Tea-Residue-Derived Klebsiella pneumoniae CGMCC 31459: Genomic Insights and Antioxidant Activity of Its Exopolysaccharides
Abstract
1. Introduction
2. Materials and Methods
2.1. Microbial Information
2.1.1. Strain Information
2.1.2. Bacterial and Nematode Culture Conditions
2.2. Genome Sequencing
2.2.1. Preparation of Sequencing Samples
2.2.2. Genome Sequencing and Quality Control
2.2.3. Genome Assembly
2.3. Genome Analysis
2.3.1. Sequence Typing Identification of K. pneumoniae CGMCC 31459
2.3.2. Carbohydrate-Active Enzyme Annotation
2.3.3. Subcellular Localization Analysis of Protein-Coding Genes
2.3.4. Functional Annotation of Protein-Coding Genes
2.3.5. Genome Circular Map Construction
2.3.6. Analysis of Virulence, Antimicrobial Resistance, and Mobile Genetic Elements
2.4. Pan-Genome Analysis
2.4.1. Klebsiella pneumoniae Genome Collection
2.4.2. Pan-Genome and Comparative Genomic Analysis
2.4.3. KEGG Pathway Analysis of K. pneumoniae CGMCC 31459
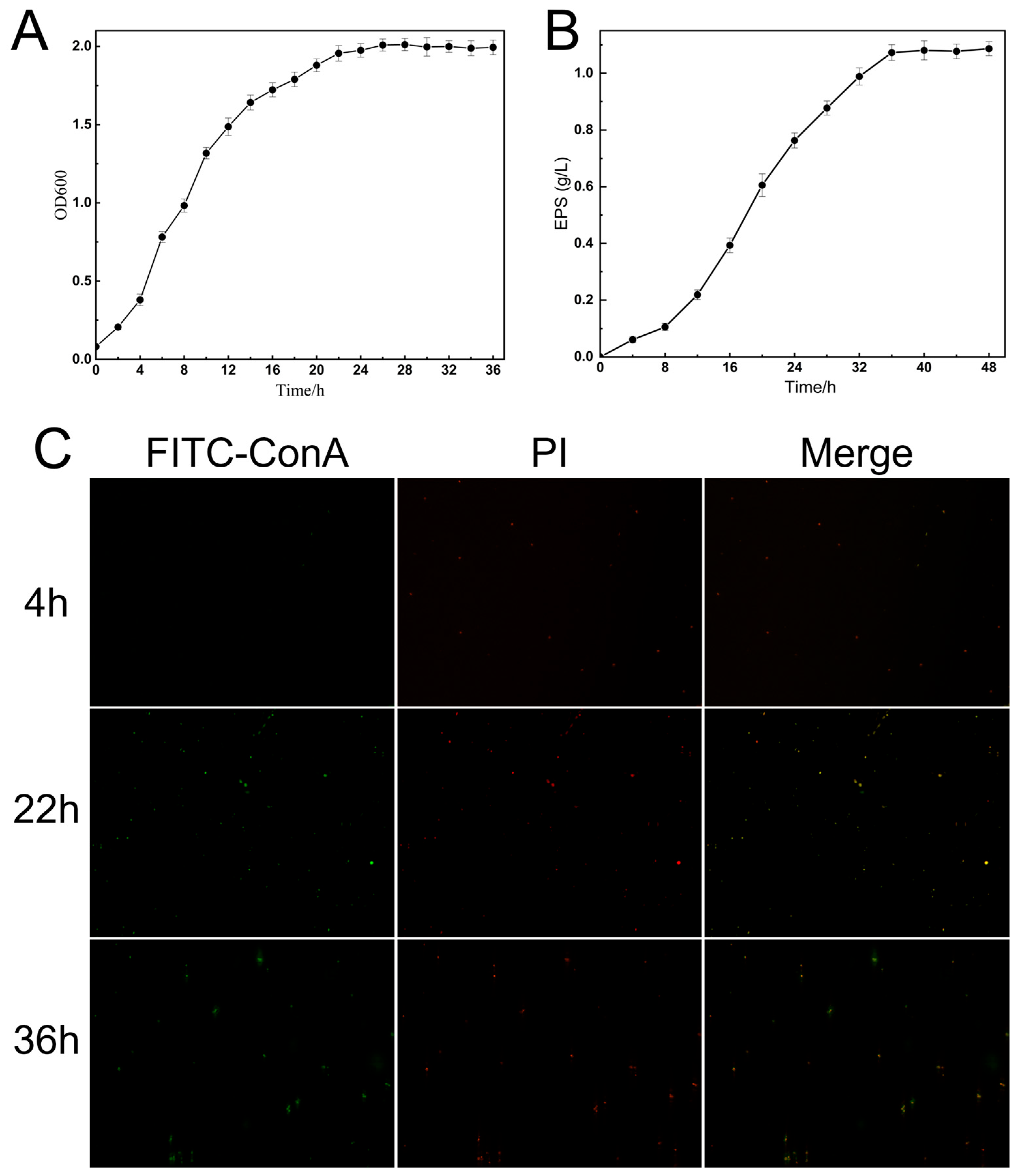
2.5. EPS Secretion Study of K. pneumoniae CGMCC 31459
2.6. Structural Characterization of EPS-KP
2.6.1. Extraction, Isolation, and Purification of EPS-KP
2.6.2. Molecular Weight Determination of EPS-KP
2.6.3. Monosaccharide Composition Analysis of EPS-KP
2.6.4. Fourier Transform Infrared Spectroscopy (FTIR) Analysis of EPS-KP
2.6.5. Methylation Analysis of EPS-KP
2.6.6. Scanning Electron Microscopy of EPS-KP
2.7. EPS-KP In Vitro Antioxidant Activity
2.7.1. Hydroxyl Radical (-OH) Scavenging Assay
2.7.2. ABTS Radical Scavenging Assay
2.7.3. DPPH Radical Scavenging Assay
2.7.4. Superoxide Anion (O2−) Radical Scavenging Assay
2.8. C. elegans Anti-Aging Experiments
2.8.1. Nematodes Synchronization
2.8.2. Lifespan Assay
2.8.3. Heat Stress and Oxidative Stress Tests
2.8.4. Lipofuscin Level Measurement
2.8.5. ROS Content Measurement
2.8.6. SOD, CAT Activity Assays
3. Results
3.1. Genome Sequencing and Analysis of K. pneumoniae CGMCC 31459
3.1.1. Functional Annotation Analysis
3.1.2. Typing and Identification of K. pneumoniae CGMCC 31459
3.1.3. Carbohydrate-Active Enzyme Analysis
3.1.4. Functional Annotation of Protein-Coding Genes
3.1.5. Subcellular Localization Analysis of Protein-Coding Genes
3.1.6. Genome Circle Plot Construction
3.1.7. Virulence, Antimicrobial Resistance, and Mobile Genetic Elements Analysis
3.2. Pan-Genome Analysis of Klebsiella pneumoniae
3.2.1. Genomic Collection of Klebsiella pneumoniae
3.2.2. Phylogenetic Analysis of Klebsiella pneumoniae
3.2.3. Pan-Genome and Core-Genome Analysis of Klebsiella pneumoniae
3.2.4. KEGG Pathway Analysis of Klebsiella pneumoniae CGMCC 31459
3.3. Observation of Bacterial Morphology and Exopolysaccharide Layer in K. pneumoniae CGMCC 31459
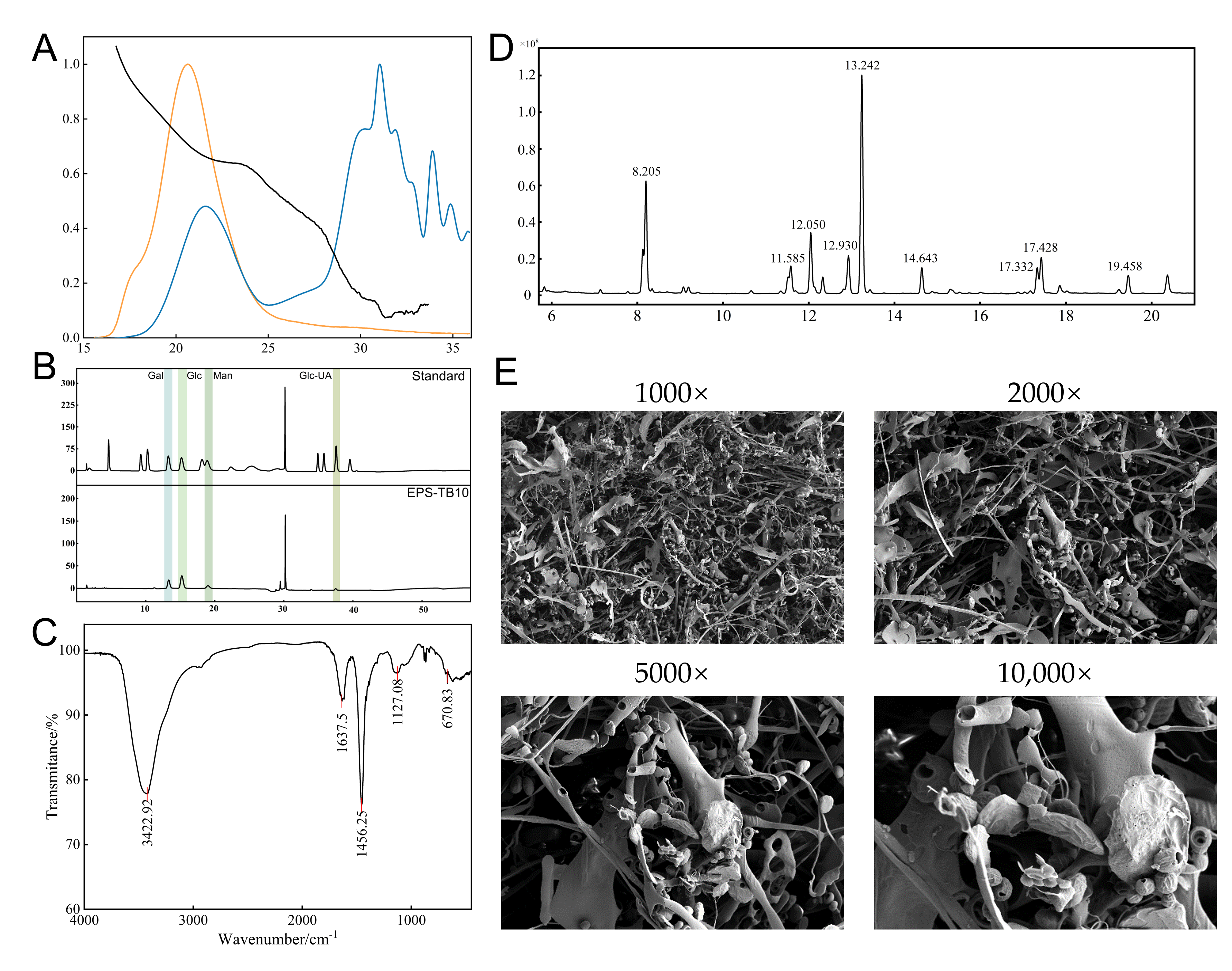
3.4. Structural Characterization of Exopolysaccharides
3.4.1. Analysis of Molecular Weight and Monosaccharide Composition of EPS-KP
3.4.2. FT-IR Spectroscopic Analysis of EPS-KP
3.4.3. Methylation Analysis of EPS-KP
3.4.4. SEM Analysis of EPS-KP
3.5. Analysis of Antioxidant Activity of EPS-KP
3.6. C. elegans Experiments
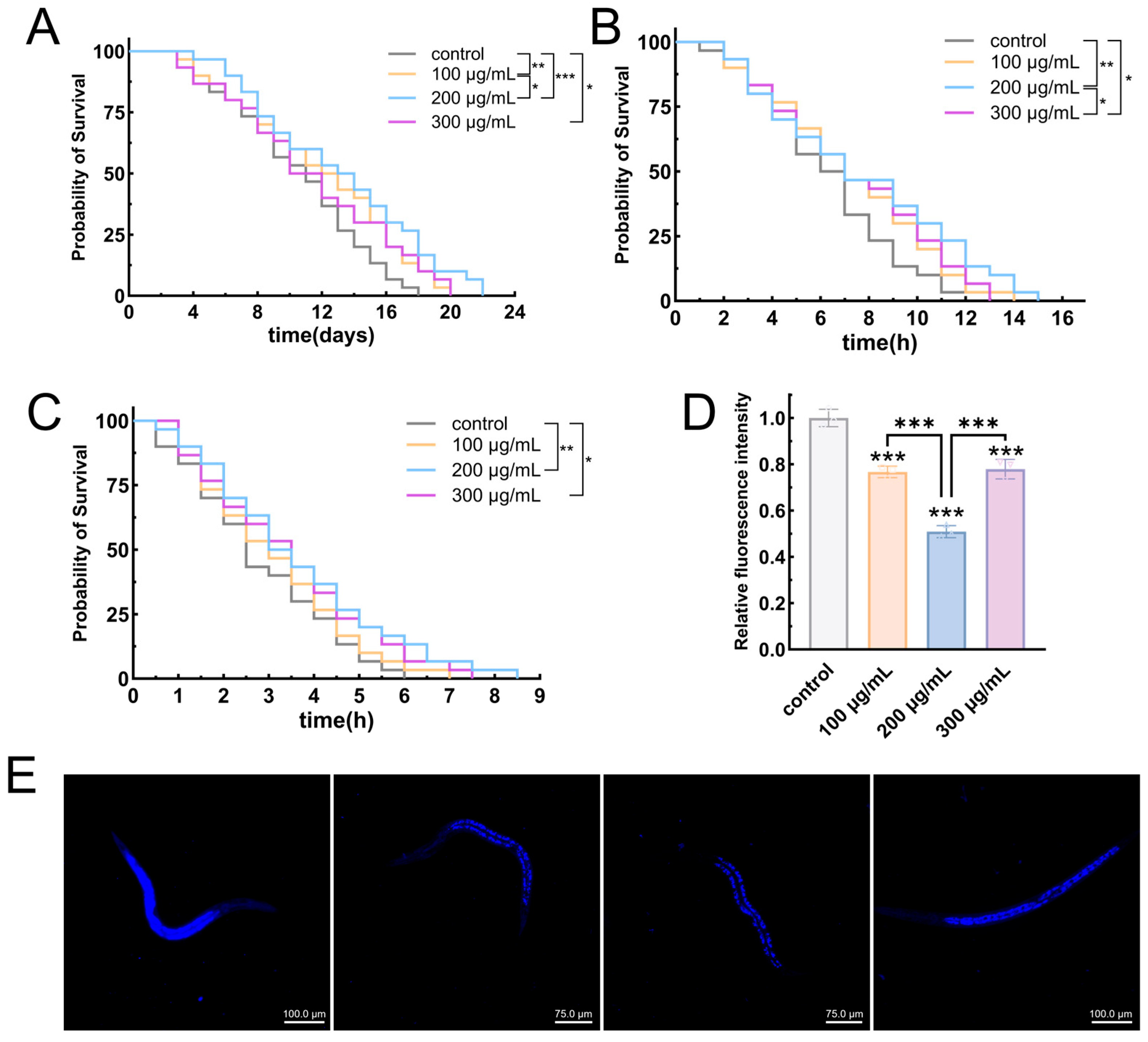
3.6.1. EPS-KP Significantly Extended the Lifespan of C. elegans
3.6.2. EPS-KP Improves Stress Resistance in C. elegans
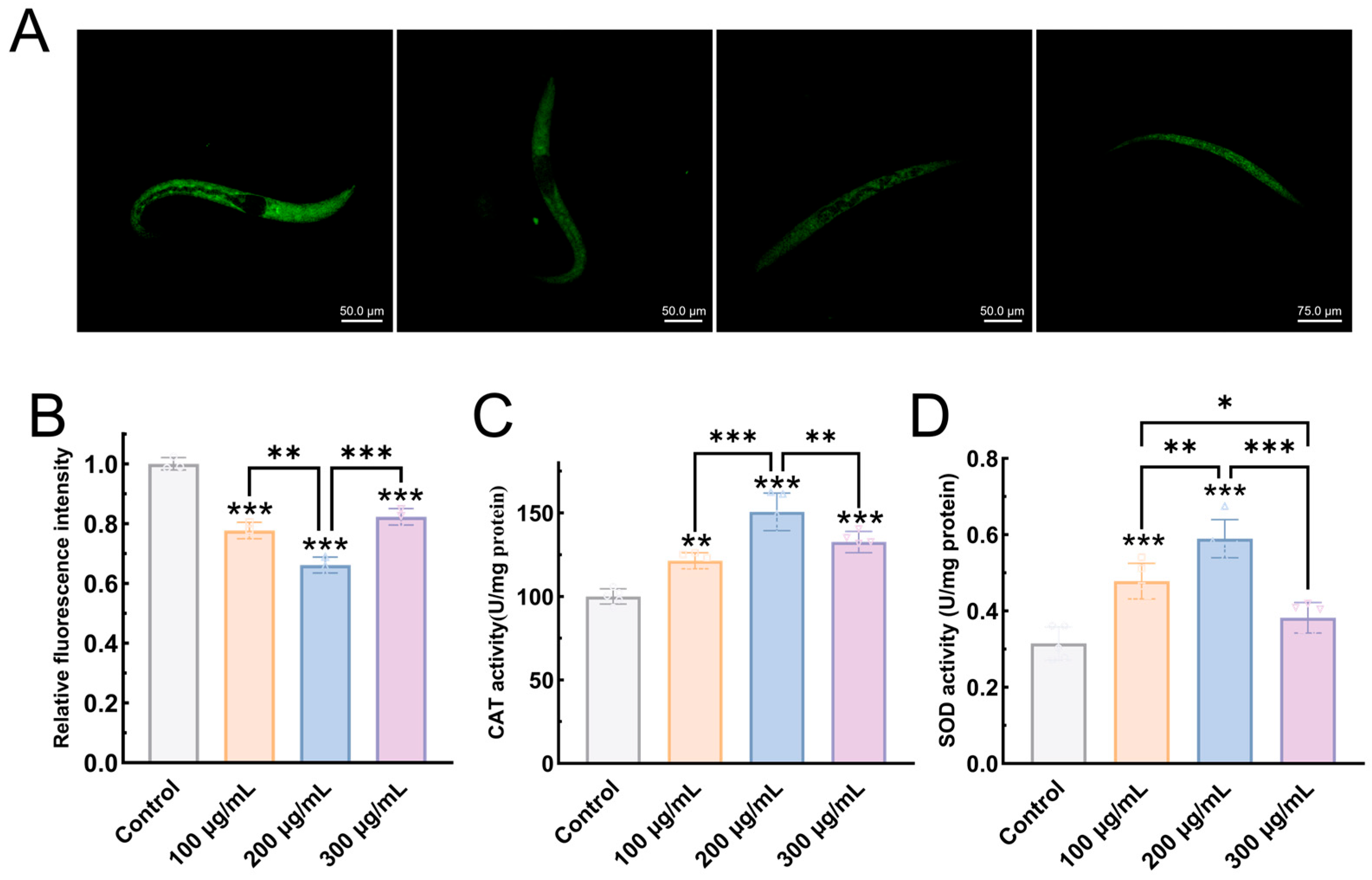
3.6.3. EPS-KP Exerts Anti-Aging Effects by Reducing Lipofuscin Accumulation
3.6.4. EPS-KP Enhances Antioxidant Capacity in C. elegans
4. Discussion
4.1. Genomic Analysis Reveals EPS Biosynthetic Capacity and Environmental Adaptability of K. pneumoniae CGMCC 31459
4.2. Structure–Activity Relationship of EPS-KP: Structural Characteristics Drive Antioxidant and Anti-Aging Functions
4.3. Structural–Functional Insights and Safe Application of EPS-KP
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EPS | Exopolysaccharides |
| EPS-KP | Exopolysaccharides of Klebsiella pneumoniae CGMCC 31459 |
| FTIR | Fourier Transform Infrared Spectroscopy |
| SEM | Scanning electron microscopy |
| HPLC | High-performance liquid chromatography |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| FITC-ConA | FITC-labeled Concanavalin A |
| PI | Propidium Iodide |
References
- Rana, S.; Upadhyay, L.S.B. Microbial Exopolysaccharides: Synthesis Pathways, Types and Their Commercial Applications. Int. J. Biol. Macromol. 2020, 157, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Luo, Z.; Zhao, Z.; Mu, Y.; Xu, J.; Dai, S.; Cui, Y.; Ying, M.; Hu, X.; Huang, L. Isolation, Structural Characterization and Multiple Activity of a Novel Exopolysaccharide Produced by Gelidibacter Sp. PG–2. Int. J. Biol. Macromol. 2025, 305, 141127. [Google Scholar] [CrossRef]
- Schmid, J.; Sieber, V.; Rehm, B. Bacterial Exopolysaccharides: Biosynthesis Pathways and Engineering Strategies. Front. Microbiol. 2015, 6, 496. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ge, M.-D.; Zhu, Y.-J.; Song, Y.; Cheung, P.C.K.; Zhang, B.-B.; Liu, L.-M. Structure, Bioactivity and Applications of Natural Hyperbranched Polysaccharides. Carbohydr. Polym. 2019, 223, 115076. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Xu, B. Skin Health Promoting Effects of Natural Polysaccharides and Their Potential Application in the Cosmetic Industry. Polysaccharides 2022, 3, 818–830. [Google Scholar] [CrossRef]
- Russo, T.A.; Marr, C.M. Hypervirulent Klebsiella pneumoniae. Clin. Microbiol. Rev. 2019, 32, e00001-19. [Google Scholar] [CrossRef]
- Kochan, T.J.; Nozick, S.H.; Valdes, A.; Mitra, S.D.; Cheung, B.H.; Lebrun-Corbin, M.; Medernach, R.L.; Vessely, M.B.; Mills, J.O.; Axline, C.M.R.; et al. Klebsiella pneumoniae Clinical Isolates with Features of Both Multidrug-Resistance and Hypervirulence Have Unexpectedly Low Virulence. Nat. Commun. 2023, 14, 7962. [Google Scholar] [CrossRef]
- Xu, L.; Li, J.; Wu, W.; Wu, X.; Ren, J. Klebsiella pneumoniae Capsular Polysaccharide: Mechanism in Regulation of Synthesis, Virulence, and Pathogenicity. Virulence 2024, 15, 2439509. [Google Scholar] [CrossRef]
- Pu, D.; Zhao, J.; Lu, B.; Zhang, Y.; Wu, Y.; Li, Z.; Zhuo, X.; Cao, B. Within-Host Resistance Evolution of a Fatal ST11 Hypervirulent Carbapenem-Resistant Klebsiella pneumoniae. Int. J. Antimicrob. Agents 2023, 61, 106747. [Google Scholar] [CrossRef]
- Wu, K.; Lin, X.; Lu, Y.; Dong, R.; Jiang, H.; Svensson, S.L.; Zheng, J.; Shen, N.; Camilli, A.; Chao, Y. RNA Interactome of Hypervirulent Klebsiella pneumoniae Reveals a Small RNA Inhibitor of Capsular Mucoviscosity and Virulence. Nat. Commun. 2024, 15, 6946. [Google Scholar] [CrossRef]
- Whitfield, C.; Kelly, S.D.; Stanton, T.D.; Wyres, K.L.; Clarke, B.R.; Forrester, T.J.B.; Kowalczyk, A. O-Antigen Polysaccharides in Klebsiella pneumoniae: Structures and Molecular Basis for Antigenic Diversity. Microbiol. Mol. Biol. Rev. 2025, 89, e00090-23. [Google Scholar] [CrossRef]
- Sun, X.; Wei, Z.; Su, Y.; Fang, R.; Fan, Y.; Zeng, D.; Ding, Q.; Miao, Y.; Liu, J.; Sun, Q. Structural Characteristics and Anti-Tumor Activities of a Novel Polysaccharide from Klebsiella sp. SXW12. Carbohydr. Polym. 2025, 356, 123368. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Kawashima, M.; Ishiguro-Watanabe, M. KEGG for Taxonomy-Based Analysis of Pathways and Genomes. Nucleic Acids Res. 2022, 51, D587–D592. [Google Scholar] [CrossRef] [PubMed]
- Parks, D.H.; Chuvochina, M.; Rinke, C.; Mussig, A.J.; Chaumeil, P.-A.; Hugenholtz, P. GTDB: An Ongoing Census of Bacterial and Archaeal Diversity through a Phylogenetically Consistent, Rank Normalized and Complete Genome-Based Taxonomy. Nucleic Acids Res. 2021, 50, D785–D794. [Google Scholar] [CrossRef]
- Emms, D.M.; Kelly, S. OrthoFinder: Phylogenetic Orthology Inference for Comparative Genomics. Genome Biol. 2019, 20, 238. [Google Scholar] [CrossRef] [PubMed]
- Tettelin, H.; Masignani, V.; Cieslewicz, M.J.; Donati, C.; Medini, D.; Ward, N.L.; Angiuoli, S.V.; Crabtree, J.; Jones, A.L.; Durkin, A.S.; et al. Genome Analysis of Multiple Pathogenic Isolates of Streptococcus agalactiae: Implications for the Microbial “Pan-Genome”. Proc. Natl. Acad. Sci. USA 2005, 102, 13950–13955. [Google Scholar] [CrossRef]
- Qin, Q.; Li, Y.; Zhang, Y.; Zhou, Z.; Zhang, W.; Chen, X.; Zhang, X.; Zhou, B.; Wang, L.; Zhang, Y. Comparative genomics reveals a deep-sea sediment-adapted life style of Pseudoalteromonas sp. SM9913. Int. Soc. Microb. Ecol. 2011, 5, 274–284. [Google Scholar] [CrossRef]
- Salvador, L.D.; Suganuma, T.; Kitahara, K.; Tanoue, H.; Ichiki, M. Monosaccharide Composition of Sweetpotato Fiber and Cell Wall Polysaccharides from Sweetpotato, Cassava, and Potato Analyzed by the High-Performance Anion Exchange Chromatography with Pulsed Amperometric Detection Method. J. Agric. Food Chem. 2000, 48, 3448–3454. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Xin, Q.; Wei, P.; Hua, Y.; Zhang, Y.; Su, Z.; She, G.; Yuan, R. Antioxidant and Anti-Aging Activities of Longan Crude and Purified Polysaccharide (LP-A) in Nematode Caenorhabditis elegans. Int. J. Biol. Macromol. 2024, 267, 131634. [Google Scholar] [CrossRef]
- Zhang, H.; Li, C.; Lai, P.F.H.; Chen, J.; Xie, F.; Xia, Y.; Ai, L. Fractionation, chemical characterization and im-munostimulatory activity of β-glucan and galactoglucan from Russula vinosa Lindblad. Carbohydr. Polym. 2021, 256, 117559. [Google Scholar] [CrossRef]
- Zhu, A.; Zheng, F.; Zhang, W.; Li, L.; Li, Y.; Hu, H.; Wu, Y.; Bao, W.; Li, G.; Wang, Q.; et al. Oxidation and Antioxidation of Natural Products in the Model Organism Caenorhabditis elegans. Antioxidants 2022, 11, 705. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.; Guo, Y.; Li, X.; Zhu, L.; Wang, H.; Wu, J.; Pan, C. Genetic Engineering of Klebsiella pneumoniae ATCC 25955 for Bioconjugate Vaccine Applications. Microorganisms 2023, 11, 1321. [Google Scholar] [CrossRef]
- Geng, X.; Yang, Y.-J.; Li, Z.; Ge, W.-B.; Xu, X.; Liu, X.-W.; Li, J.-Y. Fingolimod Inhibits Exopolysaccharide Production and Regulates Relevant Genes to Eliminate the Biofilm of K. pneumoniae. Int. J. Mol. Sci. 2024, 25, 1397. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, Y.; Chen, T.; Feng, F.; Fang, T.; Zhang, W.; Li, Y.; Ju, Y.; Xu, L.; Zhuge, X.; et al. “Precision-Guided Killer” Engineered Phage for Combating Carbapenem-Resistant Klebsiella pneumoniae Induced Inflammatory Bowel Disease. Adv. Funct. Mater. 2025, 12, e03292-23. [Google Scholar] [CrossRef]
- Pu, D.; Zhao, J.; Chang, K.; Zhuo, X.; Cao, B. “Superbugs” with Hypervirulence and Carbapenem Resistance in Klebsiella pneumoniae: The Rise of Such Emerging Nosocomial Pathogens in China. Sci. Bull. 2023, 68, 2658–2670. [Google Scholar] [CrossRef] [PubMed]
- Merla, C.; Kuka, A.; Mileto, I.; Petazzoni, G.; Gaiarsa, S.; Vitis, D.D.; Ardizzone, M.; Corbella, M.; Baldanti, F.; Cambieri, P. One-Year Surveillance for Hypervirulent Klebsiella pneumoniae Detected Carbapenem-Resistant Superbugs. Microbiol. Spectr. 2024, 12, e03292-23. [Google Scholar] [CrossRef] [PubMed]
- Diancourt, L.; Passet, V.; Verhoef, J.; Grimont, P.A.; Brisse, S. Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. J. Clin. Microbiol. 2005, 43, 4178–4182. [Google Scholar] [CrossRef]
- Lombard, V.; Ramulu, H.G.; Drula, E.; Coutinho, P.M.; Henrissat, B. The Carbohydrate-Active Enzymes Database (CAZy) in 2013. Nucleic Acids Res. 2013, 42, D490–D495. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, B.; Zheng, D.; Chen, L.; Yang, J. VFDB 2025: An integrated resource for exploring anti-virulence compounds. Nucleic Acids Res. 2024, 53, D871–D877. [Google Scholar] [CrossRef]
- Clausen, P.T.L.C.; Aarestrup, F.M.; Lund, O. Rapid and Precise Alignment of Raw Reads against Redundant Databases with KMA. BMC Bioinform. 2018, 19, 307. [Google Scholar] [CrossRef]
- Bortolaia, V.; Kaas, R.S.; Ruppe, E.; Roberts, M.C.; Schwarz, S.; Cattoir, V.; Philippon, A.; Allesoe, R.L.; Rebelo, A.R.; Florensa, A.F.; et al. ResFinder 4.0 for Predictions of Phenotypes from Genotypes. J. Antimicrob. Chemother. 2020, 75, 3491–3500. [Google Scholar] [CrossRef]
- Arndt, D.; Grant, J.R.; Marcu, A.; Sajed, T.; Pon, A.; Liang, Y.; Wishart, D.S. PHASTER: A Better, Faster Version of the PHAST Phage Search Tool. Nucleic Acids Res. 2016, 44, W16–W21. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liang, Y.; Lynch, K.H.; Dennis, J.J.; Wishart, D.S. PHAST: A Fast Phage Search Tool. Nucleic Acids Res. 2011, 39, W347–W352. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, C.; Laird, M.R.; Williams, K.P.; Lau, B.Y.; Hoad, G.; Winsor, G.L.; Brinkman, F.S. IslandViewer 4: Expanded Prediction of Genomic Islands for Larger-Scale Datasets. Nucleic Acids Res. 2017, 45, W30–W35. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, Y.; Fan, G.; Sun, D.; Zhang, X.; Yu, Z.; Wang, J.; Wu, L.; Shi, W.; Ma, J. IPGA: A Handy Integrated Prokaryotes Genome and Pan-Genome Analysis Web Service. iMeta 2022, 1, e55. [Google Scholar] [CrossRef]
- Darzi, Y.; Letunic, I.; Bork, P.; Yamada, T. iPath3.0: Interactive Pathways Explorer V3. Nucleic Acids Res. 2018, 46, W510–W513. [Google Scholar] [CrossRef]
- Kalia, M.; Yadav, V.K.; Singh, P.K.; Sharma, D.; Narvi, S.S.; Agarwal, V. Exploring the Impact of Parthenolide as Anti-Quorum Sensing and Anti-Biofilm Agent against Pseudomonas Aeruginosa. Life Sci. 2018, 199, 96–103. [Google Scholar] [CrossRef]
- Zheng, Z.; Huang, Q.; Ling, C. Water-Soluble Yeast Β-glucan Fractions with Different Molecular Weights: Extraction and Separation by Acidolysis Assisted-Size Exclusion Chromatography and Their Association with Proliferative Activity. Int. J. Biol. Macromol. 2019, 123, 269–279. [Google Scholar] [CrossRef]
- Zhu, M.; Huang, R.; Wen, P.; Song, Y.; He, B.; Tan, J.; Hao, H.; Wang, H. Structural Characterization and Immunological Activity of Pectin Polysaccharide from Kiwano (Cucumis metuliferus) Peels. Carbohydr. Polym. 2021, 254, 117371. [Google Scholar] [CrossRef]
- Guo, L.; Zhu, W.; Xu, F.; Liu, M.; Xie, Y.; Zhang, J. Optimized Ultrasonic-Assisted Extraction of Polysaccharides from Cyclina Sinensis and Evaluation of Antioxidant Activities in Vitro. CyTA—J. Food 2013, 12, 32–39. [Google Scholar] [CrossRef]
- Liao, W.; Luo, Z.; Liu, D.; Ning, Z.; Yang, J.; Ren, J. Structure Characterization of a Novel Polysaccharide from Dictyophora Indusiata and Its Macrophage Immunomodulatory Activities. J. Agric. Food Chem. 2015, 63, 535–544. [Google Scholar] [CrossRef]
- Liu, S.; Ai, Z.; Qu, F.; Chen, Y.; Ni, D. Effect of Steeping Temperature on Antioxidant and Inhibitory Activities of Green Tea Extracts against α-Amylase, α-Glucosidase and Intestinal Glucose Uptake. Food Chem. 2017, 234, 168–173. [Google Scholar] [CrossRef]
- Dong, C.-H.; Yao, Y.-J. In Vitro Evaluation of Antioxidant Activities of Aqueous Extracts from Natural and Cultured Mycelia of Cordyceps Sinensis. LWT—Food Sci. Technol. 2008, 41, 669–677. [Google Scholar] [CrossRef]
- Bai, X.; Wang, M.; Xu, T.; Zhou, S.; Chu, W. Antioxidant and Anti-Aging Activities of Acanthopanax Senticosus Polysaccharide CQ-1 in Nematode Caenorhabditis elegans. Int. J. Biol. Macromol. 2025, 297, 139925. [Google Scholar] [CrossRef] [PubMed]
- Ayuda-Durán, B.; González-Manzano, S.; González-Paramás, A.M.; Santos-Buelga, C. Caernohabditis elegans as a Model Organism to Evaluate the Antioxidant Effects of Phytochemicals. Molecules 2020, 25, 3194. [Google Scholar] [CrossRef] [PubMed]
- Conrad, R.E.; Viver, T.; Gago, J.F.; Hatt, J.K.; Venter, S.N.; Rossello-Mora, R.; Konstantinidis, K.T. Toward Quantifying the Adaptive Role of Bacterial Pangenomes during Environmental Perturbations. ISME J. 2021, 16, 1222–1234. [Google Scholar] [CrossRef]
- Low, K.E.; Howell, P.L. Gram-Negative Synthase-Dependent Exopolysaccharide Biosynthetic Machines. Curr. Opin. Struct. Biol. 2018, 53, 32–44. [Google Scholar] [CrossRef]
- Chugh, S.; Létisse, F.; Neyrolles, O. The Exometabolome as a Hidden Driver of Bacterial Virulence and Pathogenesis. Trends Microbiol. 2024, 33, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Rosconi, F.; Rudmann, E.; Li, J.; Surujon, D.; Anthony, J.; Frank, M.; Jones, D.S.; Rock, C.; Rosch, J.W.; Johnston, C.D.; et al. A Bacterial Pan-Genome Makes Gene Essentiality Strain-Dependent and Evolvable. Nat. Microbiol. 2022, 7, 1580–1592. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.-H.; Zhou, L.-Y.; Li, W.; Liu, L.; Wang, D.-D.; Zhang, W.-N.; Hussain, S.; Tian, X.-H.; Lu, Y.-M. Structural Elucidation of Three Antioxidative Polysaccharides from Tricholoma Lobayense. Carbohydr. Polym. 2017, 157, 484–492. [Google Scholar] [CrossRef]
- Chen, X.; Wu, X.; Zhang, K.; Sun, F.; Zhou, W.; Wu, Z.; Li, X. Purification, Characterization, and Emulsification Stability of High- and Low-Molecular-Weight Fractions of Polysaccharide Conjugates Extracted from Green Tea. Food Hydrocoll. 2022, 129, 107667. [Google Scholar] [CrossRef]
- Lee, Q.; Xue, Z.; Luo, Y.; Lin, Y.; Lai, M.; Xu, H.; Liu, B.; Zheng, M.; Lv, F.; Zeng, F. Low Molecular Weight Polysaccharide of Tremella Fuciformis Exhibits Stronger Antioxidant and Immunomodulatory Activities than High Molecular Weight Polysaccharide. Int. J. Biol. Macromol. 2024, 281, 136097. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, S.; Si, H.; Liu, Y.; Xie, F.; Wang, X.; Wu, S.; Chen, B.; Zhai, C.; Qiao, Y.; et al. Structure characterization and protective effect against UVB irradiation of polysaccharides isolated from the peach gums. Int. J. Biol. Macromol. 2025, 311, 143527. [Google Scholar] [CrossRef]
- Lee, K.; Kim, H.J.; Kim, S.A.; Park, S.-D.; Shim, J.-J.; Lee, J.-L. Exopolysaccharide from Lactobacillus plantarum HY7714 Protects against Skin Aging through Skin–Gut Axis Communication. Molecules 2021, 26, 1651. [Google Scholar] [CrossRef]
- Kumar, A.; Saha, K.; Kumar, V.; Bhattacharya, A.; Barge, S.; Mukherjee, K.; Kalita, C.; Khan, R. Heat-killed probiotic Levilactobacillus brevis MKAK9 and its exopolysaccharide promote longevity by modulating aging hallmarks and enhancing immune responses in Caenorhabditis elegans. Immun. Ageing 2024, 21, 52. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, Y.-R.; Yang, H.; Park, C.-H.; Yun, C.-S.; Jang, B.-C.; Hong, Y.; Park, D.-S. Potential Probiotic Lactiplantibacillus plantarum DS1800 Extends Lifespan and Enhances Stress Resistance in Caenorhabditis elegans Model. Front. Physiol. 2024, 15, 1476096. [Google Scholar] [CrossRef]
- Smolentseva, O.; Gusarov, I.; Gautier, L.; Shamovsky, I.; DeFrancesco, A.S.; Losick, R.; Nudler, E. Mechanism of Biofilm-Mediated Stress Resistance and Lifespan Extension in C. elegans. Sci. Rep. 2017, 7, 7137. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Zhang, W.; Sun, W.; Ji, X.; Zhang, S.; Qiao, K. Lentinan Extends Lifespan and Increases Oxidative Stress Resistance through DAF-16 and SKN-1 Pathways in Caenorhabditis elegans. Int. J. Biol. Macromol. 2022, 202, 286–295. [Google Scholar] [CrossRef]
- Zečić, A.; Braeckman, B.P. DAF-16/FoxO in Caenorhabditis elegans and Its Role in Metabolic Remodeling. Cells 2020, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, Q.; He, X.; Gao, X.; Wu, L.; Xiao, M.; Cai, W.; Liu, B.; Zeng, F. Antioxidant and Anti-Aging Activities of Laminaria Japonica Polysaccharide in Caenorhabditis elegans Based on Metabonomic Analysis. Int. J. Biol. Macromol. 2022, 221, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Z.; Zeng, Z.; Lin, Y.; Xiong, B.; Zheng, B.; Zhang, Y.; Pan, L. Structural Characterization of Polysaccharide from an Edible Fungus Dictyophora indusiata and the Remodel Function of Gut Microbiota in Inflammatory Mice. Carbohydr. Polym. 2025, 351, 123141. [Google Scholar] [CrossRef] [PubMed]
- Fei, Z.; Xie, H.; Xie, D.; Wang, M.; Du, Q.; Jin, P. Structural Characterization and High-Efficiency Prebiotic Activity of the Polysaccharide from Tremella Aurantialba Endophytic Bacteria. Int. J. Biol. Macromol. 2024, 260, 129347. [Google Scholar] [CrossRef]
- Wang, W.; Tian, D.; Hu, D.; Chen, W.; Zhou, Y.; Jiang, X. Different Regulatory Mechanisms of the Capsule in Hypervirulent Klebsiella Pneumonia: “Direct” wcaJ Variation vs. “Indirect” rmpA Regulation. Front. Cell. Infect. Microbiol. 2023, 13, 1108818. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-W.; Lam, I.; Chang, H.-Y.; Tsai, S.-F.; Palsson, B.O.; Charusanti, P. Capsule Deletion via a λ-Red Knockout System Perturbs Biofilm Formation and Fimbriae Expression in Klebsiella pneumoniae MGH 78578. BMC Res. Notes 2014, 7, 13. [Google Scholar] [CrossRef] [PubMed]




| Experiment Project | Group | Mean Lifespan (Days) | % of Control | Median Lifespan (Days) | MAX Lifespan (Days) |
|---|---|---|---|---|---|
| Lifetime Experiment | control | 9.84 ± 0.598 | 100 | 9.5 | 17 |
| EPS-KP-100 µg/mL | 11.83 ± 0.318 b | 120.2 b | 12 | 19 | |
| EPS-KP-200 µg/mL | 12.89 ± 0.366 c | 130.9 c | 13.5 | 21 | |
| EPS-KP-300 µg/mL | 10.79 ± 0.582 a | 116.1 a | 11 | 19 |
| Experiment Project | Group | Mean Lifespan (h) | % of Control | Median Lifespan (h) | MAX Lifespan (h) |
|---|---|---|---|---|---|
| Heat stress | control | 6.34 ± 0.069 | 100 | 6.5 | 12 |
| EPS-KP-100 µg/mL | 7.22 ± 0.302 b | 113.9 b | 7 | 13 | |
| EPS-KP-200 µg/mL | 7.98 ± 0.25 c | 125.9 c | 7 | 14 | |
| EPS-KP-300 µg/mL | 7.31 ± 0.051 c | 115.3 c | 7 | 12 | |
| Oxidative stress | control | 2.83 ± 0.200 | 100 | 2.75 | 5.5 |
| EPS-KP-100 µg/mL | 3.1 ± 0.058 | 109.5 | 3 | 6.5 | |
| EPS-KP-200 µg/mL | 3.69 ± 0.035 c | 130.4 c | 3.5 | 8 | |
| EPS-KP-300 µg/mL | 3.53 ± 0.13 c | 124.7 c | 3.5 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Shi, S.; Lin, M.; Zhang, G.; Fang, L.; Li, J.; Geng, R.; Zheng, Y.; Hao, L. Tea-Residue-Derived Klebsiella pneumoniae CGMCC 31459: Genomic Insights and Antioxidant Activity of Its Exopolysaccharides. Biomolecules 2025, 15, 1569. https://doi.org/10.3390/biom15111569
Wang Y, Shi S, Lin M, Zhang G, Fang L, Li J, Geng R, Zheng Y, Hao L. Tea-Residue-Derived Klebsiella pneumoniae CGMCC 31459: Genomic Insights and Antioxidant Activity of Its Exopolysaccharides. Biomolecules. 2025; 15(11):1569. https://doi.org/10.3390/biom15111569
Chicago/Turabian StyleWang, Yuanyuan, Shengbo Shi, Mingchun Lin, Gangrui Zhang, Longyu Fang, Jinghua Li, Rui Geng, Yuanxue Zheng, and Lujiang Hao. 2025. "Tea-Residue-Derived Klebsiella pneumoniae CGMCC 31459: Genomic Insights and Antioxidant Activity of Its Exopolysaccharides" Biomolecules 15, no. 11: 1569. https://doi.org/10.3390/biom15111569
APA StyleWang, Y., Shi, S., Lin, M., Zhang, G., Fang, L., Li, J., Geng, R., Zheng, Y., & Hao, L. (2025). Tea-Residue-Derived Klebsiella pneumoniae CGMCC 31459: Genomic Insights and Antioxidant Activity of Its Exopolysaccharides. Biomolecules, 15(11), 1569. https://doi.org/10.3390/biom15111569






