Fab N-Glycosylation in IgG: Implications in Physiological and Pathological Immune Regulation
Abstract
1. Introduction
2. Autoimmune Diseases and Fab-Glycosylated IgG
| Autoimmune Disease | Antibody with Fab Glycosylation | Glycosylation Type |
|---|---|---|
| Rheumatoid Arthritis (RA) [33] | ACPA | Complex (highly sialylated) |
| Systemic Lupus Erythematosus (SLE) [30] | anti-Smith anti-dsDNA | unknown |
| Myasthenia Gravis (MG) [38] | anti-MuSK anti-AchR | unknown |
| ANCA-associated Vasculitis (AAV) [30] | anti-PR3 anti-MPO | Complex |
| Pemphigus Vulgaris (PV) [30] | anti-Dsg3 | unknown |
| Primary Sjögren’s Syndrome (pSS) [29] | anti-SS-A/B | Complex |
| Immunoglobulin G4-related disease (IgG4-RD) [39] | IgG4 | Complex (Sialylated) |
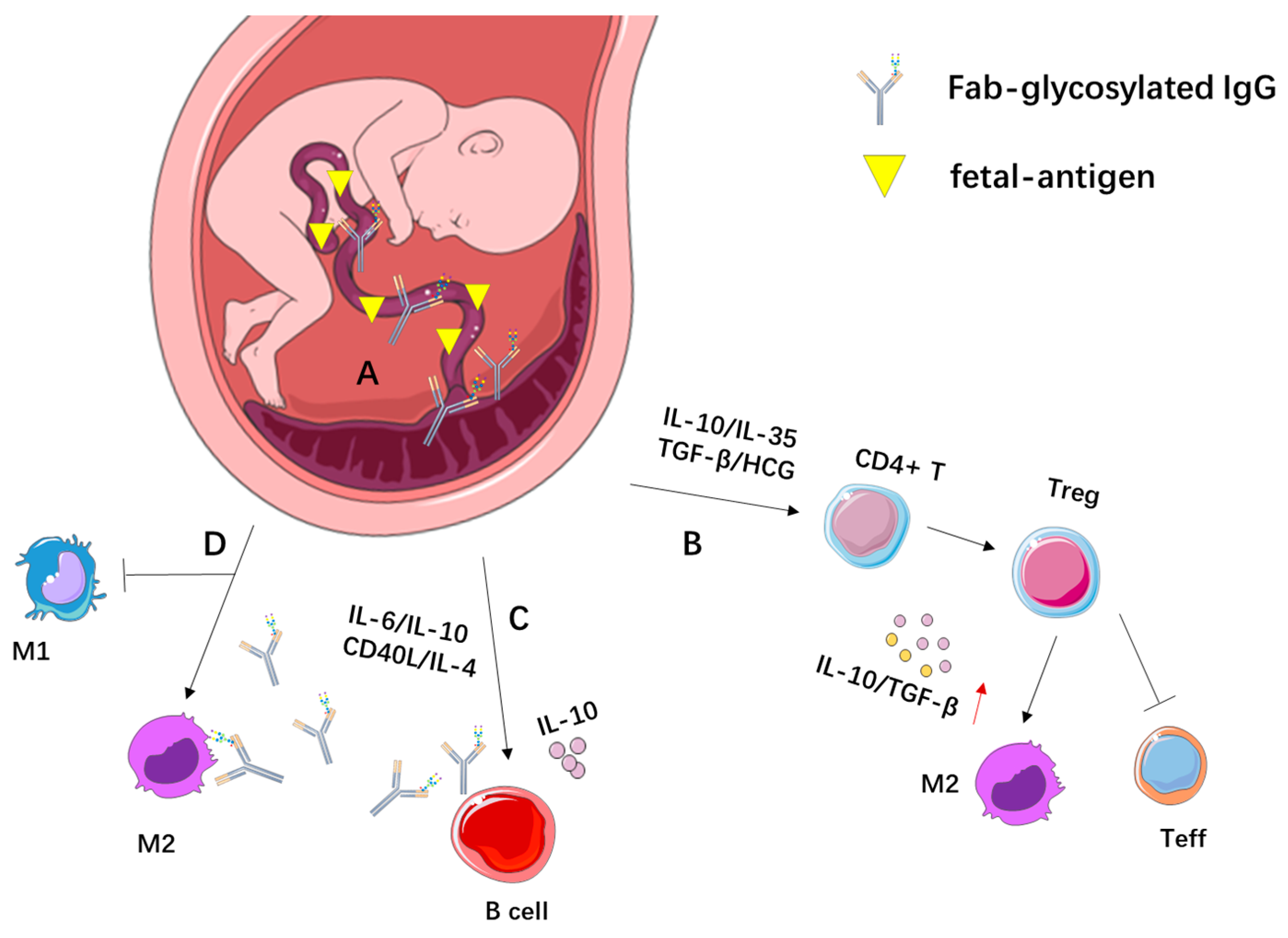
3. Immune Tolerance in Pregnancy and Fab-Glycosylated IgG
4. Fab-Glycosylated IgG in Tumor Immune Escape and Antibody Therapy
5. Association of Fab-Glycosylated IgG with IgG4
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADCC | Antibody-Dependent Cell-mediated Cytotoxicity |
| ADCP | Antibody-Dependent Cellular Phagocytosis |
| CDC | Complement-Dependent Cytotoxicity |
| N-GlcNAc | N-acetylglucosamine |
| ConA | Concanavalin A |
| CDR2 | the Second Complementarity-Determining Region |
| PSS | Primary Sjögren’s Syndrome, |
| Dsg3 | Desmoglein 3 |
| PV | Pemphigus Vulgaris |
| PR3 | Proteinase 3 |
| MPO | Myeloperoxidase |
| ANCA | Anti-Neutrophil Cytoplasmic Antibody |
| AAV | ANCA-associated Vasculitis |
| ACPA | Anti-Citrullinated Protein Antibody |
| RA | Rheumatoid Arthritis |
| MG | Myasthenia Gravis |
| MuSK | Muscle-Specific Tyrosine Kinase |
| AChR | Acetylcholine Receptor |
| SNA | Sambucus Nigra Agglutinin |
| VDG | Variable Domain Glycosylation |
| SLE | Systemic Lupus Erythematosus |
| BCR | B Cell Receptor |
| UDP-Glc | Uridine Diphosphate Glucose |
| FL | Follicular Lymphoma |
| DC-SIGN | Dendritic Cell-Specific Intercellular adhesion molecule-Grabbing Nonintegrin |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| PD-1 | Programmed Cell Death Protein 1 |
| FAE | Fab Arm Exchange |
| PD-L1 | Programmed Cell Death Ligand-1 |
| HPD | Hyperprogressive Disease |
References
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG subclasses and allotypes: From structure to effector functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef]
- Amzel, L.M.; Poljak, R.J. Three-dimensional structure of immunoglobulins. Annu. Rev. Biochem. 1979, 48, 961–997. [Google Scholar] [CrossRef]
- Huber, R.; Deisenhofer, J.; Colman, P.M.; Matsushima, M.; Palm, W. Crystallographic structure studies of an IgG molecule and an Fc fragment. Nature 1976, 264, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, G.; Rispens, T.; Vidarsson, G. Novel Concepts of Altered Immunoglobulin G Galactosylation in Autoimmune Diseases. Front. Immunol. 2018, 9, 553. [Google Scholar] [CrossRef] [PubMed]
- Nose, M.; Wigzell, H. Biological significance of carbohydrate chains on monoclonal antibodies. Proc. Natl. Acad. Sci. USA 1983, 80, 6632–6636. [Google Scholar] [CrossRef]
- Mimura, Y.; Church, S.; Ghirlando, R.; Ashton, P.R.; Dong, S.; Goodall, M.; Lund, J.; Jefferis, R. The influence of glycosylation on the thermal stability and effector function expression of human IgG1-Fc: Properties of a series of truncated glycoforms. Mol. Immunol. 2000, 37, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.L.; Lai, J.; Keck, R.; O’Connell, L.Y.; Hong, K.; Meng, Y.G.; Weikert, S.H.; Presta, L.G. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J. Biol. Chem. 2002, 277, 26733–26740. [Google Scholar] [CrossRef]
- Wang, T.T.; Ravetch, J.V. Functional diversification of IgGs through Fc glycosylation. J. Clin. Investig. 2019, 129, 3492–3498. [Google Scholar] [CrossRef]
- Walker, M.R.; Lund, J.; Thompson, K.M.; Jefferis, R. Aglycosylation of human IgG1 and IgG3 monoclonal antibodies can eliminate recognition by human cells expressing Fc gamma RI and/or Fc gamma RII receptors. Biochem. J. 1989, 259, 347–353. [Google Scholar] [CrossRef]
- Boyd, P.N.; Lines, A.C.; Patel, A.K. The effect of the removal of sialic acid, galactose and total carbohydrate on the functional activity of Campath-1H. Mol. Immunol. 1995, 32, 1311–1318. [Google Scholar] [CrossRef]
- Hodoniczky, J.; Zheng, Y.Z.; James, D.C. Control of recombinant monoclonal antibody effector functions by Fc N-glycan remodeling in vitro. Biotechnol. Prog. 2005, 21, 1644–1652. [Google Scholar] [CrossRef]
- Radcliffe, C.M.; Arnold, J.N.; Suter, D.M.; Wormald, M.R.; Harvey, D.J.; Royle, L.; Mimura, Y.; Kimura, Y.; Sim, R.B.; Inogès, S.; et al. Human follicular lymphoma cells contain oligomannose glycans in the antigen-binding site of the B-cell receptor. J. Biol. Chem. 2007, 282, 7405–7415. [Google Scholar] [CrossRef]
- Ampel, N.M.; Nelson, D.K.; Li, L.; Dionne, S.O.; Lake, D.F.; Simmons, K.A.; Pappagianis, D. The mannose receptor mediates the cellular immune response in human coccidioidomycosis. Infect. Immun. 2005, 73, 2554–2555. [Google Scholar] [CrossRef]
- Li, D.; Lou, Y.; Zhang, Y.; Liu, S.; Li, J.; Tao, J. Sialylated immunoglobulin G: A promising diagnostic and therapeutic strategy for autoimmune diseases. Theranostics 2021, 11, 5430–5446. [Google Scholar] [CrossRef]
- Hafkenscheid, L.; Bondt, A.; Scherer, H.U.; Huizinga, T.W.J.; Wuhrer, M.; Toes, R.E.M.; Rombouts, Y. Structural Analysis of Variable Domain Glycosylation of Anti-Citrullinated Protein Antibodies in Rheumatoid Arthritis Reveals the Presence of Highly Sialylated Glycans. Mol. Cell. Proteom. 2017, 16, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Canellada, A.; Gentile, T.; Dokmetjian, J.; Margni, R.A. Occurrence, properties, and function of asymmetric IgG molecules isolated from non-immune sera. Immunol. Investig. 2002, 31, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Bondt, A.; Rombouts, Y.; Selman, M.H.J.; Hensbergen, P.J.; Reiding, K.R.; Hazes, J.M.W.; Dolhain, R.J.E.M.; Wuhrer, M. Immunoglobulin G (IgG) Fab Glycosylation Analysis Using a New Mass Spectrometric High-throughput Profiling Method Reveals Pregnancy-associated Changes. Mol. Cell. Proteom. 2014, 13, 3029–3039. [Google Scholar] [CrossRef] [PubMed]
- Anumula, K.R. Quantitative glycan profiling of normal human plasma derived immunoglobulin and its fragments Fab and Fc. J. Immunol. Methods 2012, 382, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Leoni, J.; Labeta, M.; Margni, R.A. The asymmetric IgG non-precipitating antibody. Localization of the oligosaccharide involved, by concanavalin A interaction. Mol. Immunol. 1986, 23, 1397–1400. [Google Scholar] [CrossRef]
- Jefferis, R. Glycosylation of recombinant antibody therapeutics. Biotechnol. Prog. 2005, 21, 11–16. [Google Scholar] [CrossRef]
- Holland, M.; Yagi, H.; Takahashi, N.; Kato, K.; Savage, C.O.; Goodall, D.M.; Jefferis, R. Differential glycosylation of polyclonal IgG, IgG-Fc and IgG-Fab isolated from the sera of patients with ANCA-associated systemic vasculitis. Biochim. Biophys. Acta 2006, 1760, 669–677. [Google Scholar] [CrossRef]
- van de Bovenkamp, F.S.; Derksen, N.I.L.; Ooijevaar-de Heer, P.; van Schie, K.A.; Kruithof, S.; Berkowska, M.A.; van der Schoot, C.E.; H, I.J.; van der Burg, M.; Gils, A.; et al. Adaptive antibody diversification through N-linked glycosylation of the immunoglobulin variable region. Proc. Natl. Acad. Sci. USA 2018, 115, 1901–1906. [Google Scholar] [CrossRef]
- Coloma, M.J.; Trinh, R.K.; Martinez, A.R.; Morrison, S.L. Position effects of variable region carbohydrate on the affinity and in vivo behavior of an anti-(1-->6) dextran antibody. J. Immunol. 1999, 162, 2162–2170. [Google Scholar] [CrossRef]
- van de Bovenkamp, F.S.; Derksen, N.I.L.; van Breemen, M.J.; de Taeye, S.W.; Ooijevaar-de Heer, P.; Sanders, R.W.; Rispens, T. Variable Domain N-linked glycans acquired During antigen-specific immune responses can contribute to immunoglobulin G antibody stability. Front. Immunol. 2018, 9, 740. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.; Tao, M.H.; Kabat, E.A.; Morrison, S.L. Antibody variable region glycosylation: Position effects on antigen binding and carbohydrate structure. EMBO J. 1991, 10, 2717–2723. [Google Scholar] [CrossRef] [PubMed]
- Schneider, D.; Dühren-von Minden, M.; Alkhatib, A.; Setz, C.; van Bergen, C.A.M.; Benkißer-Petersen, M.; Wilhelm, I.; Villringer, S.; Krysov, S.; Packham, G.; et al. Lectins from opportunistic bacteria interact with acquired variable-region glycans of surface immunoglobulin in follicular lymphoma. Blood 2015, 125, 3287–3296. [Google Scholar] [CrossRef]
- Gutierrez, G.; Gentile, T.; Miranda, S.; Margni, R.A. Asymmetric antibodies: A protective arm in pregnancy. Chem. Immunol. Allergy 2005, 89, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Fugger, L.; Jensen, L.T.; Rossjohn, J. Challenges, Progress, and Prospects of Developing Therapies to Treat Autoimmune Diseases. Cell 2020, 181, 63–80. [Google Scholar] [CrossRef]
- Hamza, N.; Hershberg, U.; Kallenberg, C.G.; Vissink, A.; Spijkervet, F.K.; Bootsma, H.; Kroese, F.G.; Bos, N.A. Ig gene analysis reveals altered selective pressures on Ig-producing cells in parotid glands of primary Sjogren’s syndrome patients. J. Immunol. 2015, 194, 514–521. [Google Scholar] [CrossRef]
- Koers, J.; Sciarrillo, R.; Derksen, N.I.L.; Vletter, E.M.; Fillie-Grijpma, Y.E.; Raveling-Eelsing, E.; Graca, N.A.G.; Leijser, T.; Pas, H.H.; Laura van Nijen-Vos, L.; et al. Differences in IgG autoantibody Fab glycosylation across autoimmune diseases. J. Allergy Clin. Immunol. 2023, 151, 1646–1654. [Google Scholar] [CrossRef]
- Lardinois, O.M.; Deterding, L.J.; Hess, J.J.; Poulton, C.J.; Henderson, C.D.; Jennette, J.C.; Nachman, P.H.; Falk, R.J. Immunoglobulins G from patients with ANCA-associated vasculitis are atypically glycosylated in both the Fc and Fab regions and the relation to disease activity. PLoS ONE 2019, 14, e0213215. [Google Scholar] [CrossRef]
- Vletter, E.M.; Koning, M.T.; Scherer, H.U.; Veelken, H.; Toes, R.E.M. A Comparison of Immunoglobulin Variable Region N-Linked Glycosylation in Healthy Donors, Autoimmune Disease and Lymphoma. Front. Immunol. 2020, 11, 241. [Google Scholar] [CrossRef]
- Rombouts, Y.; Willemze, A.; van Beers, J.J.B.C.; Shi, J.; Kerkman, P.F.; van Toorn, L.; Janssen, G.M.C.; Zaldumbide, A.; Hoeben, R.C.; Pruijn, G.J.M.; et al. Extensive glycosylation of ACPA-IgG variable domains modulates binding to citrullinated antigens in rheumatoid arthritis. Ann. Rheum. Dis. 2016, 75, 578–585. [Google Scholar] [CrossRef]
- Lloyd, K.A.; Steen, J.; Amara, K.; Titcombe, P.J.; Israelsson, L.; Lundstrom, S.L.; Zhou, D.; Zubarev, R.A.; Reed, E.; Piccoli, L.; et al. Variable domain N-linked glycosylation and negative surface charge are key features of monoclonal ACPA: Implications for B-cell selection. Eur. J. Immunol. 2018, 48, 1030–1045. [Google Scholar] [CrossRef]
- Vergroesen, R.D.; Slot, L.M.; Hafkenscheid, L.; Koning, M.T.; van der Voort, E.I.H.; Grooff, C.A.; Zervakis, G.; Veelken, H.; Huizinga, T.W.J.; Rispens, T.; et al. B-cell receptor sequencing of anti-citrullinated protein antibody (ACPA) IgG-expressing B cells indicates a selective advantage for the introduction of N-glycosylation sites during somatic hypermutation. Ann. Rheum. Dis. 2018, 77, 956–958. [Google Scholar] [CrossRef]
- Endo, T.; Wright, A.; Morrison, S.L.; Kobata, A. Glycosylation of the variable region of immunoglobulin G--site specific maturation of the sugar chains. Mol. Immunol. 1995, 32, 931–940. [Google Scholar] [CrossRef]
- Hafkenscheid, L.; de Moel, E.; Smolik, I.; Tanner, S.; Meng, X.; Jansen, B.C.; Bondt, A.; Wuhrer, M.; Huizinga, T.W.J.; Toes, R.E.M.; et al. N-Linked Glycans in the Variable Domain of IgG Anti-Citrullinated Protein Antibodies Predict the Development of Rheumatoid Arthritis. Arthritis Rheumatol. 2019, 71, 1626–1633. [Google Scholar] [CrossRef]
- Mandel-Brehm, C.; Fichtner, M.L.; Jiang, R.; Winton, V.J.; Vazquez, S.E.; Pham, M.C.; Hoehn, K.B.; Kelleher, N.L.; Nowak, R.J.; Kleinstein, S.H.; et al. Elevated N-Linked Glycosylation of IgG V Regions in Myasthenia Gravis Disease Subtypes. J. Immunol. 2021, 207, 2005–2014. [Google Scholar] [CrossRef] [PubMed]
- Culver, E.L.; van de Bovenkamp, F.S.; Derksen, N.I.L.; Koers, J.; Cargill, T.; Barnes, E.; de Neef, L.A.; Koeleman, C.A.M.; Aalberse, R.C.; Wuhrer, M.; et al. Unique patterns of glycosylation in immunoglobulin subclass G4-related disease and primary sclerosing cholangitis. J. Gastroenterol. Hepatol. 2019, 34, 1878–1886. [Google Scholar] [CrossRef] [PubMed]
- Coelho, V.; Krysov, S.; Ghaemmaghami, A.M.; Emara, M.; Potter, K.N.; Johnson, P.; Packham, G.; Martinez-Pomares, L.; Stevenson, F.K. Glycosylation of surface Ig creates a functional bridge between human follicular lymphoma and microenvironmental lectins. Proc. Natl. Acad. Sci. USA 2010, 107, 18587–18592. [Google Scholar] [CrossRef]
- Yan, Z.; Lambert, N.C.; Guthrie, K.A.; Porter, A.J.; Loubiere, L.S.; Madeleine, M.M.; Stevens, A.M.; Hermes, H.M.; Nelson, J.L. Male microchimerism in women without sons: Quantitative assessment and correlation with pregnancy history. Am. J. Med. 2005, 118, 899–906. [Google Scholar] [CrossRef]
- Tan, X.W.; Liao, H.; Sun, L.; Okabe, M.; Xiao, Z.C.; Dawe, G.S. Fetal microchimerism in the maternal mouse brain: A novel population of fetal progenitor or stem cells able to cross the blood-brain barrier? Stem Cells 2005, 23, 1443–1452. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, H. Role of Decidual Natural Killer Cells in Human Pregnancy and Related Pregnancy Complications. Front. Immunol. 2021, 12, 728291. [Google Scholar] [CrossRef]
- Nagaeva, O.; Jonsson, L.; Mincheva-Nilsson, L. Dominant IL-10 and TGF-beta mRNA expression in gammadeltaT cells of human early pregnancy decidua suggests immunoregulatory potential. Am. J. Reprod. Immunol. 2002, 48, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Aluvihare, V.R.; Kallikourdis, M.; Betz, A.G. Regulatory T cells mediate maternal tolerance to the fetus. Nat. Immunol. 2004, 5, 266–271. [Google Scholar] [CrossRef]
- Faust, Z.; Laskarin, G.; Rukavina, D.; Szekeres-Bartho, J. Progesterone-induced blocking factor inhibits degranulation of natural killer cells. Am. J. Reprod. Immunol. 1999, 42, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Leber, A.; Zenclussen, M.L.; Teles, A.; Brachwitz, N.; Casalis, P.; El-Mousleh, T.; Jensen, F.; Woidacki, K.; Zenclussen, A.C. Pregnancy: Tolerance and suppression of immune responses. Methods Mol. Biol. 2011, 677, 397–417. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P.; Haque, N. Reproductive immunomodulatory functions of B cells in pregnancy. Int. Rev. Immunol. 2020, 39, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Zenclussen, A.C.; Gentile, T.; Kortebani, G.; Mazzolli, A.; Margni, R. Asymmetric antibodies and pregnancy. Am. J. Reprod. Immunol. 2001, 45, 289–294. [Google Scholar] [CrossRef]
- Malan Borel, I.; Gentile, T.; Angelucci, J.; Pividori, J.; Guala, M.C.; Binaghi, R.A.; Margni, R.A. IgG asymmetric molecules with antipaternal activity isolated from sera and placenta of pregnant human. J. Reprod. Immunol. 1991, 20, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Margni, R.A.; Perdigon, G.; Abatangelo, C.; Gentile, T.; Binaghi, R.A. Immunobiological behaviour of rabbit precipitating and non-precipitating (co-precipitating) antibodies. Immunology 1980, 41, 681–686. [Google Scholar]
- Volkov, M.; Van Schie, K.; Bondt, A.; Kissel, T.; Brinkhaus, M.; Bentlage, A.; Koeleman, C.; De Taeye, S.; Dolhain, R.; Wuhrer, M.; et al. AB0020 ACPA ILLUSTRATING THE IMPACT OF IGG FAB-GLYCOSYLATION ON TRANSPLACENTAL TRANSFER OF ANTIBODIES AND THEIR BINDING TO THE NEONATAL FC-RECEPTOR (FCRN). Ann. Rheum. Dis. 2021, 80, 1044. [Google Scholar] [CrossRef]
- Canellada, A.; Blois, S.; Gentile, T.; Margni Idehu, R.A. In vitro modulation of protective antibody responses by estrogen, progesterone and interleukin-6. Am. J. Reprod. Immunol. 2002, 48, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Canellada, A.; Farber, A.; Zenclussen, A.C.; Gentile, T.; Dokmetjian, J.; Keil, A.; Blois, S.; Miranda, S.; Berod, L.; Gutierrez, G.; et al. Interleukin regulation of asymmetric antibody synthesized by isolated placental B cells. Am. J. Reprod. Immunol. 2002, 48, 275–282. [Google Scholar] [CrossRef]
- Barrientos, G.; Fuchs, D.; Schrocksnadel, K.; Ruecke, M.; Garcia, M.G.; Klapp, B.F.; Raghupathy, R.; Miranda, S.; Arck, P.C.; Blois, S.M. Low levels of serum asymmetric antibodies as a marker of threatened pregnancy. J. Reprod. Immunol. 2009, 79, 201–210. [Google Scholar] [CrossRef]
- Apicella, C.; Custidiano, A.; Miranda, S.; Novoa, L.; Dokmetjian, J.; Gentile, T. Differential macrophage modulation of asymmetric IgG antibody synthesis by soluble or particulate stimuli. Immunol. Lett. 2006, 103, 177–185. [Google Scholar] [CrossRef]
- Gu, J.; Lei, Y.; Huang, Y.; Zhao, Y.; Li, J.; Huang, T.; Zhang, J.; Wang, J.; Deng, X.; Chen, Z.; et al. Fab fragment glycosylated IgG may play a central role in placental immune evasion. Hum. Reprod. 2015, 30, 380–391. [Google Scholar] [CrossRef]
- Li, J.; Korteweg, C.; Qiu, Y.; Luo, J.; Chen, Z.; Huang, G.; Li, W.; Gu, J. Two ultrastructural distribution patterns of immunoglobulin G in human placenta and functional implications. Biol. Reprod. 2014, 91, 128. [Google Scholar] [CrossRef]
- Gentile, T.; Borel, I.M.; Angelucci, J.; Miranda, S.; Margni, R.A. Preferential synthesis of asymmetric antibodies in rats immunized with paternal particulate antigens. Effect on pregnancy. J. Reprod. Immunol. 1992, 22, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer immunoediting: From immunosurveillance to tumor escape. Nat. Immunol. 2002, 3, 991–998. [Google Scholar] [CrossRef]
- Gubin, M.M.; Vesely, M.D. Cancer Immunoediting in the Era of Immuno-oncology. Clin. Cancer Res. 2022, 28, 3917–3928. [Google Scholar] [CrossRef]
- Zhu, D.; Hawkins, R.E.; Hamblin, T.J.; Stevenson, F.K. Clonal history of a human follicular lymphoma as revealed in the immunoglobulin variable region genes. Br. J. Haematol. 1994, 86, 505–512. [Google Scholar] [CrossRef]
- Liu, H.M.; Ma, L.L.; Cao, B.; Lin, J.Z.; Han, L.; Li, C.Y.; Xu, R.C.; Zhang, D.K. Progress in research into the role of abnormal glycosylation modification in tumor immunity. Immunol. Lett. 2021, 229, 8–17. [Google Scholar] [CrossRef]
- Breen, L.D.; Pucic-Bakovic, M.; Vuckovic, F.; Reiding, K.; Trbojevic-Akmacic, I.; Srajer Gajdosik, M.; Cook, M.I.; Lopez, M.J.; Wuhrer, M.; Camara, L.M.; et al. IgG and IgM glycosylation patterns in patients undergoing image-guided tumor ablation. Biochim. Biophys. Acta 2016, 1860, 1786–1794. [Google Scholar] [CrossRef]
- Amin, R.; Mourcin, F.; Uhel, F.; Pangault, C.; Ruminy, P.; Dupre, L.; Guirriec, M.; Marchand, T.; Fest, T.; Lamy, T.; et al. DC-SIGN-expressing macrophages trigger activation of mannosylated IgM B-cell receptor in follicular lymphoma. Blood 2015, 126, 1911–1920. [Google Scholar] [CrossRef]
- Klaamas, K.; Kodar, K.; Kurtenkov, O. An increased level of the Concanavalin A-positive IgG in the serum of patients with gastric cancer as evaluated by a lectin enzyme-linked immunosorbent assay (LELISA). Neoplasma 2008, 55, 143–150. [Google Scholar] [PubMed]
- Li, S.; Meng, J.; Xu, F.; Wang, Q.; Tian, X.; Li, M.; Zeng, X.; Hu, C.; Zheng, Y. IgG Glycosylation Profiling of Peripheral Artery Diseases with Lectin Microarray. J. Clin. Med. 2022, 11, 5727. [Google Scholar] [CrossRef] [PubMed]
- Stadlmann, J.; Weber, A.; Pabst, M.; Anderle, H.; Kunert, R.; Ehrlich, H.J.; Peter Schwarz, H.; Altmann, F. A close look at human IgG sialylation and subclass distribution after lectin fractionation. Proteomics 2009, 9, 4143–4153. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Chen, X.; Gu, H.; Zhao, C.; Liu, X.; Yan, M.; Deng, X.; Zhang, Z.; Gu, J. Fractionation of Fab glycosylated immunoglobulin G with concanavalin A chromatography unveils new structural properties of the molecule. Oncotarget 2016, 7, 31166–31176. [Google Scholar] [CrossRef]
- Xu, Q.; Deng, X.; Zhang, B.; Zhao, C.; Huang, T.; Zhang, Y.; Chen, Z.; Gu, J. A study of the possible role of Fab-glycosylated IgG in tumor immunity. Cancer Immunol. Immunother. CII 2021, 70, 1841–1851. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhang, S.; Tang, J.; Tian, T.; Pan, Y.; Wu, L.; Zhang, J.; Liu, Y.; Huang, J.; Dai, H.; et al. A Self-Propagating c-Met-SOX2 Axis Drives Cancer-Derived IgG Signaling That Promotes Lung Cancer Cell Stemness. Cancer Res. 2023, 83, 1866–1882. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Kiyoshi, M.; Anraku, M.; Hashii, N.; Oda-Ueda, N.; Ueda, T.; Ohkuri, T. Glycosylation decreases aggregation and immunogenicity of adalimumab Fab secreted from Pichia pastoris. J. Biochem. 2021, 169, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Oda-Ueda, N.; Ueda, T.; Ohkuri, T. Introduction of a glycosylation site in the constant region decreases the aggregation of adalimumab Fab. Biochem. Biophys. Res. Commun. 2018, 503, 752–756. [Google Scholar] [CrossRef]
- Fussl, F.; Trappe, A.; Carillo, S.; Jakes, C.; Bones, J. Comparative Elucidation of Cetuximab Heterogeneity on the Intact Protein Level by Cation Exchange Chromatography and Capillary Electrophoresis Coupled to Mass Spectrometry. Anal. Chem. 2020, 92, 5431–5438. [Google Scholar] [CrossRef]
- Qian, J.; Liu, T.; Yang, L.; Daus, A.; Crowley, R.; Zhou, Q. Structural characterization of N-linked oligosaccharides on monoclonal antibody cetuximab by the combination of orthogonal matrix-assisted laser desorption/ionization hybrid quadrupole-quadrupole time-of-flight tandem mass spectrometry and sequential enzymatic digestion. Anal. Biochem. 2007, 364, 8–18. [Google Scholar] [CrossRef]
- Janin-Bussat, M.C.; Tonini, L.; Huillet, C.; Colas, O.; Klinguer-Hamour, C.; Corvaia, N.; Beck, A. Cetuximab Fab and Fc N-glycan fast characterization using IdeS digestion and liquid chromatography coupled to electrospray ionization mass spectrometry. Methods Mol. Biol. 2013, 988, 93–113. [Google Scholar] [CrossRef]
- Giddens, J.P.; Lomino, J.V.; DiLillo, D.J.; Ravetch, J.V.; Wang, L.X. Site-selective chemoenzymatic glycoengineering of Fab and Fc glycans of a therapeutic antibody. Proc. Natl. Acad. Sci. USA 2018, 115, 12023–12027. [Google Scholar] [CrossRef]
- French, M. Serum IgG subclasses in normal adults. Monogr. Allergy 1986, 19, 100–107. [Google Scholar]
- Papadea, C.; Check, I.J. Human immunoglobulin G and immunoglobulin G subclasses: Biochemical, genetic, and clinical aspects. Crit. Rev. Clin. Lab. Sci. 1989, 27, 27–58. [Google Scholar] [CrossRef] [PubMed]
- Crescioli, S.; Correa, I.; Karagiannis, P.; Davies, A.M.; Sutton, B.J.; Nestle, F.O.; Karagiannis, S.N. IgG4 Characteristics and Functions in Cancer Immunity. Curr. Allergy Asthma Rep. 2016, 16, 7. [Google Scholar] [CrossRef] [PubMed]
- Bloom, J.W.; Madanat, M.S.; Marriott, D.; Wong, T.; Chan, S.Y. Intrachain disulfide bond in the core hinge region of human IgG4. Protein Sci. 1997, 6, 407–415. [Google Scholar] [CrossRef]
- van der Neut Kolfschoten, M.; Schuurman, J.; Losen, M.; Bleeker, W.K.; Martinez-Martinez, P.; Vermeulen, E.; den Bleker, T.H.; Wiegman, L.; Vink, T.; Aarden, L.A.; et al. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science 2007, 317, 1554–1557. [Google Scholar] [CrossRef]
- Davies, A.M.; Rispens, T.; den Bleker, T.H.; McDonnell, J.M.; Gould, H.J.; Aalberse, R.C.; Sutton, B.J. Crystal structure of the human IgG4 C(H)3 dimer reveals the role of Arg409 in the mechanism of Fab-arm exchange. Mol. Immunol. 2013, 54, 1–7. [Google Scholar] [CrossRef]
- Rispens, T.; Davies, A.M.; Ooijevaar-de Heer, P.; Absalah, S.; Bende, O.; Sutton, B.J.; Vidarsson, G.; Aalberse, R.C. Dynamics of inter-heavy chain interactions in human immunoglobulin G (IgG) subclasses studied by kinetic Fab arm exchange. J. Biol. Chem. 2014, 289, 6098–6109. [Google Scholar] [CrossRef]
- Sakthiswary, R.; Shaharir, S.S.; Wahab, A.A. Frequency and Clinical Significance of Elevated IgG4 in Rheumatoid Arthritis: A Systematic Review. Biomedicines 2022, 10, 558. [Google Scholar] [CrossRef] [PubMed]
- Koike, T. IgG4-related disease: Why high IgG4 and fibrosis? Arthritis Res. Ther. 2013, 15, 103. [Google Scholar] [CrossRef]
- Funakoshi, T.; Lunardon, L.; Ellebrecht, C.T.; Nagler, A.R.; O’Leary, C.E.; Payne, A.S. Enrichment of total serum IgG4 in patients with pemphigus. Br. J. Dermatol. 2012, 167, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Miyatani, K.; Saito, H.; Murakami, Y.; Watanabe, J.; Kuroda, H.; Matsunaga, T.; Fukumoto, Y.; Osaki, T.; Nakayama, Y.; Umekita, Y.; et al. A high number of IgG4-positive cells in gastric cancer tissue is associated with tumor progression and poor prognosis. Virchows Arch. 2016, 468, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Karagiannis, P.; Gilbert, A.E.; Josephs, D.H.; Ali, N.; Dodev, T.; Saul, L.; Correa, I.; Roberts, L.; Beddowes, E.; Koers, A.; et al. IgG4 subclass antibodies impair antitumor immunity in melanoma. J. Clin. Investig. 2013, 123, 1457–1474. [Google Scholar] [CrossRef] [PubMed]
- Jordakieva, G.; Bianchini, R.; Reichhold, D.; Piehslinger, J.; Groschopf, A.; Jensen, S.A.; Mearini, E.; Nocentini, G.; Crevenna, R.; Zlabinger, G.J.; et al. IgG4 induces tolerogenic M2-like macrophages and correlates with disease progression in colon cancer. Oncoimmunology 2021, 10, 1880687. [Google Scholar] [CrossRef]
- Harada, K.; Shimoda, S.; Kimura, Y.; Sato, Y.; Ikeda, H.; Igarashi, S.; Ren, X.S.; Sato, H.; Nakanuma, Y. Significance of immunoglobulin G4 (IgG4)-positive cells in extrahepatic cholangiocarcinoma: Molecular mechanism of IgG4 reaction in cancer tissue. Hepatology 2012, 56, 157–164. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Q.; Zhao, C.; Zhu, Z.; Zhu, X.; Zhou, J.; Zhang, S.; Yang, T.; Zhang, B.; Li, J.; et al. An immune evasion mechanism with IgG4 playing an essential role in cancer and implication for immunotherapy. J. Immunother. Cancer 2020, 8, e000661. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.J.; Wang, Y.; Collins, A.M. Human immunoglobulin classes and subclasses show variability in VDJ gene mutation levels. Immunol. Cell Biol. 2014, 92, 729–733. [Google Scholar] [CrossRef]
- Hinz, S.; Pagerols-Raluy, L.; Oberg, H.H.; Ammerpohl, O.; Grussel, S.; Sipos, B.; Grutzmann, R.; Pilarsky, C.; Ungefroren, H.; Saeger, H.D.; et al. Foxp3 expression in pancreatic carcinoma cells as a novel mechanism of immune evasion in cancer. Cancer Res. 2007, 67, 8344–8350. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, R.; Roth-Walter, F.; Ohradanova-Repic, A.; Flicker, S.; Hufnagl, K.; Fischer, M.B.; Stockinger, H.; Jensen-Jarolim, E. IgG4 drives M2a macrophages to a regulatory M2b-like phenotype: Potential implication in immune tolerance. Allergy 2019, 74, 483–494. [Google Scholar] [CrossRef]
- Bruhns, P.; Iannascoli, B.; England, P.; Mancardi, D.A.; Fernandez, N.; Jorieux, S.; Daeron, M. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood 2009, 113, 3716–3725. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Tan, S.; Jin, W.; Guan, J.; Wang, Q.; Sun, H.; Qi, J.; Yan, J.; Chai, Y.; Wang, Z.; et al. N-glycosylation of PD-1 promotes binding of camrelizumab. EMBO Rep. 2020, 21, e51444. [Google Scholar] [CrossRef]
- Wang, C.; Thudium, K.B.; Han, M.; Wang, X.T.; Huang, H.; Feingersh, D.; Garcia, C.; Wu, Y.; Kuhne, M.; Srinivasan, M.; et al. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol. Res. 2014, 2, 846–856. [Google Scholar] [CrossRef]
- Harrington, K.J.; Ferris, R.L.; Blumenschein, G., Jr.; Colevas, A.D.; Fayette, J.; Licitra, L.; Kasper, S.; Even, C.; Vokes, E.E.; Worden, F.; et al. Nivolumab versus standard, single-agent therapy of investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck (CheckMate 141): Health-related quality-of-life results from a randomised, phase 3 trial. Lancet Oncol. 2017, 18, 1104–1115. [Google Scholar] [CrossRef]
- Zhu, A.X.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmer, D.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol. 2018, 19, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Shaverdian, N.; Lisberg, A.E.; Bornazyan, K.; Veruttipong, D.; Goldman, J.W.; Formenti, S.C.; Garon, E.B.; Lee, P. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: A secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017, 18, 895–903. [Google Scholar] [CrossRef]
- Zhang, W.; Quan, Y.; Ma, X.; Zeng, L.; Li, J.; Chen, S.; Su, M.; Hong, L.; Li, P.; Wang, H.; et al. Synergistic effect of glutathione and IgG4 in immune evasion and the implication for cancer immunotherapy. Redox Biol. 2023, 60, 102608. [Google Scholar] [CrossRef]
- Koers, J.; Derksen, N.I.L.; Ooijevaar-de Heer, P.; Nota, B.; van de Bovenkamp, F.S.; Vidarsson, G.; Rispens, T. Biased N-Glycosylation Site Distribution and Acquisition across the Antibody V Region during B Cell Maturation. J. Immunol. 2019, 202, 2220–2228. [Google Scholar] [CrossRef]
- Koning, M.T.; Trollmann, I.J.M.; van Bergen, C.A.M.; Alvarez Saravia, D.; Navarrete, M.A.; Kielbasa, S.M.; Veelken, H. Peripheral IgE Repertoires of Healthy Donors Carry Moderate Mutation Loads and Do Not Overlap With Other Isotypes. Front. Immunol. 2019, 10, 1543. [Google Scholar] [CrossRef]
- Levin, M.; Levander, F.; Palmason, R.; Greiff, L.; Ohlin, M. Antibody-encoding repertoires of bone marrow and peripheral blood-a focus on IgE. J. Allergy Clin. Immunol. 2017, 139, 1026–1030. [Google Scholar] [CrossRef]
- van de Bovenkamp, F.S.; Hafkenscheid, L.; Rispens, T.; Rombouts, Y. The Emerging Importance of IgG Fab Glycosylation in Immunity. J. Immunol. 2016, 196, 1435–1441. [Google Scholar] [CrossRef]
- Rispens, T.; Meesters, J.; den Bleker, T.H.; Ooijevaar-De Heer, P.; Schuurman, J.; Parren, P.W.; Labrijn, A.; Aalberse, R.C. Fc-Fc interactions of human IgG4 require dissociation of heavy chains and are formed predominantly by the intra-chain hinge isomer. Mol. Immunol. 2013, 53, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Hammers, H.; Lipson, E.J. Nivolumab: Targeting PD-1 to bolster antitumor immunity. Future Oncol. 2015, 11, 1307–1326. [Google Scholar] [CrossRef] [PubMed]
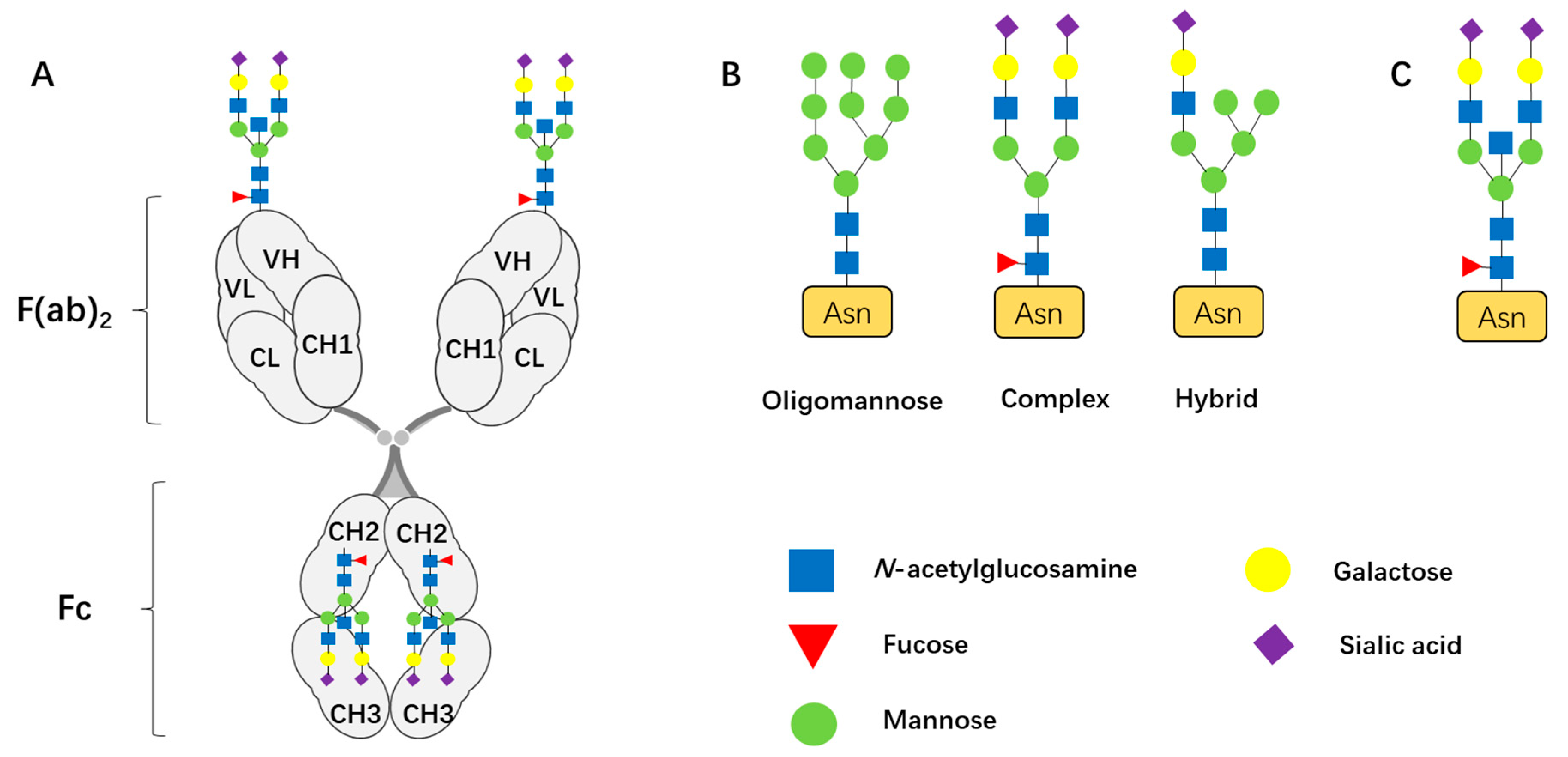

| Monosaccharide | Primary Role in N-Glycan Structure | Applications & Significance |
|---|---|---|
| N-Acetylglucosamine (GlcNAc) | Forms the core pentasaccharide (Man3GlcNAc2) of N-glycan. | Critical for the stability, solubility, and half-life [3] |
| Mannose (Man) | Forms the core pentasaccharide (Man3GlcNAc2) of N-glycan. | |
| Galactose (Gal) |
|
|
| Fucose (Fuc) | Core fucosylation | Core fucosylation on IgG drastically reduces their ADCC activity [5] |
| Sialic acid (Sia) | Terminal modification | Highly sialylated IgG enhances anti-inflammatory activity by modulating macrophage and dendritic cell functions [14] |
| Feature | Fab-Glycosylated IgG | IgG4 |
|---|---|---|
| Glycosylation Site | Fab and Fc (Asn-297) | Fab (pathological condition) and Fc (Asn-297) |
| Characteristics |
|
|
| Functional Impact | Modulates antigen binding; enhances BCR signaling; interacts with lectins to transduce signals and modulates the immune microenvironment [106]. | |
| Association with Autoimmune Diseases | Strongly correlated with specific autoantibodies, such as ACPA in RA [33]. | IgG4-related disease is characterized by massive infiltration of IgG4-positive plasma cells and fibrosis [86]. |
| Association with Cancer |
|
|
| Summary | Fab glycosylation is an acquired modification. It participates in disease development and serves as a potential biomarker for diseases. | IgG4 is a natural subclass of IgG, characterized by structural features that determine its inherent functional properties (weak effector functions and anti-inflammatory activity). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Yu, F.; Huang, B.; Liang, G.; Xu, J.; Lin, Y.; Xu, Q. Fab N-Glycosylation in IgG: Implications in Physiological and Pathological Immune Regulation. Biomolecules 2025, 15, 1508. https://doi.org/10.3390/biom15111508
Chen S, Yu F, Huang B, Liang G, Xu J, Lin Y, Xu Q. Fab N-Glycosylation in IgG: Implications in Physiological and Pathological Immune Regulation. Biomolecules. 2025; 15(11):1508. https://doi.org/10.3390/biom15111508
Chicago/Turabian StyleChen, Shuqi, Feiyuan Yu, Binliang Huang, Ganbo Liang, Jieyi Xu, Yuning Lin, and Qian Xu. 2025. "Fab N-Glycosylation in IgG: Implications in Physiological and Pathological Immune Regulation" Biomolecules 15, no. 11: 1508. https://doi.org/10.3390/biom15111508
APA StyleChen, S., Yu, F., Huang, B., Liang, G., Xu, J., Lin, Y., & Xu, Q. (2025). Fab N-Glycosylation in IgG: Implications in Physiological and Pathological Immune Regulation. Biomolecules, 15(11), 1508. https://doi.org/10.3390/biom15111508






