Regulation of Klotho Production by Mineralocorticoid Receptor Signaling in Renal Cell Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Quantitative Real-Time Polymerase Chain Reaction
2.3. Qualitative Expression Analysis
2.4. Western Blotting
2.5. Statistics
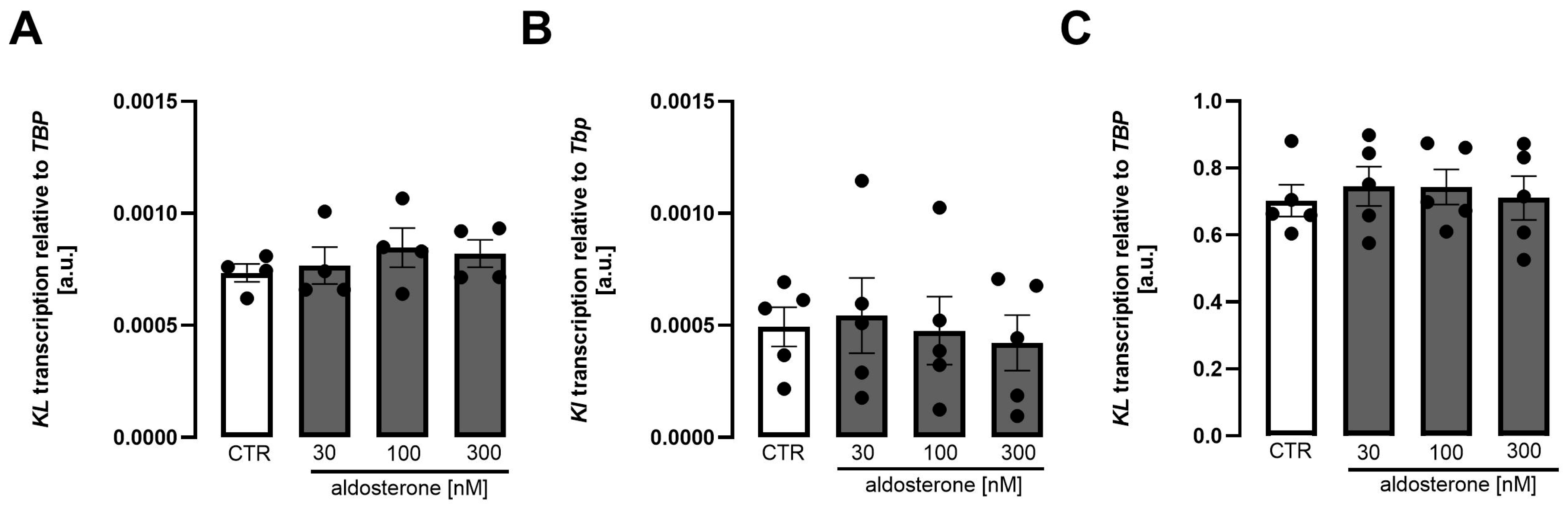
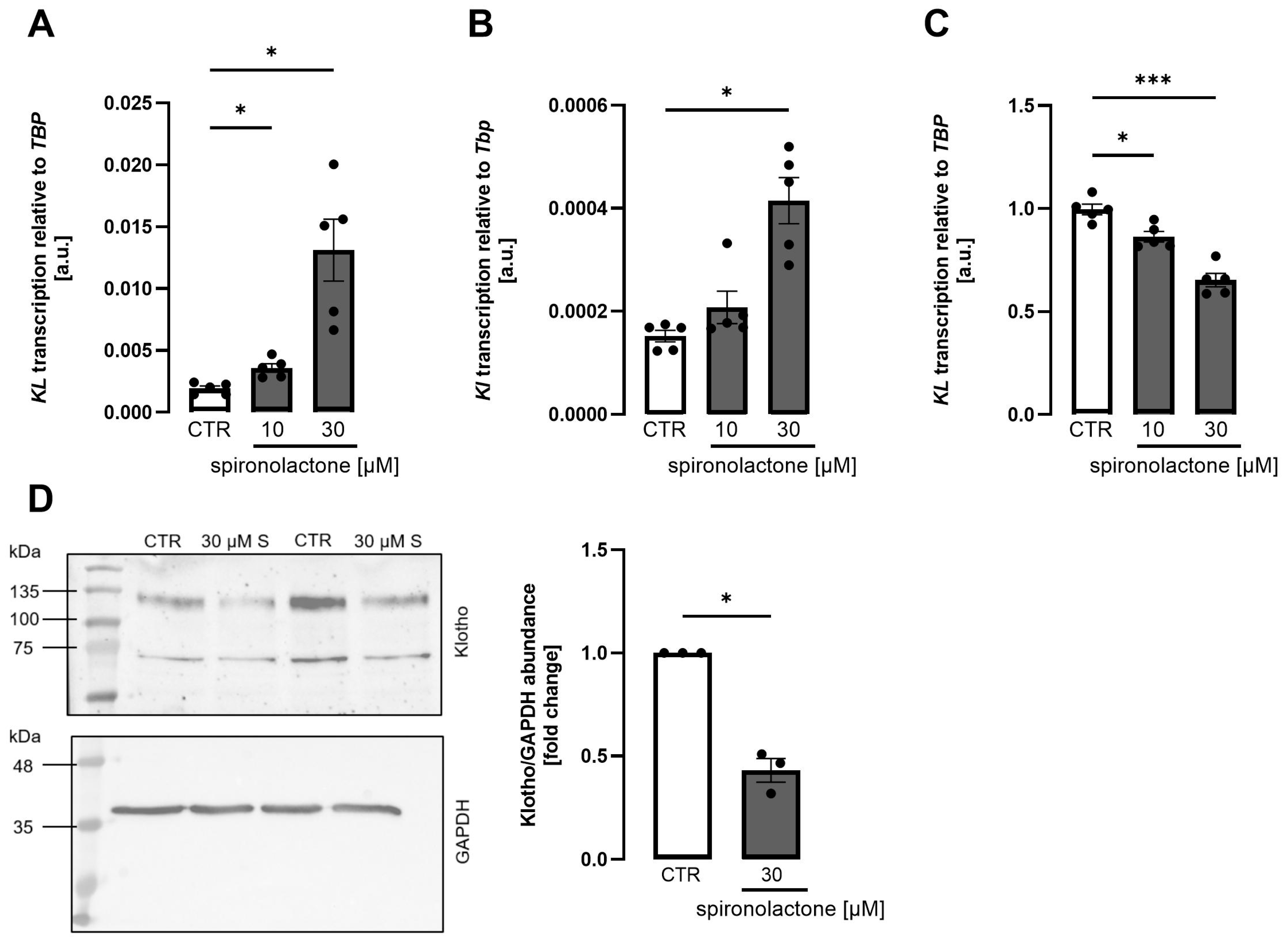
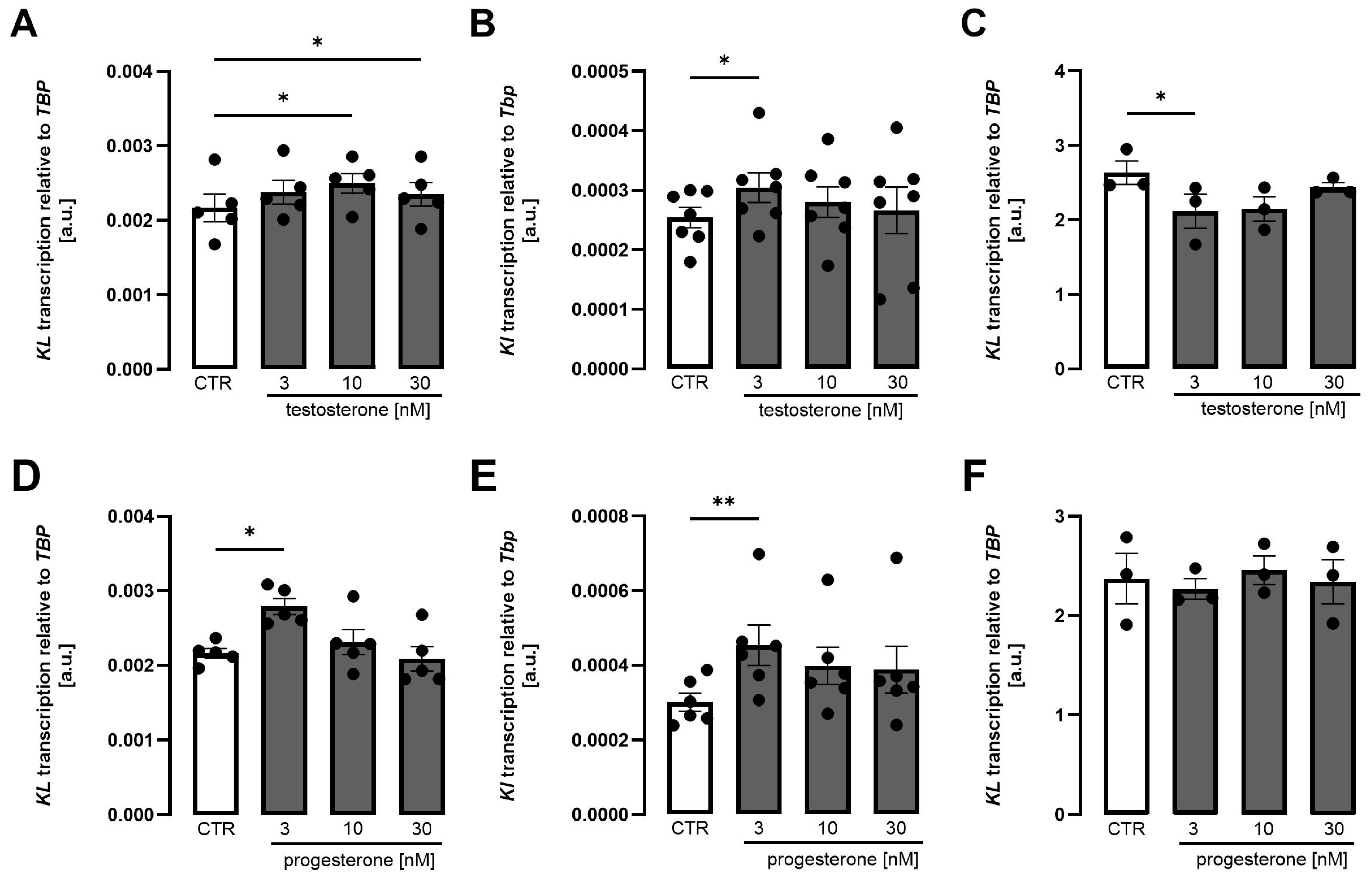
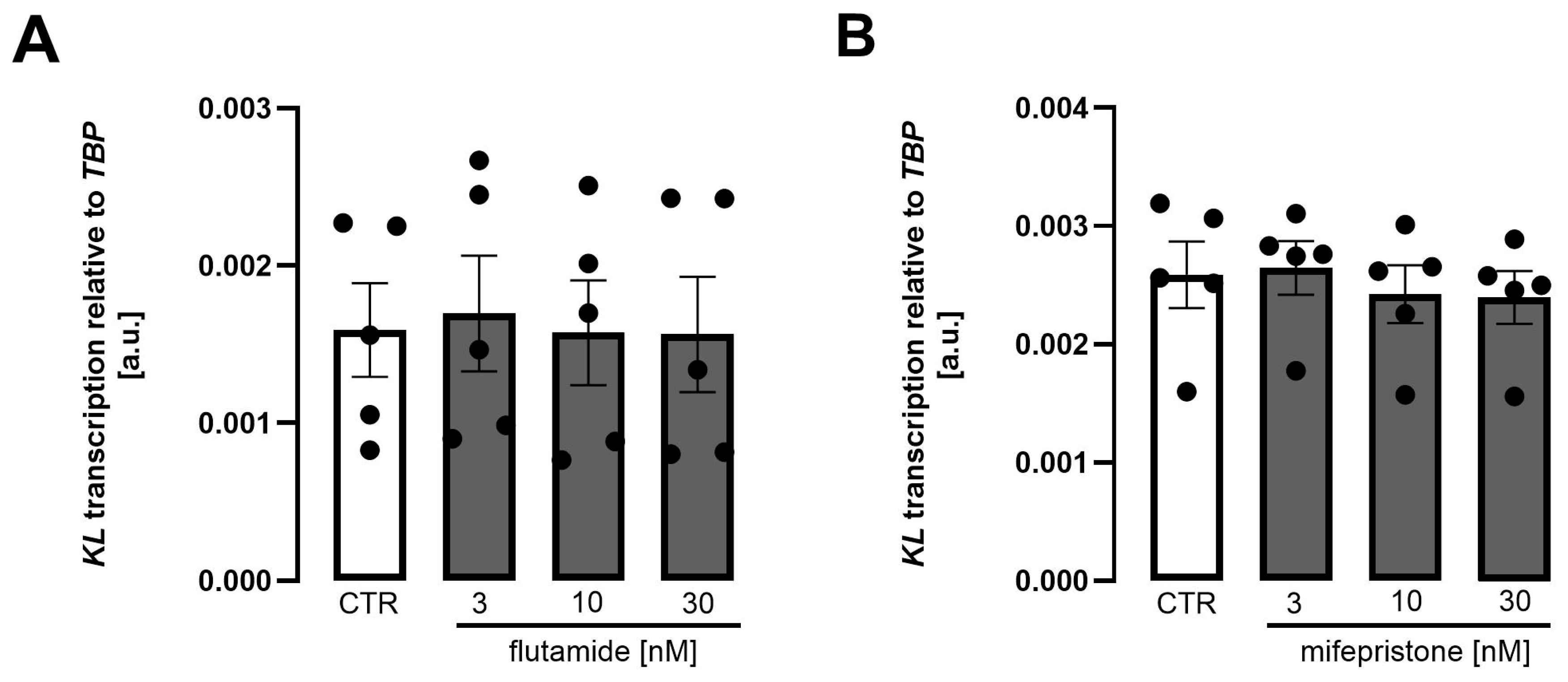
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CKD | Chronic kidney disease |
| DMEM/F-12 | Dulbecco’s Modified Eagle Medium: Nutrient Mixture F-12 |
| FBS | Fetal bovine serum |
| FGF23 | Fibroblast growth factor 23 |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| HK2 | Human kidney 2 |
| MDCK | Madin-Darby canine kidney |
| NBCS | Newborn calf serum |
| NRK-52E | Normal rat kidney, subtype 52E |
| RPTEC | Renal proximal tubule epithelial cell |
| TBP | TATA box-binding protein |
References
- Bollag, W.B. Regulation of aldosterone synthesis and secretion. Compr. Physiol. 2014, 4, 1017–1055. [Google Scholar] [CrossRef] [PubMed]
- Yanagibashi, K.; Haniu, M.; Shively, J.E.; Shen, W.H.; Hall, P. The synthesis of aldosterone by the adrenal cortex. Two zones (fasciculata and glomerulosa) possess one enzyme for 11 beta-, 18-hydroxylation, and aldehyde synthesis. J. Biol. Chem. 1986, 261, 3556–3562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, L.; Gaballa, M.M.S.; Hasan, A.A.; Xiong, Y.; Xie, L.; Klein, T.; Delic, D.; Kleuser, B.; Krämer, B.K.; et al. Impact of Salt Intake and Renin-Angiotensin-Aldosterone System Blockade on Lung Severe Acute Respiratory Syndrome Coronavirus 2 Host Factors. Kidney Blood Press. Res. 2022, 47, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Salyer, S.A.; Parks, J.; Barati, M.T.; Lederer, E.D.; Clark, B.J.; Klein, J.D.; Khundmiri , S.J. Aldosterone regulates Na (+), K (+) ATPase activity in human renal proximal tubule cells through mineralocorticoid receptor. Biochim. Biophys. Acta 2013, 1833, 2143–2152. [Google Scholar] [CrossRef]
- Terker, A.S.; Ellison, D.H. Renal mineralocorticoid receptor and electrolyte homeostasis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R1068–R1070. [Google Scholar] [CrossRef]
- Nguyen Dinh Cat, A.; Griol-Charhbili, V.; Loufrani, L.; Labat, C.; Benjamin, L.; Farman, N.; Lacolley, P.; Henrion, D.; Jaisser, F. The endothelial mineralocorticoid receptor regulates vasoconstrictor tone and blood pressure. FASEB J. 2010, 24, 2454–2463. [Google Scholar] [CrossRef]
- Lang, F. On the pleotropic actions of mineralocorticoids. Nephron Physiol. 2014, 128, 1–7. [Google Scholar] [CrossRef]
- Sun, K.; Wang, Y.-L.; Hou, C.-C.; Shang, D.; Du, L.-J.; Bai, L.; Zhang, X.-Y.; Hao, C.-M.; Duan, S.-Z. Collecting duct NCOR1 controls blood pressure by regulating mineralocorticoid receptor. J. Adv. Res. 2025, 68, 75–87. [Google Scholar] [CrossRef]
- Muto, S. Action of aldosterone on renal collecting tubule cells. Curr. Opin. Nephrol. Hypertens. 1995, 4, 31–40. [Google Scholar] [CrossRef]
- Hirasawa, G.; Sasano, H.; Takahashi, K.; Fukushima, K.; Suzuki, T.; Hiwatashi, N.; Toyota, T.; Krozowski, Z.; Nagura, H. Colocalization of 11 beta-hydroxysteroid dehydrogenase type II and mineralocorticoid receptor in human epithelia. J. Clin. Endocrinol. Metab. 1997, 82, 3859–3863. [Google Scholar] [CrossRef]
- Sasano, H.; Fukushima, K.; Sasaki, I.; Matsuno, S.; Nagura, H.; Krozowski , Z.S. Immunolocalization of mineralocorticoid receptor in human kidney, pancreas, salivary, mammary and sweat glands: A light and electron microscopic immunohistochemical study. J. Endocrinol. 1992, 132, 305–310. [Google Scholar] [CrossRef]
- Harvey, B.J.; Alzamora, R.; Stubbs, A.K.; Irnaten, M.; McEneaney, V.; Thomas , W. Rapid responses to aldosterone in the kidney and colon. J. Steroid Biochem. Mol. Biol. 2008, 108, 310–317. [Google Scholar] [CrossRef]
- Frindt, G.; Palmer, L.G. Regulation of epithelial Na+ channels by adrenal steroids: Mineralocorticoid and glucocorticoid effects. Am. J. Physiol. Ren. Physiol. 2012, 302, F20–F26. [Google Scholar] [CrossRef] [PubMed]
- Masilamani, S.; Kim, G.H.; Mitchell, C.; Wade, J.B.; Knepper , M.A. Aldosterone-mediated regulation of ENaC α, β, and γ subunit proteins in rat kidney. J. Clin. Investig. 1999, 104, R19–R23. [Google Scholar] [CrossRef] [PubMed]
- Kolkhof, P.; Bärfacker, L. 30 YEARS OF THE MINERALOCORTICOID RECEPTOR: Mineralocorticoid receptor antagonists: 60 years of research and development. J. Endocrinol. 2017, 234, T125–T140. [Google Scholar] [CrossRef] [PubMed]
- Cuthbert, J.J.; Clark, A.L. Diuretic Treatment in Patients with Heart Failure: Current Evidence and Future Directions—Part I: Loop Diuretics. Curr. Heart Fail. Rep. 2024, 21, 101–114. [Google Scholar] [CrossRef]
- Huffman, D.H.; Kampmann, J.P.; Hignite, C.E.; Azarnoff, D.L. Gynecomastia induced in normal males by spironolactone. Clin. Pharmacol. Ther. 1978, 24, 465–473. [Google Scholar] [CrossRef]
- Cumming, D.C. Use of spironolactone in treatment of hirsutism. Clevel. Clin. J. Med. 1990, 57, 285–287. [Google Scholar] [CrossRef]
- Pitt, B.; Zannad, F.; Remme, W.J.; Cody, R.; Castaigne, A.; Perez, A.; Palensky, J.; Wittes, J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N. Engl. J. Med. 1999, 341, 709–717. [Google Scholar] [CrossRef]
- Brilla, C.G.; Matsubara, L.S.; Weber, K.T. Antifibrotic effects of spironolactone in preventing myocardial fibrosis in systemic arterial hypertension. Am. J. Cardiol. 1993, 71, 12A–16A. [Google Scholar] [CrossRef]
- Bendtzen, K.; Hansen, P.R.; Rieneck, K. Spironolactone inhibits production of proinflammatory cytokines, including tumour necrosis factor-alpha and interferon-gamma, and has potential in the treatment of arthritis. Clin. Exp. Immunol. 2003, 134, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Ceron, C.S.; Castro, M.M.; Rizzi, E.; Montenegro, M.F.; Fontana, M.C.O.; Gerlach, R.F.; Tanus-Santos, J.E. Spironolactone and hydrochlorothiazide exert antioxidant effects and reduce vascular matrix metalloproteinase-2 activity and expression in a model of renovascular hypertension. Br. J. Pharmacol. 2010, 160, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Georgianos, P.I.; Agarwal, R. Mineralocorticoid Receptor Antagonism in Chronic Kidney Disease. Kidney Int. Rep. 2021, 6, 2281–2291. [Google Scholar] [CrossRef] [PubMed]
- Secora, A.M.; Shin, J.-I.; Qiao, Y.; Alexander, G.C.; Chang, A.R.; Inker, L.A.; Coresh, J.; Grams, M.E. Hyperkalemia and Acute Kidney Injury with Spironolactone Use Among Patients with Heart Failure. Mayo Clin. Proc. 2020, 95, 2408–2419. [Google Scholar] [CrossRef]
- Yang, C.-T.; Kor, C.-T.; Hsieh, Y.-P. Long-Term Effects of Spironolactone on Kidney Function and Hyperkalemia-Associated Hospitalization in Patients with Chronic Kidney Disease. J. Clin. Med. 2018, 7, 459. [Google Scholar] [CrossRef]
- Delbeck, M.; Joseph, A.; Pitt, B.; Kolkhof, P. Antifibrotic and anti-inflammatory effects of the selective nonsteroidal MR antagonist finerenone in preclinical pulmonary fibrosis. Eur. Heart J. 2021, 42, ehab724.2932. [Google Scholar] [CrossRef]
- Kintscher, U.; Edelmann, F. The non-steroidal mineralocorticoid receptor antagonist finerenone and heart failure with preserved ejection fraction. Cardiovasc. Diabetol. 2023, 22, 162. [Google Scholar] [CrossRef]
- Agarwal, R.; Joseph, A.; Anker, S.D.; Filippatos, G.; Rossing, P.; Ruilope, L.M.; Pitt, B.; Kolkhof, P.; Scott, C.; Lawatscheck, R.; et al. Hyperkalemia Risk with Finerenone: Results from the FIDELIO-DKD Trial. J. Am. Soc. Nephrol. 2022, 33, 225–237. [Google Scholar] [CrossRef]
- Arici, M.; Altun, B.; Araz, M.; Atmaca, A.; Demir, T.; Ecder, T.; Guz, G.; Yavuz, D.G.; Yildiz, A.; Yilmaz, T. The significance of finerenone as a novel therapeutic option in diabetic kidney disease: A scoping review with emphasis on cardiorenal outcomes of the finerenone phase 3 trials. Front. Med. 2024, 11, 1384454. [Google Scholar] [CrossRef]
- Bellos, I.; Marinaki, S.; Lagiou, P.; Benetou, V. Comparative Efficacy and Safety of Cardio-Renoprotective Pharmacological Interventions in Chronic Kidney Disease: An Umbrella Review of Network Meta-Analyses and a Multicriteria Decision Analysis. Biomolecules 2024, 15, 39. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Vaduganathan, M.; Claggett, B.; Jhund, P.S.; Desai, A.S.; Henderson, A.D.; Lam, C.S.P.; Pitt, B.; Senni, M.; et al. Finerenone in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2024, 391, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Kolkhof, P.; Bakris, G.; Bauersachs, J.; Haller, H.; Wada, T.; Zannad, F. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur. Heart J. 2021, 42, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Grune, J.; Beyhoff, N.; Smeir, E.; Chudek, R.; Blumrich, A.; Ban, Z.; Brix, S.; Betz, I.R.; Schupp, M.; Foryst-Ludwig, A.; et al. Selective Mineralocorticoid Receptor Cofactor Modulation as Molecular Basis for Finerenone’s Antifibrotic Activity. Hypertension 2018, 71, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Urakawa, I.; Yamazaki, Y.; Shimada, T.; Iijima, K.; Hasegawa, H.; Okawa, K.; Fujita, T.; Fukumoto, S.; Yamashita, T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 2006, 444, 770–774. [Google Scholar] [CrossRef]
- Kurosu, H.; Ogawa, Y.; Miyoshi, M.; Yamamoto, M.; Nandi, A.; Rosenblatt, K.P.; Baum, M.G.; Schiavi, S.; Hu, M.-C.; Moe, O.W.; et al. Regulation of fibroblast growth factor-23 signaling by klotho. J. Biol. Chem. 2006, 281, 6120–6123. [Google Scholar] [CrossRef]
- Toro, L.; Rojas, V.; Conejeros, C.; Ayala, P.; Parra-Lucares, A.; Ahumada, F.; Almeida, P.; Silva, M.F.; Bravo, K.; Pumarino, C.; et al. A Combined Biomarker That Includes Plasma Fibroblast Growth Factor 23, Erythropoietin, and Klotho Predicts Short- and Long-Term Morbimortality and Development of Chronic Kidney Disease in Critical Care Patients with Sepsis: A Prospective Cohort. Biomolecules 2023, 13, 1481. [Google Scholar] [CrossRef]
- Bär, L.; Stournaras, C.; Lang, F.; Föller, M. Regulation of fibroblast growth factor 23 (FGF23) in health and disease. FEBS Lett. 2019, 593, 1879–1900. [Google Scholar] [CrossRef]
- Razzaque, M.S. The FGF23-Klotho axis: Endocrine regulation of phosphate homeostasis. Nat. Rev. Endocrinol. 2009, 5, 611–619. [Google Scholar] [CrossRef]
- Miao, J.; Huang, J.; Luo, C.; Ye, H.; Ling, X.; Wu, Q.; Shen, W.; Zhou, L. Klotho retards renal fibrosis through targeting mitochondrial dysfunction and cellular senescence in renal tubular cells. Physiol. Rep. 2021, 9, e14696. [Google Scholar] [CrossRef]
- Hui, H.; Zhai, Y.; Ao, L.; Cleveland Jr, J.C.; Liu, H.; Fullerton, D.A.; Meng, X. Klotho suppresses the inflammatory responses and ameliorates cardiac dysfunction in aging endotoxemic mice. Oncotarget 2017, 8, 15663–15676. [Google Scholar] [CrossRef]
- Kuro-o, M. Klotho as a regulator of oxidative stress and senescence. Biol. Chem. 2008, 389, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Prud’homme, G.J.; Kurt, M.; Wang, Q. Pathobiology of the Klotho Antiaging Protein and Therapeutic Considerations. Front. Aging 2022, 3, 931331. [Google Scholar] [CrossRef]
- Razzaque, M.S.; Sitara, D.; Taguchi, T.; St-Arnaud, R.; Lanske, B. Premature aging-like phenotype in fibroblast growth factor 23 null mice is a vitamin D-mediated process. FASEB J. 2006, 20, 720–722. [Google Scholar] [CrossRef] [PubMed]
- Kurosu, H.; Yamamoto, M.; Clark, J.D.; Pastor, J.V.; Nandi, A.; Gurnani, P.; McGuinness, O.P.; Chikuda, H.; Yamaguchi, M.; Kawaguchi, H.; et al. Suppression of aging in mice by the hormone Klotho. Science 2005, 309, 1829–1833. [Google Scholar] [CrossRef]
- Alesutan, I.; Feger, M.; Pakladok, T.; Mia, S.; Ahmed, M.S.E.; Voelkl, J.; Lang, F. 25-Hydroxyvitamin D3 1-α-hydroxylase-dependent stimulation of renal klotho expression by spironolactone. Kidney Blood Press. Res. 2013, 37, 475–487. [Google Scholar] [CrossRef]
- Tang, C.; Pathare, G.; Michael, D.; Fajol, A.; Eichmüller, M.; Lang, F. Downregulation of Klotho expression by dehydration. Am. J. Physiol. Ren. Physiol. 2011, 301, F745–F750. [Google Scholar] [CrossRef]
- Takenaka, T.; Inoue, T.; Okada, H.; Miyazaki, T.; Suzuki, H. Decreased klotho expression in early aldosterone-induced hypertension. FASEB J. 2010, 24, lb698. [Google Scholar] [CrossRef]
- Jung, H.J.; Pham, T.D.; Su, X.-T.; Grigore, T.V.; Hoenderop, J.G.; Olauson, H.; Wall, S.M.; Ellison, D.H.; Welling , P.A.; Al-Qusairi, L. Klotho is highly expressed in the chief sites of regulated potassium secretion, and it is stimulated by potassium intake. Sci. Rep. 2024, 14, 10740. [Google Scholar] [CrossRef]
- Corvol, P.; Michaud, A.; Menard, J.; Menard, J.; Freifeld, M.; Mahoudeau, J. Antiandrogenic effect of spirolactones: Mechanism of action. Endocrinology 1975, 97, 52–58. [Google Scholar] [CrossRef]
- Goldspiel, B.R.; Kohler, D.R. Flutamide: An antiandrogen for advanced prostate cancer. DICP 1990, 24, 616–623. [Google Scholar] [CrossRef]
- Maria, B.; Stampf, F.; Goepp, A.; Ulmann, A. Termination of early pregnancy by a single dose of mifepristone (RU 486), a progesterone antagonist. Eur. J. Obstet. Gynecol. Reprod. Biol. 1988, 28, 249–255. [Google Scholar] [CrossRef]
- Hobbs, F.D.R.; McManus, R.J.; Taylor, C.J.; Jones , N.R.; Rahman, J.K.; Wolstenholme, J.; Kim, S.; Kwon, J.; Jones, L.; Hirst, J.A.; et al. Low-dose spironolactone and cardiovascular outcomes in moderate stage chronic kidney disease: A randomized controlled trial. Nat. Med. 2024, 30, 3634–3645. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.J.; Johnson, G.; Kirk, J.; Fuerstenberg, S.M.; Zager , R.A.; Torok-Storb, B. HK-2: An immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney Int. 1994, 45, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Lash, L.H.; Putt, D.A.; Matherly, L.H. Protection of NRK-52E cells, a rat renal proximal tubular cell line, from chemical-induced apoptosis by overexpression of a mitochondrial glutathione transporter. J. Pharmacol. Exp. Ther. 2002, 303, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Wieser, M.; Stadler, G.; Jennings, P.; Streubel, B.; Pfaller, W.; Ambros, P.; Riedl, C.; Katinger, H.; Grillari, J.; Grillari-Voglauer, R. hTERT alone immortalizes epithelial cells of renal proximal tubules without changing their functional characteristics. Am. J. Physiol. Ren. Physiol. 2008, 295, F1365–F1375. [Google Scholar] [CrossRef]
- Herzlinger, D.A.; Easton, T.G.; Ojakian, G.K. The MDCK epithelial cell line expresses a cell surface antigen of the kidney distal tubule. J. Cell Biol. 1982, 93, 269–277. [Google Scholar] [CrossRef]
- Le Menuet, D.; Viengchareun, S.; Penfornis, P.; Walker, F.; Zennaro, M.-C.; Lombe`s, M. Targeted oncogenesis reveals a distinct tissue-specific utilization of alternative promoters of the human mineralocorticoid receptor gene in transgenic mice. J. Biol. Chem. 2000, 275, 7878–7886. [Google Scholar] [CrossRef]
- Yang, J.; Young, M.J. The mineralocorticoid receptor and its coregulators. J. Mol. Endocrinol. 2009, 43, 53–64. [Google Scholar] [CrossRef]
- Kolkhof, P.; Joseph, A.; Kintscher, U. Nonsteroidal mineralocorticoid receptor antagonism for cardiovascular and renal disorders—New perspectives for combination therapy. Pharmacol. Res. 2021, 172, 105859. [Google Scholar] [CrossRef]
- Amazit, L.; Le Billan, F.; Kolkhof, P.; Lamribet, K.; Viengchareun, S.; Fay, M.R.; Khan, J.A.; Hillisch, A.; Lombès, M.; Rafestin-Oblin, M.E.; et al. Finerenone Impedes Aldosterone-dependent Nuclear Import of the Mineralocorticoid Receptor and Prevents Genomic Recruitment of Steroid Receptor Coactivator-1. J. Biol. Chem. 2015, 290, 21876–21889. [Google Scholar] [CrossRef]
- Rogerson, F.M.; Yao, Y.; Smith, B.J.; Fuller, P.J. Differences in the determinants of eplerenone, spironolactone and aldosterone binding to the mineralocorticoid receptor. Clin. Exp. Pharmacol. Physiol. 2004, 31, 704–709. [Google Scholar] [CrossRef]
- Seferovic, P.M.; Pelliccia, F.; Zivkovic, I.; Ristic, A.; Lalic, N.; Seferovic, J.; Simeunovic, D.; Milinkovic, I.; Rosano, G. Mineralocorticoid receptor antagonists, a class beyond spironolactone--Focus on the special pharmacologic properties of eplerenone. Int. J. Cardiol. 2015, 200, 3–7. [Google Scholar] [CrossRef]
- Steidl, M.; Pinggera, G.; Ritter, M.; Lang, F. Progesterone inhibits K conductance in plasma membrane of cultured renal epitheloid MDCK cells. Am. J. Physiol. 1991, 260, E743–E750. [Google Scholar] [CrossRef]
- Barrera-Chimal, J.; Kolkhof, P.; Lima-Posada, I.; Joachim, A.; Rossignol, P.; Jaisser, F. Differentiation between emerging non-steroidal and established steroidal mineralocorticoid receptor antagonists: Head-to-head comparisons of pharmacological and clinical characteristics. Expert Opin. Investig. Drugs 2021, 30, 1141–1157. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kohm, E.; Feger, M.; Föller, M. Regulation of Klotho Production by Mineralocorticoid Receptor Signaling in Renal Cell Lines. Biomolecules 2025, 15, 1509. https://doi.org/10.3390/biom15111509
Kohm E, Feger M, Föller M. Regulation of Klotho Production by Mineralocorticoid Receptor Signaling in Renal Cell Lines. Biomolecules. 2025; 15(11):1509. https://doi.org/10.3390/biom15111509
Chicago/Turabian StyleKohm, Elena, Martina Feger, and Michael Föller. 2025. "Regulation of Klotho Production by Mineralocorticoid Receptor Signaling in Renal Cell Lines" Biomolecules 15, no. 11: 1509. https://doi.org/10.3390/biom15111509
APA StyleKohm, E., Feger, M., & Föller, M. (2025). Regulation of Klotho Production by Mineralocorticoid Receptor Signaling in Renal Cell Lines. Biomolecules, 15(11), 1509. https://doi.org/10.3390/biom15111509




