Heteroaromatic Hybrid Benzimidazole/Oxadiazole (BZ/OZ) Ligand and Its Sm(III) Complex: Study of Their Antibacterial Activity, Toxicological Prediction and Interaction with Different Model Membranes
Abstract
1. Introduction
2. Materials and Methods
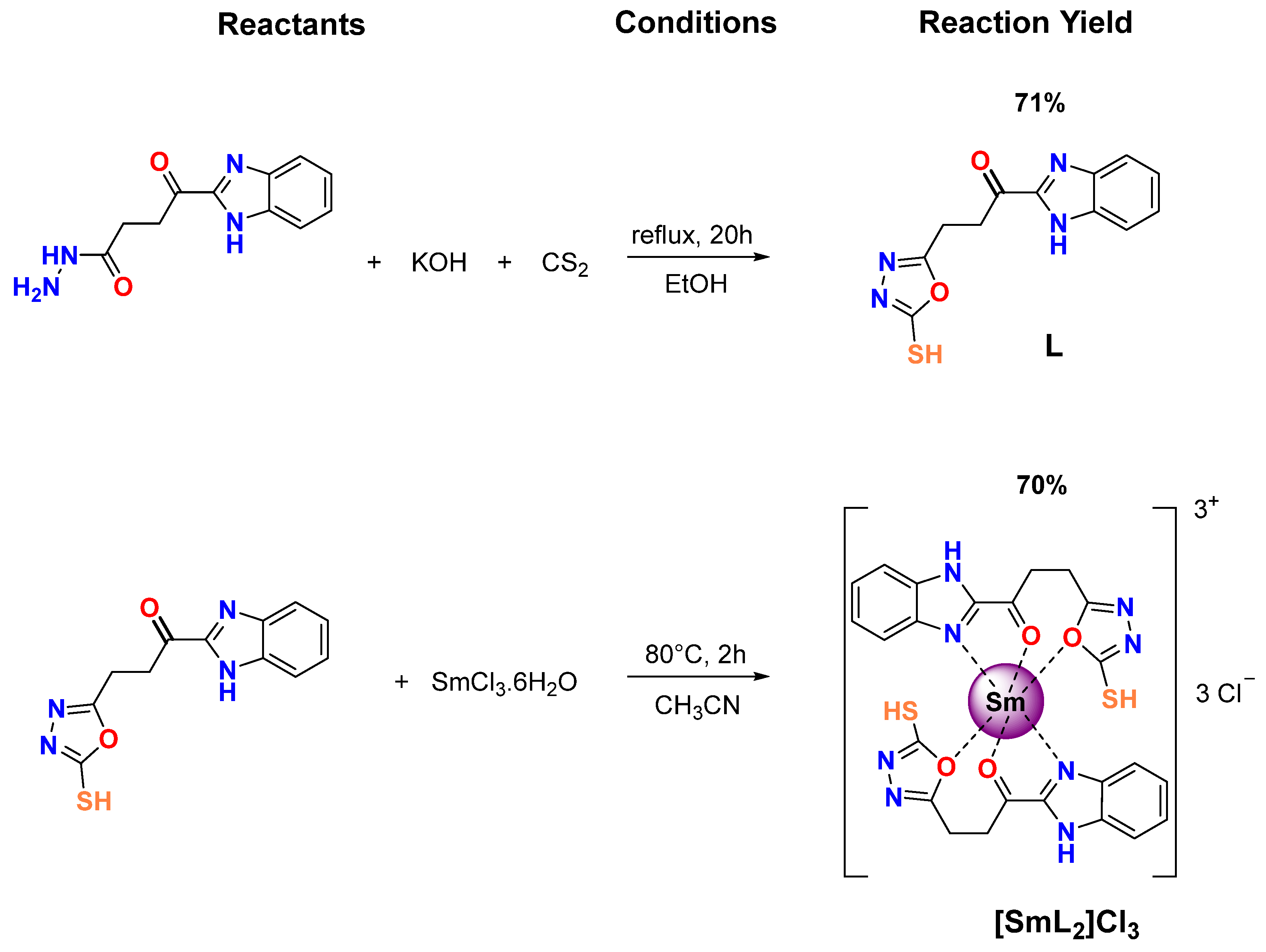
2.1. Synthesis of BZ/OZ Derivatives, L and [SmL2]Cl3
2.2. Antibacterial Activity: Determination of the Minimum Inhibitory Concentration
2.3. Model Membranes Preparation
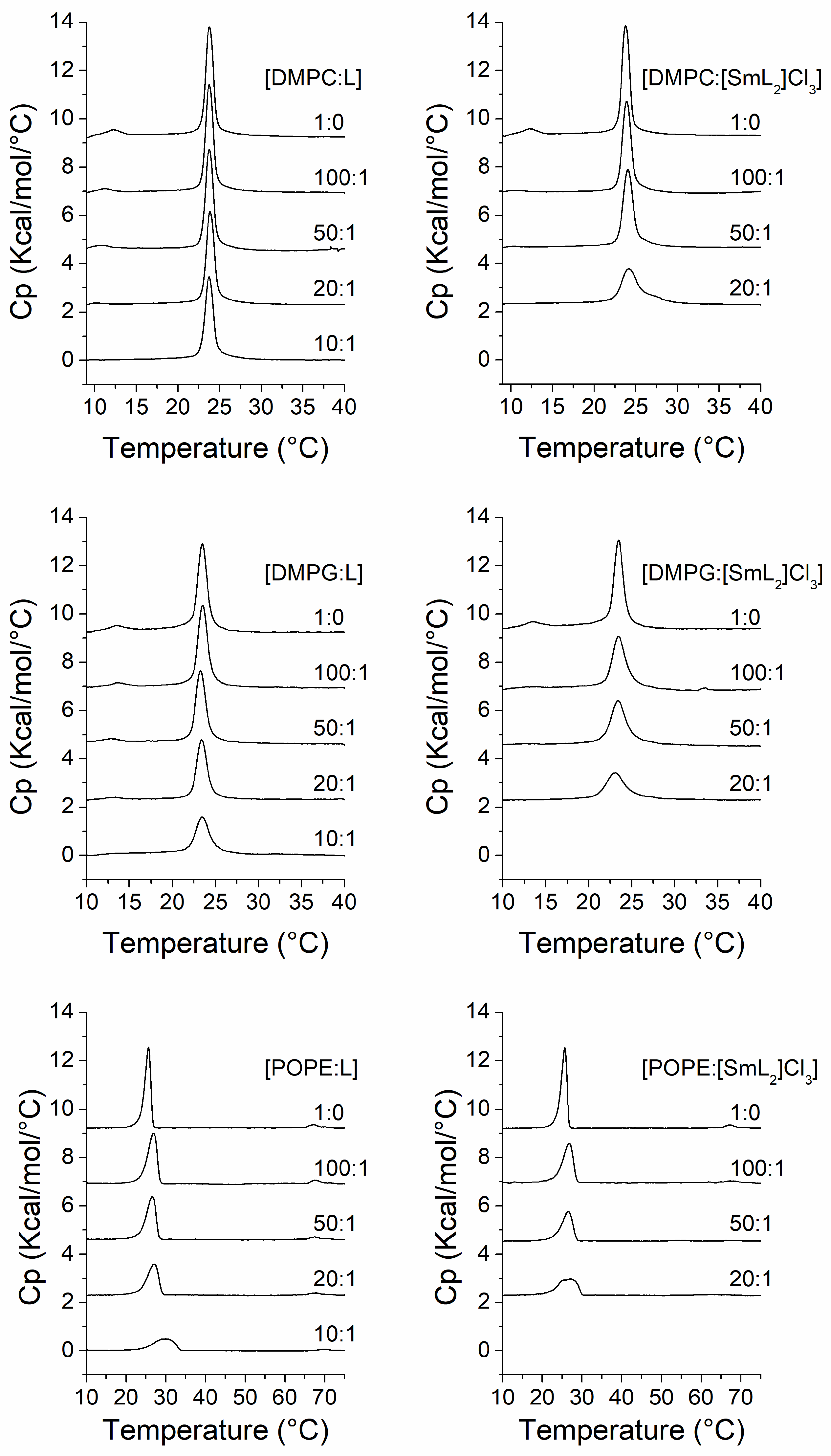
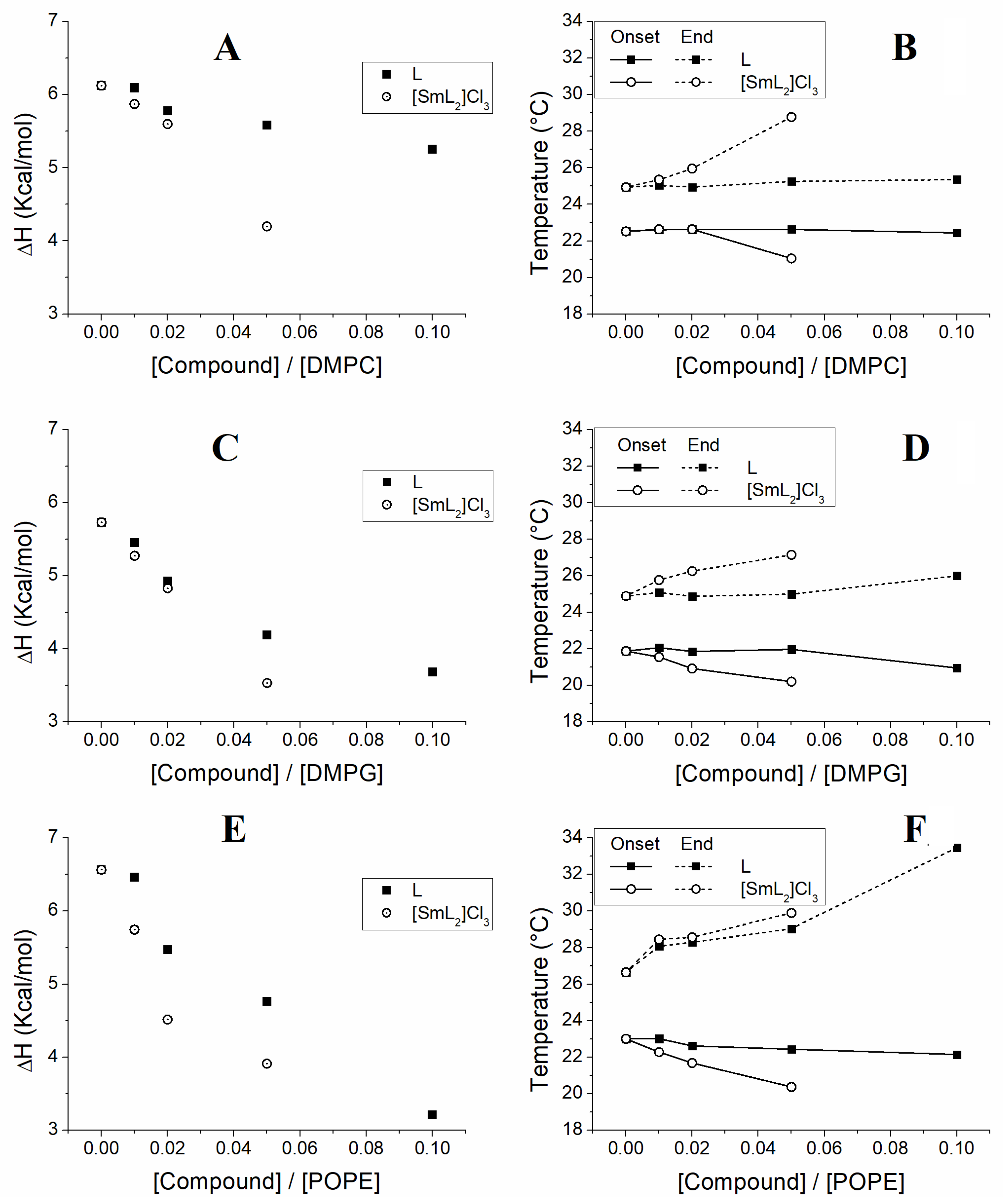
2.4. Differential Scanning Calorimetry Studies
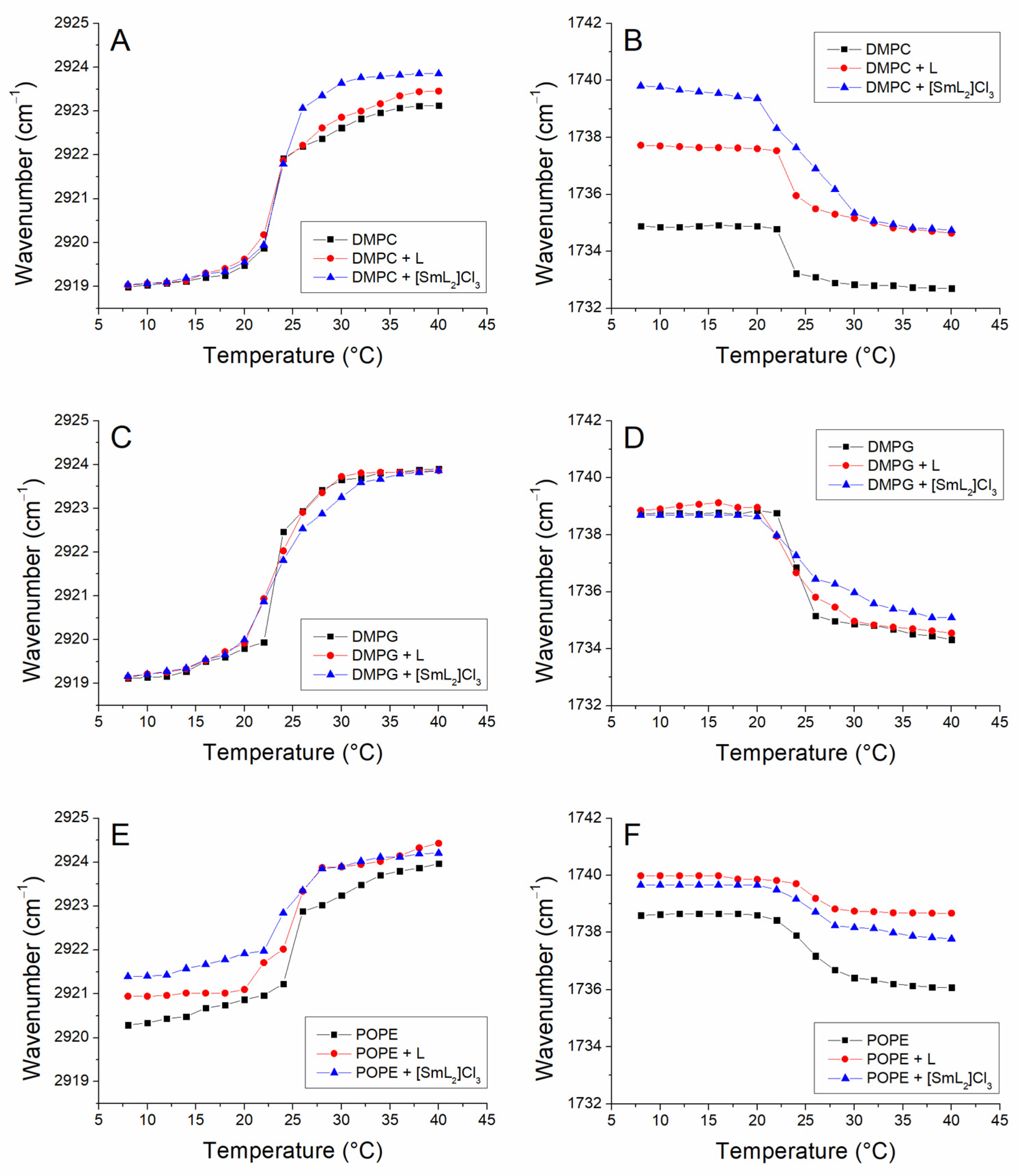
2.5. FTIR Studies
2.6. X-Ray Studies
2.7. In Silico Study
3. Results and Discussion
3.1. Synthesis and Characterization of BZ/OZ Derivatives L and [SmL2]Cl3
3.2. Antibacterial Activity
3.3. Membrane DSC Studies
3.4. Membrane FTIR Studies
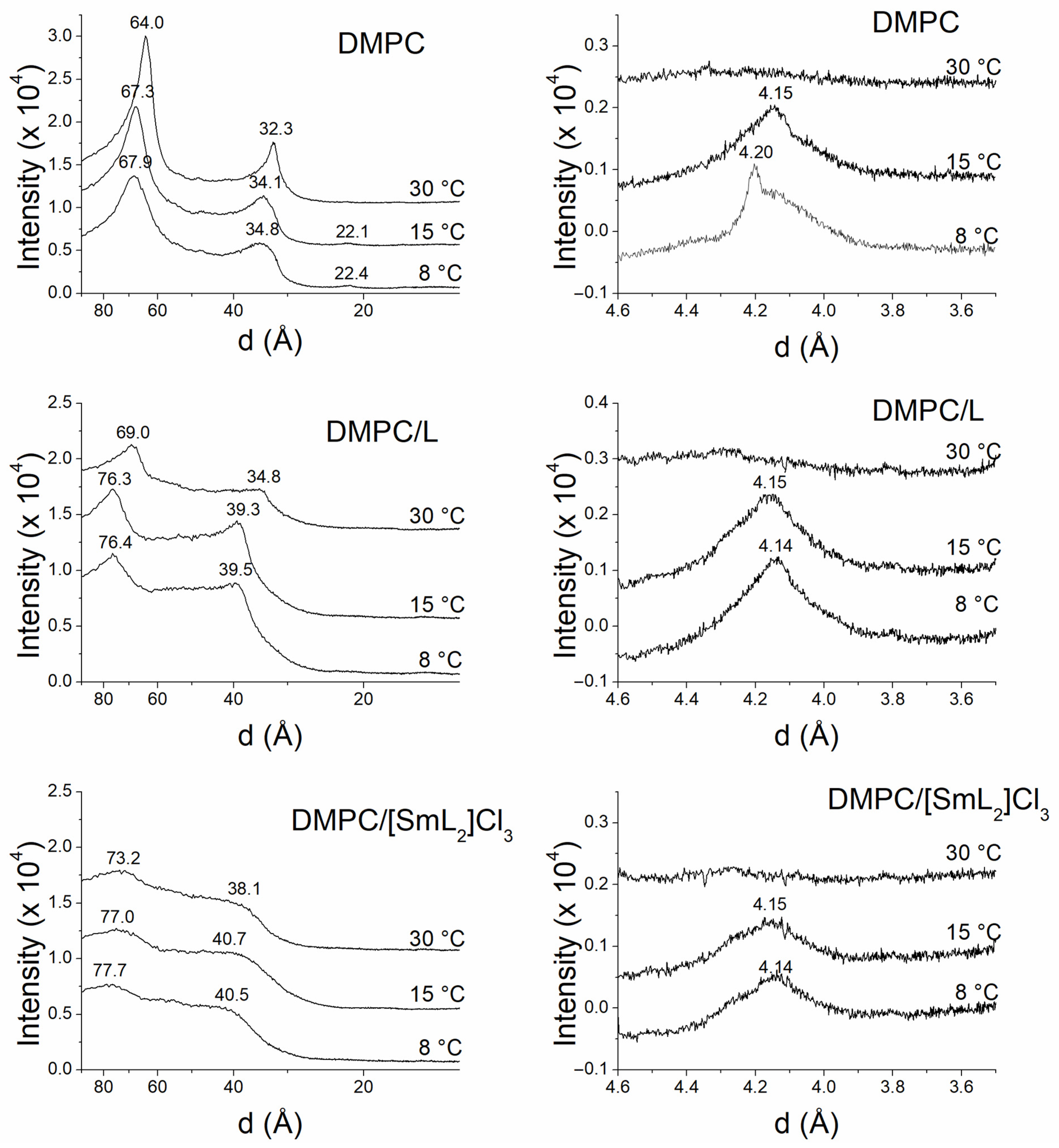
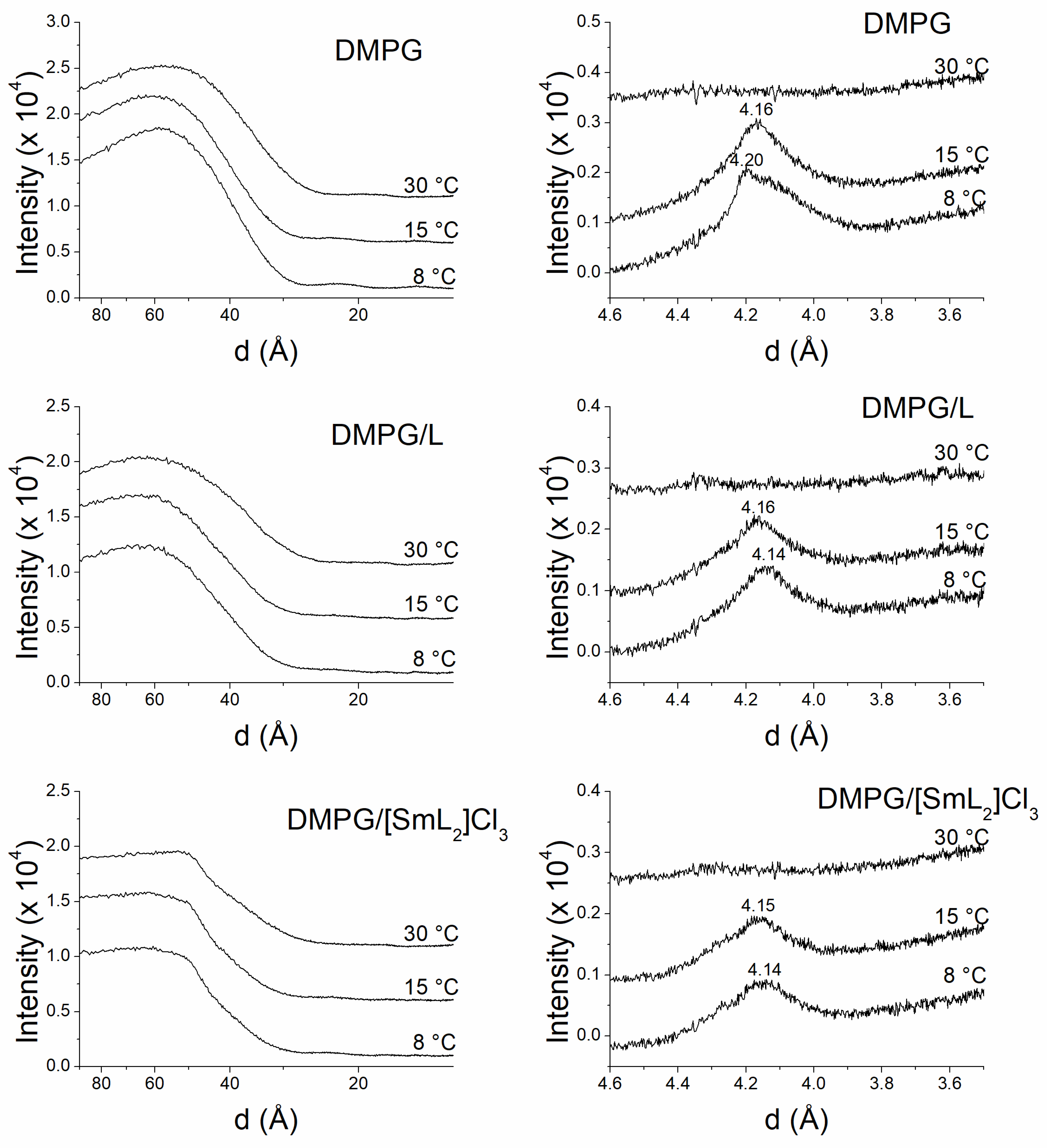
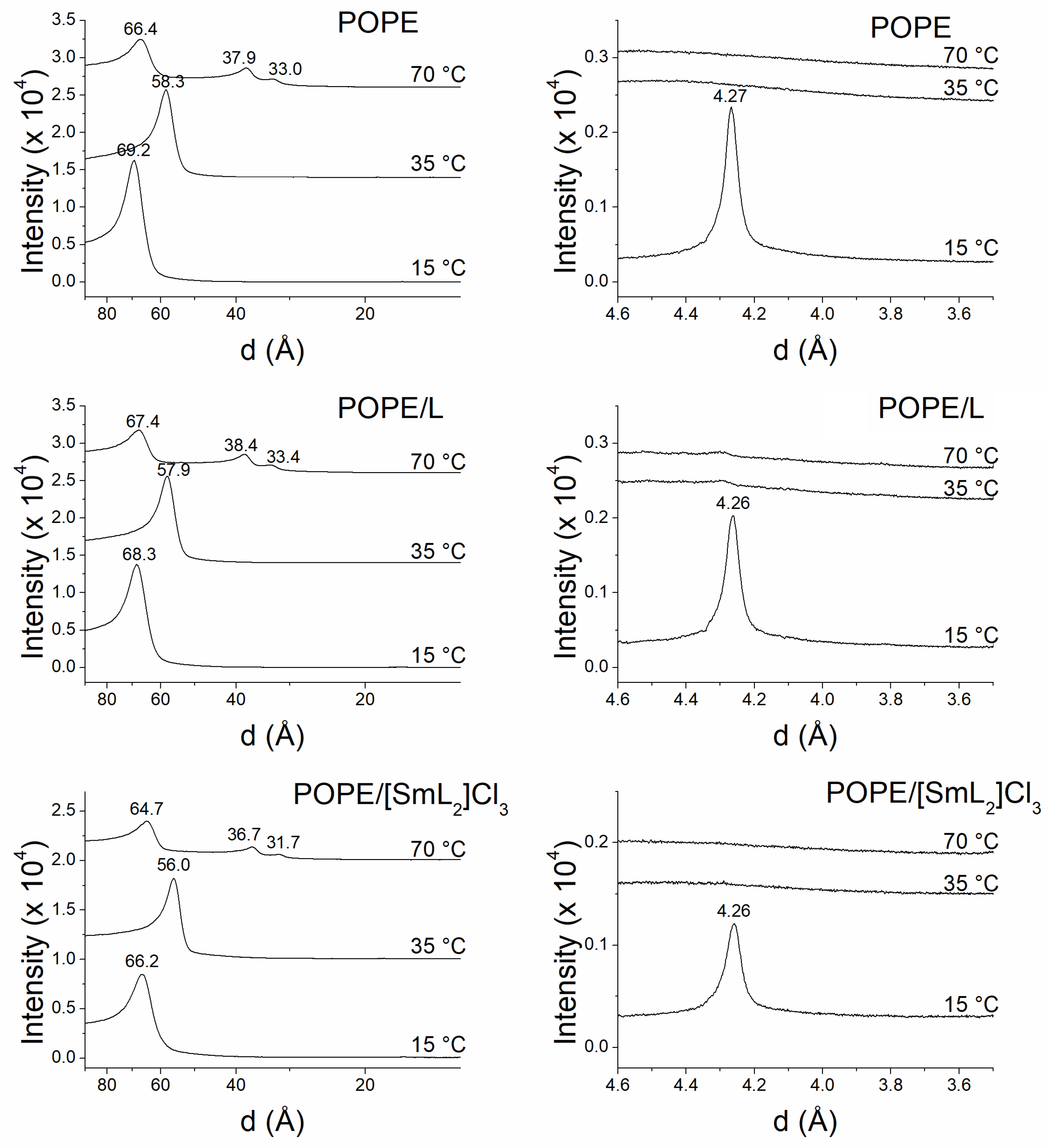
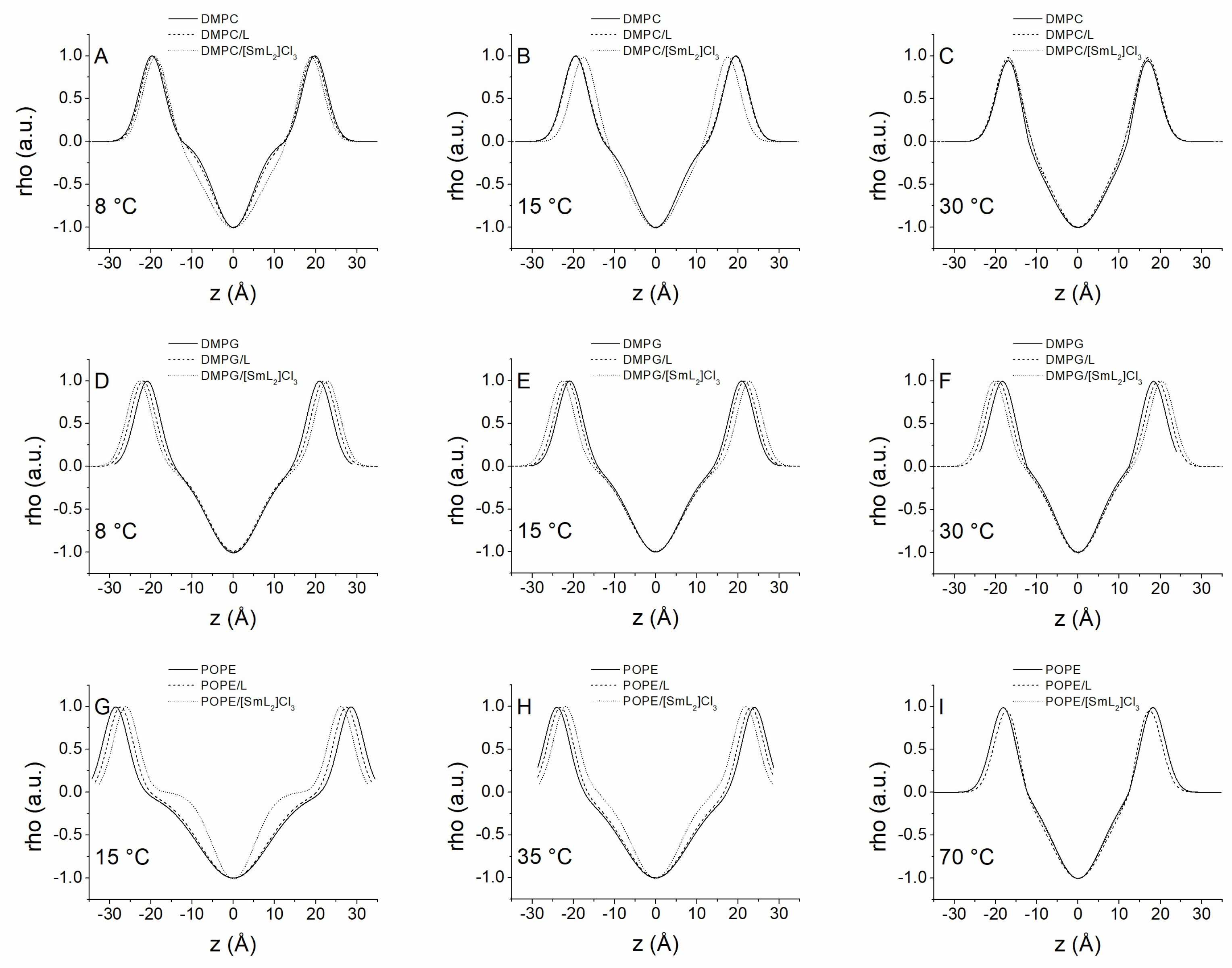
3.5. Membrane X-Ray Studies
3.6. In Silico Toxicological Predictions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ponzo, E.; De Gaetano, S.; Midiri, A.; Mancuso, G.; Giovanna, P.; Giuliana, D.; Zummo, S.; Biondo, C. The Antimicrobial Resistance Pandemic Is Here: Implementation Challenges and the Need for the One Health Approach. Hygiene 2024, 4, 297–316. [Google Scholar] [CrossRef]
- Ahmed, S.K.; Hussein, S.; Qurbani, K.; Ibrahim, R.H.; Fareeq, A.; Mahmood, K.A.; Mohamed, M.G. Antimicrobial Resistance: Impacts, Challenges, and Future Prospects. J. Med. Surg. Public Health 2024, 2, 100081. [Google Scholar] [CrossRef]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef]
- Singh, A.K.; Kumar, A.; Singh, H.; Sonawane, P.; Paliwal, H.; Thareja, S.; Pathak, P.; Grishina, M.; Jaremko, M.; Emwas, A.-H.; et al. Concept of Hybrid Drugs and Recent Advancements in Anticancer Hybrids. Pharmaceuticals 2022, 15, 1071. [Google Scholar] [CrossRef]
- Sampath Kumar, H.M.; Herrmann, L.; Tsogoeva, S.B. Structural Hybridization as a Facile Approach to New Drug Candidates. Bioorg. Med. Chem. Lett. 2020, 30, 127514. [Google Scholar] [CrossRef]
- Shabatina, T.I.; Vernaya, O.I.; Melnikov, M.Y. Hybrid Nanosystems of Antibiotics with Metal Nanoparticles—Novel Antibacterial Agents. Molecules 2023, 28, 1603. [Google Scholar] [CrossRef]
- Lungu, I.-A.; Moldovan, O.-L.; Biriș, V.; Rusu, A. Fluoroquinolones Hybrid Molecules as Promising Antibacterial Agents in the Fight against Antibacterial Resistance. Pharmaceutics 2022, 14, 1749. [Google Scholar] [CrossRef]
- Khwaza, V.; Oyedeji, O.O.; Morifi, E.; Nwamadi, M.; Fonkui, T.Y.; Ndinteh, D.T.; Aderibigbe, B.A. Design and Synthesis of Hybrid Compounds for Potential Treatment of Bacterial Co-Infections: In Vitro Antibacterial and In Silico Studies. Antibiotics 2025, 14, 582. [Google Scholar] [CrossRef]
- Elwahy, A.H.M.; Hammad, H.F.; Ibrahim, N.S.; Al-Shamiri, H.A.S.; Darweesh, A.F.; Abdelhamid, I.A. Synthesis and Antibacterial Activities of Novel Hybrid Molecules Based on Benzothiazole, Benzimidazole, Benzoxazole, and Pyrimidine Derivatives, Each Connected to N-Arylacetamide and Benzoate Groups. J. Mol. Struct. 2024, 1307, 137965. [Google Scholar] [CrossRef]
- Ghods, M.; Almasirad, A.; Tahghighi, A. Synthesis and in Vitro Anti-Bacterial Activity of Novel Quinoline-Based Aryl/Heteroaryl Amide Hybrids. J. Mol. Struct. 2025, 1335, 141923. [Google Scholar] [CrossRef]
- Műller, D.; Krakowska, A.; Zontek-Wilkowska, J.; Paczosa-Bator, B. Simple and Hybrid Materials for Antimicrobial Applications. Colloids Surf. B Biointerfaces 2025, 253, 114747. [Google Scholar] [CrossRef]
- Lin, Y.; Betts, H.; Keller, S.; Cariou, K.; Gasser, G. Recent developments of metal-based compounds against fungal pathogens. Chem. Soc. Rev. 2021, 50, 10346–10402. [Google Scholar] [CrossRef]
- Al-Jameel, S.S.; Ababutain, I.M.; Alghamdi, A.I.; Ben-Ali, A.; Al-Nasir, A.H.; Alqhtani, A.H.; Aldewely, L.K.; Alhassan, M.M.; Bakhurji, R.E.; AlGhamdi, W.M.; et al. Hybrid Organic-Inorganic Copper and Cobalt Complexes for Antimicrobial Potential Applications. Cell. Physiol. Biochem. 2024, 58, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Diaconu, D.; Mangalagiu, V.; Amariucai-Mantu, D.; Antoci, V.; Giuroiu, C.L.; Mangalagiu, I.I. Hybrid Quinoline-Sulfonamide Complexes (M2+) Derivatives with Antimicrobial Activity. Molecules 2020, 25, 2946. [Google Scholar] [CrossRef] [PubMed]
- Diaconu, D.; Antoci, V.; Mangalagiu, V.; Amariucai-Mantu, D.; Mangalagiu, I.I. Quinoline–imidazole/benzimidazole derivatives as dual-/multi-targeting hybrids inhibitors with anticancer and antimicrobial activity. Sci. Rep. 2022, 12, 16988. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.M.; Hegde, V.; Budagumpi, S.; Adimule, V.; Keri, R.S. Benzimidazole–Oxadiazole Hybrids—Development in Medicinal Chemistry: An Overview. Chem. Biol. Drug Des. 2024, 104, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Zou, Y.; Li, S.; Yang, L.; Qiu, Z.; Kong, F.; Gu, X. Discovery of Benzimidazole Substituted 1, 2, 4-Oxadiazole Compounds as Novel Anti-HBV Agents with TLR8-Agonistic Activities. Eur. J. Med. Chem. 2022, 244, 114833. [Google Scholar] [CrossRef] [PubMed]
- Soni, N.; Soni, N.; Gupta, P. Synthesis and In Vitro Anthelmintic Activity of Novel Substituted Oxadiazole Bearing Benzimidazole Derivatives. Der Pharma Chemica 2016, 8, 181–184. [Google Scholar]
- Celik, I.; Sarıaltın, S.Y.; Çoban, T.; Kılcıgil, G. Design, Synthesis, in Vitro and in Silico Studies of Benzimidazole-Linked Oxadiazole Derivatives as Anti-inflammatory Agents. ChemistrySelect 2022, 7, e202201548. [Google Scholar] [CrossRef]
- Hagar, F.F.; Abbas, S.H.; Sayed, A.M.; Gomaa, H.A.M.; Youssif, B.G.M.; Abdelhamid, D.; Abdel-Aziz, M. New Antiproliferative 1,3,4-Oxadiazole/Benzimidazole Derivatives: Design, Synthesis, and Biological Evaluation as Dual EGFR and BRAFV600E Inhibitors. Bioorg. Chem. 2025, 157, 108297. [Google Scholar] [CrossRef]
- Çevik, U.A.; Celik, I.; Görgülü, Ş.; Şahin İnan, Z.D.; Bostancı, H.E.; Karayel, A.; Özkay, Y.; Kaplancıklı, Z.A. Novel Benzimidazole–Oxadiazole Derivatives as Anticancer Agents with VEGFR2 Inhibitory Activity: Design, Synthesis, In Vitro Anticancer Evaluation, and In Silico Studies. ACS Omega 2025, 10, 6801–6813. [Google Scholar] [CrossRef]
- Çevik, U.A.; Osmaniye, D.; Çavuşoğlu, B.K.; Sağlik, B.N.; Levent, S.; Ilgin, S.; Can, N.Ö.; Özkay, Y.; Kaplancikli, Z.A. Synthesis of Novel Benzimidazole–Oxadiazole Derivatives as Potent Anticancer Activity. Med. Chem. Res. 2019, 28, 2252–2261. [Google Scholar] [CrossRef]
- Shruthi, N.; Poojary, B.; Kumar, V.; Hussain, M.M.; Rai, V.M.; Pai, V.R.; Bhat, M.; Revannasiddappa, B.C. Novel Benzimidazole–Oxadiazole Hybrid Molecules as Promising Antimicrobial Agents. RSC Adv. 2016, 6, 8303–8316. [Google Scholar] [CrossRef]
- Salahuddin; Shaharyar, M.; Mazumder, A.; Abdullah, M.M. Synthesis, Characterization and Antimicrobial Activity of 1,3,4-Oxadiazole Bearing 1H-Benzimidazole Derivatives. Arab. J. Chem. 2017, 10, S503–S508. [Google Scholar] [CrossRef]
- Patel, M.; Avashthi, G.; Gacem, A.; Alqahtani, M.S.; Park, H.-K.; Jeon, B.-H. A Review of Approaches to the Metallic and Non-Metallic Synthesis of Benzimidazole (BnZ) and Their Derivatives for Biological Efficacy. Molecules 2023, 28, 5490. [Google Scholar] [CrossRef]
- Acar Çevik, U.; Celik, I.; Görgülü, Ş.; Şahin Inan, Z.D.; Bostancı, H.E.; Özkay, Y.; Kaplacıklı, Z.A. New Benzimidazole-oxadiazole Derivatives as Potent VEGFR-2 Inhibitors: Synthesis, Anticancer Evaluation, and Docking Study. Drug Dev. Res. 2024, 85, e22218. [Google Scholar] [CrossRef]
- Tantray, M.A.; Khan, I.; Hamid, H.; Alam, M.S.; Dhulap, A.; Kalam, A. Synthesis of Benzimidazole-Based 1,3,4-Oxadiazole-1,2,3-Triazole Conjugates as Glycogen Synthase Kinase-3β Inhibitors with Antidepressant Activity in in Vivo Models. RSC Adv. 2016, 6, 43345–43355. [Google Scholar] [CrossRef]
- Acar Çevik, U.; Sağlık, B.N.; Osmaniye, D.; Levent, S.; Kaya Çavuşoğlu, B.; Karaduman, A.B.; Atlı Eklioğlu, Ö.; Özkay, Y.; Kaplancıklı, Z.A. Synthesis, Anticancer Evaluation and Molecular Docking Studies of New Benzimidazole-1,3,4-Oxadiazole Derivatives as Human Topoisomerase Types I Poison. J. Enzym. Inhib. Med. Chem. 2020, 35, 1657–1673. [Google Scholar] [CrossRef]
- Almalki, A.S.; Nazreen, S.; Elbehairi, S.E.I.; Asad, M.; Shati, A.A.; Alfaifi, M.Y.; Alhadhrami, A.; Elhenawy, A.A.; Alorabi, A.Q.; Asiri, A.M.; et al. Design, Synthesis, Anticancer Activity and Molecular Docking Studies of New Benzimidazole Derivatives Bearing 1,3,4-Oxadiazole Moieties as Potential Thymidylate Synthase Inhibitors. New J. Chem. 2022, 46, 14967–14978. [Google Scholar] [CrossRef]
- Bhasker, G.; Salahuddin; Mazumder, A.; Kumar, R.; Kumar, G.; Ahsan, M.J.; Shahar Yar, M.; Khan, F.; Kapoor, B. Hybrids of Benzimidazole-Oxadiazole: A New Avenue for Synthesis, Pharmacological Activity and Recent Patents for the Development of More Effective Ligands. Curr. Org. Synth. 2024, 21, 976–1013. [Google Scholar] [CrossRef]
- Fathi, M.A.A.; Abd El-Hafeez, A.A.; Abdelhamid, D.; Abbas, S.H.; Montano, M.M.; Abdel-Aziz, M. 1,3,4-Oxadiazole/Chalcone Hybrids: Design, Synthesis, and Inhibition of Leukemia Cell Growth and EGFR, Src, IL-6 and STAT3 Activities. Bioorg. Chem. 2019, 84, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Hagar, F.F.; Abbas, S.H.; Gomaa, H.A.M.; Youssif, B.G.M.; Sayed, A.M.; Abdelhamid, D.; Abdel-Aziz, M. Chalcone/1,3,4-Oxadiazole/Benzimidazole Hybrids as Novel Anti-Proliferative Agents Inducing Apoptosis and Inhibiting EGFR & BRAFV600E. BMC Chem. 2023, 17, 116. [Google Scholar] [CrossRef]
- Glomb, T.; Świątek, P. Antimicrobial Activity of 1,3,4-Oxadiazole Derivatives. Int. J. Mol. Sci. 2021, 22, 6979. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, H.; Alam, M.; Elhenawy, A.; Nazreen, S. Synthesis, Antimicrobial, Antiproliferative, and Docking Studies of 1,3,4-Oxadiazole Derivatives Containing Benzimidazole Scaffold. Biointerface Res. Appl. Chem. 2022, 13, 298. [Google Scholar] [CrossRef]
- Akhter, G.; Hamid, H.; Dhawan, B.; Kumar Das, A.; Tantray, M.A.; Alam, M.S.; Sharma, K. Synthesis and Design of New 1,3,4-Oxadiazole Benzimidazole Hybrids as Potential Antibacterial Agents Against MRSA by Targeting FabI. ChemistrySelect 2024, 9, e202404473. [Google Scholar] [CrossRef]
- Karaburun, A.Ç.; Kaya Çavuşoğlu, B.; Acar Çevik, U.; Osmaniye, D.; Sağlık, B.N.; Levent, S.; Özkay, Y.; Atlı, Ö.; Koparal, A.S.; Kaplancıklı, Z.A. Synthesis and Antifungal Potential of Some Novel Benzimidazole-1,3,4-Oxadiazole Compounds. Molecules 2019, 24, 191. [Google Scholar] [CrossRef]
- Sousa, C.F.; Coimbra, J.T.S.; Ferreira, M.; Pereira-Leite, C.; Reis, S.; Ramos, M.J.; Fernandes, P.A.; Gameiro, P. Passive Diffusion of Ciprofloxacin and Its Metalloantibiotic: A Computational and Experimental Study. J. Mol. Biol. 2021, 433, 166911. [Google Scholar] [CrossRef]
- Wang, J.; Ansari, M.F.; Zhou, C.-H. Unique Para-Aminobenzenesulfonyl Oxadiazoles as Novel Structural Potential Membrane Active Antibacterial Agents towards Drug-Resistant Methicillin Resistant Staphylococcus Aureus. Bioorg. Med. Chem. Lett. 2021, 41, 127995. [Google Scholar] [CrossRef]
- Hu, Z.; Marti, J. In Silico Drug Design of Benzothiadiazine Derivatives Interacting with Phospholipid Cell Membranes. Membranes 2022, 12, 331. [Google Scholar] [CrossRef]
- Aragón-Muriel, A.; Liscano, Y.; Morales-Morales, D.; Polo-Cerón, D.; Oñate-Garzón, J. A Study of the Interaction of a New Benzimidazole Schiff Base with Synthetic and Simulated Membrane Models of Bacterial and Mammalian Membranes. Membranes 2021, 11, 449. [Google Scholar] [CrossRef]
- Hussein, K.B. Synthesis, Antibacterial Activity of Sm(III) Complex with L-Phenylalanine. Zanco J. Pure Appl. Sci. 2022, 34, 51–59. [Google Scholar] [CrossRef]
- Asadpour, S.; Aramesh-Boroujeni, Z.; Jahani, S. In Vitro Anticancer Activity of Parent and Nano-Encapsulated Samarium(III) Complex towards Antimicrobial Activity Studies and FS-DNA/BSA Binding Affinity. RSC Adv. 2020, 10, 31979–31990. [Google Scholar] [CrossRef] [PubMed]
- Cota, I.; Marturano, V.; Tylkowski, B. Ln Complexes as Double Faced Agents: Study of Antibacterial and Antifungal Activity. Coord. Chem. Rev. 2019, 396, 49–71. [Google Scholar] [CrossRef]
- Ain, Q.; Pandey, S.K.; Pandey, O.P.; Sengupta, S.K. Synthesis, Spectroscopic, Thermal and Antimicrobial Studies of Neodymium(III) and Samarium(III) Complexes Derived from Tetradentate Ligands Containing N and S Donor Atoms. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 140, 27–34. [Google Scholar] [CrossRef]
- Dzulkifli, N.N.; Farina, Y.; Yamin, B.M.; Ibrahim, N. Synthesis, structural, chemical properties, and anti-bacterial screening of sm(iii) thiosemicarbazone complexes. Malays. J. Anal. Sci. 2017, 21, 560–570. [Google Scholar] [CrossRef]
- Moreno-Ramirez, M.C.; Arias-Bravo, A.S.; Aragón-Muriel, A.; Godoy, C.A.; Liscano, Y.; Garzón, J.O.; Polo-Cerón, D. Design, Synthesis and Antimicrobial Potential of Conjugated Metallopeptides Targeting DNA. Sci. Pharm. 2024, 92, 21. [Google Scholar] [CrossRef]
- Aragón-Muriel, A.; Aguilar-Castillo, B.A.; Rufino-Felipe, E.; Valdés, H.; González-Sebastián, L.; Osorio-Yáñez, R.N.; Liscano, Y.; Gómez-Benítez, V.; Polo-Cerón, D.; Morales-Morales, D. Antibacterial Activity and Molecular Studies of Non-Symmetric POCOP-Pd(II) Pincer Complexes Derived from 2,4-Dihydroxybenzaldehyde (2,4-DHBA). Polyhedron 2022, 227, 116115. [Google Scholar] [CrossRef]
- Aragón-Muriel, A.; Liscano, Y.; Upegui, Y.; Robledo, S.M.; Ramírez-Apan, M.T.; Morales-Morales, D.; Oñate-Garzón, J.; Polo-Cerón, D. In Vitro Evaluation of the Potential Pharmacological Activity and Molecular Targets of New Benzimidazole-Based Schiff Base Metal Complexes. Antibiotics 2021, 10, 728. [Google Scholar] [CrossRef]
- Aragón-Muriel, A.; Liscano-Martínez, Y.; Rufino-Felipe, E.; Morales-Morales, D.; Oñate-Garzón, J.; Polo-Cerón, D. Synthesis, Biological Evaluation and Model Membrane Studies on Metal Complexes Containing Aromatic N,O-Chelate Ligands. Heliyon 2020, 6, e04126. [Google Scholar] [CrossRef]
- Husain, A.; Rashid, M.; Mishra, R.; Parveen, S.; Shin, D.-S.; Kumar, D. Benzimidazole Bearing Oxadiazole and Triazolo-Thiadiazoles Nucleus: Design and Synthesis as Anticancer Agents. Bioorg. Med. Chem. Lett. 2012, 22, 5438–5444. [Google Scholar] [CrossRef]
- Váquiro-Reyes, I.Y.; Aragón-Muriel, A.; Polo-Cerón, D. Síntesis, Caracterización y Evaluación Farmacológica de Nuevos Complejos Metálicos Derivados de Híbridos Heteroaromáticos (Benzimidazol/Oxadiazol). Rev. Colomb. Cienc. Químico-Farm. 2019, 48, 557–588. [Google Scholar] [CrossRef]
- Koeth, L.M.; Miller, L.A. Antimicrobial Susceptibility Test Methods: Dilution and Disk Diffusion Methods. In ClinMicroNow; Wiley: Hoboken, NJ, USA, 2023; pp. 1–19. [Google Scholar]
- Boon, J.M.; Smith, B.D. Chemical Control of Phospholipid Distribution across Bilayer Membranes. Med. Res. Rev. 2002, 22, 251–281. [Google Scholar] [CrossRef] [PubMed]
- Escribá, P.V.; González-Ros, J.M.; Goñi, F.M.; Kinnunen, P.K.J.; Vigh, L.; Sánchez-Magraner, L.; Fernández, A.M.; Busquets, X.; Horváth, I.; Barceló-Coblijn, G. Membranes: A Meeting Point for Lipids, Proteins and Therapies. J. Cell Mol. Med. 2008, 12, 829–875. [Google Scholar] [CrossRef] [PubMed]
- Madigan, M.; Sattley, W.; Aiyer, J.; Stahl, D.; Buckley, D. Brock Biology of Microorganisms, Global Edition; Pearson Deutschland: Munich, Germany, 2021; ISBN 9781292404790. [Google Scholar]
- Böttcher, C.J.F.; Van Gent, C.M.; Pries, C. A Rapid and Sensitive Sub-Micro Phosphorus Determination. Anal. Chim. Acta 1961, 24, 203–204. [Google Scholar] [CrossRef]
- Ausili, A.; Gómez-Murcia, V.; Candel, A.M.; Beltrán, A.; Torrecillas, A.; He, L.; Jiang, Y.; Zhang, S.; Teruel, J.A.; Gómez-Fernández, J.C. A Comparison of the Location in Membranes of Curcumin and Curcumin-Derived Bivalent Compounds with Potential Neuroprotective Capacity for Alzheimer’s Disease. Colloids Surf. B Biointerfaces 2021, 199, 111525. [Google Scholar] [CrossRef]
- Pabst, G.; Rappolt, M.; Amenitsch, H.; Laggner, P. Structural Information from Multilamellar Liposomes at Full Hydration: Full q-Range Fitting with High Quality x-Ray Data. Phys. Rev. E 2000, 62, 4000–4009. [Google Scholar] [CrossRef]
- Ausili, A.; Martínez-Valera, P.; Torrecillas, A.; Gómez-Murcia, V.; de Godos, A.M.; Corbalán-García, S.; Teruel, J.A.; Gómez Fernández, J.C. Anticancer Agent Edelfosine Exhibits a High Affinity for Cholesterol and Disorganizes Liquid-Ordered Membrane Structures. Langmuir 2018, 34, 8333–8346. [Google Scholar] [CrossRef]
- Ramos, R.S.; Borges, R.S.; de Souza, J.S.N.; Araujo, I.F.; Chaves, M.H.; Santos, C.B.R. Identification of Potential Antiviral Inhibitors from Hydroxychloroquine and 1,2,4,5-Tetraoxanes Analogues and Investigation of the Mechanism of Action in SARS-CoV-2. Int. J. Mol. Sci. 2022, 23, 1781. [Google Scholar] [CrossRef]
- Hay, R.W. The Kinetic Background. In Reaction Mechanisms of Metal Complexes; Elsevier: Amsterdam, The Netherlands, 2000; pp. 35–57. [Google Scholar]
- Maciuca, A.-M.; Munteanu, A.-C.; Mihaila, M.; Badea, M.; Olar, R.; Nitulescu, G.M.; Munteanu, C.V.A.; Bostan, M.; Uivarosi, V. Rare-Earth Metal Complexes of the Antibacterial Drug Oxolinic Acid: Synthesis, Characterization, DNA/Protein Binding and Cytotoxicity Studies. Molecules 2020, 25, 5418. [Google Scholar] [CrossRef]
- Bistoni, G.; Rampino, S.; Scafuri, N.; Ciancaleoni, G.; Zuccaccia, D.; Belpassi, L.; Tarantelli, F. How π Back-Donation Quantitatively Controls the CO Stretching Response in Classical and Non-Classical Metal Carbonyl Complexes. Chem. Sci. 2016, 7, 1174–1184. [Google Scholar] [CrossRef]
- Patel, M.N.; Pansuriya, P.B.; Parmar, P.A.; Gandhi, D.S. Synthesis, Characterization, and Thermal and Biocidal Aspects of Drug-Based Metal Complexes. Pharm. Chem. J. 2008, 42, 687–692. [Google Scholar] [CrossRef]
- Kim, W.K.; An, J.M.; Lim, Y.J.; Kim, K.; Kim, Y.H.; Kim, D. Recent Advances in Metallodrug: Coordination-Induced Synergy between Clinically Approved Drugs and Metal Ions. Mater. Today Adv. 2025, 25, 100569. [Google Scholar] [CrossRef]
- Casal, H.L.; Mantsch, H.H. Polymorphic Phase Behaviour of Phospholipid Membranes Studied by Infrared Spectroscopy. Biochim. Biophys. Acta (BBA)-Rev. Biomembr. 1984, 779, 381–401. [Google Scholar] [CrossRef]
- Lewis, R.N.A.H.; Prenner, E.J.; Kondejewski, L.H.; Flach, C.R.; Mendelsohn, R.; Hodges, R.S.; McElhaney, R.N. Fourier Transform Infrared Spectroscopic Studies of the Interaction of the Antimicrobial Peptide Gramicidin S with Lipid Micelles and with Lipid Monolayer and Bilayer Membranes. Biochemistry 1999, 38, 15193–15203. [Google Scholar] [CrossRef] [PubMed]
- Tardieu, A.; Luzzati, V.; Reman, F.C. Structure and Polymorphism of the Hydrocarbon Chains of Lipids: A Study of Lecithin-Water Phases. J. Mol. Biol. 1973, 75, 711–733. [Google Scholar] [CrossRef] [PubMed]
- Holland, J.W.; Cullis, P.R.; Madden, T.D. Poly(Ethylene Glycol)–Lipid Conjugates Promote Bilayer Formation in Mixtures of Non-Bilayer-Forming Lipids. Biochemistry 1996, 35, 2610–2617. [Google Scholar] [CrossRef]
- Sharma, B.; Shukla, S.; Rattan, R.; Fatima, M.; Goel, M.; Bhat, M.; Dutta, S.; Ranjan, R.K.; Sharma, M. Antimicrobial Agents Based on Metal Complexes: Present Situation and Future Prospects. Int. J. Biomater. 2022, 2022, 6819080. [Google Scholar] [CrossRef]
- Lewis, R.N.A.H.; Mannock, D.A.; McElhaney, R.N. Differential Scanning Calorimetry in the Study of Lipid Phase Transitions in Model and Biological Membranes. In Methods in Membrane Lipids; Springer: Berlin/Heidelberg, Germany, 2007; pp. 171–195. [Google Scholar]
- Leekumjorn, S.; Sum, A.K. Molecular Studies of the Gel to Liquid-Crystalline Phase Transition for Fully Hydrated DPPC and DPPE Bilayers. Biochim. Biophys. Acta (BBA)-Biomembr. 2007, 1768, 354–365. [Google Scholar] [CrossRef]
- Neunert, G.; Tomaszewska-Gras, J.; Baj, A.; Gauza-Włodarczyk, M.; Witkowski, S.; Polewski, K. Phase Transitions and Structural Changes in DPPC Liposomes Induced by a 1-Carba-Alpha-Tocopherol Analogue. Molecules 2021, 26, 2851. [Google Scholar] [CrossRef]
- Lewis, R.N.; McElhaney, R.N.; Pohle, W.; Mantsch, H.H. Components of the Carbonyl Stretching Band in the Infrared Spectra of Hydrated 1,2-Diacylglycerolipid Bilayers: A Reevaluation. Biophys. J. 1994, 67, 2367–2375. [Google Scholar] [CrossRef]
- Rappolt, M.; Hickel, A.; Bringezu, F.; Lohner, K. Mechanism of the Lamellar/Inverse Hexagonal Phase Transition Examined by High Resolution X-Ray Diffraction. Biophys. J. 2003, 84, 3111–3122. [Google Scholar] [CrossRef] [PubMed]
- Rand, R.P.; Luzzati, V. X-Ray Diffraction Study in Water of Lipids Extracted from Human Erythrocytes. Biophys. J. 1968, 8, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Roessle, M.; Svergun, D.I. Small Angle X-Ray Scattering. In Encyclopedia of Biophysics; Springer: Berlin/Heidelberg, Germany, 2013; pp. 2382–2389. [Google Scholar]
- Hauser, H. Some Aspects of the Phase Behaviour of Charged Lipids. Biochim. Biophys. Acta (BBA)-Biomembr. 1984, 772, 37–50. [Google Scholar] [CrossRef]
- Pabst, G.; Danner, S.; Karmakar, S.; Deutsch, G.; Raghunathan, V.A. On the Propensity of Phosphatidylglycerols to Form Interdigitated Phases. Biophys. J. 2007, 93, 513–525. [Google Scholar] [CrossRef]
- Hamley, I.W. Diffuse Scattering from Lamellar Structures. Soft Matter 2022, 18, 711–721. [Google Scholar] [CrossRef]
- Valtersson, C.; van Duÿn, G.; Verkleij, A.J.; Chojnacki, T.; de Kruijff, B.; Dallner, G. The Influence of Dolichol, Dolichol Esters, and Dolichyl Phosphate on Phospholipid Polymorphism and Fluidity in Model Membranes. J. Biol. Chem. 1985, 260, 2742–2751. [Google Scholar] [CrossRef]
- Luzzati, V.; Gulik-Krzywicki, T.; Rivas, E.; Reiss-Husson, F.; Rand, R.P. X-Ray Study of Model Systems: Structure of the Lipid-Water Phases in Correlation with the Chemical Composition of the Lipids. J. Gen. Physiol. 1968, 51, 37–43. [Google Scholar] [CrossRef]
- Oliveira, L.P.S.; Lima, L.R.; Silva, L.B.; Cruz, J.N.; Ramos, R.S.; Lima, L.S.; Cardoso, F.M.N.; Silva, A.V.; Rodrigues, D.P.; Rodrigues, G.S.; et al. Hierarchical Virtual Screening of Potential New Antibiotics from Polyoxygenated Dibenzofurans against Staphylococcus Aureus Strains. Pharmaceuticals 2023, 16, 1430. [Google Scholar] [CrossRef]
- Halimi Syla, G.; Osmaniye, D.; Korkut Çelikateş, B.; Özkay, Y.; Kaplancıklı, Z.A. Targeting Cancer with New Morpholine-Benzimidazole-Oxadiazole Derivatives: Synthesis, Biological Evaluation, and Computational Insights. ACS Omega 2025, 10, 36134–36153. [Google Scholar] [CrossRef]
- Ding, X.; Fan, L.; Wang, L.; Zhou, M.; Wang, Y.; Zhao, Y. Designing Self-Healing Hydrogels for Biomedical Applications. Mater. Horiz. 2023, 10, 3929–3947. [Google Scholar] [CrossRef]
- Mandour, H.S.A.; Rehab, A.; Elnahrawy, M.; Salahuddin, N. Synthesis, Characterization and Bioactivity of Chalcone-Based Polybenzoxazine/Copper Oxide Nanocomposites. RSC Adv. 2025, 15, 37263–37272. [Google Scholar] [CrossRef]
- Fijałkowski, P.; Impert, O.; Pomastowski, P.; Rafińska, K.; Walczak-Skierska, J.; Fijałkowski, P.; Katafias, A.; van Eldik, R. Synthesis of Nanoparticles of Cobalt Protoporphyrin IX (Co(iii) PPIX NPs). Antiradical, Cytotoxicity and Antibacterial Properties. RSC Adv. 2025, 15, 36789–36802. [Google Scholar] [CrossRef]
- Movahedi, F.; Gu, W.; Soares, C.P.; Xu, Z.P. Encapsulating Anti-Parasite Benzimidazole Drugs into Lipid-Coated Calcium Phosphate Nanoparticles to Efficiently Induce Skin Cancer Cell Apoptosis. Front. Nanotechnol. 2021, 3, 693837. [Google Scholar] [CrossRef]
| Compound | Gram-Positive | Gram-Negative | ||||
|---|---|---|---|---|---|---|
| B. subtilis | S. aureus | E. coli | K. pneumoniae | S. dysenteriae | Salmonella | |
| L | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 |
| [SmL2]Cl3 | 500 | 500 | 250 | 250 | 250 | 500 |
| SmCl3·6H2O | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 |
| Cp 1 | <3.9 | <3.9 | <3.9 | <3.9 | <3.9 | <3.9 |
| AgNO3 1 | <7.8 | <7.8 | <7.8 | <7.8 | <7.8 | <7.8 |
| [Compound]/ [Lipid] | Molar Ratio (L:C) | DMPC/L | DMPC/[SmL2]Cl3 | DMPG/L | DMPG/[SmL2]Cl3 | POPE/L | POPE/[SmL2]Cl3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Onset | End | Onset | End | Onset | End | Onset | End | Onset | End | Onset | End | ||
| (°C) | |||||||||||||
| 0.00 | 1:0 | 22.5 | 24.9 | 22.5 | 24.9 | 21.9 | 24.9 | 21.9 | 24.9 | 23.0 | 26.7 | 23.0 | 26.7 |
| 0.01 | 100:1 | 22.6 | 25.0 | 22.6 | 25.4 | 22.1 | 25.1 | 21.5 | 25.8 | 23.0 | 28.1 | 22.3 | 28.5 |
| 0.02 | 50:1 | 22.6 | 24.9 | 22.6 | 26.0 | 21.9 | 24.9 | 20.9 | 26.3 | 22.6 | 28.3 | 21.7 | 28.6 |
| 0.05 | 20:1 | 22.6 | 25.3 | 21.0 | 28.8 | 22.0 | 25.0 | 20.2 | 27.1 | 22.4 | 29.0 | 20.4 | 29.9 |
| 0.10 | 10:1 | 22.4 | 25.4 | 21.0 | 26.0 | 22.2 | 33.5 | ||||||
| [Compound]/ [Lipid] | Molar Ratio (L:C) | DMPC/L | DMPC/[SmL2]Cl3 | DMPG/L | DMPG/[SmL2]Cl3 | POPE/L | POPE/[SmL2]Cl3 |
|---|---|---|---|---|---|---|---|
| ΔH (Kcal/mol) | |||||||
| 0.00 | 1:0 | 6.12 | 6.12 | 5.73 | 5.73 | 6.56 | 6.56 |
| 0.01 | 100:1 | 6.09 | 5.87 | 5.45 | 5.27 | 6.46 | 5.75 |
| 0.02 | 50:1 | 5.78 | 5.60 | 4.93 | 4.83 | 5.47 | 4.51 |
| 0.05 | 20:1 | 5.58 | 4.20 | 4.19 | 3.53 | 4.76 | 3.91 |
| 0.10 | 10:1 | 5.26 | 3.68 | 3.21 | |||
| -CH2 | C=O | |||
|---|---|---|---|---|
| Tm (°C) | SD | Tm (°C) | SD | |
| DMPC | 23.4 | 0.3 | 23.4 | 0.2 |
| DMPC/L | 23.5 | 0.3 | 23.9 | 0.3 |
| DMPC/[SmL2]Cl3 | 23.7 | 0.2 | 25.2 | 0.2 |
| DMPG | 23.4 | 0.2 | 25.8 | 0.6 |
| DMPG/L | 23.2 | 0.1 | 24.0 | 0.2 |
| DMPG/[SmL2]Cl3 | 23.5 | 0.2 | 25.2 | 0.4 |
| POPE | 25.3 | 0.4 | 25.5 | 0.2 |
| POPE/L | 24.7 | 0.3 | 25.5 | 0.2 |
| POPE/[SmL2]Cl3 | 24.1 | 0.3 | 25.8 | 0.3 |
| T (°C) | d (Å) | dHH (Å) | dB (Å) | dW (Å) | |
|---|---|---|---|---|---|
| DMPC | 8 | 68.6 | 39.6 | 51.6 | 17.1 |
| 15 | 67.8 | 39.0 | 51.0 | 16.8 | |
| 30 | 64.6 | 33.5 | 45.5 | 19.1 | |
| DMPC/L | 8 | 78.0 | 38.9 | 50.9 | 27.2 |
| 15 | 78.2 | 38.7 | 50.7 | 27.5 | |
| 30 | 70.4 | 33.8 | 45.8 | 24.7 | |
| DMPC/[SmL2]Cl3 | 8 | 79.5 | 37.7 | 49.7 | 29.8 |
| 15 | 79.1 | 35.0 | 47.0 | 32.1 | |
| 30 | 73.8 | 33.2 | 45.2 | 28.5 | |
| DMPG | 8 | 41.8 | 53.8 | ||
| 15 | 41.7 | 53.7 | |||
| 30 | 36.6 | 48.6 | |||
| DMPG/L | 8 | 43.9 | 55.9 | ||
| 15 | 43.1 | 55.1 | |||
| 30 | 38.7 | 50.7 | |||
| DMPG/[SmL2]Cl3 | 8 | 45.7 | 57.7 | ||
| 15 | 45.5 | 57.5 | |||
| 30 | 40.6 | 52.6 | |||
| POPE | 15 | 68.6 | 56.0 | 68.0 | 0.6 |
| 35 | 57.4 | 45.1 | 57.1 | 0.3 | |
| 70 | 69.5 | 36.2 | 48.2 | 21.3 | |
| POPE/L | 15 | 67.2 | 54.8 | 66.8 | 0.3 |
| 35 | 57.4 | 44.6 | 56.6 | 0.8 | |
| 70 | 69.9 | 34.4 | 46.4 | 23.5 | |
| POPE/[SmL2]Cl3 | 15 | 65.2 | 52.2 | 64.2 | 0.9 |
| 35 | 56.7 | 43.7 | 55.7 | 0.9 | |
| 70 | 66.8 | 36.2 | 48.2 | 18.6 |
| Molecule | CTP | PODTR | TDCP | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | RC | RI | Mouse | Rat | Rat | |||||||||
| MF | MM | RF | AM | SI | SS | OI | EC50 (mg/L) | LD50 (g/kg) | LOAEL (g/kg) | LC50 (mg/m3/h) | TD50 (mg/kg) | RMTD (g/kg) | ||
| L | C+ | C− | C− | M+ | Mild | NS | Mild | 2.74 | 0.013 | 0.201 | 2.55 × 103 | 48 | 22 | 0.215 |
| [SmL2]Cl3 | C+ | C− | C− | M− | NI | S | Mild | 0.0178 | 0.000418 | 0.0826 | 42.6 | 0.01 | 0.0292 | 0.0169 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aragón-Muriel, A.; Ausili, A.; Lima, L.S.; Santos, C.B.R.; Morales-Morales, D.; Polo-Cerón, D. Heteroaromatic Hybrid Benzimidazole/Oxadiazole (BZ/OZ) Ligand and Its Sm(III) Complex: Study of Their Antibacterial Activity, Toxicological Prediction and Interaction with Different Model Membranes. Biomolecules 2025, 15, 1568. https://doi.org/10.3390/biom15111568
Aragón-Muriel A, Ausili A, Lima LS, Santos CBR, Morales-Morales D, Polo-Cerón D. Heteroaromatic Hybrid Benzimidazole/Oxadiazole (BZ/OZ) Ligand and Its Sm(III) Complex: Study of Their Antibacterial Activity, Toxicological Prediction and Interaction with Different Model Membranes. Biomolecules. 2025; 15(11):1568. https://doi.org/10.3390/biom15111568
Chicago/Turabian StyleAragón-Muriel, Alberto, Alessio Ausili, Luciana Sampaio Lima, Cleydson B. R. Santos, David Morales-Morales, and Dorian Polo-Cerón. 2025. "Heteroaromatic Hybrid Benzimidazole/Oxadiazole (BZ/OZ) Ligand and Its Sm(III) Complex: Study of Their Antibacterial Activity, Toxicological Prediction and Interaction with Different Model Membranes" Biomolecules 15, no. 11: 1568. https://doi.org/10.3390/biom15111568
APA StyleAragón-Muriel, A., Ausili, A., Lima, L. S., Santos, C. B. R., Morales-Morales, D., & Polo-Cerón, D. (2025). Heteroaromatic Hybrid Benzimidazole/Oxadiazole (BZ/OZ) Ligand and Its Sm(III) Complex: Study of Their Antibacterial Activity, Toxicological Prediction and Interaction with Different Model Membranes. Biomolecules, 15(11), 1568. https://doi.org/10.3390/biom15111568










