engl. J. Med. 1996, 335, 1190–1196. [Google Scholar] [CrossRef]
- Hariharan, V.; Asimaki, A.; Michaelson, J.E.; Plovie, E.; MacRae, C.A.; Saffitz, J.E.; Huang, H. Arrhythmogenic right ventricular cardiomyopathy mutations alter shear response without changes in cell-cell adhesion. Cardiovasc. Res. 2014, 104, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Caspi, O.; Huber, I.; Gepstein, A.; Arbel, G.; Maizels, L.; Boulos, M.; Gepstein, L. Modeling of arrhythmogenic right ventricular cardiomyopathy with human induced pluripotent stem cells. Circ. Cardiovasc. Genet. 2013, 6, 557–568. [Google Scholar] [CrossRef]
- Garcia-Gras, E.; Lombardi, R.; Giocondo, M.J.; Willerson, J.T.; Schneider, M.D.; Khoury, D.S.; Marian, A.J. Suppression of canonical Wnt/beta-catenin signaling by nuclear plakoglobin recapitulates phenotype of arrhythmogenic right ventricular cardiomyopathy. J. Clin. Investig. 2006, 116, 2012–2021. [Google Scholar] [CrossRef]
- Campuzano, O.; Alcalde, M.; Iglesias, A.; Barahona-Dussault, C.; Sarquella-Brugada, G.; Benito, B.; Arzamendi, D.; Flores, J.; Leung, T.K.; Talajic, M.; et al. Arrhythmogenic right ventricular cardiomyopathy: Severe structural alterations are associated with inflammation. J. Clin. Pathol. 2012, 65, 1077–1083. [Google Scholar] [CrossRef]
- Asatryan, B.; Asimaki, A.; Landstrom, A.P.; Khanji, M.Y.; Odening, K.E.; Cooper, L.T.; Marchlinski, F.E.; Gelzer, A.R.; Semsarian, C.; Reichlin, T.; et al. Inflammation and immune response in arrhythmogenic cardiomyopathy: State-of-the-art review. Circulation 2021, 144, 1646–1655. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.N.; Lyne, J.; De Silva, R.; Wong, T. Myocarditic appearance of arrhythmogenic right ventricular cardiomyopathy. Circulation 2010, 122, e556–e557. [Google Scholar] [CrossRef]
- Patrianakos, A.P.; Protonotarios, N.; Nyktari, E.; Pagonidis, K.; Tsatsopoulou, A.; Parthenakis, F.I.; Vardas, P.E. Arrhythmogenic right ventricular cardiomyopathy/dysplasia and troponin release. Myocarditis or the “hot phase” of the disease? Int. J. Cardiol. 2012, 157, e26-8. [Google Scholar] [CrossRef]
- Lazaros, G.; Anastasakis, A.; Tsiachris, D.; Dilaveris, P.; Protonotarios, N.; Stefanadis, C. Naxos disease presenting with ventricular tachycardia and troponin elevation. Heart Vessel. 2009, 24, 63–65. [Google Scholar] [CrossRef]
- Monda, E.; Palmiero, G.; Rubino, M.; Verrillo, F.; Amodio, F.; Di Fraia, F.; Pacileo, R.; Fimiani, F.; Esposito, A.; Cirillo, A.; et al. Molecular basis of inflammation in the pathogenesis of cardiomyopathies. Int. J. Mol. Sci. 2020, 21, 6462. [Google Scholar] [CrossRef] [PubMed]
- Piek, A.; de Boer, R.A.; Silljé, H.H.W. The fibrosis-cell death axis in heart failure. Heart Fail. Rev. 2016, 21, 199–211. [Google Scholar] [CrossRef]
- Lubos, N.; van der Gaag, S.; Gerçek, M.; Kant, S.; Leube, R.E.; Krusche, C.A. Inflammation shapes pathogenesis of murine arrhythmogenic cardiomyopathy. Basic. Res. Cardiol. 2020, 115, 42. [Google Scholar] [CrossRef] [PubMed]
- Asimaki, A.; Tandri, H.; Duffy, E.R.; Winterfield, J.R.; Mackey-Bojack, S.; Picken, M.M.; Cooper, L.T.; Wilber, D.J.; Marcus, F.I.; Basso, C.; et al. Altered desmosomal proteins in granulomatous myocarditis and potential pathogenic links to arrhythmogenic right ventricular cardiomyopathy. Circ. Arrhythm. Electrophysiol. 2011, 4, 743–752. [Google Scholar] [CrossRef]
- Stadiotti, I.; Catto, V.; Casella, M.; Tondo, C.; Pompilio, G.; Sommariva, E. Arrhythmogenic cardiomyopathy: The guilty party in adipogenesis. J. Cardiovasc. Transl. Res. 2017, 10, 446–454. [Google Scholar] [CrossRef]
- Vencato, S.; Romanato, C.; Rampazzo, A.; Calore, M. Animal models and molecular pathogenesis of arrhythmogenic cardiomyopathy associated with pathogenic variants in intercalated disc genes. Int. J. Mol. Sci. 2024, 25, 6208. [Google Scholar] [CrossRef]
- Kohela, A.; van Rooij, E. Fibro-fatty remodelling in arrhythmogenic cardiomyopathy. Basic. Res. Cardiol. 2022, 117, 22. [Google Scholar] [CrossRef]
- Lefterova, M.I.; Haakonsson, A.K.; Lazar, M.A.; Mandrup, S. PPARγ and the global map of adipogenesis and beyond. Trends Endocrinol. Metab. 2014, 25, 293–302. [Google Scholar] [CrossRef]
- Olofsson, L.E.; Orho-Melander, M.; William-Olsson, L.; Sjöholm, K.; Sjöström, L.; Groop, L.; Carlsson, B.; Carlsson, L.M.S.; Olsson, B. CCAAT/enhancer binding protein alpha (C/EBPalpha) in adipose tissue regulates genes in lipid and glucose metabolism and a genetic variation in C/EBPalpha is associated with serum levels of triglycerides. J. Clin. Endocrinol. Metab. 2008, 93, 4880–4886. [Google Scholar] [CrossRef] [PubMed]
- d’Amati, G.; di Gioia, C.R.T.; Giordano, C.; Gallo, P. Myocyte transdifferentiation. Arch. Pathol. Lab. Med. 2000, 124, 287–290. [Google Scholar] [CrossRef]
- Fujita, S.; Terasaki, F.; Otsuka, K.; Katashima, T.; Kanzaki, Y.; Kawamura, K.; Tanaka, T.; Kitaura, Y. Markedly increased intracellular lipid droplets and disruption of intercellular junctions in biopsied myocardium from a patient with arrhythmogenic right ventricular cardiomyopathy. Heart Vessel. 2008, 23, 440–444. [Google Scholar] [CrossRef]
- Lyon, R.C.; Mezzano, V.; Wright, A.T.; Pfeiffer, E.; Chuang, J.; Banares, K.; Castaneda, A.; Ouyang, K.; Cui, L.; Contu, R.; et al. Connexin defects underlie arrhythmogenic right ventricular cardiomyopathy in a novel mouse model. Hum. Mol. Genet. 2014, 23, 1134–1150. [Google Scholar] [CrossRef] [PubMed]
- Dorn, T.; Kornherr, J.; Parrotta, E.I.; Zawada, D.; Ayetey, H.; Santamaria, G.; Iop, L.; Mastantuono, E.; Sinnecker, D.; Goedel, A.; et al. Interplay of cell-cell contacts and RhoA/MRTF-A signaling regulates cardiomyocyte identity. EMBO J. 2018, 37, e98133. [Google Scholar] [CrossRef] [PubMed]
- Smits, A.M.; Dronkers, E.; Goumans, M.-J. The epicardium as a source of multipotent adult cardiac progenitor cells: Their origin, role and fate. Pharmacol. Res. 2018, 127, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, S.; Lodder, E.M.; Verkerk, A.O.; Wolswinkel, R.; Beekman, L.; Pilichou, K.; Basso, C.; Remme, C.A.; Thiene, G.; Bezzina, C.R. Intercalated disc abnormalities, reduced Na(+) current density, and conduction slowing in desmoglein-2 mutant mice prior to cardiomyopathic changes. Cardiovasc. Res. 2012, 95, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.S.; van Opbergen, C.J.M.; van Veen, T.A.B.; Delmar, M. The intercalated disc: A unique organelle for electromechanical synchrony in cardiomyocytes. Physiol. Rev. 2023, 103, 2271–2319. [Google Scholar] [CrossRef]
- Cerrone, M.; Lin, X.; Zhang, M.; Agullo-Pascual, E.; Pfenniger, A.; Chkourko Gusky, H.; Novelli, V.; Kim, C.; Tirasawadichai, T.; Judge, D.P.; et al. Missense mutations in plakophilin-2 cause sodium current deficit and associate with a Brugada syndrome phenotype. Circulation 2014, 129, 1092–1103. [Google Scholar] [CrossRef]
- Rook, M.B.; Evers, M.M.; Vos, M.A.; Bierhuizen, M.F.A. Biology of cardiac sodium channel Nav1.5 expression. Cardiovasc. Res. 2012, 93, 12–23. [Google Scholar] [CrossRef]
- Sato, P.Y.; Musa, H.; Coombs, W.; Guerrero-Serna, G.; Patiño, G.A.; Taffet, S.M.; Isom, L.L.; Delmar, M. Loss of plakophilin-2 expression leads to decreased sodium current and slower conduction velocity in cultured cardiac myocytes. Circ. Res. 2009, 105, 523–526. [Google Scholar] [CrossRef]
- Severs, N.J.; Coppen, S.R.; Dupont, E.; Yeh, H.-I.; Ko, Y.-S.; Matsushita, T. Gap junction alterations in human cardiac disease. Cardiovasc. Res. 2004, 62, 368–377. [Google Scholar] [CrossRef]
- Söhl, G.; Willecke, K. Gap junctions and the connexin protein family. Cardiovasc. Res. 2004, 62, 228–232. [Google Scholar] [CrossRef]
- Solan, J.L.; Lampe, P.D. Connexin43 phosphorylation: Structural changes and biological effects. Biochem. J. 2009, 419, 261–272. [Google Scholar] [CrossRef]
- Oxford, E.M.; Musa, H.; Maass, K.; Coombs, W.; Taffet, S.M.; Delmar, M. Connexin43 remodeling caused by inhibition of plakophilin-2 expression in cardiac cells. Circ. Res. 2007, 101, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Asimaki, A.; Tandri, H.; Huang, H.; Halushka, M.K.; Gautam, S.; Basso, C.; Thiene, G.; Tsatsopoulou, A.; Protonotarios, N.; McKenna, W.J.; et al. A new diagnostic test for arrhythmogenic right ventricular cardiomyopathy. N. Engl. J. Med. 2009, 360, 1075–1084. [Google Scholar] [CrossRef]
- Calkins, H.; Corrado, D.; Marcus, F. Risk stratification in arrhythmogenic right ventricular cardiomyopathy. Circulation 2017, 136, 2068–2082. [Google Scholar] [CrossRef]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. 2023 ESC Guidelines for the management of cardiomyopathies. Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef]
- Corrado, D.; Basso, C.; Rizzoli, G.; Schiavon, M.; Thiene, G. Does sports activity enhance the risk of sudden death in adolescents and young adults? J. Am. Coll. Cardiol. 2003, 42, 1959–1963. [Google Scholar] [CrossRef]
- Gasperetti, A.; Targetti, M.; Olivotto, I. Antiarrhythmic drugs in arrhythmogenic right ventricular cardiomyopathy: The importance of optimal beta-blocker dose titration. Int. J. Cardiol. 2021, 338, 150–151. [Google Scholar] [CrossRef]
- Kreimer, F.; Saguner, A.M.; Akin, I.; Milting, H.; Eckardt, L.; El-Battrawy, I. Arrhythmogenic right ventricular cardiomyopathy: Diagnosis, risk stratification, and treatment. Dtsch. Arztebl. Int. 2025, 122, 229–234. [Google Scholar]
- Jordà, P.; Bosman, L.P.; Gasperetti, A.; Mazzanti, A.; Gourraud, J.B.; Davies, B.; Frederiksen, T.C.; Weidmann, Z.M.; Di Marco, A.; Roberts, J.D.; et al. Arrhythmic risk prediction in arrhythmogenic right ventricular cardiomyopathy: External validation of the arrhythmogenic right ventricular cardiomyopathy risk calculator. Eur. Heart J. 2022, 43, 3041–3052. [Google Scholar] [CrossRef]
- Garcia-Montero, M.; Fanous, Y.; Krahn, A.D.; Davies, B.; Cadrin-Tourigny, J.; Roberts, J.D. New insights into genetic right ventricular cardiomyopathies. Can. J. Cardiol. 2025, 41, 1023–1037. [Google Scholar] [CrossRef] [PubMed]
- Lundstrom, K. Viral vectors in gene therapy. Diseases 2018, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Büning, H.; Srivastava, A. Capsid modifications for targeting and improving the efficacy of AAV vectors. Mol. Ther. Methods Clin. Dev. 2019, 12, 248–265. [Google Scholar] [CrossRef] [PubMed]
- Mundisugih, J.; Ravindran, D.; Kizana, E. Exploring the therapeutic potential of gene therapy in Arrhythmogenic Right Ventricular Cardiomyopathy. Biomedicines 2024, 12, 1351. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Nakamura, S.; Higo, S.; Shiba, M.; Kohama, Y.; Kondo, T.; Kameda, S.; Tabata, T.; Okuno, S.; Ikeda, Y.; et al. Modeling reduced contractility and impaired desmosome assembly due to plakophilin-2 deficiency using isogenic iPS cell-derived cardiomyocytes. Stem Cell Rep. 2022, 17, 337–351. [Google Scholar] [CrossRef]
- Bradford, W.H.; Zhang, J.; Gutierrez-Lara, E.J.; Liang, Y.; Do, A.; Wang, T.-M.; Nguyen, L.; Mataraarachchi, N.; Wang, J.; Gu, Y.; et al. Plakophilin 2 gene therapy prevents and rescues arrhythmogenic right ventricular cardiomyopathy in a mouse model harboring patient genetics. Nat. Cardiovasc. Res. 2023, 2, 1246–1261. [Google Scholar] [CrossRef]
- Kyriakopoulou, E.; Versteeg, D.; de Ruiter, H.; Perini, I.; Seibertz, F.; Döring, Y.; Zentilin, L.; Tsui, H.; van Kampen, S.J.; Tiburcy, M.; et al. Therapeutic efficacy of AAV-mediated restoration of PKP2 in arrhythmogenic cardiomyopathy. Nat. Cardiovasc. Res. 2023, 2, 1262–1276. [Google Scholar] [CrossRef]
- Wu, I.; Zeng, A.; Greer-Short, A.; Aycinena, J.A.; Tefera, A.E.; Shenwai, R.; Farshidfar, F.; Van Pell, M.; Xu, E.; Reid, C.; et al. AAV9:PKP2 improves heart function and survival in a Pkp2-deficient mouse model of arrhythmogenic right ventricular cardiomyopathy. Commun. Med. 2024, 4, 38. [Google Scholar]
- van Opbergen, C.J.M.; Narayanan, B.; Sacramento, C.B.; Stiles, K.M.; Mishra, V.; Frenk, E.; Ricks, D.; Chen, G.; Zhang, M.; Yarabe, P.; et al. AAV-mediated delivery of plakophilin-2a arrests progression of arrhythmogenic right ventricular cardiomyopathy in Murine hearts: Preclinical evidence supporting gene therapy in humans. Circ. Genom. Precis. Med. 2024, 17, e004305. [Google Scholar]
- Lexeo Therapeutics, Inc. Form 10-K, Annual Report for the Fiscal Year Ended December 31, 2024; U.S. Securities and Exchange Commission: Washington, DC, USA, 2025.
- Therapeutics, T. Tenaya Therapeutics Provides Update on RIDGE-1 Gene Therapy Clinical Trial for PKP2-Associated ARVC. Tenaya Therapeutics Investor Relations. Available online: https://investors.tenayatherapeutics.com/node/8511/pdf (accessed on 8 October 2025).
- Rocket Pharmaceuticals, Inc. Rocket Pharmaceuticals Announces Preliminary Positive Results from Phase 1 Trial of RP-A601 for PKP2-Associated Arrhythmogenic Cardiomyopathy. Rocket Pharmaceuticals Investor Relations. Available online: https://ir.rocketpharma.com/node/12551/pdf (accessed on 8 October 2025).
- Beurel, E.; Grieco, S.F.; Jope, R.S. Glycogen synthase kinase-3 (GSK3): Regulation, actions, and diseases. Pharmacol. Ther. 2015, 148, 114–131. [Google Scholar] [CrossRef]
- Eldar-Finkelman, H.; Martinez, A. GSK-3 inhibitors: Preclinical and clinical focus on CNS. Front. Mol. Neurosci. 2011, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- Coghlan, M.P.; Culbert, A.A.; Cross, D.A.; Corcoran, S.L.; Yates, J.W.; Pearce, N.J.; Rausch, O.L.; Murphy, G.J.; Carter, P.S.; Roxbee Cox, L.; et al. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem. Biol. 2000, 7, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Snitow, M.E.; Bhansali, R.S.; Klein, P.S. Lithium and therapeutic targeting of GSK-3. Cells 2021, 10, 255. [Google Scholar] [CrossRef]
- Hamstra, S.I.; Kurgan, N.; Baranowski, R.W.; Qiu, L.; Watson, C.J.F.; Messner, H.N.; MacPherson, R.E.K.; MacNeil, A.J.; Roy, B.D.; Fajardo, V.A. Low-dose lithium feeding increases the SERCA2a-to-phospholamban ratio, improving SERCA function in murine left ventricles. Exp. Physiol. 2020, 105, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Šeflová, J.; Cruz-Cortés, C.; Guerrero-Serna, G.; Robia, S.L.; Espinoza-Fonseca, L.M. Mechanisms for cardiac calcium pump activation by its substrate and a synthetic allosteric modulator using fluorescence lifetime imaging. PNAS Nexus 2024, 3, gad453. [Google Scholar] [CrossRef] [PubMed]
- Chelko, S.P.; Asimaki, A.; Andersen, P.; Bedja, D.; Amat-Alarcon, N.; DeMazumder, D.; Jasti, R.; MacRae, C.A.; Leber, R.; Kleber, A.G.; et al. Central role for GSK3β in the pathogenesis of arrhythmogenic cardiomyopathy. JCI Insight 2016, 1, e85923. [Google Scholar] [CrossRef] [PubMed]
| ACM Variant | Predominant Ventricular Involvement | Key Clinical Features |
|---|---|---|
| ARVC | Right ventricle | RV fibro-fatty replacement, ventricular arrhythmias, typical onset in adolescence or early adulthood. |
| BiV-ACM | Both ventricles | Early LV involvement alongside RV, higher risk of heart failure. |
| ALVC | Left ventricle | LV arrhythmias, subepicardial fibrosis, and misdiagnosis as DCM are common. |
| Genes | Protein Encoded | Evidence Level |
|---|---|---|
| PKP2 | Plakophilin-2 | Strong [36,37,38,39] |
| DSG2 | Desmoglein-2 | Strong [38,40] |
| DSC2 | Desmocollin-2 | Strong [38,40,41] |
| JUP | Plakoglobin | Strong [38,42] |
| DSP | Desmoplakin | Strong [38,43] |
| TMEM43 | Transmembrane protein 43 | Strong [38,44] |
| DES | Desmin | Moderate [38,45] |
| PLB(PLN) | Phospholamban | Moderate [38,46] |
| CDH2 | N-cadherin | Limited [38,47] |
| SCN5A | Sodium voltage-gated channel alpha subunit 5 | Limited [38,48] |
| TGF3β | Transforming growth factor 3 beta | Limited [38,49] |
| LMNA | Lamin A/C | Limited [38,50] |
| FLNC | Filamin C | Not defined yet [38,51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popa, E.; Hostiuc, S. Molecular Pathogenesis of Arrhythmogenic Cardiomyopathy: Mechanisms and Therapeutic Perspectives. Biomolecules 2025, 15, 1512. https://doi.org/10.3390/biom15111512
Popa E, Hostiuc S. Molecular Pathogenesis of Arrhythmogenic Cardiomyopathy: Mechanisms and Therapeutic Perspectives. Biomolecules. 2025; 15(11):1512. https://doi.org/10.3390/biom15111512
Chicago/Turabian StylePopa, Eliza, and Sorin Hostiuc. 2025. "Molecular Pathogenesis of Arrhythmogenic Cardiomyopathy: Mechanisms and Therapeutic Perspectives" Biomolecules 15, no. 11: 1512. https://doi.org/10.3390/biom15111512
APA StylePopa, E., & Hostiuc, S. (2025). Molecular Pathogenesis of Arrhythmogenic Cardiomyopathy: Mechanisms and Therapeutic Perspectives. Biomolecules, 15(11), 1512. https://doi.org/10.3390/biom15111512







