From Native Glycosaminoglycans to Mimetics: Design, Mechanisms, and Biomedical Applications
Abstract
1. Introduction
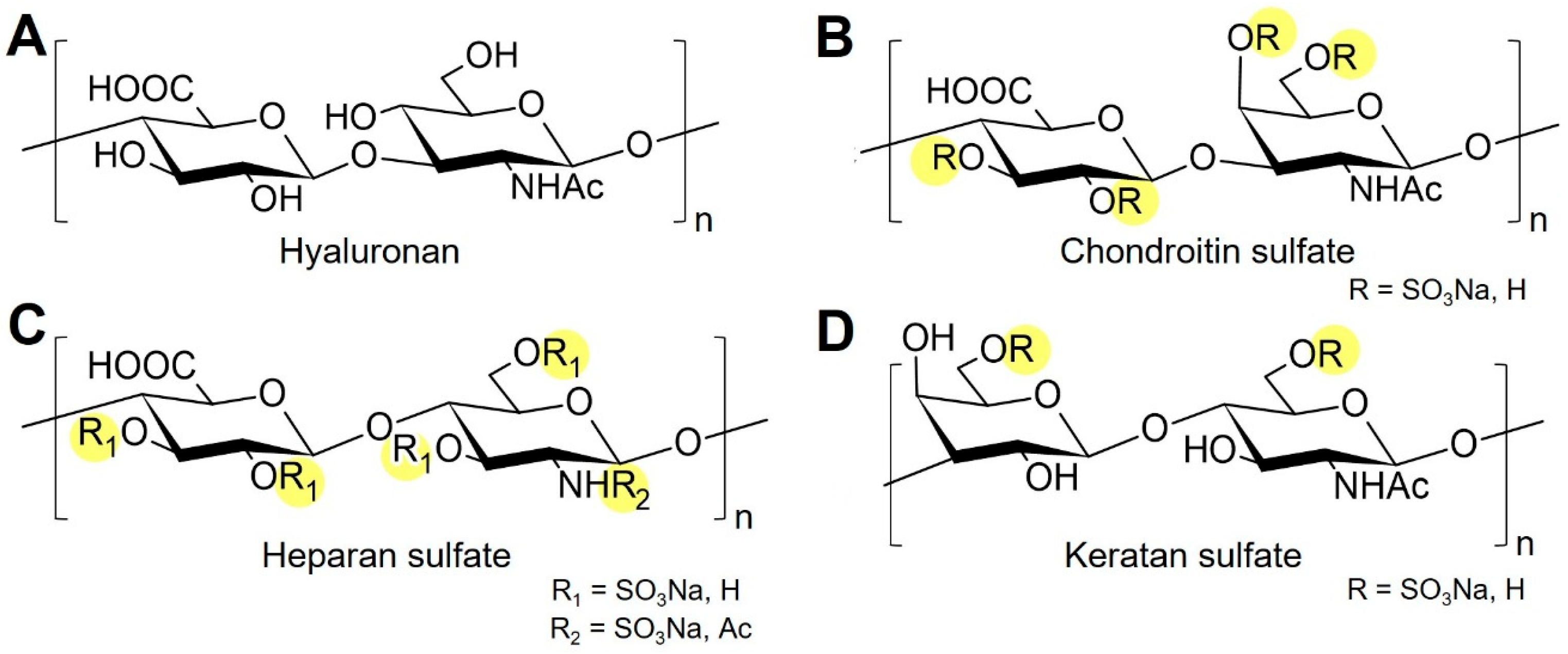
2. Learning from Nature: Synthesis and Interaction of Native GAGs
3. Rationale for GAG Mimetics and Synthetic and Bioengineering Strategies
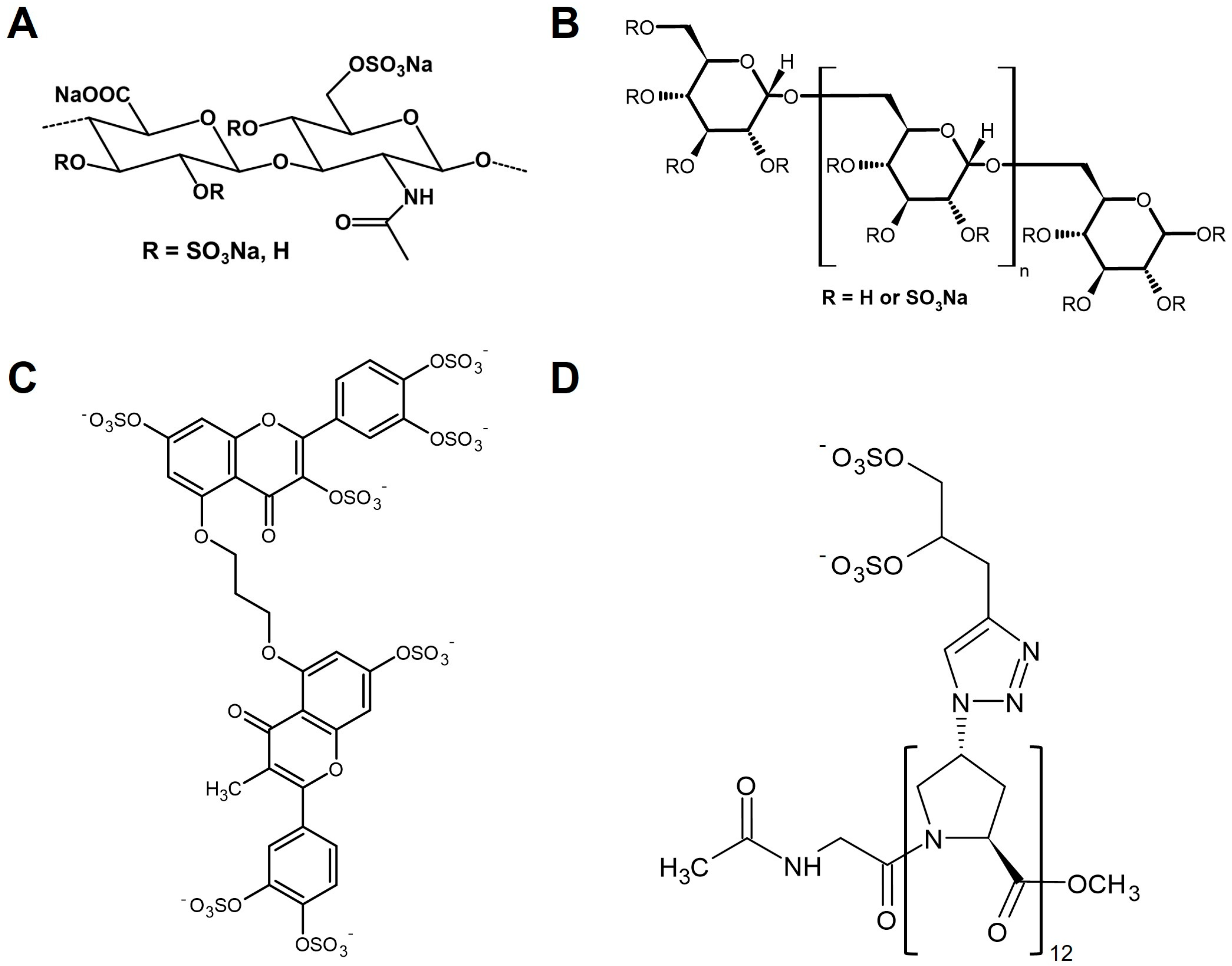
3.1. HS Mimetics Based on Modified Polysaccharides
3.2. Non-Saccharide-Based HS Mimetics
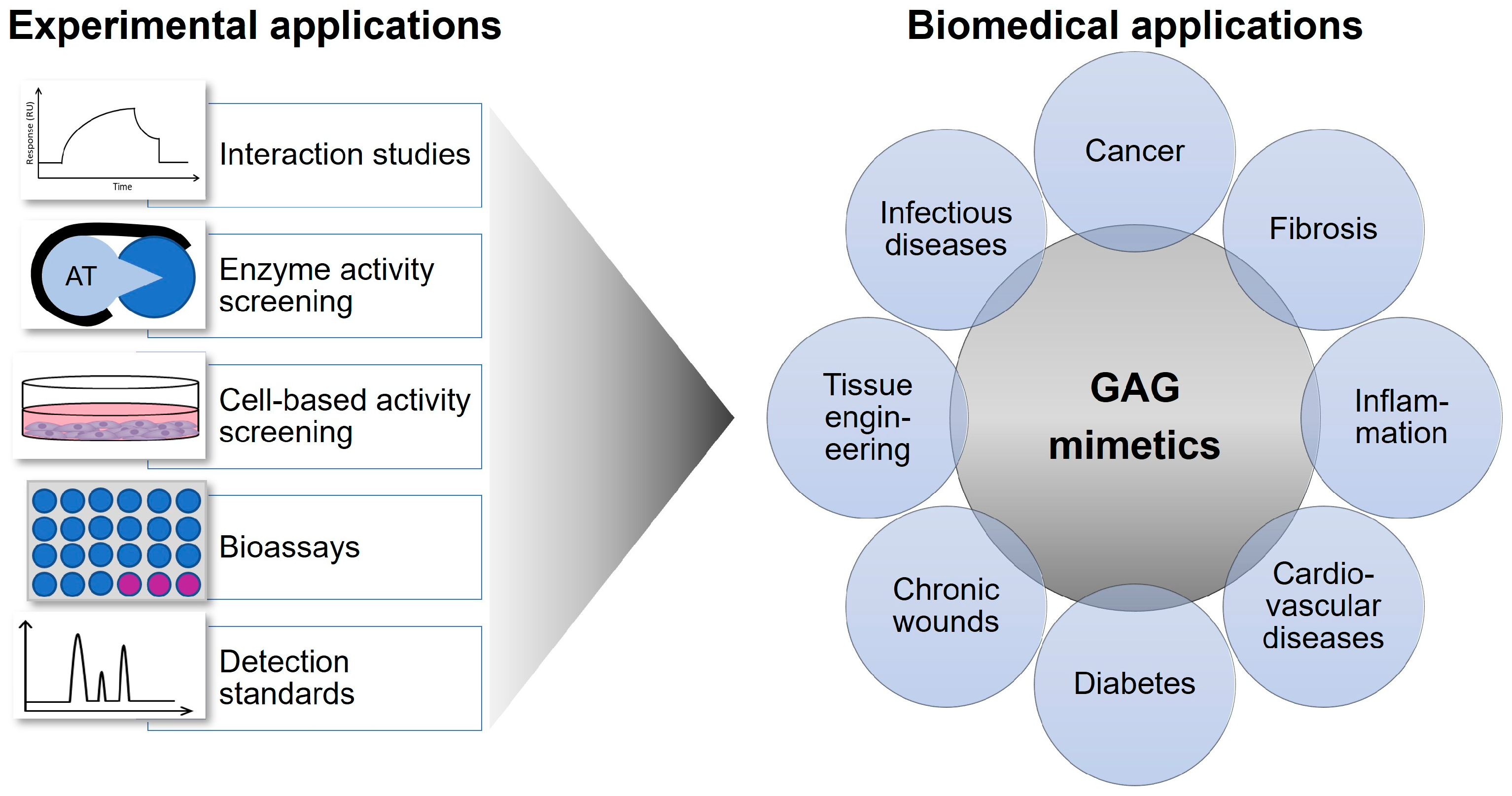
4. Selected Biomedical Applications of GAG Mimetics
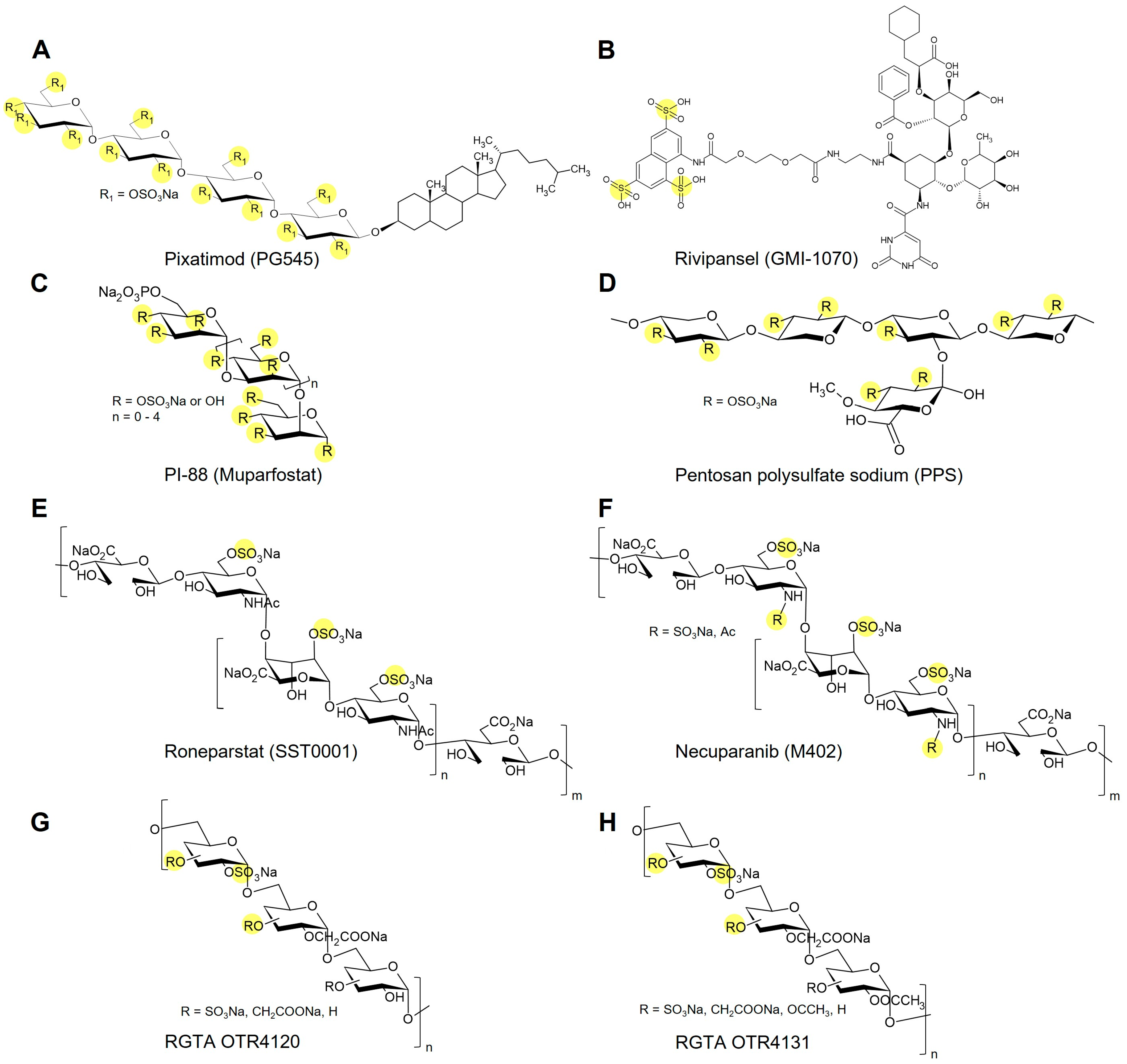
5. Glycomimetics in Late-Stage Development and Clinical Translation
6. Safety and Immunogenicity Considerations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Köwitsch, A.; Zhou, G.; Groth, T. Medical Application of Glycosaminoglycans: A Review. J. Tissue Eng. Regen. Med. 2017, 12, e23–e41. [Google Scholar] [CrossRef]
- Xu, D.; Esko, J.D. Demystifying Heparan Sulfate-Protein Interactions. Annu. Rev. Biochem. 2014, 83, 129–157. [Google Scholar] [CrossRef]
- Esko, J.D.; Selleck, S.B. Order Out of Chaos: Assembly of Ligand Binding Sites in Heparan Sulfate. Annu. Rev. Biochem. 2002, 71, 435–471. [Google Scholar] [CrossRef]
- Pessentheiner, A.R.; Ducasa, G.M.; Gordts, P.L.S.M. Proteoglycans in Obesity-Associated Metabolic Dysfunction and Meta-Inflammation. Front. Immunol. 2020, 11, 769. [Google Scholar] [CrossRef] [PubMed]
- Chittum, J.E.; Thompson, A.; Desai, U.R. Glycosaminoglycan Microarrays for Studying Glycosaminoglycan-Protein Systems. Carbohydr. Polym. 2024, 335, 122106. [Google Scholar] [CrossRef]
- Portillo, G.; Li, D.R.; Goyal, Y.; Rowan, N.; Al-Horani, B.G.; Anbalagan, R.A.; Heparin, M.; Gatica Portillo, D.R.; Li, Y.; Goyal, N.; et al. Heparin, Heparin-like Molecules, and Heparin Mimetics in Breast Cancer: A Concise Review. Biomolecules 2025, 15, 1034. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Sheng, A.; Chi, L. Glycosaminoglycan-Protein Interactions and Their Roles in Human Disease. Front. Mol. Biosci. 2021, 8, 639666. [Google Scholar] [CrossRef] [PubMed]
- Vallet, S.D.; Clerc, O.; Ricard-Blum, S. Glycosaminoglycan-Protein Interactions: The First Draft of the Glycosaminoglycan Interactome. J. Histochem. Cytochem. 2021, 69, 93–104. [Google Scholar] [CrossRef]
- Le Pennec, J.; Picart, C.; Vivès, R.R.; Migliorini, E. Sweet but Challenging: Tackling the Complexity of GAGs with Engineered Tailor-Made Biomaterials. Adv. Mater. 2024, 36, 2312154. [Google Scholar] [CrossRef]
- Zhang, L. Glycosaminoglycan (GAG) Biosynthesis and GAG-Binding Proteins. Prog. Mol. Biol. Transl. Sci. 2010, 93, 1–17. [Google Scholar] [CrossRef]
- Sarrazin, S.; Lamanna, W.C.; Esko, J.D. Heparan Sulfate Proteoglycans. Cold Spring Harb. Perspect. Biol. 2011, 3, a004952. [Google Scholar] [CrossRef] [PubMed]
- Shojania, A.M.; Tetreault, J.; Turnbull, G. The Variations between Heparin Sensitivity of Different Lots of Activated Partial Thromboplastin Time Reagent Produced by the Same Manufacturer. Am. J. Clin. Pathol. 1988, 89, 19–23. [Google Scholar] [CrossRef]
- Mende, M.; Bednarek, C.; Wawryszyn, M.; Sauter, P.; Biskup, M.B.; Schepers, U.; Bräse, S. Chemical Synthesis of Glycosaminoglycans. Chem. Rev. 2016, 116, 8193–8255. [Google Scholar] [CrossRef]
- Deangelis, P.L.; Liu, J.; Linhardt, R.J. Chemoenzymatic Synthesis of Glycosaminoglycans: Re-Creating, Re-Modeling and Re-Designing Nature’s Longest or Most Complex Carbohydrate Chains. Glycobiology 2013, 23, 764–777. [Google Scholar] [CrossRef]
- DeAngelis, P.L. Glycosaminoglycan Polysaccharide Biosynthesis and Production: Today and Tomorrow. Appl. Microbiol. Biotechnol. 2012, 94, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Morla, S.; Ravikumar, O.; O’Hara, C.; Boothello, R.; Vera, A.; Abdelfadiel, E.I.; Fayyad, R.; Afosah, D.K.; Sharon, C.; Fernandez, L.; et al. Designing Synthetic, Sulfated Glycosaminoglycan Mimetics That Are Orally Bioavailable and Exhibiting In Vivo Anticancer Activity. J. Med. Chem. 2023, 66, 1321–1338. [Google Scholar] [CrossRef]
- Khachigian, L.M.; Parish, C.R. Phosphomannopentaose Sulfate (PI-88): Heparan Sulfate Mimetic with Clinical Potential in Multiple Vascular Pathologies. Cardiovasc. Drug Rev. 2004, 22, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Francis, D.J.; Parish, C.R.; McGarry, M.; Santiago, F.S.; Lowe, H.C.; Brown, K.J.; Bingley, J.A.; Hayward, I.P.; Cowden, W.B.; Campbell, J.H.; et al. Blockade of Vascular Smooth Muscle Cell Proliferation and Intimal Thickening after Balloon Injury by the Sulfated Oligosaccharide PI-88: Phosphomannopentaose Sulfate Directly Binds FGF-2, Blocks Cellular Signaling, and Inhibits Proliferation. Circ. Res. 2003, 92, 8. [Google Scholar] [CrossRef] [PubMed]
- Morla, S. Glycosaminoglycans and Glycosaminoglycan Mimetics in Cancer and Inflammation. Int. J. Mol. Sci. 2019, 20, 1963. [Google Scholar] [CrossRef]
- Chhabra, M.; Ferro, V. PI-88 and Related Heparan Sulfate Mimetics. Adv. Exp. Med. Biol. 2020, 1221, 473–491. [Google Scholar] [CrossRef]
- Kuhnast, B.; el Hadri, A.; Boisgard, R.; Hinnen, F.; Richard, S.; Caravano, A.; Nancy-Portebois, V.; Petitou, M.; Tavitian, B.; Dollé, F. Synthesis, Radiolabeling with Fluorine-18 and Preliminary in Vivo Evaluation of a Heparan Sulphate Mimetic as Potent Angiogenesis and Heparanase Inhibitor for Cancer Applications. Org. Biomol. Chem. 2016, 14, 1915–1920. [Google Scholar] [CrossRef] [PubMed]
- Matou, S.; Colliec-Jouault, S.; Galy-Fauroux, I.; Ratiskol, J.; Sinquin, C.; Guezennec, J.; Fischer, A.M.; Helley, D. Effect of an Oversulfated Exopolysaccharide on Angiogenesis Induced by Fibroblast Growth Factor-2 or Vascular Endothelial Growth Factor in Vitro. Biochem. Pharmacol. 2005, 69, 751–759. [Google Scholar] [CrossRef]
- Schiraldi, C.; Cimini, D.; de Rosa, M. Production of Chondroitin Sulfate and Chondroitin. Appl. Microbiol. Biotechnol. 2010, 87, 1209–1220. [Google Scholar] [CrossRef]
- Bedini, E.; Decastro, C.; Derosa, M.; Dinola, A.; Iadonisi, A.; Restaino, O.F.; Schiraldi, C.; Parrilli, M. A Microbiological–Chemical Strategy to Produce Chondroitin Sulfate A,C. Angew. Chem. Int. Ed. 2011, 50, 6160–6163. [Google Scholar] [CrossRef] [PubMed]
- Corsuto, L.; Rother, S.; Koehler, L.; Bedini, E.; Moeller, S.; Schnabelrauch, M.; Hintze, V.; Schiraldi, C.; Scharnweber, D. Sulfation Degree Not Origin of Chondroitin Sulfate Derivatives Modulates Keratinocyte Response. Carbohydr. Polym. 2018, 191, 53–64. [Google Scholar] [CrossRef]
- Badri, A.; Williams, A.; Awofiranye, A.; Datta, P.; Xia, K.; He, W.; Fraser, K.; Dordick, J.S.; Linhardt, R.J.; Koffas, M.A.G. Complete Biosynthesis of a Sulfated Chondroitin in Escherichia Coli. Nat. Commun. 2021, 12, 1389. [Google Scholar] [CrossRef]
- Couto, M.R.; Rodrigues, J.L.; Rodrigues, L.R. Heterologous Production of Chondroitin. Biotechnol. Rep. 2022, 33, e00710. [Google Scholar] [CrossRef]
- Domínguez-Rodríguez, P.; Reina, J.J.; Gil-Caballero, S.; Nieto, P.M.; de Paz, J.L.; Rojo, J. Glycodendrimers as Chondroitin Sulfate Mimetics: Synthesis and Binding to Growth Factor Midkine. Chemistry 2017, 23, 11338–11345. [Google Scholar] [CrossRef]
- García-Jiménez, M.J.; Gil-Caballero, S.; Maza, S.; Corzana, F.; Juárez-Vicente, F.; Miles, J.R.; Sakamoto, K.; Kadomatsu, K.; García-Domínguez, M.; de Paz, J.L.; et al. Midkine Interaction with Chondroitin Sulfate Model Synthetic Tetrasaccharides and Their Mimetics: The Role of Aromatic Interactions. Chem.—A Eur. J. 2021, 27, 12395–12409. [Google Scholar] [CrossRef] [PubMed]
- Sheng, G.J.; Oh, Y.I.; Chang, S.K.; Hsieh-Wilson, L.C. Tunable Heparan Sulfate Mimetics for Modulating Chemokine Activity. J. Am. Chem. Soc. 2013, 135, 10898–10901. [Google Scholar] [CrossRef]
- Hintze, V.; Schnabelrauch, M.; Rother, S. Chemical Modification of Hyaluronan and Their Biomedical Applications. Front. Chem. 2022, 10, 830671. [Google Scholar] [CrossRef]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic Acid Hydrogels for Biomedical Applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef] [PubMed]
- Hintze, V.; Miron, A.; Moeller, S.; Schnabelrauch, M.; Wiesmann, H.-P.; Worch, H.; Scharnweber, D. Sulfated Hyaluronan and Chondroitin Sulfate Derivatives Interact Differently with Human Transforming Growth Factor-Β1 (TGF-Β1). Acta Biomater. 2012, 8, 2144–2152. [Google Scholar] [CrossRef] [PubMed]
- Hintze, V.; Samsonov, S.A.; Anselmi, M.; Moeller, S.; Becher, J.; Schnabelrauch, M.; Scharnweber, D.; Pisabarro, M.T. Sulfated Glycosaminoglycans Exploit the Conformational Plasticity of Bone Morphogenetic Protein 2 (BMP-2) and Alter the Interaction Pro Fi Le with Its Receptor. Biomacromolecules 2014, 15, 3083–3092. [Google Scholar] [CrossRef] [PubMed]
- Hintze, V.; Moeller, S.; Schnabelrauch, M.; Bierbaum, S.; Viola, M.; Worch, H.; Scharnweber, D. Modifications of Hyaluronan Influence the Interaction with Human Bone Morphogenetic Protein-4 (HBMP-4). Biomacromolecules 2009, 10, 3290–3297. [Google Scholar] [CrossRef]
- Koehler, L.; Ruiz-Gómez, G.; Balamurugan, K.; Rother, S.; Freyse, J.; Möller, S.; Schnabelrauch, M.; Köhling, S.; Djordjevic, S.; Scharnweber, D.; et al. Dual Action of Sulfated Hyaluronan on Angiogenic Processes in Relation to Vascular Endothelial Growth Factor-A. Sci. Rep. 2019, 9, 18143. [Google Scholar] [CrossRef]
- Thönes, S.; Rother, S.; Wippold, T.; Blaszkiewicz, J.; Balamurugan, K.; Moeller, S.; Ruiz-gómez, G.; Schnabelrauch, M.; Scharnweber, D.; Saalbach, A.; et al. Hyaluronan/Collagen Hydrogels Containing Sulfated Hyaluronan Improve Wound Healing by Sustained Release of Heparin-Binding EGF-like Growth Factor. Acta Biomater. 2019, 86, 135–147. [Google Scholar] [CrossRef]
- Rother, S.; Samsonov, S.A.; Hofmann, T.; Blaszkiewicz, J.; Köhling, S.; Moeller, S.; Schnabelrauch, M.; Rademann, J.; Kalkhof, S.; von Bergen, M.; et al. Structural and Functional Insights into the Interaction of Sulfated Glycosaminoglycans with Tissue Inhibitor of Metalloproteinase-3—A Possible Regulatory Role on Extracellular Matrix Homeostasis. Acta Biomater. 2016, 45, 143–154. [Google Scholar] [CrossRef]
- Salbach-Hirsch, J.; Kraemer, J.; Rauner, M.; Samsonov, S.A.; Pisabarro, M.T.; Moeller, S.; Schnabelrauch, M.; Scharnweber, D.; Hofbauer, L.C.; Hintze, V. The Promotion of Osteoclastogenesis by Sulfated Hyaluronan through Interference with Osteoprotegerin and Receptor Activator of NF-ΚB Ligand/Osteoprotegerin Complex Formation. Biomaterials 2013, 34, 7653–7661. [Google Scholar] [CrossRef]
- Salbach-Hirsch, J.; Samsonov, S.A.; Hintze, V.; Hofbauer, C.; Picke, A.; Rauner, M.; Gehrcke, J.-P.; Moeller, S.; Schnabelrauch, M.; Scharnweber, D.; et al. Structural and Functional Insights into Sclerostin-Glycosaminoglycan Interactions in Bone. Biomaterials 2015, 67, 335–345. [Google Scholar] [CrossRef]
- Shi, J.; Kanoya, R.; Tani, Y.; Ishikawa, S.; Maeda, R.; Suzuki, S.; Kawanami, F.; Miyagawa, N.; Takahashi, K.; Oku, T.; et al. Sulfated Hyaluronan Binds to Heparanase and Blocks Its Enzymatic and Cellular Actions in Carcinoma Cells. Int. J. Mol. Sci. 2022, 23, 5055. [Google Scholar] [CrossRef]
- Guimond, S.E.; Mycroft-West, C.J.; Gandhi, N.S.; Tree, J.A.; Le, T.T.; Spalluto, C.M.; Humbert, M.v.; Buttigieg, K.R.; Coombes, N.; Elmore, M.J.; et al. Synthetic Heparan Sulfate Mimetic Pixatimod (PG545) Potently Inhibits SARS-CoV-2 by Disrupting the Spike–ACE2 Interaction. ACS Cent. Sci. 2022, 8, 527–545. [Google Scholar] [CrossRef]
- Friand, V.; Haddad, O.; Papy-Garcia, D.; Hlawaty, H.; Vassy, R.; Hamma-Kourbali, Y.; Perret, G.Y.; Courty, J.; Baleux, F.; Oudar, O.; et al. Glycosaminoglycan Mimetics Inhibit SDF-1/CXCL12-Mediated Migration and Invasion of Human Hepatoma Cells. Glycobiology 2009, 19, 1511–1524. [Google Scholar] [CrossRef] [PubMed]
- Wolf, H.; Graßmann, A.; Bester, R.; Hossinger, A.; Möhl, C.; Paulsen, L.; Groschup, M.H.; Schätzl, H.; Vorberg, I. Modulation of Glycosaminoglycans Affects PrPSc Metabolism but Does Not Block PrPSc Uptake. J. Virol. 2015, 89, 9853–9864. [Google Scholar] [CrossRef]
- Ramasundaram, S.; Saravanakumar, G.; Sobha, S.; Oh, T.H. Dextran Sulfate Nanocarriers: Design, Strategies and Biomedical Applications. Int. J. Mol. Sci. 2022, 24, 355. [Google Scholar] [CrossRef] [PubMed]
- Barritault, D.; Gilbert-Sirieix, M.; Rice, K.L.; Siñeriz, F.; Papy-Garcia, D.; Baudouin, C.; Desgranges, P.; Zakine, G.; Saffar, J.L.; van Neck, J. RGTA® or ReGeneraTing Agents Mimic Heparan Sulfate in Regenerative Medicine: From Concept to Curing Patients. Glycoconj. J. 2017, 34, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Arlov, Ø.; Aachmann, F.L.; Sundan, A.; Espevik, T.; Skjåk-Bræk, G. Heparin-like Properties of Sulfated Alginates with Defined Sequences and Sulfation Degrees. Biomacromolecules 2014, 15, 2744–2750. [Google Scholar] [CrossRef]
- Arlov, Ø.; Aachmann, F.L.; Feyzi, E.; Sundan, A.; Skjåk-Bræk, G. The Impact of Chain Length and Flexibility in the Interaction between Sulfated Alginates and HGF and FGF-2. Biomacromolecules 2015, 16, 3417–3424. [Google Scholar] [CrossRef]
- Stone, A.L.; Melton, D.J.; Lewis, M.S. Structure-Function Relations of Heparin-Mimetic Sulfated Xylan Oligosaccharides: Inhibition of Human Immunodeficiency Virus-1 Infectivity in Vitro. Glycoconj. J. 1998, 15, 697–712. [Google Scholar] [CrossRef]
- Zayed, A.; Avila-Peltroche, J.; El-Aasr, M.; Ulber, R. Sulfated Galactofucans: An Outstanding Class of Fucoidans with Promising Bioactivities. Mar. Drugs 2022, 20, 412. [Google Scholar] [CrossRef]
- Maatouk, B.; Jaffa, M.A.; Karam, M.; Fahs, D.; Nour-Eldine, W.; Hasan, A.; Jaffa, A.A.; Mhanna, R. Sulfated Alginate/Polycaprolactone Double-Emulsion Nanoparticles for Enhanced Delivery of Heparin-Binding Growth Factors in Wound Healing Applications. Colloids Surf. B Biointerfaces 2021, 208, 112105. [Google Scholar] [CrossRef]
- Erginer, M.; Akcay, A.; Coskunkan, B.; Morova, T.; Rende, D.; Bucak, S.; Baysal, N.; Ozisik, R.; Eroglu, M.S.; Agirbasli, M.; et al. Sulfated Levan from Halomonas Smyrnensis as a Bioactive, Heparin-Mimetic Glycan for Cardiac Tissue Engineering Applications. Carbohydr. Polym. 2016, 149, 289–296. [Google Scholar] [CrossRef]
- Sahraneshin-Samani, F.; Kazemi-Ashtiani, M.; Karimi, H.; Shiravandi, A.; Baharvand, H.; Daemi, H. Regioselective Sulfated Chitosan Produces a Biocompatible and Antibacterial Wound Dressing with Low Inflammatory Response. Biomater. Adv. 2022, 139, 213020. [Google Scholar] [CrossRef]
- Gan, H.-Q.; Gui, W.-Z.; Hu, X.-L.; Zheng, Y.-J.; He, X.-P. Trinary Dressing Material Formed between Polysaccharides and Gold Nanoparticles for Synergistic Wound Disinfection and Repair. ACS Appl. Bio Mater. 2025, 8, 9072–9080. [Google Scholar] [CrossRef] [PubMed]
- Freeman, I.; Kedem, A.; Cohen, S. The Effect of Sulfation of Alginate Hydrogels on the Specific Binding and Controlled Release of Heparin-Binding Proteins. Biomaterials 2008, 29, 3260–3268. [Google Scholar] [CrossRef]
- Gionet-Gonzales, M.; Casella, A.; Diloretto, D.; Ginnell, C.; Griffin, K.H.; Bigot, A.; Leach, J.K. Sulfated Alginate Hydrogels Prolong the Therapeutic Potential of MSC Spheroids by Sequestering the Secretome. Adv. Heal. Mater. 2021, 10, e2101048. [Google Scholar] [CrossRef]
- Madkhali, O.A.; Sivagurunathan Moni, S.; Sultan, M.H.; Bukhary, H.A.; Ghazwani, M.; Alhakamy, N.A.; Meraya, A.M.; Alshahrani, S.; Alqahtani, S.S.; Bakkari, M.A.; et al. Formulation and Evaluation of Injectable Dextran Sulfate Sodium Nanoparticles as a Potent Antibacterial Agent. Sci. Rep. 2021, 11, 9914. [Google Scholar] [CrossRef] [PubMed]
- Afosah, D.K.; Al-Horani, R.A. Sulfated Non-Saccharide Glycosaminoglycan Mimetics as Novel Drug Discovery Platform for Various Pathologies. Curr. Med. Chem. 2020, 27, 3412. [Google Scholar] [CrossRef]
- Freeman, C.; Liu, L.; Banwell, M.G.; Brown, K.J.; Bezos, A.; Ferro, V.; Parish, C.R. Use of Sulfated Linked Cyclitols as Heparan Sulfate Mimetics to Probe the Heparin/Heparan Sulfate Binding Specificity of Proteins. J. Biol. Chem. 2005, 280, 8842–8849. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cui, L. Discovery and Development of Small-Molecule Heparanase Inhibitors. Bioorg. Med. Chem. 2023, 90, 117335. [Google Scholar] [CrossRef]
- Raman, K.; Karuturi, R.; Swarup, V.P.; Desai, U.R.; Kuberan, B. Discovery of Novel Sulfonated Small Molecules That Inhibit Vascular Tube Formation. Bioorg. Med. Chem. Lett. 2012, 22, 4467–4470. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.J.; Karuturi, R.; Al-Horani, R.A.; Baranwal, S.; Patel, J.; Desai, U.R.; Patel, B.B. Synthetic, Non-Saccharide, Glycosaminoglycan Mimetics Selectively Target Colon Cancer Stem Cells. ACS Chem. Biol. 2014, 9, 1826–1833. [Google Scholar] [CrossRef]
- Boothello, R.S.; Patel, N.J.; Sharon, C.; Abdelfadiel, E.I.; Morla, S.; Brophy, D.F.; Robert Lippman, H.; Desai, U.R.; Patel, B.B. A Unique Nonsaccharide Mimetic of Heparin Hexasaccharide Inhibits Colon Cancer Stem Cells via P38 MAP Kinase Activation. Mol. Cancer Ther. 2019, 18, 51–61. [Google Scholar] [CrossRef]
- Lim, T.C.; Cai, S.; Huber, R.G.; Bond, P.J.; Siew Chia, P.X.; Khou, S.L.; Gao, S.; Lee, S.S.; Lee, S.G. Facile Saccharide-Free Mimetics That Recapitulate Key Features of Glycosaminoglycan Sulfation Patterns. Chem. Sci. 2018, 9, 7940–7947. [Google Scholar] [CrossRef]
- Schuksz, M.; Fuster, M.M.; Brown, J.R.; Crawford, B.E.; Ditto, D.P.; Lawrence, R.; Glass, C.A.; Wang, L.; Tor, Y.; Esko, J.D. Surfen, a Small Molecule Antagonist of Heparan Sulfate. Proc. Natl. Acad. Sci. USA 2008, 105, 13075–13080. [Google Scholar] [CrossRef]
- Mohamed, S.; Coombe, D.R. Heparin Mimetics: Their Therapeutic Potential. Pharmaceuticals 2017, 10, 78. [Google Scholar] [CrossRef] [PubMed]
- Lanzi, C.; Cassinelli, G. Heparan Sulfate Mimetics in Cancer Therapy: The Challenge to Define Structural Determinants and the Relevance of Targets for Optimal Activity. Molecules 2018, 23, 2915. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.N.; Mao, Z.X.; Wu, Y.; Liang, M.X.; Wang, D.D.; Chen, X.; Chang, P.A.; Zhang, W.; Tang, J.H. The Anti-Cancer Properties of Heparin and Its Derivatives: A Review and Prospect. Cell Adhes. Migr. 2020, 14, 118–128. [Google Scholar] [CrossRef]
- Salbach, J.; Rachner, T.D.; Rauner, M.; Hempel, U.; Anderegg, U.; Franz, S.; Simon, J.C.; Hofbauer, L.C. Regenerative Potential of Glycosaminoglycans for Skin and Bone. J. Mol. Med. 2012, 90, 625–635. [Google Scholar] [CrossRef]
- Salbach-Hirsch, J.; Rauner, M.; Hofbauer, C.; Hofbauer, L.C. New Insights into the Role of Glycosaminoglycans in the Endosteal Bone Microenvironment. Biol. Chem. 2021, 402, 1415–1425. [Google Scholar] [CrossRef]
- Anderegg, U.; Halfter, N.; Schnabelrauch, M.; Hintze, V. Collagen/Glycosaminoglycan-Based Matrices for Controlling Skin Cell Responses. Biol. Chem. 2021, 402, 1325–1335. [Google Scholar] [CrossRef]
- Scharnweber, D.; Hübner, L.; Rother, S.; Hempel, U.; Anderegg, U.; Samsonov, S.A.; Pisabarro, M.T.; Hofbauer, L.; Schnabelrauch, M.; Franz, S.; et al. Glycosaminoglycan Derivatives: Promising Candidates for the Design of Functional Biomaterials. J. Mater. Sci. Mater. Med. 2015, 26, 232. [Google Scholar] [CrossRef]
- van der Smissen, A.; Samsonov, S.; Hintze, V.; Scharnweber, D.; Moeller, S.; Schnabelrauch, M.; Pisabarro, M.T.; Anderegg, U. Artificial Extracellular Matrix Composed of Collagen I and Highly Sulfated Hyaluronan Interferes with TGFβ1 Signaling and Prevents TGFβ1-Induced Myofibroblast Differentiation. Acta Biomater. 2013, 9, 7775–7786. [Google Scholar] [CrossRef] [PubMed]
- Syanda, A.M.; Kringstad, V.I.; Blackford, S.J.I.; Kjesbu, J.S.; Ng, S.S.; Ma, L.; Xiao, F.; Coron, A.E.; Rokstad, A.M.A.; Modi, S.; et al. Sulfated Alginate Reduces Pericapsular Fibrotic Overgrowth on Encapsulated CGMP-Compliant HPSC-Hepatocytes in Mice. Front. Bioeng. Biotechnol. 2022, 9, 816542. [Google Scholar] [CrossRef]
- Nonaka, M.; Bao, X.; Matsumura, F.; Götze, S.; Kandasamy, J.; Kononov, A.; Broide, D.H.; Nakayama, J.; Seeberger, P.H.; Fukuda, M. Synthetic Di-Sulfated Iduronic Acid Attenuates Asthmatic Response by Blocking T-Cell Recruitment to Inflammatory Sites. Proc. Natl. Acad. Sci. USA 2014, 111, 8173–8178. [Google Scholar] [CrossRef]
- Koliesnik, I.O.; Kuipers, H.F.; Medina, C.O.; Zihsler, S.; Liu, D.; Van Belleghem, J.D.; Bollyky, P.L. The Heparan Sulfate Mimetic PG545 Modulates T Cell Responses and Prevents Delayed-Type Hypersensitivity. Front. Immunol. 2020, 11, 132. [Google Scholar] [CrossRef]
- Sutton, A.; Friand, V.; Papy-Garcia, D.; Dagouassat, M.; Martin, L.; Vassy, R.; Haddad, O.; Sainte-Catherine, O.; Kraemer, M.; Saffar, L.; et al. Glycosaminoglycans and Their Synthetic Mimetics Inhibit RANTES-Induced Migration and Invasion of Human Hepatoma Cells. Mol. Cancer Ther. 2007, 6, 2948–2958. [Google Scholar] [CrossRef]
- al Matari, N.; Deeb, G.; Mshiek, H.; Sinjab, A.; Kadara, H.; Abou-Kheir, W.; Mhanna, R. Anti-Tumor Effects of Biomimetic Sulfated Glycosaminoglycans on Lung Adenocarcinoma Cells in 2D and 3D In Vitro Models. Molecules 2020, 25, 2595. [Google Scholar] [CrossRef]
- Ferro, V.; Liu, L.; Johnstone, K.D.; Wimmer, N.; Karoli, T.; Handley, P.; Rowley, J.; Dredge, K.; Li, C.P.; Hammond, E.; et al. Discovery of PG545: A Highly Potent and Simultaneous Inhibitor of Angiogenesis, Tumor Growth, and Metastasis. J. Med. Chem. 2012, 55, 3804–3813. [Google Scholar] [CrossRef] [PubMed]
- Lemech, C.; Dredge, K.; Bampton, D.; Hammond, E.; Clouston, A.; Waterhouse, N.J.; Stanley, A.C.; Leveque-El Mouttie, L.; Chojnowski, G.M.; Haydon, A.; et al. Phase Ib Open-Label, Multicenter Study of Pixatimod, an Activator of TLR9, in Combination with Nivolumab in Subjects with Microsatellite-Stable Metastatic Colorectal Cancer, Metastatic Pancreatic Ductal Adenocarcinoma and Other Solid Tumors. J. Immunother. Cancer 2023, 11, e006136. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Rausell-Palamos, F.; Zhang, J.; Zheng, Z.; Zhang, T.; Valle, S.; Rosselot, C.; Berrouet, C.; Conde, P.; Spindler, M.P.; et al. Dextran Sulfate Protects Pancreatic β-Cells, Reduces Autoimmunity, and Ameliorates Type 1 Diabetes. Diabetes 2020, 69, 1692–1707. [Google Scholar] [CrossRef]
- Chevalier, F.; Lavergne, M.; Negroni, E.; Ferratge, S.; Carpentier, G.; Gilbert-Sirieix, M.; Siñeriz, F.; Uzan, G.; Albanese, P. Glycosaminoglycan Mimetic Improves Enrichment and Cell Functions of Human Endothelial Progenitor Cell Colonies. Stem Cell Res. 2014, 12, 703–715. [Google Scholar] [CrossRef]
- Picke, A.K.; Salbach-Hirsch, J.; Hintze, V.; Rother, S.; Rauner, M.; Kascholke, C.; Möller, S.; Bernhardt, R.; Rammelt, S.; Pisabarro, M.T.; et al. Sulfated Hyaluronan Improves Bone Regeneration of Diabetic Rats by Binding Sclerostin and Enhancing Osteoblast Function. Biomaterials 2016, 96, 11–23. [Google Scholar] [CrossRef]
- Frescaline, G.; Bouderlique, T.; Huynh, M.B.; Papy-Garcia, D.; Courty, J.; Albanese, P. Glycosaminoglycans Mimetics Potentiate the Clonogenicity, Proliferation, Migration and Differentiation Properties of Rat Mesenchymal Stem Cells. Stem Cell Res. 2012, 8, 180–192. [Google Scholar] [CrossRef][Green Version]
- Rother, S.; Samsonov, S.A.; Hempel, U.; Vogel, S.; Moeller, S.; Blaszkiewicz, J.; Köhling, S.; Schnabelrauch, M.; Rademann, J.; Pisabarro, M.T.; et al. Sulfated Hyaluronan Alters the Interaction Profile of TIMP-3 with the Endocytic Receptor LRP-1 Clusters II and IV and Increases the Extracellular TIMP-3 Level of Human Bone Marrow Stromal Cells. Biomacromolecules 2016, 17, 3252–3261. [Google Scholar] [CrossRef]
- Groah, S.L.; Libin, A.; Spungen, M.; Nguyen, K.L.; Woods, E.; Nabili, M.; Ramella-Roman, J.; Barritault, D. Regenerating Matrix-Based Therapy for Chronic Wound Healing: A Prospective within-Subject Pilot Study. Int. Wound J. 2011, 8, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.P.; Molina, A.; Tran, N.; Collins, G.; Arinzeh, T.L. Investigating Cellulose Derived Glycosaminoglycan Mimetic Scaffolds for Cartilage Tissue Engineering Applications. J. Tissue Eng. Regen. Med. 2018, 12, e592–e603. [Google Scholar] [CrossRef] [PubMed]
- Menezes, R.; Hashemi, S.; Vincent, R.; Collins, G.; Meyer, J.; Foston, M.; Arinzeh, T.L. Investigation of Glycosaminoglycan Mimetic Scaffolds for Neurite Growth. Acta Biomater. 2019, 90, 169–178. [Google Scholar] [CrossRef]
- Chang, J.; Patton, J.T.; Sarkar, A.; Ernst, B.; Magnani, J.L.; Frenette, P.S. GMI-1070, a Novel Pan-Selectin Antagonist, Reverses Acute Vascular Occlusions in Sickle Cell Mice. Blood 2010, 116, 1779–1786. [Google Scholar] [CrossRef]
- Telen, M.J.; Wun, T.; McCavit, T.L.; de Castro, L.M.; Krishnamurti, L.; Lanzkron, S.; Hsu, L.L.; Smith, W.R.; Rhee, S.; Magnani, J.L.; et al. Randomized Phase 2 Study of GMI-1070 in SCD: Reduction in Time to Resolution of Vaso-Occlusive Events and Decreased Opioid Use. Blood 2015, 125, 2656–2664. [Google Scholar] [CrossRef]
- Dampier, C.D.; Telen, M.J.; Wun, T.; Brown, R.C.; Desai, P.; El Rassi, F.; Fuh, B.; Kanter, J.; Pastore, Y.; Rothman, J.; et al. A Randomized Clinical Trial of the Efficacy and Safety of Rivipansel for Sickle Cell Vaso-Occlusive Crisis. Blood 2023, 141, 168–179. [Google Scholar] [CrossRef]
- de Boer, C.; Armstrong, Z.; Lit, V.A.J.; Barash, U.; Ruijgrok, G.; Boyango, I.; Weitzenberg, M.M.; Schroder, S.P.; Sarris, A.J.C.; Meeuwenoord, N.J.; et al. Mechanism-Based Heparanase Inhibitors Reduce Cancer Metastasis in Vivo. Proc. Natl. Acad. Sci. USA 2022, 119, e2203167119. [Google Scholar] [CrossRef]
- Lee, E.C.; Nguyen, C.T.H.; Strounina, E.; Davis-Poynter, N.; Ross, B.P. Structure-Activity Relationships of GAG Mimetic-Functionalized Mesoporous Silica Nanoparticles and Evaluation of Acyclovir-Loaded Antiviral Nanoparticles with Dual Mechanisms of Action. ACS Omega 2018, 3, 1689–1699. [Google Scholar] [CrossRef]
- Tansik, G.; Kilic, E.; Beter, M.; Demiralp, B.; Kiziltas Sendur, G.; Can, N.; Ozkan, H.; Ergul, E.; Guler, M.O.; Tekinay, A.B. A Glycosaminoglycan Mimetic Peptide Nanofiber Gel as an Osteoinductive Scaffold. Biomater. Sci. 2016, 4, 1328–1339. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Xu, Z.; Hao, T.; Wang, P.G.; Zhao, W.; Li, T. Design and Synthesis of Neutralizable Fondaparinux. JACS Au 2022, 2, 2791–2799. [Google Scholar] [CrossRef]
- Samama, M.M.; Gerotziafas, G.T. Evaluation of the Pharmacological Properties and Clinical Results of the Synthetic Pentasaccharide (Fondaparinux). Thromb. Res. 2003, 109, 1–11. [Google Scholar] [CrossRef]
- Ferro, V.; Dredge, K.; Liu, L.; Hammond, E.; Bytheway, I.; Li, C.; Johnstone, K.; Karoli, T.; Davis, K.; Copeman, E.; et al. PI-88 and Novel Heparan Sulfate Mimetics Inhibit Angiogenesis. Semin. Thromb. Hemost. 2007, 33, 557–562. [Google Scholar] [CrossRef]
- Brennan, T.V.; Lin, L.; Brandstadter, J.D.; Rendell, V.R.; Dredge, K.; Huang, X.; Yang, Y. Heparan Sulfate Mimetic PG545-Mediated Antilymphoma Effects Require TLR9-Dependent NK Cell Activation. J. Clin. Investig. 2016, 126, 207–219. [Google Scholar] [CrossRef]
- Bendersky, V.; Yang, Y.; Brennan, T.V. Immunomodulatory Activities of the Heparan Sulfate Mimetic PG545. Adv. Exp. Med. Biol. 2020, 1221, 461–470. [Google Scholar] [CrossRef]
- Weissmann, M.; Bhattacharya, U.; Feld, S.; Hammond, E.; Ilan, N.; Vlodavsky, I. The Heparanase Inhibitor PG545 Is a Potent Anti-Lymphoma Drug: Mode of Action. Matrix Biol. 2019, 77, 58–72. [Google Scholar] [CrossRef]
- Xeroudaki, M.; Peebo, B.; Germundsson, J.; Fagerholm, P.; Lagali, N. RGTA in Corneal Wound Healing after Transepithelial Laser Ablation in a Rabbit Model: A Randomized, Blinded, Placebo-Controlled Study. Acta Ophthalmol. 2016, 94, 685–691. [Google Scholar] [CrossRef]
- Nickel, J.C.; Herschorn, S.; Whitmore, K.E.; Forrest, J.B.; Hu, P.; Friedman, A.J.; Baseman, A.S. Pentosan Polysulfate Sodium for Treatment of Interstitial Cystitis/Bladder Pain Syndrome: Insights from a Randomized, Double-Blind, Placebo Controlled Study. J. Urol. 2015, 193, 857–862. [Google Scholar] [CrossRef]
- van Ophoven, A.; Vonde, K.; Koch, W.; Auerbach, G.; Maag, K.P. Efficacy of Pentosan Polysulfate for the Treatment of Interstitial Cystitis/Bladder Pain Syndrome: Results of a Systematic Review of Randomized Controlled Trials. Curr. Med. Res. Opin. 2019, 35, 1495–1503. [Google Scholar] [CrossRef]
- Galli, M.; Chatterjee, M.; Grasso, M.; Specchia, G.; Magen, H.; Einsele, H.; Celeghini, I.; Barbieri, P.; Paoletti, D.; Pace, S.; et al. Phase I Study of the Heparanase Inhibitor Roneparstat: An Innovative Approach for Ultiple Myeloma Therapy. Haematologica 2018, 103, e469–e472. [Google Scholar] [CrossRef]
- O’Reilly, E.M.; Roach, J.; Miller, P.; Yu, K.H.; Tjan, C.; Rosano, M.; Krause, S.; Avery, W.; Wolf, J.; Flaherty, K.; et al. Safety, Pharmacokinetics, Pharmacodynamics, and Antitumor Activity of Necuparanib Combined with Nab-Paclitaxel and Gemcitabine in Patients with Metastatic Pancreatic Cancer: Phase I Results. Oncologist 2017, 22, 1429. [Google Scholar] [CrossRef]
- Biemond, B.J.; Tombak, A.; Kilinc, Y.; Al-Khabori, M.; Abboud, M.; Nafea, M.; Inati, A.; Wali, Y.; Kristensen, J.; Kowalski, J.; et al. Sevuparin for the Treatment of Acute Pain Crisis in Patients with Sickle Cell Disease: A Multicentre, Randomised, Double-Blind, Placebo-Controlled, Phase 2 Trial. Lancet Haematol. 2021, 8, e334–e343. [Google Scholar] [CrossRef]
- Maitland, K.; Hamaluba, M.; Obonyo, N.; Oguda, E.; Mogoka, C.; Williams, T.N.; Chaponda, M.; Miti, S.; Kamavu, L.K.; Jonathan Gwasupika, J.; et al. SEVUparin as a Potential Adjunctive Treatment in Children with Severe Malaria: A Phase I Trial Safety and Dose Finding Trial (SEVUSMAART). Wellcome Open Res. 2024, 8, 484. [Google Scholar] [CrossRef]
- Kishimoto, T.K.; Viswanathan, K.; Ganguly, T.; Elankumaran, S.; Smith, S.; Pelzer, K.; Lansing, J.C.; Sriranganathan, N.; Zhao, G.; Galcheva-Gargova, Z.; et al. Contaminated Heparin Associated with Adverse Clinical Events and Activation of the Contact System. New Engl. J. Med. 2008, 358, 2457–2467. [Google Scholar] [CrossRef]
- Blossom, D.B.; Kallen, A.J.; Patel, P.R.; Elward, A.; Robinson, L.; Gao, G.; Langer, R.; Perkins, K.M.; Jaeger, J.L.; Kurkjian, K.M.; et al. Outbreak of Adverse Reactions Associated with Contaminated Heparin. New Engl. J. Med. 2008, 359, 2674–2684. [Google Scholar] [CrossRef]
- Hauck, S.; Zager, P.; Halfter, N.; Wandel, E.; Torregrossa, M.; Kakpenova, A.; Rother, S.; Ordieres, M.; Räthel, S.; Berg, A.; et al. Collagen/Hyaluronan Based Hydrogels Releasing Sulfated Hyaluronan Improve Dermal Wound Healing in Diabetic Mice via Reducing Inflammatory Macrophage Activity. Bioact. Mater. 2021, 6, 4342–4359. [Google Scholar] [CrossRef]
- Franz, S.; Allenstein, F.; Kajahn, J.; Forstreuter, I.; Hintze, V.; Möller, S.; Simon, J.C. Artificial Extracellular Matrices Composed of Collagen i and High-Sulfated Hyaluronan Promote Phenotypic and Functional Modulation of Human pro-Inflammatory M1 Macrophages. Acta Biomater. 2013, 9, 5621–5629. [Google Scholar] [CrossRef] [PubMed]
- Jouy, F.; Lohmann, N.; Wandel, E.; Ruiz-Gómez, G.; Pisabarro, M.T.; Beck-Sickinger, A.G.; Schnabelrauch, M.; Möller, S.; Simon, J.C.; Kalkhof, S.; et al. Sulfated Hyaluronan Attenuates Inflammatory Signaling Pathways in Macrophages Involving Induction of Antioxidants. Proteomics 2017, 17, 1700082. [Google Scholar] [CrossRef] [PubMed]
- Hammond, E.; Haynes, N.M.; Cullinane, C.; Brennan, T.V.; Bampton, D.; Handley, P.; Karoli, T.; Lanksheer, F.; Lin, L.; Yang, Y.; et al. Immunomodulatory Activities of Pixatimod: Emerging Nonclinical and Clinical Data, and Its Potential Utility in Combination with PD-1 Inhibitors. J. Immunother. Cancer 2018, 6, 54. [Google Scholar] [CrossRef]
- Garcia-Filipe, S.; Barbier-Chassefiere, V.; Alexakis, C.; Huet, E.; Ledoux, D.; Kerros, M.E.; Petit, E.; Barritault, D.; Caruelle, J.P.; Kern, P. RGTA OTR4120, a Heparan Sulfate Mimetic, Is a Possible Long-Term Active Agent to Heal Burned Skin. J. Biomed. Mater. Res. A 2007, 80, 75–84. [Google Scholar] [CrossRef]
- Zakine, G.; Barbier, V.; Garcia-Filipe, S.; Luboinski, J.; Papy-Garcia, D.; Chachques, J.C.; Carpentier, A.; Barritault, D. Matrix Therapy with RGTA OTR4120 Improves Healing Time and Quality in Hairless Rats with Deep Second-Degree Burns. Plast. Reconstr. Surg. 2011, 127, 541–550. [Google Scholar] [CrossRef] [PubMed]
| Target Protein | GAG Mimetic | Cells/Model | Outcome/Limitation | Ref. |
|---|---|---|---|---|
| Fibrosis | ||||
| TGF-β1 | sHA |
|
| [73] |
| - | Sulfated alginate |
|
| [74] |
| Inflammation | ||||
| CCL20, L-selectin | 2,4-O-di- sulfated iduronic acid (Di-S-IdoA) |
|
| [75] |
| ERK1/2 signaling | PG545 |
|
| [76] |
| Cancer | ||||
| CCL5 | Carboxylated dextran sulfates OTR4120, OTR4131 |
|
| [77] |
| Growth factors (not specified) | Sulfated alginate (degree of substitution 22.0, 2.7) |
|
| [78] |
| FGF-1, FGF-2, VEGF-A, heparinase, TLR9 | PG545 |
|
| [79,80] |
| Diabetes | ||||
| Proinflammatory cytokines (IL-1β, TNF-α, IFN-γ) | Low- molecular- weight dextran sulfate |
|
| [81] |
| Cardiovascular diseases | ||||
| FGF-2, VEGF-A | Dextran sulfate OTR4131 |
|
| [82] |
| Infectious diseases | ||||
| SARS-CoV-2 | PG545 |
|
| [42] |
| Bone regeneration | ||||
| Sclerostin | High- sulfated HA (sHA3) |
|
| [83] |
| FGF-2 | Dextran sulfate OTR4131, OTR4120 |
|
| [84] |
| Chronic wounds | ||||
| TIMP-3 | sHA3, over- sulfated CS |
|
| [38,85] |
| GAG-binding growth factors (not specified) | Cacipliq20 (RGTA) |
|
| [86] |
| Cartilage tissue engineering | ||||
| TGF-β3, lysozyme | Fully sulfated sodium cellulose sulfate |
|
| [87] |
| Neural tissue engineering | ||||
| Nerve growth factor (NGF) | Fully and partially sulfated cellulose sulfate |
|
| [88] |
| Target Protein | GAG Mimetic | Cells/Model | Outcome/Limitation | Ref. |
|---|---|---|---|---|
| Inflammation | ||||
| E-selectin | GMI-1070 (rivipansel) |
|
| [89,90,91] |
| Cancer | ||||
| Heparinase | Cyclo- phellitol- derived heparinase inhibitors |
|
| [92] |
| Infectious diseases | ||||
| Herpes simplex virus (HSV) | Benzene sulfonate- functionalized mesoporous silica nanoparticles |
|
| [93] |
| Bone regeneration | ||||
| GAG-binding growth factors (especially BMP-2) | Peptide amphiphile molecule (Lauryl- VVAGEGD (Kp- sulfo- benzoate)S) -Am) |
|
| [94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Junker, F.; Rother, S. From Native Glycosaminoglycans to Mimetics: Design, Mechanisms, and Biomedical Applications. Biomolecules 2025, 15, 1518. https://doi.org/10.3390/biom15111518
Junker F, Rother S. From Native Glycosaminoglycans to Mimetics: Design, Mechanisms, and Biomedical Applications. Biomolecules. 2025; 15(11):1518. https://doi.org/10.3390/biom15111518
Chicago/Turabian StyleJunker, Fabian, and Sandra Rother. 2025. "From Native Glycosaminoglycans to Mimetics: Design, Mechanisms, and Biomedical Applications" Biomolecules 15, no. 11: 1518. https://doi.org/10.3390/biom15111518
APA StyleJunker, F., & Rother, S. (2025). From Native Glycosaminoglycans to Mimetics: Design, Mechanisms, and Biomedical Applications. Biomolecules, 15(11), 1518. https://doi.org/10.3390/biom15111518








