Re-Examination of Inflammation in Major Depressive Disorder: Bridging Systemic and Neuroinflammatory Insights
Abstract
1. Introduction
2. Inflammatory Responses in Human MDD Patients
2.1. Systemic Immune Responses in MDD Patients
| Author | Subject Condition | Inflammatory Marker | Results |
|---|---|---|---|
| Foley et al. [13] | Meta-analysis: depression diagnosed clinically | Peripheral blood composition. | Increased counts of total white blood cells, monocytes, neutrophils, granulocytes, natural killer cells, CD19+ B cells, and CD4+ T helper cells. Decreased percentage of lymphocytes. |
| Alvaarez-Mon et al. [22] | Psychiatrist-confirmed MDD with a minimum score of 14 points on the Hamilton Rating Scale for Depression, aged between 18 and 65. | Peripheral CD4+ T lymphocyte subset distribution, activation, and differentiation states | Increased serum levels of IL-17 and TNF-α, and increased Th17 differentiation in circulating CD4+ T cells of MDD patients. |
| Li et al. [23] | Clinically diagnosed with MDD for the first time, and without previous use of antidepressants. | T helper cell cytokine and CD4+CD25+ T regulatory cell levels | Increased Th1/Th2 ratio and decreased CD4+CD25+ T regulatory cell levels. |
| Tannous et al. [18] | Volunteers aged between 18 and 65 meeting the DSM-IV diagnosis of MDD. | CRP, IL-6 levels in the blood | Significantly higher IL-6 levels and a trend for increased CRP levels. |
| Pitharouli et al. [24] | Diagnosed with MDD from the Composite International Diagnostic Interview | Serum CRP level | Significantly higher levels of CRP. Depression is significantly associated with increased Log CRP levels. |
| Gattaneo et al. [25] | Patients aged between 19 and 72, suffering from moderate to severe unipolar depression. | IL-1α, IL-1β, IL-4, IL-6, IL-7, IL-8, IL-10, MIF, and TNF-α levels in the blood. | Higher mRNA levels of IL-1β, IL-6, MIF, and TNF-α. Lower levels of IL-4. No significant change in IL-1α, IL-7, IL-8, and IL-10. Antidepressant non-responders have higher baseline mRNA levels of IL-1β, MIF, and TNF-α. |
| Yin et al. [19] | Patients aged between 18 and 72, and first-time diagnosed with depression according to ICD-10. | IL-1, IL-6, IL-10, TNF-α, and hs-CRP in the peripheral blood. | Significantly higher levels of IL-1, IL-6, IL-10, TNF-α, and hs-CRP. |
| Kobayashi et al. [30] | Diagnosed with MDD using DSM-IV criteria by psychiatrists. | Blood mRNA levels of IL-1β, IL-6, TNF-α, IL-10, IL-1RA, SOCS1, SOCS2, and SOCS3. Blood protein levels of IL-1β and IL-6. | mRNA levels of SOCS1, SOCS2 and SOCS3 are decreased. No increase in IL-1β, IL-6, TNF-α, IL-10, or IL-1RA. |
| Galecka et al. [21] | Patients aged between 20 and 67 and diagnosed with MDD according to ICD-10. | Serum protein and RNA levels of IL-17, IL-21, IL-23, IL-35, and Foxp3. | Gene expression levels of IL-17, IL-21, IL-23, and IL-35 are significantly increased. |
| Merendino et al. [20] | Female patients suffering from moderate-severe depression. | Serum IL-18 and CD30. | Significantly higher IL-18 levels and no change in CD30 levels. |
2.2. Neuroimmune Responses in MDD Patients
2.2.1. Neuroimaging and Cerebrospinal Fluid (CSF) Cytokine Profile in MDD Patients
2.2.2. Cytokine Expression and Glia Activation in Postmortem Brains
| Author | Tissue Type | Inflammatory Marker | Results |
|---|---|---|---|
| Holmes et al. [33] | PET study | TSPO | TSPO availability significantly increased in the anterior cingulate cortex. |
| Setiawan et al. [42] | PET study | TSPO | TSPO distribution volume significantly increased during major depressive episodes. |
| Hannestad et al. [34] | PET study | TSPO | No significant difference in TSPO levels. |
| Sasayama et al. [44] | CSF | IL-6 | Significantly higher levels of IL-6. |
| Hestad et al. [45] | CSF | IL-6, TNF-α, and MCP-1 | No significant difference in IL-6, TNF-α, or MCP-1 levels. |
| Torres-Platas et al. [37] | Post-mortem (dorsal anterior cingulate cortex) | MCP-1, IBA-1 | Gene expression of IBA1 and MCP-1 significantly increased. IBA1-positive microglia density did not change, but increased proportion of primed microglia. |
| Dean et al. [38] | Post-mortem (frontal cortex) | Transmembrane TNF and soluble TNF | Transmembrane TNF is significantly increased, but no change in soluble TNF. |
| Clark et al. [39] | Post-mortem (ventrolateral prefrontal cortex) | IL-1β, IL-2, IL-4, IL-5, IL-6, IL-13, IL-33, IFN-γ, TNF-α, CCL2, COX2. | Significantly decreased levels of IL-33, IFN-γ, and TNF-α. Expression levels of other cytokines did not change. |
| Pantazatos et al. [40] | Post-mortem (dorsal lateral prefrontal cortex) | IL-8, MCP-1 | Gene expression levels of IL-8 and MCP-1 significantly decreased. |
| Brisch et al. [46] | Post-mortem (dorsal raphe nucleus) | HLA-DR | No significant change in microglia density. |
| Cobb et al. [47] | Post-mortem (hippocampus) | GFAP | GFAP+ astrocyte density decreased in the left hippocampi. |
| Williams et al. [48] | Post-mortem (substantia nigra) | GFAP | No change in GFAP+ astrocyte. |
| Davis et al. [49] | Post-mortem (dorsolateral prefrontal cortex and the anterior cingulate cortex) | GFAP | Significantly increased GFAP reactivity in the dorsolateral prefrontal cortex layer I. |
| Hayashi et al. [50] | Post-mortem (frontopolar cortex) | Olig2 | The number of olig2+ nuclei is significantly reduced. |
| Rajkowska et al. [51] | Post-mortem (white matter from the ventral prefrontal cortex) | CNPase | No significant change in the density of CNPase+ oligodendrocytes. |
2.3. Inflammation and the Heterogeneity of MDD
2.4. The Desynchrony of Systemic and Neuroinflammation in MDD Patients
2.5. Possible Sources of Systemic Inflammation in MDD Patients
2.5.1. The HPA Axis
2.5.2. The Sympathetic Nervous System (SNS)
2.5.3. Gut Microbiota
2.5.4. “Pathogen Host Defense” Hypothesis
2.5.5. Damage-Associated Molecular Patterns (DAMPs)
3. Inflammatory Responses in the Rodent Model of Depression
3.1. Animal Studies Confirmed the Bidirectional Relationship Between Inflammation and Depression
3.2. Changes in Brain Lymphatic Function in Animal Models of Depression
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP | Adenosine triphosphate |
| AP | Area postrema |
| BBB | Blood–brain barrier |
| cAMP | Cyclic adenosine monophosphate |
| CCL2 | C-C motif chemokine ligand 2 |
| CD4 | Cluster of differentiation 4 |
| CD19 | Cluster of differentiation 19 |
| CD14 | Cluster of differentiation 14 |
| CD16 | Cluster of differentiation 16 |
| CD25 | Cluster of differentiation 25 |
| CD30 | Cluster of differentiation 30 |
| CD47 | Cluster of differentiation 47 |
| CD200 | CD200 molecule |
| CNPase | 2′,3′-cyclic-nucleotide 3′-phosphodiesterase |
| CMS | Chronic mild stress |
| CNS | Central nervous system |
| COX2 | cycloocygenase-2 |
| CP | Choroid plexus |
| CRP | C-reactive protein |
| CSF | Cerebrospinal fluid |
| CUMS | Chronic unpredictable mild stress |
| CX3CL1 | C-X3-C motif chemokine ligand 1 |
| CX3CR1 | C-X3-C motif chemokine receptor 1 |
| DAMP | Damage-associated molecular pattern |
| Foxp3 | Forkhead box protein P3 |
| GFAP | Glial fibrillary acidic protein |
| GR | Glucocorticoid receptor |
| HLA-DR | Human leukocyte antigen, DR isotypes |
| HMGB1 | High mobility group box 1 |
| HPA | Hypothalamic–pituitary–adrenal |
| hs-CRP | high-sensitivity C-reactive protein |
| HSP | Heat shock protein |
| IBA1 | Ionized calcium-binding adapter molecule 1 |
| IFN-α | Interferon alfa |
| IFN-γ | Interferon gamma |
| IL-1α | Interleukin-1α |
| IL-1β | Interleukin-1β |
| IL-1RA | Interleukin 1 receptor antagonist |
| IL-2 | Interleukin-2 |
| IL-4 | Interleukin-4 |
| IL-5 | Interleukin-5 |
| IL-6 | Interleukin-6 |
| IL-7 | Interleukin-7 |
| IL-8 | Interleukin-8 |
| IL-10 | Interleukin-10 |
| IL-12 | Interleukin-12 |
| IL-13 | Interleukin-13 |
| IL-17 | Interleukin-17 |
| IL-18 | Interleukin-18 |
| IL-21 | Interleukin-21 |
| IL-23 | Interleukin-23 |
| IL-33 | Interleukin-33 |
| IL-35 | Interleukin-35 |
| IP-10 | Interferon-inducible protein 10 |
| LPS | Lipopolysaccharide |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MDD | Major depressive disorder |
| MIP-1β | macrophage inflammatory protein 1 beta |
| MRI | Magnetic resonance imaging |
| NF-κB | Nuclear factor kappa B |
| Olig2 | Oligodendrocyte transcription factor 2 |
| PET | Positron emission tomography |
| PNS | Parasympathetic nervous system |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| RAGE | Receptor for advanced glycation end products |
| SCFA | Short-chain fatty acids |
| SFO | Subfornical organ |
| SNS | Sympathetic nervous system |
| SOCS1 | Suppressor of cytokine signaling 1 |
| SOCS2 | Suppressor of cytokine signaling 2 |
| SOCS3 | Suppressor of cytokine signaling 3 |
| SSRI | Selective serotonin reuptake inhibitor |
| Th1/Th2 | T helper type 1 (Th1) and T helper type 2 (Th2) |
| Th17 | T helper 17 cell |
| TLR | Toll-like receptor |
| TNF-α | Tumor necrosis factor-alpha |
| TSPO | Translocator protein |
| VOLT | Vascular organ of lamina terminalis |
References
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef]
- Brigitta, B. Pathophysiology of depression and mechanisms of treatment. Dialogues Clin. Neurosci. 2002, 4, 7–20. [Google Scholar] [CrossRef]
- Al-Harbi, K.S. Treatment-resistant depression: Therapeutic trends, challenges, and future directions. Patient Prefer. Adherence 2012, 6, 369–388. [Google Scholar] [CrossRef] [PubMed]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A meta-analysis of cytokines in major depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Raison, C.L.; Miller, A.H. Is Depression an Inflammatory Disorder? Curr. Psychiatry Rep. 2011, 13, 467–475. [Google Scholar] [CrossRef]
- Enache, D.; Pariante, C.M.; Mondelli, V. Markers of central inflammation in major depressive disorder: A systematic review and meta-analysis of studies examining cerebrospinal fluid, positron emission tomography and post-mortem brain tissue. Brain Behav. Immun. 2019, 81, 24–40. [Google Scholar] [CrossRef] [PubMed]
- Mazza, M.G.; De Lorenzo, R.; Conte, C.; Poletti, S.; Vai, B.; Bollettini, I.; Melloni, E.M.T.; Furlan, R.; Ciceri, F.; Rovere-Querini, P.; et al. Anxiety and depression in COVID-19 survivors: Role of inflammatory and clinical predictors. Brain Behav. Immun. 2020, 89, 594–600. [Google Scholar] [CrossRef]
- Zainal, N.H.; Newman, M.G. Inflammation mediates depression and generalized anxiety symptoms predicting executive function impairment after 18 years. J. Affect. Disord. 2022, 296, 465–475. [Google Scholar] [CrossRef]
- Beurel, E.; Toups, M.; Nemeroff, C.B. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron 2020, 107, 234–256. [Google Scholar] [CrossRef]
- Jiao, W.; Lin, J.; Deng, Y.; Ji, Y.; Liang, C.; Wei, S.; Jing, X.; Yan, F. The immunological perspective of major depressive disorder: Unveiling the interactions between central and peripheral immune mechanisms. J. Neuroinflamm. 2025, 22, 10. [Google Scholar] [CrossRef]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef]
- Duivis, H.E.; Vogelzangs, N.; Kupper, N.; de Jonge, P.; Penninx, B.W.J.H. Differential association of somatic and cognitive symptoms of depression and anxiety with inflammation: Findings from the Netherlands Study of Depression and Anxiety (NESDA). Psychoneuroendocrinology 2013, 38, 1573–1585. [Google Scholar] [CrossRef]
- Foley, É.M.; Parkinson, J.T.; Mitchell, R.E.; Turner, L.; Khandaker, G.M. Peripheral blood cellular immunophenotype in depression: A systematic review and meta-analysis. Mol. Psychiatry 2023, 28, 1004–1019. [Google Scholar] [CrossRef]
- Maes, M.; Van der Planken, M.; Stevens, W.J.; Peeters, D.; DeClerck, L.S.; Bridts, C.H.; Schotte, C.; Cosyns, P. Leukocytosis, monocytosis and neutrophilia: Hallmarks of severe depression. J. Psychiatr. Res. 1992, 26, 125–134. [Google Scholar] [CrossRef]
- Álvarez Casiani, R.I.; Grendas, L.N.; Olaviaga, A.; Chiapella, L.C.; Arena, Á.R.; Tifner, V.; Prokopez, C.R.; López-Carvajal, J.E.; Robetto, J.; Carrera Silva, E.A.; et al. Monocyte profiles and their association with depression severity and functional disability. J. Psychiatr. Res. 2025, 184, 272–278. [Google Scholar] [CrossRef]
- Baumeister, D.; Russell, A.; Pariante, C.M.; Mondelli, V. Inflammatory biomarker profiles of mental disorders and their relation to clinical, social and lifestyle factors. Soc. Psychiatry Psychiatr. Epidemiol. 2014, 49, 841–849. [Google Scholar] [CrossRef]
- Kim, K.; Abramishvili, D.; Du, S.; Papadopoulos, Z.; Cao, J.; Herz, J.; Smirnov, I.; Thomas, J.-L.; Colonna, M.; Kipnis, J. Meningeal lymphatics-microglia axis regulates synaptic physiology. Cell 2025, 188, 2705–2719.e23. [Google Scholar] [CrossRef] [PubMed]
- Tannous, J.; Godlewska, B.R.; Tirumalaraju, V.; Soares, J.C.; Cowen, P.J.; Selvaraj, S. Stress, inflammation and hippocampal subfields in depression: A 7 Tesla MRI Study. Transl. Psychiatry 2020, 10, 78. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Zhou, H.; Li, J.; Wang, L.; Zhu, M.; Wang, N.; Yang, P.; Yang, Z. The change of inflammatory cytokines after antidepressant treatment and correlation with depressive symptoms. J. Psychiatr. Res. 2025, 184, 418–423. [Google Scholar] [CrossRef]
- Merendino, R.A.; Di Rosa, A.E.; Di Pasquale, G.; Minciullo, P.L.; Mangraviti, C.; Costantino, A.; Ruello, A.; Gangemi, S. Interleukin-18 and CD30 serum levels in patients with moderate-severe depression. Mediat. Inflamm. 2002, 11, 265–267. [Google Scholar] [CrossRef] [PubMed]
- Gałecka, M.; Bliźniewska-Kowalska, K.; Orzechowska, A.; Szemraj, J.; Maes, M.; Berk, M.; Su, K.-P.; Gałecki, P. Inflammatory versus Anti-Inflammatory Profiles in Major Depressive Disorders—The Role of IL-17, IL-21, IL-23, IL-35 and Foxp3. J. Pers. Med. 2021, 11, 66. [Google Scholar] [CrossRef]
- Alvarez-Mon, M.A.; Gómez-Lahoz, A.M.; Orozco, A.; Lahera, G.; Diaz, D.; Ortega, M.A.; Albillos, A.; Quintero, J.; Aubá, E.; Monserrat, J.; et al. Expansion of CD4 T Lymphocytes Expressing Interleukin 17 and Tumor Necrosis Factor in Patients with Major Depressive Disorder. J. Pers. Med. 2021, 11, 220. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, B.; Qiu, W.; Yang, L.; Hu, B.; Tian, X.; Yang, H. Altered expression of CD4+ CD25+ regulatory T cells and its 5-HT1a receptor in patients with major depression disorder. J. Affect. Disord. 2010, 124, 68–75. [Google Scholar] [CrossRef]
- Pitharouli, M.C.; Hagenaars, S.P.; Glanville, K.P.; Coleman, J.R.I.; Hotopf, M.; Lewis, C.M.; Pariante, C.M. Elevated C-Reactive Protein in Patients With Depression, Independent of Genetic, Health, and Psychosocial Factors: Results From the UK Biobank. Am. J. Psychiatry 2021, 178, 522–529. [Google Scholar] [CrossRef]
- Cattaneo, A.; Gennarelli, M.; Uher, R.; Breen, G.; Farmer, A.; Aitchison, K.J.; Craig, I.W.; Anacker, C.; Zunsztain, P.A.; McGuffin, P.; et al. Candidate genes expression profile associated with antidepressants response in the GENDEP study: Differentiating between baseline “predictors” and longitudinal “targets”. Neuropsychopharmacology 2013, 38, 377–385. [Google Scholar] [CrossRef]
- Hannestad, J.; DellaGioia, N.; Bloch, M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: A meta-analysis. Neuropsychopharmacology 2011, 36, 2452–2459. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, F.; Monteleone, P.; Maj, M. Interleukin-1β and tumor necrosis factor-α in children with major depressive disorder or dysthymia. J. Affect. Disord. 2004, 78, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Marquesdeak, A.; Neto, F.; Dominguez, W.; Solis, A.; Kurcgant, D.; Sato, F.; Ross, J.; Prado, E. Cytokine profiles in women with different subtypes of major depressive disorder. J. Psychiatr. Res. 2007, 41, 152–159. [Google Scholar] [CrossRef]
- Danese, A.; Moffitt, T.E.; Pariante, C.M.; Ambler, A.; Poulton, R.; Caspi, A. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch. Gen. Psychiatry 2008, 65, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Shinagawa, S.; Nagata, T.; Shigeta, M.; Kondo, K. Suppressors of Cytokine Signaling Are Decreased in Major Depressive Disorder Patients. J. Pers. Med. 2022, 12, 1040. [Google Scholar] [CrossRef]
- Lindqvist, D.; Janelidze, S.; Hagell, P.; Erhardt, S.; Samuelsson, M.; Minthon, L.; Hansson, O.; Björkqvist, M.; Träskman-Bendz, L.; Brundin, L. Interleukin-6 Is Elevated in the Cerebrospinal Fluid of Suicide Attempters and Related to Symptom Severity. Biol. Psychiatry 2009, 66, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Werry, E.L.; Bright, F.M.; Piguet, O.; Ittner, L.M.; Halliday, G.M.; Hodges, J.R.; Kiernan, M.C.; Loy, C.T.; Kril, J.J.; Kassiou, M. Recent Developments in TSPO PET Imaging as A Biomarker of Neuroinflammation in Neurodegenerative Disorders. Int. J. Mol. Sci. 2019, 20, 3161. [Google Scholar] [CrossRef]
- Holmes, S.E.; Hinz, R.; Conen, S.; Gregory, C.J.; Matthews, J.C.; Anton-Rodriguez, J.M.; Gerhard, A.; Talbot, P.S. Elevated Translocator Protein in Anterior Cingulate in Major Depression and a Role for Inflammation in Suicidal Thinking: A Positron Emission Tomography Study. Biol. Psychiatry 2018, 83, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Hannestad, J.; DellaGioia, N.; Gallezot, J.-D.; Lim, K.; Nabulsi, N.; Esterlis, I.; Pittman, B.; Lee, J.-Y.; O’Connor, K.C.; Pelletier, D.; et al. The neuroinflammation marker translocator protein is not elevated in individuals with mild-to-moderate depression: A [11C]PBR28 PET study. Brain Behav. Immun. 2013, 33, 131–138. [Google Scholar] [CrossRef] [PubMed]
- LACAPERE, J.-J.; DUMA, L.; FINET, S.; KASSIOU, M.; PAPADOPOULOS, V. Insight into structural features of TSPO: Implications for drug development. Trends Pharmacol. Sci. 2020, 41, 110–122. [Google Scholar] [CrossRef]
- Bansal, Y.; Kuhad, A. Mitochondrial Dysfunction in Depression. Curr. Neuropharmacol. 2016, 14, 610–618. [Google Scholar] [CrossRef]
- Torres-Platas, S.G.; Cruceanu, C.; Chen, G.G.; Turecki, G.; Mechawar, N. Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain Behav. Immun. 2014, 42, 50–59. [Google Scholar] [CrossRef]
- Dean, B.; Tawadros, N.; Scarr, E.; Gibbons, A.S. Regionally-specific changes in levels of tumour necrosis factor in the dorsolateral prefrontal cortex obtained postmortem from subjects with major depressive disorder. J. Affect. Disord. 2010, 120, 245–248. [Google Scholar] [CrossRef]
- Clark, S.M.; Pocivavsek, A.; Nicholson, J.D.; Notarangelo, F.M.; Langenberg, P.; McMahon, R.P.; Kleinman, J.E.; Hyde, T.M.; Stiller, J.; Postolache, T.T.; et al. Reduced kynurenine pathway metabolism and cytokine expression in the prefrontal cortex of depressed individuals. J. Psychiatry Neurosci. 2016, 41, 386–394. [Google Scholar] [CrossRef]
- Pantazatos, S.P.; Huang, Y.-Y.; Rosoklija, G.B.; Dwork, A.J.; Arango, V.; Mann, J.J. Whole-transcriptome brain expression and exon-usage profiling in major depression and suicide: Evidence for altered glial, endothelial and ATPase activity. Mol. Psychiatry 2017, 22, 760–773. [Google Scholar] [CrossRef]
- Tonelli, L.H.; Stiller, J.; Rujescu, D.; Giegling, I.; Schneider, B.; Maurer, K.; Schnabel, A.; Möller, H.-J.; Chen, H.H.; Postolache, T.T. Elevated cytokine expression in the orbitofrontal cortex of victims of suicide. Acta Psychiatr. Scand. 2008, 117, 198–206. [Google Scholar] [CrossRef]
- Setiawan, E.; Wilson, A.A.; Mizrahi, R.; Rusjan, P.M.; Miler, L.; Rajkowska, G.; Suridjan, I.; Kennedy, J.L.; Rekkas, P.V.; Houle, S.; et al. Role of Translocator Protein Density, a Marker of Neuroinflammation, in the Brain During Major Depressive Episodes. JAMA Psychiatry 2015, 72, 268–275. [Google Scholar] [CrossRef]
- Schnieder, T.P.; Trencevska, I.; Rosoklija, G.; Stankov, A.; Mann, J.J.; Smiley, J.; Dwork, A.J. Microglia of Prefrontal White Matter in Suicide. J. Neuropathol. Exp. Neurol. 2014, 73, 880–890. [Google Scholar] [CrossRef]
- Sasayama, D.; Hattori, K.; Wakabayashi, C.; Teraishi, T.; Hori, H.; Ota, M.; Yoshida, S.; Arima, K.; Higuchi, T.; Amano, N.; et al. Increased cerebrospinal fluid interleukin-6 levels in patients with schizophrenia and those with major depressive disorder. J. Psychiatr. Res. 2013, 47, 401–406. [Google Scholar] [CrossRef]
- Hestad, K.A.; Engedal, K.; Whist, J.E.; Aukrust, P.; Farup, P.G.; Mollnes, T.E.; Ueland, T. Patients with depression display cytokine levels in serum and cerebrospinal fluid similar to patients with diffuse neurological symptoms without a defined diagnosis. Neuropsychiatr. Dis. Treat. 2016, 12, 817–822. [Google Scholar] [CrossRef]
- Brisch, R.; Steiner, J.; Mawrin, C.; Krzyżanowska, M.; Jankowski, Z.; Gos, T. Microglia in the dorsal raphe nucleus plays a potential role in both suicide facilitation and prevention in affective disorders. Eur. Arch. Psychiatry Clin. Neurosci. 2017, 267, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Cobb, J.A.; O’Neill, K.; Milner, J.; Mahajan, G.J.; Lawrence, T.J.; May, W.L.; Miguel-Hidalgo, J.; Rajkowska, G.; Stockmeier, C.A. Density of GFAP-immunoreactive astrocytes is decreased in left hippocampi in major depressive disorder. Neuroscience 2016, 316, 209–220. [Google Scholar] [CrossRef]
- Williams, M.R.; Galvin, K.; O’Sullivan, B.; MacDonald, C.D.; Ching, E.W.K.; Turkheimer, F.; Howes, O.D.; Pearce, R.K.B.; Hirsch, S.R.; Maier, M. Neuropathological changes in the substantia nigra in schizophrenia but not depression. Eur. Arch. Psychiatry Clin. Neurosci. 2014, 264, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Davis, S. Glial fibrillary acidic protein in late life major depressive disorder: An immunocytochemical study. J. Neurol. Neurosurg. Psychiatry 2002, 73, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Nihonmatsu-Kikuchi, N.; Yu, X.; Ishimoto, K.; Hisanaga, S.-I.; Tatebayashi, Y. A novel, rapid, quantitative cell-counting method reveals oligodendroglial reduction in the frontopolar cortex in major depressive disorder. Mol. Psychiatry 2011, 16, 1155–1158. [Google Scholar] [CrossRef]
- Rajkowska, G.; Mahajan, G.; Maciag, D.; Sathyanesan, M.; Iyo, A.H.; Moulana, M.; Kyle, P.B.; Woolverton, W.L.; Miguel-Hidalgo, J.J.; Stockmeier, C.A.; et al. Oligodendrocyte Morphometry and Expression of Myelin—Related mRNA in Ventral Prefrontal White Matter in Major Depressive Disorder. J. Psychiatr. Res. 2015, 65, 53–62. [Google Scholar] [CrossRef]
- Kofod, J.; Elfving, B.; Nielsen, E.H.; Mors, O.; Köhler-Forsberg, O. Depression and inflammation: Correlation between changes in inflammatory markers with antidepressant response and long-term prognosis. Eur. Neuropsychopharmacol. 2022, 54, 116–125. [Google Scholar] [CrossRef]
- Kapfhammer, H.-P. Somatic symptoms in depression. Dialogues Clin. Neurosci. 2006, 8, 227–239. [Google Scholar] [CrossRef]
- Maes, M.; Kubera, M.; Leunis, J.-C. The gut-brain barrier in major depression: Intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuro Endocrinol. Lett. 2008, 29, 117–124. [Google Scholar]
- Galea, I.; Bechmann, I.; Perry, V.H. What is immune privilege (not)? Trends Immunol. 2007, 28, 12–18. [Google Scholar] [CrossRef]
- Koren, T.; Yifa, R.; Amer, M.; Krot, M.; Boshnak, N.; Ben-Shaanan, T.L.; Azulay-Debby, H.; Zalayat, I.; Avishai, E.; Hajjo, H.; et al. Insular cortex neurons encode and retrieve specific immune responses. Cell 2021, 184, 5902–5915.e17. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, C.; Le, T.; Swain, M.G. Cerebral Microglia Recruit Monocytes into the Brain in Response to Tumor Necrosis Factorα Signaling during Peripheral Organ Inflammation. J. Neurosci. 2009, 29, 2089–2102. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Yang, X.; He, Y.; Duan, C.; Sun, N. Psychological stress induces depressive-like behavior associated with bone marrow-derived monocyte infiltration into the hippocampus independent of blood-brain barrier disruption. J. Neuroinflamm. 2022, 19, 208. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H.; Raison, C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef]
- Levine, J.; Barak, Y.; Chengappa, K.N.; Rapoport, A.; Rebey, M.; Barak, V. Cerebrospinal cytokine levels in patients with acute depression. Neuropsychobiology 1999, 40, 171–176. [Google Scholar] [CrossRef]
- Felger, J.C.; Haroon, E.; Patel, T.A.; Goldsmith, D.R.; Wommack, E.C.; Woolwine, B.J.; Le, N.-A.; Feinberg, R.; Tansey, M.G.; Miller, A.H. What does plasma CRP tell us about peripheral and central inflammation in depression? Mol. Psychiatry 2020, 25, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Schubert, J.J.; Veronese, M.; Fryer, T.D.; Manavaki, R.; Kitzbichler, M.G.; Nettis, M.A.; Mondelli, V.; Pariante, C.M.; Bullmore, E.T.; NIMA Consortium. A Modest Increase in 11C-PK11195-Positron Emission Tomography TSPO Binding in Depression Is Not Associated With Serum C-Reactive Protein or Body Mass Index. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 716–724. [Google Scholar] [CrossRef]
- Tapp, Z.M.; Godbout, J.P.; Kokiko-Cochran, O.N. A Tilted Axis: Maladaptive Inflammation and HPA Axis Dysfunction Contribute to Consequences of TBI. Front. Neurol. 2019, 10, 345. [Google Scholar] [CrossRef]
- Biber, K.; Neumann, H.; Inoue, K.; Boddeke, H.W.G.M. Neuronal “On” and “Off” signals control microglia. Trends Neurosci. 2007, 30, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Pocock, J.M.; Kettenmann, H. Neurotransmitter receptors on microglia. Trends Neurosci. 2007, 30, 527–535. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, T.; Meng, D.; Sun, L.; Yang, G.; He, Y.; Zhang, C. Involvement of CX3CL1/CX3CR1 in depression and cognitive impairment induced by chronic unpredictable stress and relevant underlying mechanism. Behav. Brain Res. 2020, 381, 112371. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, C.; Lan, X.; Li, H.; Chao, Z.; Ning, Y. Plasma inflammatory cytokines and treatment-resistant depression with comorbid pain: Improvement by ketamine. J. Neuroinflamm. 2021, 18, 200. [Google Scholar] [CrossRef]
- Johnson, J.D.; Campisi, J.; Sharkey, C.M.; Kennedy, S.L.; Nickerson, M.; Greenwood, B.N.; Fleshner, M. Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience 2005, 135, 1295–1307. [Google Scholar] [CrossRef]
- Dutcher, E.G.; Pama, E.A.C.; Lynall, M.-E.; Khan, S.; Clatworthy, M.R.; Robbins, T.W.; Bullmore, E.T.; Dalley, J.W. Early-Life Stress and Inflammation: A Systematic Review of a Key Experimental Approach in Rodents. Brain Neurosci. Adv. 2020, 4, 2398212820978049. [Google Scholar] [CrossRef]
- Turkheimer, F.E.; Veronese, M.; Mondelli, V.; Cash, D.; Pariante, C.M. Sickness behaviour and depression: An updated model of peripheral-central immunity interactions. Brain Behav. Immun. 2023, 111, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Althubaity, N.; Schubert, J.; Martins, D.; Yousaf, T.; Nettis, M.A.; Mondelli, V.; Pariante, C.; Harrison, N.A.; Bullmore, E.T.; Dima, D.; et al. Choroid plexus enlargement is associated with neuroinflammation and reduction of blood brain barrier permeability in depression. NeuroImage Clin. 2021, 33, 102926. [Google Scholar] [CrossRef]
- Carloni, S.; Bertocchi, A.; Mancinelli, S.; Bellini, M.; Erreni, M.; Borreca, A.; Braga, D.; Giugliano, S.; Mozzarelli, A.M.; Manganaro, D.; et al. Identification of a choroid plexus vascular barrier closing during intestinal inflammation. Science 2021, 374, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Turkheimer, F.E.; Althubaity, N.; Schubert, J.; Nettis, M.A.; Cousins, O.; Dima, D.; Mondelli, V.; Bullmore, E.T.; Pariante, C.; Veronese, M. Increased serum peripheral C-reactive protein is associated with reduced brain barriers permeability of TSPO radioligands in healthy volunteers and depressed patients: Implications for inflammation and depression. Brain Behav. Immun. 2021, 91, 487–497. [Google Scholar] [CrossRef]
- Greene, C.; Hanley, N.; Campbell, M. Blood-brain barrier associated tight junction disruption is a hallmark feature of major psychiatric disorders. Transl. Psychiatry 2020, 10, 373. [Google Scholar] [CrossRef] [PubMed]
- Eiff, B.; Bullmore, E.T.; Clatworthy, M.R.; Fryer, T.D.; Pariante, C.M.; Mondelli, V.; Maccioni, L.; Hadjikhani, N.; Loggia, M.L.; Moskowitz, M.A.; et al. Extra-axial inflammatory signal and its relationship to peripheral and central immunity in depression. Brain 2024, 148, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Lynall, M.-E.; Kigar, S.L.; Lehmann, M.L.; DePuyt, A.E.; Tuong, Z.K.; Listwak, S.J.; Elkahloun, A.G.; Bullmore, E.T.; Herkenham, M.; Clatworthy, M.R. B-cells are abnormal in psychosocial stress and regulate meningeal myeloid cell activation. Brain Behav. Immun. 2021, 97, 226–238. [Google Scholar] [CrossRef]
- Hadjikhani, N.; Albrecht, D.S.; Mainero, C.; Ichijo, E.; Ward, N.; Granziera, C.; Zürcher, N.R.; Akeju, O.; Bonnier, G.; Price, J.; et al. Extra-Axial Inflammatory Signal in Parameninges in Migraine with Visual Aura. Ann. Neurol. 2020, 87, 939–949. [Google Scholar] [CrossRef]
- Correia, A.S.; Vale, N. Advancements Exploring Major Depressive Disorder: Insights on Oxidative Stress, Serotonin Metabolism, BDNF, HPA Axis Dysfunction, and Pharmacotherapy Advances. Int. J. Transl. Med. 2024, 4, 176–196. [Google Scholar] [CrossRef]
- Pariante, C.M.; Lightman, S.L. The HPA axis in major depression: Classical theories and new developments. Trends Neurosci. 2008, 31, 464–468. [Google Scholar] [CrossRef]
- Pariante, C.M.; Miller, A.H. Glucocorticoid receptors in major depression: Relevance to pathophysiology and treatment. Biol. Psychiatry 2001, 49, 391–404. [Google Scholar] [CrossRef]
- Amasi-Hartoonian, N.; Sforzini, L.; Cattaneo, A.; Pariante, C.M. Cause or consequence? Understanding the role of cortisol in the increased inflammation observed in depression. Curr. Opin. Endocr. Metab. Res. 2022, 24, 100356. [Google Scholar] [CrossRef]
- Munhoz, C.D.; Lepsch, L.B.; Kawamoto, E.M.; Malta, M.B.; Lima, L.d.S.; Avellar, M.C.W.; Sapolsky, R.M.; Scavone, C. Chronic unpredictable stress exacerbates lipopolysaccharide-induced activation of nuclear factor-kappaB in the frontal cortex and hippocampus via glucocorticoid secretion. J. Neurosci. 2006, 26, 3813–3820. [Google Scholar] [CrossRef] [PubMed]
- Niraula, A.; Wang, Y.; Godbout, J.P.; Sheridan, J.F. Corticosterone Production during Repeated Social Defeat Causes Monocyte Mobilization from the Bone Marrow, Glucocorticoid Resistance, and Neurovascular Adhesion Molecule Expression. J. Neurosci. 2018, 38, 2328–2340. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.G.; Thompson, B.M.; Watkins, L.R.; Maier, S.F. Glucocorticoids mediate stress-induced priming of microglial pro-inflammatory responses. Brain Behav. Immun. 2012, 26, 337–345. [Google Scholar] [CrossRef]
- Chhatar, S.; Lal, G. Role of adrenergic receptor signalling in neuroimmune communication. Curr. Res. Immunol. 2021, 2, 202–217. [Google Scholar] [CrossRef] [PubMed]
- Tanner, M.A.; Maitz, C.A.; Grisanti, L.A. Immune cell β2-adrenergic receptors contribute to the development of heart failure. Am. J. Physiol. Heart Circ. Physiol. 2021, 321, H633–H649. [Google Scholar] [CrossRef]
- Spengler, R.N.; Allen, R.M.; Remick, D.G.; Strieter, R.M.; Kunkel, S.L. Stimulation of alpha-adrenergic receptor augments the production of macrophage-derived tumor necrosis factor. J. Immunol. 1990, 145, 1430–1434. [Google Scholar] [CrossRef]
- Borger, P.; Hoekstra, Y.; Esselink, M.T.; Postma, D.S.; Zaagsma, J.; Vellenga, E.; Kauffman, H.F. Beta-adrenoceptor-mediated inhibition of IFN-gamma, IL-3, and GM-CSF mRNA accumulation in activated human T lymphocytes is solely mediated by the beta2-adrenoceptor subtype. Am. J. Respir. Cell Mol. Biol. 1998, 19, 400–407. [Google Scholar] [CrossRef]
- Grisanti, L.A.; Evanson, J.; Marchus, E.; Jorissen, H.; Woster, A.P.; DeKrey, W.; Sauter, E.R.; Combs, C.K.; Porter, J.E. Pro-inflammatory responses in human monocytes are beta1-adrenergic receptor subtype dependent. Mol. Immunol. 2010, 47, 1244–1254. [Google Scholar] [CrossRef]
- Tomozawa, Y.; Yabuuchi, K.; Inoue, T.; Satoh, M. Participation of cAMP and cAMP-dependent protein kinase in beta-adrenoceptor-mediated interleukin-1 beta mRNA induction in cultured microglia. Neurosci. Res. 1995, 22, 399–409. [Google Scholar] [CrossRef]
- Blandino, P.; Barnum, C.J.; Deak, T. The involvement of norepinephrine and microglia in hypothalamic and splenic IL-1beta responses to stress. J. Neuroimmunol. 2006, 173, 87–95. [Google Scholar] [CrossRef]
- Evrensel, A.; Ceylan, M.E. The Gut-Brain Axis: The Missing Link in Depression. Clin. Psychopharmacol. Neurosci. 2015, 13, 239–244. [Google Scholar] [CrossRef]
- Kamada, N.; Chen, G.Y.; Inohara, N.; Núñez, G. Control of pathogens and pathobionts by the gut microbiota. Nat. Immunol. 2013, 14, 685–690. [Google Scholar] [CrossRef]
- Sarkar, A.; Lehto, S.M.; Harty, S.; Dinan, T.G.; Cryan, J.F.; Burnet, P.W.J. Psychobiotics and the Manipulation of Bacteria–Gut–Brain Signals. Trends Neurosci. 2016, 39, 763–781. [Google Scholar] [CrossRef]
- Haghikia, A.; Jörg, S.; Duscha, A.; Berg, J.; Manzel, A.; Waschbisch, A.; Hammer, A.; Lee, D.-H.; May, C.; Wilck, N.; et al. Dietary Fatty Acids Directly Impact Central Nervous System Autoimmunity via the Small Intestine. Immunity 2015, 43, 817–829. [Google Scholar] [CrossRef]
- Kaur, H.; Das, C.; Mande, S.S. In Silico Analysis of Putrefaction Pathways in Bacteria and Its Implication in Colorectal Cancer. Front. Microbiol. 2017, 8, 2166. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Jin, K.; Jiang, C.; Pan, F.; Wu, J.; Luan, H.; Zhao, Z.; Chen, J.; Mou, T.; Wang, Z.; et al. A pilot exploration of multi-omics research of gut microbiome in major depressive disorders. Transl. Psychiatry 2022, 12, 8. [Google Scholar] [CrossRef]
- Calarge, C.A.; Devaraj, S.; Shulman, R.J. Gut permeability and depressive symptom severity in unmedicated adolescents. J. Affect. Disord. 2019, 246, 586–594. [Google Scholar] [CrossRef]
- Campisi, J.; Fleshner, M. Role of extracellular HSP72 in acute stress-induced potentiation of innate immunity in active rats. J. Appl. Physiol. 2003, 94, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Asea, A.; Kraeft, S.K.; Kurt-Jones, E.A.; Stevenson, M.A.; Chen, L.B.; Finberg, R.W.; Koo, G.C.; Calderwood, S.K. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat. Med. 2000, 6, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, A.; Verma, A.K.; Srivastava, M.; Srivastava, R. Oxidative Stress and Major Depression. J. Clin. Diagn. Res. JCDR 2014, 8, CC04–CC07. [Google Scholar] [CrossRef]
- Maes, M.; Mihaylova, I.; Kubera, M.; Uytterhoeven, M.; Vrydags, N.; Bosmans, E. Increased plasma peroxides and serum oxidized low density lipoprotein antibodies in major depression: Markers that further explain the higher incidence of neurodegeneration and coronary artery disease. J. Affect. Disord. 2010, 125, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Naik, E.; Dixit, V.M. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J. Exp. Med. 2011, 208, 417–420. [Google Scholar] [CrossRef]
- Wu, T.-H.; Lin, C.-H. IL-6 mediated alterations on immobile behavior of rats in the forced swim test via ERK1/2 activation in specific brain regions. Behav. Brain Res. 2008, 193, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Choi, B.; Lee, E.H.; Choi, K.S.; Sohn, S. Immobilization stress induces cell death through production of reactive oxygen species in the mouse cerebral cortex. Neurosci. Lett. 2006, 392, 27–31. [Google Scholar] [CrossRef]
- Grippo, A.J.; Francis, J.; Beltz, T.G.; Felder, R.B.; Johnson, A.K. Neuroendocrine and cytokine profile of chronic mild stress-induced anhedonia. Physiol. Behav. 2005, 84, 697–706. [Google Scholar] [CrossRef]
- Yu, X.; Yao, H.; Zhang, X.; Liu, L.; Liu, S.; Dong, Y. Comparison of LPS and MS-Induced Depressive Mouse Model: Behavior, Inflammation and Biochemical Changes. BMC Psychiatry. 2022, 22, 590. [Google Scholar] [CrossRef]
- Goshen, I.; Yirmiya, R. Interleukin-1 (IL-1): A central regulator of stress responses. Front. Neuroendocrinol. 2009, 30, 30–45. [Google Scholar] [CrossRef]
- Ding, L.; Xu, X.; Li, C.; Wang, Y.; Xia, X.; Zheng, J.C. Glutaminase in microglia: A novel regulator of neuroinflammation. Brain Behav. Immun. 2021, 92, 139–156. [Google Scholar] [CrossRef]
- Ji, C.; Tang, Y.; Zhang, Y.; Li, C.; Liang, H.; Ding, L.; Xia, X.; Xiong, L.; Qi, X.-R.; Zheng, J.C. Microglial glutaminase 1 deficiency mitigates neuroinflammation associated depression. Brain Behav. Immun. 2022, 99, 231–245. [Google Scholar] [CrossRef]
- Chen, H.; Fu, S.; Li, X.; Shi, M.; Qian, J.; Zhao, S.; Yuan, P.; Ding, L.; Xia, X.; Zheng, J.C. Microglial glutaminase 1 mediates chronic restraint stress-induced depression-like behaviors and synaptic damages. Signal Transduct. Target. Ther. 2023, 8, 452. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Fan, X.; Zhang, Y.; Yao, M.; Wang, G.; Dong, Y.; Liu, J.; Song, W. The glymphatic system: A new perspective on brain diseases. Front. Aging Neurosci. 2023, 15, 1179988. [Google Scholar] [CrossRef] [PubMed]
- Aspelund, A.; Antila, S.; Proulx, S.T.; Karlsen, T.V.; Karaman, S.; Detmar, M.; Wiig, H.; Alitalo, K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 2015, 212, 991–999. [Google Scholar] [CrossRef]
- Sun, B.-L.; Wang, L.; Yang, T.; Sun, J.; Mao, L.; Yang, M.; Yuan, H.; Colvin, R.A.; Yang, X. Lymphatic Drainage System of the Brain: A Novel Target for Intervention of Neurological Diseases. Prog. Neurobiol. 2018, 163–164, 118–143. [Google Scholar] [CrossRef] [PubMed]
- Jessen, N.A.; Munk, A.S.F.; Lundgaard, I.; Nedergaard, M. The Glymphatic System—A Beginner’s Guide. Neurochem. Res. 2015, 40, 2583–2599. [Google Scholar] [CrossRef]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 2012, 4, 147ra111. [Google Scholar] [CrossRef]
- Yamada, K.; Iwatsubo, T. Involvement of the glymphatic/meningeal lymphatic system in Alzheimer’s disease: Insights into proteostasis and future directions. Cell. Mol. Life Sci. CMLS 2024, 81, 192. [Google Scholar] [CrossRef]
- Barlattani, T.; Grandinetti, P.; Di Cintio, A.; Montemagno, A.; Testa, R.; D’Amelio, C.; Olivieri, L.; Tomasetti, C.; Rossi, A.; Pacitti, F.; et al. Glymphatic System and Psychiatric Disorders: A Rapid Comprehensive Scoping Review. Curr. Neuropharmacol. 2024, 22, 2016–2033. [Google Scholar] [CrossRef]
- Chang, J.; Guo, B.; Gao, Y.; Li, W.; Tong, X.; Feng, Y.; Abumaria, N. Characteristic Features of Deep Brain Lymphatic Vessels and Their Regulation by Chronic Stress. Research 2023, 6, 0120. [Google Scholar] [CrossRef]
- Liu, X.; Hao, J.; Yao, E.; Cao, J.; Zheng, X.; Yao, D.; Zhang, C.; Li, J.; Pan, D.; Luo, X.; et al. Polyunsaturated fatty acid supplement alleviates depression-incident cognitive dysfunction by protecting the cerebrovascular and glymphatic systems. Brain Behav. Immun. 2020, 89, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Yang, M.; Xia, P.; Xiao, C.; Huang, S.; Zhang, Z.; Cheng, X.; Li, W.; Jin, J.; Zhang, J.; et al. A functional role of meningeal lymphatics in sex difference of stress susceptibility in mice. Nat. Commun. 2022, 13, 4825. [Google Scholar] [CrossRef]
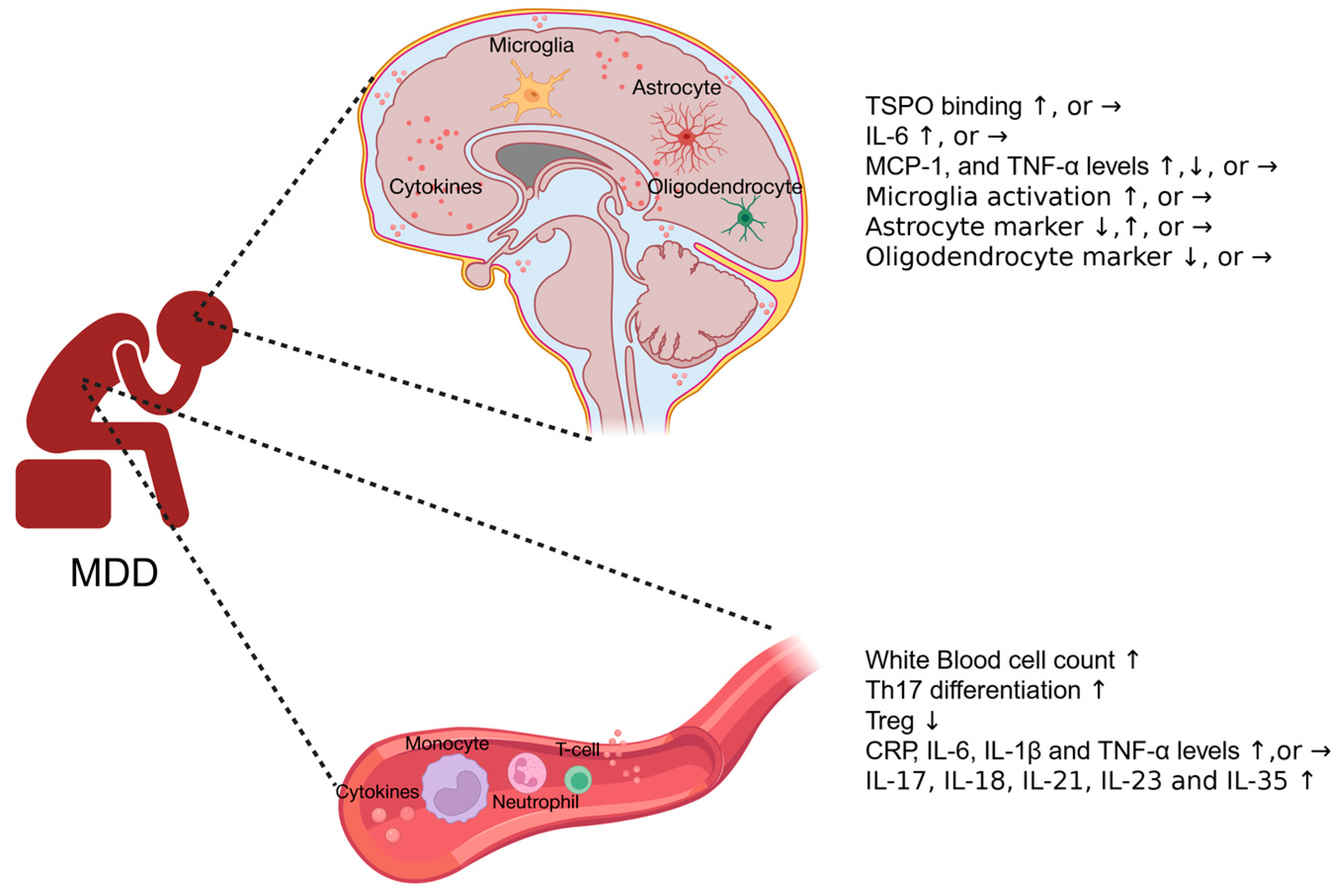
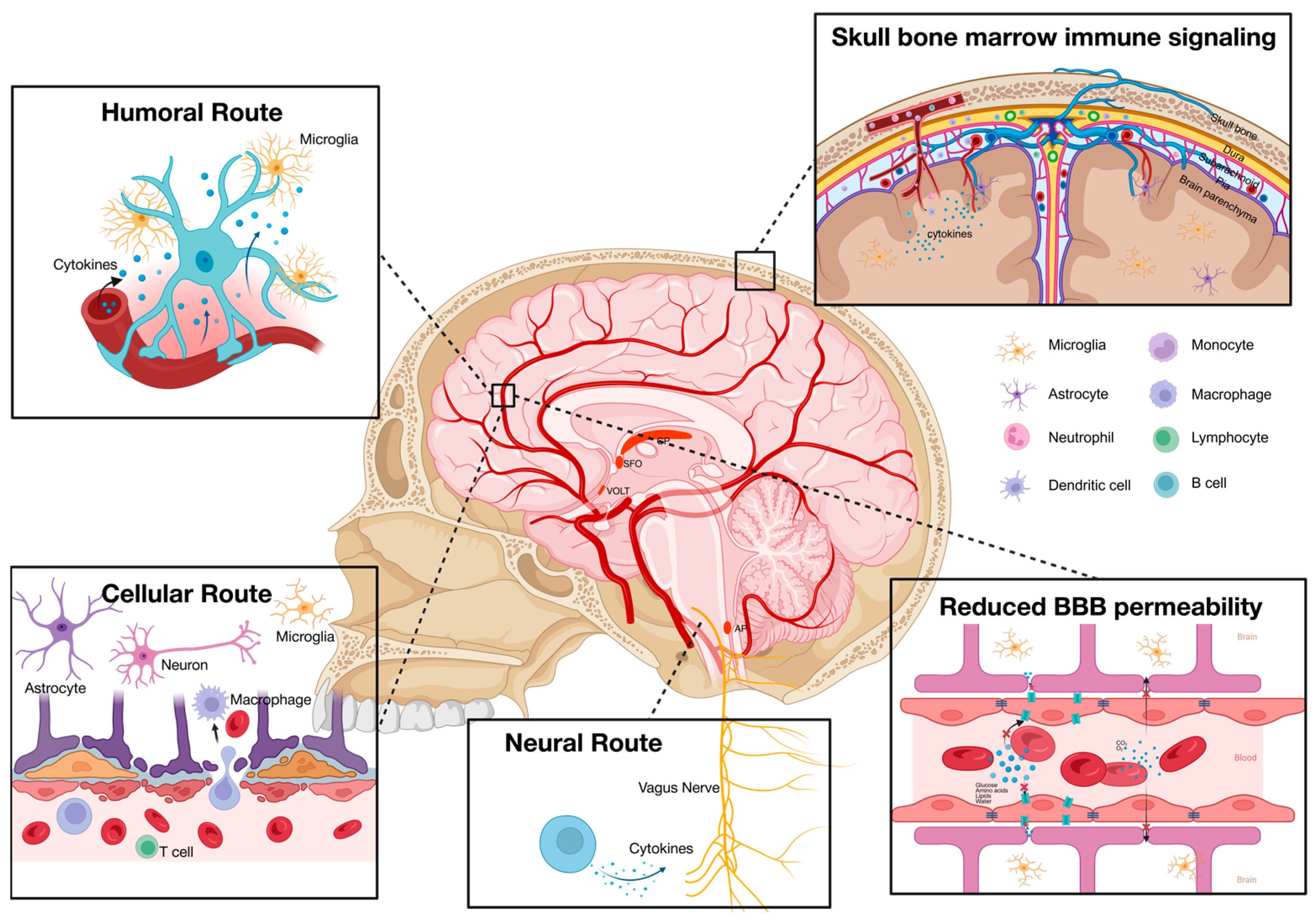
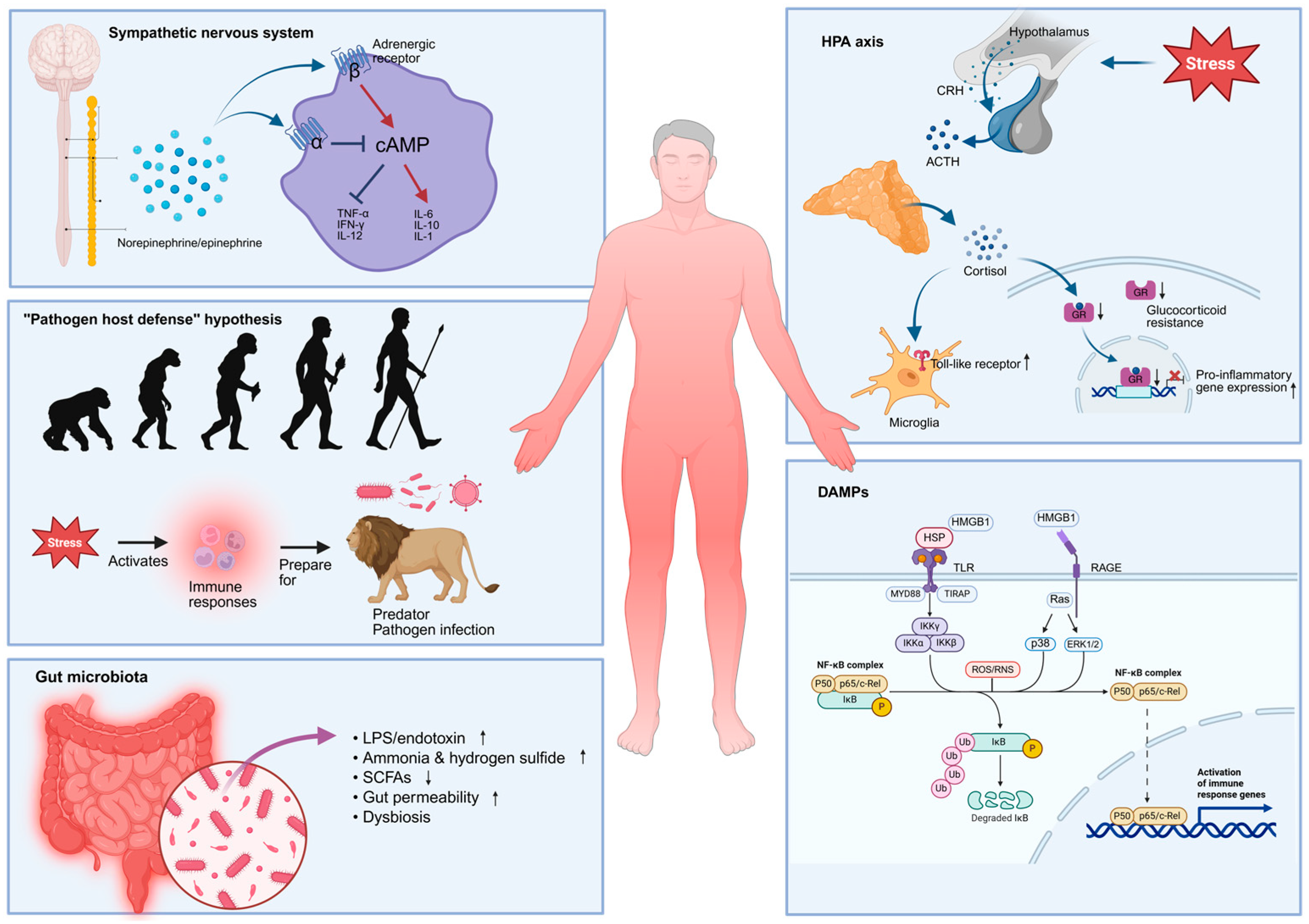
| Author | Peripheral Blood Inflammatory Marker | Neuroinflammation Marker | Correlation |
|---|---|---|---|
| Hannestad et al. [34] | hsCRP | TSPO binding | No |
| Holmes et al. [33] | IFN-γ, TNF-α, IL-6, IL-8, IL-1β and CRP | TSPO binding | No |
| Lindqvist et al. [31] | IL-6, IL-1β, IL-8, and TNF-α | IL-6, IL-1β, IL-8, and TNF-α in CSF | No |
| Sasayama et al. [44] | IL-6 | IL-6 in CSF | No |
| Setiawan et al. [42] | IL-1β, IL-6, TNF-α, and CRP | TSPO binding | No |
| Hestad et al. [45] | Eotaxin, IP-10, MIP-1β | Eotaxin, IP-10, MIP-1β in CSF | High correlation (>0.4) |
| Levine et al. [60] | TNF | IL-1β in CSF | Significant positive correlation |
| Felger et al. [61] | CRP | CRP in CSF | Strong correlation (r = 0.855, p < 0.001) |
| Schubert et al. [62] | CRP | TSPO binding | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, X.; Ho, Y.-S.; Chang, R.C.-C. Re-Examination of Inflammation in Major Depressive Disorder: Bridging Systemic and Neuroinflammatory Insights. Biomolecules 2025, 15, 1556. https://doi.org/10.3390/biom15111556
Ye X, Ho Y-S, Chang RC-C. Re-Examination of Inflammation in Major Depressive Disorder: Bridging Systemic and Neuroinflammatory Insights. Biomolecules. 2025; 15(11):1556. https://doi.org/10.3390/biom15111556
Chicago/Turabian StyleYe, Xinyu, Yuen-Shan Ho, and Raymond Chuen-Chung Chang. 2025. "Re-Examination of Inflammation in Major Depressive Disorder: Bridging Systemic and Neuroinflammatory Insights" Biomolecules 15, no. 11: 1556. https://doi.org/10.3390/biom15111556
APA StyleYe, X., Ho, Y.-S., & Chang, R. C.-C. (2025). Re-Examination of Inflammation in Major Depressive Disorder: Bridging Systemic and Neuroinflammatory Insights. Biomolecules, 15(11), 1556. https://doi.org/10.3390/biom15111556






