Kynurenic Acid Protects Against Myocardial Ischemia/Reperfusion Injury by Activating GPR35 Receptors and Preserving Mitochondrial Structure and Function
Abstract
1. Introduction
2. Materials and Methods
2.1. H9c2 Cell Culture
2.2. Experimental Design
2.2.1. Simulated Ischemia/Reperfusion
2.2.2. KYNA Treatment
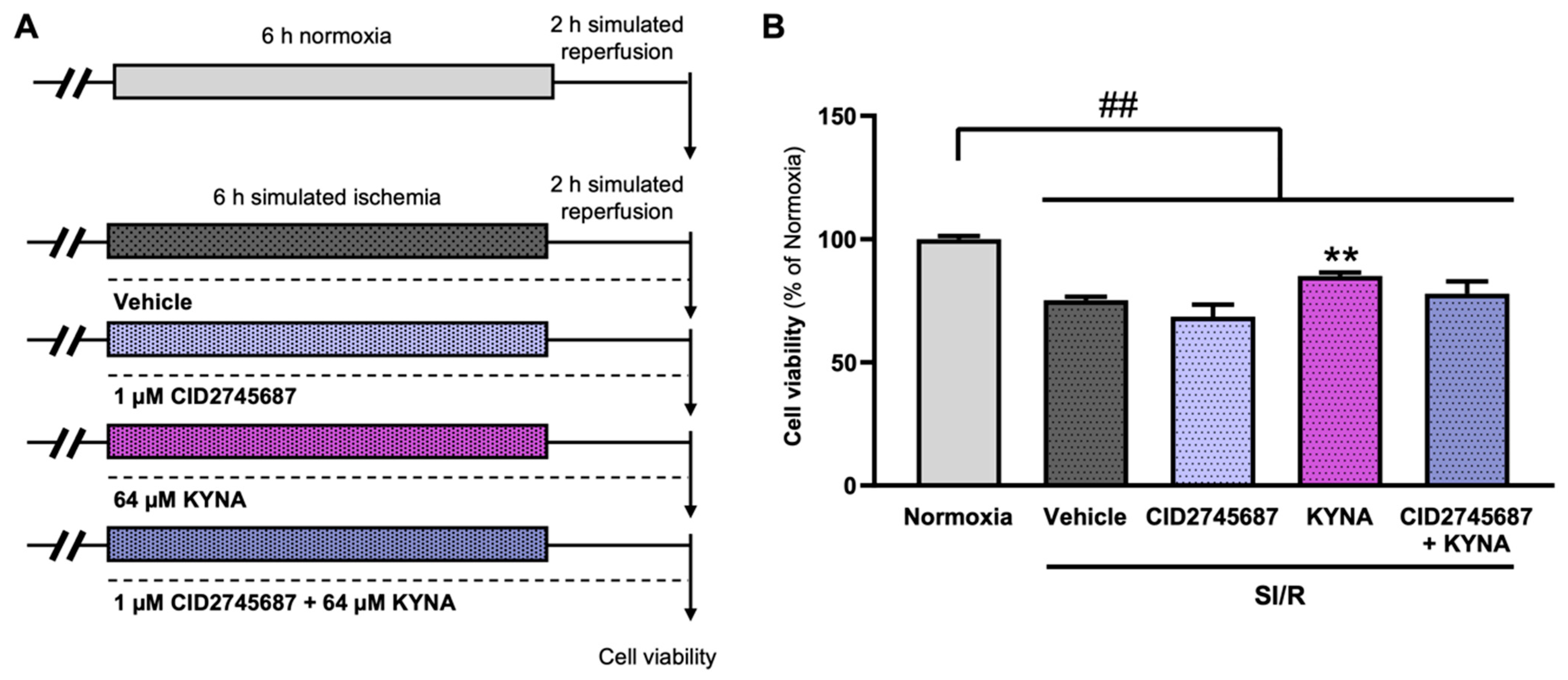
2.2.3. Modulation of GPR35 Receptor Activity
2.3. Electron Microscopy
2.4. Observation of Mitochondrial Morphology and Distribution by Mitotracking
2.5. RNA Isolation and Polymerase Chain Reaction (qPCR)
2.6. Western Blotting
2.7. Measurement of Mitochondria-Derived Superoxide Levels
2.8. Estimation of Mitochondrial Membrane Potential Using JC-1 Staining
2.9. Assessment of Mitochondrial Respiration
2.10. Determination of Metabolic Cell Phenotype Using the Seahorse Analyzer
2.11. Measurement of Cell Viability
2.12. Statistical Analysis
3. Results
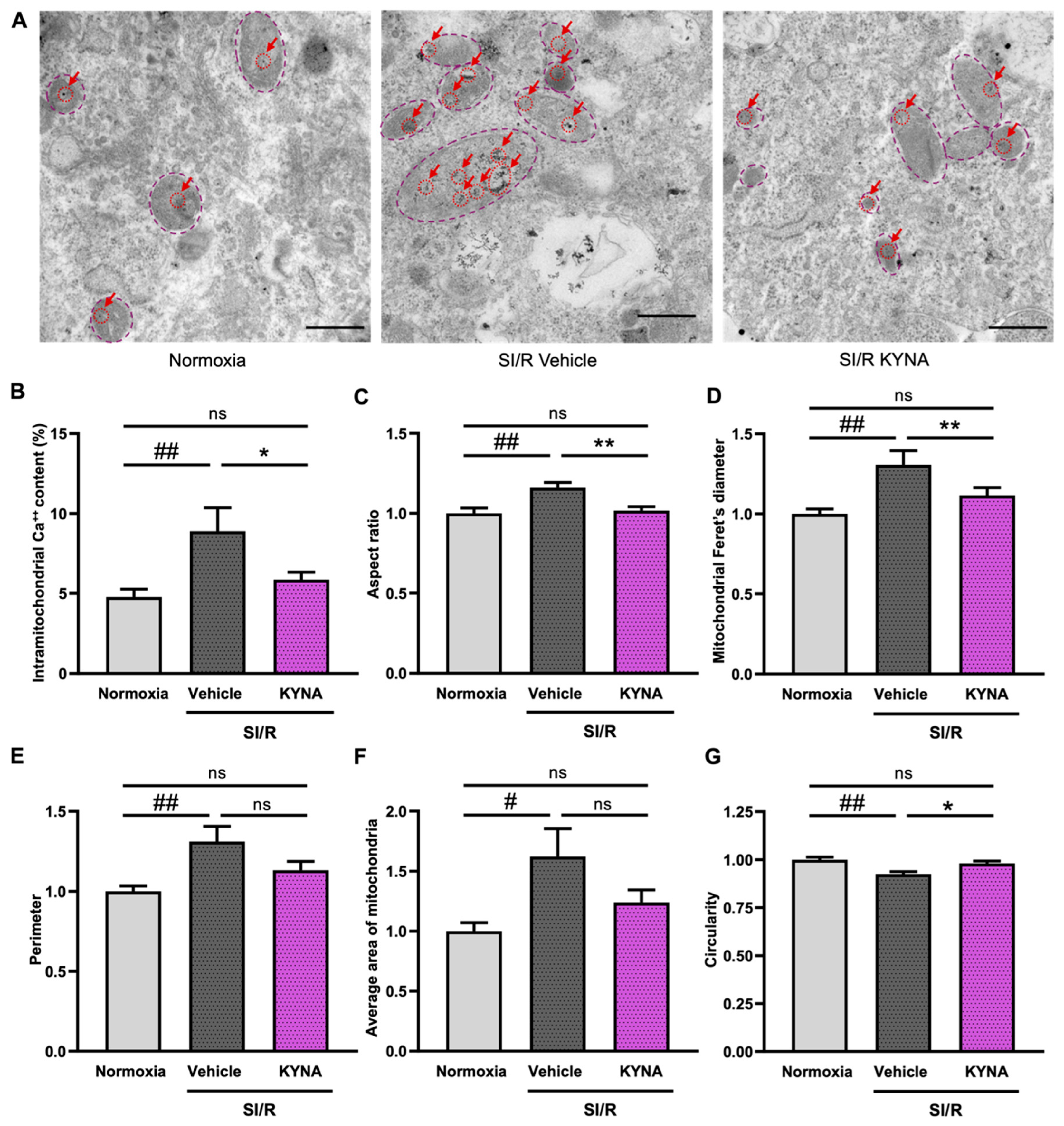
3.1. KYNA Treatment Reduced the Rate of Intramitochondrial Calcium Accumulation and Improved Mitochondrial Ultrastructure in Cells Exposed to SI/R
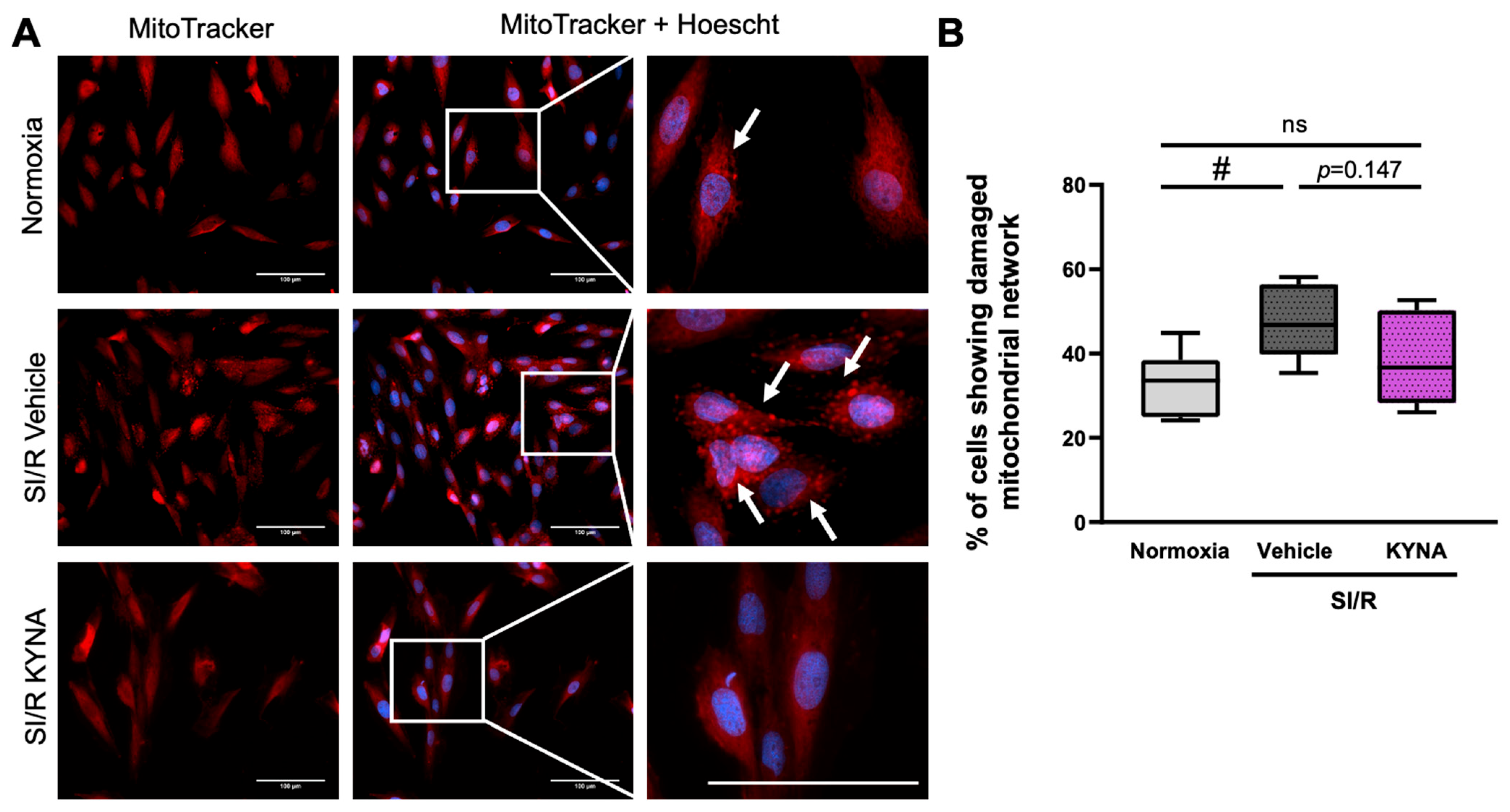
3.2. Treatment with KYNA Seemed to Prevent the SI/R-Induced Alterations in the Mitochondrial Network
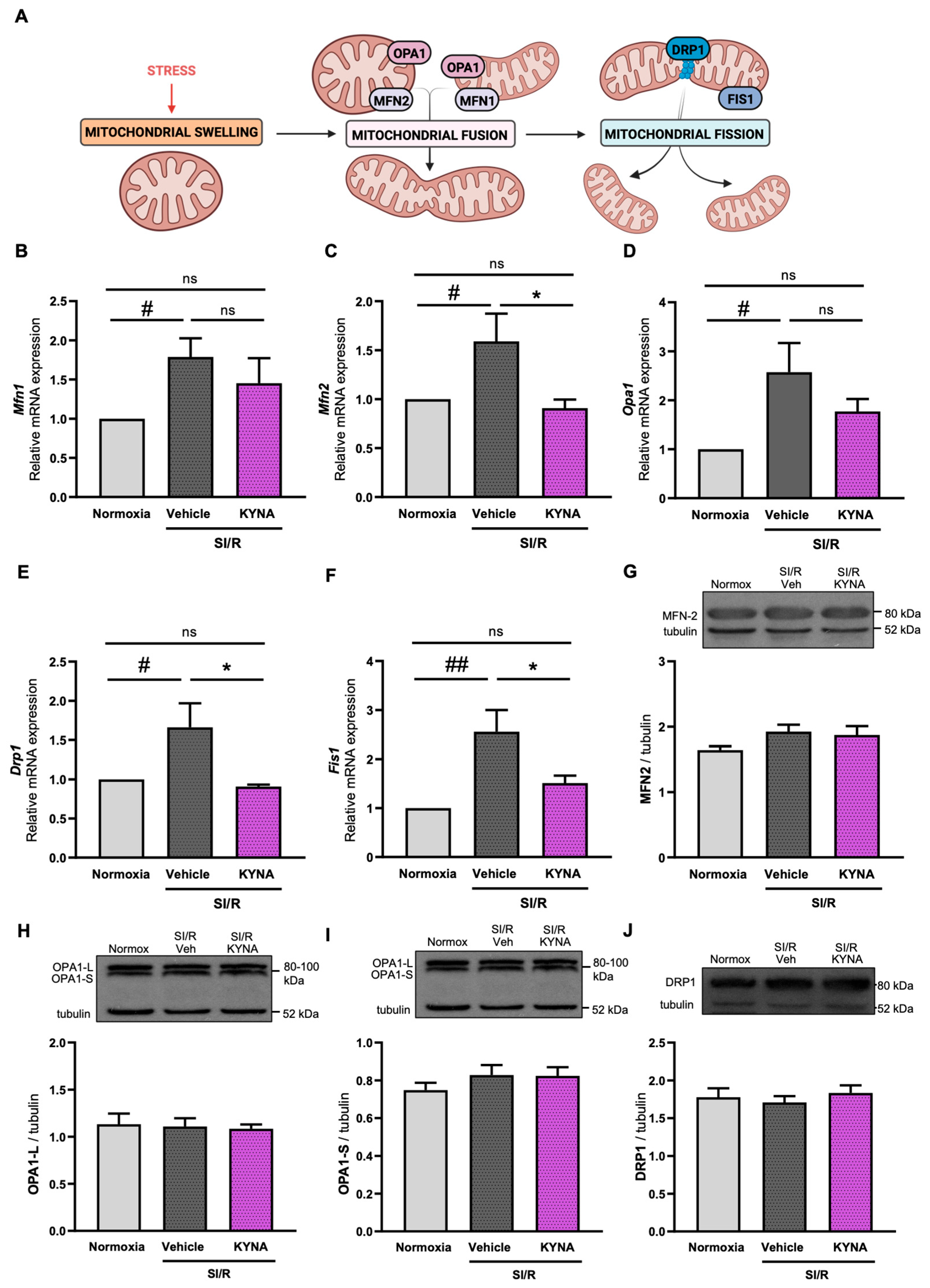
3.3. Administration of KYNA Upregulated the Transcription of Mitochondrial Fusion- and Fission-Related Genes, Without Altering Protein Expression Levels
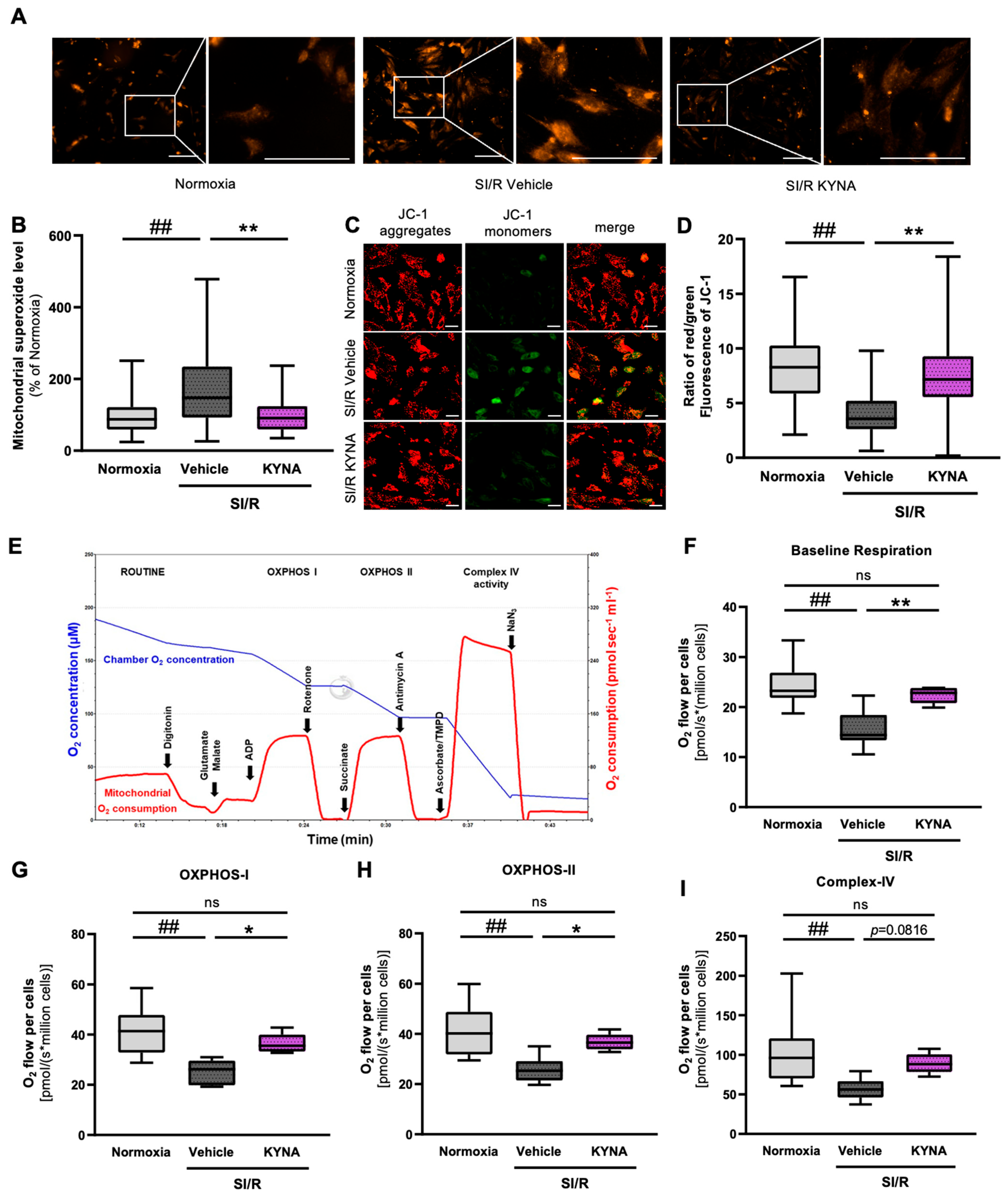
3.4. KYNA Administration Reduced the Mitochondrial Superoxide Production and Preserved the Mitochondrial Membrane Potential in Cardiac Cells Exposed to SI/R
3.5. KYNA Treatment Improved Parameters of Mitochondrial Respiration in Cardiac Cells Undergoing SI/R
3.6. The Cardiocytoprotective Effect of KYNA Involves GPR35 Receptor Activation
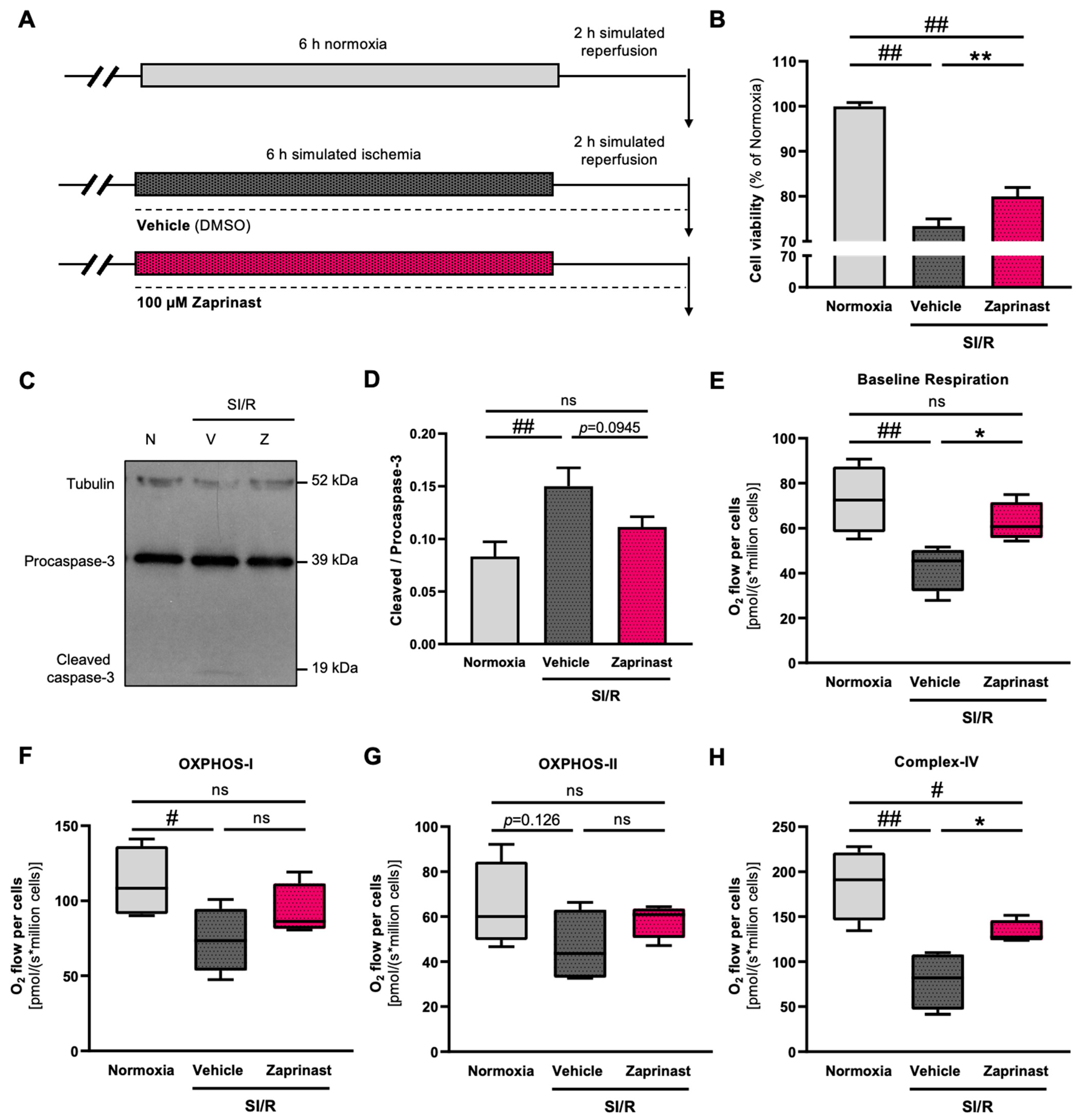
3.7. GPR35 Receptor Activation by Zaprinast Improved the Survival and Mitochondrial Function of Cardiac Cells Subjected to SI/R
4. Discussion
4.1. Future Perspectives and Translational Considerations
4.2. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADP | adenylate-diphosphate |
| AhR | aryl hydrocarbon receptor |
| AMI | acute myocardial infarction |
| AMPA | α-amino-3-hydroxy-5-methyl-4o-isoxazolepropionic acid |
| GPR35 | G-protein coupled receptor 35 |
| I/R | ischemia/reperfusion |
| KYNA | kynurenic acid |
| NMDA | N-methyl-D-aspartic acid |
| OXPHOS | oxidative phosphorylation |
| SI/R | simulated ischemia/reperfusion |
References
- Dorweiler, B.; Pruefer, D.; Andrasi, T.B.; Maksan, S.M.; Schmiedt, W.; Neufang, A.; Vahl, C.F. Ischemia-Reperfusion Injury: Pathophysiology and Clinical Implications. Eur. J. Trauma Emerg. Surg. 2007, 33, 600–612. [Google Scholar] [CrossRef]
- Anderson, J.L.; Morrow, D.A. Acute Myocardial Infarction. N. Engl. J. Med. 2017, 376, 2053–2064. [Google Scholar] [CrossRef]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Ischemia/Reperfusion. Compr. Physiol. 2016, 7, 113–170. [Google Scholar] [CrossRef]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Cell Biology of Ischemia/Reperfusion Injury. Int. Rev. Cell Mol. Biol. 2012, 298, 229–317. [Google Scholar] [CrossRef] [PubMed]
- Lesnefsky, E.J.; Moghaddas, S.; Tandler, B.; Kerner, J.; Hoppel, C.L. Mitochondrial Dysfunction in Cardiac Disease: Ischemia–Reperfusion, Aging, and Heart Failure. J. Mol. Cell. Cardiol. 2001, 33, 1065–1089. [Google Scholar] [CrossRef]
- Badawy, A.A.-B. Kynurenine Pathway of Tryptophan Metabolism: Regulatory and Functional Aspects. Int. J. Tryptophan. Res. 2017, 10, 1178646917691938. [Google Scholar] [CrossRef]
- Moroni, F.; Cozzi, A.; Sili, M.; Mannaioni, G. Kynurenic Acid: A Metabolite with Multiple Actions and Multiple Targets in Brain and Periphery. J. Neural. Transm. 2012, 119, 133–139. [Google Scholar] [CrossRef]
- Tóth, F.; Cseh, E.K.; Vécsei, L. Natural Molecules and Neuroprotection: Kynurenic Acid, Pantethine and α-Lipoic Acid. Int. J. Mol. Sci. 2021, 22, 403. [Google Scholar] [CrossRef] [PubMed]
- Vamos, E.; Pardutz, A.; Klivenyi, P.; Toldi, J.; Vecsei, L. The Role of Kynurenines in Disorders of the Central Nervous System: Possibilities for Neuroprotection. J. Neurol. Sci. 2009, 283, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Andiné, P.; Lehmann, A.; Ellrén, K.; Wennberg, E.; Kjellmer, I.; Nielsen, T.; Hagberg, H. The Excitatory Amino Acid Antagonist Kynurenic Acid Administered after Hypoxic-Ischemia in Neonatal Rats Offers Neuroprotection. Neurosci. Lett. 1988, 90, 208–212. [Google Scholar] [CrossRef]
- Nahomi, R.B.; Nam, M.-H.; Rankenberg, J.; Rakete, S.; Houck, J.A.; Johnson, G.C.; Stankowska, D.L.; Pantcheva, M.B.; MacLean, P.S.; Nagaraj, R.H. Kynurenic Acid Protects Against Ischemia/Reperfusion-Induced Retinal Ganglion Cell Death in Mice. Int. J. Mol. Sci. 2020, 21, 1795. [Google Scholar] [CrossRef]
- Urenjak, J.; Obrenovitch, T.P. Neuroprotective Potency of Kynurenic Acid against Excitotoxicity. Neuroreport 2000, 11, 1341–1344. [Google Scholar] [CrossRef]
- Sun, T.; Xie, R.; He, H.; Xie, Q.; Zhao, X.; Kang, G.; Cheng, C.; Yin, W.; Cong, J.; Li, J.; et al. Kynurenic Acid Ameliorates NLRP3 Inflammasome Activation by Blocking Calcium Mobilization via GPR35. Front. Immunol. 2022, 13, 1019365. [Google Scholar] [CrossRef] [PubMed]
- Agudelo, L.Z.; Ferreira, D.M.S.; Cervenka, I.; Bryzgalova, G.; Dadvar, S.; Jannig, P.R.; Pettersson-Klein, A.T.; Lakshmikanth, T.; Sustarsic, E.G.; Porsmyr-Palmertz, M.; et al. Kynurenic Acid and Gpr35 Regulate Adipose Tissue Energy Homeostasis and Inflammation. Cell Metab. 2018, 27, 378–392.e5. [Google Scholar] [CrossRef]
- Zhen, D.; Liu, J.; Zhang, X.D.; Song, Z. Kynurenic Acid Acts as a Signaling Molecule Regulating Energy Expenditure and Is Closely Associated With Metabolic Diseases. Front. Endocrinol. 2022, 13, 847611. [Google Scholar] [CrossRef] [PubMed]
- Wirthgen, E.; Hoeflich, A.; Rebl, A.; Günther, J. Kynurenic Acid: The Janus-Faced Role of an Immunomodulatory Tryptophan Metabolite and Its Link to Pathological Conditions. Front. Immunol. 2017, 8, 1957. [Google Scholar] [CrossRef]
- Cosi, C.; Mannaioni, G.; Cozzi, A.; Carlà, V.; Sili, M.; Cavone, L.; Maratea, D.; Moroni, F. G-Protein Coupled Receptor 35 (GPR35) Activation and Inflammatory Pain: Studies on the Antinociceptive Effects of Kynurenic Acid and Zaprinast. Neuropharmacology 2011, 60, 1227–1231. [Google Scholar] [CrossRef]
- Wang, D.; Li, D.; Zhang, Y.; Chen, J.; Zhang, Y.; Liao, C.; Qin, S.; Tian, Y.; Zhang, Z.; Xu, F. Functional Metabolomics Reveal the Role of AHR/GPR35 Mediated Kynurenic Acid Gradient Sensing in Chemotherapy-Induced Intestinal Damage. Acta Pharm. Sin. B 2021, 11, 763–780. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Z.; Shen, P.; Zhao, C.; Liu, B.; Shu, C.; Hu, X.; Fu, Y. Kynurenic Acid Ameliorates Lipopolysaccharide-Induced Endometritis by Regulating the GRP35/NF-κB Signaling Pathway. Toxicol. Appl. Pharmacol. 2022, 438, 115907. [Google Scholar] [CrossRef]
- Pyun, D.H.; Kim, T.J.; Kim, M.J.; Hong, S.A.; Abd El-Aty, A.M.; Jeong, J.H.; Jung, T.W. Endogenous Metabolite, Kynurenic Acid, Attenuates Nonalcoholic Fatty Liver Disease via AMPK/Autophagy- and AMPK/ORP150-Mediated Signaling. J. Cell. Physiol. 2021, 236, 4902–4912. [Google Scholar] [CrossRef]
- Marciniak, S.; Wnorowski, A.; Smolińska, K.; Walczyna, B.; Turski, W.; Kocki, T.; Paluszkiewicz, P.; Parada-Turska, J. Kynurenic Acid Protects against Thioacetamide-Induced Liver Injury in Rats. Anal. Cell. Pathol. 2018, 2018, 1270483. [Google Scholar] [CrossRef] [PubMed]
- Olenchock, B.A.; Moslehi, J.; Baik, A.H.; Davidson, S.M.; Williams, J.; Gibson, W.J.; Chakraborty, A.A.; Pierce, K.A.; Miller, C.M.; Hanse, E.A.; et al. EGLN1 Inhibition and Rerouting of α-Ketoglutarate Suffice for Remote Ischemic Protection. Cell 2016, 164, 884–895. [Google Scholar] [CrossRef]
- Gáspár, R.; Nógrádi-Halmi, D.; Demján, V.; Diószegi, P.; Igaz, N.; Vincze, A.; Pipicz, M.; Kiricsi, M.; Vécsei, L.; Csont, T. Kynurenic Acid Protects against Ischemia/Reperfusion Injury by Modulating Apoptosis in Cardiomyocytes. Apoptosis 2024, 29, 1483–1498. [Google Scholar] [CrossRef]
- Gáspár, R.; Pipicz, M.; Hawchar, F.; Kovács, D.; Djirackor, L.; Görbe, A.; Varga, Z.V.; Kiricsi, M.; Petrovski, G.; Gácser, A.; et al. The Cytoprotective Effect of Biglycan Core Protein Involves Toll-like Receptor 4 Signaling in Cardiomyocytes. J. Mol. Cell. Cardiol. 2016, 99, 138–150. [Google Scholar] [CrossRef]
- Choi, J.-H.; Kim, D.-H.; Yun, I.-J.; Chang, J.-H.; Chun, B.-G.; Choi, S.-H. Zaprinast Inhibits Hydrogen Peroxide-Induced Lysosomal Destabilization and Cell Death in Astrocytes. Eur. J. Pharmacol. 2007, 571, 106–115. [Google Scholar] [CrossRef]
- Schütte, H.; Witzenrath, M.; Mayer, K.; Weissmann, N.; Schell, A.; Rosseau, S.; Seeger, W.; Grimminger, F. The PDE Inhibitor Zaprinast Enhances NO-Mediated Protection against Vascular Leakage in Reperfused Lungs. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 279, L496–L502. [Google Scholar] [CrossRef]
- Mayhew, T.M. A Review of Recent Advances in Stereology for Quantifying Neural Structure. J. Neurocytol. 1992, 21, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Pajer, K.; Bellák, T.; Grósz, T.; Nógrádi, B.; Patai, R.; Sinkó, J.; Vinay, L.; Liabeuf, S.; Erdélyi, M.; Nógrádi, A. Riluzole Treatment Modulates KCC2 and EAAT-2 Receptor Expression and Ca2+ Accumulation Following Ventral Root Avulsion Injury. Eur. J. Cell Biol. 2023, 102, 151317. [Google Scholar] [CrossRef] [PubMed]
- Nogradi, B.; Meszlenyi, V.; Patai, R.; Polgar, T.F.; Spisak, K.; Kristof, R.; Siklos, L. Diazoxide Blocks or Reduces Microgliosis When Applied Prior or Subsequent to Motor Neuron Injury in Mice. Brain Res. 2020, 1741, 146875. [Google Scholar] [CrossRef]
- Demeter-Haludka, V.; Kovács, M.; Petrus, A.; Patai, R.; Muntean, D.M.; Siklós, L.; Végh, Á. Examination of the Role of Mitochondrial Morphology and Function in the Cardioprotective Effect of Sodium Nitrite Administered 24 h Before Ischemia/Reperfusion Injury. Front. Pharmacol. 2018, 9, 286. [Google Scholar] [CrossRef]
- Errea, O.; Moreno, B.; Gonzalez-Franquesa, A.; Garcia-Roves, P.M.; Villoslada, P. The Disruption of Mitochondrial Axonal Transport Is an Early Event in Neuroinflammation. J. Neuroinflamm. 2015, 12, 152. [Google Scholar] [CrossRef]
- Johnson, D.; Nehrke, K. Mitochondrial Fragmentation Leads to Intracellular Acidification in Caenorhabditis Elegans and Mammalian Cells. Mol. Biol. Cell 2010, 21, 2191–2201. [Google Scholar] [CrossRef]
- Kuznetsov, A.V.; Javadov, S.; Margreiter, R.; Grimm, M.; Hagenbuchner, J.; Ausserlechner, M.J. The Role of Mitochondria in the Mechanisms of Cardiac Ischemia-Reperfusion Injury. Antioxidants 2019, 8, 454. [Google Scholar] [CrossRef]
- Djafarzadeh, S.; Jakob, S.M. High-Resolution Respirometry to Assess Mitochondrial Function in Permeabilized and Intact Cells. J. Vis. Exp. 2017, 120, 54985. [Google Scholar] [CrossRef]
- Bertero, E.; Popoiu, T.-A.; Maack, C. Mitochondrial Calcium in Cardiac Ischemia/Reperfusion Injury and Cardioprotection. Basic Res. Cardiol. 2024, 119, 569–585. [Google Scholar] [CrossRef]
- Pham, N.A.; Robinson, B.H.; Hedley, D.W. Simultaneous Detection of Mitochondrial Respiratory Chain Activity and Reactive Oxygen in Digitonin-Permeabilized Cells Using Flow Cytometry. Cytometry 2000, 41, 245–251. [Google Scholar] [CrossRef]
- Wyant, G.A.; Yu, W.; Doulamis, I.P.; Nomoto, R.S.; Saeed, M.Y.; Duignan, T.; McCully, J.D.; Kaelin, W.G. Mitochondrial Remodeling and Ischemic Protection by G Protein-Coupled Receptor 35 Agonists. Science 2022, 377, 621–629. [Google Scholar] [CrossRef]
- Kamel, R.; Baetz, D.; Gueguen, N.; Lebeau, L.; Barbelivien, A.; Guihot, A.-L.; Allawa, L.; Gallet, J.; Beaumont, J.; Ovize, M.; et al. Kynurenic Acid: A Novel Player in Cardioprotection against Myocardial Ischemia/Reperfusion Injuries. Pharmaceuticals 2023, 16, 1381. [Google Scholar] [CrossRef] [PubMed]
- Marin, W.; Marin, D.; Ao, X.; Liu, Y. Mitochondria as a Therapeutic Target for Cardiac Ischemia-reperfusion Injury (Review). Int. J. Mol. Med. 2021, 47, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.-I.; Jou, M.-J. Oxidative Stress Caused by Mitochondrial Calcium Overload. Ann. N. Y. Acad. Sci. 2010, 1201, 183–188. [Google Scholar] [CrossRef]
- Smaili, S.S.; Hsu, Y.T.; Youle, R.J.; Russell, J.T. Mitochondria in Ca2+ Signaling and Apoptosis. J. Bioenerg. Biomembr. 2000, 32, 35–46. [Google Scholar] [CrossRef]
- Montes de Oca Balderas, P.; Aguilera, P. A Metabotropic-Like Flux-Independent NMDA Receptor Regulates Ca2+ Exit from Endoplasmic Reticulum and Mitochondrial Membrane Potential in Cultured Astrocytes. PLoS ONE 2015, 10, e0126314. [Google Scholar] [CrossRef]
- Kwong, J.Q.; Molkentin, J.D. Physiological and Pathological Roles of the Mitochondrial Permeability Transition Pore in the Heart. Cell Metab. 2015, 21, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Javadov, S.; Chapa-Dubocq, X.; Makarov, V. Different Approaches to Modeling Analysis of Mitochondrial Swelling. Mitochondrion 2018, 38, 58–70. [Google Scholar] [CrossRef]
- Federico, M.; Portiansky, E.L.; Sommese, L.; Alvarado, F.J.; Blanco, P.G.; Zanuzzi, C.N.; Dedman, J.; Kaetzel, M.; Wehrens, X.H.T.; Mattiazzi, A.; et al. Calcium-Calmodulin-Dependent Protein Kinase Mediates the Intracellular Signalling Pathways of Cardiac Apoptosis in Mice with Impaired Glucose Tolerance. J. Physiol. 2017, 595, 4089–4108. [Google Scholar] [CrossRef]
- Karbowski, M.; Youle, R.J. Dynamics of Mitochondrial Morphology in Healthy Cells and during Apoptosis. Cell Death Differ. 2003, 10, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Linn, B.S.; Zhang, Y.; Ren, J. Mitophagy and Mitochondrial Integrity in Cardiac Ischemia-Reperfusion Injury. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 2293–2302. [Google Scholar] [CrossRef] [PubMed]
- Atici, A.E.; Crother, T.R.; Noval Rivas, M. Mitochondrial Quality Control in Health and Cardiovascular Diseases. Front. Cell Dev. Biol. 2023, 11, 1290046. [Google Scholar] [CrossRef]
- Serasinghe, M.N.; Chipuk, J.E. Mitochondrial Fission in Human Diseases. Handb. Exp. Pharmacol. 2017, 240, 159–188. [Google Scholar] [CrossRef]
- Wang, J.; Toan, S.; Zhou, H. New Insights into the Role of Mitochondria in Cardiac Microvascular Ischemia/Reperfusion Injury. Angiogenesis 2020, 23, 299–314. [Google Scholar] [CrossRef]
- Rafelski, S.M. Mitochondrial Network Morphology: Building an Integrative, Geometrical View. BMC Biol. 2013, 11, 71. [Google Scholar] [CrossRef]
- Glancy, B.; Kim, Y.; Katti, P.; Willingham, T.B. The Functional Impact of Mitochondrial Structure Across Subcellular Scales. Front. Physiol. 2020, 11, 541040. [Google Scholar] [CrossRef]
- Cogliati, S.; Enriquez, J.A.; Scorrano, L. Mitochondrial Cristae: Where Beauty Meets Functionality. Trends Biochem. Sci. 2016, 41, 261–273. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial ROS-Induced ROS Release: An Update and Review. Biochim. Biophys. Acta 2006, 1757, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-Y.; Yiang, G.-T.; Liao, W.-T.; Tsai, A.P.-Y.; Cheng, Y.-L.; Cheng, P.-W.; Li, C.-Y.; Li, C.-J. Current Mechanistic Concepts in Ischemia and Reperfusion Injury. Cell. Physiol. Biochem. 2018, 46, 1650–1667. [Google Scholar] [CrossRef]
- Baran, H.; Staniek, K.; Kepplinger, B.; Gille, L.; Stolze, K.; Nohl, H. Kynurenic Acid Influences the Respiratory Parameters of Rat Heart Mitochondria. Pharmacology 2001, 62, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Poles, M.Z.; Nászai, A.; Gulácsi, L.; Czakó, B.L.; Gál, K.G.; Glenz, R.J.; Dookhun, D.; Rutai, A.; Tallósy, S.P.; Szabó, A.; et al. Kynurenic Acid and Its Synthetic Derivatives Protect Against Sepsis-Associated Neutrophil Activation and Brain Mitochondrial Dysfunction in Rats. Front. Immunol. 2021, 12, 717157. [Google Scholar] [CrossRef]
- Bratek-Gerej, E.; Ziembowicz, A.; Godlewski, J.; Salinska, E. The Mechanism of the Neuroprotective Effect of Kynurenic Acid in the Experimental Model of Neonatal Hypoxia–Ischemia: The Link to Oxidative Stress. Antioxidants 2021, 10, 1775. [Google Scholar] [CrossRef] [PubMed]
- Sharmin, O.; Abir, A.H.; Potol, A.; Alam, M.; Banik, J.; Rahman, A.F.M.T.; Tarannum, N.; Wadud, R.; Habib, Z.F.; Rahman, M. Activation of GPR35 Protects against Cerebral Ischemia by Recruiting Monocyte-Derived Macrophages. Sci. Rep. 2020, 10, 9400. [Google Scholar] [CrossRef]
- Chen, K.; He, L.; Li, Y.; Li, X.; Qiu, C.; Pei, H.; Yang, D. Inhibition of GPR35 Preserves Mitochondrial Function After Myocardial Infarction by Targeting Calpain 1/2. J. Cardiovasc. Pharmacol. 2020, 75, 556–563. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, P.; Fan, H.; Zhang, C.; Yu, P.; Liang, X.; Chen, Y. GPR35 Acts a Dual Role and Therapeutic Target in Inflammation. Front. Immunol. 2023, 14, 1254446. [Google Scholar] [CrossRef]
- Li, Z.; Yu, Z.; Cui, S.; Hu, S.; Li, B.; Chen, T.; Qu, C.; Yang, B. AMPA Receptor Inhibition Alleviates Inflammatory Response and Myocardial Apoptosis after Myocardial Infarction by Inhibiting TLR4/NF-κB Signaling Pathway. Int. Immunopharmacol. 2024, 133, 112080. [Google Scholar] [CrossRef]
- Braidy, N.; Grant, R.; Brew, B.J.; Adams, S.; Jayasena, T.; Guillemin, G.J. Effects of Kynurenine Pathway Metabolites on Intracellular NAD Synthesis and Cell Death in Human Primary Astrocytes and Neurons. Int. J. Tryptophan. Res. 2009, 2, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Turski, W.A.; Małaczewska, J.; Marciniak, S.; Bednarski, J.; Turski, M.P.; Jabłoński, M.; Siwicki, A.K. On the Toxicity of Kynurenic Acid in Vivo and in Vitro. Pharmacol. Rep. 2014, 66, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Papp, A.; Hartwell, R.; Evans, M.; Ghahary, A. The Safety and Tolerability of Topically Delivered Kynurenic Acid in Humans. A Phase 1 Randomized Double-Blind Clinical Trial. J. Pharm. Sci. 2018, 107, 1572–1576. [Google Scholar] [CrossRef]
- Turska, M.; Paluszkiewicz, P.; Turski, W.A.; Parada-Turska, J. A Review of the Health Benefits of Food Enriched with Kynurenic Acid. Nutrients 2022, 14, 4182. [Google Scholar] [CrossRef] [PubMed]
- Murphy, N.; Ramakrishnan, N.; Vo-Le, B.; Vo-Le, B.; Smith, M.A.; Iqbal, T.; Swann, A.C.; Mathew, S.J.; Lijffijt, M. A Randomized Cross-over Trial to Define Neurophysiological Correlates of AV-101 N-Methyl-D-Aspartate Receptor Blockade in Healthy Veterans. Neuropsychopharmacology 2021, 46, 820–827. [Google Scholar] [CrossRef]
| Genes | Forward Primers (5′→3′) | Reverse Primers (5′→3′) |
|---|---|---|
| Mfn1 | GAAGGCCTGTCCAGAACTGA | CCGGGTTCCTGTATGTTGCT |
| Mfn2 | CCCTTACCAGCTAGAAACGAGA | GACAAAGTGCTTGAGAGGGGA |
| Opa1 | TGCTGTTGGAGGTGGCTATAC | GGTGTACCCGCAGTGAAGAA |
| Drp1 | CGTAGTGGGAACTCAGAGCAG | ACCCCATTCTTCTGCTTCAACT |
| Fis1 | GGTTGCGTGGTAAGGGATGA | CAAACTGCGTGCTCTTGGAC |
| Gpr35 | GCTCTTTGCAGGTTGTGACTG | GCACGGCTGAAGATGTTTCG |
| Protein | Manufacturer, Catalog No. | Conditions |
|---|---|---|
| MFN2 | CST, Cat#11925 (RRID: AB_2750893) | 1:1000, 4 °C, ON |
| OPA1 | CST, Cat#80471 (RRID: AB_2734117) | 1:1000, 4 °C, ON |
| DRP1 | CST, Cat#8570 (RRID: AB_10950498) | 1:1000, 4 °C, ON |
| Caspase-3 | CST, Cat#14220 (RRID: AB_2798429) | 1:1000, 4 °C, ON |
| Cleaved caspase-3 | CST, Cat#9664 (RRID: AB_2070042) | 1:750, 4 °C, ON |
| α-tubulin | CST, Cat#2114 (RRID: AB_2210548) | 1:2000, 4 °C, ON |
| Investigated Parameter | Effects of KYNA on SI/R-Induced Changes | Effects of Zaprinast on SI/R-Induced Changes |
|---|---|---|
| Cell viability | ↑ # | ↑ |
| Apoptotic activity | ↓ # | ↓ |
| Mitochondrial function: | improved | improved |
| (1) Baseline respiration | ↑ | ↑ |
| (2) OXPHOS-I | ↑ | ↑ (ns) |
| (3) OXPHOS-II | ↑ | ↑ (ns) |
| (4) Complex-IV activity | ↑ (ns) | ↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nógrádi-Halmi, D.; Erdélyi-Furka, B.; Csóré, D.; Plechl, É.; Igaz, N.; Juhász, L.; Poles, M.Z.; Nógrádi, B.; Patai, R.; Polgár, T.F.; et al. Kynurenic Acid Protects Against Myocardial Ischemia/Reperfusion Injury by Activating GPR35 Receptors and Preserving Mitochondrial Structure and Function. Biomolecules 2025, 15, 1481. https://doi.org/10.3390/biom15101481
Nógrádi-Halmi D, Erdélyi-Furka B, Csóré D, Plechl É, Igaz N, Juhász L, Poles MZ, Nógrádi B, Patai R, Polgár TF, et al. Kynurenic Acid Protects Against Myocardial Ischemia/Reperfusion Injury by Activating GPR35 Receptors and Preserving Mitochondrial Structure and Function. Biomolecules. 2025; 15(10):1481. https://doi.org/10.3390/biom15101481
Chicago/Turabian StyleNógrádi-Halmi, Dóra, Barbara Erdélyi-Furka, Dóra Csóré, Éva Plechl, Nóra Igaz, László Juhász, Marietta Zita Poles, Bernát Nógrádi, Roland Patai, Tamás Ferenc Polgár, and et al. 2025. "Kynurenic Acid Protects Against Myocardial Ischemia/Reperfusion Injury by Activating GPR35 Receptors and Preserving Mitochondrial Structure and Function" Biomolecules 15, no. 10: 1481. https://doi.org/10.3390/biom15101481
APA StyleNógrádi-Halmi, D., Erdélyi-Furka, B., Csóré, D., Plechl, É., Igaz, N., Juhász, L., Poles, M. Z., Nógrádi, B., Patai, R., Polgár, T. F., Kiricsi, M., Vécsei, L., Gáspár, R., & Csont, T. (2025). Kynurenic Acid Protects Against Myocardial Ischemia/Reperfusion Injury by Activating GPR35 Receptors and Preserving Mitochondrial Structure and Function. Biomolecules, 15(10), 1481. https://doi.org/10.3390/biom15101481








