T7-Synthesized Double-Stranded RNA Mimicking miR-71 Induces Termite RNAi and Increases Fungal Efficacy
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Entomopathogen Cultivation
2.3. Synthesis of miRNA Mimics and dsRNA
2.4. RNA Injection and Fungal Contamination
2.5. RNA Extraction, Library Preparation, and RNA-Seq
2.6. RT-qPCR Analysis
2.7. TMT-Based Quantitative Proteomics
2.8. Statistical Analysis
3. Results
3.1. Effects of T7-Synthesized miR-71 Mimic Vs. Mir-71 Agomir on Fungal Efficacy
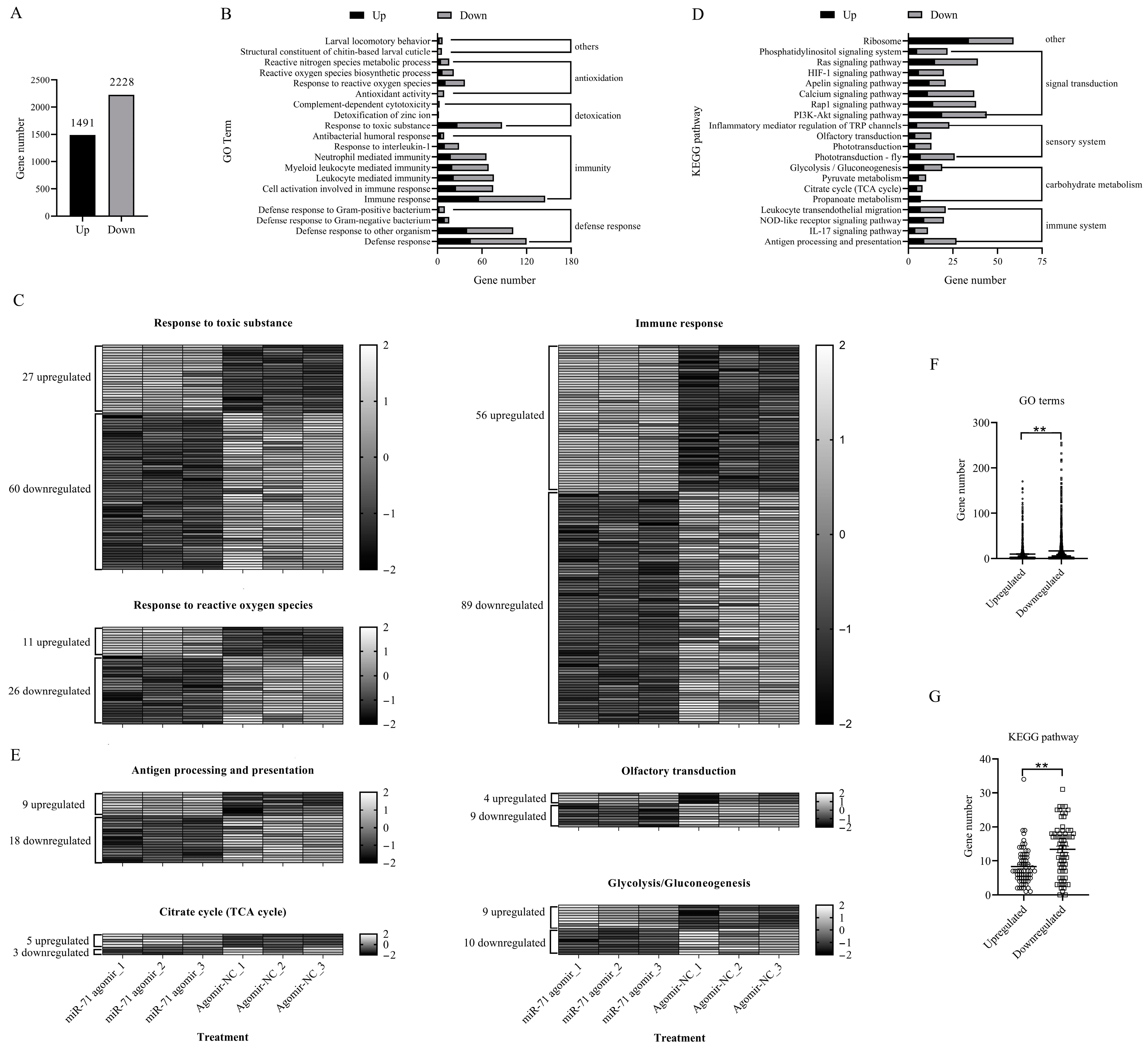
3.2. Effects of miR-71 Agomir on Termite Gene Expression Profiles
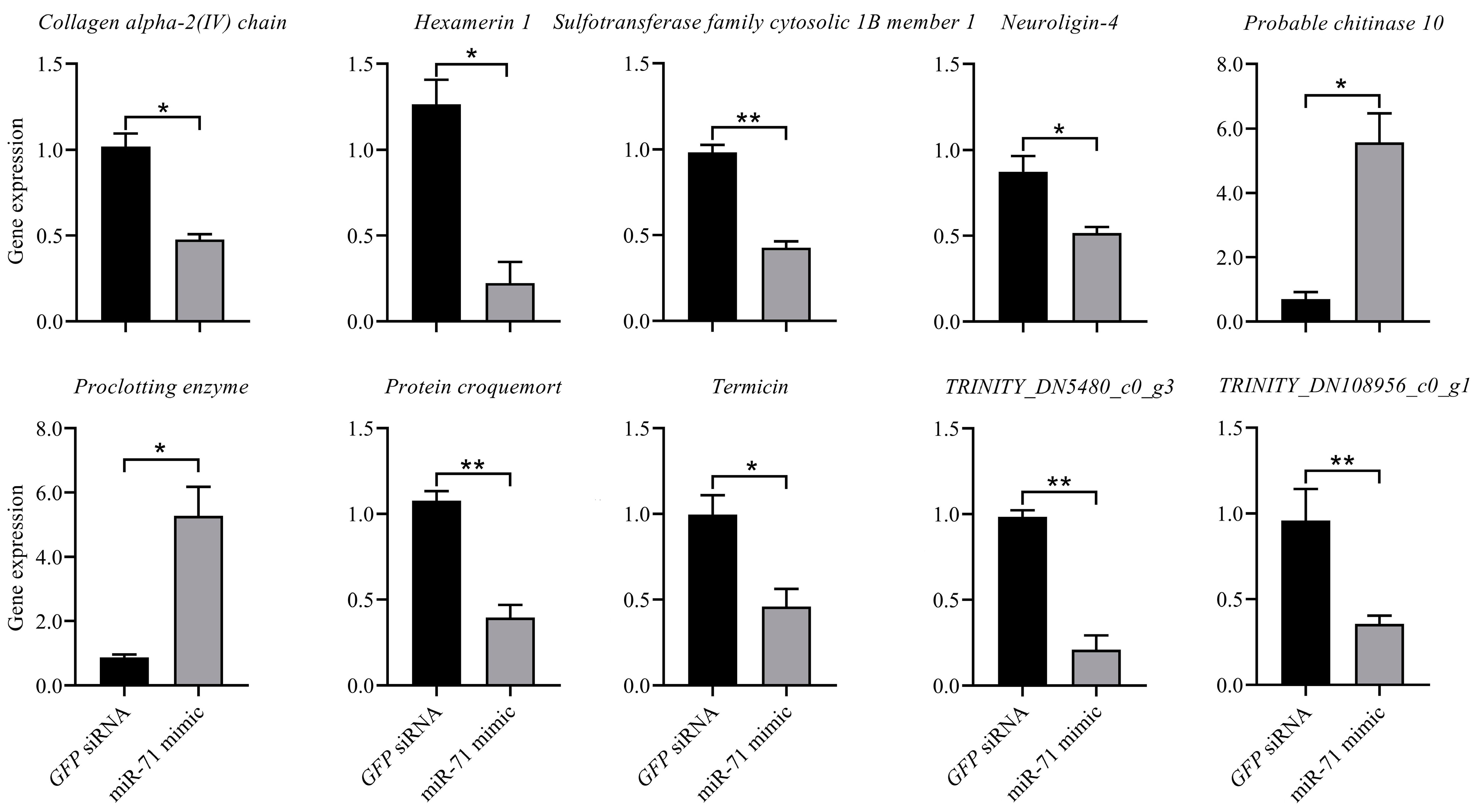
3.3. RT-qPCR Verification for RNAi Mediated by T7-Synthesized miR-71 Mimic
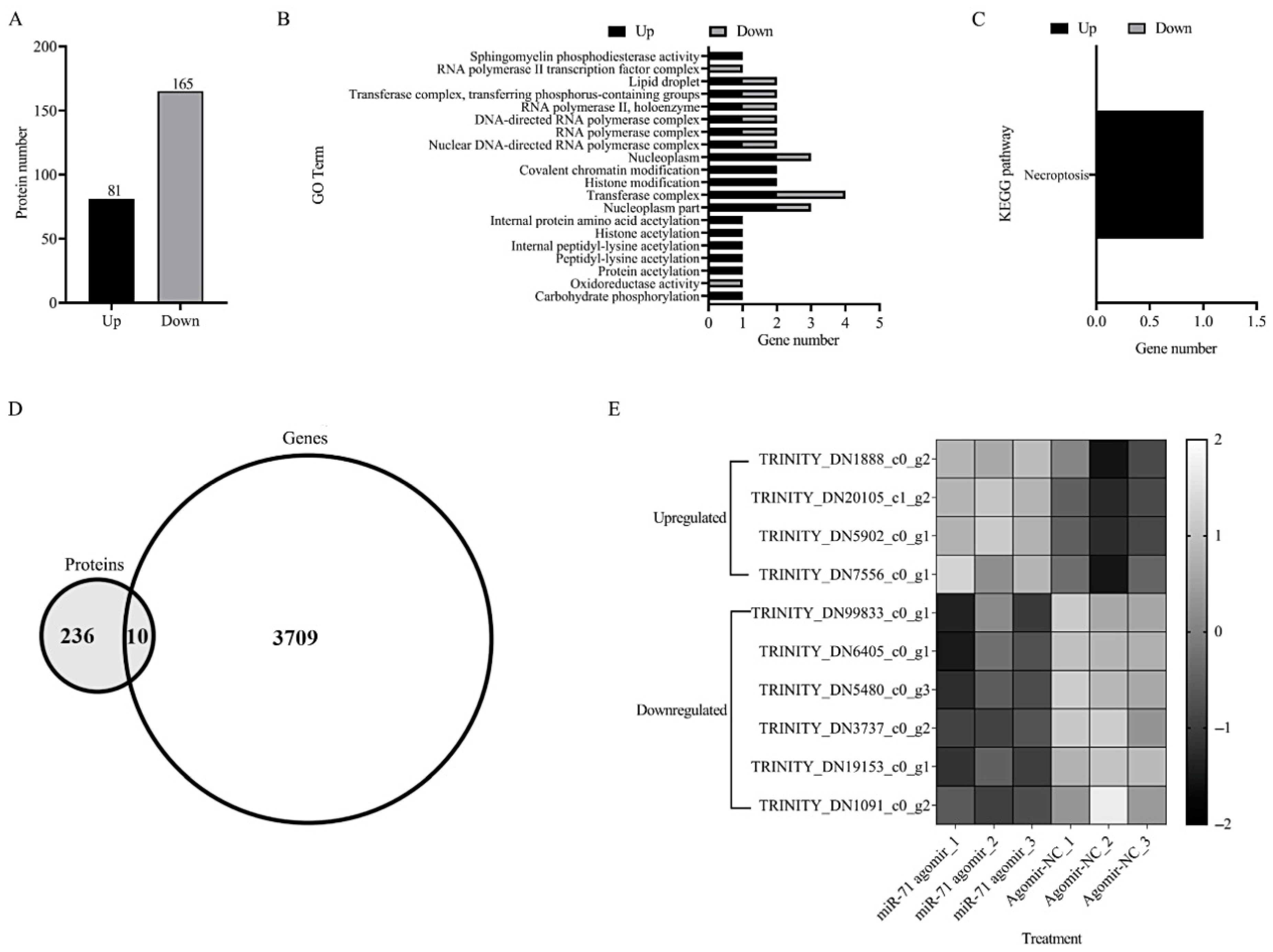
3.4. Further Verification by Tandem Mass Tag (TMT)-Based Quantitative Proteomics
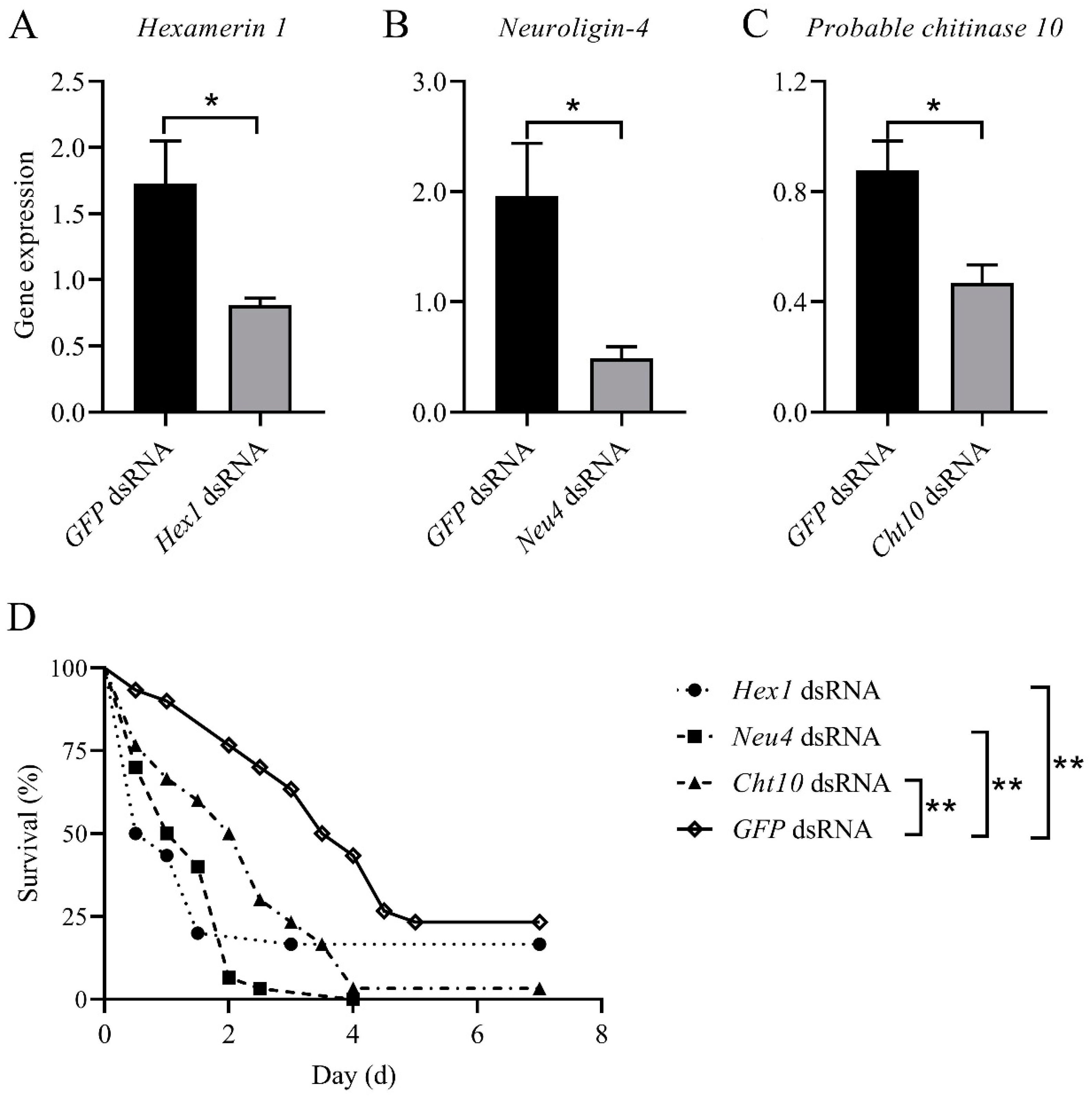
3.5. Effects of the Genes Targeted by miR-71 on Fungal Efficacy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Riegler, M. Insect threats to food security. Science 2018, 361, 846. [Google Scholar] [CrossRef]
- Zanne, A.E.; Flores-Moreno, H.; Powell, J.R.; Cornwell, W.K.; Dalling, J.W.; Austin, A.T.; Classen, A.T.; Eggleton, P.; Okada, K.-i.; Parr, C.L.; et al. Termite sensitivity to temperature affects global wood decay rates. Science 2022, 377, 1440–1444. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Zeng, Y.; Ning, J.; Wu, X.; Wang, W.; Ren, J.; Wu, Q.; Yang, X.; Wang, S.; Guo, Z.; et al. A plant virus manipulates both its host plant and the insect that facilitates its transmission. Sci. Adv. 2025, 11, eadr4563. [Google Scholar] [CrossRef] [PubMed]
- Kasai, S.; Itokawa, K.; Uemura, N.; Takaoka, A.; Furutani, S.; Maekawa, Y.; Kobayashi, D.; Imanishi-Kobayashi, N.; Amoa-Bosompem, M.; Murota, K.; et al. Discovery of super–insecticide-resistant dengue mosquitoes in Asia: Threats of concomitant knockdown resistance mutations. Sci. Adv. 2022, 8, eabq7345. [Google Scholar] [CrossRef]
- Wan, N.-F.; Fu, L.; Dainese, M.; Kiær, L.P.; Hu, Y.-Q.; Xin, F.; Goulson, D.; Woodcock, B.A.; Vanbergen, A.J.; Spurgeon, D.J.; et al. Pesticides have negative effects on non-target organisms. Nat. Commun. 2025, 16, 1360. [Google Scholar] [CrossRef]
- Scholte, E.-J.; Ng’habi, K.; Kihonda, J.; Takken, W.; Paaijmans, K.; Abdulla, S.; Killeen, G.F.; Knols, B.G.J. An Entomopathogenic Fungus for Control of Adult African Malaria Mosquitoes. Science 2005, 308, 1641–1642. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Hong, B.; Reichelt, M.; Luck, K.; Mai, D.T.; Jiang, X.; Gershenzon, J.; Vassão, D.G. Metabolism of plant-derived toxins from its insect host increases the success of the entomopathogenic fungus Beauveria bassiana. ISME J. 2023, 17, 1693–1704. [Google Scholar] [CrossRef]
- Cui, C.; Wang, Y.; Liu, J.; Zhao, J.; Sun, P.; Wang, S. A fungal pathogen deploys a small silencing RNA that attenuates mosquito immunity and facilitates infection. Nat. Commun. 2019, 10, 4298. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, J.; Kim, D.S.; Zhang, J. The essential role of gut microbiota in dsRNA-mediated pest control of the phytophagous ladybird beetle, Henosepilachna vigintioctopunctata. NPJ Biofilms. Microbiomes 2025, 11, 141. [Google Scholar] [CrossRef]
- Cui, C.; Wang, Y.; Li, Y.; Sun, P.; Jiang, J.; Zhou, H.; Liu, J.; Wang, S. Expression of mosquito miRNAs in entomopathogenic fungus induces pathogen-mediated host RNA interference and increases fungal efficacy. Cell Rep. 2022, 41, 111527. [Google Scholar] [CrossRef]
- Chendrimada, T.P.; Finn, K.J.; Ji, X.; Baillat, D.; Gregory, R.I.; Liebhaber, S.A.; Pasquinelli, A.E.; Shiekhattar, R. MicroRNA silencing through RISC recruitment of eIF6. Nature 2007, 447, 823–828. [Google Scholar] [CrossRef]
- Dang, C.; Xiao, S.; Wang, F.; Fang, Q.; Yao, H.; He, K.; Li, F.; Xue, D.; Ye, G. miRNA-mediated insect-resistant transgenic rice poses no risk to a non-target parasitoid, Cotesia chilonis, via direct feeding or through its target host. Insect Sci. 2025, 32, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Sheng, C.W.; Karthi, S.; Jiang, N.; Zhang, C.; Du, H.; Zhao, K.; Liu, S.; Li, M.Y.; Chen, J. New Insights into Expanding the Insecticidal Spectrum of dsRNA Mediated by the High Sequence Identity between dsRNA and Nontarget mRNA. J. Agric. Food Chem. 2025, 73, 4605–4616. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; He, H.; Zhao, C.; Lei, C.; Li, J.; Yan, F.M. Potential of Virus-Mediated RNAi of Insect Genes in Plants to Control Aphids. J. Agric. Food Chem. 2025, 73, 7716–7724. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yan, F.M.; Zhao, C.C.; Su, L.J.; Huang, Q.Y.; Tang, Q.B. microRNAs shape social immunity: A potential target for biological control of the termite Reticulitermes chinensis. J. Pest Sci. 2023, 96, 265–279. [Google Scholar] [CrossRef]
- Song, X.; Li, Y.; Cao, X.; Qi, Y. MicroRNAs and Their Regulatory Roles in Plant–Environment Interactions. Annu. Rev. Plant Biol. 2019, 70, 489–525. [Google Scholar] [CrossRef]
- Tian, M.M.; Zhang, Y.Q.; Zhu, X.K.; Wen, D.; Li, Y.F.; Qiao, Z.Z.; Xu, Z.; Ma, W.H.; Zhu, Z.H.; Hua, H.X. MicroRNA miR-7-5p|-1|0| regulates wing development in brown planthoppers, Nilaparvata lugens. Pest Manag. Sci. 2025, 81, 7012–7018. [Google Scholar] [CrossRef]
- Khan, A.; Smagghe, G.; Li, S.; Shakeel, M.; Yang, G.; Ahmed, N. Insect metamorphosis and chitin metabolism under miRNA regulation: A review with current advances. Pest Manag. Sci. 2025, 81, 3437–3451. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, D.Q.; Li, C.; Yang, W.J.; Xu, K.K. Disruption of microRNA pathway core genes inhibits molting and reproduction of the cigarette beetle, Lasioderma serricorne. Pest Manag. Sci. 2024, 80, 4543–4552. [Google Scholar] [CrossRef]
- Liu, H.; Shen, E.; Wu, H.; Ma, W.; Chen, H.; Lin, Y. Trans-kingdom expression of an insect endogenous microRNA in rice enhances resistance to striped stem borer Chilo suppressalis. Pest Manag. Sci. 2022, 78, 770–777. [Google Scholar] [CrossRef]
- Zhang, L.F.; Lou, J.T.; Lu, M.H.; Gao, C.; Zhao, S.; Li, B.; Liang, S.; Li, Y.; Li, D.; Liu, M.F. Suppression of miR-199a maturation by HuR is crucial for hypoxia-induced glycolytic switch in hepatocellular carcinoma. EMBO J. 2015, 34, 2671–2685. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, Z.; Sun, S.; Wu, J.; Li, R.; Wang, H.; Cui, X. SISTER OF TM3 activates FRUITFULL1 to regulate inflorescence branching in tomato. Hortic. Res. 2021, 8, 251. [Google Scholar] [CrossRef]
- Betti, F.; Ladera-Carmona, M.J.; Weits, D.A.; Ferri, G.; Iacopino, S.; Novi, G.; Svezia, B.; Kunkowska, A.B.; Santaniello, A.; Piaggesi, A.; et al. Exogenous miRNAs induce post-transcriptional gene silencing in plants. Nat. Plants 2021, 7, 1379–1388. [Google Scholar] [CrossRef]
- Liu, L.; Wang, D.H.; Zhao, C.C.; Yan, F.M.; Lei, C.L.; Su, L.J.; Zhang, Y.C.; Huang, Q.Y.; Tang, Q.B. Transcriptomics Reveals the Killing Mechanism by Which Entomopathogenic Fungi Manipulate the RNA Expression Profiles of Termites and Provides Inspiration for Green Pest Management. J. Agric. Food Chem. 2023, 71, 7152–7162. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, C.-C.; Zhao, X.-Y.; Guan, J.-X.; Lei, C.-L.; Huang, Q.-Y. Isocitrate dehydrogenase-mediated metabolic disorders disrupt active immunization against fungal pathogens in eusocial termites. J. Pest Sci. 2020, 93, 291–301. [Google Scholar] [CrossRef]
- Shang, R.; Lee, S.; Senavirathne, G.; Lai, E.C. microRNAs in action: Biogenesis, function and regulation. Nat. Rev. Genet. 2023, 24, 816–833. [Google Scholar] [CrossRef]
- Mao, Y.B.; Liu, Y.Q.; Chen, D.Y.; Chen, F.Y.; Fang, X.; Hong, G.J.; Wang, L.J.; Wang, J.W.; Chen, X.Y. Jasmonate response decay and defense metabolite accumulation contributes to age-regulated dynamics of plant insect resistance. Nat. Commun. 2017, 8, 13925. [Google Scholar] [CrossRef]
- Dai, Z.; Tan, J.; Zhou, C.; Yang, X.; Yang, F.; Zhang, S.; Sun, S.; Miao, X.; Shi, Z. The OsmiR396-OsGRF8-OsF3H-flavonoid pathway mediates resistance to the brown planthopper in rice (Oryza sativa). Plant Biotechnol. J. 2019, 17, 1657–1669. [Google Scholar] [CrossRef]
- Fan, D.; Ran, L.; Hu, J.; Ye, X.; Xu, D.; Li, J.; Su, H.; Wang, X.; Ren, S.; Luo, K. miR319a/TCP module and DELLA protein regulate trichome initiation synergistically and improve insect defenses in Populus tomentosa. New Phytol. 2020, 227, 867–883. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X.; Xiao, X.; He, X.C.; He, B.D.; Liu, C.M.; Teng, Z.Q. Agomir-331 Suppresses Reactive Gliosis and Neuroinflammation after Traumatic Brain Injury. Cells 2023, 12, 2429. [Google Scholar] [CrossRef]
- He, X.C.; Wang, J.; Du, H.Z.; Liu, C.M.; Teng, Z.Q. Intranasal Administration of Agomir-let-7i Improves Cognitive Function in Mice with Traumatic Brain Injury. Cells 2022, 11, 1348. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Qiu, F.; Cao, H.; Li, H.; Dai, G.; Ma, T.; Gong, Y.; Luo, W.; Zhu, D.; Qiu, Z.; et al. Therapeutic delivery of microRNA-125a-5p oligonucleotides improves recovery from myocardial ischemia/reperfusion injury in mice and swine. Theranostics 2023, 13, 685–703. [Google Scholar] [CrossRef]
- Connaghan-Jones, K.D.; Moody, A.D.; Bain, D.L. Quantitative DNase footprint titration: A tool for analyzing the energetics of protein-DNA interactions. Nat. Protoc. 2008, 3, 900–914. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, Y.; Wang, Y.; Shi, C.; Li, C.; Li, Q.; Linhardt, R.J. Bacteriophage T7 transcription system: An enabling tool in synthetic biology. Biotechnol. Adv. 2018, 36, 2129–2137. [Google Scholar] [CrossRef] [PubMed]
- Quilez-Molina, A.I.; Niño Sanchez, J.; Merino, D. The role of polymers in enabling RNAi-based technology for sustainable pest management. Nat. Commun. 2024, 15, 9158. [Google Scholar] [CrossRef]
- Zhong, X.; Yu, X.; Zhang, J.; Xu, J.; Qin, M.; Cao, M.; Francis, F.; Xia, L. RNAi technologies for insect control in crop protection. Crop J. 2025, 13, 1009–1021. [Google Scholar] [CrossRef]
- Zhu, L.; Li, H.; Tao, Z.; Ma, F.; Wu, S.; Miao, X.; Cao, L.; Shi, Z. The microRNA OsmiR393 regulates rice brown planthopper resistance by modulating the auxin-ROS signaling cross-talk. Sci. Adv. 2025, 11, eadu6722. [Google Scholar] [CrossRef]
- Bai, S.; Yao, Z.; Cai, Z.; Ma, Q.; Guo, Q.; Zhang, P.; Zhou, Q.; Gu, J.; Liu, S.; Lemaitre, B.; et al. Bacterial-induced Duox-ROS regulates the Imd immune pathway in the gut by modulating the peritrophic matrix. Cell Rep. 2025, 44, 115404. [Google Scholar] [CrossRef]
- Dolezal, T.; Krejcova, G.; Bajgar, A.; Nedbalova, P.; Strasser, P. Molecular regulations of metabolism during immune response in insects. Insect Biochem. Mol. Biol. 2019, 109, 31–42. [Google Scholar] [CrossRef]
- Arrese, E.L.; Soulages, J.L. Insect Fat Body: Energy, Metabolism, and Regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef]
- Zheng, R.; Xie, M.; Keyhani, N.O.; Xia, Y. An insect chemosensory protein facilitates locust avoidance to fungal pathogens via recognition of fungal volatiles. Int. J. Biol. Macromol. 2023, 253, 127389. [Google Scholar] [CrossRef]
- Takeuchi, H.; Kurahashi, T. Suppression of olfactory signal transduction by insecticides. NPJ Sci. Food 2019, 3, 9. [Google Scholar] [CrossRef]
- Chen, Y.; Ou, J.; Liu, Y.; Wu, Q.; Wen, L.; Zheng, S.; Li, S.; Feng, Q.; Liu, L. Transcriptomic analysis of the testicular fusion in Spodoptera litura. BMC Genom. 2020, 21, 171. [Google Scholar] [CrossRef]
- Moussian, B. Recent advances in understanding mechanisms of insect cuticle differentiation. Insect Biochem. Mol. Biol. 2010, 40, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lu, Z.; Keyhani, N.O.; Deng, J.; Zhao, X.; Huang, S.; Luo, Z.; Jin, K.; Zhang, Y. Insect fungal pathogens secrete a cell wall-associated glucanase that acts to help avoid recognition by the host immune system. PLoS Pathog. 2023, 19, e1011578. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, M.; Feng, K.; Sun, H.; Tang, F. The Function of Termicin from Odontotermes formosanus (Shiraki) in the Defense against Bacillus thuringiensis (Bt) and Beauveria bassiana (Bb) Infection. Insects 2024, 15, 360. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Lu, H.; Cheng, R.; Zhao, P.; Zhang, G.; Chen, H.; Tang, Q.; Liu, L. T7-Synthesized Double-Stranded RNA Mimicking miR-71 Induces Termite RNAi and Increases Fungal Efficacy. Biomolecules 2025, 15, 1517. https://doi.org/10.3390/biom15111517
Zhao C, Lu H, Cheng R, Zhao P, Zhang G, Chen H, Tang Q, Liu L. T7-Synthesized Double-Stranded RNA Mimicking miR-71 Induces Termite RNAi and Increases Fungal Efficacy. Biomolecules. 2025; 15(11):1517. https://doi.org/10.3390/biom15111517
Chicago/Turabian StyleZhao, Chenchen, Hang Lu, Ruotian Cheng, Pengfei Zhao, Gaoling Zhang, Hongsong Chen, Qingbo Tang, and Long Liu. 2025. "T7-Synthesized Double-Stranded RNA Mimicking miR-71 Induces Termite RNAi and Increases Fungal Efficacy" Biomolecules 15, no. 11: 1517. https://doi.org/10.3390/biom15111517
APA StyleZhao, C., Lu, H., Cheng, R., Zhao, P., Zhang, G., Chen, H., Tang, Q., & Liu, L. (2025). T7-Synthesized Double-Stranded RNA Mimicking miR-71 Induces Termite RNAi and Increases Fungal Efficacy. Biomolecules, 15(11), 1517. https://doi.org/10.3390/biom15111517






