Structure–Activity Relationships of N-Acyl Dopamines in Inhibiting Myofibroblast Transdifferentiation of Retinal Pigment Epithelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Porcine and Human RPE Cells
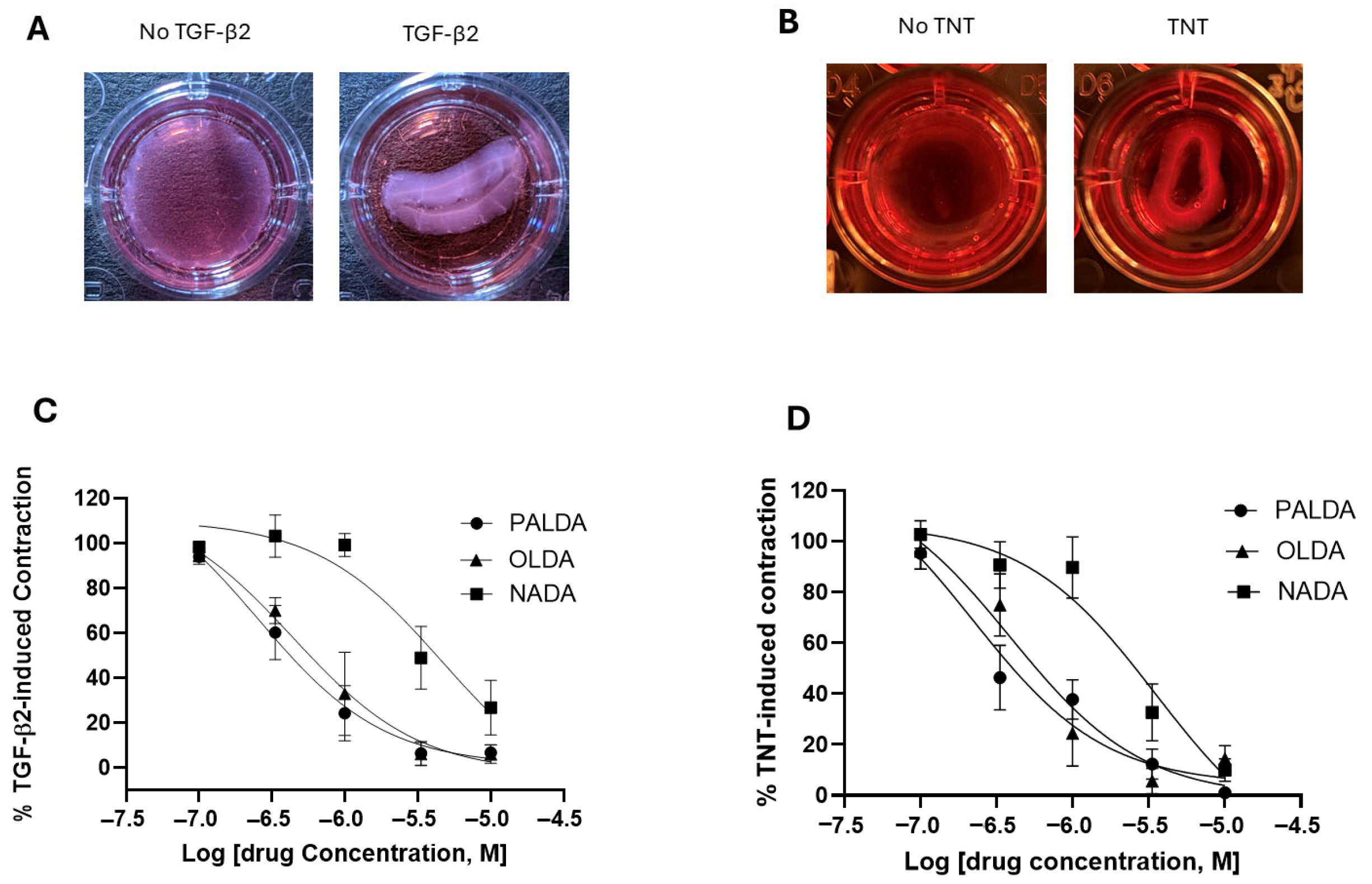
2.2. Contraction Assays
2.3. Western Blot Analysis
2.4. Immunocytochemistry
2.5. Cell Proliferation Assay
2.6. Statistical Analysis
3. Results
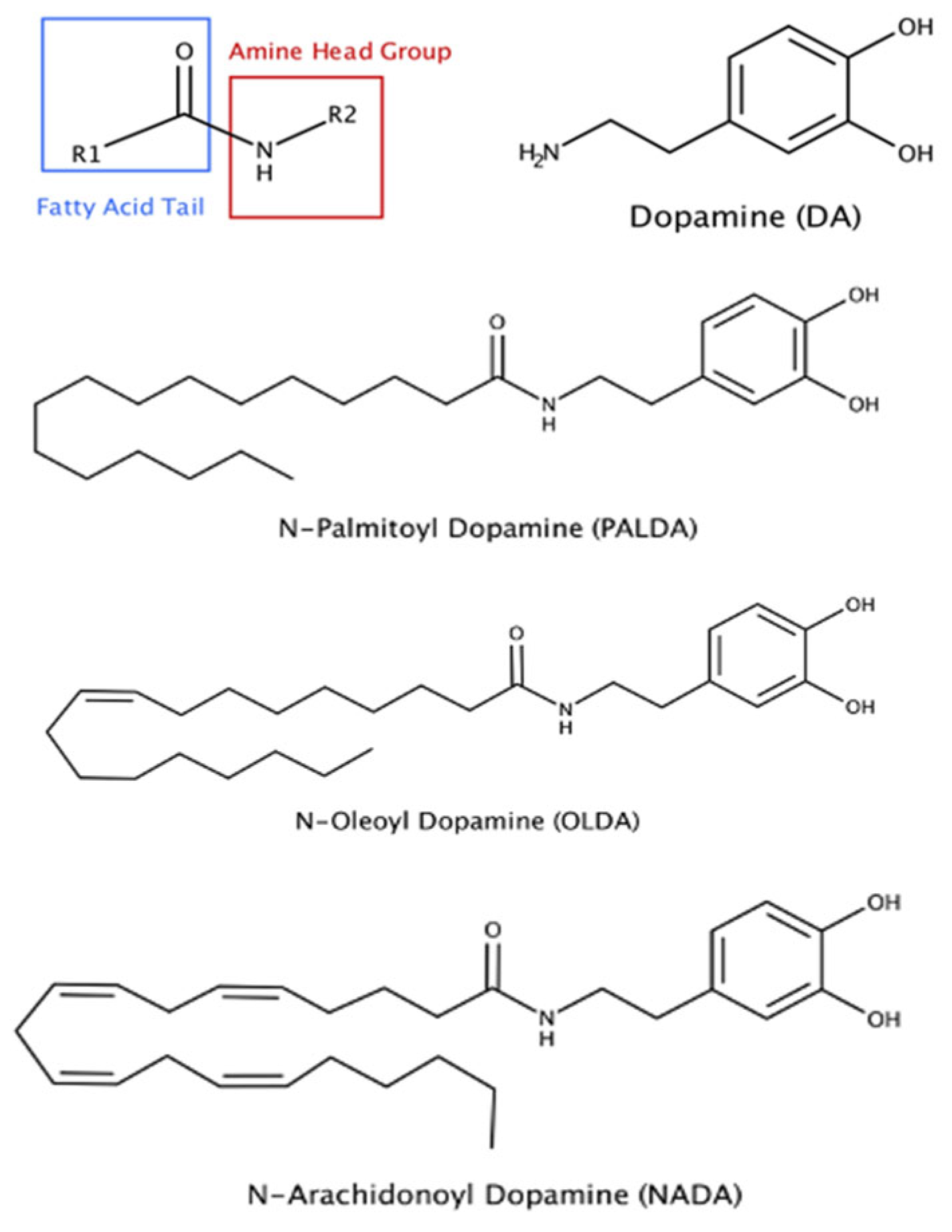
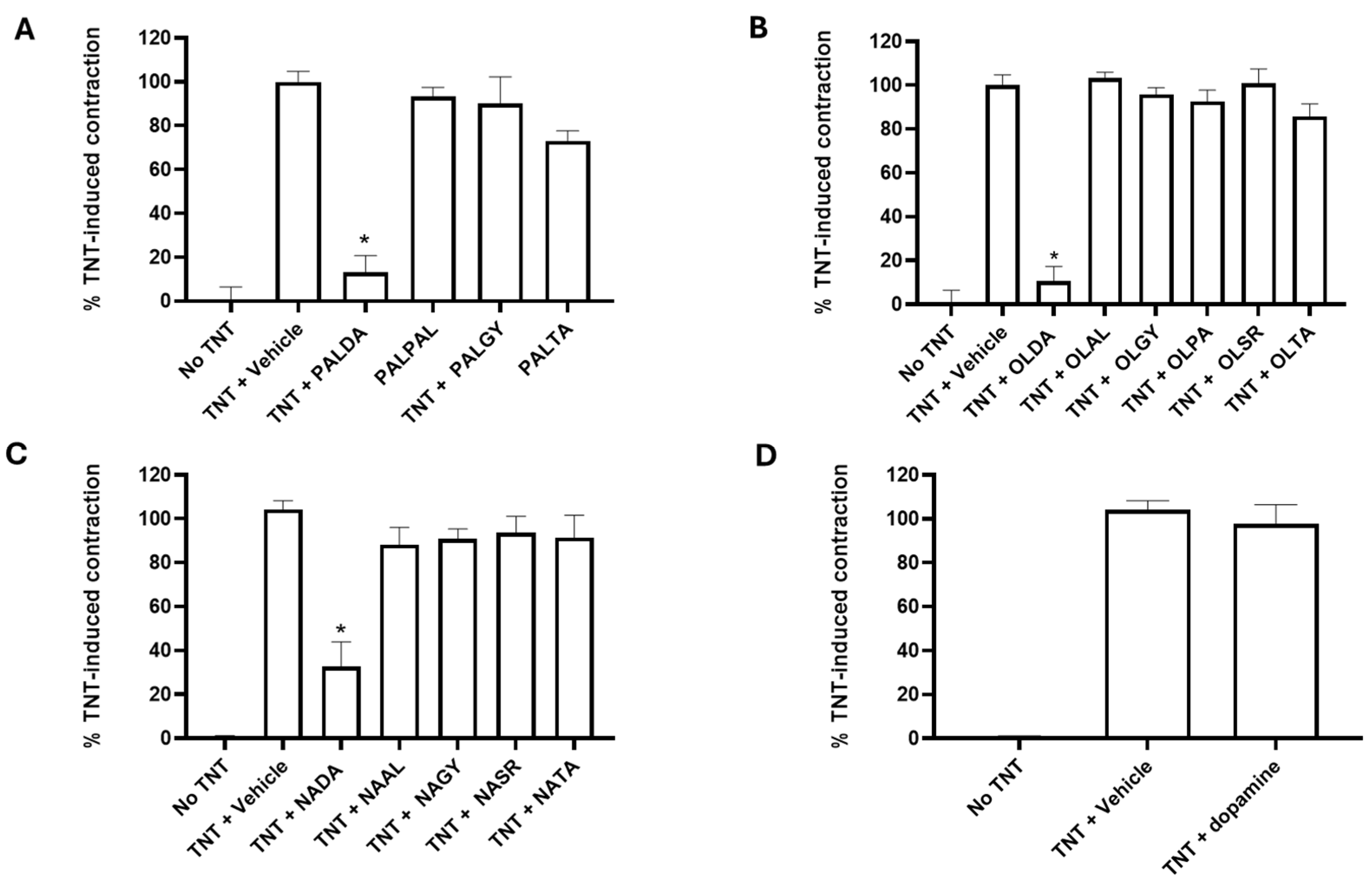
3.1. The Impact of Substitution of Different Fatty Acid Side Chains on the Inhibitory Effects of N-Acyl Dopamines on RPE Cell-Mediated Matrix Contraction
3.2. The Impact of Different Head Groups on the Effects of N-Acyl Amide Derivatives on RPE Cell-Mediated Matrix Contraction
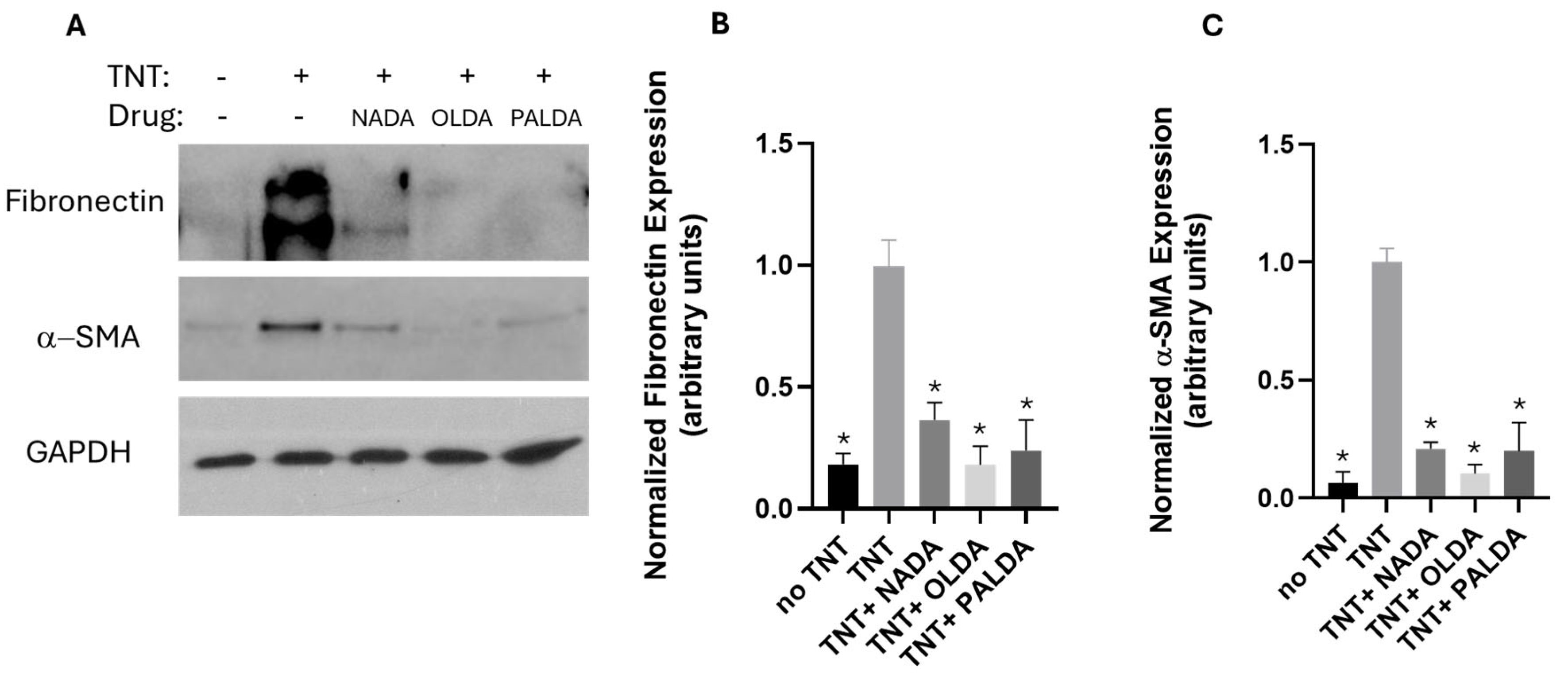
3.3. The Effects of N-Acyl Dopamines on α-SMA and Fibronectin Expression in Transdifferentiated Human RPE Cells
3.4. The Effects of N-Acyl Dopamines on α-SMA Expression in F-Actin Stress Fibers of Transdifferentiated Human RPE Cells
3.5. The Effects of N-Acyl Dopamines on RPE Cell Proliferation
4. Discussion
4.1. Structure–Activity Relationship of N-Acyl Amide Derivatives on Inhibiting Myofibroblast Transdifferentiation of RPE Cells
4.2. Functional Significance and Potential Molecular Targets of N-Acyl Dopamines as Inhibitors of Myofibroblast Transdifferentiation of RPE Cells
4.3. Novelty and Limitations of the Study and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RPE | Retinal pigment epithelial |
| PVR | Proliferative vitreoretinopathy |
| PALDA | N-palmitoyl dopamine |
| OLDA | N-oleoyl dopamine |
| NADA | N-arachidonoyl dopamine |
References
- Ku, J.C.; Raiten, J.; Li, Y. Understanding fibrosis: Mechanisms, clinical implications, current therapies, and prospects for future interventions. Biomed. Eng. Adv. 2024, 7, 100118. [Google Scholar] [CrossRef]
- Darby, I.A.; Hewitson, T.D. Fibroblast differentiation in wound healing and fibrosis. Int. Rev. Cytol. 2007, 257, 143–179. [Google Scholar] [CrossRef] [PubMed]
- Idrees, S. Proliferative Vitreoretinopathy: A Review. Int. Ophthalmol. Clin. 2019, 59, 221–240. [Google Scholar] [CrossRef] [PubMed]
- Ferro Desideri, L.; Artemiev, D.; Zandi, S.; Zinkernagel, M.S.; Anguita, R. Proliferative vitreoretinopathy: An update on the current and emerging treatment options. Graefes Arch. Clin. Exp. Ophthalmol. 2024, 262, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Suarez, J.; Kim, Y.J.; Miller, W.P.; Kim, L.A. Recent advances in pharmacological treatments of proliferative vitreoretinopathy. Curr. Opin. Ophthalmol. 2025, 36, 253–261. [Google Scholar] [CrossRef]
- Tamiya, S.; Kaplan, H.J. Role of epithelial-mesenchymal transition in proliferative vitreoretinopathy. Exp. Eye Res. 2016, 142, 26–31. [Google Scholar] [CrossRef]
- Li, X.; Zhao, M.; He, S. RPE epithelial-mesenchymal transition plays a critical role in the pathogenesis of proliferative vitreoretinopathy. Ann. Transl. Med. 2020, 8, 263. [Google Scholar] [CrossRef]
- Aebersold, A.; Duff, M.; Sloan, L.; Song, Z.H. Cannabidiol Signaling in the Eye and Its Potential as an Ocular Therapeutic Agent. Cell Physiol. Biochem. 2021, 55, 1–14. [Google Scholar] [CrossRef]
- Lu, H.C.; Mackie, K. Review of the Endocannabinoid System. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 607–615. [Google Scholar] [CrossRef]
- Di Marzo, V. Endocannabinoid System. In eLS; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Shrader, S.H.; Song, Z.H. Discovery of endogenous inverse agonists for G protein-coupled receptor 6. Biochem. Biophys. Res. Commun. 2020, 522, 1041–1045. [Google Scholar] [CrossRef]
- Connor, M.; Vaughan, C.W.; Vandenberg, R.J. N-acyl amino acids and N-acyl neurotransmitter conjugates: Neuromodulators and probes for new drug targets. Br. J. Pharmacol. 2010, 160, 1857–1871. [Google Scholar] [CrossRef]
- Akimov, M.; Bezuglov, V. N-Acylated Dopamines: A New Life for the Old Dopamine. In Dopamine: Functions, Regulation and Health Effects; Nova Science Publishers: New York, NY, USA, 2012; pp. 49–80. [Google Scholar]
- Sloan, L.J.; Funk, K.M.; Tamiya, S.; Song, Z.H. Effect of N-oleoyl dopamine on myofibroblast trans-differentiation of retinal pigment epithelial cells. Biochem. Biophys. Res. Commun. 2023, 667, 127–131. [Google Scholar] [CrossRef]
- Umazume, K.; Tsukahara, R.; Liu, L.; Fernandez de Castro, J.P.; McDonald, K.; Kaplan, H.J.; Tamiya, S. Role of retinal pigment epithelial cell β-catenin signaling in experimental proliferative vitreoretinopathy. Am. J. Pathol. 2014, 184, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Boles, N.C.; Fernandes, M.; Swigut, T.; Srinivasan, R.; Schiff, L.; Rada-Iglesias, A.; Wang, Q.; Saini, J.S.; Kiehl, T.; Stern, J.H.; et al. Epigenomic and Transcriptomic Changes During Human RPE EMT in a Stem Cell Model of Epiretinal Membrane Pathogenesis and Prevention by Nicotinamide. Stem Cell Rep. 2020, 14, 631–647. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.; Nunn, B.M.; Chauhan, R.; McDonald, K.; Kaplan, H.J.; O’Toole, M.G.; Tamiya, S. Sustained dasatinib treatment prevents early fibrotic changes following ocular trauma. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Umazume, K.; Barak, Y.; McDonald, K.; Liu, L.; Kaplan, H.J.; Tamiya, S. Proliferative vitreoretinopathy in the Swine-a new model. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4910–4916. [Google Scholar] [CrossRef]
- Umazume, K.; Liu, L.; Scott, P.A.; de Castro, J.P.; McDonald, K.; Kaplan, H.J.; Tamiya, S. Inhibition of PVR with a tyrosine kinase inhibitor, dasatinib, in the swine. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1150–1159. [Google Scholar] [CrossRef]
- Charteris, D.G.; Sethi, C.S.; Lewis, G.P.; Fisher, S.K. Proliferative vitreoretinopathy-developments in adjunctive treatment and retinal pathology. Eye 2002, 16, 369–374. [Google Scholar] [CrossRef]
- Chu, C.J.; Huang, S.M.; De Petrocellis, L.; Bisogno, T.; Ewing, S.A.; Miller, J.D.; Zipkin, R.E.; Daddario, N.; Appendino, G.; Di Marzo, V.; et al. N-oleoyldopamine, a novel endogenous capsaicin-like lipid that produces hyperalgesia. J. Biol. Chem. 2003, 278, 13633–13639. [Google Scholar] [CrossRef]
- Bezuglov, V.; Bobrov, M.; Gretskaya, N.; Gonchar, A.; Zinchenko, G.; Melck, D.; Bisogno, T.; Di Marzo, V.; Kuklev, D.; Rossi, J.C.; et al. Synthesis and biological evaluation of novel amides of polyunsaturated fatty acids with dopamine. Bioorg. Med. Chem. Lett. 2001, 11, 447–449. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Chu, C.J.; Moriello, A.S.; Kellner, J.C.; Walker, J.M.; Di Marzo, V. Actions of two naturally occurring saturated N-acyldopamines on transient receptor potential vanilloid 1 (TRPV1) channels. Br. J. Pharmacol. 2004, 143, 251–256. [Google Scholar] [CrossRef]
- Huang, S.M.; Bisogno, T.; Trevisani, M.; Al-Hayani, A.; De Petrocellis, L.; Fezza, F.; Tognetto, M.; Petros, T.J.; Krey, J.F.; Chu, C.J.; et al. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc. Natl. Acad. Sci. USA 2002, 99, 8400–8405. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, H.B.; Walker, J.M. The expanding field of cannabimimetic and related lipid mediators. Br. J. Pharmacol. 2005, 144, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Liu, H.; Ke, B.; Jiang, J.; Wu, B. The peripheral CB(1) receptor antagonist JD5037 attenuates liver fibrosis via a CB(1) receptor/beta-arrestin1/Akt pathway. Br. J. Pharmacol. 2020, 177, 2830–2847. [Google Scholar] [CrossRef] [PubMed]
- Teixeira-Clerc, F.; Julien, B.; Grenard, P.; Tran Van Nhieu, J.; Deveaux, V.; Li, L.; Serriere-Lanneau, V.; Ledent, C.; Mallat, A.; Lotersztajn, S. CB1 cannabinoid receptor antagonism: A new strategy for the treatment of liver fibrosis. Nat. Med. 2006, 12, 671–676. [Google Scholar] [CrossRef]
- Wei, Y. Presence and regulation of cannabinoid receptors in human retinal pigment epithelial cells. Mol. Vis. 2009, 15, 1243–1251. [Google Scholar]
- Wei, Y.; Wang, X.; Zhao, F.; Zhao, P.Q.; Kang, X.L. Cannabinoid receptor 1 blockade protects human retinal pigment epithelial cells from oxidative injury. Mol. Vis. 2013, 19, 357–366. [Google Scholar]
- Brito, R.; Sheth, S.; Mukherjea, D.; Rybak, L.P.; Ramkumar, V. TRPV1: A Potential Drug Target for Treating Various Diseases. Cells 2014, 3, 517–545. [Google Scholar] [CrossRef]
- Benítez-Angeles, M.; Morales-Lázaro, S.L.; Juárez-González, E.; Rosenbaum, T. TRPV1: Structure, Endogenous Agonists, and Mechanisms. Int. J. Mol. Sci. 2020, 21, 3421. [Google Scholar] [CrossRef]
- Peng, G.; Tang, X.; Gui, Y.; Yang, J.; Ye, L.; Wu, L.; Ding, Y.h.; Wang, L. Transient receptor potential vanilloid subtype 1: A potential therapeutic target for fibrotic diseases. Front. Physiol. 2022, 13, 951980. [Google Scholar] [CrossRef]
- Križaj, D.; Cordeiro, S.; Strauß, O. Retinal TRP channels: Cell-type-specific regulators of retinal homeostasis and multimodal integration. Prog. Retin. Eye Res. 2023, 92, 101114. [Google Scholar] [CrossRef]

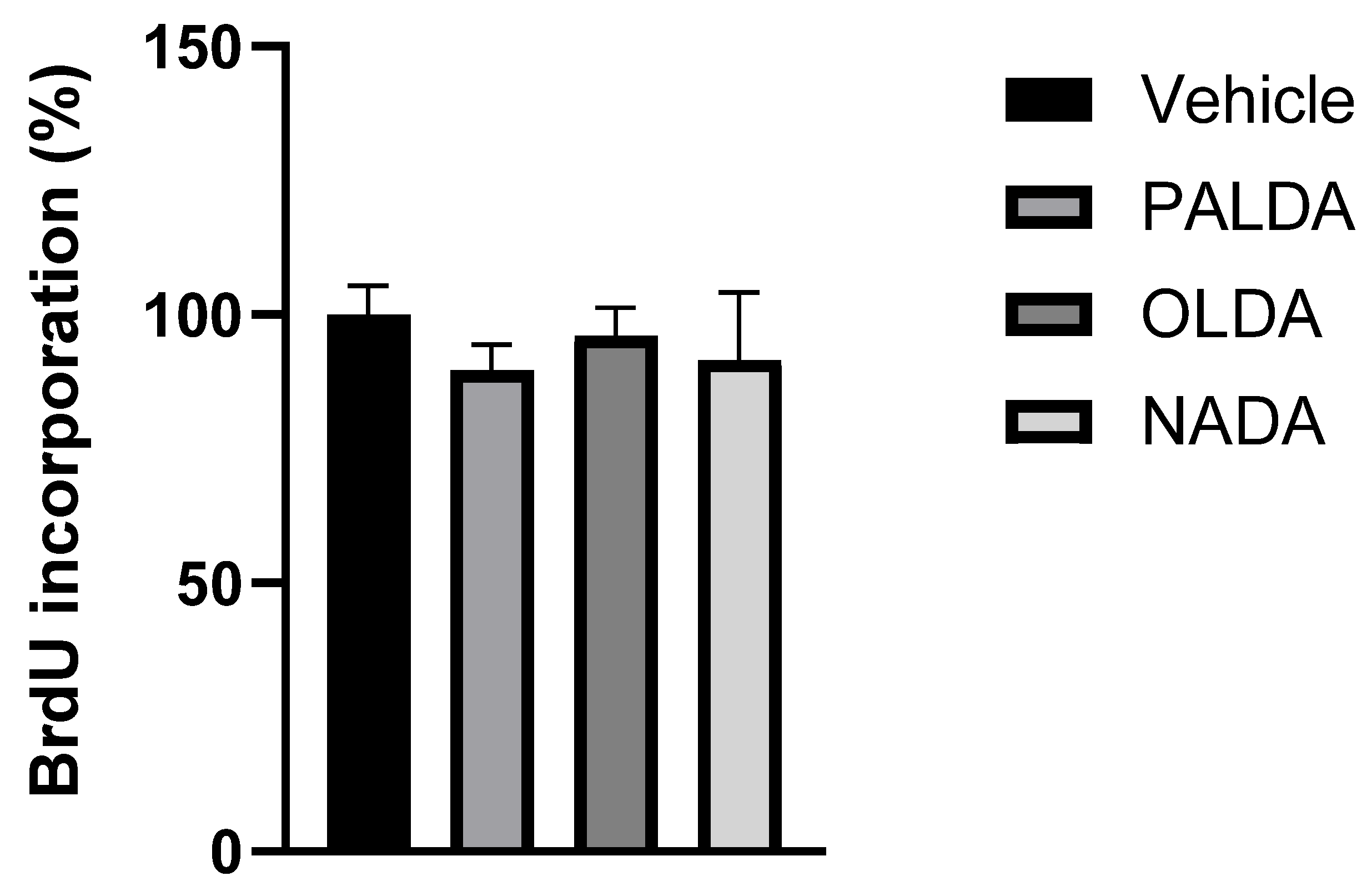
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, D.; Motheramgari, V.; Freudenberger, R.; Shrader, S.H.; Sloan, L.J.; Lung, Z.; Wang, W.; Tamiya, S.; Song, Z.-H. Structure–Activity Relationships of N-Acyl Dopamines in Inhibiting Myofibroblast Transdifferentiation of Retinal Pigment Epithelial Cells. Biomolecules 2025, 15, 1526. https://doi.org/10.3390/biom15111526
Zhao D, Motheramgari V, Freudenberger R, Shrader SH, Sloan LJ, Lung Z, Wang W, Tamiya S, Song Z-H. Structure–Activity Relationships of N-Acyl Dopamines in Inhibiting Myofibroblast Transdifferentiation of Retinal Pigment Epithelial Cells. Biomolecules. 2025; 15(11):1526. https://doi.org/10.3390/biom15111526
Chicago/Turabian StyleZhao, Dandan, Vishaka Motheramgari, Riley Freudenberger, Sarah H. Shrader, Lucy J. Sloan, Zoe Lung, Wei Wang, Shigeo Tamiya, and Zhao-Hui Song. 2025. "Structure–Activity Relationships of N-Acyl Dopamines in Inhibiting Myofibroblast Transdifferentiation of Retinal Pigment Epithelial Cells" Biomolecules 15, no. 11: 1526. https://doi.org/10.3390/biom15111526
APA StyleZhao, D., Motheramgari, V., Freudenberger, R., Shrader, S. H., Sloan, L. J., Lung, Z., Wang, W., Tamiya, S., & Song, Z.-H. (2025). Structure–Activity Relationships of N-Acyl Dopamines in Inhibiting Myofibroblast Transdifferentiation of Retinal Pigment Epithelial Cells. Biomolecules, 15(11), 1526. https://doi.org/10.3390/biom15111526





