Comprehensive Identification and Abscisic Acid-Responsive Expression Profiling of NAC Transcription Factor in Triterpenoid Saponin in Hedera helix
Abstract
1. Introduction
2. Materials and Methods
2.1. Acquisition and Experimental Treatments of H. helix Plants
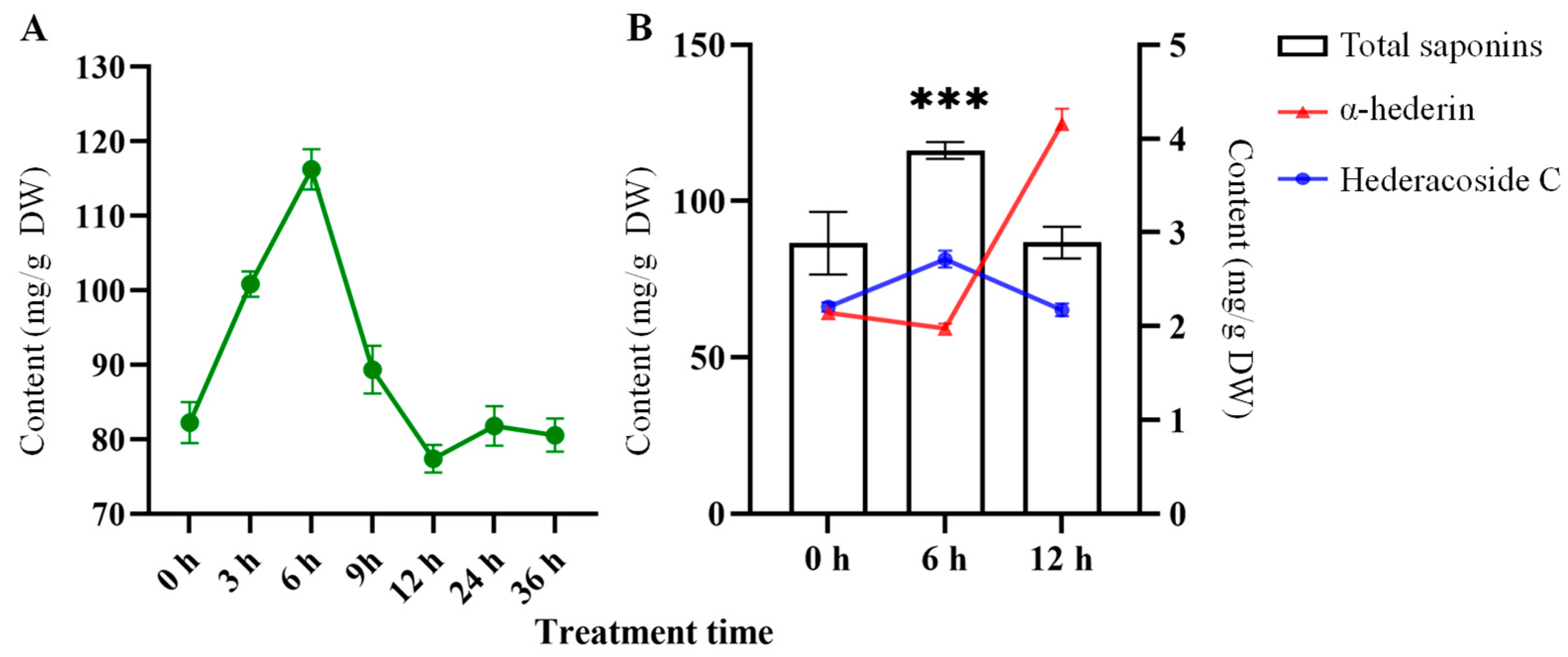
2.2. Quantification and Composition of Triterpenoid Saponins in H. helix
2.3. Transcriptome Sequencing and Bioinformatic Analyses
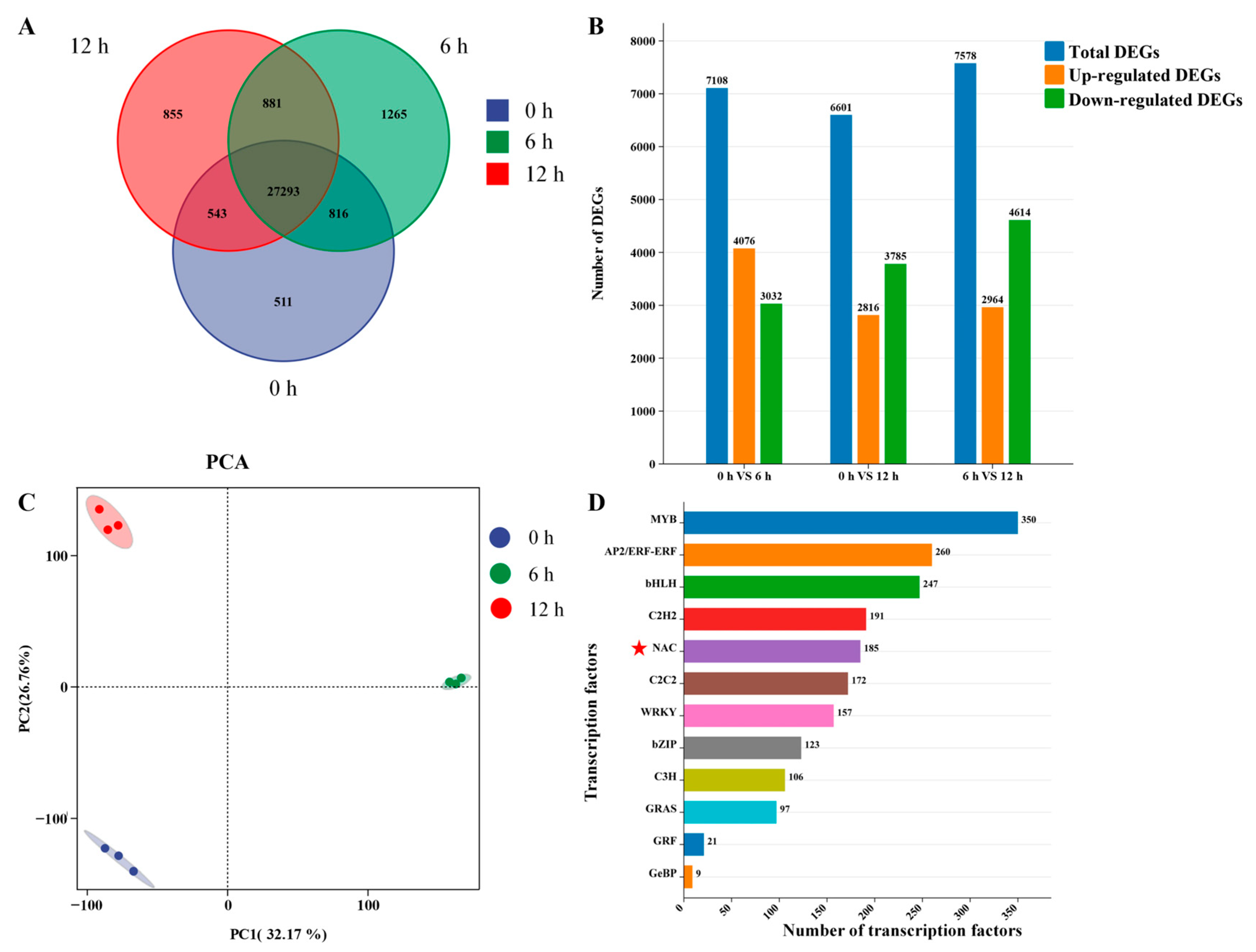
2.4. Identification of DEGs and PCA Plot
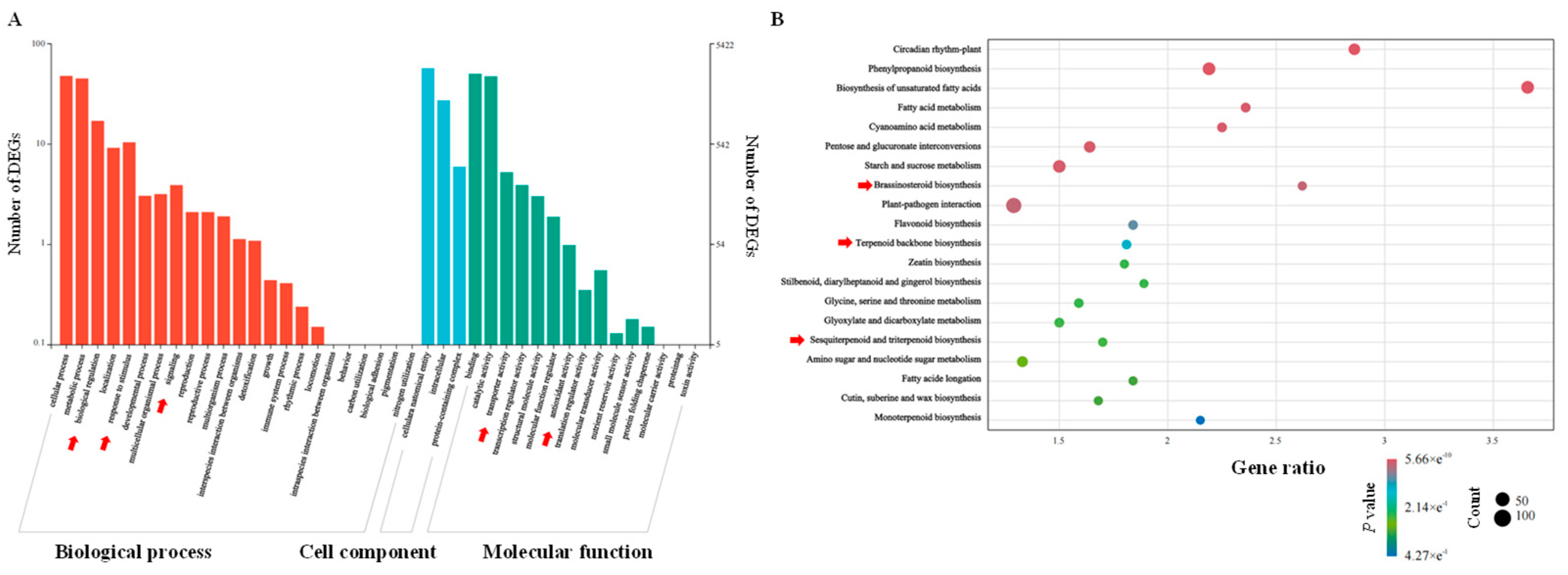
2.5. Functional Enrichment and Pathway Analysis
2.6. Comprehensive Identification of NAC Family Members in H. helix
2.7. Phylogenetic Tree, and Multi-Species Collinearity Analysis of HhNAC Proteins
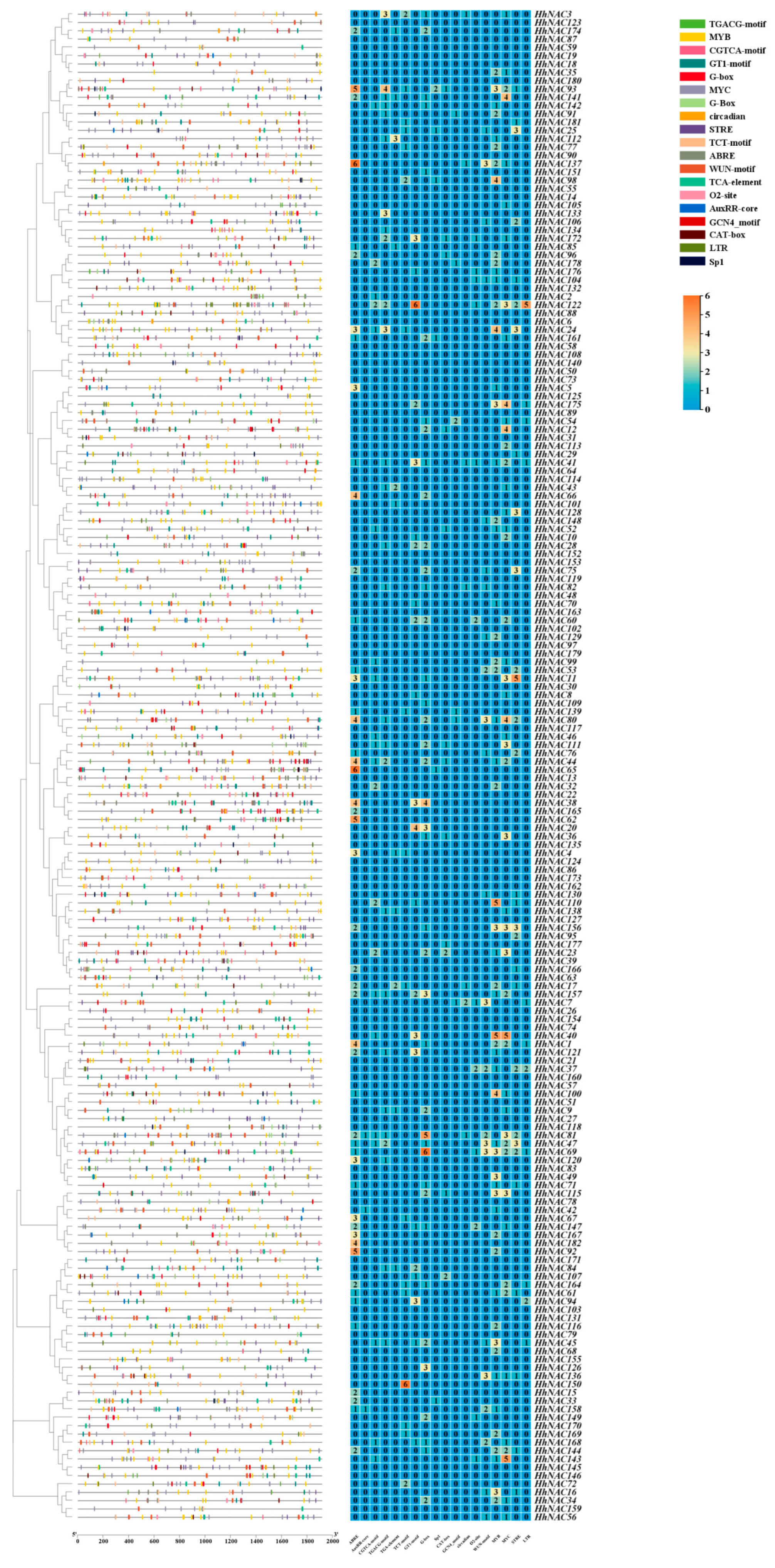
2.8. Exon-Intron Structure, Conserved Motifs, and Cis-Regulatory Elements Analyses
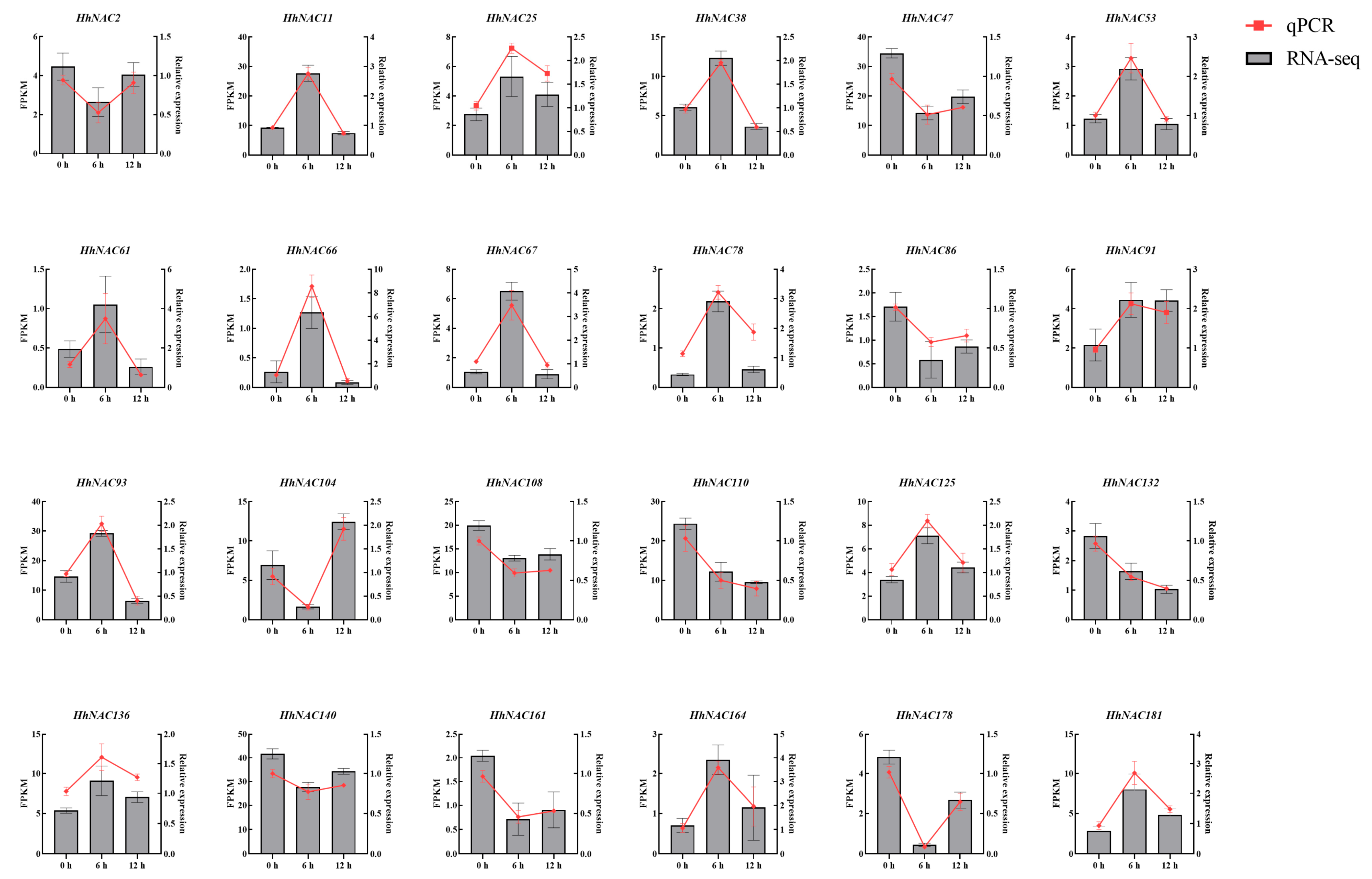
2.9. Total RNA Extraction and qPCR Analysis
3. Results
3.1. Saponin Accumulation in H. helix Under Exogenous ABA Treatment
3.2. Transcriptome Analysis of H. helix Under Exogenous ABA Treatment
3.3. Transcription Factors Exhibiting Differential Expression in Response to Exogenous ABA
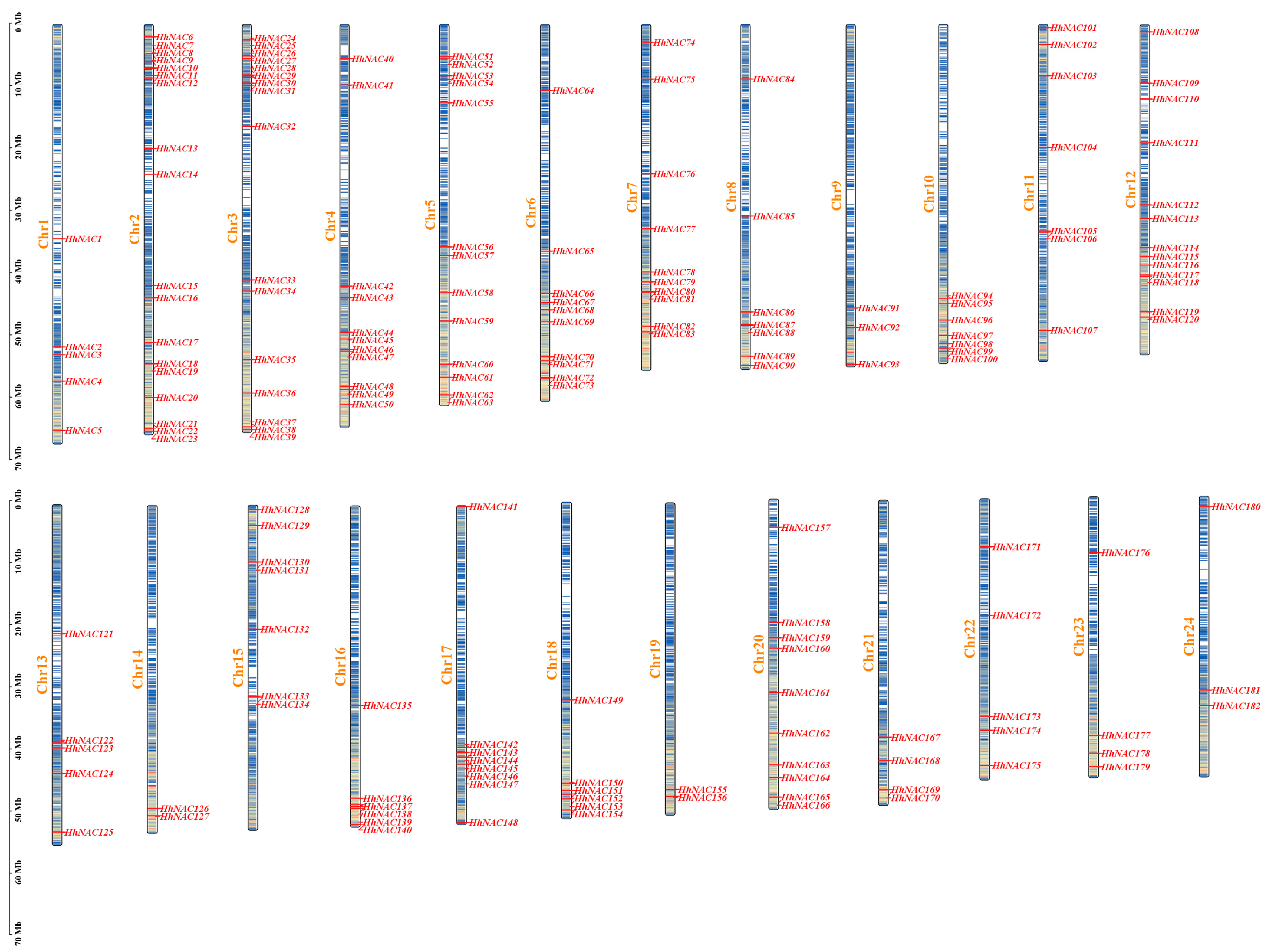
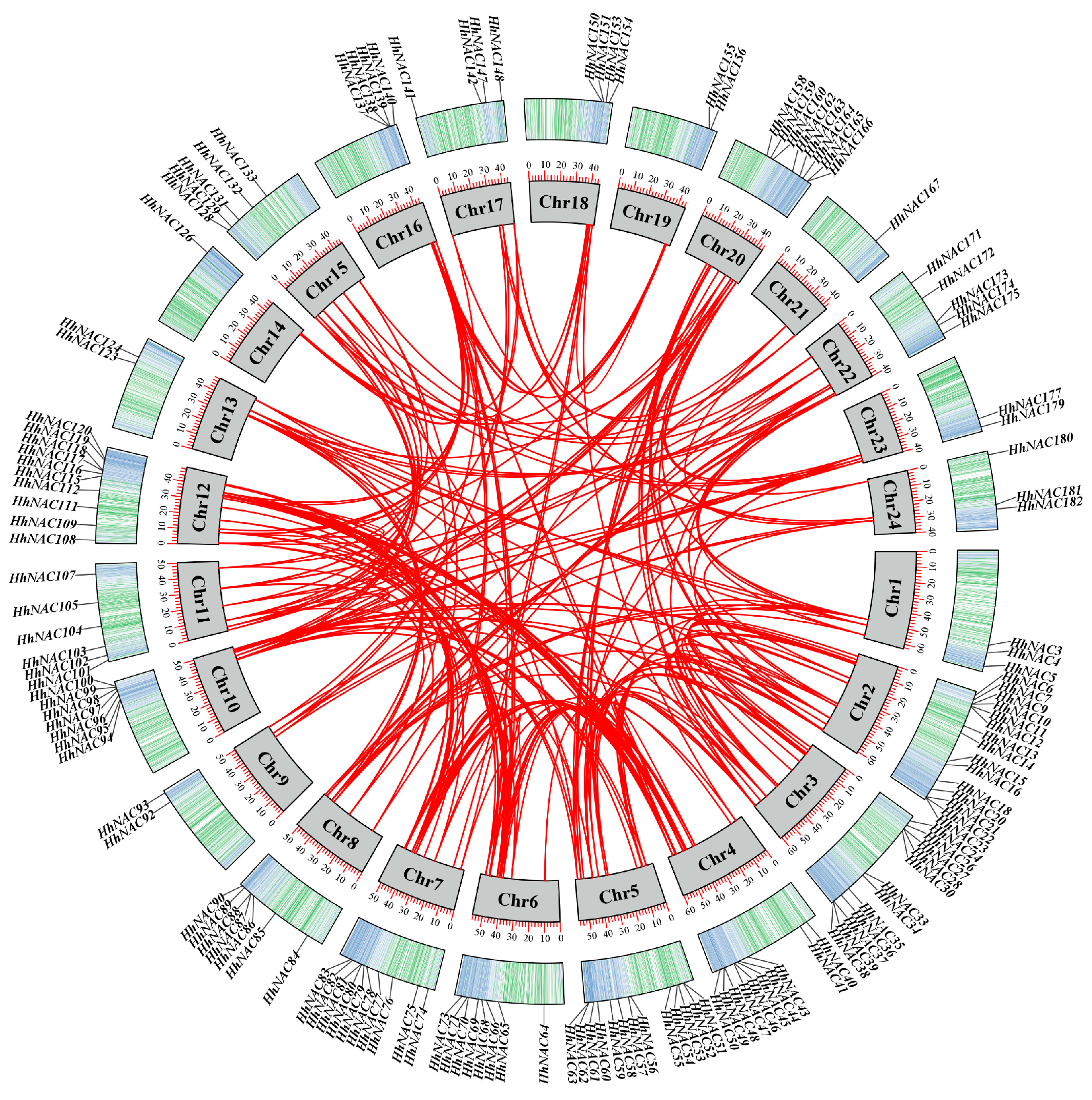
3.4. Identification and Chromosome Localization of NAC TF in H. helix
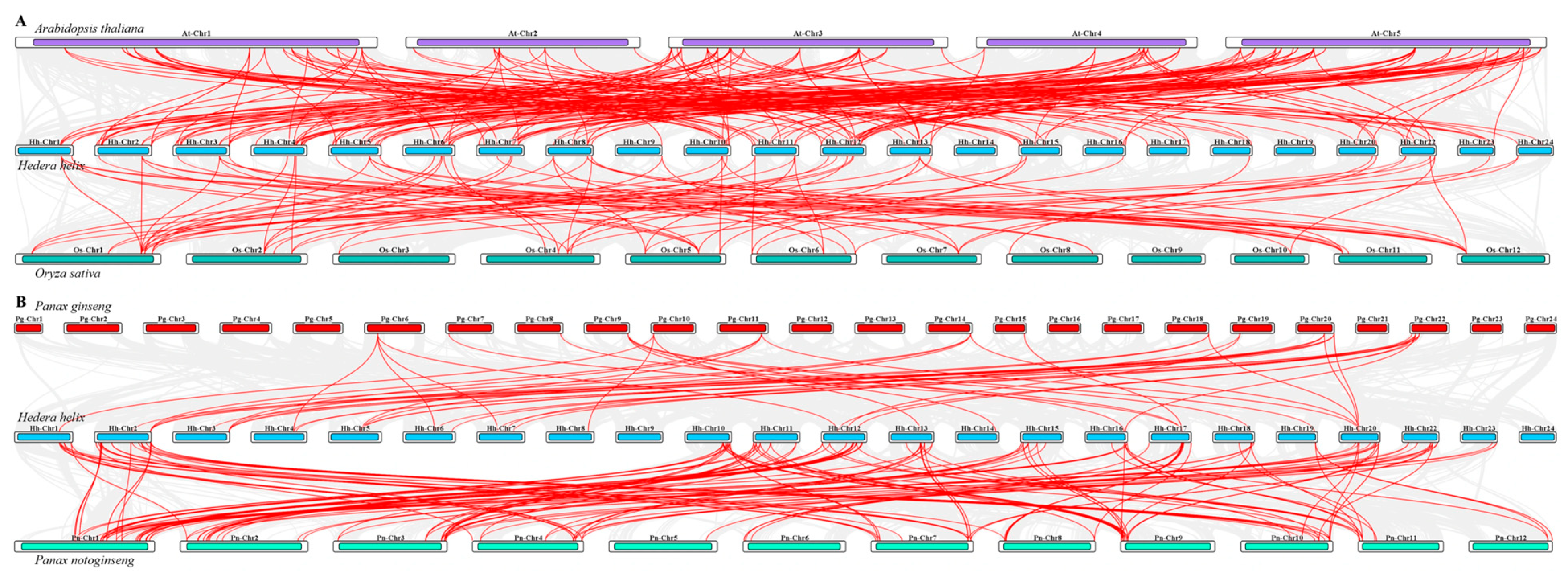
3.5. Collinearity Analysis of HhNAC TF in H. helix
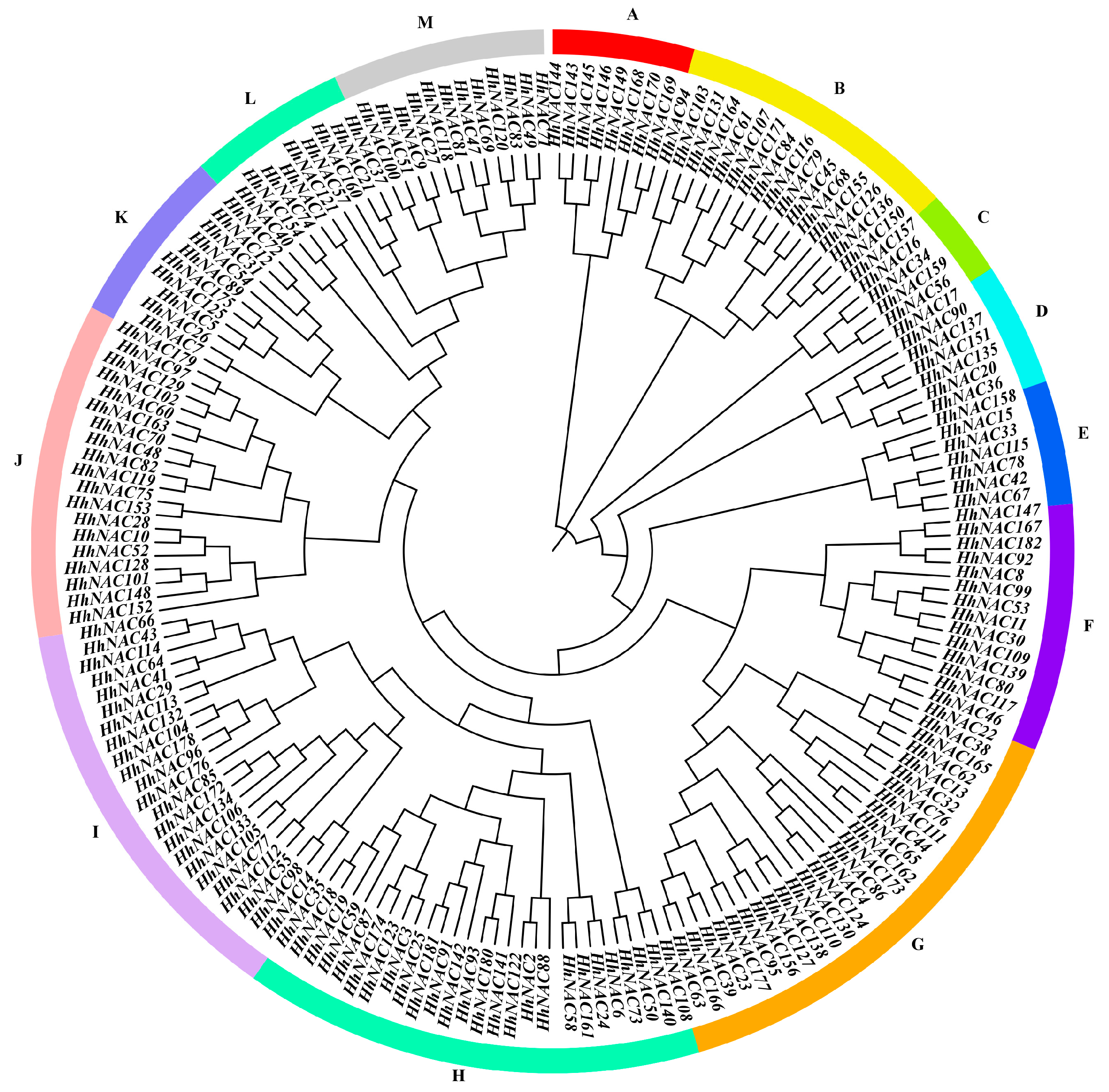
3.6. Phylogenetic Analysis of HhNACs and Other Homologous NAC Proteins
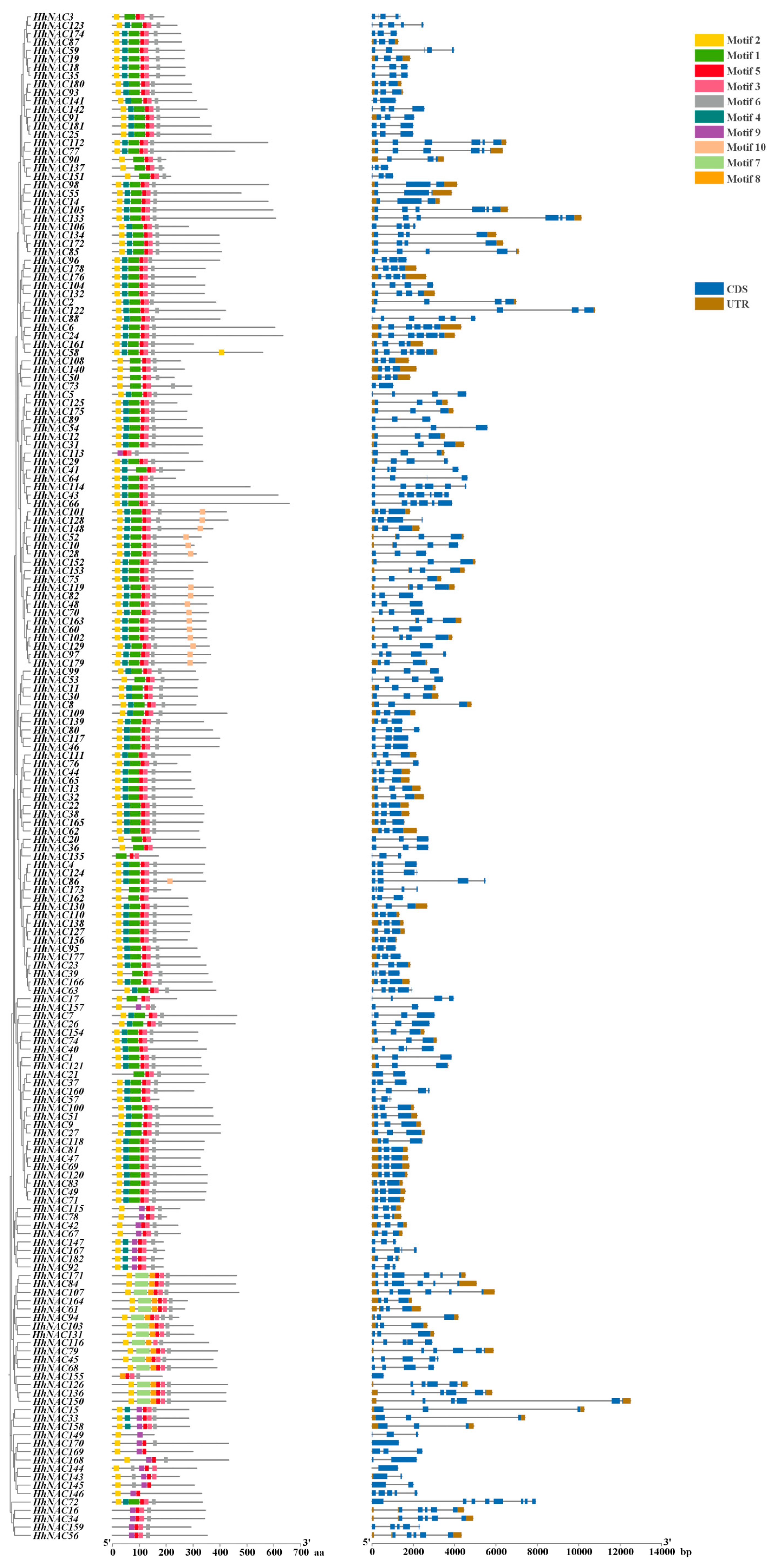
3.7. Conserved Motifs and Gene Architecture of HhNAC Family Members
3.8. Analysis of Cis-Regulatory Elements in the Promoter Sequences of HhNACs
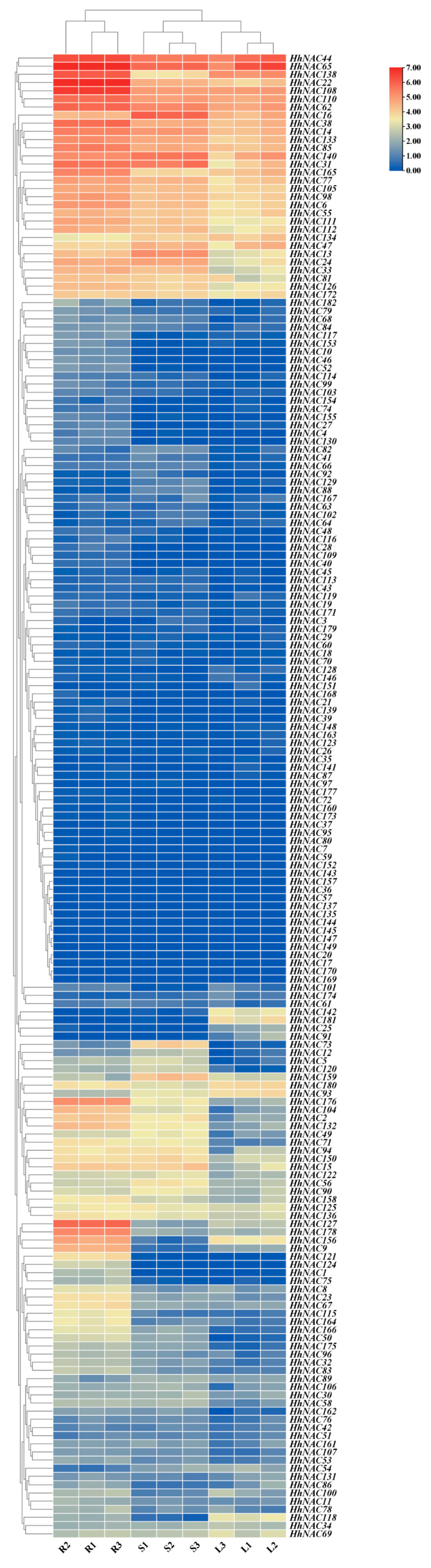
3.9. Expression Profiling of HhNACs in H. helix Different Organs
3.10. Expression Profiling of HhNACs Under ABA Treatment
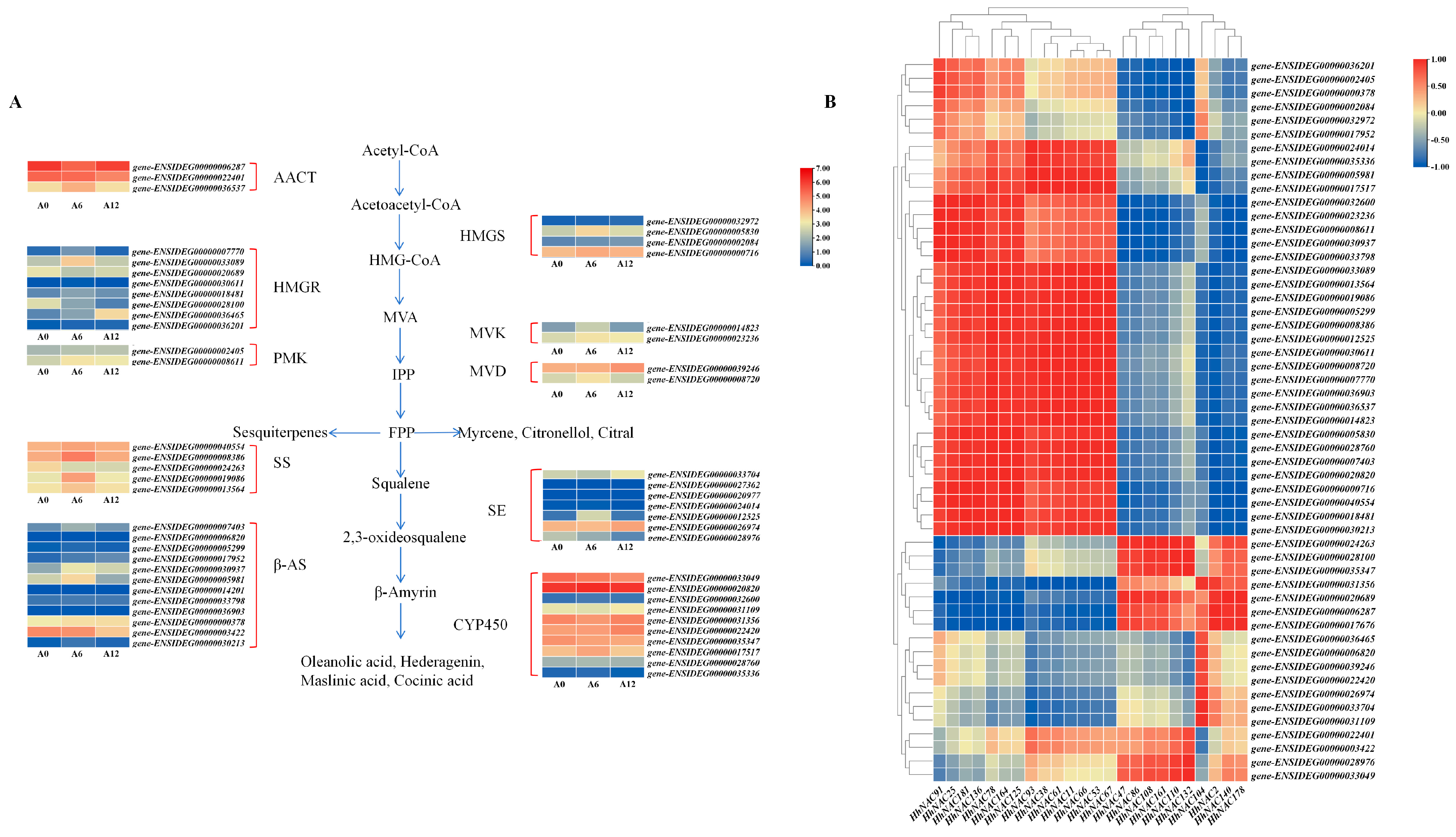
3.11. Expression Profiling of Saponin Biosynthetic Genes Under ABA Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bezruk, I.; Marksa, M.; Georgiyants, V.; Ivanauskas, L.; Raudone, L. Phytogeographical profiling of ivy leaf (Hedera helix L.). Ind. Crops Prod. 2020, 154, 112713. [Google Scholar] [CrossRef]
- Belmehdi, O.; Taha, D.; Abrini, J.; Ming, L.C.; Khalid, A.; Abdalla, A.N.; Algarni, A.S.; Hermansyah, A.; Bouyahya, A. Anticancer properties and mechanism insights of α-hederin. Biomed. Pharmacother. 2023, 165, 115205. [Google Scholar] [CrossRef]
- Sierocinski, E.; Holzinger, F.; Chenot, J.F. Ivy leaf (Hedera helix) for acute upper respiratory tract infections: An updated systematic review. Eur. J. Clin. Pharmacol. 2021, 77, 1113–1122. [Google Scholar] [CrossRef]
- Leong, F.; Hua, X.; Wang, M.; Chen, T.; Song, Y.; Tu, P.; Chen, X.J. Quality standard of traditional Chinese medicines: Comparison between European pharmacopoeia and Chinese pharmacopoeia and recent advances. Chin. Med. 2020, 15, 76. [Google Scholar] [CrossRef]
- Pham, H.N.; Tran, C.A.; Trinh, T.D.; Nguyen, T.N.L.; Tran, P.H.N.; Le, V.N.; Le, N.H.; Phung, V.T. UHPLC-Q-TOF-MS/MS dereplication to identify chemical constituents of Hedera helix leaves in Vietnam. J. Anal. Methods. Chem. 2022, 8, 1167265. [Google Scholar] [CrossRef]
- Yao, L.; Lu, J.; Wang, J.; Gao, W.Y. Advances in biosynthesis of triterpenoid saponins in medicinal plants. Chin. J. Nat. Med. 2020, 18, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.J.; Wang, D.K.; Wu, K.J.; Wang, F.F.; Yang, Q.W.; Han, R.L.; Liang, Z.S.; Jia, Q.J. Cloning and characterization of the gene encoding HMGS synthase in Polygonatum sibiricum. Biomed. Res. Int. 2022, 7, 7441296. [Google Scholar] [CrossRef]
- Ye, J.; Xie, Y.; Zhang, P.; Khan, A.; Zhou, Z.; Liu, L. Chemical composition and pharmacological activity of seco-prezizaane-type sesquiterpenes. Chin. Herb. Med. 2024, 16, 70–81. [Google Scholar]
- Bian, X.Y.; Wu, T.K.; Qiang, R.R.; Deng, Z.; Rehman, F.; Han, Q.Y.; Xu, D.; Yuan, Y.; Wang, X.B.; An, Z.W.; et al. Genome-wide identification and expression profile of farnesyl pyrophosphate synthase (FPS) gene family in Euphorbia hirta L. Int. J. Mol. Sci. 2025, 18, 798. [Google Scholar] [CrossRef]
- Dong, L.M.; Pollier, J.; Bassard, J.E.; Ntallas, G.; Almeida, A.; Lazaridi, E.; Khakimov, B.; Arendt, P.; de Oliveira, L.S.; Lota, F.; et al. Co-expression of squalene epoxidases with triterpene cyclases boosts production of triterpenoids in plants and yeast. Metab. Eng. 2018, 49, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dinday, S.; Ghosh, S. Recent advances in triterpenoid pathway elucidation and engineering. Biotechnol. Adv. 2023, 68, 108214. [Google Scholar] [CrossRef]
- Wei, Y.S.; Javed, T.; Liu, T.T.; Ali, A.; Gao, S.J. Mechanisms of abscisic acid (ABA)-mediated plant defense responses: An updated review. Plant Stress 2025, 15, 100724. [Google Scholar] [CrossRef]
- Wang, H.; Hu, S.Q.; Li, T.; Qu, X.J.; Zhang, J.Q.; Wang, B.S.; Sun, Y.X.; Cao, R.T.; Yan, Y.T.; Song, Z.; et al. Comparative transcriptome analysis reveals abscisic acid-induced bHLH transcription factors involved in saikosaponin biosynthesis in Bupleurum chinense DC. Plant Signal. Behav. 2025, 20, 2495301. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, Y.; Hu, Z.X.; Li, J.R. An HD-ZIP I transcription factor DZHDZ32 upregulates diosgenin biosynthesis in Dioscorea zingiberensis. Int. J. Mol. Sci. 2025, 26, 4185. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, Q.; Zeng, Z.X.; Wang, Y.; Zhao, M.Z.; Wang, K.Y.; Zhang, M.P. The AP2/ERF transcription factor PgERF120 regulates ginsenoside biosynthesis in ginseng. Biomolecules 2024, 14, 345. [Google Scholar] [CrossRef]
- Man, J.H.; Shi, Y.; Huang, Y.Y.; Zhang, X.Q.; Wang, X.; Liu, S.H.; He, G.J.; An, K.L.; Han, D.R.; Wang, X.H.; et al. PnMYB4 negatively modulates saponin biosynthesis in Panax notoginseng through interplay with PnMYB1. Hortic. Res. 2023, 10, uhad134. [Google Scholar] [CrossRef]
- Xiong, H.Y.; He, H.D.; Chang, Y.; Miao, B.B.; Liu, Z.W.; Wang, Q.Q.; Dong, F.M.; Xiong, L.Z. Multiple roles of NAC transcription factors in plant development and stress responses. J. Integr. Plant. Biol. 2025, 67, 510–538. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.J.; Sun, S.M.; Han, B.; Yang, X.Y. NAC domain transcription factor gene GhNAC3 confers drought tolerance in plants. Plant Physiol. Biochem. 2023, 195, 114–123. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, M.Z.; Ma, H.D.; Zhang, Y.; Liu, Q.; Liu, S.Z.; Wang, Y.F.; Wang, K.Y.; Zhang, M.P.; Wang, Y. The NAC transcription factor PgNAC41-2 gene involved in the regulation of ginsenoside biosynthesis in Panax ginseng. Int. J. Mol. Sci. 2023, 24, 11946. [Google Scholar] [CrossRef]
- Geng, L.F.; Yu, S.; Zhang, Y.C.; Su, L.; Lu, W.P.; Zhu, H.; Jiang, X.Q. Transcription factor RcNAC091 enhances rose drought tolerance through the abscisic acid-dependent pathway. Plant Physiol. 2023, 193, 1695–1712. [Google Scholar] [CrossRef]
- Yang, D.Y.; Chen, Y.Z.; Wang, R.; He, Y.M.; Ma, X.F.; Shen, J.C.; He, Z.L.; Lai, H.G. Effects of exogenous abscisic acid on the physiological and biochemical responses of Camellia oleifera seedlings under drought stress. Plants 2024, 13, 225. [Google Scholar] [CrossRef]
- Jiang, T.; Zhang, Y.; Zuo, G.G.; Luo, T.; Wang, H.; Zhang, R.; Luo, Z.Y. Transcription factor PgNAC72 activates DAMMARENEDIOL SYNTHASE expression to promote ginseng saponin biosynthesis. Plant Physiol. 2024, 195, 2952–2969. [Google Scholar] [CrossRef]
- Ni, L.J.; Zhu, G.M.; Gao, L.L.; Hu, J.N.; Jin, H.T.; Jin, Z.W. HPLC-MS/MS analysis of chemical constituents in Hedera nepalensisvar and Hedera helix. Chin. Trad. Pat. Med. 2021, 43, 1094–1099. [Google Scholar]
- Liu, S.Y.; Wang, Z.T.; Zhu, R.H.; Wang, F.Y.; Cheng, Y.X.; Liu, Y.Q. Three differential expression analysis methods for RNA sequencing: Limma, edgeR, dESeq2. J. Vis. Exp. 2021, 18, 175. [Google Scholar]
- Bu, D.C.; Luo, H.T.; Huo, P.P.; Wang, Z.H.; Zhang, S.; He, Z.H.; Wu, Y.; Zhao, L.H.; Liu, J.J.; Guo, J.; et al. KOBAS-i: Intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis. Nucleic Acids Res. 2021, 49, W317–W325. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.H.; Xu, J.; Liu, Y.L.; Feng, J.T.; Chen, H.; He, Y.H.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Xu, Z.T.; Zheng, F.X.; Deng, X.J.; Sun, Y.M.; Yu, Z.M.; Shen, X.X. Genome-wide identification and analysis of GATA gene family in Dendrobium officinale under methyl jasmonate and salt stress. Plants 2025, 14, 1576. [Google Scholar] [CrossRef]
- Zheng, F.X.; Xu, Z.T.; Deng, X.J.; Wang, X.Y.; Sun, Y.M.; Shen, X.X.; Yu, Z.M. Genome-wide transcriptome analysis reveals GRF transcription factors involved in methyl jasmonate-induced flavonoid biosynthesis in Hedera helix. Plants 2025, 14, 2094. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zou, J.; Hao, Z.; Gao, M.; Zhang, G.; Liu, M. Identification and functional characterization of a new flavonoid glycosyltransferase from Rheum palmatum. Chin. Herb. Med. 2025, 17, 307-314. [Google Scholar]
- Wang, S.; Zhao, X.L.; Li, C.; Dong, J.; Ma, J.C.; Long, Y.H.; Xing, Z.B. DNA methylation regulates the secondary metabolism of saponins to improve the adaptability of Eleutherococcus senticosus during drought stress. BMC Genom. 2024, 25, 330. [Google Scholar] [CrossRef]
- Bezruk, I.; Materiienko, A.; Gubar, S.; Proskurina, K.; Budanova, L.; Ivanauskas, L.; Georgiyants, V. Estimation of the influence of the environmental factors on the accumulation of phytochemicals and antioxidant capacity in the ivy leaves (Hedera helix L.). Nat. Prod. Res. 2022, 36, 1014–1019. [Google Scholar] [CrossRef]
- Kong, L.Y.; Chen, P.; Chang, C. Drought resistance and ginsenosides biosynthesis in response to abscisic acid in Panax ginseng C. A. Meyer. Int. J. Mol. Sci. 2023, 24, 9194. [Google Scholar] [CrossRef]
- Lu, L.; Fang, J.B.; Xia, N.; Zhang, J.; Diao, Z.J.; Wang, X.; Liu, Y.; Tang, D.Z.; Li, S.P. Phosphorylation of the transcription factor OsNAC29 by OsMAPK3 activates diterpenoid genes to promote rice immunity. Plant Cell 2024, 37, koae320. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Kim, S.Y.; Hyeon, D.Y.; Kim, D.H.; Dong, T.; Park, Y.; Jin, J.B.; Joo, S.H.; Kim, S.K.; Hong, J.C.; et al. The Arabidopsis NAC transcription factor ANAC096 cooperates with bZIP-type transcription factors in dehydration and osmotic stress responses. Plant Cell 2013, 25, 4708–4724. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.Q.; Xu, Y.N.; Sun, Y.Q.; Li, N.N.; Zhang, S.Y.; Li, G.L. Identification of the NTL gene family in Beta vulgaris L. and functional role of BvNTL2 in drought resistance. Plants 2025, 14, 1528. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.L.; Liu, D.F.; Luo, W.; Li, Z.T.; Feng, J.L.; Guo, Y.L.; Chong, K.; Xu, Y.Y. Domestication-selected COG4-OsbZIP23 module regulates chilling tolerance in rice. Cell Rep. 2024, 43, 114965. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, X.; Zheng, F.; Xu, Z.; Mao, X.; Yu, Z.; Shen, X. Comprehensive Identification and Abscisic Acid-Responsive Expression Profiling of NAC Transcription Factor in Triterpenoid Saponin in Hedera helix. Biomolecules 2025, 15, 1557. https://doi.org/10.3390/biom15111557
Deng X, Zheng F, Xu Z, Mao X, Yu Z, Shen X. Comprehensive Identification and Abscisic Acid-Responsive Expression Profiling of NAC Transcription Factor in Triterpenoid Saponin in Hedera helix. Biomolecules. 2025; 15(11):1557. https://doi.org/10.3390/biom15111557
Chicago/Turabian StyleDeng, Xiaoji, Feixiong Zheng, Zhangting Xu, Xiaoping Mao, Zhenming Yu, and Xiaoxia Shen. 2025. "Comprehensive Identification and Abscisic Acid-Responsive Expression Profiling of NAC Transcription Factor in Triterpenoid Saponin in Hedera helix" Biomolecules 15, no. 11: 1557. https://doi.org/10.3390/biom15111557
APA StyleDeng, X., Zheng, F., Xu, Z., Mao, X., Yu, Z., & Shen, X. (2025). Comprehensive Identification and Abscisic Acid-Responsive Expression Profiling of NAC Transcription Factor in Triterpenoid Saponin in Hedera helix. Biomolecules, 15(11), 1557. https://doi.org/10.3390/biom15111557