Polyphenol-Based Therapeutic Strategies for Mitochondrial Dysfunction in Aging
Abstract
1. Introduction
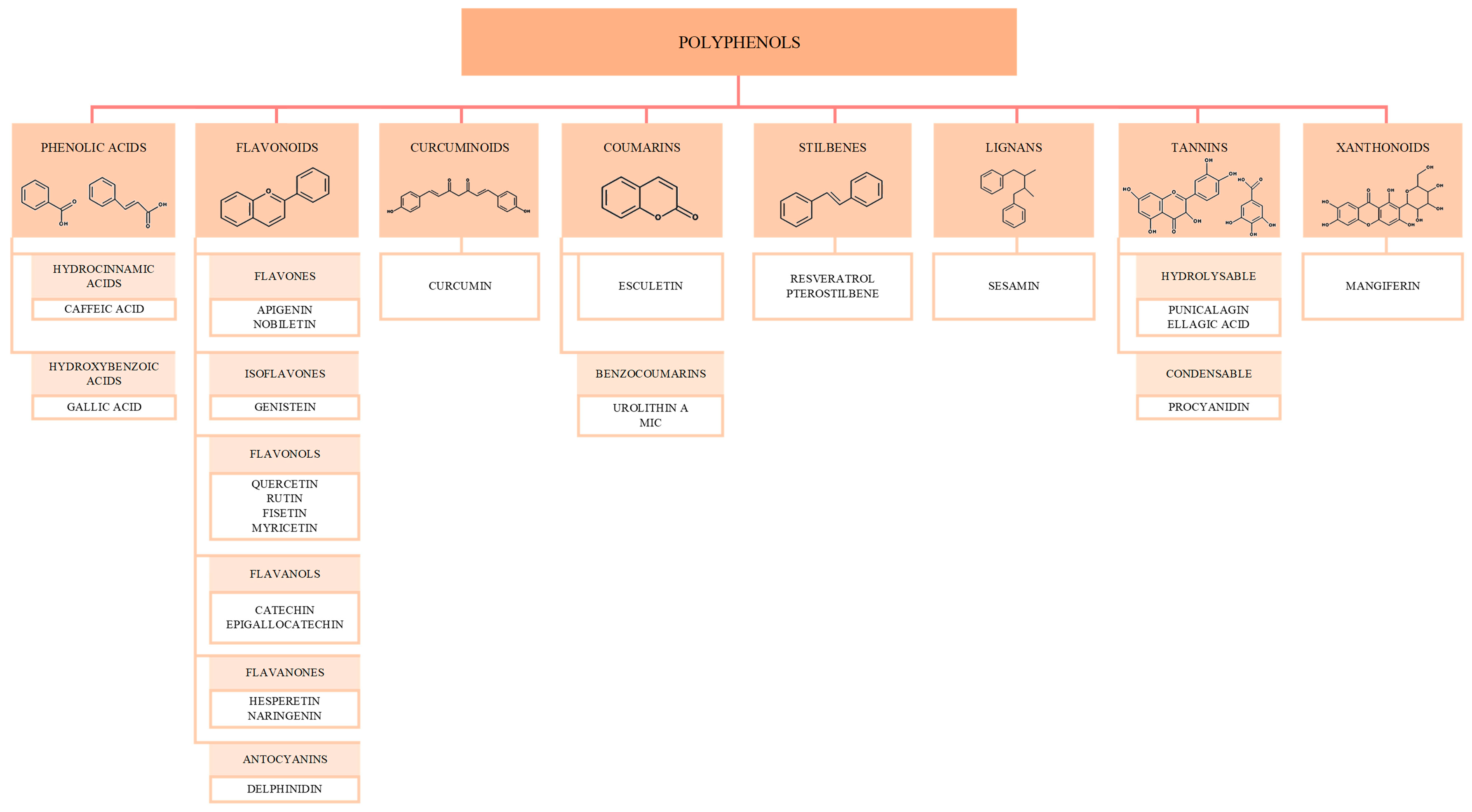
2. Polyphenols: Mechanisms and Anti-Aging Effects
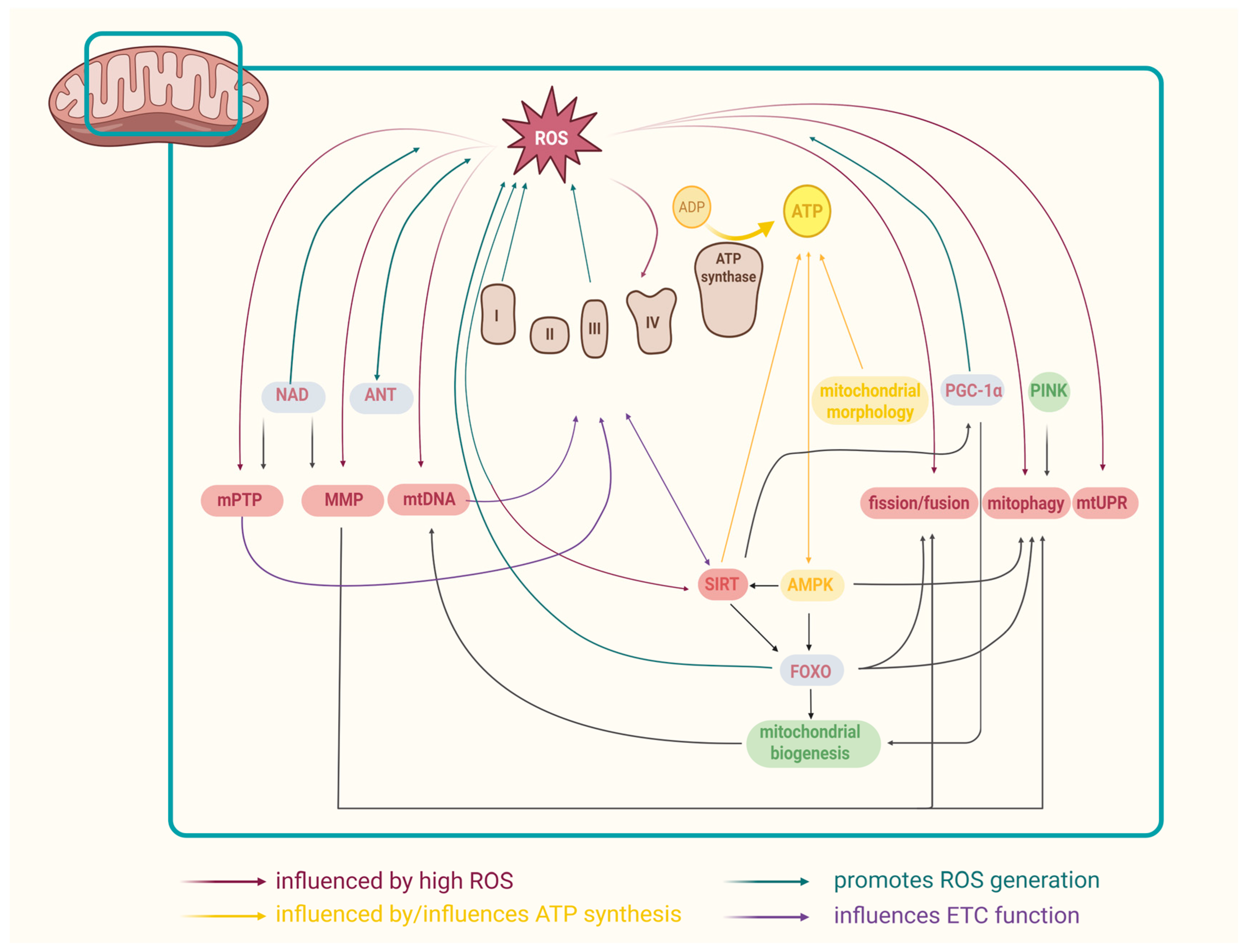
3. Mitochondria and Aging: Mechanisms Involved
3.1. ROS and Aging
3.2. Mitochondrial ETC and Aging
3.3. Mitochondrial DNA and Aging
3.4. Mitochondrial Dynamics and Aging
3.5. Mitochondrial Morphology and Aging
3.6. Mitochondrial Proteostasis and Aging
3.7. Mitochondrial Permeability Transition Pore (mPTP)
3.8. Mitochondrial Membrane Potential (MMP)
3.9. Mitochondrial Adenine Nucleotide Translocase (ANT) and Aging
3.10. NAD+ and Aging
3.11. Mitochondrial Regulators
3.11.1. Sirtuins and Aging
3.11.2. FOXO and Aging
3.11.3. PINK1 and Aging
3.11.4. AMPK and Aging
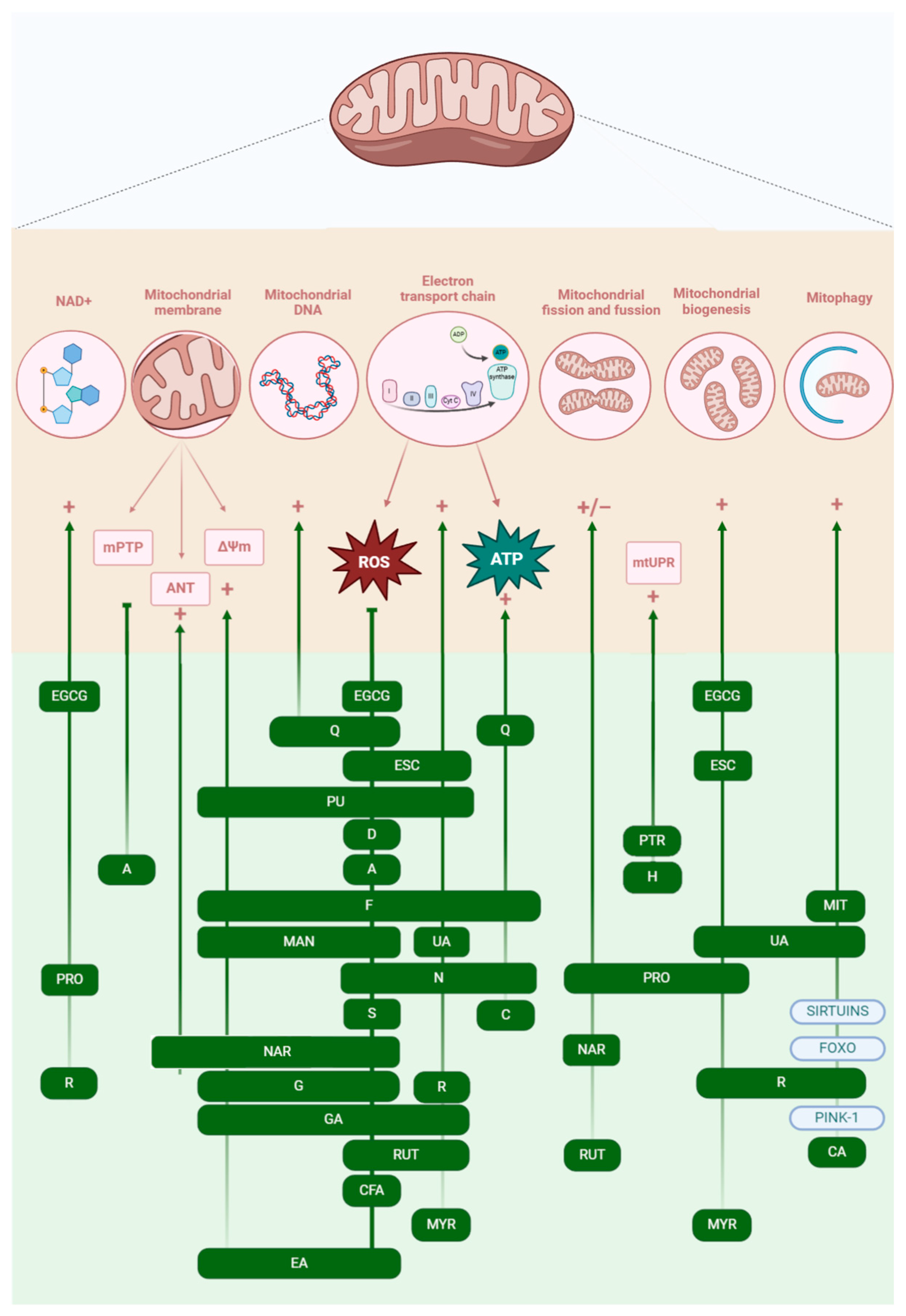
4. Targeting Mitochondria with Polyphenols to Mitigate Aging-Related Decline
4.1. Phenolic Acids
4.1.1. Caffeic Acid
4.1.2. Gallic Acid
4.2. Flavonoids
4.2.1. Apigenin
4.2.2. Nobiletin
4.2.3. Genistein
4.2.4. Quercetin
4.2.5. Rutin
4.2.6. Fisetin
4.2.7. Myricetin
4.2.8. Catechinic Acid
4.2.9. Epigallocatechin 3-Gallate
4.2.10. Hesperetin
4.2.11. Naringenin
4.2.12. Delphinidin
4.3. Curcuminoids—Curcumin
4.4. Coumarins
4.4.1. Esculetin
4.4.2. Urolithin A
4.4.3. Mitophagy-Inducing Coumarin
4.5. Stilbenes
4.5.1. Resveratrol
4.5.2. Pterostilbene
4.6. Lignans—Sesamin
4.7. Tannins
4.7.1. Punicalagin
4.7.2. Ellagic Acid
4.7.3. Procyanidins
4.8. Xantonoids—Mangiferin
4.9. Mixtures of Polyphenols
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMP | Adenosine monophosphate |
| AMPK | AMP-activated protein kinase |
| ANT | Adenine nucleotide translocase |
| ATP | Adenosine triphosphate |
| BEC-1 | Beclin-1 |
| CI | Complex I |
| CII | Complex II |
| CIII | Complex III |
| CIV | Complex IV |
| CV | Complex V (ATP-synthase) |
| EGCG | Epigallocatechin 3-gallate |
| ETC | Electron transport chain |
| FMN | Flavin mononucleotide |
| IMM | Inner mitochondrial membrane |
| MIC | Mitophagy-inducing coumarin |
| mtDNA | Mitochondrial DNA |
| MMP | Mitochondrial membrane potential |
| mPTP | Mitochondrial permeability transition pore |
| mtUPR | Mitochondrial unfolded protein response |
| OMM | Outer mitochondrial membrane |
| PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PTEN | Phosphatase and tensin homolog |
| ROS | Reactive oxygen species |
| SIRT | Sirtuin |
| SOD | Superoxide dismutase |
| SDHB | Succinate dehydrogenase complex subunit B |
| SDHC | Succinate dehydrogenase complex subunit C |
| TPP+ | Triphenyl phosphonium |
References
- Li, Y.; Tian, X.; Luo, J.; Bao, T.; Wang, S.; Wu, X. Molecular Mechanisms of Aging and Anti-Aging Strategies. Cell Commun. Signal. 2024, 22, 285. [Google Scholar] [CrossRef] [PubMed]
- Rottenberg, H. The Reduction in the Mitochondrial Membrane Potential in Aging: The Role of the Mitochondrial Permeability Transition Pore. Int. J. Mol. Sci. 2023, 24, 12295. [Google Scholar] [CrossRef] [PubMed]
- Amorim, J.A.; Coppotelli, G.; Rolo, A.P.; Palmeira, C.M.; Ross, J.M.; Sinclair, D.A. Mitochondrial and Metabolic Dysfunction in Ageing and Age-Related Diseases. Nat. Rev. Endocrinol. 2022, 18, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S. The Mitochondrial Basis of Aging and Age-Related Disorders. Genes 2017, 8, 398. [Google Scholar] [CrossRef]
- Liu, Y.; Weng, W.; Gao, R.; Liu, Y. New Insights for Cellular and Molecular Mechanisms of Aging and Aging-Related Diseases: Herbal Medicine as Potential Therapeutic Approach. Oxid. Med. Cell. Longev. 2019, 2019, 1–25. [Google Scholar] [CrossRef]
- Nielsen, J.L.; Bakula, D.; Scheibye-Knudsen, M. Clinical Trials Targeting Aging. Front. Aging 2022, 3, 820215. [Google Scholar] [CrossRef]
- Liao, P.; Yan, B.; Wang, C.; Lei, P. Telomeres: Dysfunction, Maintenance, Aging and Cancer. Aging Dis. 2023, 15, 2595–2631. [Google Scholar] [CrossRef]
- LeBrasseur, N.K. Hungry for Biomarkers of Aging. Aging Cell 2024, 23, e14158. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of Aging: An Expanding Universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Protasoni, M.; Zeviani, M. Mitochondrial Structure and Bioenergetics in Normal and Disease Conditions. Int. J. Mol. Sci. 2021, 22, 586. [Google Scholar] [CrossRef]
- Yan, C.; Duanmu, X.; Zeng, L.; Liu, B.; Song, Z. Mitochondrial DNA: Distribution, Mutations, and Elimination. Cells 2019, 8, 379. [Google Scholar] [CrossRef]
- Chocron, E.S.; Munkácsy, E.; Pickering, A.M. Cause or Casualty: The Role of Mitochondrial DNA in Aging and Age-Associated Disease. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2019, 1865, 285–297. [Google Scholar] [CrossRef]
- Harrington, J.S.; Ryter, S.W.; Plataki, M.; Price, D.R.; Choi, A.M.K. Mitochondria in Health, Disease, and Aging. Physiol. Rev. 2023, 103, 2349–2422. [Google Scholar] [CrossRef]
- Milan, A.; Mioc, M.; Mioc, A.; Gogulescu, A.; Mardale, G.; Avram, Ș.; Maksimović, T.; Mara, B.; Șoica, C. Cytotoxic Potential of Betulinic Acid Fatty Esters and Their Liposomal Formulations: Targeting Breast, Colon, and Lung Cancer Cell Lines. Molecules 2024, 29, 3399. [Google Scholar] [CrossRef]
- Chen, G.; Kroemer, G.; Kepp, O. Mitophagy: An Emerging Role in Aging and Age-Associated Diseases. Front. Cell Dev. Biol. 2020, 8, 200. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, M.; Geltinger, F.; Verwanger, T.; Weiss, R.; Richter, K.; Rinnerthaler, M. Lipid Droplets Protect Aging Mitochondria and Thus Promote Lifespan in Yeast Cells. Front. Cell Dev. Biol. 2021, 9, 774985. [Google Scholar] [CrossRef] [PubMed]
- Stefanatos, R.; Sanz, A. The Role of Mitochondrial ROS in the Aging Brain. FEBS Lett. 2018, 592, 743–758. [Google Scholar] [CrossRef] [PubMed]
- Madreiter-Sokolowski, C.T.; Hiden, U.; Krstic, J.; Panzitt, K.; Wagner, M.; Enzinger, C.; Khalil, M.; Abdellatif, M.; Malle, E.; Madl, T.; et al. Targeting Organ-Specific Mitochondrial Dysfunction to Improve Biological Aging. Pharmacol. Ther. 2024, 262, 108710. [Google Scholar] [CrossRef]
- Russo, G.L.; Spagnuolo, C.; Russo, M.; Tedesco, I.; Moccia, S.; Cervellera, C. Mechanisms of Aging and Potential Role of Selected Polyphenols in Extending Healthspan. Biochem. Pharmacol. 2020, 173, 113719. [Google Scholar] [CrossRef]
- Hano, C.; Tungmunnithum, D. Plant Polyphenols, More than Just Simple Natural Antioxidants: Oxidative Stress, Aging and Age-Related Diseases. Medicines 2020, 7, 26. [Google Scholar] [CrossRef]
- Brimson, J.M.; Prasanth, M.I.; Malar, D.S.; Thitilertdecha, P.; Kabra, A.; Tencomnao, T.; Prasansuklab, A. Plant Polyphenols for Aging Health: Implication from Their Autophagy Modulating Properties in Age-Associated Diseases. Pharmaceuticals 2021, 14, 982. [Google Scholar] [CrossRef]
- Rudrapal, M.; Rakshit, G.; Singh, R.P.; Garse, S.; Khan, J.; Chakraborty, S. Dietary Polyphenols: Review on Chemistry/Sources, Bioavailability/Metabolism, Antioxidant Effects, and Their Role in Disease Management. Antioxidants 2024, 13, 429. [Google Scholar] [CrossRef]
- Sandoval-Acuña, C.; Ferreira, J.; Speisky, H. Polyphenols and Mitochondria: An Update on Their Increasingly Emerging ROS-Scavenging Independent Actions. Arch. Biochem. Biophys. 2014, 559, 75–90. [Google Scholar] [CrossRef]
- Luo, J.; Si, H.; Jia, Z.; Liu, D. Dietary Anti-Aging Polyphenols and Potential Mechanisms. Antioxidants 2021, 10, 283. [Google Scholar] [CrossRef]
- Gogulescu, A.; Blidisel, A.; Soica, C.; Mioc, A.; Voicu, A.; Jojic, A.; Voicu, M.; Banciu, C. Neurological Side Effects of TNF-α Inhibitors Revisited: A Review of Case Reports. Med. (B Aires) 2024, 60, 1409. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Guo, P.; Wang, P.; Zheng, S.; Qu, Z.; Liu, N. The Review of Anti-Aging Mechanism of Polyphenols on Caenorhabditis Elegans. Front. Bioeng. Biotechnol. 2021, 9, 635768. [Google Scholar] [CrossRef]
- Li, Y.; Chen, F.; Wei, A.; Bi, F.; Zhu, X.; Yin, S.; Lin, W.; Cao, W. Klotho Recovery by Genistein via Promoter Histone Acetylation and DNA Demethylation Mitigates Renal Fibrosis in Mice. J. Mol. Med. 2019, 97, 541–552. [Google Scholar] [CrossRef]
- Sodagam, L.; Lewinska, A.; Kwasniewicz, E.; Kokhanovska, S.; Wnuk, M.; Siems, K.; Rattan, S.I.S. Phytochemicals Rosmarinic Acid, Ampelopsin, and Amorfrutin-A Can Modulate Age-Related Phenotype of Serially Passaged Human Skin Fibroblasts in Vitro. Front. Genet. 2019, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- da Luz, P.L.; Tanaka, L.; Brum, P.C.; Dourado, P.M.M.; Favarato, D.; Krieger, J.E.; Laurindo, F.R.M. Red Wine and Equivalent Oral Pharmacological Doses of Resveratrol Delay Vascular Aging but Do Not Extend Life Span in Rats. Atherosclerosis 2012, 224, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Riordan, S.M.; Heruth, D.P.; Grigoryev, D.N.; Zhang, L.Q.; Ye, S.Q. A Critical Role of Nicotinamide Phosphoribosyltransferase in Human Telomerase Reverse Transcriptase Induction by Resveratrol in Aortic Smooth Muscle Cells. Oncotarget 2015, 6, 10812–10824. [Google Scholar] [CrossRef]
- Pirmoradi, S.; Fathi, E.; Farahzadi, R.; Pilehvar-Soltanahmadi, Y.; Zarghami, N. Curcumin Affects Adipose Tissue-Derived Mesenchymal Stem Cell Aging Through TERT Gene Expression. Drug Res. 2018, 68, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Pereira, Q.C.; dos Santos, T.W.; Fortunato, I.M.; Ribeiro, M.L. The Molecular Mechanism of Polyphenols in the Regulation of Ageing Hallmarks. Int. J. Mol. Sci. 2023, 24, 5508. [Google Scholar] [CrossRef]
- Menicacci, B.; Cipriani, C.; Margheri, F.; Mocali, A.; Giovannelli, L. Modulation of the Senescence-Associated Inflammatory Phenotype in Human Fibroblasts by Olive Phenols. Int. J. Mol. Sci. 2017, 18, 2275. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, X.; Pang, X.; Zhao, Z.; Yu, H.; Zhou, H. Genistein Protects against Ox-LDL-Induced Senescence through Enhancing SIRT1/LKB1/AMPK-Mediated Autophagy Flux in HUVECs. Mol. Cell. Biochem. 2019, 455, 127–134. [Google Scholar] [CrossRef]
- Sharma, R.; Kumar, R.; Sharma, A.; Goel, A.; Padwad, Y. Long-Term Consumption of Green Tea EGCG Enhances Murine Health Span by Mitigating Multiple Aspects of Cellular Senescence in Mitotic and Post-Mitotic Tissues, Gut Dysbiosis, and Immunosenescence. J. Nutr. Biochem. 2022, 107, 109068. [Google Scholar] [CrossRef]
- Matos, L.; Gouveia, A.M.; Almeida, H. Resveratrol Attenuates Copper-Induced Senescence by Improving Cellular Proteostasis. Oxid. Med. Cell. Longev. 2017, 2017, 3793817. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fang, M.; Tu, X.; Mo, X.; Zhang, L.; Yang, B.; Wang, F.; Kim, Y.-B.; Huang, C.; Chen, L.; et al. Dietary Polyphenols as Anti-Aging Agents: Targeting the Hallmarks of Aging. Nutrients 2024, 16, 3305. [Google Scholar] [CrossRef]
- Maldonado, E.; Morales-Pison, S.; Urbina, F.; Solari, A. Aging Hallmarks and the Role of Oxidative Stress. Antioxidants 2023, 12, 651. [Google Scholar] [CrossRef]
- Leyane, T.S.; Jere, S.W.; Houreld, N.N. Oxidative Stress in Ageing and Chronic Degenerative Pathologies: Molecular Mechanisms Involved in Counteracting Oxidative Stress and Chronic Inflammation. Int. J. Mol. Sci. 2022, 23, 7273. [Google Scholar] [CrossRef]
- Scialo, F.; Sanz, A. Coenzyme Q Redox Signalling and Longevity. Free Radic. Biol. Med. 2021, 164, 187–205. [Google Scholar] [CrossRef] [PubMed]
- Brunetta, H.S.; Holwerda, A.M.; van Loon, L.J.C.; Holloway, G.P. Mitochondrial ROS and Aging: Understanding Exercise as a Preventive Tool. J. Sci. Sport Exerc. 2020, 2, 15–24. [Google Scholar] [CrossRef]
- Signorile, A.; De Rasmo, D. Mitochondrial Complex I, a Possible Sensible Site of CAMP Pathway in Aging. Antioxidants 2023, 12, 221. [Google Scholar] [CrossRef]
- van de Ven, R.A.H.; Santos, D.; Haigis, M.C. Mitochondrial Sirtuins and Molecular Mechanisms of Aging. Trends Mol. Med. 2017, 23, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Guan, T.; Shafiq, K.; Yu, Q.; Jiao, X.; Na, D.; Li, M.; Zhang, G.; Kong, J. Mitochondrial Dysfunction in Aging. Ageing Res. Rev. 2023, 88, 101955. [Google Scholar] [CrossRef]
- Yang, J.; Luo, J.; Tian, X.; Zhao, Y.; Li, Y.; Wu, X. Progress in Understanding Oxidative Stress, Aging, and Aging-Related Diseases. Antioxidants 2024, 13, 394. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, H.; Liang, R.; Huang, Y.; Tang, Q. Aging through the Lens of Mitochondrial DNA Mutations and Inheritance Paradoxes. Biogerontology 2025, 26, 33. [Google Scholar] [CrossRef]
- Akbari, M.; Kirkwood, T.B.L.; Bohr, V.A. Mitochondria in the Signaling Pathways That Control Longevity and Health Span. Ageing Res. Rev. 2019, 54, 100940. [Google Scholar] [CrossRef] [PubMed]
- Mas-Bargues, C.; García-Domínguez, E.; Borrás, C. Recent Approaches to Determine Static and Dynamic Redox State-Related Parameters. Antioxidants 2022, 11, 864. [Google Scholar] [CrossRef]
- Rusu, M.E.; Fizeșan, I.; Vlase, L.; Popa, D.-S. Antioxidants in Age-Related Diseases and Anti-Aging Strategies. Antioxidants 2022, 11, 1868. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive Oxygen Species, Toxicity, Oxidative Stress, and Antioxidants: Chronic Diseases and Aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Santos, A.L.; Sinha, S.; Lindner, A.B. The Good, the Bad, and the Ugly of ROS: New Insights on Aging and Aging-Related Diseases from Eukaryotic and Prokaryotic Model Organisms. Oxid. Med. Cell. Longev. 2018, 2018, 1941285. [Google Scholar] [CrossRef]
- Miwa, S.; Kashyap, S.; Chini, E.; von Zglinicki, T. Mitochondrial Dysfunction in Cell Senescence and Aging. J. Clin. Investig. 2022, 132, e158447. [Google Scholar] [CrossRef]
- Prasert, S.; Gavin, S.; Sawaek, W. Oxidative Stress and Inflammation: The Root Causes of Aging. Explor. Med. 2023, 4, 127–156. [Google Scholar] [CrossRef]
- Tatarková, Z.; Kuka, S.; Račay, P.; Lehotský, J.; Dobrota, D.; Mištuna, D.; Kaplán, P. Effects of Aging on Activities of Mitochondrial Electron Transport Chain Complexes and Oxidative Damage in Rat Heart. Physiol. Res. 2011, 60, 281–289. [Google Scholar] [CrossRef]
- Hur, J.H.; Stork, D.A.; Walker, D.W. Complex-I-Ty in Aging. J. Bioenerg. Biomembr. 2014, 46, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, A.; Gogulescu, A.; Șoica, C.; Mioc, A.; Mioc, M.; Milan, A.; Lukinich-Gruia, A.T.; Pricop, M.-A.; Jianu, C.; Banciu, C.; et al. In Vitro and In Silico Evaluation of Syzygium Aromaticum Essential Oil: Effects on Mitochondrial Function and Cytotoxic Potential Against Cancer Cells. Plants 2024, 13, 3443. [Google Scholar] [CrossRef]
- Goetzman, E.; Gong, Z.; Zhang, B.; Muzumdar, R. Complex II Biology in Aging, Health, and Disease. Antioxidants 2023, 12, 1477. [Google Scholar] [CrossRef]
- Bowman, A.; Birch-Machin, M.A. Age-Dependent Decrease of Mitochondrial Complex II Activity in Human Skin Fibroblasts. J. Investig. Dermatol. 2016, 136, 912–919. [Google Scholar] [CrossRef]
- Purhonen, J.; Banerjee, R.; Wanne, V.; Sipari, N.; Mörgelin, M.; Fellman, V.; Kallijärvi, J. Mitochondrial Complex III Deficiency Drives C-MYC Overexpression and Illicit Cell Cycle Entry Leading to Senescence and Segmental Progeria. Nat. Commun. 2023, 14, 2356. [Google Scholar] [CrossRef] [PubMed]
- Hurtado-Villagómez, A.V.; Olmos-Orizaba, E.; Saavedra-Molina, A.; Rodriguez-Orozco, A.R.; Calderon-Cortes, E.; Cortes-Rojo, C. Calorie Restriction Delays the Aging of Saccharomyces Cerevisiae by Improving Complex III Activity via Glutathione Peroxidase 2 (Gpx2). FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Dolcini, J.; Wu, H.; Nwanaji-Enwerem, J.C.; Kiomourtozlogu, M.-A.; Cayir, A.; Sanchez-Guerra, M.; Vokonas, P.; Schwarz, J.; Baccarelli, A.A. Mitochondria and Aging in Older Individuals: An Analysis of DNA Methylation Age Metrics, Leukocyte Telomere Length, and Mitochondrial DNA Copy Number in the VA Normative Aging Study. Aging 2020, 12, 2070–2083. [Google Scholar] [CrossRef]
- Li, C.; White, S.H.; Warren, L.K.; Wohlgemuth, S.E. Skeletal Muscle from Aged American Quarter Horses Shows Impairments in Mitochondrial Biogenesis and Expression of Autophagy Markers. Exp. Gerontol. 2018, 102, 19–27. [Google Scholar] [CrossRef]
- Bao, H.; Cao, J.; Chen, M.; Chen, M.; Chen, W.; Chen, X.; Chen, Y.; Chen, Y.; Chen, Y.; Chen, Z.; et al. Biomarkers of Aging. Sci. China Life Sci. 2023, 66, 893–1066. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Smith, H.J.; Yao, P.; Mair, W.B. Causal Roles of Mitochondrial Dynamics in Longevity and Healthy Aging. EMBO Rep. 2019, 20, e48395. [Google Scholar] [CrossRef]
- Liu, Y.J.; McIntyre, R.L.; Janssens, G.E.; Houtkooper, R.H. Mitochondrial Fission and Fusion: A Dynamic Role in Aging and Potential Target for Age-Related Disease. Mech. Ageing Dev. 2020, 186, 111212. [Google Scholar] [CrossRef]
- Spurlock, B.; Tullet, J.; Hartman, J.L.; Mitra, K. Interplay of Mitochondrial Fission-Fusion with Cell Cycle Regulation: Possible Impacts on Stem Cell and Organismal Aging. Exp. Gerontol. 2020, 135, 110919. [Google Scholar] [CrossRef] [PubMed]
- De Gaetano, A.; Gibellini, L.; Zanini, G.; Nasi, M.; Cossarizza, A.; Pinti, M. Mitophagy and Oxidative Stress: The Role of Aging. Antioxidants 2021, 10, 794. [Google Scholar] [CrossRef]
- Biala, A.K.; Dhingra, R.; Kirshenbaum, L.A. Mitochondrial Dynamics: Orchestrating the Journey to Advanced Age. J. Mol. Cell. Cardiol. 2015, 83, 37–43. [Google Scholar] [CrossRef]
- Faitg, J.; Leduc-Gaudet, J.-P.; Reynaud, O.; Ferland, G.; Gaudreau, P.; Gouspillou, G. Effects of Aging and Caloric Restriction on Fiber Type Composition, Mitochondrial Morphology and Dynamics in Rat Oxidative and Glycolytic Muscles. Front. Physiol. 2019, 10, 420. [Google Scholar] [CrossRef] [PubMed]
- Leduc-Gaudet, J.-P.; Picard, M.; Pelletier, F.S.-J.; Sgarioto, N.; Auger, M.-J.; Vallée, J.; Robitaille, R.; St-Pierre, D.H.; Gouspillou, G. Mitochondrial Morphology Is Altered in Atrophied Skeletal Muscle of Aged Mice. Oncotarget 2015, 6, 17923–17937. [Google Scholar] [CrossRef]
- Zimmermann, A.; Madreiter-Sokolowski, C.; Stryeck, S.; Abdellatif, M. Targeting the Mitochondria-Proteostasis Axis to Delay Aging. Front. Cell Dev. Biol. 2021, 9, 656201. [Google Scholar] [CrossRef]
- Moehle, E.A.; Shen, K.; Dillin, A. Mitochondrial Proteostasis in the Context of Cellular and Organismal Health and Aging. J. Biol. Chem. 2019, 294, 5396–5407. [Google Scholar] [CrossRef]
- Shpilka, T.; Haynes, C.M. The Mitochondrial UPR: Mechanisms, Physiological Functions and Implications in Ageing. Nat. Rev. Mol. Cell Biol. 2018, 19, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Carvajal, F.; Sanhueza, M. The Mitochondrial Unfolded Protein Response: A Hinge Between Healthy and Pathological Aging. Front. Aging Neurosci. 2020, 12, 581849. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Rivero, J.M.; Pastor-Maldonado, C.J.; Romero-González, A.; Gómez-Fernandez, D.; Povea-Cabello, S.; Álvarez-Córdoba, M.; Villalón-García, I.; Talaverón-Rey, M.; Suárez-Carrillo, A.; Munuera-Cabeza, M.; et al. Pterostilbene in Combination with Mitochondrial Cofactors Improve Mitochondrial Function in Cellular Models of Mitochondrial Diseases. Front. Pharmacol. 2022, 13, 862085. [Google Scholar] [CrossRef]
- Rottenberg, H.; Hoek, J.B. The Mitochondrial Permeability Transition: Nexus of Aging, Disease and Longevity. Cells 2021, 10, 79. [Google Scholar] [CrossRef]
- Kanoi, R.; Loachan, P.; Das, S.; Rao, B.S.S. Mangiferin, a Naturally Occurring Polyphenol, Mitigates Oxidative Stress Induced Premature Senescence in Human Dermal Fibroblast Cells. Mol. Biol. Rep. 2021, 48, 457–466. [Google Scholar] [CrossRef]
- Mongelli, A.; Mengozzi, A.; Geiger, M.; Gorica, E.; Mohammed, S.A.; Paneni, F.; Ruschitzka, F.; Costantino, S. Mitochondrial Epigenetics in Aging and Cardiovascular Diseases. Front. Cardiovasc. Med. 2023, 10, 1204483. [Google Scholar] [CrossRef] [PubMed]
- Lima, T.; Li, T.Y.; Mottis, A.; Auwerx, J. Pleiotropic Effects of Mitochondria in Aging. Nat. Aging 2022, 2, 199–213. [Google Scholar] [CrossRef]
- Poljšak, B.; Kovač, V.; Špalj, S.; Milisav, I. The Central Role of the NAD+ Molecule in the Development of Aging and the Prevention of Chronic Age-Related Diseases: Strategies for NAD+ Modulation. Int. J. Mol. Sci. 2023, 24, 2959. [Google Scholar] [CrossRef]
- Grabowska, W.; Sikora, E.; Bielak-Zmijewska, A. Sirtuins, a Promising Target in Slowing down the Ageing Process. Biogerontology 2017, 18, 447–476. [Google Scholar] [CrossRef]
- Ji, Z.; Liu, G.-H.; Qu, J. Mitochondrial Sirtuins, Metabolism, and Aging. J. Genet. Genom. 2022, 49, 287–298. [Google Scholar] [CrossRef]
- Peng, X.; Ni, H.; Kuang, B.; Wang, Z.; Hou, S.; Gu, S.; Gong, N. Sirtuin 3 in Renal Diseases and Aging: From Mechanisms to Potential Therapies. Pharmacol. Res. 2024, 206, 107261. [Google Scholar] [CrossRef]
- Tseng, A.H.H.; Shieh, S.-S.; Wang, D.L. SIRT3 Deacetylates FOXO3 to Protect Mitochondria against Oxidative Damage. Free Radic. Biol. Med. 2013, 63, 222–234. [Google Scholar] [CrossRef]
- He, L.; Liu, Q.; Cheng, J.; Cao, M.; Zhang, S.; Wan, X.; Li, J.; Tu, H. SIRT4 in Ageing. Biogerontology 2023, 24, 347–362. [Google Scholar] [CrossRef]
- Sah, P.; Rai, A.K.; Syiem, D. Sirtuin Activators as an Anti-Aging Intervention for Longevity. Explor. Drug Sci. 2025, 3, 100881. [Google Scholar] [CrossRef]
- Cui, Z.; Zhao, X.; Amevor, F.K.; Du, X.; Wang, Y.; Li, D.; Shu, G.; Tian, Y.; Zhao, X. Therapeutic Application of Quercetin in Aging-Related Diseases: SIRT1 as a Potential Mechanism. Front. Immunol. 2022, 13, 943321. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Cruzat, V.F.; Newsholme, P.; Cheng, J.; Chen, Y.; Lu, Y. Regulation of SIRT1 in Aging: Roles in Mitochondrial Function and Biogenesis. Mech. Ageing Dev. 2016, 155, 10–21. [Google Scholar] [CrossRef]
- Jerome, M.S.; Kuthethur, R.; Kabekkodu, S.P.; Chakrabarty, S. Regulation of Mitochondrial Function by Forkhead Transcription Factors. Biochimie 2022, 198, 96–108. [Google Scholar] [CrossRef]
- Cheng, Z. FoxO Transcription Factors in Mitochondrial Homeostasis. Biochem. J. 2022, 479, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Tia, N.; Singh, A.K.; Pandey, P.; Azad, C.S.; Chaudhary, P.; Gambhir, I.S. Role of Forkhead Box O (FOXO) Transcription Factor in Aging and Diseases. Gene 2018, 648, 97–105. [Google Scholar] [CrossRef]
- Martins, R.; Lithgow, G.J.; Link, W. Long Live FOXO: Unraveling the Role of FOXO Proteins in Aging and Longevity. Aging Cell 2016, 15, 196–207. [Google Scholar] [CrossRef]
- Kitagishi, Y.; Nakano, N.; Ogino, M.; Ichimura, M.; Minami, A.; Matsuda, S. PINK1 Signaling in Mitochondrial Homeostasis and in Aging (Review). Int. J. Mol. Med. 2017, 39, 3–8. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, Y.; Ren, Y.; Wang, W. PINK1/Parkin-Mediated Mitophagy Ameliorates Mitochondrial Dysfunction in Lacrimal Gland Acinar Cells During Aging. Investig. Ophthalmol. Vis. Sci. 2024, 65, 12. [Google Scholar] [CrossRef] [PubMed]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of Metabolism and Mitochondrial Homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Zhou, M.; Chen, C.; Wu, X.; Wang, X. Role of AMPK Mediated Pathways in Autophagy and Aging. Biochimie 2022, 195, 100–113. [Google Scholar] [CrossRef]
- Wu, W.; Mi, Y.; Meng, Q.; Li, N.; Li, W.; Wang, P.; Hou, Y. Natural Polyphenols as Novel Interventions for Aging and Age-Related Diseases: Exploring Efficacy, Mechanisms of Action and Implications for Future Research. Chin. Herb. Med. 2025, 17, 279–291. [Google Scholar] [CrossRef]
- Saenno, R.; Suwannakot, K.; Prajit, R.; Sirichoat, A.; Aranarochana, A.; Sritawan, N.; Pannangrong, W.; Wigmore, P.; Welbat, J.U. Caffeic Acid Attenuates Neuronal Apoptosis, Oxidative Stress, and Memory Deficits via Antioxidant Properties in Aging Rats Induced by D-Galactose. Mol. Neurobiol. 2025, 62, 5143–5155. [Google Scholar] [CrossRef]
- Sheng, X.; Zhu, Y.; Zhou, J.; Yan, L.; Du, G.; Liu, Z.; Chen, H. Antioxidant Effects of Caffeic Acid Lead to Protection of Drosophila Intestinal Stem Cell Aging. Front. Cell Dev. Biol. 2021, 9, 735483. [Google Scholar] [CrossRef] [PubMed]
- Rahimifard, M.; Baeeri, M.; Bahadar, H.; Moini-Nodeh, S.; Khalid, M.; Haghi-Aminjan, H.; Mohammadian, H.; Abdollahi, M. Therapeutic Effects of Gallic Acid in Regulating Senescence and Diabetes; an In Vitro Study. Molecules 2020, 25, 5875. [Google Scholar] [CrossRef]
- Shan, H.; Geng, L.; Jiang, X.; Song, M.; Wang, J.; Liu, Z.; Zhuo, X.; Wu, Z.; Hu, J.; Ji, Z.; et al. Large-Scale Chemical Screen Identifies Gallic Acid as a Geroprotector for Human Stem Cells. Protein Cell 2022, 13, 532–539. [Google Scholar] [CrossRef]
- Oyebode, O.T.; Abolaji, A.O.; Oluwadare, J.O.; Adedara, A.O.; Olorunsogo, O.O. Apigenin Ameliorates D-Galactose-Induced Lifespan Shortening Effects via Antioxidative Activity and Inhibition of Mitochondrial-Dependent Apoptosis in Drosophila Melanogaster. J. Funct. Foods 2020, 69, 103957. [Google Scholar] [CrossRef]
- Wang, H.-H.; Sun, Y.-N.; Qu, T.-Q.; Sang, X.-Q.; Zhou, L.-M.; Li, Y.-X.; Ren, F.-Z. Nobiletin Prevents D-Galactose-Induced C2C12 Cell Aging by Improving Mitochondrial Function. Int. J. Mol. Sci. 2022, 23, 11963. [Google Scholar] [CrossRef]
- Nohara, K.; Mallampalli, V.; Nemkov, T.; Wirianto, M.; Yang, J.; Ye, Y.; Sun, Y.; Han, L.; Esser, K.A.; Mileykovskaya, E.; et al. Nobiletin Fortifies Mitochondrial Respiration in Skeletal Muscle to Promote Healthy Aging against Metabolic Challenge. Nat. Commun. 2019, 10, 3923. [Google Scholar] [CrossRef] [PubMed]
- Savoia, P.; Raina, G.; Camillo, L.; Farruggio, S.; Mary, D.; Veronese, F.; Graziola, F.; Zavattaro, E.; Tiberio, R.; Grossini, E. Anti-Oxidative Effects of 17 β-Estradiol and Genistein in Human Skin Fibroblasts and Keratinocytes. J. Dermatol. Sci. 2018, 92, 62–77. [Google Scholar] [CrossRef]
- Deepika; Maurya, P.K. Health Benefits of Quercetin in Age-Related Diseases. Molecules 2022, 27, 2498. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Liu, Z.; Zhang, H.; Liu, Q.; Weng, Z.; Wang, D.; Guo, W.; Xu, J.; Wang, D.; Jiang, Z.; et al. Bisphenol S Decreased Lifespan and Healthspan via Insulin/IGF-1-like Signaling-against Mitochondrial Stress in Caenorhabditis Elegans. Ecotoxicol. Environ. Saf. 2024, 285, 117136. [Google Scholar] [CrossRef] [PubMed]
- Girsang, E.; Lister, I.N.E.; Ginting, C.N.; Sholihah, I.A.; Raif, M.A.; Kunardi, S.; Million, H.; Widowati, W. Antioxidant and Antiaging Activity of Rutin and Caffeic Acid. Pharmaciana 2020, 10, 147. [Google Scholar] [CrossRef]
- Li, T.; Chen, S.; Feng, T.; Dong, J.; Li, Y.; Li, H. Rutin Protects against Aging-Related Metabolic Dysfunction. Food Funct. 2016, 7, 1147–1154. [Google Scholar] [CrossRef]
- Li, S.; Li, J.; Pan, R.; Cheng, J.; Cui, Q.; Chen, J.; Yuan, Z. Sodium Rutin Extends Lifespan and Health Span in Mice Including Positive Impacts on Liver Health. Br. J. Pharmacol. 2022, 179, 1825–1838. [Google Scholar] [CrossRef]
- Park, S.; Kim, B.-K.; Park, S.-K. Effects of Fisetin, a Plant-Derived Flavonoid, on Response to Oxidative Stress, Aging, and Age-Related Diseases in Caenorhabditis Elegans. Pharmaceuticals 2022, 15, 1528. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Liang, Y.; Li, Y.; Zhao, Y.; Zhang, Y.; Li, Z.; Li, Z.; Wu, Z. Fisetin Delays Postovulatory Oocyte Aging by Regulating Oxidative Stress and Mitochondrial Function through Sirt1 Pathway. Molecules 2023, 28, 5533. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.-Y.; Lee, D.; Ryu, H.G.; Choi, B.-H.; Go, Y.; Lee, N.; Lee, D.; Son, H.G.; Jeon, J.; Kim, S.-H.; et al. Myricetin Improves Endurance Capacity and Mitochondrial Density by Activating SIRT1 and PGC-1α. Sci. Rep. 2017, 7, 6237. [Google Scholar] [CrossRef]
- Wu, X.; Al-Amin, M.; Zhao, C.; An, F.; Wang, Y.; Huang, Q.; Teng, H.; Song, H. Catechinic Acid, a Natural Polyphenol Compound, Extends the Lifespan of Caenorhabditis Elegans via Mitophagy Pathways. Food Funct. 2020, 11, 5621–5634. [Google Scholar] [CrossRef]
- Schmitt, F.; Eckert, G.P. Caenorhabditis Elegans as a Model for the Effects of Phytochemicals on Mitochondria and Aging. Biomolecules 2022, 12, 1550. [Google Scholar] [CrossRef]
- Xiong, L.-G.; Chen, Y.-J.; Tong, J.-W.; Gong, Y.-S.; Huang, J.-A.; Liu, Z.-H. Epigallocatechin-3-Gallate Promotes Healthy Lifespan through Mitohormesis during Early-to-Mid Adulthood in Caenorhabditis Elegans. Redox Biol. 2018, 14, 305–315. [Google Scholar] [CrossRef]
- Yessenkyzy, A.; Saliev, T.; Zhanaliyeva, M.; Masoud, A.-R.; Umbayev, B.; Sergazy, S.; Krivykh, E.; Gulyayev, A.; Nurgozhin, T. Polyphenols as Caloric-Restriction Mimetics and Autophagy Inducers in Aging Research. Nutrients 2020, 12, 1344. [Google Scholar] [CrossRef]
- Wang, R.-J.; Ni, Y.-J.; Liu, Y.-Q. Hesperetin Increases Lifespan and Antioxidant Ability Correlating with IIS, HSP, MtUPR, and JNK Pathways of Chronic Oxidative Stress in Caenorhabditis Elegans. Int. J. Mol. Sci. 2024, 25, 13148. [Google Scholar] [CrossRef]
- Yeh, C.-H.; Shen, Z.-Q.; Wang, T.-W.; Kao, C.-H.; Teng, Y.-C.; Yeh, T.-K.; Lu, C.-K.; Tsai, T.-F. Hesperetin Promotes Longevity and Delays Aging via Activation of Cisd2 in Naturally Aged Mice. J. Biomed. Sci. 2022, 29, 53. [Google Scholar] [CrossRef] [PubMed]
- Testai, L.; Piragine, E.; Piano, I.; Flori, L.; Da Pozzo, E.; Miragliotta, V.; Pirone, A.; Citi, V.; Di Cesare Mannelli, L.; Brogi, S.; et al. The Citrus Flavonoid Naringenin Protects the Myocardium from Ageing-Dependent Dysfunction: Potential Role of SIRT1. Oxid. Med. Cell. Longev. 2020, 2020, 4650207. [Google Scholar] [CrossRef]
- Chen, G.; Zeng, L.; Yan, F.; Liu, J.; Qin, M.; Wang, F.; Zhang, X. Long-Term Oral Administration of Naringenin Counteracts Aging-Related Retinal Degeneration via Regulation of Mitochondrial Dynamics and Autophagy. Front. Pharmacol. 2022, 13, 919905. [Google Scholar] [CrossRef]
- Wang, K.; Chen, Z.; Huang, L.; Meng, B.; Zhou, X.; Wen, X.; Ren, D. Naringenin Reduces Oxidative Stress and Improves Mitochondrial Dysfunction via Activation of the Nrf2/ARE Signaling Pathway in Neurons. Int. J. Mol. Med. 2017, 40, 1582–1590. [Google Scholar] [CrossRef]
- Chen, Y.; Ge, Z.; Huang, S.; Zhou, L.; Zhai, C.; Chen, Y.; Hu, Q.; Cao, W.; Weng, Y.; Li, Y. Delphinidin Attenuates Pathological Cardiac Hypertrophy via the AMPK/NOX/MAPK Signaling Pathway. Aging 2020, 12, 5362–5383. [Google Scholar] [CrossRef]
- Naaz, A.; Zhang, Y.; Faidzinn, N.A.; Yogasundaram, S.; Dorajoo, R.; Alfatah, M. Curcumin Inhibits TORC1 and Prolongs the Lifespan of Cells with Mitochondrial Dysfunction. Cells 2024, 13, 1470. [Google Scholar] [CrossRef]
- Zhen, A.X.; Piao, M.J.; Kang, K.A.; Fernando, P.D.S.M.; Kang, H.K.; Koh, Y.S.; Hyun, J.W. Esculetin Prevents the Induction of Matrix Metalloproteinase-1 by Hydrogen Peroxide in Skin Keratinocytes. J. Cancer Prev. 2019, 24, 123–128. [Google Scholar] [CrossRef]
- Karnewar, S.; Vasamsetti, S.B.; Gopoju, R.; Kanugula, A.K.; Ganji, S.K.; Prabhakar, S.; Rangaraj, N.; Tupperwar, N.; Kumar, J.M.; Kotamraju, S. Mitochondria-Targeted Esculetin Alleviates Mitochondrial Dysfunction by AMPK-Mediated Nitric Oxide and SIRT3 Regulation in Endothelial Cells: Potential Implications in Atherosclerosis. Sci. Rep. 2016, 6, 24108. [Google Scholar] [CrossRef] [PubMed]
- Ryu, D.; Mouchiroud, L.; Andreux, P.A.; Katsyuba, E.; Moullan, N.; Nicolet-dit-Félix, A.A.; Williams, E.G.; Jha, P.; Lo Sasso, G.; Huzard, D.; et al. Urolithin A Induces Mitophagy and Prolongs Lifespan in C. Elegans and Increases Muscle Function in Rodents. Nat. Med. 2016, 22, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Andreux, P.A.; Blanco-Bose, W.; Ryu, D.; Burdet, F.; Ibberson, M.; Aebischer, P.; Auwerx, J.; Singh, A.; Rinsch, C. The Mitophagy Activator Urolithin A Is Safe and Induces a Molecular Signature of Improved Mitochondrial and Cellular Health in Humans. Nat. Metab. 2019, 1, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Chamoli, M.; Rane, A.; Foulger, A.; Chinta, S.J.; Shahmirzadi, A.A.; Kumsta, C.; Nambiar, D.K.; Hall, D.; Holcom, A.; Angeli, S.; et al. A Drug-like Molecule Engages Nuclear Hormone Receptor DAF-12/FXR to Regulate Mitophagy and Extend Lifespan. Nat. Aging 2023, 3, 1529–1543. [Google Scholar] [CrossRef]
- Varghese, N.; Werner, S.; Grimm, A.; Eckert, A. Dietary Mitophagy Enhancer: A Strategy for Healthy Brain Aging? Antioxidants 2020, 9, 932. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Lin, C. Effect of Resveratrol and Pterostilbene on Aging and Longevity. BioFactors 2018, 44, 69–82. [Google Scholar] [CrossRef]
- Gherardi, G.; Corbioli, G.; Ruzza, F.; Rizzuto, R. CoQ10 and Resveratrol Effects to Ameliorate Aged-Related Mitochondrial Dysfunctions. Nutrients 2022, 14, 4326. [Google Scholar] [CrossRef]
- Serino, A.; Salazar, G. Protective Role of Polyphenols against Vascular Inflammation, Aging and Cardiovascular Disease. Nutrients 2018, 11, 53. [Google Scholar] [CrossRef]
- Park, S.-J.; Ahmad, F.; Philp, A.; Baar, K.; Williams, T.; Luo, H.; Ke, H.; Rehmann, H.; Taussig, R.; Brown, A.L.; et al. Resveratrol Ameliorates Aging-Related Metabolic Phenotypes by Inhibiting CAMP Phosphodiesterases. Cell 2012, 148, 421–433. [Google Scholar] [CrossRef]
- Smoliga, J.; Blanchard, O. Enhancing the Delivery of Resveratrol in Humans: If Low Bioavailability Is the Problem, What Is the Solution? Molecules 2014, 19, 17154–17172. [Google Scholar] [CrossRef] [PubMed]
- Smoliga, J.M.; Colombo, E.S.; Campen, M.J. A Healthier Approach to Clinical Trials Evaluating Resveratrol for Primary Prevention of Age-Related Diseases in Healthy Populations. Aging 2013, 5, 495–506. [Google Scholar] [CrossRef]
- Semba, R.D.; Ferrucci, L.; Bartali, B.; Urpí-Sarda, M.; Zamora-Ros, R.; Sun, K.; Cherubini, A.; Bandinelli, S.; Andres-Lacueva, C. Resveratrol Levels and All-Cause Mortality in Older Community-Dwelling Adults. JAMA Intern. Med. 2014, 174, 1077. [Google Scholar] [CrossRef]
- McCormack, D.; McFadden, D. A Review of Pterostilbene Antioxidant Activity and Disease Modification. Oxid. Med. Cell. Longev. 2013, 2013, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Le, T.; Nakahara, Y.; Ueda, M.; Okumura, K.; Hirai, J.; Sato, Y.; Takemoto, D.; Tomimori, N.; Ono, Y.; Nakai, M.; et al. Sesamin Suppresses Aging Phenotypes in Adult Muscular and Nervous Systems and Intestines in a Drosophila Senescence-Accelerated Model. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1826–1839. [Google Scholar] [CrossRef]
- Shimoyoshi, S.; Takemoto, D.; Ono, Y.; Kitagawa, Y.; Shibata, H.; Tomono, S.; Unno, K.; Wakabayashi, K. Sesame Lignans Suppress Age-Related Cognitive Decline in Senescence-Accelerated Mice. Nutrients 2019, 11, 1582. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, N.; Saifi, A.; Chaudhary, A.; Tripathi, P.N.; Chaudhary, A.; Sharma, A. Multifaceted Neuroprotective Role of Punicalagin: A Review. Neurochem. Res. 2024, 49, 1427–1436. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Chen, F.; Lei, J.; Zhou, B. Pomegranate Polyphenol Punicalagin Improves Learning Memory Deficits, Redox Homeostasis, and Neuroinflammation in Aging Mice. Phytother. Res. 2023, 37, 3655–3674. [Google Scholar] [CrossRef]
- Clementi, M.E.; Maulucci, G.; Bianchetti, G.; Pizzoferrato, M.; Sampaolese, B.; Tringali, G. Cytoprotective Effects of Punicalagin on Hydrogen–Peroxide–Mediated Oxidative Stress and Mitochondrial Dysfunction in Retinal Pigment Epithelium Cells. Antioxidants 2021, 10, 192. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Yan, Y.; Jiang, Y.; Meng, X. Ellagic Acid and Its Anti-Aging Effects on Central Nervous System. Int. J. Mol. Sci. 2022, 23, 10937. [Google Scholar] [CrossRef] [PubMed]
- Hseu, Y.-C.; Chou, C.-W.; Senthil Kumar, K.J.; Fu, K.-T.; Wang, H.-M.; Hsu, L.-S.; Kuo, Y.-H.; Wu, C.-R.; Chen, S.-C.; Yang, H.-L. Ellagic Acid Protects Human Keratinocyte (HaCaT) Cells against UVA-Induced Oxidative Stress and Apoptosis through the Upregulation of the HO-1 and Nrf-2 Antioxidant Genes. Food Chem. Toxicol. 2012, 50, 1245–1255. [Google Scholar] [CrossRef]
- Alverina, C. Procyanidin and Its Benefits on Aging: A Literature Review. Int. J. Med. Sci. Clin. Res. Stud. 2022, 2, 762–769. [Google Scholar] [CrossRef]
- Reutzel, M.; Grewal, R.; Silaidos, C.; Zotzel, J.; Marx, S.; Tretzel, J.; Eckert, G.P. Effects of Long-Term Treatment with a Blend of Highly Purified Olive Secoiridoids on Cognition and Brain ATP Levels in Aged NMRI Mice. Oxid. Med. Cell. Longev. 2018, 2018, 4070935. [Google Scholar] [CrossRef]
- Chen, Y.; Onken, B.; Chen, H.; Zhang, X.; Driscoll, M.; Cao, Y.; Huang, Q. Healthy Lifespan Extension Mediated by Oenothein B Isolated from Eucalyptus Grandis × Eucalyptus Urophylla GL9 in Caenorhabditis Elegans. Food Funct. 2020, 11, 2439–2450. [Google Scholar] [CrossRef]
- Chen, Y.; Onken, B.; Chen, H.; Xiao, S.; Liu, X.; Driscoll, M.; Cao, Y.; Huang, Q. Mechanism of Longevity Extension of Caenorhabditis Elegans Induced by Pentagalloyl Glucose Isolated from Eucalyptus Leaves. J. Agric. Food Chem. 2014, 62, 3422–3431. [Google Scholar] [CrossRef]
- Asseburg, H.; Schäfer, C.; Müller, M.; Hagl, S.; Pohland, M.; Berressem, D.; Borchiellini, M.; Plank, C.; Eckert, G.P. Effects of Grape Skin Extract on Age-Related Mitochondrial Dysfunction, Memory and Life Span in C57BL/6J Mice. Neuromolecular Med. 2016, 18, 378–395. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, G.; Zhang, X.; Xu, D.; Gao, J.; Fan, J.; Zhou, Z. Anthocyanins from Black Chokeberry (Aroniamelanocarpa Elliot) Delayed Aging-Related Degenerative Changes of Brain. J. Agric. Food Chem. 2017, 65, 5973–5984. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Drouet, S.; Hano, C. Flavonoids from Sacred Lotus Stamen Extract Slows Chronological Aging in Yeast Model by Reducing Oxidative Stress and Maintaining Cellular Metabolism. Cells 2022, 11, 599. [Google Scholar] [CrossRef] [PubMed]
- Amigoni, L.; Stuknytė, M.; Ciaramelli, C.; Magoni, C.; Bruni, I.; De Noni, I.; Airoldi, C.; Regonesi, M.E.; Palmioli, A. Green Coffee Extract Enhances Oxidative Stress Resistance and Delays Aging in Caenorhabditis Elegans. J. Funct. Foods 2017, 33, 297–306. [Google Scholar] [CrossRef]
- Dilberger, B.; Passon, M.; Asseburg, H.; Silaidos, C.V.; Schmitt, F.; Schmiedl, T.; Schieber, A.; Eckert, G.P. Polyphenols and Metabolites Enhance Survival in Rodents and Nematodes—Impact of Mitochondria. Nutrients 2019, 11, 1886. [Google Scholar] [CrossRef]
- Ayyadurai, V.; Deonikar, P. Attenuation of Aging-Related Oxidative Stress Pathways by Phytonutrients: A Computational Systems Biology Analysis. Nutrients 2023, 15, 3762. [Google Scholar] [CrossRef] [PubMed]
- Popescu, I.; Deelen, J.; Illario, M.; Adams, J. Challenges in Anti-aging Medicine–Trends in Biomarker Discovery and Therapeutic Interventions for a Healthy Lifespan. J. Cell. Mol. Med. 2023, 27, 2643–2650. [Google Scholar] [CrossRef]
- Lang, Y.; Gao, N.; Zang, Z.; Meng, X.; Lin, Y.; Yang, S.; Yang, Y.; Jin, Z.; Li, B. Classification and Antioxidant Assays of Polyphenols: A Review. J. Future Foods 2024, 4, 193–204. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Polyphenols as Antioxidant/Pro-Oxidant Compounds and Donors of Reducing Species: Relationship with Human Antioxidant Metabolism. Processes 2023, 11, 2771. [Google Scholar] [CrossRef]
- Rolt, A.; Cox, L.S. Structural Basis of the Anti-Ageing Effects of Polyphenolics: Mitigation of Oxidative Stress. BMC Chem. 2020, 14, 50. [Google Scholar] [CrossRef]
- Santos, S.C.; Fortes, G.A.C.; Camargo, L.T.F.M.; Camargo, A.J.; Ferri, P.H. Antioxidant Effects of Polyphenolic Compounds and Structure-Activity Relationship Predicted by Multivariate Regression Tree. LWT 2021, 137, 110366. [Google Scholar] [CrossRef]
- Cao, D.; Wang, M.; Qiu, X.; Liu, D.; Jiang, H.; Yang, N.; Xu, R.-M. Structural Basis for Allosteric, Substrate-Dependent Stimulation of SIRT1 Activity by Resveratrol. Genes Dev. 2015, 29, 1316–1325. [Google Scholar] [CrossRef]
- You, W.; Zheng, W.; Weiss, S.; Chua, K.F.; Steegborn, C. Structural Basis for the Activation and Inhibition of Sirtuin 6 by Quercetin and Its Derivatives. Sci. Rep. 2019, 9, 19176. [Google Scholar] [CrossRef] [PubMed]
- Naoi, M.; Wu, Y.; Shamoto-Nagai, M.; Maruyama, W. Mitochondria in Neuroprotection by Phytochemicals: Bioactive Polyphenols Modulate Mitochondrial Apoptosis System, Function and Structure. Int. J. Mol. Sci. 2019, 20, 2451. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Sun, Z.; Shen, Q.; Huang, Z.; Wang, S.; Yang, N.; Li, G.; Wu, Q.; Wang, W.; Li, L.; et al. Rational Design of Nanocarriers for Mitochondria-Targeted Drug Delivery. Chin. Chem. Lett. 2022, 33, 4146–4156. [Google Scholar] [CrossRef]
- Guan, Y.; Li, L.; Yang, R.; Lu, Y.; Tang, J. Targeting Mitochondria with Natural Polyphenols for Treating Neurodegenerative Diseases: A Comprehensive Scoping Review from Oxidative Stress Perspective. J. Transl. Med. 2025, 23, 572. [Google Scholar] [CrossRef]
- Oprean, C.; Zambori, C.; Borcan, F.; Soica, C.; Zupko, I.; Minorics, R.; Bojin, F.; Ambrus, R.; Muntean, D.; Danciu, C.; et al. Anti-Proliferative and Antibacterial in Vitro Evaluation of the Polyurethane Nanostructures Incorporating Pentacyclic Triterpenes. Pharm. Biol. 2016, 54, 2714–2722. [Google Scholar] [CrossRef]
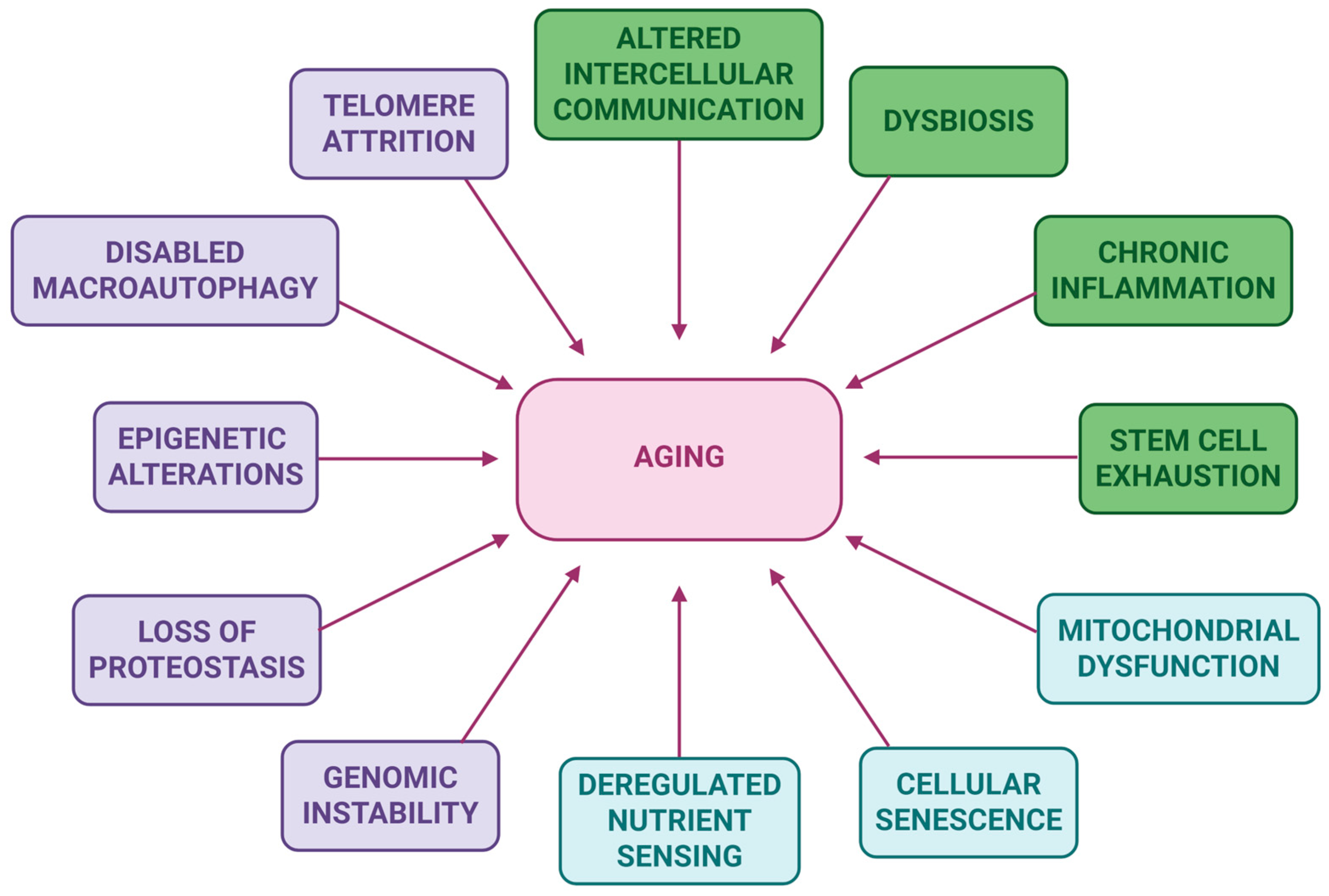
| Polyphenolic Compound(s) | Sample Type | Mechanism of Action | Anti-Aging Effects | Reference |
|---|---|---|---|---|
| Epigenetic alterations | ||||
| Genistein | Mouse fibrotic kidneys | ↓ histone 3 deacetylation of Klotho ↓ DNMT1, DNMT3a | ↓ renal fibrosis, a typical renal aging feature | [27] |
| Rosmarinic acid | Human Skin Fibroblasts | ↑ 5-mC in late passage cells | Aging-modulatory effects | [28] |
| Telomere attrition | ||||
| Resveratrol, red wine | Male Wistar rats | ↑ telomere length and activity | Delayed vascular aging, no lifespan effect | [29] |
| Resveratrol | Human aortic smooth muscle cells | Telomerase activation via NAMPT-SIRT4 | Potential anti-aging effect on aortic smooth muscle cells | [30] |
| Rosmarinic acid | Serially passaged Human Skin Fibroblasts | ↓ rate of telomeres loss | Aging-modulatory effects | [28] |
| Curcumin | Mesenchymal stem cells derived from adipose tissue | ↑ telomerase reverse transcriptase expression | Improved lifespan of cells | [31] |
| Stem cell exhaustion | ||||
| Oleuropein | Human bone marrow mesenchymal stem cell progenitors | ↑ osteoblastogenesis ↓ adipogenesis | Slowing skeletal aging | [32] |
| Curcumin | Mesenchymal stem cells derived from adipose tissue | Proliferation of stem cells | ↓ cell aging | [31] |
| Cellular senescence | ||||
| Oleuropein | Human lung cells Neonatal human dermal fibroblasts | ↓ number of prosenescent cells | Potential beneficial effects on aging | [33] |
| Oleuropein | Human embryonic fibroblast IMR90 | Delayed senescence morphology development | Prolonged lifespan | [32] |
| Genistein | Human umbilical vein endothelial cells | Inhibited senescence ↓ p16, p21, SA-β-gal | Potential aging-delaying effects | [34] |
| Altered intercellular communication | ||||
| Resveratrol | Aged mice | ↓ TNF-α and IL-1ß | ↓ of age-related proinflammatory pattern | [32] |
| Epigalocathecin | Male Swiss albino mice | ↓ TNF-α and IL-1ß | Enhanced lifespan and healthspan | [35] |
| Deregulated nutrient sensing | ||||
| Catechins | Aged rats’ hippocampus | ↑ SIRT 1 | Improved cognitive abilities | [32] |
| Epigalocathecin | Male Swiss albino mice | ↓AMPK, AKT ↑ SIRT 3,5 | Enhanced lifespan and healthspan | [35] |
| Loss of proteostasis | ||||
| Resveratrol | CuSO4-SIPS WI-38 fibroblasts | Improved proteostasis | Possible prevention of age-associated cellular dysfunction | [36] |
| Dysbiosis | ||||
| Epigalocathecin | Male Swiss albino mice | Preserved microbial diversity ↓ pathogenic/opportunistic pathogenic species | Enhanced lifespan and healthspan | [35] |
| Quercetin | Mice with pulmonary fibrosis | Improved intestinal flora imbalance | Delayed the aging process of alveolar epithelial cells | [37] |
| Chronic inflammation | ||||
| Pomegranate | Aged mice | ↓ IL-6, IL-1β, IL-18, TNF-α, | Improved memory and learning deficits | [37] |
| Polyphenolic Compounds | Origin | Sample Type | Mechanism of Action | Anti-Aging Effects | Ref. |
|---|---|---|---|---|---|
| Oleuropein, oleurosid, and hydroxytyrosol | Olive oil | NMRI mice | Increased the age-declined level of ATP in brain cells | Improved spatial working memory | [147] |
| Oenothein B and pentagalloyl glucose | Eucalyptus polyphenols | C. elegans | Regulated the ETC by influencing the ETC encoding gene, isp-1 | Extended the lifespan | [26,148,149] |
| Catechin, epicatechin, proanthocyanidins, and trans-resveratrol | Grape skin extract | C57BL/6J mice aged brain | Increased moderately the ATP levels Partly improved mitochondrial respiration | Shift in survival curve toward higher survival rates | [150] |
| Anthocyanins | Aronia melanocarpa | Aged mice | Increased the level of SOD and glutathione peroxidase (GPx) | Inhibited age-related cognitive decline | [151] |
| Myricetin, quercetin, kaempferol, and isorhamnerin glycosides | Nelumbo nucifera’s stamen extract | Saccharomyces cerevisiae | Upregulates SIRT and SOD → reduced oxidative stress Preserved MMP, contributing to the maintenance of mitochondrial functions | Delayed chronological aging | [152] |
| Hydroxicinammic acid derivates (5-O-caffeoylquinic and 5-O-feruloylquinic acids) | Green coffee extract | C.elegans. | Prevented oxidative stress | Delayed aging Lifespan prolongation | [153] |
| 21 compounds (protocatechuic acid) | Pre-fermented polyphenol mixture | Mice, C.elegans | Increased the activity of CI, II, and IV Increased MMP and ATP concentration | Increased the median lifespan in both species | [154] |
| Rutin, ellagic acid, kaempherol, cyanidin, malvidin, and delphinidin | Fruit/berry/vegetable juice powder | Reduced ROS Increased SOD, catalase, GPx, and heme oxygenase-1 levels | Attenuates aging | [155] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maksimović, T.; Gădău, C.; Antal, G.; Čoban, M.; Eșanu, O.; Atyim, E.; Mioc, A.; Șoica, C. Polyphenol-Based Therapeutic Strategies for Mitochondrial Dysfunction in Aging. Biomolecules 2025, 15, 1116. https://doi.org/10.3390/biom15081116
Maksimović T, Gădău C, Antal G, Čoban M, Eșanu O, Atyim E, Mioc A, Șoica C. Polyphenol-Based Therapeutic Strategies for Mitochondrial Dysfunction in Aging. Biomolecules. 2025; 15(8):1116. https://doi.org/10.3390/biom15081116
Chicago/Turabian StyleMaksimović, Tamara, Carmen Gădău, Gabriela Antal, Mihaela Čoban, Oana Eșanu, Elisabeta Atyim, Alexandra Mioc, and Codruța Șoica. 2025. "Polyphenol-Based Therapeutic Strategies for Mitochondrial Dysfunction in Aging" Biomolecules 15, no. 8: 1116. https://doi.org/10.3390/biom15081116
APA StyleMaksimović, T., Gădău, C., Antal, G., Čoban, M., Eșanu, O., Atyim, E., Mioc, A., & Șoica, C. (2025). Polyphenol-Based Therapeutic Strategies for Mitochondrial Dysfunction in Aging. Biomolecules, 15(8), 1116. https://doi.org/10.3390/biom15081116






