Abstract
Alzheimer’s disease (AD) is a progressive neurodegenerative condition with increasing global prevalence. As early diagnosis becomes critical for timely symptomatic management, noninvasive and easily accessible biomarkers are needed. Given the shared embryologic origins between the eye and the brain, ocular imaging has emerged as a promising diagnostic technique. This review summarizes the associations between AD, ocular imaging and fluid biomarkers in the anterior and posterior segment. We also describe the underlying pathophysiology that explains the connections between each ocular structure and the brain in the context of AD. Optical coherence tomography (OCT), OCT angiography, and fundus photography are the most common imaging modalities utilized in AD research. However, these techniques may or may not be feasible in primary care or neurologic clinical settings. Compared to plasma biomarker analysis, which is minimally invasive and nearing clinical implementation, ocular biomarkers remain primarily valuable in research investigations.
1. Introduction
Alzheimer’s disease (AD) is the most common type of dementia worldwide, with a projected global prevalence of 131.5 million by 2050 [1]. It is currently the seventh leading cause of death in the United States, with no known cure [2]. The earliest symptom of AD is typically short-term memory loss, often followed by progressive decline in executive functioning, language, and visuospatial skills as well as neuropsychiatric symptoms in moderate to late stages [2].
The pathogenesis of AD is characterized by the loss of cholinergic neurons and the extracellular accumulation of amyloid-β (Aβ) peptide resulting from the impaired degradation of amyloid precursor protein (APP) [2]. Histopathological examination of the brain in AD demonstrates hallmark features such as neuritic plaques (extracellular Aβ deposits), intracellular neurofibrillary tangles of hyperphosphorylated tau (pTau), and widespread cortical neuronal degeneration [2]. These neuropathological changes can begin decades before the onset of clinical symptoms, which often limits the effectiveness of treatment initiated later in the disease course. Diagnosis is primarily based on clinical evaluation, functional assessment, and neuropsychological testing. Confirmation of AD pathology can be achieved with fluorodeoxyglucose–positron emission technology and cerebrospinal fluid (CSF) analysis [2]. However, these diagnostic techniques are invasive, costly, and largely confined to academic health centers and research settings. This highlights the urgent need for alternative diagnostic approaches that can enable earlier detection and therapeutic intervention.
Given that the eye and the brain share a common embryological origin [3,4,5], neurodegenerative disease processes associated with AD may be observed in ocular tissue [6]. This narrative review aims to explore the role of ocular protein biomarkers and imaging in the detection of AD. We examine structures within both the anterior and posterior segments of the eye, as well as ocular fluids including tear secretions, aqueous humor, and vitreous humor.
Ocular Anatomy and Function
Figure 1 demonstrates the structures of the eye. The conjunctiva is a thin, vascularized mucous membrane that lines the eyelids and globe, and functions to protect the ocular surface, support immune surveillance, and produce mucin for the tear film for lubrication and barrier function [7]. The cornea provides the majority of the eye’s refractive power while maintaining a transparent, avascular barrier [8]. The cornea is comprised of five layers, including an epithelium with tight junctions, Bowman’s layer, stroma, Descemet’s membrane, and endothelium [8]. These layers regulate hydration and block pathogens [8]. The cornea is heavily innervated, leading to blink reflexes, wound healing, and tear film production [9]. The ocular surface is coated by a tear fluid which is made of secretions containing lipids, proteins, and electrolytes from the lacrimal gland, meibomian gland, and conjunctival goblet cells [10]. This tear film lubricates, nourishes, and protects the surface, which can influence vision quality [10].
The iris is a muscle that modulates the pupillary diameter via sphincter and dilator muscles, as well as signals from the autonomic nervous system, and helps reduce optical aberrations [11,12,13]. Behind the iris, the crystalline lens is a transparent, avascular structure composed of fiber cells surrounded by a capsule [14]. The lens is responsible for refraction and accommodation [14]. These structures, from the cornea to the lens, form the anterior chamber of the eye, which is full of aqueous humor, a fluid secreted by the ciliary body [15]. This fluid exits the eye via the trabecular meshwork–Schlemm’s canal pathway and the uveoscleral pathway [15]. Decreased fluid drainage can cause irreversible vision loss in glaucoma [15].
The posterior chamber (the space between the lens and retina) is composed of vitreous humor, a collagenous substance that provides mechanical support for the globe [16]. Over time, this fluid liquefies and the collagen network aggregates, predisposing aging individuals to posterior vitreous detachment and other retinal complications [16]. The retina converts photons of light to neural signals via phototransduction in rod and cone photoreceptor cells [17]. The retina has other specialized cells, including bipolar, horizontal, amacrine, and ganglion cells, for detecting contrast and motion [17]. The neural signals are transmitted to the retinal ganglion cell axons which come together to form the optic nerve [18]. The optic nerve is connected to areas of the brain including the lateral geniculate nucleus in the thalamus and the superior colliculus in the midbrain [18].
The choroid is deep to the retina and composed of the choriocapillaris, Sattler’s and Haller’s layers, Bruch’s membrane, and the suprachoroid [19]. The choroid provides blood flow to the outer retina and retinal pigment epithelium, and removes metabolic waste from the photoreceptor cells [19].
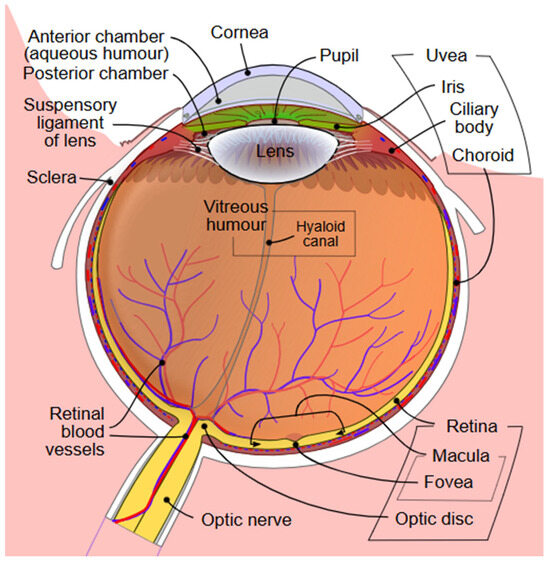
Figure 1.
Diagram of the anterior and posterior chambers of the eye [20].
Table 1 summarizes the associations between each ocular structure and AD that will be discussed in this review.

Table 1.
Associations Between Ocular Tissue and AD.
2. Anterior Segment
2.1. Conjunctiva
To our knowledge, there exists one report regarding the association between conjunctival microcirculation and AD. Smith et al. compared vascular parameters of the conjunctival microcirculation between patients with and without AD using computer-assisted intravital microscopy, and found that participants with AD had a significantly higher whole blood viscosity and greater severity of vasculopathy [21]. This suggests that AD pathology is associated with microvascular abnormalities in the external eye, though the underlying mechanism and temporal relationship remain unclear.
2.2. Cornea
Nerve growth factor is responsible for nourishing cholinergic neurons in the basal forebrain. In the pathogenesis of AD, the degeneration of cholinergic neurons is accompanied by decreases in nerve growth factor. The cornea contains high levels of both nerve growth factor and acetylcholine, so neuronal degeneration in the forebrain may also be reflected in the cornea, leading to changes in corneal nerve fibers [22,23,24]. In fact, corneal changes in sensitivity and morphology have been associated with the presence of AD, and proposed as biomarkers for AD detection.
Four in vivo studies have revealed variable but significant changes in corneal nerve and cells among patients with AD compared to cognitively normal controls based on confocal microscopy and esthesiometry assessment. Örnek et al. reported a significantly lower corneal sensitivity and increased dendritic cell density in patients with AD compared to age- and sex-matched control patients [25]. Ponirakis also noted a stepwise decrease in corneal nerve fiber density, branch density, and fiber length between healthy controls, patients with mild cognitive impairment, and those with AD [26]. Similarly, Gundogan et al. found significantly lower corneal nerve fiber length, fiber density, branch density, and corneal sensitivity in patients with AD compared to controls [22]. In contrast, Dehghani et al. reported no significant differences in corneal nerve fiber length, fiber density, branch density, and diameter between AD and cognitively normal patients [27]. The discrepancy in findings could be due to the small, heterogenous patient cohorts and cross-sectional study designs.
Overall, these studies are limited by a relatively low sample size and cross-sectional nature, and may be confounded by the presence of other ocular and systemic conditions that impact corneal nerve fiber parameters. Observational cohort clinical trials are necessary to evaluate the effectiveness of changes in corneal nerve fiber parameters as diagnostic indicators of AD.
2.3. Iris/Pupillary Reaction
Prior research shows that pupillary reaction is associated with cognitive processing, including working memory span tasks [28]. This is due to the innervation of the pupillary sphincter muscle with parasympathetic neurons, including cholinergic neurons, and the muscle’s constriction in response to acetylcholine. Therefore, patients with AD may also present with changes in pupillary reaction and increased pupillary size.
Frost et al. reported that carriers with a mutation in the APP gene had a 75% longer recovery time to a bright flash of light than non-carriers [29]. Additionally, Frost et al. found that pupillary flash response (to white light stimuli) parameters classified AD with a relatively high sensitivity (84.1%), specificity (78.3%), and area under the curve (AUC) (89.6%) [30]. El Haj et al. demonstrated lower variability in pupil size during forward and backward memory span tasks, as well as during counting among patients with AD compared to controls [28]. Fotiou et al. found that pupillary light reflex parameters, including percentage recovery-redilation, latency, time for maximum velocity, time for maximum constriction, maximum constriction velocity, maximum constriction acceleration, amplitude, and ratio of baseline pupil diameter to the minimum pupil diameter after exposure to light stimuli after 3.5 s, were significantly lower among patients with AD compared to cognitively normal patients [31]. Prettyman et al. similarly demonstrated decreased resting pupil diameter, amplitude, and maximum dilational velocity of the darkness reflex in AD patients compared to age- and sex-matched control patients [32]. Lustig-Barzelay et al. developed a chromatic pupilloperimetry-based machine learning model which discriminated between patients at high risk for developing AD based on positive family history and cognitively normal patients. Transient pupil response latency demonstrated an area under the curve (AUC) of 0.90 ± 0.051 (left-eye) and 0.87 ± 0.048 (right-eye) in this discrimination task [33].
In contrast to the aforementioned studies on pupillary response, Kawasaki et al. found that patients with AD did not have a statistically significantly different pupillary contraction amplitude to red and blue light stimuli compared to control patients, and these study groups had a similar post-illumination pupillary response [34]. Similarly, Van Stavern et al. did not report any significantly different pupil flash response parameters between preclinical AD and normal-aging study groups [35]. It is possible that early-stage AD does not alter pupil responses, but further studies evaluating patients with preclinical AD are needed to confirm this.
Based on studies published to date, pupillary changes have the potential to serve as a noninvasive marker of AD pathology, but there are equivocal findings regarding the use of pupillary parameters in discriminating patients with and without AD. Further studies must be conducted to evaluate the diagnostic and prognostic accuracy of artificial intelligence algorithms for AD trained on pupillo-perimetry data.
2.4. Crystalline Lens
The crystalline lens has been identified as a site of neurodegenerative protein deposition in AD, and this process is thought to mimic insoluble protein deposition in cataract formation. Goldstein et al. reported that beta-amyloid protein was located in the cytosol of supranuclear/dense cortical lens fiber cells in patients with AD using slit lamp photomicroscopy, mass spectrometry, and immunohistochemistry [36]. Furthermore, patients with AD but not controls had equatorial supranuclear cataracts which showed birefringent Congo red staining, characteristic of amyloid protein [36]. In their later work, Moncaster et al. used ELISA to demonstrate increased accumulation of beta-amyloid in the lenses of patients with Down Syndrome, which results in three copies of the APP gene located on chromosome 21. This study also showed supranuclear opacification and beta-amyloid deposits in the cytosol of lens fiber cells [37]. Similarly, Kerbage et al. found that a fluorescent signature indicating the presence of a ligand bound to amyloid-β in the lens could discriminate probable AD patients from age-matched healthy patients with a relatively high sensitivity (85%) and specificity (95%) [38,39].
In contrast to the aforementioned studies, Michael et al. did not find Congo red staining or beta-amyloid immunohistochemical staining consistent with AD in lenses from postmortem donors with and without AD [40]. In later work, they confirmed an absence of amyloid-beta and tau in the postmortem lenses of AD donors using confocal Raman microspectroscopy [41]. Ho et al. also found negative immunostaining for beta-amyloid and phosphorylated tau, and negative Congo red staining in the postmortem eyes of AD patients [42]. Additionally, Williams et al. did not find any tau or beta-amyloid inclusions in the lens of patients with AD using immunohistochemistry [43].
Given the presence of lens opacities in the postmortem eyes of AD patients in some studies, Bei et al. evaluated whether cataract grade or lens opacities were associated with the risk of AD among patients with and without biomarkers for AD based on positron emission tomography and cerebrospinal fluid levels of Aβ42 [44]. While participants with positive biomarkers had more advanced cataracts and cortical light scattering compared to those with negative biomarkers, these differences were not statistically significant after adjusting for age [44]. Based on these results, the authors concluded that cataract grade and lens opacities could not be used to predict the risk of AD development [44].
Given the equivocal findings regarding the use of neurodegenerative protein deposition in the crystalline lens, further work is needed to determine why some lenses of patients with AD demonstrate beta-amyloid deposition while others do not. The variability in studies evaluating neuropathologic protein deposition in the lens could be due to differences in specimen preparation and staining, postmortem vs. in vivo examinations, time interval between death and specimen processing, and confounding lens opacities [45].
3. Posterior Segment
3.1. Retina
3.1.1. Cell Layers
Optical coherence tomography (OCT) (Figure 2) [46] is a commonly used device to assess retinal tissue structure in clinical practice, and plays a central role in diagnosing and monitoring retinal disease progression and response to therapy in macular and optic nerve disorders. This non-invasive imaging technique captures cross-sectional, high-resolution images of the retinal cell layers at the macula and optic nerve. Given that these techniques allow for the analysis of changes in retinal thickness, blood flow velocity, and other structural parameters, they have been widely used in AD research.
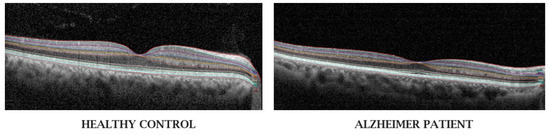
Figure 2.
Optical coherence tomography of the retina of a healthy patient and that of a patient with Alzheimer’s disease from Garcia-Martin et al. [46]. Eyes of patients with AD have inner retinal layer thinning (involving the retinal nerve fiber layer, ganglion cell layer, and ganglion cell-inner plexiform layer) compared to those of healthy controls.
There exist three main types of OCT. Time-domain OCT is one of the earliest forms of OCT, and is not widely used in clinical practice due to prolonged time for image acquisition, low axial resolution, and more recent technological advances to higher resolution [47] spectral domain-OCT (SD-OCT). SD-OCT detects the pattern of light reflected from each retinal cell layer, and is most frequently used in clinical practice today. Swept source-OCT (SS-OCT), which uses a tunable laser source, photodetector, and long-wavelength emission to capture images with greater resolution, is more expensive and mostly available to medical retina and vitreoretinal specialists in the research setting [47]. Furthermore, analysis of SS-OCT is limited by its lack of a normative database [47].
The impact of AD pathogenesis on the retina is multifactorial. Chronic activation of neuroinflammation decreases the integrity of blood-retinal barrier. Pro-inflammatory cytokines induce neuronal loss by damaging retinal ganglion cells and their axons [48]. Additionally, vascular insufficiency causes retinal ischemia, leading to cellular apoptosis [49,50]. Chronic gliosis of Müller cells leads to structural remodeling, including thinning of the inner retinal layers, which is detected by OCT imaging [51].
Meta-analyses of existing literature demonstrate inner retinal thinning in AD, particularly in the nerve fiber layer (RNFL), ganglion cell layer (GCL), and ganglion cell-inner plexiform complex (GC-IPL) [52,53]. These cell layers are likely affected by neurodegenerative disease processes because they are composed of nervous tissue [54]. The RNFL consists of axons (similar to white matter in the brain) of retinal ganglion cells (RGCs) while the GC-IPL consists of neuronal cell bodies and dendrites (similar to gray matter in the brain) of RGCs [54]. Thinning of the RNFL and GC-IPL occurs in all quadrants [55,56].
In contrast, Snyder et al. reported an increase in volume of the inner plexiform layer (IPL) among cognitively normal patients with positive family history and patient-reported memory deficits [57]. They also found that IPL volume positively correlated with the surface area of retinal inclusion bodies containing beta-amyloid [57]. They hypothesized that the IPL thickening could be due to decreased cholinergic activity in the IPL, which then causes beta-amyloid deposition, mimicking AD pathology in the neocortex [57]. Other studies have also reported deposition of AD neuropathologic proteins, including Aβ40, Aβ42, and pTau in the retina, with levels positively correlating with amyloid in the brain [58,59,60,61].
Given the extensive literature associating retinal structural changes with AD, OCT assessment of the retina may be used for diagnostic purposes for AD. Existing studies on artificial intelligence models trained on OCT data to distinguish patients with and without AD appear promising [62,63,64,65]. Therefore, patients with retinal thinning not explained by retinal pathology may be identified by their OCT findings and may benefit from neurocognitive workup for the early detection and symptomatic treatment of AD. Moreover, retinal conditions including diabetic retinopathy and retinal vein occlusions that are routinely monitored with OCT have been associated with an increased risk of AD [66,67].
Despite these promising studies, the clinical utility of OCT as a diagnostic tool for AD remains limited due to comorbid eye diseases, such as age-related macular degeneration, glaucoma, and diabetic retinopathy [68]. These ocular conditions can cause retinal thinning and vascular abnormalities that confound OCT interpretations in dementia [69,70,71]. These diseases are significantly associated with the presence of dementia [66], and affect similar demographic groups, which also limits the ability to isolate retinal changes secondary to dementia. Additionally, while OCT examination is relatively cheap and noninvasive for patients, the widespread implementation of this imaging modality in primary care or neurology clinics is limited by the relatively high cost of SD-OCT machines, which can range from $40,000 to $150,000 [72]. Additionally, there is no standardized protocol or consensus on retinal thickness thresholds for OCT-based AD diagnosis [73].
3.1.2. Vasculature
The retinal microvasculature is similar in structure and function to cerebral small vessels, and therefore may parallel changes in cerebral vasculature in neurodegenerative disease processes [54]. Retinal vasculature is assessed with color fundus photography which is a non-invasive imaging technique commonly used in clinical practice to monitor disease progression. Fundus photography (Figure 3) [74] has been used to evaluate vascular parameters including caliber, fractal dimension, curvature tortuosity, number of first branching vessels, branching coefficients, junctional exponent deviation, and length-to-diameter ratios of retinal arterioles and venules in patients with dementia in research [75]. However, the analysis of imaging parameters is limited by the impact of magnification, refractive error, and variability in the quantitative measurements of retinal vascular parameters between software packages and within the same software package [75].
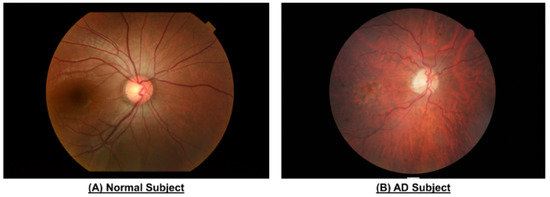
Figure 3.
Color fundus photography of the eyes of patients with and without AD from Chan et al. [74]. Reproduced with permission from the Journal of Visualized Experiments. Those with AD demonstrate narrower vasculature, reduced vascular fractal dimensions, and greater vessel tortuosity.
Retinal vasculature can also be assessed with OCT angiography (OCTA) (Figure 4) [76], which is a more recent software addition to SD-OCT used to diagnose vascular conditions in the retina, optic nerve, and choroid [77,78]. Based on the detection of the speed of red blood cells flowing through vessels, retinal OCTA produces a 2-dimensional en face image of vascular density and perfusion of a specific tissue layer, such as the superficial and deep capillary plexuses [77,78]. From the patient perspective, this is a superior modality to fluorescein angiography, which requires an invasive intravenous injection of contrast material [77,78]. However, this modality is limited by artifacts from eye movements during image acquisition, confounding from media opacities, and projection artifacts from light reflected by the retinal pigment epithelium [77,78].
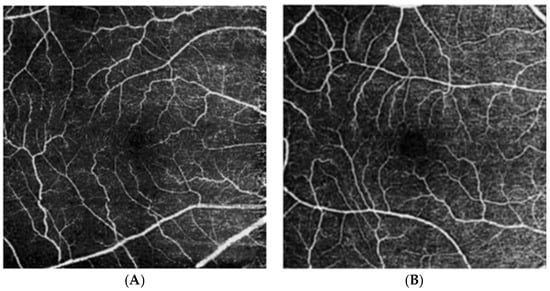
Figure 4.
Optical coherence tomography angiography of the superficial vascular plexus of an eye from a cognitively unimpaired individual (A) and an individual with Alzheimer’s disease dementia (B) from Marquié et al. [76]. Those with AD demonstrate reduced vascular and perfusion density, as well as increased tortuosity.
Systematic reviews and meta-analyses using OCTA and fundus photography indicate that retinal vessels in AD patients are lower in density and branching complexity, and may be narrowed or widened compared to cognitively normal patients [75,79]. Ma et al. reported that apolipoprotein E (APOE) ε4 carriers had a significantly lower perfusion density of the retina in the 6-mm Early Treatment of Diabetic Retinopathy Study circle and outer macular ring compared to noncarriers [80]. Li et al. found reduced vessel length density and vessel perfusion density in AD compared to control patients [81]. Cheung et al. found increased tortuosity of both retinal arterioles and venules in AD in a case–control study [82]. While most studies found no significant difference in the foveal avascular zone (FAZ) between patients with and without AD [83], O’ Bryhim et al. found a significantly greater FAZ in biomarker-positive preclinical AD compared to biomarker-negative patients [84]. Similarly, Bulut et al., Zabel et al., and Wu et al. reported a significantly greater FAZ in AD patients compared to controls [85,86,87]. This is likely due to variability in OCTA acquisition, including the scan size, layer definition, and artifact handling.
While existing literature points to differences in retinal vasculature parameters between AD and cognitively normal controls, further work is required to validate the software used to provide quantitative measurements from fundus photography. Given that digital fundus photography is a cost-effective imaging technique for screening that is preferred by patients and may increase compliance with scheduled visits [88], the use of retinal vascular parameters to distinguish patients with and without AD warrants further investigation after the refinement of software.
Recently, investigators have been increasingly interested in artificial intelligence approaches for the noninvasive identification of patients at risk for AD using ocular fundus imaging. Fundus-based AD screening is supported by epidemiologic studies demonstrating a link between retinal imaging features and incident dementia, such as Rebouças et al. [89]. This study found that retinal vascular caliber and tortuosity were significantly associated with mixed and vascular dementia in the Three-City-Alienor cohort [89]. A systematic review and meta-analysis of machine learning models trained with OCT, OCTA, and fundus photography to detect AD and Parkinson’s disease demonstrated moderate accuracy (AUC = 0.726) [90]. However, authors noted heterogeneity in the definition of early disease between studies and observational study designs as limitations, highlighting the gap between research use and implementation of AI systems in clinical practice. Wisely et al. developed a convolutional neural network trained on GC-IPL thickness maps, OCTA, ultra-widefield color fundus photography, and fundus autofluorescence images to detect AD [91]. They found that GC-IPL maps were the strongest inputs for higher diagnostic accuracy, and models trained with images only had a similar performance to models trained with imaging and patient data [91]. Similarly, Luengnaruemitchai et al. developed a convolutional neural network trained on color fundus photography that demonstrated a 96% accuracy, 99% sensitivity, and 90% specificity in discriminating between patients with and without AD and mild cognitive impairment [92].
If standardized protocols for OCT and OCTA-based AD diagnosis are established, AI-based risk stratification could be implemented in clinical practice. Changes in inner retinal thickness and vascular density could be used to flag patients of ophthalmology clinics who may be at high-risk for developing AD. These patients can then be referred to their primary care provider for a neurology referral and cognitive assessment.
3.2. Optic Nerve
The optic nerve has a direct anatomical and functional connection to the central nervous system, and can be assessed with similar neuroimaging modalities used to examine the brain in AD, including positron emission technology and magnetic resonance imaging. These techniques allow for high-resolution visualization and quantification of structural and metabolic brain changes associated with AD. However, they are costly, involve long image acquisition times, and may not be available in low-resource areas. Non-invasive and cheaper alternatives to these techniques that have also been used to assess disc parameters in AD include computed tomography (CT) and OCT of the optic nerve head.
Wu et al. compared the CT density and beta-amyloid level of the intraorbital optic nerve using CT imaging and 18F-flutemetamol amyloid positron emission tomography, respectively [93]. They found that the intraorbital optic nerve standardized uptake value ratio was significantly greater and optic nerve density was significantly lower in AD patients compared to cognitively normal patients [93]. Additionally, these optic nerve parameters were able to distinguish AD from mild cognitive impairment (MCI) and MCI from cognitively normal patients with an AUC of 0.9167 and 0.8951, respectively [93].
Changes in the optic nerve head have also been associated with AD severity and duration. Tsai et al. reported that higher pallor area-to-disc ratios were associated with higher Alzheimer’s Disease Assessment Scale scores and duration of disease using fundus photography and an optic nerve head analyzer [94]. Additionally, patients with AD had a significantly increased cup-to-disc ratio and cup volume and decreased disc rim area compared to age- and race-matched cognitively normal control patients [94]. In contrast, Kurna et al. reported no significant difference in the rim area, rim volume, linear cup-to-disc ratio, height variation contour, and cup shape measure between AD and control patients using confocal scanning laser tomography [95].
Peripapillary nerve fiber loss is hypothesized to be associated with neurodegenerative conditions including AD [95]. Several studies reported thinning of the peripapillary RNFL in AD, particularly in the superior and inferior quadrants [96,97,98]. However, Gharbiya et al. and van de Kreeke et al. found no significant difference in the peripapillary RNFL between patients with and without AD and preclinical AD, respectively [99,100].
Using magnetic resonance imaging and in vivo volumetry, Kusbeci et al. reported significantly lower optic nerve volume in AD compared to cognitively healthy participants [101]. However, Davies et al. found no significant difference in the mean cross-sectional area of the optic nerve, myelinated axon surface density, total axon number, or mean cross-sectional axon area in the postmortem intraorbital optic nerve segments of patients with AD compared to controls [102]. The postmortem specimen in Davies et al. may have been susceptible to atrophy and decreased axonal integrity following death.
Cesareo et al. reported glaucoma-like findings among patients with AD, which may be related to an underlying association between glaucoma and AD [66,103]. Despite a lower intraocular pressure, those with AD had a worse mean deviation, pattern standard deviation, and altered Glaucoma Hemifield Test compared to age- and sex-matched controls. These measures are used in clinical practice to diagnose and monitor glaucoma progression, suggesting their utility in AD detection as well.
Optic nerve pallor may be a proxy for axonal loss, which can be quantified using colorimetric analysis of fundus photography [104]. Salobrar-García et al. reported no significant difference in the hemoglobin levels of the optic nerve head tissue using a postmortem analysis [105]. In contrast, Bambo et al. reported that mean hemoglobin percentage and hemoglobin content in the neuroretinal rim were reduced in AD compared to age- and sex-matched controls using fundus photography and colorimetric assessment [104]. Axonal loss at the optic nerve head in AD can also be confirmed with histological staining. Syed et al. reported decreased axonal density at the central and peripheral optic nerve with loss of smaller-sized axons in AD compared to control patients [106].
These studies highlight the growing interest in the optic nerve as a potential ocular biomarker for AD diagnosis. While prior studies demonstrate structural and functional changes in the optic nerve head, peripapillary nerve fiber layer, and infraorbital nerve volume in AD, there is variability across imaging techniques that may be due to differences in resolution, sample size, disease severity, and methodology. Among the imaging modalities used to assess the optic nerve, OCT is the most widely used and seems the most promising in serving a non-invasive diagnostic imaging technique. Future studies could further explore the clinical utility of optic nerve imaging in AD diagnosis with standardized protocols and larger, diverse cohorts.
3.3. Choroid
Similarly to the retina, the choroid demonstrates morphological and vascular changes in AD. The choriocapillaris provides oxygen to the outer retina and vitreous chamber [107]. Choroidal ischemia is linked to changes in choroidal thickness, and these parameters are often abnormal in retinal degenerative conditions including age-related macular degeneration. Changes in the choroidal vasculature and blood flow are hypothesized to mimic cerebrovascular changes in AD [107]. Choroidal structure and vasculature can be assessed with SS-OCT and OCTA.
Choroidal thinning in AD has been established in several studies [53,81,99,105,107,108,109,110,111]. Li et al. also found that decreased subfoveal choroidal thickness was predictive of AD [107] in an in vivo analysis of 71 patients using enhanced depth imaging OCT. In contrast, Asanad et al. reported choroidal thickening in the central macula using histopathological techniques in postmortem AD compared to controls and this thickening was associated with stromal vessel number [112]. Similarly to Asanad et al., Castilla-Martí et al. demonstrated choroidal thickening in the inner nasal and inferior macula, as well as the outer temporal, nasal, and inferior macula in AD compared to cognitively normal controls [113].
Corradetti et al. reported increased choriocapillaris flow deficits in patients with presymptomatic AD compared to healthy patients [114]. While there was no difference in the choroidal vascularity index, total choroidal area and luminal area were significantly higher in AD in Robbins et al. [109]. In contrast, Ma et al. reported no significant differences in total choroidal area, luminal area, or choroidal vascularity index between APOE ε4 allele carriers and non-carriers [80]. Kwapong et al. reported that a lower choriocapillaris density on SS-OCT angiography (SS-OCTA) was associated with increased neuropathologic proteins in AD including Aβ40, Aβ42, and pTau181 [115]. Burke et al. found that increased total choroidal area and vessel area was associated with the presence of APOE ε4 allele or a positive family history for AD [116]. Di Pippo et al. found a significantly lower choriocapillaris flow area in AD compared to controls [117].
Collectively, these studies demonstrate that the choroid undergoes structural changes in AD that mirror pathologic changes in the retina and the brain. Choroidal thinning is the most consistently-reported finding, and may serve as a potential biomarker for AD diagnosis similar to inner retinal thinning. However, there is equivocal evidence in changes in vascular parameters, and the variability in findings may be attributed to genetic and systemic factors such as APOE ε4 status, as well as eye diseases that can impact choroidal thickness including high myopia (thinner choroid) or central serous chorioretinopathy (thicker choroid). The association of choriocapillaris parameters with AD-specific proteins including beta-amyloid strengthens the association between ocular vasculature and AD pathophysiology.
4. Ocular Fluids
In addition to central nervous system imaging, quantification of neuropathologic proteins in CSF serves as the gold standard for underlying AD pathology detection [118]. However, CSF sample collection involves a lumbar puncture, which is an invasive procedure. The routine use of CSF analysis is also limited by issues with transportation, storage, and cost [119]. Similar to the detection of AD neuropathologic proteins in CSF, emerging studies have identified the presence of beta amyloid and tau proteins in ocular fluids. In fact, Sampani et al. measured relative concentrations of Aβ40, Aβ42, total Tau, ptau181, glial fibrillary acidic protein (GFAP), and neurofilament light chain (NfL), and found that concentrations were higher in eye fluids including tear secretions, aqueous humor, and vitreous humor, compared to plasma [120].
4.1. Tear Secretions
In contrast to vitreous and aqueous humor collection, tear fluid biomarkers can be sampled non-invasively, and therefore, there is a particular interest in the use of tear secretions in AD diagnosis. Tear fluids are most commonly collected via Schirmer strips, which involve placing the strip in the lower cul-de-sac, lower conjunctival fornix, external canthus of eyelids, or lower conjunctival sacs for up to 5 min, and capillary collection, which involves placing microcapillary tubes into the ventral cul-de-sac or lacrimal lake at the inner or external canthi for up to 5 min [121]. While Schirmer strips are inexpensive and simple to use, their use in AD research is limited by a low volume of tears collected, evaporation [122], and by the inclusion of highly abundant proteins from the ocular surface epithelium that may interfere with neuropathologic protein analysis [121].
While Kalló et al. reported that participants with AD had a significantly higher protein concentration and flow rate compared to controls with capillary collection, Kenny et al. reported no significant differences in these parameters between study groups with Schirmer strips [123,124]. Additionally, Kalló et al. reported that the combination of lipocalin-1, dermcidin, lysozyme-C, and lacritin in tears was diagnostic of AD with a sensitivity of 81% and specificity of 77% [123]. Örnek et al. found decreased tear break-up time and Schirmer’s 1 test scores in AD compared to controls [25].
Gijs et al. found that AD participants had relatively higher levels of Aβ38, Aβ40, Aβ42, and total Tau compared to healthy controls [125]. In contrast, Gharbiya et al. found significantly lower Aβ42 levels in tears of AD participants compared to healthy controls, and hypothesized that this could parallel the lower Aβ42 in CSF due to sequestration in the brain [126]. They also hypothesized that beta-amyloid could be sequestered in lacrimal gland cells in AD participants [126]. While the aforementioned studies directly compared AD participants with healthy controls, Del Prete reported the presence of Aβ42 in tear samples of two cognitively normal participants with a family history of AD, and the absence of this neurodegeneration biomarker in one healthy participant with no family history [127].
Kenny et al. reported increased levels of microRNA among participants with AD, likely due to the cellular disruption and neurodegeneration that characterize AD [124]. They particularly found that microRNA-200b-5p was significantly elevated in the tear fluid of AD participants, which has not been previously reported, and is associated with reducing cognitive impairment via insulin signaling in murine models [128]. Interestingly, Kenny et al. also detected elF4E, a translation initiation factor involved in protein synthesis, in tear samples among AD participants, but not among those of healthy controls [124].
In summary, tear fluid analysis represents a potentially emerging diagnostic method for AD, offering non-invasive, accessible, and scalable neuropathologic protein detection. Existing literature shows that differences in proteins, including AD-specific neuropathologic proteins, and microRNAs can be used to distinguish patients with and without AD. The detection of beta-amyloid in tears of cognitively normal patients with a positive family history raises the potential for early detection and risk stratification. However, the variability in results and tear collection methods, as well as limitations in tear collection methods require further investigation before tears can be used to diagnose AD in clinical practice.
In clinical practice, translation of tear-based biomarkers could be appropriate in primary care, neurology, geriatric, and optometry or ophthalmology settings. Given that tear sampling is relatively quick, cheap, and noninvasive, this could potentially be offered routinely to high-risk older patients with a positive family history for AD. Prior to implementation, further research is needed to standardize assays for measuring tear biomarkers, establish diagnostic windows for normal vs. pathologic levels, and determine the sensitivity and specificity of these windows for detecting neurodegenerative diseases. After these steps, assay results for each protein could be combined into high-, intermediate-, or low-risk categories based on pre-defined thresholds. Patients in the high- and intermediate-risk categories could then be referred to neurology for a full cognitive assessment for potential AD or other dementia.
4.2. Aqueous Humor
Aqueous humor is the circulating and regenerative watery fluid found in the anterior chamber of the eye, secreted by the ciliary body and absorbed into the trabecular meshwork [129]. This fluid regulates the intraocular volume and pressure, and pathology involving variations in intraocular pressure includes ocular hypertension and glaucoma [129]. Given that glaucoma is associated with AD [66] and that glaucoma and AD share retinal structural changes including ganglion cell thinning, aqueous humor may have a potential role in AD diagnosis. However, aqueous humor collection requires anterior chamber paracentesis, which is an invasive procedure and has potential for complications, such as damage to the iris and lens, as well as endophthalmitis and corneal abscess [130].
There are limited studies demonstrating the presence of AD neuropathologic proteins in aqueous humor. Inoue et al. reported that the aqueous humor of patients with primary open-angle glaucoma had significantly higher levels of apolipoprotein (Apo) AI, ApoCIII, ApoE, and α2-macroglobulin compared to that of patients with cataracts [131]. However, Cappelli et al. did not find significantly different levels of Aβ42 in the aqueous humor of patients with glaucoma and that of control patients [132]. Similarly, Janciauskiene et al. found no significant differences in the levels of Aß38, Aß40, or Aß42 between patients with cataracts alone, cataract and glaucoma, cataract and pseudoexfoliation syndrome, and cataract and diabetic retinopathy [133].
Patel et al. reported that pTau181 in the aqueous humor was positively correlated with plasma pTau181, and negatively correlated with Montreal Cognitive Assessment scores (commonly used in dementia evaluation) among patients who were not previously diagnosed with cognitive impairment [134]. Bai et al. detected NfL, Aβ40, Aβ42, GFAP, and pTau181 in aqueous humor samples of patients with neovascular age-related macular degeneration (nAMD) or senile cataracts. NfL levels in the aqueous humor of patients with nAMD were negatively correlated with lower Mini-Mental State Examination (MMSE) scores [135].
Although aqueous humor is not as easily accessible as tear secretions, existing literature suggests that this fluid contains AD-specific neuropathologic proteins, particularly among patients with glaucoma. Shared structural retinal changes justify the exploration of the diagnostic role of aqueous humor in AD. While some studies demonstrate beta-amyloid, hyperphosphorylated tau, and neurofilament light chain in the aqueous humor, the results are overall inconsistent with other reports finding no significant difference between patients with and without AD. However, the correlation between hyperphosphorylated tau and cognitive scores in patients without known dementia indicates the utility of aqueous humor analysis in early or preclinical detection. That being said, the invasive nature of aqueous humor collection, coupled with its potential complications, limits the clinical utility of aqueous humor in routine AD diagnosis compared to other techniques such as OCT and OCTA.
4.3. Vitreous Humor
Vitreous humor is a gel-like, non-regenerative, embryological remnant fluid that fills the posterior chamber of the eye and helps maintain the structure of the globe [136]. This fluid is involved in pathology including vitreous hemorrhage and posterior vitreous detachment. Prior studies have evaluated the vitreous humor for neurodegenerative biomarkers given the association between retinal pathology, including diabetic retinopathy and age-related macular degeneration, and AD [66]. Similarly to aqueous humor collection, vitreous humor collection requires an invasive procedure (pars plana vitrectomy). However, vitreous humor may be of interest in AD research because this fluid is isolated from systemic circulation and therefore may reflect both retinal and central nervous system pathology.
To our knowledge, there exists one study evaluating vitreous neuropathologic proteins in patients with AD compared to cognitively normal participants. Vig et al. reported significantly higher total Tau levels in postmortem vitreous humor in those with pathologically confirmed AD compared to controls [137]. Other studies have indirectly evaluated the association between AD-associated biomarkers in vitreous humor and neurocognitive defects. Wright et al. reported that lower levels of Aβ40, Aβ42, and total Tau were significantly associated with lower MMSE scores in cognitively normal participants [138]. While Subramanian et al. did not find any associations between APOE ε2 or ε4 genotypes and vitreous NfL, they did report vitreous NfL levels to be positively correlated with vitreous Aβ40, Aβ42, and total Tau [139].
The limited evidence evaluating the diagnostic potential of vitreous humor in AD shows that the vitreous has elevated neuropathologic proteins in confirmed postmortem AD patients, and these proteins correlate to cognitive scores in healthy living patients. Furthermore, neurodegenerative proteins are present at a higher concentration in vitreous humor than plasma [120]. While these studies indicate the potential involvement of vitreous humor in AD detection, vitreous humor collection is invasive and may only be indicated among patients undergoing standard-of-care vitrectomy for retinal pathology. Patients incidentally found to have elevated neuropathologic biomarkers may benefit from closer monitoring for cognitive pathology.
5. Plasma Biomarkers
While ocular imaging and fluid biomarkers, particularly OCT-based markers, offer a noninvasive option for AD diagnosis, plasma biomarkers are currently closer to clinical implementation for AD diagnosis. Plasma levels of phosphorylated tau, beta-amyloid, and NfL have shown strong associations with the presence of AD [140,141,142]. Plasma assays for neuropathologic proteins are relatively low-cost, minimally invasive, and scalable for use in primary care, as specimens may be collected during routine blood testing, making these assays feasible for population-level screening [143]. However, there exist several barriers to implementation in clinical practice. Patient factors, including comorbidities, medications, and demographics, can confound the concentration of neuropathologic proteins [144]. Reference ranges need to be adjusted for these factors. Furthermore, the recommended two-cutoff thresholding (which defines positive, intermediate, and negative) categories based on beta-amyloid creates an indeterminate (intermediate) category which can require further confirmatory testing with PET and CSF, delaying referral for further cognitive testing [144]. Additionally, pre-analytical sample handling involves steps that must be performed precisely [145]. Details such as the correct primary collection tube type, delays before centrifugation and freezing, and storage temperature have been shown to impact beta-amyloid protein levels, and may be difficult to control in a real-world setting [145]. Beyond this, there exists analytical variability across platforms, which limits the comparability between assays in the absence of standardized reference ranges that can be applied to multiple platforms.
In contrast, the implementation of ocular imaging techniques such as OCT and OCTA in primary care and neurology practices is limited by a high machine cost and need for trained personnel [72]. Moreover, co-existing ocular conditions as well as the absence of standardized diagnostic thresholds or protocols also limit their clinical utility [68]. While individuals with eye diseases are more likely to undergo ocular imaging than those without ocular conditions, population-level screening with blood-based biomarkers in primary care settings would capture those with and without eye disease. Overall, ocular imaging-based biomarkers may serve as adjunct diagnostic tools, but plasma biomarkers are currently more feasible for implementation in clinical practice.
6. Conclusions
In conclusion, the shared embryological origins between the eye and the brain may result in shared pathophysiological ocular and neurological changes in AD. This is evident by the deposition of AD neuropathologic proteins throughout the anterior and posterior segments of the eye. Imaging modalities including OCT and OCTA most strongly demonstrate the presence of AD biomarkers in retinal cell layers and vasculature. AD neuropathologic proteins are detectable in ocular fluids, including tear secretions, aqueous humor, and vitreous humor, but warrant further research into their diagnostic and predictive ability. However, some fluids, including aqueous and vitreous humor, involve invasive collection procedures that limit their utility in clinical practice for AD diagnosis. Overall, ocular biomarkers for AD represent a relatively less invasive and potentially cheaper modality for diagnosing AD. Based on the sources of discrepancies between studies evaluating similar associations between ocular structures and AD, future research should establish and implement protocols for commonly used imaging techniques including OCT and OCTA. These protocols should specify scan size, slab definitions, artifact handling, and minimum quality thresholds to ensure comparable data between studies. Furthermore, given the limitations of Schirmer strips, future studies should use the capillary collection technique for higher-quality analysis of tear secretions. When possible, research should pair in-vivo slit-lamp and fluorescent ligand imaging with postmortem assays among the same donors, with a longitudinal study design and standardized time-to-fixation, staining, and other processing steps to better establish the presence of neuropathologic proteins in the lens. Overall, research evaluating the association between eye and AD is limited by cross-sectional study designs and small cohorts. Longitudinal, prospective studies with larger sample sizes would be more appropriate in establishing the causal and temporal relationships required to understand the underlying pathophysiology driving changes in the eye and brain during neurodegenerative disease development. Future studies could evaluate the performance of artificial intelligence models trained on multimodal ophthalmic information in diagnosing AD.
Author Contributions
Conceptualization, M.L.S. and M.P.; writing—original draft preparation, M.P.; writing—review and editing, M.P., M.L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AD | Alzheimer’s disease |
| APOE | Apolipoprotein E |
| APP | Amyloid precursor protein |
| AUC | Area under the curve |
| CSF | Cerebrospinal fluid |
| CT | Computed tomography |
| GC-IPL | Ganglion cell-inner plexiform layer |
| GCL | Ganglion cell layer |
| GFAP | Glial fibrillary acidic protein |
| IPL | Inner plexiform layer |
| MCI | Mild cognitive impairment |
| MMSE | Mini-Mental State Examination |
| NfL | Neurofilament light chain |
| OCT | Optical coherence tomography |
| OCTA | Optical coherence tomography angiography |
| pTau | Hyperphosphorylated tau |
| RGC | Retinal ganglion cell |
| RNFL | Retinal nerve fiber layer |
| SD-OCT | Spectral domain optical coherence tomography |
| SS-OCT | Swept source optical coherence tomography |
| SS-OCTA | Swept source optical coherence tomography angiography |
References
- Chaitanuwong, P.; Singhanetr, P.; Chainakul, M.; Arjkongharn, N.; Ruamviboonsuk, P.; Grzybowski, A. Potential Ocular Biomarkers for Early Detection of Alzheimer’s Disease and Their Roles in Artificial Intelligence Studies. Neurol. Ther. 2023, 12, 1517–1532. [Google Scholar] [CrossRef]
- Kumar, A.; Sidhu, J.; Lui, F.; Tsao, J.W. Alzheimer Disease. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2025. [Google Scholar]
- Bales, T.R.; Lopez, M.J.; Clark, J. Embryology, Eye. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2025. [Google Scholar]
- Singh, R.; Munakomi, S. Embryology, Neural Tube. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2025. [Google Scholar]
- Marchesi, N.; Fahmideh, F.; Boschi, F.; Pascale, A.; Barbieri, A. Ocular Neurodegenerative Diseases: Interconnection between Retina and Cortical Areas. Cells 2021, 10, 2394. [Google Scholar] [CrossRef]
- Hussain, A.; Sheikh, Z.; Subramanian, M. The Eye as a Diagnostic Tool for Alzheimer’s Disease. Life 2023, 13, 726. [Google Scholar] [CrossRef] [PubMed]
- Shumway, C.L.; Motlagh, M.; Wade, M. Anatomy, Head and Neck, Eye Conjunctiva. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2025. [Google Scholar]
- Eghrari, A.O.; Riazuddin, S.A.; Gottsch, J.D. Overview of the Cornea: Structure, Function, and Development. Prog. Mol. Biol. Transl. Sci. 2015, 134, 7–23. [Google Scholar] [CrossRef]
- Medeiros, C.S.; Santhiago, M.R. Corneal nerves anatomy, function, injury and regeneration. Exp. Eye Res. 2020, 200, 108243. [Google Scholar] [CrossRef]
- Downie, L.E.; Bandlitz, S.; Bergmanson, J.P.G.; Craig, J.P.; Dutta, D.; Maldonado-Codina, C.; Ngo, W.; Siddireddy, J.S.; Wolffsohn, J.S. CLEAR—Anatomy and physiology of the anterior eye. Cont. Lens Anterior Eye 2021, 44, 132–156. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, K.; Jin, Y.; Niu, Y.; Zuo, T. Changes of higher order aberration with various pupil sizes in the myopic eye. J. Refract. Surg. 2003, 19, S270–S274. [Google Scholar] [CrossRef]
- McDougal, D.H.; Gamlin, P.D. Autonomic control of the eye. Compr. Physiol. 2015, 5, 439–473. [Google Scholar] [CrossRef] [PubMed]
- Bloom, J.; Motlagh, M.; Czyz, C.N. Anatomy, Head and Neck: Eye Iris Sphincter Muscle. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2025. [Google Scholar]
- Hejtmancik, J.F.; Shiels, A. Overview of the Lens. Prog. Mol. Biol. Transl. Sci. 2015, 134, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Goel, M.; Picciani, R.G.; Lee, R.K.; Bhattacharya, S.K. Aqueous humor dynamics: A review. Open Ophthalmol. J. 2010, 4, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Le Goff, M.M.; Bishop, P.N. Adult vitreous structure and postnatal changes. Eye 2008, 22, 1214–1222. [Google Scholar] [CrossRef]
- Hoon, M.; Okawa, H.; Della Santina, L.; Wong, R.O. Functional architecture of the retina: Development and disease. Prog. Retin. Eye Res. 2014, 42, 44–84. [Google Scholar] [CrossRef]
- Erskine, L.; Herrera, E. Connecting the retina to the brain. ASN Neuro 2014, 6, 1759091414562107 . [Google Scholar] [CrossRef]
- Zhang, W.; Kaser-Eichberger, A.; Fan, W.; Platzl, C.; Schrödl, F.; Heindl, L.M. The structure and function of the human choroid. Ann. Anat. 2024, 254, 152239. [Google Scholar] [CrossRef]
- Jmarchn, R.A. Schematic Diagram of the Human Eye. Available online: https://commons.wikimedia.org/wiki/File:Schematic_diagram_of_the_human_eye_en.svg (accessed on 30 September 2025).
- Smith, M.M.; Chen, P.C.; Li, C.S.; Ramanujam, S.; Cheung, A.T. Whole blood viscosity and microvascular abnormalities in Alzheimer’s Disease. Clin. Hemorheol. Microcirc. 2009, 41, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Gundogan, A.O.; Oltulu, R.; Belviranli, S.; Tezcan, A.; Adam, M.; Mirza, E.; Altaş, M.; Okka, M. Corneal innervation changes ın Alzheimer’s: Implications for sensory dysfunction. Int. Ophthalmol. 2024, 44, 270. [Google Scholar] [CrossRef] [PubMed]
- Lambiase, A.; Manni, L.; Bonini, S.; Rama, P.; Micera, A.; Aloe, L. Nerve growth factor promotes corneal healing: Structural, biochemical, and molecular analyses of rat and human corneas. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1063–1069. [Google Scholar]
- Müller, L.J.; Marfurt, C.F.; Kruse, F.; Tervo, T.M. Corneal nerves: Structure, contents and function. Exp. Eye Res. 2003, 76, 521–542. [Google Scholar] [CrossRef]
- Örnek, N.; Dağ, E.; Örnek, K. Corneal sensitivity and tear function in neurodegenerative diseases. Curr. Eye Res. 2015, 40, 423–428. [Google Scholar] [CrossRef]
- Ponirakis, G.; Al Hamad, H.; Sankaranarayanan, A.; Khan, A.; Chandran, M.; Ramadan, M.; Tosino, R.; Gawhale, P.V.; Alobaidi, M.; AlSulaiti, E.; et al. Association of corneal nerve fiber measures with cognitive function in dementia. Ann. Clin. Transl. Neurol. 2019, 6, 689–697. [Google Scholar] [CrossRef]
- Dehghani, C.; Frost, S.; Jayasena, R.; Fowler, C.; Masters, C.L.; Kanagasingam, Y.; Jiao, H.; Lim, J.K.H.; Chinnery, H.R.; Downie, L.E. Morphometric Changes to Corneal Dendritic Cells in Individuals with Mild Cognitive Impairment. Front. Neurosci. 2020, 14, 556137. [Google Scholar] [CrossRef]
- El Haj, M.; Chapelet, G.; Moustafa, A.A.; Boutoleau-Bretonnière, C. Pupil size as an indicator of cognitive activity in mild Alzheimer’s disease. Excli J. 2022, 21, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Frost, S.M.; Kanagasingam, Y.; Sohrabi, H.R.; Taddei, K.; Bateman, R.; Morris, J.; Benzinger, T.; Goate, A.; Masters, C.L.; Martins, R.N. Pupil response biomarkers distinguish amyloid precursor protein mutation carriers from non-carriers. Curr. Alzheimer Res. 2013, 10, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Frost, S.; Kanagasingam, Y.; Sohrabi, H.; Bourgeat, P.; Villemagne, V.; Rowe, C.C.; Macaulay, S.L.; Szoeke, C.; Ellis, K.A.; Ames, D.; et al. Pupil response biomarkers for early detection and monitoring of Alzheimer’s disease. Curr. Alzheimer Res. 2013, 10, 931–939. [Google Scholar] [CrossRef]
- Fotiou, D.F.; Brozou, C.G.; Haidich, A.B.; Tsiptsios, D.; Nakou, M.; Kabitsi, A.; Giantselidis, C.; Fotiou, F. Pupil reaction to light in Alzheimer’s disease: Evaluation of pupil size changes and mobility. Aging Clin. Exp. Res. 2007, 19, 364–371. [Google Scholar] [CrossRef]
- Prettyman, R.; Bitsios, P.; Szabadi, E. Altered pupillary size and darkness and light reflexes in Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 1997, 62, 665–668. [Google Scholar] [CrossRef] [PubMed]
- Lustig-Barzelay, Y.; Sher, I.; Sharvit-Ginon, I.; Feldman, Y.; Mrejen, M.; Dallasheh, S.; Livny, A.; Schnaider Beeri, M.; Weller, A.; Ravona-Springer, R.; et al. Machine learning for comprehensive prediction of high risk for Alzheimer’s disease based on chromatic pupilloperimetry. Sci. Rep. 2022, 12, 9945. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, A.; Ouanes, S.; Crippa, S.V.; Popp, J. Early-Stage Alzheimer’s Disease Does Not Alter Pupil Responses to Colored Light Stimuli. J. Alzheimers Dis. 2020, 75, 1273–1282. [Google Scholar] [CrossRef]
- Van Stavern, G.P.; Bei, L.; Shui, Y.B.; Huecker, J.; Gordon, M. Pupillary light reaction in preclinical Alzheimer’s disease subjects compared with normal ageing controls. Br. J. Ophthalmol. 2019, 103, 971–975. [Google Scholar] [CrossRef]
- Goldstein, L.E.; Muffat, J.A.; Cherny, R.A.; Moir, R.D.; Ericsson, M.H.; Huang, X.; Mavros, C.; Coccia, J.A.; Faget, K.Y.; Fitch, K.A.; et al. Cytosolic beta-amyloid deposition and supranuclear cataracts in lenses from people with Alzheimer’s disease. Lancet 2003, 361, 1258–1265. [Google Scholar] [CrossRef]
- Moncaster, J.A.; Pineda, R.; Moir, R.D.; Lu, S.; Burton, M.A.; Ghosh, J.G.; Ericsson, M.; Soscia, S.J.; Mocofanescu, A.; Folkerth, R.D.; et al. Alzheimer’s disease amyloid-beta links lens and brain pathology in Down syndrome. PLoS ONE 2010, 5, e10659. [Google Scholar] [CrossRef]
- Kerbage, C.; Sadowsky, C.H.; Tariot, P.N.; Agronin, M.; Alva, G.; Turner, F.D.; Nilan, D.; Cameron, A.; Cagle, G.D.; Hartung, P.D. Detection of Amyloid β Signature in the Lens and Its Correlation in the Brain to Aid in the Diagnosis of Alzheimer’s Disease. Am. J. Alzheimers Dis. Other Demen 2015, 30, 738–745. [Google Scholar] [CrossRef]
- Kerbage, C.; Sadowsky, C.H.; Jennings, D.; Cagle, G.D.; Hartung, P.D. Alzheimer’s disease diagnosis by detecting exogenous fluorescent signal of ligand bound to Beta amyloid in the lens of human eye: An exploratory study. Front. Neurol. 2013, 4, 62. [Google Scholar] [CrossRef]
- Michael, R.; Rosandić, J.; Montenegro, G.A.; Lobato, E.; Tresserra, F.; Barraquer, R.I.; Vrensen, G.F. Absence of beta-amyloid in cortical cataracts of donors with and without Alzheimer’s disease. Exp. Eye Res. 2013, 106, 5–13. [Google Scholar] [CrossRef]
- Michael, R.; Otto, C.; Lenferink, A.; Gelpi, E.; Montenegro, G.A.; Rosandić, J.; Tresserra, F.; Barraquer, R.I.; Vrensen, G.F. Absence of amyloid-beta in lenses of Alzheimer patients: A confocal Raman microspectroscopic study. Exp. Eye Res. 2014, 119, 44–53. [Google Scholar] [CrossRef]
- Ho, C.Y.; Troncoso, J.C.; Knox, D.; Stark, W.; Eberhart, C.G. Beta-amyloid, phospho-tau and alpha-synuclein deposits similar to those in the brain are not identified in the eyes of Alzheimer’s and Parkinson’s disease patients. Brain Pathol. 2014, 24, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.A.; McGuone, D.; Frosch, M.P.; Hyman, B.T.; Laver, N.; Stemmer-Rachamimov, A. Absence of Alzheimer Disease Neuropathologic Changes in Eyes of Subjects With Alzheimer Disease. J. Neuropathol. Exp. Neurol. 2017, 76, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Bei, L.; Shui, Y.B.; Bai, F.; Nelson, S.K.; Van Stavern, G.P.; Beebe, D.C. A test of lens opacity as an indicator of preclinical Alzheimer Disease. Exp. Eye Res. 2015, 140, 117–123. [Google Scholar] [CrossRef]
- Tian, T.; Zhang, B.; Jia, Y.; Li, Z. Promise and challenge: The lens model as a biomarker for early diagnosis of Alzheimer’s disease. Dis. Markers 2014, 2014, 826503. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martin, E.; Jimeno-Huete, D.; Dongil-Moreno, F.J.; Boquete, L.; Sánchez-Morla, E.M.; Miguel-Jiménez, J.M.; López-Dorado, A.; Vilades, E.; Fuertes, M.I.; Pueyo, A.; et al. Differential Study of Retinal Thicknesses in the Eyes of Alzheimer’s Patients, Multiple Sclerosis Patients and Healthy Subjects. Biomedicines 2023, 11, 3126. [Google Scholar] [CrossRef]
- Zeppieri, M.; Marsili, S.; Enaholo, E.S.; Shuaibu, A.O.; Uwagboe, N.; Salati, C.; Spadea, L.; Musa, M. Optical Coherence Tomography (OCT): A Brief Look at the Uses and Technological Evolution of Ophthalmology. Medicina 2023, 59, 2114. [Google Scholar] [CrossRef]
- Fernández-Albarral, J.A.; Salobrar-García, E.; Martínez-Páramo, R.; Ramírez, A.I.; de Hoz, R.; Ramírez, J.M.; Salazar, J.J. Retinal glial changes in Alzheimer’s disease—A review. J. Optom. 2019, 12, 198–207. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Q.; Tao, R.; Lu, H.; Xiao, Z.; Zheng, L.; Ding, D.; Ding, S.; Ma, Y.; Lu, Z.; et al. Decreased Retinal Vascular Density in Alzheimer’s Disease (AD) and Mild Cognitive Impairment (MCI): An Optical Coherence Tomography Angiography (OCTA) Study. Front. Aging Neurosci. 2020, 12, 572484. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.P.; Grewal, D.S.; Thompson, A.C.; Polascik, B.W.; Dunn, C.; Burke, J.R.; Fekrat, S. Retinal Microvascular and Neurodegenerative Changes in Alzheimer’s Disease and Mild Cognitive Impairment Compared with Control Participants. Ophthalmol. Retin. 2019, 3, 489–499. [Google Scholar] [CrossRef]
- Bringmann, A.; Pannicke, T.; Grosche, J.; Francke, M.; Wiedemann, P.; Skatchkov, S.N.; Osborne, N.N.; Reichenbach, A. Müller cells in the healthy and diseased retina. Prog. Retin. Eye Res. 2006, 25, 397–424. [Google Scholar] [CrossRef]
- den Haan, J.; Verbraak, F.D.; Visser, P.J.; Bouwman, F.H. Retinal thickness in Alzheimer’s disease: A systematic review and meta-analysis. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2017, 6, 162–170. [Google Scholar] [CrossRef]
- Bayhan, H.A.; Aslan Bayhan, S.; Celikbilek, A.; Tanık, N.; Gürdal, C. Evaluation of the chorioretinal thickness changes in Alzheimer’s disease using spectral-domain optical coherence tomography. Clin. Exp. Ophthalmol. 2015, 43, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Shi, C.; Shen, M.; Lu, F. Advances in retina imaging as potential biomarkers for early diagnosis of Alzheimer’s disease. Transl. Neurodegener. 2021, 10, 6. [Google Scholar] [CrossRef]
- Chan, V.T.T.; Sun, Z.; Tang, S.; Chen, L.J.; Wong, A.; Tham, C.C.; Wong, T.Y.; Chen, C.; Ikram, M.K.; Whitson, H.E.; et al. Spectral-Domain OCT Measurements in Alzheimer’s Disease: A Systematic Review and Meta-analysis. Ophthalmology 2019, 126, 497–510. [Google Scholar] [CrossRef]
- Cheung, C.Y.; Ong, Y.T.; Hilal, S.; Ikram, M.K.; Low, S.; Ong, Y.L.; Venketasubramanian, N.; Yap, P.; Seow, D.; Chen, C.L.; et al. Retinal ganglion cell analysis using high-definition optical coherence tomography in patients with mild cognitive impairment and Alzheimer’s disease. J. Alzheimers Dis. 2015, 45, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Snyder, P.J.; Johnson, L.N.; Lim, Y.Y.; Santos, C.Y.; Alber, J.; Maruff, P.; Fernández, B. Nonvascular retinal imaging markers of preclinical Alzheimer’s disease. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2016, 4, 169–178. [Google Scholar] [CrossRef]
- de Ruyter, F.J.H.; Morrema, T.H.J.; den Haan, J.; Twisk, J.W.R.; de Boer, J.F.; Scheltens, P.; Boon, B.D.C.; Thal, D.R.; Rozemuller, A.J.; Verbraak, F.D.; et al. Phosphorylated tau in the retina correlates with tau pathology in the brain in Alzheimer’s disease and primary tauopathies. Acta Neuropathol. 2023, 145, 197–218. [Google Scholar] [CrossRef]
- Koronyo, Y.; Rentsendorj, A.; Mirzaei, N.; Regis, G.C.; Sheyn, J.; Shi, H.; Barron, E.; Cook-Wiens, G.; Rodriguez, A.R.; Medeiros, R.; et al. Retinal pathological features and proteome signatures of Alzheimer’s disease. Acta Neuropathol. 2023, 145, 409–438. [Google Scholar] [CrossRef]
- Dumitrascu, O.M.; Lyden, P.D.; Torbati, T.; Sheyn, J.; Sherzai, A.; Sherzai, D.; Sherman, D.S.; Rosenberry, R.; Cheng, S.; Johnson, K.O.; et al. Sectoral segmentation of retinal amyloid imaging in subjects with cognitive decline. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2020, 12, e12109. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, N.; Shi, H.; Oviatt, M.; Doustar, J.; Rentsendorj, A.; Fuchs, D.T.; Sheyn, J.; Black, K.L.; Koronyo, Y.; Koronyo-Hamaoui, M. Alzheimer’s Retinopathy: Seeing Disease in the Eyes. Front. Neurosci. 2020, 14, 921. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.; Silva, G.; Duque, C.; Januário, C.; Santana, I.; Ambrósio, A.F.; Castelo-Branco, M.; Bernardes, R. Retinal texture biomarkers may help to discriminate between Alzheimer’s, Parkinson’s, and healthy controls. PLoS ONE 2019, 14, e0218826. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Smith, G.; Guo, H.; Liu, B.; Pan, Z.; Wang, Z.; Xiong, S.; Fang, R. Modular machine learning for Alzheimer’s disease classification from retinal vasculature. Sci. Rep. 2021, 11, 238. [Google Scholar] [CrossRef]
- Cheung, C.Y.; Ran, A.R.; Wang, S.; Chan, V.T.T.; Sham, K.; Hilal, S.; Venketasubramanian, N.; Cheng, C.Y.; Sabanayagam, C.; Tham, Y.C.; et al. A deep learning model for detection of Alzheimer’s disease based on retinal photographs: A retrospective, multicentre case-control study. Lancet Digit. Health 2022, 4, e806–e815. [Google Scholar] [CrossRef]
- Wang, X.; Jiao, B.; Liu, H.; Wang, Y.; Hao, X.; Zhu, Y.; Xu, B.; Xu, H.; Zhang, S.; Jia, X.; et al. Machine learning based on Optical Coherence Tomography images as a diagnostic tool for Alzheimer’s disease. CNS Neurosci. Ther. 2022, 28, 2206–2217. [Google Scholar] [CrossRef]
- Lee, C.S.; Larson, E.B.; Gibbons, L.E.; Lee, A.Y.; McCurry, S.M.; Bowen, J.D.; McCormick, W.C.; Crane, P.K. Associations between recent and established ophthalmic conditions and risk of Alzheimer’s disease. Alzheimer’s Dement. 2019, 15, 34–41. [Google Scholar] [CrossRef]
- Prasad, M.; Goodman, D.; Gutta, S.; Sheikh, Z.; Cabral, H.J.; Shunyakova, J.; Sanjiv, N.; Curley, C.; Yarala, R.R.; Tsai, L.; et al. Associations Between Retinal Vascular Occlusions and Dementia. Healthcare 2024, 12, 2371. [Google Scholar] [CrossRef]
- Xu, Y.; Phu, J.; Aung, H.L.; Hesam-Shariati, N.; Keay, L.; Tully, P.J.; Booth, A.; Anderson, C.S.; Anstey, K.J.; Peters, R. Frequency of coexistent eye diseases and cognitive impairment or dementia: A systematic review and meta-analysis. Eye 2023, 37, 3128–3136. [Google Scholar] [CrossRef]
- Chua, J.; Sim, R.; Tan, B.; Wong, D.; Yao, X.; Liu, X.; Ting, D.S.W.; Schmidl, D.; Ang, M.; Garhöfer, G.; et al. Optical Coherence Tomography Angiography in Diabetes and Diabetic Retinopathy. J. Clin. Med. 2020, 9, 1723. [Google Scholar] [CrossRef]
- Bussel, I.I.; Wollstein, G.; Schuman, J.S. OCT for glaucoma diagnosis, screening and detection of glaucoma progression. Br. J. Ophthalmol. 2014, 98, ii15–ii19. [Google Scholar] [CrossRef]
- Heckenlaible, N.J.; Toomey, C.B.; Handa, J.T. OCT Changes Observed during the Progression of Early Age-Related Macular Degeneration. Ophthalmol. Sci. 2025, 5, 100615. [Google Scholar] [CrossRef]
- Price, H.B.; Song, G.; Wang, W.; O’Kane, E.; Lu, K.; Jelly, E.; Miller, D.A.; Wax, A. Development of next generation low-cost OCT towards improved point-of-care retinal imaging. Biomed. Opt. Express 2025, 16, 748–759. [Google Scholar] [CrossRef]
- Alber, J.; Arthur, E.; Sinoff, S.; DeBuc, D.C.; Chew, E.Y.; Douquette, L.; Hatch, W.V.; Hudson, C.; Kashani, A.; Lee, C.S.; et al. A recommended “minimum data set” framework for SD-OCT retinal image acquisition and analysis from the Atlas of Retinal Imaging in Alzheimer’s Study (ARIAS). Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2020, 12, e12119. [Google Scholar] [CrossRef] [PubMed]
- Chan, V.T.T.; Tso, T.H.K.; Tang, F.; Tham, C.; Mok, V.; Chen, C.; Wong, T.Y.; Cheung, C.Y. Using Retinal Imaging to Study Dementia. J. Vis. Exp. 2017, 129, e56137. [Google Scholar] [CrossRef] [PubMed]
- McGrory, S.; Cameron, J.R.; Pellegrini, E.; Warren, C.; Doubal, F.N.; Deary, I.J.; Dhillon, B.; Wardlaw, J.M.; Trucco, E.; MacGillivray, T.J. The application of retinal fundus camera imaging in dementia: A systematic review. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2017, 6, 91–107. [Google Scholar] [CrossRef] [PubMed]
- Marquié, M.; Valero, S.; Martínez, J.; Alarcón-Martín, E.; García-Sánchez, A.; de Rojas, I.; Castilla-Martí, M.; Castilla-Martí, L.; Hernández, I.; Rosende-Roca, M.; et al. Differences in macular vessel density in the superficial plexus across cognitive impairment: The NORFACE cohort. Sci. Rep. 2022, 12, 16938. [Google Scholar] [CrossRef] [PubMed]
- López-Cuenca, I.; Salobrar-García, E.; Elvira-Hurtado, L.; Fernández-Albarral, J.A.; Sánchez-Puebla, L.; Salazar, J.J.; Ramírez, J.M.; Ramírez, A.I.; de Hoz, R. The Value of OCT and OCTA as Potential Biomarkers for Preclinical Alzheimer’s Disease: A Review Study. Life 2021, 11, 712. [Google Scholar] [CrossRef]
- Hagag, A.M.; Gao, S.S.; Jia, Y.; Huang, D. Optical coherence tomography angiography: Technical principles and clinical applications in ophthalmology. Taiwan J. Ophthalmol. 2017, 7, 115–129. [Google Scholar] [CrossRef]
- Wu, H.; Wang, C.; Chen, C.; Xu, X.; Zhu, Y.; Sang, A.; Jiang, K.; Dong, J. Association between Retinal Vascular Geometric Changes and Cognitive Impairment: A Systematic Review and Meta-Analysis. J. Clin. Neurol. 2020, 16, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.P.; Robbins, C.B.; Lee, J.M.; Soundararajan, S.; Stinnett, S.S.; Agrawal, R.; Plassman, B.L.; Lad, E.M.; Whitson, H.; Grewal, D.S.; et al. Longitudinal Analysis of the Retina and Choroid in Cognitively Normal Individuals at Higher Genetic Risk of Alzheimer Disease. Ophthalmol. Retin. 2022, 6, 607–619. [Google Scholar] [CrossRef]
- Li, Z.B.; Lin, Z.J.; Li, N.; Yu, H.; Wu, Y.L.; Shen, X. Evaluation of retinal and choroidal changes in patients with Alzheimer’s type dementia using optical coherence tomography angiography. Int. J. Ophthalmol. 2021, 14, 860–868. [Google Scholar] [CrossRef]
- Cheung, C.Y.; Ong, Y.T.; Ikram, M.K.; Ong, S.Y.; Li, X.; Hilal, S.; Catindig, J.A.; Venketasubramanian, N.; Yap, P.; Seow, D.; et al. Microvascular network alterations in the retina of patients with Alzheimer’s disease. Alzheimer’s Dement. 2014, 10, 135–142. [Google Scholar] [CrossRef]
- Vagiakis, I.; Bakirtzis, C.; Andravizou, A.; Pirounides, D. Unlocking the Potential of Vessel Density and the Foveal Avascular Zone in Optical Coherence Tomography Angiography as Biomarkers in Alzheimer’s Disease. Healthcare 2024, 12, 1589. [Google Scholar] [CrossRef] [PubMed]
- O’Bryhim, B.E.; Lin, J.B.; Van Stavern, G.P.; Apte, R.S. OCT Angiography Findings in Preclinical Alzheimer’s Disease: 3-Year Follow-Up. Ophthalmology 2021, 128, 1489–1491. [Google Scholar] [CrossRef]
- Bulut, M.; Kurtuluş, F.; Gözkaya, O.; Erol, M.K.; Cengiz, A.; Akıdan, M.; Yaman, A. Evaluation of optical coherence tomography angiographic findings in Alzheimer’s type dementia. Br. J. Ophthalmol. 2018, 102, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Zabel, P.; Kaluzny, J.J.; Wilkosc-Debczynska, M.; Gebska-Toloczko, M.; Suwala, K.; Zabel, K.; Zaron, A.; Kucharski, R.; Araszkiewicz, A. Comparison of Retinal Microvasculature in Patients with Alzheimer’s Disease and Primary Open-Angle Glaucoma by Optical Coherence Tomography Angiography. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3447–3455. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, X.; Azhati, G.; Li, T.; Xu, G.; Liu, F. Retinal microvascular attenuation in mental cognitive impairment and Alzheimer’s disease by optical coherence tomography angiography. Acta Ophthalmol. 2020, 98, e781–e787. [Google Scholar] [CrossRef]
- Weng, C.Y.; Maguire, M.G.; Flaxel, C.J.; Jain, N.; Kim, S.J.; Patel, S.; Smith, J.R.; Kim, L.A.; Yeh, S. Effectiveness of Conventional Digital Fundus Photography-Based Teleretinal Screening for Diabetic Retinopathy and Diabetic Macular Edema: A Report by the American Academy of Ophthalmology. Ophthalmology 2024, 131, 927–942. [Google Scholar] [CrossRef]
- Rebouças, S.C.L.; Cougnard-Gregoire, A.; Arnould, L.; Delyfer, M.N.; Schweitzer, C.; Korobelnik, J.F.; Foubert-Samier, A.; Cheung, C.Y.; Wong, T.Y.; Delcourt, C.; et al. Retinal microvasculature and incident dementia over 10 years: The Three-City-Alienor cohort. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2023, 15, e12480. [Google Scholar] [CrossRef]
- Ganji, Z.; Nikparast, F.; Shoeibi, N.; Shoeibi, A.; Zare, H.; Sharak, N.A. Retinal imaging and artificial intelligence: A systematic review and meta-analysis of diagnostic techniques for neurodegenerative diseases. Photodiagnosis Photodyn. Ther. 2025, 55, 104788. [Google Scholar] [CrossRef] [PubMed]
- Wisely, C.E.; Wang, D.; Henao, R.; Grewal, D.S.; Thompson, A.C.; Robbins, C.B.; Yoon, S.P.; Soundararajan, S.; Polascik, B.W.; Burke, J.R.; et al. Convolutional neural network to identify symptomatic Alzheimer’s disease using multimodal retinal imaging. Br. J. Ophthalmol. 2022, 106, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Luengnaruemitchai, G.; Kaewmahanin, W.; Munthuli, A.; Phienphanich, P.; Puangarom, S.; Sangchocanonta, S.; Jariyakosol, S.; Hirunwiwatkul, P.; Tantibundhit, C. Alzheimer’s Together with Mild Cognitive Impairment Screening Using Polar Transformation of Middle Zone of Fundus Images Based Deep Learning. In Proceedings of the 45th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Sydney, Australia, 24–27 July 2023; pp. 1–4. [Google Scholar] [CrossRef]
- Wu, H.; Lei, Z.; Ou, Y.; Shi, X.; Xu, Q.; Shi, K.; Ding, J.; Zhao, Q.; Wang, X.; Cai, X.; et al. Computed Tomography Density and β-Amyloid Deposition of Intraorbital Optic Nerve May Assist in Diagnosing Mild Cognitive Impairment and Alzheimer’s Disease: A (18)F-Flutemetamol Positron Emission Tomography/Computed Tomography Study. Front. Aging Neurosci. 2022, 14, 836568. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.S.; Ritch, R.; Schwartz, B.; Lee, S.S.; Miller, N.R.; Chi, T.; Hsieh, F.Y. Optic nerve head and nerve fiber layer in Alzheimer’s disease. Arch. Ophthalmol. 1991, 109, 199–204. [Google Scholar] [CrossRef]
- Kurna, S.A.; Akar, G.; Altun, A.; Agirman, Y.; Gozke, E.; Sengor, T. Confocal scanning laser tomography of the optic nerve head on the patients with Alzheimer’s disease compared to glaucoma and control. Int. Ophthalmol. 2014, 34, 1203–1211. [Google Scholar] [CrossRef]
- Berisha, F.; Feke, G.T.; Trempe, C.L.; McMeel, J.W.; Schepens, C.L. Retinal abnormalities in early Alzheimer’s disease. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2285–2289. [Google Scholar] [CrossRef]
- Kirbas, S.; Turkyilmaz, K.; Anlar, O.; Tufekci, A.; Durmus, M. Retinal nerve fiber layer thickness in patients with Alzheimer disease. J. Neuroophthalmol. 2013, 33, 58–61. [Google Scholar] [CrossRef]
- Lu, Y.; Li, Z.; Zhang, X.; Ming, B.; Jia, J.; Wang, R.; Ma, D. Retinal nerve fiber layer structure abnormalities in early Alzheimer’s disease: Evidence in optical coherence tomography. Neurosci. Lett. 2010, 480, 69–72. [Google Scholar] [CrossRef]
- Gharbiya, M.; Trebbastoni, A.; Parisi, F.; Manganiello, S.; Cruciani, F.; D’Antonio, F.; De Vico, U.; Imbriano, L.; Campanelli, A.; De Lena, C. Choroidal thinning as a new finding in Alzheimer’s disease: Evidence from enhanced depth imaging spectral domain optical coherence tomography. J. Alzheimers Dis. 2014, 40, 907–917. [Google Scholar] [CrossRef] [PubMed]
- van de Kreeke, J.A.; Nguyen, H.T.; den Haan, J.; Konijnenberg, E.; Tomassen, J.; den Braber, A.; Ten Kate, M.; Collij, L.; Yaqub, M.; van Berckel, B.; et al. Retinal layer thickness in preclinical Alzheimer’s disease. Acta Ophthalmol. 2019, 97, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Kusbeci, T.; Kusbeci, O.Y.; Mas, N.G.; Karabekir, H.S.; Yavas, G.; Yucel, A. Stereological Evaluation of the Optic Nerve Volume in Alzheimer Disease. J. Craniofac Surg. 2015, 26, 1683–1686. [Google Scholar] [CrossRef] [PubMed]
- Davies, D.C.; McCoubrie, P.; McDonald, B.; Jobst, K.A. Myelinated axon number in the optic nerve is unaffected by Alzheimer’s disease. Br. J. Ophthalmol. 1995, 79, 596–600. [Google Scholar] [CrossRef]
- Cesareo, M.; Martucci, A.; Ciuffoletti, E.; Mancino, R.; Cerulli, A.; Sorge, R.P.; Martorana, A.; Sancesario, G.; Nucci, C. Association Between Alzheimer’s Disease and Glaucoma: A Study Based on Heidelberg Retinal Tomography and Frequency Doubling Technology Perimetry. Front. Neurosci. 2015, 9, 479. [Google Scholar] [CrossRef]
- Bambo, M.P.; Garcia-Martin, E.; Gutierrez-Ruiz, F.; Pinilla, J.; Perez-Olivan, S.; Larrosa, J.M.; Polo, V.; Pablo, L. Analysis of optic disk color changes in Alzheimer’s disease: A potential new biomarker. Clin. Neurol. Neurosurg. 2015, 132, 68–73. [Google Scholar] [CrossRef]
- Salobrar-Garcia, E.; Méndez-Hernández, C.; Hoz, R.; Ramírez, A.I.; López-Cuenca, I.; Fernández-Albarral, J.A.; Rojas, P.; Wang, S.; García-Feijoo, J.; Gil, P.; et al. Ocular Vascular Changes in Mild Alzheimer’s Disease Patients: Foveal Avascular Zone, Choroidal Thickness, and ONH Hemoglobin Analysis. J. Pers. Med. 2020, 10, 231. [Google Scholar] [CrossRef]
- Syed, A.B.; Armstrong, R.A.; Smith, C.U. A quantitative analysis of optic nerve axons in elderly control subjects and patients with Alzheimer’s disease. Folia Neuropathol. 2005, 43, 1–6. [Google Scholar]
- Li, M.; Li, R.; Lyu, J.H.; Chen, J.H.; Wang, W.; Gao, M.L.; Li, W.J.; De, J.; Mu, H.Y.; Pan, W.G.; et al. Relationship Between Alzheimer’s Disease and Retinal Choroidal Thickness: A Cross-Sectional Study. J. Alzheimers Dis. 2021, 80, 407–419. [Google Scholar] [CrossRef]
- Cunha, J.P.; Proença, R.; Dias-Santos, A.; Melancia, D.; Almeida, R.; Águas, H.; Santos, B.O.; Alves, M.; Ferreira, J.; Papoila, A.L.; et al. Choroidal thinning: Alzheimer’s disease and aging. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2017, 8, 11–17. [Google Scholar] [CrossRef]
- Robbins, C.B.; Grewal, D.S.; Thompson, A.C.; Powers, J.H.; Soundararajan, S.; Koo, H.Y.; Yoon, S.P.; Polascik, B.W.; Liu, A.; Agrawal, R.; et al. Choroidal Structural Analysis in Alzheimer Disease, Mild Cognitive Impairment, and Cognitively Healthy Controls. Am. J. Ophthalmol. 2021, 223, 359–367. [Google Scholar] [CrossRef]
- López-de-Eguileta, A.; Lage, C.; López-García, S.; Pozueta, A.; García-Martínez, M.; Kazimierczak, M.; Bravo, M.; de Arcocha-Torres, M.; Banzo, I.; Jimenez-Bonilla, J.; et al. Evaluation of choroidal thickness in prodromal Alzheimer’s disease defined by amyloid PET. PLoS ONE 2020, 15, e0239484. [Google Scholar] [CrossRef]
- Trebbastoni, A.; Marcelli, M.; Mallone, F.; D’Antonio, F.; Imbriano, L.; Campanelli, A.; de Lena, C.; Gharbiya, M. Attenuation of Choroidal Thickness in Patients With Alzheimer Disease: Evidence From an Italian Prospective Study. Alzheimer Dis. Assoc. Disord. 2017, 31, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Asanad, S.; Ross-Cisneros, F.N.; Barron, E.; Nassisi, M.; Sultan, W.; Karanjia, R.; Sadun, A.A. The retinal choroid as an oculovascular biomarker for Alzheimer’s dementia: A histopathological study in severe disease. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2019, 11, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Castilla-Martí, L.; García-Sánchez, A.; Martínez, J.; Rosende-Roca, M.; Vargas, L.; Tartari, J.P.; Casales, F.; Rodríguez, J.N.; Bein, N.; Alegret, M.; et al. Changes in choroidal thickness quantified by Optical Coherence Tomography across cognitive impairment: Data from the NORFACE cohort. Alzheimers Res. Ther. 2024, 16, 249. [Google Scholar] [CrossRef]
- Corradetti, G.; Oncel, D.; Kadomoto, S.; Arakaki, X.; Kloner, R.A.; Sadun, A.A.; Sadda, S.R.; Chan, J.W. Choriocapillaris and Retinal Vascular Alterations in Presymptomatic Alzheimer’s Disease. Investig. Ophthalmol. Vis. Sci. 2024, 65, 47. [Google Scholar] [CrossRef] [PubMed]
- Kwapong, W.R.; Tang, F.; Liu, P.; Zhang, Z.; Cao, L.; Feng, Z.; Yang, S.; Shu, Y.; Xu, H.; Lu, Y.; et al. Choriocapillaris reduction accurately discriminates against early-onset Alzheimer’s disease. Alzheimers Dement. 2024, 20, 4185–4198. [Google Scholar] [CrossRef]
- Burke, J.; Gibbon, S.; Low, A.; Hamid, C.; Reid-Schachter, M.; Muniz-Terrera, G.; Ritchie, C.W.; Dhillon, B.; O’Brien, J.T.; King, S.; et al. Association between choroidal microvasculature in the eye and Alzheimer’s disease risk in cognitively healthy mid-life adults: A pilot study. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2025, 17, e70075. [Google Scholar] [CrossRef]
- Di Pippo, M.; Cipollini, V.; Giubilei, F.; Scuderi, G.; Abdolrahimzadeh, S. Retinal and Choriocapillaris Vascular Changes in Early Alzheimer Disease Patients Using Optical Coherence Tomography Angiography. J. Neuroophthalmol. 2024, 44, 184–189. [Google Scholar] [CrossRef]
- Arnerić, S.P.; Batrla-Utermann, R.; Beckett, L.; Bittner, T.; Blennow, K.; Carter, L.; Dean, R.; Engelborghs, S.; Genius, J.; Gordon, M.F.; et al. Cerebrospinal Fluid Biomarkers for Alzheimer’s Disease: A View of the Regulatory Science Qualification Landscape from the Coalition Against Major Diseases CSF Biomarker Team. J. Alzheimers Dis. 2017, 55, 19–35. [Google Scholar] [CrossRef]
- Anoop, A.; Singh, P.K.; Jacob, R.S.; Maji, S.K. CSF Biomarkers for Alzheimer’s Disease Diagnosis. Int. J. Alzheimers Dis. 2010, 2010, 606802. [Google Scholar] [CrossRef] [PubMed]
- Sampani, K.; Ness, S.; Tuz-Zahra, F.; Aytan, N.; Spurlock, E.E.; Alluri, S.; Chen, X.; Siegel, N.H.; Alosco, M.L.; Xia, W.; et al. Neurodegenerative biomarkers in different chambers of the eye relative to plasma: An agreement validation study. Alzheimers Res. Ther. 2024, 16, 192. [Google Scholar] [CrossRef]
- Pieczyński, J.; Szulc, U.; Harazna, J.; Szulc, A.; Kiewisz, J. Tear fluid collection methods: Review of current techniques. Eur. J. Ophthalmol. 2021, 31, 2245–2251. [Google Scholar] [CrossRef]
- Green-Church, K.B.; Nichols, K.K.; Kleinholz, N.M.; Zhang, L.; Nichols, J.J. Investigation of the human tear film proteome using multiple proteomic approaches. Mol. Vis. 2008, 14, 456–470. [Google Scholar]
- Kalló, G.; Emri, M.; Varga, Z.; Ujhelyi, B.; Tőzsér, J.; Csutak, A.; Csősz, É. Changes in the Chemical Barrier Composition of Tears in Alzheimer’s Disease Reveal Potential Tear Diagnostic Biomarkers. PLoS ONE 2016, 11, e0158000. [Google Scholar] [CrossRef]
- Kenny, A.; Jiménez-Mateos, E.M.; Zea-Sevilla, M.A.; Rábano, A.; Gili-Manzanaro, P.; Prehn, J.H.M.; Henshall, D.C.; Ávila, J.; Engel, T.; Hernández, F. Proteins and microRNAs are differentially expressed in tear fluid from patients with Alzheimer’s disease. Sci. Rep. 2019, 9, 15437. [Google Scholar] [CrossRef]
- Gijs, M.; Ramakers, I.; Visser, P.J.; Verhey, F.R.J.; van de Waarenburg, M.P.H.; Schalkwijk, C.G.; Nuijts, R.; Webers, C.A.B. Association of tear fluid amyloid and tau levels with disease severity and neurodegeneration. Sci. Rep. 2021, 11, 22675. [Google Scholar] [CrossRef]
- Gharbiya, M.; Visioli, G.; Trebbastoni, A.; Albanese, G.M.; Colardo, M.; D’Antonio, F.; Segatto, M.; Lambiase, A. Beta-Amyloid Peptide in Tears: An Early Diagnostic Marker of Alzheimer’s Disease Correlated with Choroidal Thickness. Int. J. Mol. Sci. 2023, 24, 2590. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, S.; Marasco, D.; Sabetta, R.; Del Prete, A.; Marino, F.Z.; Franco, R.; Troisi, S.; Troisi, M.; Cennamo, G. Tear Liquid for Predictive Diagnosis of Alzheimer’s Disease. Reports 2021, 4, 26. [Google Scholar] [CrossRef]
- Higaki, S.; Muramatsu, M.; Matsuda, A.; Matsumoto, K.; Satoh, J.I.; Michikawa, M.; Niida, S. Defensive effect of microRNA-200b/c against amyloid-beta peptide-induced toxicity in Alzheimer’s disease models. PLoS ONE 2018, 13, e0196929. [Google Scholar] [CrossRef]
- Sunderland, D.K.; Sapra, A. Physiology, Aqueous Humor Circulation. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2025. [Google Scholar]
- Cheung, C.M.; Durrani, O.M.; Murray, P.I. The safety of anterior chamber paracentesis in patients with uveitis. Br. J. Ophthalmol. 2004, 88, 582–583. [Google Scholar] [CrossRef]
- Inoue, T.; Kawaji, T.; Tanihara, H. Elevated levels of multiple biomarkers of Alzheimer’s disease in the aqueous humor of eyes with open-angle glaucoma. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5353–5358. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, F.; Caudano, F.; Marenco, M.; Testa, V.; Masala, A.; Sindaco, D.; Macrì, A.; Traverso, C.E.; Iester, M.; Ricciarelli, R. Evaluating the Correlation between Alzheimer’s Amyloid-β Peptides and Glaucoma in Human Aqueous Humor. Transl. Vis. Sci. Technol. 2020, 9, 21. [Google Scholar] [CrossRef]
- Janciauskiene, S.; Westin, K.; Grip, O.; Krakau, T. Detection of Alzheimer peptides and chemokines in the aqueous humor. Eur. J. Ophthalmol. 2011, 21, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Wisely, C.E.; Robbins, C.B.; Parker, D.; Challa, P.; Grewal, D.S.; Fekrat, S. Aqueous and Plasma Levels of Phosphorylated Tau 181 in Individuals with Normal Cognition. J. Alzheimers Dis. 2024, 100, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Wan, Z.; Wang, M.; Wu, X.; Wang, T.; Zhang, Y.; Xue, Y.; Xu, H.; Peng, Q. Association of cognitive function with Neurofilament light chain in the aqueous humor of human eye. Front. Aging Neurosci. 2022, 14, 1027705. [Google Scholar] [CrossRef]
- Jena, S.; Tripathy, K. Vitreous Hemorrhage. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2025. [Google Scholar]
- Vig, V.; Garg, I.; Tuz-Zahra, F.; Xu, J.; Tripodis, Y.; Nicks, R.; Xia, W.; Alvarez, V.E.; Alosco, M.L.; Stein, T.D.; et al. Vitreous Humor Biomarkers Reflect Pathological Changes in the Brain for Alzheimer’s Disease and Chronic Traumatic Encephalopathy. J. Alzheimers Dis. 2023, 93, 1181–1193. [Google Scholar] [CrossRef]
- Wright, L.M.; Stein, T.D.; Jun, G.; Chung, J.; McConnell, K.; Fiorello, M.; Siegel, N.; Ness, S.; Xia, W.; Turner, K.L.; et al. Association of Cognitive Function with Amyloid-β and Tau Proteins in the Vitreous Humor. J. Alzheimers Dis. 2019, 68, 1429–1438. [Google Scholar] [CrossRef]
- Subramanian, M.L.; Vig, V.; Chung, J.; Fiorello, M.G.; Xia, W.; Zetterberg, H.; Blennow, K.; Zetterberg, M.; Shareef, F.; Siegel, N.H.; et al. Neurofilament light chain in the vitreous humor of the eye. Alzheimers Res. Ther. 2020, 12, 111. [Google Scholar] [CrossRef]
- Pais, M.V.; Forlenza, O.V.; Diniz, B.S. Plasma Biomarkers of Alzheimer’s Disease: A Review of Available Assays, Recent Developments, and Implications for Clinical Practice. J. Alzheimers Dis. Rep. 2023, 7, 355–380. [Google Scholar] [CrossRef]
- Cai, H.; Pang, Y.; Fu, X.; Ren, Z.; Jia, L. Plasma biomarkers predict Alzheimer’s disease before clinical onset in Chinese cohorts. Nat. Commun. 2023, 14, 6747. [Google Scholar] [CrossRef]
- Moscoso, A.; Grothe, M.J.; Ashton, N.J.; Karikari, T.K.; Lantero Rodríguez, J.; Snellman, A.; Suárez-Calvet, M.; Blennow, K.; Zetterberg, H.; Schöll, M.; et al. Longitudinal Associations of Blood Phosphorylated Tau181 and Neurofilament Light Chain With Neurodegeneration in Alzheimer Disease. JAMA Neurol. 2021, 78, 396–406. [Google Scholar] [CrossRef]
- Giacomucci, G.; Mazzeo, S.; Ingannato, A.; Crucitti, C.; Bagnoli, S.; Padiglioni, S.; Romano, L.; Galdo, G.; Emiliani, F.; Frigerio, D.; et al. Future perspective and clinical applicability of the combined use of plasma phosphorylated tau 181 and neurofilament light chain in Subjective Cognitive Decline and Mild Cognitive Impairment. Sci. Rep. 2024, 14, 11307. [Google Scholar] [CrossRef] [PubMed]
- Schöll, M.; Vrillon, A.; Ikeuchi, T.; Quevenco, F.C.; Iaccarino, L.; Vasileva-Metodiev, S.Z.; Burnham, S.C.; Hendrix, J.; Epelbaum, S.; Zetterberg, H.; et al. Cutting through the noise: A narrative review of Alzheimer’s disease plasma biomarkers for routine clinical use. J. Prev. Alzheimers Dis. 2025, 12, 100056. [Google Scholar] [CrossRef] [PubMed]
- Verberk, I.M.W.; Gouda, M.; Antwi-Berko, D.; van Leeuwenstijn, M.; Bongers, B.; Houtkamp, I.M.; van der Flier, W.M.; Janelidze, S.; Hansson, O.; Bastard, N.L.; et al. Evidence-based standardized sample handling protocol for accurate blood-based Alzheimer’s disease biomarker measurement: Results and consensus of the Global Biomarker Standardization Consortium. Alzheimers Dement. 2025, 21, e70752. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).